Abstract
Background
Following traumatic bone loss or removal of bone tumors, the failure of bone allograft transplantation for large bone defect repair remains a significant problem in orthopedics. Therefore, new strategies that can efficiently enhance allograft healing and long-term incorporation are critically needed.
Method
In this study, we first injected Notch-activating Jagged1 peptide to mice and then isolated bone marrow tissues and cells for proliferation and differentiation assays. Femur bone allograft surgery was also performed in Jagged1 pre-treated mice, and bone defect healing process were monitored by histology, Micro-CT and biomechanical testing.
Result
Our results showed that Jagged1 therapeutic injection is sufficient to maximally activate Notch and promote bone marrow stromal cell proliferation in vivo, while no effects on bone structure were observed. More importantly, Jagged1 pre-treatment significantly promoted bone callus formation and increased bone mechanical strength during allograft healing in a femur bone defect mouse model.
Conclusion
This study reveals that Notch in vivo activation can be induced by injection of Jagged1 peptide for expansion of local native stromal cells that will significantly enhance bone callus formation.
The Translational potential of this Article
The clinical uses of this therapeutic strategy would be immediately applicable for chronic long bone defect repair. More importantly, this devised strategy for expansion of endogenous BMSCs can also be applied to enhance other tissue and organ repair.
Keywords: Jagged1, Notch signaling, Stromal cells, Bone allograft
1. Introduction
Following traumatic bone loss or removal of bone tumors, the failure of critical-sized bone defects to self-repair remains a significant problem in orthopedics. In the United States, over 3 million graft surgeries are performed each year using autologous grafts, allografts, and bone substitutes to restitute non-healing bone defects in the diverse fields of orthopedic, neurocranial, plastic, and oral surgery [1]. Outstanding experimental and clinical research has demonstrated the superiority of autologous grafts to processed allografts [2]. However, the limited size and availability of autologous bone grafts, in addition to the chronic pain at the donor site [3,4], has hindered wider use of processed allografts in bone grafting surgery. Nearly 33% of all bone grafts used in North America are now allografts [5]. The reduced formation of new bone in allografts, reflecting lower angiogenesis and osteogenesis, may contribute to an unacceptably high, 60% 10-year post-implantation failure rate [6,7]. Therefore, new strategies that can efficiently enhance allograft healing and long-term incorporation are still critically needed.
As the abundance of mesenchymal stromal cells (MSCs) within the injuryrepair site is critical for the success of new bone formation, delivery of MSCs to injury sites has, for many years, been experimentally used in bone tissue engineering [8]. Although this approach has shown promising results, several limitations remain. First, harvesting of a patient's own stromal cells is often associated with donor site morbidity and pain and requires additional patient hospitalizations [9]. Second, in vitro stromal cell purification and expansion has been expensive and time-consuming with several associated safety concerns, including mutagenesis and infection. Third, many adult tissues (e.g., bone and muscle) have stromal cells which exist in their native ‘niche’ in a quiescent state which need to be activated by injury or inflammatory conditions for tissue repair [10]. Therefore, it may be possible to circumvent cell harvesting and amplification steps by developing strategies which can mobilize and expand native endogenous stromal cells to repair injured tissues. Stromal cell-derived factor 1 (SDF-1) has been shown to stimulate migration of MSCs in vivo, but has limited effects on MSC proliferation [11]. Although the growth factor bone morphogenetic protein 2 (BMP2) has been clinically used to enhance MSC differentiation toward bone-forming cells during spine fusion surgery, several adverse effects still remain including ectopic bone formation, life-threatening inflammatory edema, and carcinogenesis when high doses of BMP2 have been used [12]. BMP2 is also extremely costly; therefore, development of an alternative bioactive agent(s) which can reduce or replace BMP2 or be used in combination with BMP2, could be very valuable in clinical bone repair.
We recently identified the Notch pathway as a significant molecular regulator of MSC maintenance and proliferation. In particular, we demonstrated that Notch loss-of-function led to enhanced limb bud MSC chondrogenic and osteogenic differentiation, whereas sustained Notch activation induced MSC proliferation and the arrest of cell differentiation [13]. Furthermore, we discovered that the Notch signaling ligand Jagged1 (JAG1) is an important inducer of bone marrow derived MSC (BMSC) proliferation [14], and that JAG1-mediated Notch activation significantly inhibits cellular senescence in BMSC cultures [15]. These previous studies demonstrate that Notch signaling is a “master regulator” that controls the MSC pool in the skeletal system which could be clinically exploited for development of therapeutic treatments. The goal of our current study was to investigate whether Notch-activating Jagged1 peptide induces endogenous bone marrow stromal cell expansion and if Notch-expanded stromal cells enhance bone defect repair.
2. Materials and methods
2.1. Animal study
Twelve-week old C57BL/6J mice from Jackson Laboratory (Bar Harbor, ME) were used in this study. All mouse experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of LSU Health - Shreveport. Soluble Notch signaling JAG1 peptide (CDDYYYGFGCNKFCRPR) or control scrambled peptide (RCGPDCFDNYGRYKYCF) (New England Peptides, Gardner, MA) were intraperitoneally injected into mice daily at a dose of 25 μg/g for up to 14 days. Bromodeoxyuridine/5-bromo-2′-deoxyuridine (BrdU) labeling solution (100 mg/kg BrdU in sterile PBS) was intraperitoneally injected 12 h prior to tissue collection to evaluate MSC proliferation in vivo. Femurs from each group of mice (n = 8) were harvested on days 0, 3, 5, 7, and 14 for total bone marrow cells isolation and histological analysis. BMSCs were further isolated and seeded for Colony Fibroblastic Unit Forming (CFU–F), proliferation and osteogenic differentiation assays (see below for details).
For femur bone allograft surgeries, 7-day JAG1 injected C57BL/6J mice (n = 8) were anesthetized with 2% isoflurane and the back of the right leg shaved prior to aseptic surgeries. A 7 mm long incision was made, and the mid-shaft of the femur exposed by blunt muscle dissection. A 4 mm long bone segment within the mid-shaft of femur was removed using a Dremel 545 diamond-tipped cutting tool. This section of bone was replaced with a processed 4 mm long bone allograft, which was stabilized using a 26-gauge metal pin fixed within the intramedullary marrow cavity as previously described [16]. The incision was then closed with sutures. This group of mice were euthanized at 8 weeks post-surgery and femur samples were harvested for histology, biomechanical testing, and Micro-CT.
2.2. Bone marrow cells isolation and culture
BMSCs were harvested from femurs and tibia as previously described [16]. Briefly, femurs and tibia were dissected. Bone marrow was collected by flushing with Dulbecco's Modified Eagle media (DMEM) with a 25 gauge needle. A single cell suspension was obtained by filtering the collected bone marrow through a 70 μm strainer. Obtained bone marrow cells were then incubated in α-Minimum Essential Medium containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin for CFU-F assay. Unattached cells were washed out with PBS after 72-h culture. Attached cells were sub-cultured on day 5 as passage 1 (P1) for expansion. Passage 2 (P2) cells were used for subsequent in vitro assays. To activate Notch signaling in cultured cells, JAG1 peptide-coated plates were generated using the protocol previously described [15]. Briefly, culture plates (Nunc, polystyrene) were incubated in a solution containing JAG1 peptides (10 mg/ml) at 4 °C overnight before drying for cell culture. The same concentrations of scrambled peptides were used to coat the plates as the controls. All P2 BMSCs were cultured on JAG1- or control peptide-coated plates for 24 h with or without the Notch gamma secretase inhibitor N-(3,5-difluorophenylacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT; 1 μM; Calbiochem) treatments prior to for RNA extraction.
2.3. Flow cytometry analysis
P2 BMSC “stemness” was evaluated by flow cytometry. Cells were incubated with FITC conjugated-CD29, -Sca-1, and -CD45; APC conjugated-CD34 and -CD105, and PE-conjugated-CD44 for 30 min, and then analyzed by LSR-II SORP flow cytometry (BD Biosciences, San Diego, CA) using DIVA 8.02 software (BD Biosciences).
2.4. Cell proliferation and differentiation assays
BMSC proliferation was determined by using a cell counting kit 8 (CCK-8 kit, Abcam, Cambridge, MA) according to the manufacturer's instructions. Briefly, BMSCs at a concentration of 2 × 103 cells/100 μl were seeded into 96-well plates. Cell proliferation was assayed at 24, 48, and 72 h after seeding. The plates were read at OD 450 nm using a Microplate Reader (Biotek, Winooski, VT, USA). For osteogenic differentiation, cells were grown in 12 well/plates and incubated with osteogenic induction medium: DMEM supplemented with 10%FBS, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone. Osteogenic differentiation was determined by alkaline phosphatase (ALP) and Alizarin Red staining, as previously described [17].
2.5. Histology and immunohistochemistry (IHC) staining
Femurs were fixed in 10% normal buffered formalin (NBF), decalcified with 10% ethylene diaminetetraacetic acid (EDTA, pH 7.4), dehydrated and embedded in paraffin. Bone tissue sections (5 μm) were used for histology and immunohistochemistry (IHC) analysis. H&E staining was used to evaluate bone structure and marrow cell density. The bone volume fraction vs. total tissue volume (BV/TV) was determined as the number of pixels representing the bone area divided by the number of pixels representing the combined bone and marrow area. Sections were stained with Notch signaling pathway target Hes1 as described [15], using anti-Hes1 antibody (A-12, sc-166378) from Santa Cruz (Santa Cruz, CA). Bone marrow cell proliferation was monitored by BrdU staining using antibody (sc-32323) from Santa Cruz. Stained slides were examined under EVOS™ Core Imaging System (Thermo Fisher Scientific, Waltham, MA).
2.6. Quantitative RT-PCR and western blot
Total RNA was isolated from total bone marrow cells and BMSCs using RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RT-PCR was performed on ABI 7500 Real-time PCR System (Life Technologies, Grand Island, NY, USA) using primers for JAG1, Hes1, Hey1, Notch1, Col1a1, and Runx2. All the primers were purchased from Integrated DNA Technologies (IDT, Austin, TX) and primer sequences are available upon request. β-actin expression was determined and used as the housekeeping gene. Quantification of the relative mRNA expression of the target genes was achieved by normalizing to β-actin using the 2−ΔΔCt method. For western blot, mouse femurs were first harvested on day 3, 5, 7, 14 after injection of JAG1 and control peptide daily, then the total protein was extracted from bone marrow cells using lysed in RIPA buffer [10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 0.1% sodium deoxycholate, 1 mM EDTA, 1% NP-40, complete protease inhibitors (Roche, Indianapolis, IN), phosphatase inhibitor cocktail 1 and 2 (Sigma–Aldrich)]. Protein expression of Hes1 in cells was detected by SDS-PAGE, transfer to PVDF membranes (Bio-Rad, Hercules, CA), incubation with Hes1 antibody from Abcam (Cambridge, MA, USA), Collagen type I and Runx2 antibody from Novus Biologicals (Centennial, CO, USA) and signal detection followed the standard procedures using ECL detection (Amersham Biosciences, Piscataway, NJ). Anti-β-actin antibody (Sigma) was used as a control for equal protein loading. The quantitative analysis of the immunoblot data was performed using the ImageJ 1.40g software.
2.7. Micro-computed tomography (Micro-CT) bone imaging analyses
Micro-CT image analysis was performed at 8 weeks (n = 6) after allograft implantation. Briefly, after subject anesthesia, reconstructed femurs were scanned by a Micro-CT system (Viva CT 40, Scanco Medical) with high-resolution (10.5 microns) X-ray energy settings of 55 kVp and 145 mA. Quantification of bone volume and total volume of calls were evaluated as previously described using the Scanco analysis software [18].
2.8. Biomechanical torsion testing
Micro-CT scanned femur bone samples were harvested and fixed in aluminum square tubes (0.5 cm) filled with bone cement to ensure that fracture lines were in the middle of the interval. Specimens were then mounted on an EnduraTec Test Bench™ system (Bose Corp., Minnetonka, MN) with a 200 N mm torque cell and tested for torsion at a rate of 1°/s until failure to determine the bone strength by ultimate torque in torsion tests.
2.9. Statistical analysis
Data are presented as mean ± standard error (SE) and analyzed by Analysis of Variance or student t-tests by computer software Prism (Version 7, GraphPad). A probability level <0.05 was set as statistically significant.
3. Results
3.1. JAG1 injection induces notch signaling in bone marrow
JAG1 is a cell surface ligand that interacts with Notch receptors to activate downstream signaling. Our previous study demonstrated that JAG1-induced Notch activation and could promote MSC proliferation in cultures [14]. To determine if JAG1 injection could be used to stimulate Notch activation in vivo, soluble JAG1 peptide and control scrambled peptide were intraperitoneally administered to C57BL/6 mice daily for up to 14 days. Mice were sacrificed on days 0, 3, 5, 7, and 14, and total bone marrow cells were collected for RNA and protein extraction. As seen in our RT-PCR data, the Notch target gene Hes1 expression significantly increased between day 3–7 compared to day 0 (before the 1st injection) but Hes1 levels dropped significantly at day 14, compared to peak expression at day 7 (Fig. 1A). Consistent with Hes1, the other two Notch signaling related molecules Hey1 (Fig. 1B) and Notch1 (Fig. 1C) were also highly expressed at day 7, and expressed in low level at day 14. More importantly, our western blot data (Fig. 1D and E) further confirmed that the Hes1 protein expression level was gradually increased from day 3 to day 7 with decreased expression at day 14 in JAG1-injected mice, while the control scrambled peptide had no effect on the Hes1 expression suggesting that the short-term 7-day injection of JAG1 is an effective approach to activate Notch signaling in vivo. Based on this, we then decided to limit our JAG1 injection to 7 days for other experiments going forward.
Fig. 1.
JAG1 injection induces Notch activation in vivo. (A) qRT-PCR analysis shows that Notch target gene Hes1 significantly increased in total bone marrow cells isolated from mice before and after JAG1 injection. (B) qRT-PCR analysis shows that Notch target gene Hey1 is highly expressed at day 7 in bone marrow cells from JAG1 injected mice. (C) qRT-PCR analysis shows that Notch receptor Notch1 is highly expressed at day 7 in bone marrow cells isolated from JAG1 injected mice. (D) Immunoblot analyses of the Notch target Hes1 expression. Protein samples from mouse bone marrow cells at 3, 5, 7, 14 days after JAG1 or scrambled peptide injection were analyzed. β-actin was a loading control. (E) Quantification of Hes1 immunoblot assay by ImageJ software. The normalized ratio is relative to the β-actin control. The data (mean ± SE.) were from three independent experiments.∗, p < 0.01.
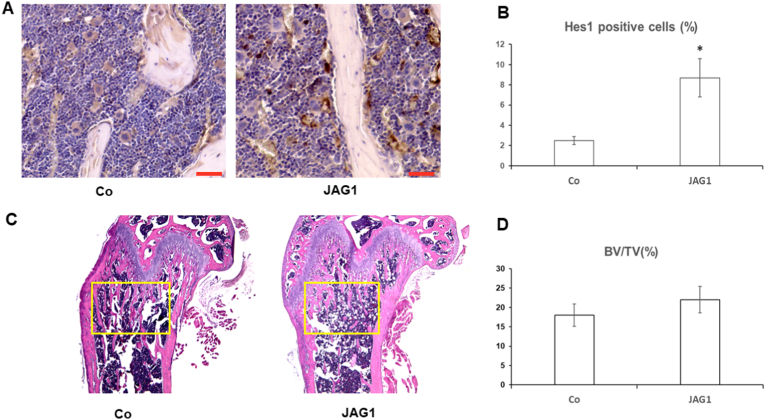
To further confirm the in vivo activation of Notch signaling, mouse femur bone tissues were obtained for histology and immunostaining after a 7-day injection of JAG1. Expression of the Notch target gene Hes1 in JAG1-injected mice was significantly increased compared to the control peptide-injected mice based on stronger immunostaining for Hes1 in femur bone marrow cells (Fig. 2A and B). Conversely, our histology results showed no significant differences in the BV/TV ratio in the femur growth plate between control peptide- and JAG1-injected mice (Fig. 2C and D), suggesting that bone structure was not altered by Notch activation in vivo at least within the short-term.
Fig. 2.
Short-term 7-day injection of JAG1 has no effect on bone structures. (A) Immunohistochemical staining against Hes1 in the bone marrow section from control and JAG1 injected mice. (B) Percentage of Hes1 positive cells versus total cells in bone marrow from control and JAG1 treated mice. (C) Light micrographs of H&E staining performed on trabecular bone sections from proximal femora of mice. Yellow Square indicates area used for calculation of BV/TV in (D). (Scale bar: 20 μm).
3.2. Notch signaling activation promotes cell proliferation in bone marrow
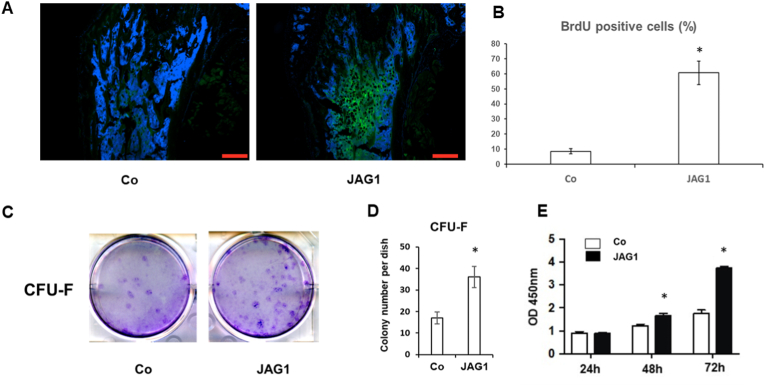
To test whether JAG1 could induce bone marrow cell proliferation, bromodeoxyuridine was given to mice 12 h before tissue collection. BrdU is an analog of thymidine used to detect proliferating cells in living tissue. As expected, JAG1-injected mice showed a significant increase in the number of BrdU positive cells inside the bone marrow space compared to control mice, demonstrating a greater proliferation index (Fig. 3A and B). These results clearly indicate that JAG1 can stimulate endogenous bone marrow cell proliferation, including mesenchymal stem cells.
Fig. 3.
Effects of JAG1 injection on proliferation of MSCs. (A) Representative image of Anti-BrdU staining of bone marrow section from control and JAG1 injected mice. Bar indicates 200 um. (B) Quantification of percentage of BrdU-labeled green positive cells versus total cells. (C) Colonies formed in cells collected from bone marrow of femur and tibia. (D) Quantification of colonies (>50 cells/colony) in total bone marrow cells seeded in 10-cm culture plates. Data represent the mean ± SE; n = 5. (E) BMSCs from JAG1 injected mice showed higher proliferation rate at 48 and 72 h in culture when compared to BMSCs from control mice.
To further determine the effect of JAG1 on proliferation of MSCs in vivo, we performed bone marrow MSCs isolation from femur and tibia in both JAG1 treated and control mice. The same total numbers of bone marrow cells were recovered from the mice when these cells were cultured on plastic dish in media promoting stem cell growth. After 10 days of culture, we observed more CFU colonies in cells from JAG1 treated mice when compared to cells from control mice (Fig. 3C and D). Cell proliferation was determined at 24, 48, and 72 h in these P2 BMSCs. Our results showed that BMSC proliferation was significantly higher in cells from JAG1-treated animals than those from scramble peptide-treated animals at 48 and 72 h of culture (Fig. 3E) suggesting JAG1-expanded BMSCs exhibit higher capacity for expansion.
The stemness of bone marrow-derived MCSs was also evaluated by flow cytometry for several stem cells markers: CD29, CD44, Sca-1, CD105, and negative markers: CD34, CD45. The results of these studies are shown in Fig. 4A and B which clearly show that there are more CD105+ cells in JAG1-treated mice when compared to control mice, but there were no significant differences observed in CD29+, CD44+ and Sca-1+ staining. More importantly, no CD34+ and CD45+ staining was observed in MSCs from either JAG1 peptide or control peptide-treated mice indicating high purity of stromal cells in culture. To further elucidate the possible molecular mechanism of JAG1-mediated cell proliferation, we first cultured wild type mouse BMSCs on JAG1 peptide-coated plates to activate Notch signaling in vitro and extracted total RNA for real time PCR. Here we focused on several cell cycle associated genes since we have previously demonstrated that JAG1 inhibits cellular senescence in human bone marrow derived MSCs by regulation of cyclin dependent kinase D1(CCD1), and cyclin dependent kinase inhibitors p16 and p21 [15]. As expected, JAG1 indeed induced the expression of CCD1 and decreased the p16 and p21 expression after 24 h in culture (Fig. 4C). In contrast, when these cells were treated with Notch inhibitor DAPT, the expression of CCD1 was reduced, whereas the p16 and p21 expression was increased. More importantly, the inductive and reductive effect of JAG1 on the expression of CCD1, p16 and p21 was largely blocked by Notch inhibitor DAPT treatment strongly suggest that JAG1 induces cell proliferation through the regulation of cell cycle associated genes in a Notch signaling dependent manner.
Fig. 4.
Phenotype of bone marrow derived stromal cells from control and JAG1 injected mice. (A): Expression profile for CD29, CD44, CD105 and Sca-1 positive population. (B) Quantification of positive population in BMSCs from flow cytometry data. (C) Gene expression profile for cell cycle associated proteins (p16, p21, CCD1) in BMSC cultures treated with JAG1 and/or DAPT for 24 h. (D) Alkaline phosphatase (ALP) and Alizarin red staining showed that BMSCs from JAG1 injected mice had higher osteogenic potential when compared to BMSCs from control mice. (E) qRT-PCR analysis showed that osteogenic markers type I collagen (Col1a1) and Runx2 highly expressed in BMSCs from JAG1 injected mice. Data represent the mean ± SE from three independent experiments. (F) Western blot data showed that the protein level of type I collagen (Col1a1) and Runx2 is higher in BMSCs from JAG1 injected mice.
3.3. JAG1 pretreatment increases callus formation and mechanical strength of bone allograft healing
To determine the osteogenic potential of BMSCs from JAG1 peptide-treated animals, cells from JAG1-or control peptide-injected mice were cultured in osteogenic media for 14 days. Staining for ALP and Alizarin Red were used to monitor cell osteogenic differentiation and calcified nodule formation. We found stronger ALP and Alizarin Red staining in cells from JAG1 peptide-treated mice compared to cells from scramble peptide-treated mice (Fig. 4D). mRNA expression for Col1a1 and Runx2 was also higher in cells from JAG1 peptide-treated mice compared to scramble peptide-treated mice (Fig. 4E). Western blot data (Fig. 4F) from these cells further confirmed the increased expression of Col1a1 and Runx2 in protein level.
To determine whether JAG1 expanded BMSCs could be used to enhance bone allograft healing in vivo, we monitored the femur bone allograft healing process in mice treated with JAG1 for 7 days prior to the allograft surgery. Bone callus formation was evaluated at 8 weeks after surgery by H&E staining in femur sections. As shown in Fig. 5A, more interlaced bone was observed surrounding the allograft in JAG1-treated mice compared to scramble peptide-treated mice consistent with more rapid bone callus formation in this allograft healing. Furthermore, Micro-CT findings also clearly showed an increased BV/TV in callus formed in JAG1 treated mice when compared to that in control mice (Fig. 5B and C). Results of biomechanical testing showed that the maximum torque was significantly increased at 8 weeks in JAG1 pre-treatment group compared to controls (Fig. 5D), demonstrating that the enhanced callus formation was translated to enhanced bone mechanical strength.
Fig. 5.
JAG1 pretreatment promotes bone callus formation in a mouse femur allograft model. Bone allograft transplantation procedure was conducted in 12-week-old mice pre-treated with JAG1 or control peptides. (A) H&E stained histology analysis was performed in mice 8 weeks after surgery. (B, C) Micro-CT analysis showed that bone volume and BV/TV were increased in callus tissues 8 weeks after surgery in the mice pre-treated with JAG1. (D) Mechanical testing showed that maximal torque was increased in JAG1 pre-treatment mice 8 weeks after surgery. Data are presented as means ± SE. ∗p < 0.05, n = 8. # indicates the allografts.
4. Discussion
MSCs are becoming an increasingly attractive option for tissue engineering and regenerative medicine due to their availability, self-renewal capacity, multi-lineage differentiation potential, and anti-inflammatory properties. The ability of MSCs to differentiate into chondrocytes, osteocytes, and myocytes holds great promise for skeletal tissue regeneration, especially for bone and cartilage. BMSCs and other tissue-derived MSCs have been extensively studied for the treatment of a variety of diseases in experimental animals, including large cartilage and bone defect repairs [16,19,20], and in spinal cord injury [21,22]. Therefore, stem cell-based therapy represents a major breakthrough in regenerative medicine which overcomes many limitations to regeneration often posed by massive tissue loss.
Although bone marrow is an excellent source of MSCs, relatively few pure MSCs can be isolated from bone marrow aspirates [23]. Because large numbers of MSCs are typically needed to produce the amount of extracellular matrix needed for rapid tissue repair, in vitro expansion of MSCs has been widely applied to obtain sufficient cells for in vivo transplantation. However, an important current limitations to MSC culture technology is that expanded stem cells often fail to proliferate well and progressively lose their regenerative capacity in vivo [18]. Therefore, there is still a pressing need to develop strategies that can mobilize and expand native endogenous stem cells for injured tissue repair.
We have identified the Notch pathway as a novel molecular regulator of MSC maintenance and differentiation. Particularly, we found that the Notch2 receptor is an important target of Notch activation induced by JAG1, a ligand of Notch signaling, during MSC proliferation and differentiation in mouse skeletogenesis, and that Notch2-expressing MSCs proliferate and potently differentiate along an osteogenic lineage [14]. Although these data clearly indicate that Notch signaling can be used to expand and maintain stemness of MSCs in cultures, it is still unknown whether or how this pathway could be used to increase endogenous MSC numbers and translate them into subsequent tissue repair.
In the present study, we used Notch activation to rapidly enhance bone marrow MSC in vivo expansion and promote subsequent bone repair in a mouse femoral bone defect model. In vivo Notch activation was achieved by injecting soluble JAG1 peptide into study animals, demonstrated by increased expression of Notch target gene Hes1, Hey1 and Notch1. Interestingly, longer term (14-day) injection of JAG1 showed a loss of Notch signaling compared to short-term injection of 7-day, indicating that short-term 7-day injection is optimal for activating Notch in mice. As a negative autoregulation feedback loop has been reported in regulation of Notch Hes1 expression [24], the possible explanation for the loss of Notch signaling after day 7 is that the JAG1 induced Hes1 expression reached to the threshold level, which triggered a rapid degradation of Hes1 protein by the ubiquitin-proteasome system. More importantly, short-term JAG1-induced Notch activation had no independent effects on bone structure, a finding which further confirms the relative safety of short-term JAG1 peptide administration. It is also worth noting that the influence of JAG1 on lung, heart, kidney after 2-week injection was also monitored in tissue histological slides, while no significant toxic effects and tissue structure changes were observed. This is also consistent to a previous report that 14-day injection of JAG1 showed no side-effects in Akt mice [25]. Since we only observed the changes in two weeks after injection, we cannot rule out the possible influence of JAG1 on bone and other organs when injected for longer than 2 weeks.
The therapeutic potential of bone marrow-derived MSCs has been well documented. Not only these cells are able to migrate to injured tissues, but they are also able to differentiate into several different phenotypes similar to the local tissues in which they are placed [9,23]. To further determine whether Notch activation can be used to expand the absolute numbers of endogenous stromal cells, BMSCs were then isolated from bone marrow mononuclear cells and tested for stemness and osteogenic potential. Consistent with previous findings, our data clearly indicated in vivo activation of Notch signaling by JAG1 peptide administration increases the absolute number of endogenous BMSCs by showing increased stromal cell colony formation and CD105+ expression. Interestingly, CD105 (endoglin) is highly expressed in vascular endothelial cells as an accessory receptor for TGF-beta superfamily ligands [26]. This might suggest that JAG1 may help drive angiogenesis in vascular endothelial cells within tissues to aid tissue healing. Since the local stem cells are essential to create new tissues and their proliferation rate is determined by the duration of the cell cycle, so reduce the transition from one phase to the next become a useful approach to increase cell numbers. Therefore, we further analyzed the effect of JAG1 on the genes associated with cell cycle progression. Consistent with our previous finding in human MSCs [15], the PCR data showed that the expressions of cell cycle responsive gene CCD1, p16 and p21 are all affected by JAG1 treatment, while the Notch inhibitor DAPT blocks this effect, indicating that the Notch signal is a critical inducer of stem cell proliferation by regulation of cell cycle progression.
More importantly, these JAG1 in vivo expanded MSCs exhibit higher osteogenic potential, as demonstrated by stronger ALP and Alizarin Red staining and increased Col1a1 and Runx2 expression, which are central mediators of bone tissue repair. Given the importance of local BMSCs in bone tissue repair, we next evaluated whether Notch signaling expanded endogenous BMSCs could be used for bone defect repair. In this experiment, we first injected JAG1 for 7 days to expand native BMSCs in bone marrow, and then we created a large bone defect in femur and reconstructed with bone allograft for repair. As expected, JAG1 pre-treatment significantly improved bone allograft healing by promoting more callus in the surrounding allografts and by enhancing mechanical strength of the repair. These findings further confirmed that JAG1 induced Notch signaling can not only be used to expand in vivo BMSC numbers, but also can significantly promote subsequent bone tissue repair by increasing the abundance of BMSC in the bone marrow.
5. Conclusions
In summary, our findings show that proliferation and differentiation of pre-existing BMSCs are crucial for bone regeneration. We demonstrate that Notch in vivo activation can be induced by injection of JAG1 peptide for expansion of local native BMSCs that will significantly enhance bone callus formation. Thus, the clinical uses of this therapeutic strategy would be immediately applicable for chronic long bone defect repair, including periprosthetic bone loss in adult bone reconstruction, bone loss secondary to infectious or oncologic etiologies, and pediatric skeletal deformities and repairs. More importantly, this devised strategy for expansion of endogenous BMSCs cannot only promote bone tissue repair, but can also be applied to enhance other tissue and organ repair.
Author contributions
G.W., Y.D. designed all experiments; G.W., H.Z. performed most experiments and data. collection, C.K., S.B. obtained the funding; G.W., J.Y. wrote the manuscript. P.M., J.A., C.K., S.B., and Y.D. analyzed data and proof reading the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported in part by the following grants: a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Grant No. R01AR073277-01A1) to S.B. This work was also supported by an Institutional Development Award (IDeA) from the National Institutes of General Medical Sciences of the National Institutes of Health under grant number P20GM121307 and National Institutes of Health grant number HL149264 to C.G. Kevil.
References
- 1.Stevenson S. Biology of bone grafts. Orthop Clin N Am. 1999;30(4):543–552. doi: 10.1016/s0030-5898(05)70107-3. [eng] [DOI] [PubMed] [Google Scholar]
- 2.Stevenson S., Shaffer J.W., Goldberg V.M. The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res. 1996;326:86–95. doi: 10.1097/00003086-199605000-00011. [eng] [DOI] [PubMed] [Google Scholar]
- 3.Stevenson S., Emery S.E., Goldberg V.M. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [eng] [DOI] [PubMed] [Google Scholar]
- 4.Younger E.M., Chapman M.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [eng] [DOI] [PubMed] [Google Scholar]
- 5.Yazici C., Takahata M., Reynolds D.G., Xie C., Samulski R.J., Samulski J. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011;19(8):1416–1425. doi: 10.1038/mt.2010.294. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrey B.H., Lord C.F., Gebhardt M.C., Mankin H.J. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72(6):825–833. [eng] [PubMed] [Google Scholar]
- 7.Lord C.F., Gebhardt M.C., Tomford W.W., Mankin H.J. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70(3):369–376. [eng] [PubMed] [Google Scholar]
- 8.Colnot C. Cell sources for bone tissue engineering: insights from basic science. Tissue Eng B Rev. 2011;17(6):449–457. doi: 10.1089/ten.teb.2011.0243. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan A.I., Reuben D., Haynesworth S.E. Cell-based tissue engineering therapies: the influence of whole body physiology. Adv Drug Deliv Rev. 1998;33(1–2):3–14. doi: 10.1016/s0169-409x(98)00016-7. [eng] [DOI] [PubMed] [Google Scholar]
- 10.Ji J.F., He B.P., Dheen S.T., Tay S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cell. 2004;22(3):415–427. doi: 10.1634/stemcells.22-3-415. [eng] [DOI] [PubMed] [Google Scholar]
- 11.Abbott J.D., Huang Y., Liu D., Hickey R., Krause D.S., Giordano F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [eng] [DOI] [PubMed] [Google Scholar]
- 12.James A.W., LaChaud G., Shen J., Asatrian G., Nguyen V., Zhang X. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng B Rev. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y., Jesse A.M., Kohn A., Gunnell L.M., Honjo T., Zuscik M.J. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137(9):1461–1471. doi: 10.1242/dev.042911. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y., Long T., Wang C., Mirando A.J., Chen J., O'Keefe R.J. NOTCH-mediated maintenance and expansion of human bone marrow stromal/stem cells: a technology designed for orthopedic regenerative medicine. Stem Cells Transl Med. 2014;3(12):1456–1466. doi: 10.5966/sctm.2014-0034. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Y., Xu Y., Xue T., Chen L., Shi B., Shu B. Notch activation enhances mesenchymal stem cell sheet osteogenic potential by inhibition of cellular senescence. Cell Death Dis. 2017;8(2) doi: 10.1038/cddis.2017.2. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long T., Zhu Z., Awad H.A., Schwarz E.M., Hilton M.J., Dong Y. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials. 2014;35(9):2752–2759. doi: 10.1016/j.biomaterials.2013.12.039. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang X., Luo Z., Wang X., Jaeblon T., Marymont J.V., Dong Y. Deletion of RBPJK in mesenchymal stem cells enhances osteogenic activity by up-regulation of BMP signaling. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0135971. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang X., Shu B., Wang Y., Luo Z., Wang G., Barton S. Human mesenchymal stromal cell sheet enhances allograft repair in a mouse model. Sci Rep. 2017;7(1):7982. doi: 10.1038/s41598-017-08804-2. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroda R., Ishida K., Matsumoto T., Akisue T., Fujioka H., Mizuno K. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15(2):226–231. doi: 10.1016/j.joca.2006.08.008. [eng] [DOI] [PubMed] [Google Scholar]
- 20.Lee K.B., Hui J.H., Song I.C., Ardany L., Lee E.H. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cell. 2007;25(11):2964–2971. doi: 10.1634/stemcells.2006-0311. [eng] [DOI] [PubMed] [Google Scholar]
- 21.Hopf A., Schaefer D.J., Kalbermatten D.F., Guzman R., Madduri S. Schwann cell-like cells: origin and usability for repair and regeneration of the peripheral and central nervous system. Cells. 2020;9(9) doi: 10.3390/cells9091990. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liau L.L., Looi Q.H., Chia W.C., Subramaniam T., Ng M.H., Law J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:112. doi: 10.1186/s13578-020-00475-3. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marolt Presen D., Traweger A., Gimona M., Redl H. Mesenchymal stromal cell-based bone regeneration therapies: from cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front Bioeng Biotechnol. 2019;7:352. doi: 10.3389/fbioe.2019.00352. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata H., Yoshiura S., Ohtsuka T., Bessho Y., Harada T., Yoshikawa K. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298(5594):840–843. doi: 10.1126/science.1074560. [eng] [DOI] [PubMed] [Google Scholar]
- 25.Kerr B.A., West X.Z., Kim Y.W., Zhao Y., Tischenko M., Cull R.M. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun. 2016;7:10960. doi: 10.1038/ncomms10960. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C.S., Xin Z.C., Dai J., Lue T.F. Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol. 2013;28(9):1109–1116. doi: 10.14670/hh-28.1109. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]