Abstract
mRNA-based COVID-19 vaccines are effective; however, persistent vaccine hesitancy is partly due to a misperception of their potential adverse events. Non-specific sensory symptoms (NSSS) following immunization are thought to be mediated by stress-related responses. In this case-control study, we evaluated NSSS from a cohort of 7,812,845 BNT162b2 first-dose recipients, of whom 10,929 reported an adverse event following immunization (AEFI). We found an overall frequency of 3.4% (377 cases) or 4.8 cases per 100,000 doses administered. Anatomically, the arms (61%) and face/neck region (36.2%) were the most commonly affected sites. The control group had significantly higher rates of reactogenicity-associated symptoms, suggesting that NSSS are reactogenicity-independent; in multivariable analysis, healthcare workers reported sensory symptoms less frequently (aOR 0.54; 95% CI 0.40–0.72; p < 0.001). This is the first study describing the topography and associated factors for developing NSSS among BNT162b2 recipients. The benign nature of these symptoms may help dissipate hesitation towards this vaccine.
Keywords: AEFI, Adverse event, Immunization, BNT162b2, COVID-19, Immunization stress-related response, Paresthesia, SARS-CoV-2
1. Introduction
Massive immunization against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently the most effective strategy to end the coronavirus disease 2019 (COVID-19) pandemic. BNT162b2, an mRNA-based vaccine, is effective in reducing disease severity and mortality [1], [2], [3], [4]; however, safety remains a public concern, in part due to mistrust, misunderstanding, or misinformation. Multiple local and systemic adverse events following immunization (AEFI) have been reported, including neurologic ones [5], [6]. The development of non-specific signs, symptoms, or syndromes that do not necessarily have a causal association with the vaccine are known as immunization stress-related responses, a particular subtype of AEFI usually presenting within the first 30 min after immunization [7]. However, the evidence of sensory abnormalities following vaccination is scarce and mostly limited to a few series among influenza vaccines recipients, with a reported frequency of up to 15% [8], [9]. Despite the extensive number of COVID-19 vaccines that have been administered worldwide (>3.4 billion as of the writing of this manuscript), little is known about this particular AEFI among mRNA vaccines recipients. Here we aimed to describe the frequency of transient sensory symptoms, the bodily sites affected by this event, and explore potentially associated factors linked to their development among first dose recipients of the BNT162b2 mRNA COVID-19 vaccine who reported an AEFI in Mexico.
2. Methods
2.1. Study design and population
Data for this case-control study on sensory symptoms as an AEFI following the first dose of the BNT162b2 mRNA COVID-19 vaccine from December 24, 2020, to May 27, 2021, in Mexico were obtained from the General Board of Epidemiology, Mexican Ministry of Health. The study protocol was revised and approved by the Ethics and Research Committees of Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán and the Mexican Ministry of Health (Ref. NER-3903-21-23-1). Due to the observational nature and the use of de-identified databases, committees waived the need for signed informed consent.
The Mexican Epidemiological Surveillance System is a passive system where AEFI are reported either by the detecting health institution or directly by the vaccine recipient. After reports are received, all AEFI are characterized as either serious or non-serious according to the World Health Organization operational definition at the local level [6], [10]. In addition, an ad-hoc committee evaluates all potentially serious events. Operational details on this system and case definitions have been published elsewhere [6], [11].
For the present analysis, we included adult (≥18 years) recipients who reported an AEFI following the first dose of the BNT162b2 mRNA COVID-19 vaccine. Cases of AEFI associated with the development of new-onset sensory symptoms such as any subtype of acute stroke, Guillain-Barré syndrome, acute transverse myelitis, peripheral facial palsy (Bell’s palsy), anaphylaxis, and bronchospasm were excluded; also, recipients diagnosed with severe COVID-19 within the first ten days after vaccination and those without an accurate description of sensory symptoms or topography.
2.2. Data collection and variables
We obtained a de-identified dataset of all AEFI, including age, sex, occupation, history of recent non-SARS-CoV-2 infection ≤ 15 days before vaccination, allergies of any kind, or confirmed SARS-CoV-2 infection either real-time reverse transcription-polymerase chain reaction (RT-PCR) or antigen testing; vaccine-associated adverse events as assessed by the attending physician; the interval in minutes from vaccine administration to first symptom onset; and clinical outcome. Comorbidities were not routinely recorded on the dataset, limiting their use for the current analysis. From clinical notes, we extracted the frequency and topography of all sensory symptoms, included the following: paresthesia, dysesthesia, numbness, pinprick, tingling, or a combination thereof; these symptoms, resolved within the first 48 h after their development, according to the clinical notes. At least two researchers reviewed all data, and a third researcher adjudicated any difference in interpretation between the primary reviewers.
2.3. Statistical analysis
We compared the characteristics between vaccine recipients who developed sensory symptoms following immunization and those who did not in a 2:1 control-to-case ratio matched by age and sex. Controls were randomly selected vaccine recipients included in the dataset who developed an AEFI (either neurologic or non-neurologic) not associated with the development of sensory symptoms, as described above. Categorical variables are reported as frequencies and proportions; continuous variables as median with interquartile range (IQR) or as mean with standard deviation (SD). Analyses of categorical variables were performed with the χ 2 or Fisher’s exact tests, and continuous variables with the Student’s t-test or Mann-Whitney U test, as appropriate. Multiple comparison problem was addressed by false discovery rate corrections using the Benjamini-Hochberg procedure (q-value = 0.05). To determine associated factors with the development of sensory symptoms, we performed a binary logistic regression analysis adjusted for age (dichotomized by its mean value), sex, occupation (healthcare workers classification included physicians, nurses, technicians, or other healthcare professionals); history of allergies; history of confirmed SARS-CoV-2 infection; and history of non-SARS-CoV-2 infection in the 15 days preceding vaccination. The model adjustment was evaluated using the Hosmer-Lemeshow goodness of fit test and considered reliable when the p-value was ≥ 0.20; its results are reported as adjusted odds ratios (aOR) with a 95% confidence interval (CI). All p-values were two-tailed and considered significant with a value ≤ 0.05. All analyses were performed with IBM SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA).
3. Results
During the study period, the Mexican Epidemiological Surveillance System received and processed 10,929 reports of AEFI among 7,812,845 first dose recipients of the BNT162b2 mRNA COVID-19 vaccine (0.14%; 139.9/100,000 doses); of all reports, we excluded 127 (1.16%) who developed a serious non-neurologic event potentially associated to sensory symptoms, including 26 (0.24%) with anaphylactic reactions, 97 (0.88%) with bronchospasm, and 36 (0.33%), with a serious neurologic AEFI. We identified 377 reports of new-onset transient sensory symptoms for a frequency of 3.4% (95% CI 3.1% to 3.8%) among all AEFI or 4.8 cases per 100,000 doses administered. After evaluation, 23 cases were excluded due to missing data on topography.
We included 1,062 reports for the final analysis, 354 cases, and 708 controls; 898 (84.6%) were women and 164 (15.4%) male. The mean age was 39.9 ± 12.3 years (baseline characteristics of the sample can be found in Table 1 and Supplementary Fig. 1). The proportion of healthcare workers was significantly higher among the control group. There were no differences in the history of recent infection rates, including both, SARS-CoV-2 or non-SARS-CoV-2 infections. The timing from vaccination to the first AEFI symptom was shorter in patients with sensory symptoms than in those without; overall, those with sensory symptoms reported other symptoms less frequently.
Table 1.
Baseline characteristics and reported adverse events following immunization.
| Cases (n = 354) |
Control (n = 708) |
Total (n = 1,062) |
p-value | q-value | |
|---|---|---|---|---|---|
| Age, mean (±SD), years | 40 (12.5) | 39.8 (12.3) | 39.9 (12.3) | 0.844 | 0.043 |
| Age > 40 years, n (%) | 156 (44.1) | 306 (43.2) | 462 (43.5) | 0.793 | 0.041 |
| Sex, n (%) | 0.952 | 0.048 | |||
| Female | 299 (84.5) | 599 (84.6) | 898 (84.6) | ||
| Male | 55 (15.5) | 109 (15.4) | 164 (15.4) | ||
| Healthcare workers, n (%) | 232 (65.5) | 552 (78) | 784 (73.8) | <0.001 | 0.017* |
| Medical history, n (%) | |||||
| Allergies (any) | 233 (65.8) | 511 (72.2) | 744 (70.1) | 0.033 | 0.026 |
| Non-SARS-CoV-2 infection ≤ 15 days | 6 (1.7) | 11 (1.6) | 17 (1.6) | 0.863 | 0.045 |
| History of confirmed SARS-CoV-2 infection | 105 (29.7) | 209 (29.5) | 314 (29.6) | 0.962 | 0.05 |
| Time to AEFI report, median (IQR), minutes | 20 (10–180) | 60 (15–720) | 30 (10–600) | <0.001 | 0.002* |
| Reported symptoms, n (%) | |||||
| Fever, ≥38 °C | 35 (9.9) | 140 (19.8) | 175 (16.5) | <0.001 | 0.019* |
| Headache | 140 (39.5) | 416 (58.8) | 556 (52.4) | <0.001 | 0.007* |
| Injection site pain | 145 (41) | 332 (46.9) | 477 (44.9) | 0.067 | 0.029 |
| Fatigue | 76 (21.5) | 262 (37) | 338 (31.8) | <0.001 | 0.012* |
| Malaise | 55 (15.5) | 172 (24.3) | 227 (21.4) | 0.001 | 0.021* |
| Dizziness | 99 (28) | 213 (30.1) | 312 (29.4) | 0.475 | 0.036 |
| Chills | 42 (11.9) | 201 (28.4) | 243 (22.9) | <0.001 | 0.005* |
| Joint pain | 48 (13.6) | 207 (29.2) | 255 (24) | <0.001 | 0.01* |
| Muscle pain | 68 (19.2) | 236 (33.3) | 304 (28.6) | <0.001 | 0.014* |
| Tachycardia | 55 (15.5) | 101 (14.3) | 156 (14.7) | 0.581 | 0.038 |
| Nausea | 69 (19.5) | 191 (27) | 260 (24.5) | 0.007 | 0.024* |
| Vomiting | 18 (5.1) | 45 (6.4) | 63 (5.9) | 0.408 | 0.033 |
| Diarrhea | 25 (7.1) | 39 (5.5) | 64 (6) | 0.316 | 0.031 |
Abbreviations: SD, standard deviation; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. *Significant p-value after false discovery rate correction.
Regarding topography of symptoms, arms were the most commonly affected site in 216 (61%) cases, of which 120 (55.5%) occurred in the vaccine-recipient arm. Face/neck was the second most commonly involved region, with sensory symptoms in 128 (36.2%) cases (Fig. 1 ). Fifty-nine (16.4%) reported involvement of two or more locations. In 19 cases (5.4%), the symptoms followed a hemi-body distribution, and when accounting for only the extremities in 67 cases (26.9%), the symptoms were bilateral, involving more frequently legs than arms (35/67, 52.2% vs. 32/182, 17.6%; p < 0.001). After adjusting for potential confounding factors by multivariable analysis (Table 2 ), the odds for reporting sensory symptoms were significantly lower among healthcare workers (aOR 0.54; 95% CI 0.40–0.72; p < 0.001). None of the other included variables were associated with developing sensory symptoms.
Fig. 1.
Topography of the sensory symptoms reported by first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine.
Table 2.
Associated factors with the development of transient neurologic symptoms among first dose recipients of the BNT162b2 mRNA COVID-19 vaccine.
| Bivariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Female sex | 0.99 (0.7-–1.41) | 0.952 | 1.12 (0.78–1.61) | 0.554 |
| Age > 40 years | 1.04 (0.80–1.34) | 0.793 | 0.95 (0.73–1.24) | 0.718 |
| Healthcare workers | 0.54 (0.41–0.71) | <0.001 | 0.54 (0.40–0.72) | <0.001 |
| Allergies (any) | 0.74 (0.56–0.98) | 0.033 | 0.79 (0.59–1.04) | 0.094 |
| Non-SARS-CoV-2 infection ≤ 15 days | 1.09 (0.40–2.98) | 0.863 | 1.13 (0.41–3.11) | 0.818 |
| History of confirmed SARS-CoV-2 infection | 1.01 (0.76–1.33) | 0.962 | 1.04 (0.79–1.38) | 0.778 |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Hosmer-Lemeshow goodness of fit test: χ2 8.219, 8 df, p = 0.412).
4. Discussion
In this study, the frequency of sensory symptoms among 10,929 first dose recipients of the BNT162b2 mRNA COVID-19 vaccine who reported an AEFI in Mexico was 3.4% (95% CI 3.1% to 3.8%), consistent with a previously reported frequency of 3.5% [6], and comparable to the numbness and tingling (2.8% to 4.8%, respectively) reported in a cross-sectional survey study among 803 healthcare worker recipients of BNT162b2 in the United States [4]. We found that arms were the most commonly involved body site and that the vaccinated arm was affected in more than half of the cases. This is consistent with the reported frequency of sensory symptoms in a study among recipients of the AS03-adjuvanted A(H1N1)pdm09 vaccine during the 2009 influenza pandemic in Canada [8]; interestingly, in that study, sensory symptoms were the third most commonly reported AEFI (15%, or 7.5 cases per 100,000 doses administered). Differences in frequency may be related to methodological definitions, nature of surveillance between detection systems, mechanism of action, as well as differential biological and chemical effects of vectors and adjuvants.
The mechanisms for sensory symptoms following immunization are still unknown, but links to depressive and anxiety symptoms have been reported in non-COVID-19 vaccine recipients [7]. This particular type of AEFI, which includes other events such as immediate vasovagal-responses and non-epileptic seizures following vaccination, named immunization stress-related responses, usually develops within minutes to hours after vaccination [10], as evidenced in this study, where symptoms among the cases were reported significantly earlier than among controls.
Stress responses are complex and vary from person to person; they involve a combination of physiological factors, biological vulnerability, psychological resources, patterns of coping, mood, social context, nature of the stressor, knowledge, and preparedness [12]. Also, the COVID-19 outbreak has been associated with fear and grief, further increasing the risk for mental health problems, particularly anxiety and depression, underlying psychological factors that may affect the perception and interpretation of symptoms. In this regard, a study among mRNA vaccine recipients using data from the Vaccine Adverse Event Reporting System (VAERS), subjects with a history of anxiety or depression had a 2.6 fold increased odds for reporting a neurologic AEFI [13]. Therefore, psychological guidance should be emphasized, especially in this population.
Furthermore, since the development and mass implementation of the COVID-19 vaccines, hesitancy towards most (especially for mRNA-based ones), promoted by misleading news reports and social media information about immunization, may play a role in the development of non-specific sensory symptoms among COVID-19 vaccine recipients [14], [15], [16]. Moreover, these transient symptoms may be related to concerns about developing a severe illness from the vaccine; anxiety activates the amygdala, a key step in activating the limbic system, potentially amplifying sensory somatic perception [17], [18].
The significantly higher rates of other, local and systemic, vaccine reactogenicity-associated events such as injection site pain, fever, headache, fatigue, malaise, and muscle pain we observed in the control group support, in addition to the non-specific pattern of involved body sites, supports our hypothesis that these transient, non-specific sensory symptoms may be part of the immunization stress-related response [9], [19]. In our population, being a healthcare worker decreased the odds of reporting a sensory symptom; this may be related to the rapidly increasing knowledge about non-serious AEFI, efficacy, and safety profiles of mRNA-based vaccines by this group [3], [4].
Our study has limitations, including the passive nature of the surveillance system, where some adverse events may be underreported. We used a case-control design trying to overcome the subjective nature and relatively low frequency of sensory events; however, our study has the strength that both study groups derive from a population-based dataset composed of first dose recipients of the BNT162b2 mRNA COVID-19 vaccine who reported an AEFI. Also, we relied on limited data, such as the interval from vaccination to sensory symptom onset or accurate duration; the lack of other relevant data such as comorbidities (reported only for a minority of cases), psychological/psychiatric evaluations, and data for recipients without AEFI to evaluate other potential associations. Furthermore, our results may be biased because our studied sample mainly consisted of healthcare workers. This overrepresentation may be explained because our national COVID-19 vaccination initially focused on first-line healthcare workers [20]. Therefore, as the general population is currently being actively vaccinated, we plan to perform a larger study, analyzing the multiple vaccines available in Mexico, and not limit our inclusion to first-dose recipients.
5. Conclusions
Here, we describe the topography of transient sensory symptoms, as well as associated factors for their development among first dose recipients of the BNT162b2 mRNA COVID-19 vaccine. In our population, the frequency of this AEFI was lower than that previously reported among influenza vaccine recipients during the 2009 H1N1 pandemic in other countries. Emphasizing vaccine safety and not only efficacy among SARS-CoV-2 vaccine recipients is likely to reduce immunization stress-related response and decrease the occurrence of this type of AEFI.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología, Mexico (COVID-19 Fund [F0005-2020-01]; grant 311790), to SIV-F]. The funder had no role in the study design, data collection, data analysis, data interpretation, writing, or revision of the report.
Data sharing
The manuscript provides all the data collected. After approval by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán and the Mexican Ministry of Health Ethics and Research Committees, de-identified data to replicate our results will be available to qualified researchers upon written request to the corresponding author.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Sergio Ivan Valdes-Ferrer reports financial support was provided by Consejo Nacional de Ciencia y Tecnología.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.10.058.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
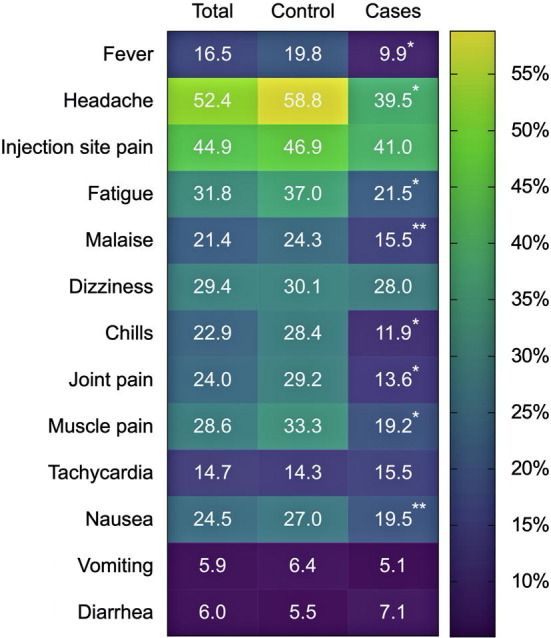
Reported systemic symptoms and their relative frequency among first dose recipients of the BNT162b2 mRNA COVID-19 vaccine.
References
- 1.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. LancetLond Engl. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadali R.A.K., Janagama R., Peruru S., Gajula V., Madathala R.R., Chennaiahgari N., et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J MedVirol. 2021;93(7):4420–4429. doi: 10.1002/jmv.v93.710.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renoud L., Khouri C., Revol B., Lepelley M., Perez J., Roustit M., et al. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern Med. 2021;181(9):1243. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Grimshaw M., Ceballos-Liceaga S.E., Hernández-Vanegas L.E., Núñez I., Hernández-Valdivia N., Carrillo-García D.A., et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin Immunol Orlando Fla. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold M.S., MacDonald N.E., McMurtry C.M., Balakrishnan M.R., Heininger U., Menning L., et al. Immunization stress-related response - Redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine. 2020;38(14):3015–3020. doi: 10.1016/j.vaccine.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 8.De Serres G., Rouleau I., Skowronski D.M., Ouakki M., Lacroix K., Bédard F., et al. Paresthesia and sensory disturbances associated with 2009 pandemic vaccine receipt: Clinical features and risk factors. Vaccine. 2015;33(36):4464–4471. doi: 10.1016/j.vaccine.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M.A., Naus M., Singer J., Valiquette L., Coleman B.L., De Serres G., et al. Investigating the association of receipt of seasonal influenza vaccine with occurrence of anesthesia/paresthesia and severe headaches, Canada 2012/13-2016/17, the Canadian Vaccine Safety Network. Vaccine. 2020;38(19):3582–3590. doi: 10.1016/j.vaccine.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Immunization stress-related responses: a synopsis of the manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization; 2019. https://apps.who.int/iris/bitstream/handle/10665/330277/9789241515948-eng.pdf?sequence=1&isAllowed=y [accessed May 21, 2021].
- 11.García-Grimshaw M., Michel-Chávez A., Vera-Zertuche J.M., Galnares-Olalde J.A., Hernández-Vanegas L.E., Figueroa-Cucurachi M., et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol Orlando Fla. 2021;230:108818. doi: 10.1016/j.clim.2021.108818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneiderman N., Ironson G., Siegel S.D. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1(1):607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G., Li X., Sun M., Zhou Y., Yin M., Zhao B., et al. COVID-19 mRNA Vaccines Are Generally Safe in the Short Term: A Vaccine Vigilance Real-World Study Says. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirumbolo S. Vaccination hesitancy and the “myth” on mRNA-based vaccines in Italy in the COVID-19 era: Does urgency meet major safety criteria? J MedVirol. 2021;93(7):4049–4053. doi: 10.1002/jmv.26922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas N., Mustapha T., Khubchandani J., Price J.H. The Nature and Extent of COVID-19 Vaccination Hesitancy in Healthcare Workers. J Commun Health. 2021 doi: 10.1007/s10900-021-00984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGlone F., Vallbo A.B., Olausson H., Loken L., Wessberg J. Discriminative touch and emotional touch. Can J Exp Psychol Rev Can Psychol Exp. 2007;61:173–183. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- 18.Rodic D., Meyer A.H., Lieb R., Meinlschmidt G. The Association of Sensory Responsiveness with Somatic Symptoms and Illness Anxiety. Int J Behav Med. 2016;23(1):39–48. doi: 10.1007/s12529-015-9483-1. [DOI] [PubMed] [Google Scholar]
- 19.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019;4(1) doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secretaría de Salud, Gobierno de México. Política nacional rectora de vacunación contra el SARS-CoV-2 para la prevención de la COVID-19 en México; 2021. https://coronavirus.gob.mx/wp-content/uploads/2021/05/11May2021_PNVx_COVID.pdf [accessed July 1, 2021].



