Abstract
Legionella longbeachae is almost as frequent a cause of legionellosis in Australia as Legionella pneumophila, but epidemiological investigation of possible environmental sources and clinical cases has been limited by the lack of a discriminatory subtyping method. The purpose of this study was to examine the genetic variability among Australian isolates of L. longbeachae serogroup 1. Pulsed-field gel electrophoresis (PFGE) of SfiI fragments revealed three distinct pulsotypes among 57 clinical and 11 environmental isolates and the ATCC control strains of L. longbeachae serogroups 1 and 2. Each pulsotype differed by four bands, corresponding to <65% similarity. A clonal subgroup within each pulsotype was characterized by >88% similarity. The largest major cluster was pulsotype A, which included 43 clinical isolates and 9 environmental isolates and was divided into five subgroups. Pulsotypes B and C comprised smaller numbers of clinical and environmental isolates, which could each be further divided into three subgroups. The ATCC type strain of L. longbeachae serogroup 1 was classified as pulsotype B, subtype B3, while the ATCC type strain of L. longbeachae serogroup 2 was identified as a different pulsotype, LL2. SfiI macrorestriction analysis followed by PFGE showed that the Australian L. longbeachae strains are not a single clonal population as previously reported.
Legionellae are environmental organisms that can cause disease in humans (2). Clinical manifestations of legionella infection range from no symptoms to potentially fatal pneumonia and multisystem disease. There are 42 species in the genus Legionella (5), more than half of which have been implicated in human disease (2).
Transmission of the bacteria from the environment to humans occurs via inhalation or aspiration of Legionella-containing aerosols (6, 8). A suspected cluster or outbreak of cases of legionellosis requires careful epidemiological investigation to identify possible sources of infection. Such investigations also require a sensitive and discriminatory subtyping technique to identify similarities and differences between possibly related strains (3).
Legionella longbeachae is an uncommon pathogen in most parts of the world (17) but causes up to half the cases of legionellosis in many regions in Australia (1, 11). The reason for this is not clear. It has commonly been isolated from soil and decomposing materials, such as bark or sawdust used in potting mixes (33), and has been detected occasionally in water (29). L. longbeachae has caused at least two outbreaks of legionellosis in Australia, one in Western Australia (7) and the other in South Australia (19). Studies of Australian clinical strains of L. longbeachae by multienzyme electrophoresis (19), ribotyping (19), and random amplified polymorphic DNA (RAPD) typing (9) have suggested that L. longbeachae serogroup 1 strains are largely clonal. This similarity between strains has thwarted attempts to develop a discriminatory subtyping method, which would be useful to link environmental isolates to cases of clinical disease.
Legionella pneumophila serogroup 1 causes up to 95% of the cases of legionellosis worldwide and most outbreaks and sporadic cases in Australia (1, 11). For this reason, it has been the focus of most subtyping techniques, including typing with different panels of monoclonal antibodies (20), plasmid analysis (10, 26), multienzyme or alloenzyme electrophoresis (18), restriction fragment length polymorphism (15, 16), ribotyping (18), arbitrary primed PCR (13, 14), RAPD typing (30), and macrorestriction enzyme digestion followed by pulsed-field gel electrophoresis (PFGE) (28, 31). At present, PFGE, following restriction digestion with the enzymes SfiI or NotI, is the most discriminatory method. This technique has also been used to subtype epidemiologically linked strains of L. pneumophila serogroup 6 (24), Legionella bozemanii (22), and Legionella micdadei (21) but was not used previously to type isolates of L. longbeachae.
In this study we used macrorestriction enzyme digestion followed by PFGE to investigate the genetic variability of clinical and environmental isolates of L. longbeachae serogroup 1 from five states in Australia over a period of 10 years.
MATERIALS AND METHODS
Bacterial isolates.
The 68 isolates of L. longbeachae serogroup 1 investigated in this study included 24 clinical isolates from New South Wales, 16 clinical and 5 environmental isolates from Queensland, 1 clinical isolate from Tasmania, 4 clinical isolates from South Australia, and 12 clinical and 6 environmental isolates from Western Australia (Table 1). One environmental isolate which had been identified as L. longbeachae serogroup 2 from Western Australia was also tested. Controls were selected from the American Type Culture Collection (ATCC): L. pneumophila serogroup 1 (Philadelphia 1; ATCC 33152), L. longbeachae serogroup 1 (Long Beach 4; ATCC 33264), and L. longbeachae serogroup 2 (Tucker 1; ATCC 33484).
TABLE 1.
Clinical and environmental isolates of L. pneumophila and L. longbeachae used in this studya
| Species and ICPMR reference no. | Country or state of origin | Source | Yr of isolation | Pulsotype | Estimated genome size (kb) |
|---|---|---|---|---|---|
| Legionella pneumophila subsp. pneumophila Brenner ATCC 33152* (type strain, Philadelphia 1) | USA | Human lung | 1979 | Not assigned | 3,629 |
| Legionella longbeachae McKinney | |||||
| ATCC 33462* (type strain, Long Beach 4) | USA | Human lung | 1981 | B3 | 3,436 |
| ATCC 33484* (type strain, Tucker 1, Georgia) | USA | Human lung | 1981 | LL2 | 3,514 |
| 147, 150, 287*, 288, 273, 460 | NSW | Clinical | 1992–1998 | A1 | 3,569 |
| 149, 158, 280, 283, 284 | NSW | Clinical | 1990–1992 | A2 | |
| 144, 272*, 275 | NSW | Clinical | 1992 | A3 | 3,514 |
| 143 | NSW | Clinical | 1993 | B1 | |
| 459* | NSW | Sputum | 1998 | B2 | 4,041 |
| 159, 279, 281*, 285, 286 | NSW | Clinical | 1992–1993 | C2 | 3,495 |
| 142*, 274, 282 | NSW | Clinical | 1992–1994 | C3 | 3,674 |
| 151, 157, 160, 163, 164, 302, 303*, 354 | QLD | Clinical | 1988–1992 | A1 | 3,429 |
| 155 | QLD | Environmental | 1992 | A2 | |
| 156, 165 | QLD | Clinical | 1992 | A2 | |
| 153* | QLD | Environmental | 1992 | A3 | 3,723 |
| 162, 304, 305, 306, 356 | QLD | Clinical | 1989–1995 | A4 | |
| 141, 154 | QLD | Environmental | 1992 | A4 | |
| 161* | QLD | Clinical | 1992 | A5 | 3,673 |
| 152* | QLD | Environmental | 1992 | B1 | 3,297 |
| 260 | TAS | Clinical | 1993 | A1 | |
| 389 | SA | Clinical | 1988 | A3 | |
| 387, 401* | SA | Clinical | 1983 | B1 | 3,297 |
| 390* | SA | Clinical | 1992 | C3 | 3,655 |
| 361, 362, 363, 364, 365, 370, 371, 374, 375 | WA | Clinical | 1992–1993 | A1 | |
| 367, 368, 376 | WA | Potting mix | 1992–1994 | A1 | |
| 377* | WA | Potting mix | 1994 | A2 | 3,531 |
| 378 | WA | Sputum | 1994 | A3 | |
| 366* | WA | BAL | 1992 | A4 | 3,436 |
| 359* | WA | Potting mix | 1992 | A4 | 3,436 |
| 373* | WA | Potting mix | 1993 | C1 | 3,468 |
| 379* | WA | Sputum | 1994 | C1 | 3,591 |
| 369* (atypical strain) | WA | Potting mix | 1993 | Not assigned | 2,421 |
BAL, bronchoalveolar lavage; NSW, New South Wales; SA, South Australia; QLD, Queensland; TAS, Tasmania; WA, Western Australia; ICPMR, Institute of Clinical Pathology and Medical Research, Westmead Hospital, Westmead, NSW. All L. longbeachae isolates were serogroup 1 except ATCC 33484 and 369 (atypical strain), which were serogroup 2. The asterisks denote isolates for which an estimated genome size is given in the right-hand column.
Bacterial cultures were grown for 48 h on buffered charcoal yeast extract agar with alpha ketoglutarate (BCYEα agar; Oxoid, Ltd., Basingstoke, Hampshire, England) and incubated in a humidified atmosphere with 5% CO2 at 35°C (31).
Identification of isolates.
A rapid latex test (Serobact; Disposable Products, Adelaide, South Australia) was used for presumptive identification of the isolates as L. longbeachae serogroup 1. These results were confirmed by direct immunofluorescence with a panel of pooled monovalent Legionella antibodies (MarDx Diagnostics, Scotch Plains, N.J.) and a monoclonal antibody to L. pneumophila groups 1 to 14 (Genetic Systems, Seattle, Wash.) according to the manufacturers’ recommendations. Direct immunofluorescence with eight species- or serogroup-specific monovalent antibodies, including L. longbeachae serogroups 1 and 2 (MarDx Diagnostics), was also performed according the manufacturer’s protocol. Isolate identification was confirmed as L. longbeachae with a positive reaction to reagents L. species b to j and L. omni species b to p and a negative reaction to both L. pneumophila reagents 1 to 6 and L. pneumophila 1 to 14, in addition to a positive reaction to L. longbeachae-specific monovalent antibody.
Preparation of PFGE plugs.
PFGE plugs were prepared according to a modified version of the methods of Smith and Cantor (32) and Gautom (12). Briefly, bacterial cells were harvested into approximately 3 ml of Pett IV buffer (1.0 M NaCl, 10 mM Tris-HCl [pH 7.6]) and the bacterial suspensions were adjusted to exactly 20% transmittance (equivalent to 3 × 1010 organisms/ml) with a calibrated bacterial nephelometer (Vitek colorimeter; Hach Company, Loveland, Colo.). After centrifugation, a 400-μl aliquot of the bacterial suspension was concentrated to half its volume and mixed with an equal volume of molten 2.4% low-melting-point agarose (Bio-Rad, Hercules, Calif.) in Pett IV buffer and dispensed into a disposable plug mold (Bio-Rad). The final concentration of the bacterial DNA in the plug was 10 μg (1 μg of DNA/plug slice). The usually recommended preliminary RNase and lysozyme digestion step at 37°C (4, 23, 31) was omitted, as it was found that this step did not affect digestion with the enzyme SfiI. The bacterial plugs were incubated in 2-ml Eppendorf tubes containing 1.5 ml of ESP solution (0.5 M EDTA [pH 8.0], 1.0% N-lauryl sarcosine, 2 mg of proteinase K/ml) and incubated overnight at 55°C.
PFGE plug digestion and electrophoresis.
Prior to digestion, the plugs were incubated in a solution of 2 ml of 10 mM Tris–0.1 M EDTA and 1.0 mM phenylmethylsulfonyl fluoride (pH 7.5) (Sigma-Aldrich, St. Louis, Mo.) for 1 h at room temperature, washed in 1× TE (10 mM Tris, 0.1 mM EDTA [pH 7.5]), and then stored in 1× TE at 4°C until required. For restriction enzyme digestion, plug slices were digested overnight at 50°C for SfiI or 37°C for NotI in a 50-μl reaction mixture which contained 2.5 U of SfiI or NotI restriction enzyme/ml of buffer (New England Biolabs, Beverly, Mass.). L. longbeachae serogroup 1 (ATCC 33462) and L. pneumophila serogroup 1 (ATCC 33152) were used as internal controls and digested in parallel with the test organisms. These were included as controls in every gel along with at least three lanes of Saccharomyces cerevisiae chromosomes (catalog no. 345; Promega, Madison, Wis.) as fragment size standards. The fragments were electrophoretically separated by PFGE with a contour-clamped homogeneous electric field system (Bio-Rad Chef Mapper) in 1% PFGE grade agarose (Bio-Rad) and 0.5× TBE running buffer (45 mM Tris, 45 mM boric acid, 1.0 mM EDTA [pH 8.0]). The initial pulse time of 3.51 s was increased linearly to a final switch time of 93.56 s over 24 h at 6 V/cm at 14°C. The gels were then stained with 0.5 g of ethidium bromide/ml for 10 min, destained in water, and photographed under UV transillumination.
Evaluation of reproducibility of the PFGE results.
Electrophoretic bands for the PFGE restriction fragments were sized and compared with the software program GelCompar version 4.1 (Applied Maths, Kortrijk, Belgium). Computer comparison was based on the algorithm of the unweighted pair group method for arithmetic averages and the Dice coefficient (25) with 3.2% band tolerance. Band tolerance statistics were calculated on the basis of differences in band positions of a list of identical internal control patterns with the GelCompar program. The lowest band tolerance required to have identical isolates typed as identical by the GelCompar program was 3.2%, and this value was applied to the entire band-matching comparison. No other computer-enhanced optimization or smoothing was used.
RESULTS
Analysis of PFGE typing.
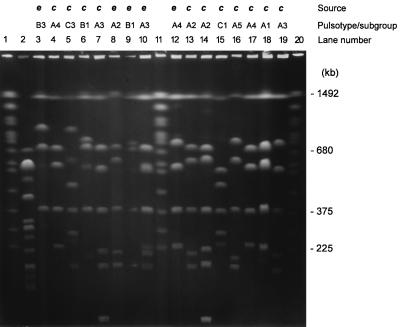
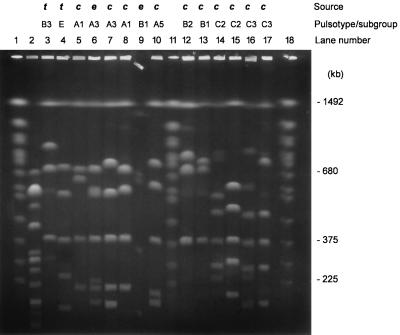
Preliminary results showed that digestion with NotI produced too few (three to four) restriction fragments to allow discrimination between strains of L. longbeachae (results not shown). Further analyses were confined to PFGE with SfiI. The number of SfiI fragments varied from four to seven, which ranged in size from approximately 400 to 1,500 kb (Fig. 1, 2, and 3). Interpolation of the known chromosome sizes of S. cerevisiae gave a standard reference curve for the comparison of sample fragment sizes. The genome sizes of the L. longbeachae isolates were calculated by adding the sizes of individual fragments for each strain. They ranged from 3,300 to 4,300 kb (Table 1). A fragment of 1,493 kb was common to all L. longbeachae serogroup 1 isolates and both serogroups 1 and 2 ATCC strains but was absent from the one environmental isolate that had been identified as L. longbeachae serogroup 2 and from the L. pneumophila control strain. The next most common fragments of the L. longbeachae isolates were 389 (94% of isolates) and 701 kb (79% of isolates).
FIG. 1.
PFGE of SfiI-cleaved DNAs from L. longbeachae serogroup 1 isolates from Australia. Lanes: 2, L. pneumophila serogroup 1 (ATCC 33152); 3, L. longbeachae serogroup 1 (ATCC 33462); 4, 141; 5, 142; 6, 143; 7, 144; 8, 149; 9, 152; 10, 153; 12, 154; 13, 155; 14, 158; 15, 159; 16, 161; 17, 162; 18, 163; 19, 272. Lanes 1, 11, and 20 contained S. cerevisiae chromosomes as a molecular size standard. e, environmental isolate; c, clinical isolate. The letters A to C and the numbers 1 to 5 indicate pulsotypes and subgroups, respectively.
FIG. 2.
PFGE of SfiI-cleaved DNAs from L. longbeachae serogroup 1 isolates from Australia. Lanes: 2, L. pneumophila serogroup 1 (ATCC 33152); 3, L. longbeachae serogroup 1 (ATCC 33462); 4, L. longbeachae serogroup 2 (ATCC 33484); 5, 287; 6, 153; 7, 272; 8, 303; 9, 152; 10, 161; 12, 459; 13, 401; 14, 159; 15, 379; 16, 142; 17, 390. Lanes 1, 11, and 18 contained S. cerevisiae chromosomes as a molecular size standard. t, type strains; e, environmental isolate; c, clinical isolate. The letters A to C and the numbers 1 to 5 indicate pulsotypes and subgroups, respectively. The DNA block in lane 9 moved from its original position on the comb prior to the gel being run.
FIG. 3.
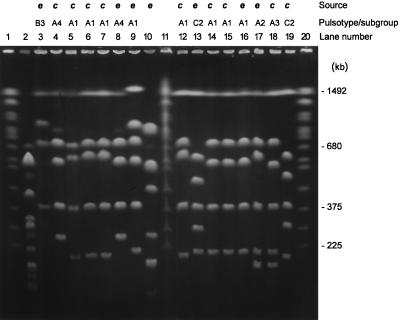
PFGE of SfiI-cleaved DNAs from L. longbeachae serogroup 1 isolates from Western Australia. Lanes: 2, L. pneumophila serogroup 1 (ATCC 33152); 3, L. longbeachae serogroup 1 (ATCC 33462); 4, 359; 5, 361; 6, 364; 7, 365; 8, 366; 9, 368; 10, 369; 12, 371; 13, 373; 14, 374; 15, 375; 16, 376; 17, 377; 18, 378. Lane 19, isolate 369, was typed by the MIDI system as an atypical L. longbeachae strain. Lanes 1, 11, and 20 contained S. cerevisiae chromosomes as a molecular size standard. e, environmental isolate; c, clinical isolate. The letters A to C and the numbers 1 to 4 indicate pulsotypes and subgroups, respectively.
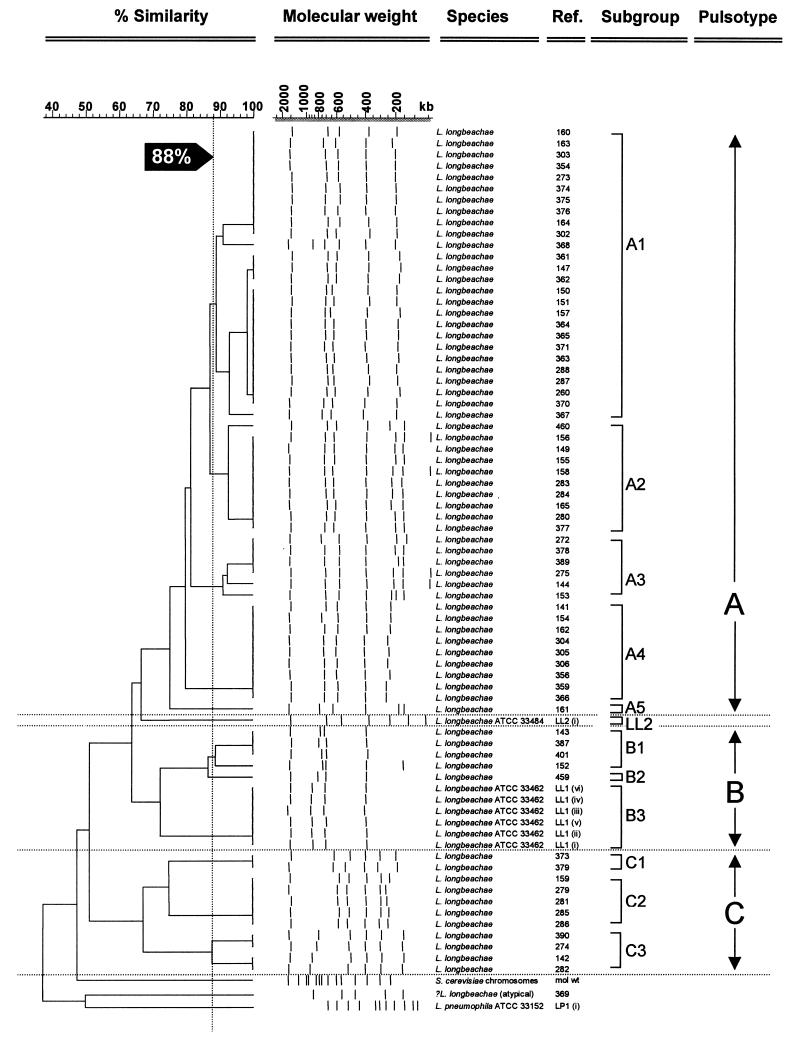
The two L. longbeachae ATCC serogroup 1 and 2 control strains showed patterns that were distinguishable from each other, with the former being similar to the pulsotypes obtained from the Australian L. longbeachae serogroup 1 strains. The type strains of L. longbeachae serogroups 1 and 2 (ATCC 33462 and 33484) showed a similarity of 64%, using the Dice coefficient. L. longbeachae and L. pneumophila serogroup 1 (Philadelphia 1) were <40% similar, and the Australian L. longbeachae serogroup 1 strains showed a similarity of 52%. When the Australian L. longbeachae serogroup 1 isolates were considered together, there were three distinct patterns, resulting in three dendrogram clades that could be separated by a four-band difference and <65% similarity with the Dice coefficient (Fig. 4). The percentage of similarity between different pulsotypes varied from 52 to 65%. Type A was the commonest pattern, with 52 of 68 isolates, and was divided into five subgroups, A1 to A5, which differed in one or two bands. The number of fragments shared between subgroups within a pulsotype varied from five to seven fragments. Within each of these subgroups, the fragment patterns were >88% similar by the Dice coefficient and could be clearly distinguished from each other on a PFGE gel.
FIG. 4.
Cluster dendrogram of Australian L. longbeachae isolates generated from SfiI restriction fragments separated by PFGE and analyzed with the program GelCompar ver 4.1. Similarity of >65% and more than a four-band difference in the band pattern divide the different types and clades. Subtype divisions have >88% similarity and band pattern differences of two or three bands. The type strain for L. longbeachae serogroup 1 was designated pulsotype B3, and the type strain for L. long-beachae serogroup 2 was designated pulsotype LL2. Isolates LL1 (i to vi) are representative internal controls of ATCC 33462, which were run on separate PFGE gels.
Most pulsotype A subgroups were found over periods of several years, and some were widely distributed geographically (Fig. 2 and Table 1). Type A1 was represented by 27 isolates from four states (Table 1). It was isolated repeatedly from two patients (isolates 361 to 365 and 370 to 371), and there were single isolates from individual patients presented over a period of at least 10 years, which suggests that the PFGE patterns are genetically stable. Types A2, A3, and A4 were represented by nine, six, and nine isolates, respectively, and there was one clinical isolate in pulsotype A5. The designation LL2 was given to the type strain of L. longbeachae serogroup 2, which showed 65% similarity to the pulsotype A clade of the Australian clinical L. longbeachae serogroup 1 isolates.
Six L. longbeachae isolates were designated pulsotype B. Four of the six isolates, which were geographically widespread, were designated pulsotype B1. There was only a single representative of pulsotype B2. Pulsotype B was most similar (74% similarity) to the L. longbeachae ATCC serogroup 1 control strain, which was designated pulsotype B3.
Eleven L. longbeachae isolates designated pulsotype C were divided into three subgroups: pulsotype C1 was found only in Western Australia (two isolates), pulsotype C2 was found only in New South Wales (five isolates), and pulsotype C3 was found in South Australia (one isolate) and in New South Wales (three isolates).
The Western Australian isolates included 12 clinical isolates from six patients and 6 environmental isolates (Fig. 3), which belonged to pulsotypes A and C. Five isolates from patient 1 (361 to 365), two from patient 3 (370 and 371), two isolates from patient 4 (374 and 375), and two potting mix isolates (368 and 376) showed pattern A1. Pulsotype A4 was found in one patient isolate (366; patient 2) and from a potting mix sample (359). Pulsotype C1 (379) was isolated from one patient (patient 6) and was also represented among potting mix isolates. Thus, all four subtypes isolated from Western Australian patients were also found among the environmental isolates over periods of 1 to 3 years.
One Western Australian environmental isolate (369) did not fit any of the three pulsotypes (Fig. 3 and 4), and its genome size was smaller (2,421 kb). None of the major fragments was present in any other isolate investigated. The 1,493-kb band, common to all other L. longbeachae isolates, was absent. Initial investigation of this isolate by routine methods with direct and indirect immunofluorescence indicated that it was L. longbeachae but was only weakly reactive with serogroup 2 antiserum. PFGE also showed this strain to be very different (38% similarity by the Dice coefficient) (Fig. 4) from other L. longbeachae isolates but more similar to L. pneumophila. Further tests to confirm its identification were undertaken. It failed to react with both L. pneumophila monovalent serum pools and reacted weakly with monovalent immunofluorescence pooled sera. When this isolate was tested with the MIDI bacterial identification system (Sherlock MIS; MIDI Inc., Newark, Del.) with bacterial fatty acid analysis and compared to a commercial database, it was identified as L. longbeachae, but based on its similarity index (0.437) it was an atypical strain.
DISCUSSION
The purpose of our study was to examine the genetic variability of Australian L. longbeachae serogroup 1 isolates and develop a practicable method for subtyping. Using macrorestriction digestion with SfiI followed by PFGE, we demonstrated three distinct patterns (dendrogram clades) that could be separated by four-band differences and <65% similarity with the Dice coefficient (Fig. 1) among 68 clinical and environmental isolates. The three major L. longbeachae serogroup 1 pulsotypes were subdivided into 11 subgroups, most of which were widely distributed geographically throughout Australia and over significant periods.
These results are in contrast to those of previous studies, in which alloenzyme electrophoresis, ribotyping, and RAPD analyses failed to distinguish among Australian strains of L. longbeachae serogroup 1 and showed only minor differences among strains of L. longbeachae serogroup 2 (9, 19). The results of previous studies have been interpreted as indicating widespread distribution of a single clone of L. longbeachae in Australia (9, 19).
However, by adding the sizes of fragments obtained after digestion (Table 1), we estimated that the genome sizes of different strains varied from 3,300 to 4,300 kb. This is similar to the degree of variation in genome size among strains of L. pneumophila serogroup 1, which has been reported to range from 2,600 to 3,900 kb (31). A previous study had also reported the genome size of L. pneumophila Philadelphia 1 as 3,900 kb (4). This suggests that L. longbeachae is more variable than was previously believed.
This apparent variability is unlikely to be due to incomplete lysis of bacteria or digestion of DNA, since consistent results were obtained on repeat testing up to five times. The largest (1,493-kb) fragment was present in all L. longbeachae serogroup 1 strains and both L. longbeachae ATCC control strains, and the number of sizes of fragments did not vary when lower concentrations of DNA were used (data not shown). Moreover, the L. pneumophila PFGE internal control strain, which was processed in the same way as L. longbeachae isolates, gave the same number and size of fragments as previously described (31).
The appearance of the same pulsotypes and subgroups in different parts of Australia over a period of 10 years may be explained by a low mutation rate of L. longbeachae strains (19). Alternatively, it could be due to the widespread distribution of a common vehicle, such as potting mix, with persistence of L. longbeachae in the environment or in unused potting mix. It has been shown that L. longbeachae is able to survive in potting mix for up to 7 months (33).
Ideally, for PFGE, a restriction enzyme should be chosen that will generate at least 10 fragments per isolate (34). The enzyme NotI generated too few fragments to produce a useful profile, and SfiI generated only four to seven fragments from L. longbeachae DNA. However, in defining their criteria for the use of PFGE for bacterial subtyping, Tenover et al. (34) conceded that modification of the criteria may be necessary for defining pulsotypes among large numbers of isolates over extended periods. We suggest that modification of the criteria is justified to extend the use of SfiI for PFGE to L. longbeachae as well as L. pneumophila (31), L. micdadei (21), and L. bozemanii (22), for which its use has been described. PFGE typing of L. bozemanii with SfiI produced a similar number of fragments (four to eight), and pulsotypes were defined by criteria similar to those used in our study, namely, four or more band differences and <65% band similarity by the Dice coefficient (22).
The routine use of PFGE as a typing method is often limited by the fact that it is time-consuming and labor intensive (12). The standard procedure (23) was reduced to 3 days by using a turbidity standard rather than an optical density reading to estimate bacterial numbers for agarose plugs, and the omission of the lysozyme digestion step eliminated an overnight incubation. Although interpretation of subtyping data is most useful when multiple techniques are used (31), no other sufficiently discriminatory method for subtyping L. longbeachae has been described. However, it is likely that the use of other restriction enzymes, which cleave at different sites within the genome, could provide complementary patterns for subtyping.
SfiI digestion followed by PFGE showed that the Australian L. longbeachae strains are not a single clonal population. The results of this study contribute to an understanding of the distribution of L. longbeachae serogroup 1 strains in Australia. A computer database with a number of mainly unrelated environmental and clinical isolates from five Australian states was established and can now be used in, and supplemented by, investigations of future cases and outbreaks. The PFGE method developed is discriminatory, could be applied to other Legionella species, and is sufficiently rapid to allow a timely investigation of a potential outbreak of legionellosis.
ACKNOWLEDGMENTS
We thank the following individuals and institutions who provided the samples of L. longbeachae used in this study: Jan Lanser and Norma Sangster, Infectious Disease Laboratories, Institute of Medical and Veterinary Science, Adelaide, South Australia; Alistair McGregor and Rob Peterson, Department of Microbiology, Royal Hobart Hospital, Hobart, Tasmania; Bruce Grey, Public Health Microbiology, Queensland Health Scientific Services, Cooper’s Plains, Queensland; Todd Gorsuch, Department of Microbiology, Concord Hospital, Concord, New South Wales; Thomas Riley, Department of Microbiology, Queen Elizabeth II Medical Centre, Nedlands, Western Australia; Graeme Nimmo and Jacqueline Schooneueldt, Microbiology Department, Princess Alexandra Hospital, Brisbane, Queensland; Michelle Worthington, Clinical Pathology Department, South Western Area Pathology Service, Liverpool, New South Wales; and Robert Chiew and Leanne Hicks, Department of Clinical Microbiology, ICPMR, Westmead Hospital, Westmead, New South Wales.
The work presented here was undertaken in the Department of Clinical Microbiology and Infectious Diseases, Institute of Clinical Pathology and Medical Research, with funds provided by the Westmead Research Foundation, the Sydney University Post-Graduate Student support fund, and NH&MRC Dora Lush Biomedical Scholarship 977453 to J.M.-P.
REFERENCES
- 1.Anonymous. Legionellosis. Commun Dis Intell. 1997;21:137. [Google Scholar]
- 2.Bangsborg J M. Antigenic and genetic characterization of Legionella proteins: contributions to taxonomy, diagnosis and pathogenesis. APMIS Suppl. 1997;70:1–53. [PubMed] [Google Scholar]
- 3.Barbaree J M. Selecting a subtyping technique for use in investigations of legionellosis epidemics. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 169–172. [Google Scholar]
- 4.Bender L, Ott M, Marre R, Hacker J. Genome analysis of Legionella ssp. by orthogonal field alternation gel electrophoresis (OFAGE) FEMS Microbiol Lett. 1990;60:253–257. doi: 10.1016/0378-1097(90)90313-f. [DOI] [PubMed] [Google Scholar]
- 5.Benson R F, Fields B S. Classification of the genus Legionella. Semin Respir Infect. 1998;13:90–99. [PubMed] [Google Scholar]
- 6.Blatt S P, Parkinson M D, Pace E, Hoffman P, Dolan D, Lauderdale P, Zajac R A, Melcher G P. Nosocomial Legionnaires’ disease: aspiration as a primary mode of disease acquisition. Am J Med. 1993;95:16–22. doi: 10.1016/0002-9343(93)90227-g. [DOI] [PubMed] [Google Scholar]
- 7.Brennan R. A review of notified cases of legionellosis in Western Australia, 1994. Commun Dis Intell. 1995;19:514–517. [Google Scholar]
- 8.Broome C V. Epidemiologic assessment of methods of transmission of legionellosis. Zentbl Bakteriol Mikrobiol Hyg A. 1983;255:52–57. [PubMed] [Google Scholar]
- 9.Bull J, Nimmo G. Genetic diversity and clonal population structure of L. longbeachae serogroup 1 in Australia. Microbiol Aust. 1997;18:A120. [Google Scholar]
- 10.Castellani Pastoris M, Mingrone M G, Passi C. Plasmid profiles of Legionella spp. isolates, Italy. Eur J Epidemiol. 1987;3:261–264. doi: 10.1007/BF00149734. [DOI] [PubMed] [Google Scholar]
- 11.Doyle R M, Steele T W, McLennan A M, Parkinson I H, Manning P A, Heuzenroeder M W. Sequence analysis of the mip gene of the soilborne pathogen Legionella longbeachae. Infect Immun. 1998;66:1492–1499. doi: 10.1128/iai.66.4.1492-1499.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautom R K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Lus P, Fields B S, Benson R F, Martin W T, O’Connor S P, Black C M. Comparison of arbitrarily primed polymerase chain reaction, ribotyping, and monoclonal antibody analysis for subtyping Legionella pneumophila serogroup 1. J Clin Microbiol. 1993;31:1940–1942. doi: 10.1128/jcm.31.7.1940-1942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grattard F, Berthelot P, Reyrolle M, Ros A, Etienne J, Pozzetto B. Molecular typing of nosocomial strains of Legionella pneumophila by arbitrarily primed PCR. J Clin Microbiol. 1996;34:1595–1598. doi: 10.1128/jcm.34.6.1595-1598.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haertl R, Bandlow G. Subtyping of Legionella pneumophila serogroup 1 isolates by small-fragment restriction endonuclease analysis. Eur J Clin Microbiol Infect Dis. 1991;10:630–635. doi: 10.1007/BF01975814. [DOI] [PubMed] [Google Scholar]
- 16.Harrison T G, Saunders N A, Haththotuwa A, Doshi N, Taylor A G. Typing of Legionella pneumophila serogroups 2-14 strains by analysis of restriction fragment length polymorphisms. Lett Appl Microbiol. 1990;11:189–192. doi: 10.1111/j.1472-765x.1990.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 17.Lang R, Wiler Z, Manor J, Kazak R, Boldur I. Legionella longbeachae pneumonia in a patient splenectomized for hairy-cell leukemia. Infection. 1990;18:31–32. doi: 10.1007/BF01644179. [DOI] [PubMed] [Google Scholar]
- 18.Lanser J, Adams M, Doyle R, Hewitt P, Sangster N. Genetic characterization of Legionella pneumophila serogroup 1 associated with respiratory disease in Australia. Appl Environ Microbiol. 1992;58:706–708. doi: 10.1128/aem.58.2.706-708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanser J A, Adams M, Doyle R, Sangster N, Steele T W. Genetic relatedness of Legionella longbeachae isolates from human and environmental sources in Australia. Appl Environ Microbiol. 1990;56:2784–2790. doi: 10.1128/aem.56.9.2784-2790.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luck P C, Birtles R J, Helbig J H. Correlation of MAb subgroups with genotype in closely related Legionella pneumophila serogroup 1 strains from a cooling tower. J Med Microbiol. 1995;43:50–54. doi: 10.1099/00222615-43-1-50. [DOI] [PubMed] [Google Scholar]
- 21.Luck P C, Helbig J H, Drasar V, Bornstein N, Fallon R J, Castellani-Pastoris M. Genomic heterogenicity amongst phenotypically similar Legionella micdadei strains. FEMS Microbiol Lett. 1995;126:49–54. doi: 10.1111/j.1574-6968.1995.tb07389.x. [DOI] [PubMed] [Google Scholar]
- 22.Luck P C, Helbig J H, Hagedorn H J, Ehret W. DNA fingerprinting by pulsed-field gel electrophoresis to investigate a nosocomial pneumonia caused by Legionella bozemanii serogroup 1. Appl Environ Microbiol. 1995;61:2759–2761. doi: 10.1128/aem.61.7.2759-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maslow J N, Slutsky A M, Arbeit R. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F, White T, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 24.Mitchell D H, Hicks L J, Chiew R, Montanaro J C, Chen S C. Pseudoepidemic of Legionella pneumophila serogroup 6 associated with contaminated bronchoscopes. J Hosp Infect. 1997;37:19–23. doi: 10.1016/s0195-6701(97)90069-4. [DOI] [PubMed] [Google Scholar]
- 25.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolte F S, Conlin C A, Roisin A J, Redmond S R. Plasmids as epidemiological markers in nosocomial Legionnaires’ disease. J Infect Dis. 1984;149:251–256. doi: 10.1093/infdis/149.2.251. [DOI] [PubMed] [Google Scholar]
- 27.Pruckler J M, Mermel L A, Benson R F, Giorgio C, Cassiday P K, Breiman R F, Whitney C G, Fields B S. Comparison of Legionella pneumophila isolates by arbitrarily primed PCR and pulsed-field gel electrophoresis: analysis from seven epidemic investigations. J Clin Microbiol. 1995;33:2872–2875. doi: 10.1128/jcm.33.11.2872-2875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riffard S, Lo Presti F, Vandenesch F, Forey F, Reyrolle M, Etienne J. Comparative analysis of infrequent-restriction-site PCR and pulsed-field gel electrophoresis for epidemiological typing of Legionella pneumophila serogroup 1 strains. J Clin Microbiol. 1998;36:161–167. doi: 10.1128/jcm.36.1.161-167.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint C P. A colony based confirmation assay for Legionella and Legionella pneumophila employing the EnviroAmp Legionella system and seroagglutination. Lett Appl Microbiol. 1998;26:377–381. doi: 10.1046/j.1472-765x.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandery M, Coble J, McKersie-Donnolley S. Random amplified polymorphic DNA (RAPD) profiling of Legionella pneumophila. Lett Appl Microbiol. 1994;19:184–187. doi: 10.1111/j.1472-765x.1994.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoonmaker D, Heimberger T, Birkhead G. Comparison of ribotyping and restriction enzyme analysis using pulsed-field gel electrophoresis for distinguishing Legionella pneumophila isolates obtained during a nosocomial outbreak. J Clin Microbiol. 1992;30:1491–1498. doi: 10.1128/jcm.30.6.1491-1498.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith C L, Cantor C R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- 33.Steele T W, Moore C V, Sangster N. Distribution of Legionella longbeachae serogroup 1 and other Legionella in potting soils in Australia. Appl Environ Microbiol. 1990;56:2984–2988. doi: 10.1128/aem.56.10.2984-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]