Abstract
Introduction:
Treatment effectiveness for major depressive disorder (MDD) is often affected by client non-adherence, dropout, and non-response. Identification of client characteristics predicting successful treatment completion and/or response (i.e., symptom reduction) may be an important tool to increase intervention effectiveness. It is unclear whether neural attenuations in reward processing associated with MDD predict behavioral treatment outcome.
Methods:
This study aimed to determine whether blunted neural responses to reward at baseline differentiate MDD (n=60; 41 with comorbid anxiety) and healthy control (HC; n=40) groups; and predict MDD completion of and response to 7-10 sessions of behavior therapy. Participants completed a monetary incentive delay (MID) task. The N200, P300, contingent negative variation (CNV) event related potentials (ERPs) and behavioral responses (reaction time [RT], correct hits) were quantified and extracted for cross-sectional group analyses. ERPs and behavioral responses demonstrating group differences were then used to predict therapy completion and response within MDD.
Results:
MDD exhibited faster RT and smaller P300 amplitudes than HC across conditions. Within the MDD group, treatment completers (n=37) exhibited larger P300 amplitudes than non-completers (n=21).
Limitations:
This study comprises secondary analyses of EEG data; thus task parameters are not optimized to examine further ERPs from the paradigm. We did not examine heterogenous presentations of MDD; however, severity and comorbidity did not influence findings.
Conclusions:
Previous studies suggest that P300 is an index of motivational salience and stimulus resource allocation. In sum, individuals who deploy greater neural resources to task demands are more likely to persevere in behavioral therapy.
Keywords: P300, Depression, treatment outcome, behavioral therapy, event-related potential
Introduction
Major depressive disorder (MDD) is a chronic illness linked to substantial personal disability and socioeconomic burden (Greenberg et al., 2015; lancu et al., 2020). Current front line treatments for MDD are only moderately effective (e.g., Ekers et al., 2007) and research has yet to establish reliable matches between patient characteristics and successful treatment outcomes that could serve to optimize outcomes (Kemp et al., 2008). Many individuals with MDD experience anhedonia and show biobehavioral deficits in appetitive, reward seeking behaviors (e.g., Baskin-Sommers & Foti, 2015; Klawohn et al., 2019; Pizzagalli, 2014). Thus, individual differences in reward system function may relate to one’s level of motivation to engage in treatment and/or one’s likelihood of success in behavioral treatments aiming to enhance reward system engagement to reduce depressive symptoms (e.g., Nagy et al., 2018).
Electroencephalography (EEG) is a cost-effective and objective psychophysiological tool capturing electrical brain responses during anticipation and receipt of appetitive or pleasant stimuli (Glazer et al., 2018; Proudfit et al., 2015) that can be leveraged to: (a) differentiate clinical groups from healthy controls (HC); and (b) predict future treatment response and/or symptom change in clinical populations (Hajcak et al., 2019; Matsen et al., 2020; Olbrich & Arns, 2013; Wade & losifescu, 2016). Although functional magnetic resonance imaging (fMRI) literature suggests that MDD patients tend to show lower frontostriatal brain responses to reward anticipation and/or feedback than HC (Borsini et al., 2020), low temporal resolution of fMRI limits knowledge of precisely where in the stimulus processing stream groups diverge. EEG possesses high temporal resolution and can be leveraged to complement fMRI spatial resolution, providing a more comprehensive picture of where and when brain functions show impairment in MDD. This knowledge may inform the degree to which individuals with these reward impairments engage in and respond to behavioral interventions.
The monetary incentive delay (MID) task (Knutson et al., 2001) enables researchers to record brain responses to cues signaling the opportunity for potential future gains and losses separately from gain/loss feedback, thereby disentangling the timing of opportunity for positive outcomes from the positive outcomes themselves. Event related potentials (ERPs), EEG segments time-locked to stimuli averaged across trials, have shown initial promise in predicting future depressive symptom change and response to cognitive behavioral therapy as well as antidepressants (Burkhouse et al., 2016; Burkhouse et al., 2019; Webb et al., 2020). Two ERP components relating to evaluation and categorization of cues are particularly relevant for studying reward anticipation. The cue N200 is a negative frontocentral deflection peaking between 200-350 msec post-stimulus that indexes selective attention to stimulus characteristics (see: Hajcak et al., 2012). For HC samples on the MID task, N200 is typically larger in amplitude for neutral and loss cues than gain cues, reflecting a mismatch or deviation from gains, thought to be considered the preferred stimulus type (Glazer et al., 2018; Novak & Foti, 2015). The cue P300 is a positive parietal deflection peaking between 300-600 msec post-stimulus that is often larger in amplitude for gain and/or loss cues than neutral cues in HC samples, thought to reflect stimulus categorization, and potentiation interpreted as in indicator of stimulus salience (Angus et al., 2017; Broyd et al., 2012; Glazer et al., 2018; Gu et al., 2017; Novak & Foti, 2015; Novak et al., 2016; Oumeziane et al., 2017; but see Flores et al., 2015).
N200 amplitude attenuations have been reported as a function of MDD diagnosis or depressive symptom severity in non-reward paradigms such as the flanker task (e.g., Alderman et al., 2015; Clawson et al., 2013) and color-word Stroop task (e.g., Holmes & Pizzagalli, 2008). It remains unclear whether this observed tendency for individuals with MDD to allocate less neural resources than HC to processing visual mismatches extends to reward-specific contexts. Similarly, multiple studies indicate that MDD patients exhibit smaller P300 amplitudes than HC during non-reward tasks, such as the continuous performance task (Diner et al., 1985) and flanker task (Klawohn et al., 2020). Moreover, smaller P300 amplitudes are linked to higher depression symptom severity among MDD patients (oddball task: Nan et al., 2018) and in female youth two years later (flanker task: Santopetro et al., 2020), suggesting that blunted P300 amplitude in MDD may not be specific to the context (e.g., reward potential) of stimulus presentation, but instead reflect a general reduction in neural resources allocated to stimulus categorization/evaluation. Thus, the N200 and P300 to cue stimuli in the MID task may offer functional insight into processing differences observed in the MID task anticipation phase.
Another ERP component relevant to reward anticipation is the contingent negative variation (CNV), which is elicited by a cue signaling that a response is needed to an upcoming target stimulus. The CNV is a negative-going slow wave maximal fronto-centrally that indexes anticipatory attention and motor preparation for motivationally salient targets (Glazer et al., 2018). Previous studies of reward anticipation among HC show that CNV amplitude is larger after gain and/or loss cues than neutral cues (Novak et al., 2016, Oumeziane et al., 2017). However, not all studies show CNV amplitude differences as a function of reward (Broyd et al., 2012). Prior work demonstrates that MDD exhibit smaller CNV amplitudes than HC across emotional and neutral faces (Vanderhasselt et al., 2014; Zhao et al., 2019). It is unclear whether this blunted CNV responses would generalize to reward and/or loss cues on the MID. CNV differences or lack thereof across reward and loss conditions may provide specificity to insights regarding the functional disruptions associated with MDD (i.e., general blunted anticipation vs. impaired modulation of anticipation during opportunity for reward).
This project focuses on secondary analysis of ERP data from one study focused on healthy control participants and two larger clinical trials examining (1) predictors and mechanisms of response to behavioral activatin (BA) for depression and (2) differential predictors and mechanisms of response to BA versus exposure therapy (EXP) for individuals with generalized anxiety disorder (GAD; a large proportion of whom also met criteria for current or lifetime MDD). ERPs provide a low-cost objective metric that may inform the potential effectiveness of behavior treatments for individuals with MDD. This study examines the anticipation phase of the MID task in MDD (with and without comorbid anxiety disorders) and HC groups during simultaneous EEG-fMRI recording prior to patient enrollment in treatment. Although the treatments offer different rationales and strategies for behavior change, both seek to increase behavioral engagement of patients either through increased activity (BA) or reduction of avoidance (EXP). Thus, measurement of cognitive function during opportunities to gain reward and/or avoid loss (i.e., MID anticipation) is particularly relevant for both treatment approaches.The primary goals of this project are twofold: (a) identify whether individuals with MDD show smaller ERP amplitudes (cue N200, cue P300, CNV) than HC during reward and/or loss anticipation; and (b) whether smaller ERP amplitudes in MDD predict future therapy completion and behavioral treatment response across behavior therapy interventions based on previous ERP research demonstrating reduced ERP amplitude associated with depression (e.g., Nan et al., 2018; Santopetro et al., 2020) and poorer treatment outcomes in pharmacologic treatments (e.g., Olbrich & Arns, 2013). For cross-sectional analyses, we hypothesize that: (1) MDD will show smaller N200 and P300 amplitudes to gain cues than HC, reflecting reduced resources devoted to reward categorization; and (2) MDD will exhibit smaller CNV amplitudes across gain, loss, and neutral cues than HC, reflective of an overarching deficit in attention preparation for target stimuli. For longitudinal analyses, we predict that P300 amplitude to gain cues as a marker of adaptive attentional engagement will be positively associated with behavioral therapy attendance and post-treatment decreases in depression symptoms.A secondary goal is to determine whether differences between MDD and HC groups are driven by individuals with pure MDD versus comorbid MDD and anxiety disorders, as prior work suggests that these groups may show divergent patterns of brain activity (e.g., MacNamara et al., 2016).
Methods
Participants
This investigation compared two groups of participants: (1) individuals meeting criteria for lifetime MDD (MDD) with or without comorbid anxiety disorders; and (2) healthy individuals with no lifetime psychiatric diagnoses or illicit substance use problems (HC). For the MDD group, the present study combined data collected as part of two larger clinical trials focused on identifying whether neuroimaging and behavioral indices related to approach-avoidance behavior were predictive of treatment response in: (1) a randomized, unblinded clinical trial wherein enrolled participants had clinically-significant generalized anxiety disorder (GAD) symptoms and were randomized to complete either BA or EXP (Santiago et al., 2020) (ClinicalTrials.gov: # NCT02807480; Study 1); and (2) a non-randomized, unblinded clinical trial in which all enrolled participants had clinically-significant depression and completed BA therapy (ClinicalTrials.gov: #NCT02602340; Study 2). The HC group in the present analysis consisted of 40 healthy control participants enrolled in the Tulsa 1000 study, a naturalistic longitudinal study of treatment-seeking individuals with psychiatric symptoms as well as individuals without a history of psychiatric illness (Victor et al., 2018; NCT02450240). Both clinical trials and the T1000 study took place at the Laureate Institute for Brain Research, were approved by the Western Institutional Review Board, and were carried out in accordance with the Declaration of Helsinki. Participants provided informed written consent and participant confidentiality was ensured.
Participants were recruited from radio, internet, and paper advertisements. Individuals verbally consented to complete a screen by trained staff to assess preliminary study eligibility. Eligible participants were scheduled for a clinical interview session wherein trained staff administered informed consent and the MINI International Neuropsychiatric Interview (version 6.0 or 7.0)[Sheehan & Lecrubier, 2010; Sheehan et al., 2015] to assess lifetime disorders using Diagnostic and Statistical Manual of Mental Disorders, 4th Edition or 5th Edition (DSM-IV-TR or DSM-5) criteria [American Psychiatric Association 2000, 2013]. Initial exclusion criteria for all groups were: (1) positive urine/breathalyzer screen for alcohol and drugs of abuse; (2) lifetime bipolar and schizophrenia spectrum disorders; (3) moderate-to-severe traumatic brain injury; (4) significant or unstable medical disturbance not controlled by medication; (5) current suicide plan/intent; and (6) fMRI contraindications (e.g., metal in body, pregnancy). Figure 1 depicts the CONSORT diagram. Other than symptom requirements relating to GAD and MDD, the inclusion/exclusion criteria were identical across the two clinical studies. However, we only included individuals in the present analysis if they met diagnostic criteria for lifetime MDD at baseline. All participants included in the present analysis were required to have usable EEG data for the MID task based on EEG quality checks described below. The final MDD group consisted of 60 participants, 52% (n = 31) meeting criteria for a current major depressive episode and 98% (n = 59) meeting criteria for at least one past major depressive episode. With respect to comorbid diagnoses within the MDD group, 68% (n = 41) met criteria for at least one anxiety and/or stress disorder (see Table 2), while 7% met criteria for lifetime alcohol use disorder (n = 4). MDD participant medication information can be found in Table 3.
Figure 1.
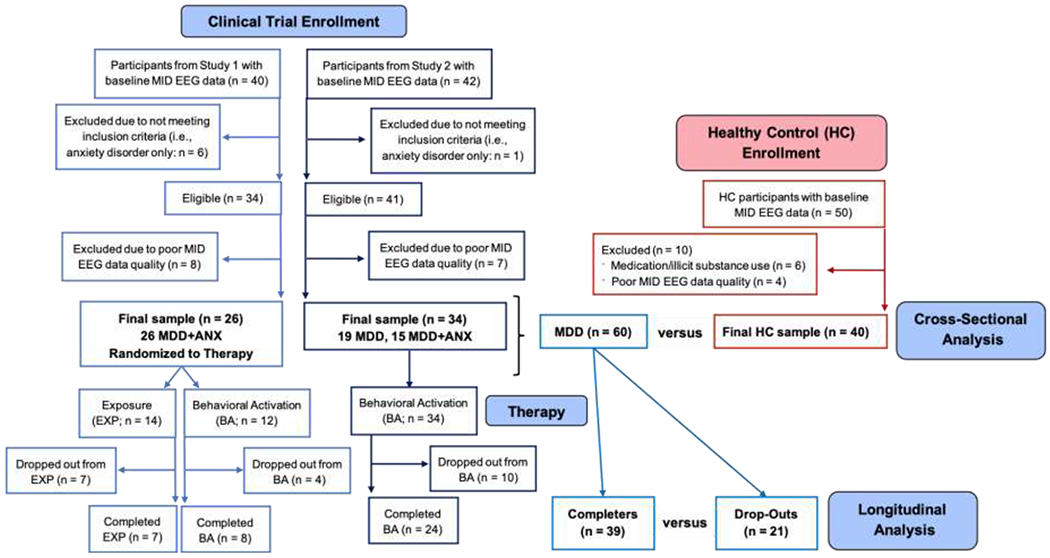
CONSORT diagram of participant recruitment and inclusion/exclusion criteria.
Table 2.
Baseline characteristics as a function of MDD therapy completion (n = 60).
| Completers (n = 39) | Non-completers (n = 21) | ||
|---|---|---|---|
|
| |||
| Frequency | Frequency | Statistics | |
|
| |||
| Study (S) 1 or 2 | 15 S1, 24 S2 | 11 S1, 10 S2 | χ2(1) = 1.08, p = 0.30 |
|
| |||
| Therapy Type | 32 BA, 7 EXP | 14 BA, 7 EXP | χ2(1) = 1.81, p = 0.18 |
| Sex: Female | 26 (67%) | 16 (76%) | χ2(1) = 0.59, p = 0.44 |
| Education (Categories) | Fisher’s Exact Test p = .65 | ||
| High School, GED or Less | 8 (21%) | 2 (10%) | |
| Some College | 14 (36%) | 9 (43%) | |
| Graduated 4-Year College | 11 (28%) | 5 (24%) | |
| Advanced Degree | 6 (15%) | 5 (24%) | |
| Therapy Type | 32 BA, 7 EXP | 14 BA, 7 EXP | χ2(1) = 1.80, p = .18 |
| Current MDE | 21 (54%) | 10 (48%) | χ2(1) = 0.21, p = .65 |
| Past MDE | 38 (97%) | 21 (100%) | Fisher’s Exact Test p = 1 |
| Any Comorbid Anxiety Disorder | 27 (69%) | 14 (67%) | χ2(1) = 0.04, p = .84 |
| Lifetime GAD | 21 (54%) | 13 (62%) | |
| Lifetime SAD | 10 (26%) | 4 (19%) | |
| Lifetime PTSD | 4 (10%) | 3 (14%) | |
| Lifetime PD | 7 (18%) | 3 (14%) | |
| Lifetime OCD | 0 (0%) | 1 (5%) | |
| Lifetime Alcohol Use Disorder | 2 (5%) | 2 (10%) | Fisher’s Exact Test p = .61 |
| Baseline Questionnaire Scores | M (SD) | M (SD) | Statistics |
| PROMIS Depression | 59.31 (6.87) | 59.12 (5.82) | Welch’s t(36) = 0.10, p = .92 |
| PROMIS Anxiety | 61.23 (6.82) | 63.23 (4.93) | Welch’s t(42) = 1.21, p = .23 |
| Behavioral Activation Scale (BAS) | 36.79 (7.01) | 39.90 (6.99) | Welch’s t(41) = 1.64, p = .11 |
| Behavioral Inhibition Scale (BIS) | 23.55 (4.75) | 23.24 (4.12) | W = 359, p = .52 |
| Beck Depression Inventory II | 20.90 (9.84) | 21.57 (6.19) | Welch’s t(56) = 0.32, p = .75 |
| Baseline MID P300 Amplitude * | 2.08 (2.53) | 0.54 (1.93) | Welch’s t(51) = 2.61, p = .01 |
| Baseline MID RT * | 240.69 (28.97) | 238.68 (31.89) | Welch’s t(38) = 0.27, p = .79 |
Note.
These metrics showed a significant difference between MDD and HC in cross-sectional analyses; raw RT scores are presented for group means and standard deviations but RT was log-transformed prior to t-test entry.
MDD = major depressive disorder. BA = behavioral activation. EXP = exposure. MDE = major depressive episode. GAD = generalized anxiety disorder. SAD = social anxiety disorder. PTSD = posttraumatic stress disorder. PD = panic disorder. OCD = obsessive compulsive disorder. PROMIS = Patient-Reported Outcomes Measurement Information System. MID = monetary incentive delay. RT = reaction time. Missing data: (1) PROMIS Depression: Completers: n = 1; Non-completers: n = 4; and (2) PROMIS Anxiety: Completers n = 3 and Non-completers n = 4. Outliers removed from: (1) RT analyses: n = 1 Completer and n = 1 Drop-Out; and (2) P300 amplitude analyses: n = 1 Completer and n = 1 Drop-Out.
Table 3.
Relationship of variables to reliable change index (RCI)-based treatment response within MDD therapy completers with post-treatment data (n = 38).
| Responders* (n = 19) | Non-Responders (n = 19) | ||
|---|---|---|---|
|
| |||
| Frequency | Frequency | Statistics | |
| Study (S) 1 or 2 | 5 S1, 14 S2 | 10 S1, 9 S2 | χ2(1) = 2.75, p = .10 |
| Therapy Type | 17 BA, 2 EXP | 14 BA, 5 EXP | Fisher’s Exact Test p = .40 |
| M (SD) | M (SD) | Statistics | |
| Baseline BDI-II score | 23.37 (10.57) | 18.63 (8.93) | Welch’s t(35) = 1.49, p = .15 |
| Post-Treatment BDI-II score | 7.53 (5.08) | 18.32 (11.71) | W = 289, p < .01 |
| Baseline MID P300 Amplitude ** | 2.64 (2.23) | 1.84 (2.48) | Welch’s t(34) = 1.02, p = .32 |
| Baseline MID RT ** | 241.02 (26.52) | 242.33 (31.36) | Welch’s t(35) = 0.10, p = .92 |
Note.
Treatment response was defined using the reliable change index (RCI) formula = (subject post-treatment BDI-II score - baseline BDI-II score)/(standard error of the difference [SEdiff] between the two BDI-II scores across subjects). SEdiff = standard deviation of the difference between the two BDI-II scores across subjects [SDdiff]*square root(1 − reliability [r] of the BDI-II). Subjects with BDI-II RCI scores > +1.96 were categorized as treatment responders. According to Beck et al. (1996), BDI-II r = .92, and within this sample, SDdiff = 10.54, so SEdiff = 2.98.
These metrics showed a significant difference between MDD and HC in cross-sectional analyses; RT was log-transformed prior to group statistics. MID = monetary incentive delay. RT = reaction time. The following outliers were removed: (1) P300 amplitude: n = 1 responder and n = 1 non-responder; and (2) RT: n = 1 responder.
While cross-sectional analyses compared MDD and HC groups, longitudinal analyses focused on MDD participants who enrolled in therapy (n = 60, 100%). Longitudinal analyses: (1) compared MDD participants who completed at least 7 out of 10 weeks of group based therapy (n = 39) versus those who dropped out prior to completion (n = 21); and (2) therapy completers considered treatment responders (reflected by the reliable change index (RCI) formula, n = 19) to non-responders (n = 19) who did not show this decrease.
Baseline Clinical Interview and Questionnaires
Participants completed a demographic questionnaire, the MINI for DSM-IV or DSM-5 (Sheehan & Lecrubier, 2010; Sheehan et al., 2015), PROMIS depression and anxiety subscales (Cella et al., 2010), and the Behavioral Inhibition/Behavioral Activation (BIS/BAS) Scales (Carver & White, 1994) prior to simultaneous EEG-fMRI recording. MDD participants completed the Beck Depression Inventory II (BDI-II; Beck et al., 1996) at baseline, and individuals who completed therapy also filled out the BDI-II post-treatment. Both treatments consisted of manualized, ten-session interventions and were delivered in a group format (6-10 participants per group) for 90 min weekly. Details regarding therapy supervision, consultation, and fidelity can be found in supplemental material.
MID Task
Participants completed two runs (562 s; 45 per run) of the MID task (Knutson et al., 2001) during simultaneous EEG-fMRI recording. On each trial, participants were first presented with a fixation circle (duration: 2-6 s), followed by a cue (duration: 2 s) indicating potential gain (circle), loss (square), or no gain/loss (circle or square) and a subsequent 2 s delay wherein a cross appeared on the screen. Degree of potential gain/loss varied as a function of the location of a horizontal line within the cue and was also indicated with text: (1) bottom of the cue = no gain/loss (+/−$0); (2) the middle of the cue = intermediate gain/loss (+/−$1); and (3) line at the top of the cue = highest gain/loss (+/−$5). To earn a gain or avoid a loss, participants were required to press a button within a certain duration of time (callibrated to individual reaction time [RT]) following onset of a white triangle (target). Task difficulty was based on RT collected during practice prior to the neuroimaging scan and was calibrated to a success rate of two-thirds of trials. After participants made their response, the computer screen went blank (interstimulus interval between delay and blank screen = 2 s). Then feedback was presented for 2 s indicating the result (+0, −0, +1, −1, +5, −5) on the trial (15 trials per run per conditions, i.e., 30 trials per condition overall). Trial order was pseudo-randomized, and all participants completed the runs in the same order. Participants earned $30 on average, which they were paid post-scan.
Simultaneous EEG and fMRI Data Collection
Details of fMRI image acquisitionare not reported in the current study but can be found in the parent study protocol paper (Santiago et al., 2020). EEG was recorded during fMRI using a 32-channel MR-compatible EEG system (Brain Products GmbH; Munich, Germany) referenced online to FCz. The electrodes were arranged according to the international 10–20 system. One electrode was placed on the back for recording the electrocardiogram (ECG) signal. A Brain Products’ SyncBox device was used to synchronize the EEG system clock with the 10 MHz MRI scanner clock. Data acquisition sampling rate was 5 kHz, measurement resolution was 0.1 μV. EEG signals were hardware-filtered throughout data acquisition between 0.016 and 250 Hz.
EEG Preprocessing
EEG processing was completed using Brain Vision Analyzer (BVA) 2.1 (Brain Products GmbH, Munich, Germany). Gradient artifact in the EEG data from the fMRI recording was removed in accordance with recommendations from Debener et al. (2007). EEG were re-referenced offline to linked mastoids (e.g., Novak et al., 2015), down-sampled to 250 Hz, and bandpass filtered from .01-30 Hz with a 12db/oct roll-off. Notch filters were applied (1 Hz bandwidth) to remove fMRI slice timing frequency (19.5 Hz and harmonics; see: Mayeli et al., 2021) and 60 Hz line noise (see: Luck, 2014). Ballistocardiogram (BCG) artifacts were reduced using average artifact subtraction (AAS) employing a semi-automated routine in BVA to detect R peaks in the ECG channel and create a template for BCG correction. Additionally, independent components analysis (ICA) using the extended infomax routine was employed to isolate remaining BCG artifact in addition to ocular artifacts (i.e., blinks,saccades). Automated routines were used to identify and exclude from analysis artifacts defined as more than 50 μV change between any two data points; trials with absolute fluctuations exceeding 200 μV in any 200 ms interval and/or any change of less than 0.5μV in a 200 ms period (i.e., flatlining) (e.g., Angus et al., 2017). Participants were required to have at least 15 out of 30 usable cue trials (50% usable data; Bennett et al., 2017; Landes et al., 2018) to create single-participant averages for each of the three conditions (gain, loss, no gain/loss) and be included in analyses. Wilcoxin rank sum tests indicated groups did not differ in usable trials for gains (W = 1303, p = .47, median MDD = 27, median HC = 27), losses (W = 1256, p = .70, median MDD = 27, median HC = 28), or no gains/losses (W = 1372, p = .22, median MDD = 27, median HC = 28).
ERP Quantification and Extraction
Figure 3 depicts grand average ERP waveforms stimulus-locked to cue onset across participants (n = 100) as a function of three conditions: (1) gain (+$,1 +$5 trials); (2) loss (−$1, −$5 trials); and (3) no gain/loss (+$0, −$0 trials). Measurement windows were based on prior studies (e.g., Broyd et al., 2012; Novak & Foti, 2015; Novak et al., 2015). Waveforms were baselined to 200 ms prior to cue onset (Bennett et al., 2019; Broyd et al., 2012; Landes et al., 2018). Mean amplitudes were extracted for further statistical analysis using the following windows time-locked to cue onset: (1) N200 = 300-400 ms at Fz and Cz; (2) P300 = 300-600 ms at Pz and POz; and (3) CNV = 4050-4250 ms at Fz and Cz.
Figure 3.
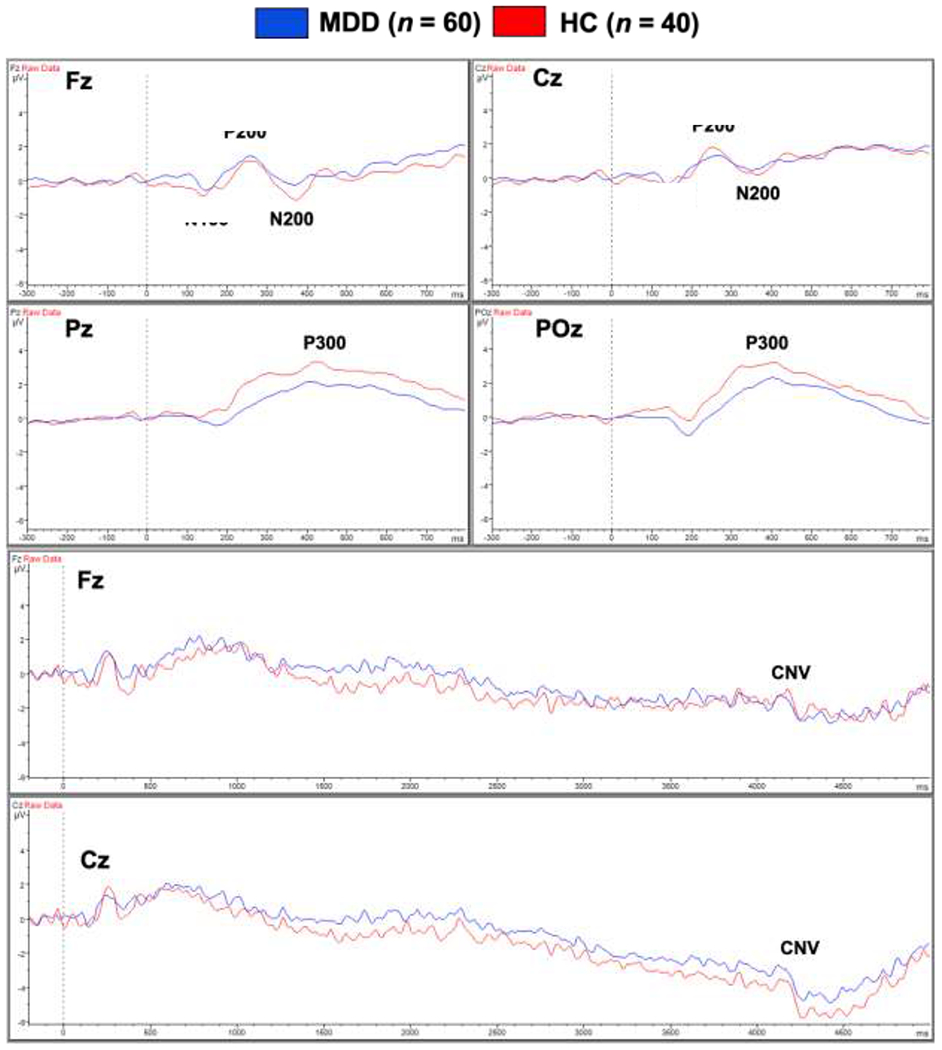
Grand average waveforms for the anticipation phase of the MID task, stimulus-locked to the onset of gain, loss, and no gain/loss cues for major depressive disorder (MDD; n = 60) and healthy control (HC; n = 40) groups.
Statistical Analyses
Statistical analyses were performed in R Studio 1.1.463 using functions within car, ggplot2, gmodels, nlme, psych, and multcomp packages and code guided by Field et al. (2012). For dimensional variables, histograms were plotted to evaluate univariate normality, and z-score transformations were used to detect outliers z > |3.291| (Tabachnick & Fidell, 2007). Outliers on a particular variable were removed entirely from analyses that included this variable. The following number of participants were indexed as ERP outliers and removed from respective analyses: (1) N200: n = 1 MDD; (2) P300: n = 1 HC and n = 2 MDD; and (3) CNV: n = 5 MDD. Levene’s test was used to confirm the group homogeneity of variance assumption prior to computation of parametric tests. Log transformations were used to normalize variables with substantial skew. For categorical variables, chi-square tests were computed between groups unless small cell sizes required the use of Fisher’s exact test. For continuous dependent variables compared between groups, Welch’s two-sample t-test was computed when assumptions were upheld, otherwise Wilcoxon rank-sum tests were used.
Cross-Sectional Analyses
Demographics and clinical characteristics.
Categorical differences in education, sex, and race/ethnicity were compared between MDD and HC. Welch’s two-sample t-test examined group differences in age, whereas depression, anxiety, and BIS/BAS scores were compared between groups using Wilcoxon rank sum non-parametric tests due to violated assumptions of normality and homogeneity of variance.
Behavioral performance.
Linear mixed effects (LME) models were computed for the two behavioral dependent variables (correct hits, log-transformed RT). Group (MDD, HC) was the between-subjects variable, condition (gain, loss, no gain/loss) was the within-subjects variable. Maximized log-likelihood was employed for analysis. Participant was included as a random effect.
ERPs.
LME models were computed for each of the three dependent variables (N200, P300, and CNV mean amplitudes). Group (MDD, HC) was the between-subjects variable, while condition (gain, loss, no gain/loss) and electrode (Fz and Cz for all ERPs except P300, which used Pz and POz) were within-subjects variables. ERP mean amplitude was the dependent variable. Maximized log-likelihood was employed for analysis. Participant was included as a random effect. Cohen’s d effect size was computed for significant effects. Simultaneous post-hoc tests for significant effects were computed, using the Holm-Bonferroni method to adjust p values for multiple comparisons.
Longitudinal Analyses
MDD participants were classified as completers if they attended at least 7 out of 10 therapy sessions (n = 39) and non-completers if they attended less than 7 sessions (n = 21). These MDD subgroups were compared on study assignment (1 or 2) and baseline characteristics including sex, education, clinical diagnoses, questionnaire scores, average RT, and average P300 amplitude (Pz and POz). RT and P300 amplitude were chosen because they significantly differentiated MDD and HC groups in cross-sectional analyses. Within MDD therapy completers with post-treatment questionnaire data (n = 38), treatment responders using RCI, see: Table 3;n = 19) were compared to non-responders (n = 19) on ERP amplitude and RT.
Results
Cross-Sectional Analyses
Demographics and clinical characteristics (Table 1).
Table 1.
Group demographic and clinical characteristics.
| MDD (n = 60) | HC (n = 40) | ||
|---|---|---|---|
|
| |||
| Frequency | Frequency | Statistics | |
| Sex: Female | 42 (70%) | 20 (50%) | χ2(1) = 4.07, p = .04 |
| Race/Ethnicity: Non-White | 14 (23%) | 11 (28%) | χ2(1) = 0.22, p = .64 |
| Asian | 1 (2%) | 1 (2%) | |
| Black | 5 (8%) | 1 (2%) | |
| Hispanic | 0 (0%) | 1 (2%) | |
| Native American | 4 (7%) | 6 (15%) | |
| Other | 4 (7%) | 2 (5%) | |
| White | 46 (76%) | 29 (74%) | |
| Education (Categories) | Fisher’s Exact Test p = .01 | ||
| High School, GED or Less | 10 (17%) | 6 (15%) | |
| Some College | 23 (38%) | 19 (48%) | |
| Graduated 4-Year College | 16 (27%) | 15 (37%) | |
| Advanced Degree | 11 (18%) | 0 (0%) | |
| M (SD) | M (SD) | Statistics | |
| Age (Years) | 34.12 (10.87) | 31.19 (10.41) | Welch’s t(86) = −1.35, p = .18 |
| PROMIS Depression (T score) | 59.25 (6.51) | 43.90 (6.33) | W = 88, p < .001 |
| PROMIS Anxiety (T score) | 61.88 (6.28) | 45.76 (7.41) | W = 98, p < .001 |
| Behavioral Activation Scale (BAS) | 37.90 (7.10) | 40.58 (4.28) | W = 1429, p = .08 |
| Behavioral Inhibition Scale (BIS) | 23.48 (4.47) | 17.38 (3.22) | W = 311, p < .001 |
Note. MDD = major depressive disorder. HC = healthy control. GED = general educational development. PROMIS = Patient-Reported Outcomes Measurement Information System. Missing data: (1) PROMIS Depression: MDD n = 5; (2) PROMIS Anxiety: MDD n = 8; and (3) BAS and BIS (MDD n = 1).
Groups did not differ as a function of non-White race/ethnicity (p = .64), or age (p = .18). However, groups differed in: (1) sex: the MDD group included more females than the HC group (χ2(1) = 4.07, p = .04); and (2) education (Fisher’s exact test p = .01): more MDD participants (n = 11, 18%) had attained masters or doctoral degrees than HC participants (0%). MDD reported higher PROMIS depression (W = 88, p < .001) and anxiety (W = 98, p < .001) symptoms than HC. Although MDD endorsed higher BIS scores than HC (W = 311, p < .001), groups did not differ on BAS scores (p = .08).
Behavioral performance (Table S1, post-hoc tests are listed in Table S3).
For RT, the group main effect F(1, 95) = 4.06, p < .05) indicated that MDD were faster than HC across conditions (d = .39, Figure 4A), while the condition main effect (F(2, 190) = 51.50, p < .001) showed that participants responded more quickly to gains and losses than no gains/losses (both post-hoc p < .001 and d = 0.79 and 0.80, Figure 4B). Post-hoc tests examining each MDD subgroup and HC indicated that both pure and comorbid MDD groups exhibited faster RT than HC (p < .01 and p = .02, respectively). No significant effects emerged for correct hits (all p > .40). The influence of sex and education as well as current vs. past MDD were evaluated using linear mixed models and are reported in supplemental material.
Figure 4.
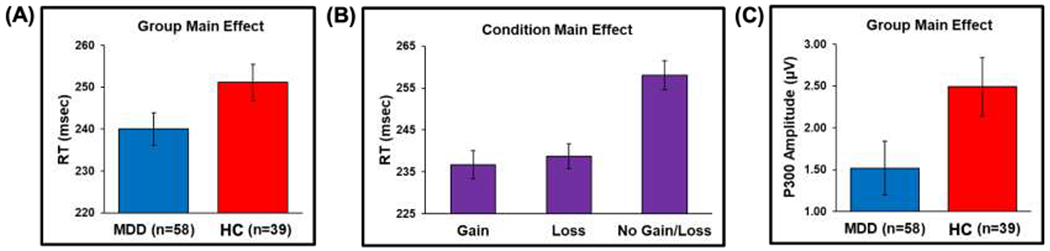
(A) Group main effect showing that MDD exhibited faster reaction time (RT) than HC across conditions; (B) Condition main effect indicating that gains and losses elicited faster RT than no gains/losses; and (C) Group main effect demonstrating that MDD exhibit smaller P300 amplitude than HC. Error bars reflect ±1 standard error.
Mean ERP amplitudes. (Table S2, post-hoc tests with are listed in Table S3).
Between-group differences did not emerge for N200, or CNV amplitudes. For the N200 a main effect of electrode (F(1, 485) = 21.45, p < .001) depicted in Figure S1 indicated that N200 amplitudes were larger at Fz (M = −0.26, SD = 2.06) than Cz (M=0.48, SD=1.98; d=.47 For the CNV a main effect of electrode (F(1, 465) = 29.65, p < .001) illustrated in Figure S2 showed that CNV amplitudes were larger at Cz (M = −3.56, SD = 3.18) than Fz (M = −1.75, SD = 3.03; d = 57). A main effect of group emerged for P300 amplitude (F(1, 95) = 4.06, p < .05), and Figure 4C demonstrates that MDD displayed smaller P300 amplitudes than HC (d = 0.42). To determine whether the MDD-HC difference in P300 amplitude was driven by participants with pure (n = 18) versus comorbid MDD (n = 40), post-hoc tests computed between each MDD subgroup and HC indicated that both pure MDD and comorbid MDD groups showed smaller P300 amplitudes than HC (p = .03 and p < .001, respectively). Although a main effect of condition emerged for P300 amplitude (F(2, 475) = 8.05, p < .001) illustrated in Figure S3, post-hoc tests indicated that no condition differences were significant after adjustment for multiple comparisons (all p ≥ .16). The influence of sex and education as well as current vs. past MDD were evaluated using linear mixed models and are reported in supplemental material, the results of which maintained support for group differences reported above.
Longitudinal Analyses
Table 2 and Figure 5 indicate that MDD therapy completers exhibited higher baseline P300 amplitudes (Welch’s t(51) = 2.61, p = .01, Cohen’s d = .69) than non-completers. Flowever, completers and non-completers did not differ as a function of study assignment, therapy type, sex, education, frequency of major depressive episodes or comorbid anxiety disorders, baseline internalizing symptoms, or baseline RT. Table 3 shows that BDI-II treatment responders and non-responders did not differ on study assignment, therapy type, baseline BDI-II scores, P300 amplitudes, or RT.
Figure 5.
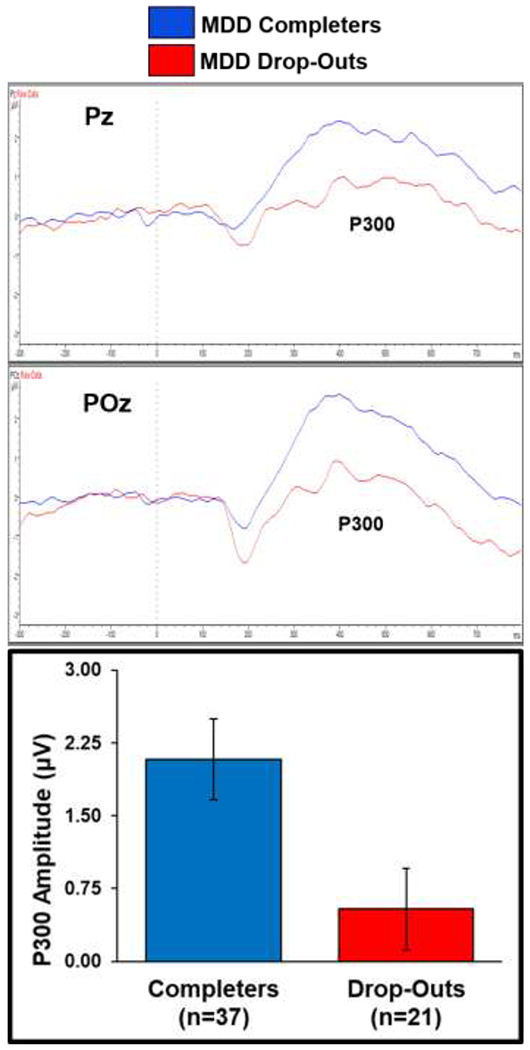
P300 grand average waveforms across MID cue conditions are overlaid for MDD therapy completers and non-completers at Pz and POz electrode sites. Completers exhibited higher baseline P300 amplitudes than non-completers across conditions and electrodes. Error bars reflect ±1 standard error.
Discussion
The goal of the current study was to determine utility of ERPs recorded during the anticipation phase of the MID task in: (1) distinguishing MDD from HC; and (2) predicting treatment outcomes (attendance and symptom reduction) for MDD completing behavior therapy. First, results indicate that individuals with MDD do in fact display lower P300 amplitude responses to cue stimuli relative to individuals with no mental health conditions. The group difference was not modulated by the condition of the cue, suggesting broad attention blunting to stimuli regardless of relevance for upcoming opportunities to obtain gains or avoid losses. This finding is similar to other studies reporting links between blunted P300 amplitude and depression using other cognitive paradigms such as the flanker task (e.g., Santopetro et al., 2020) and oddball task (Nan et al., 2018). Second, the hypothesis of blunted N200 amplitude to cues among the MDD group was not supported. This distinguishes the current pattern of results from previous studies showing blunted N200 among individuals with MDD in the flanker task (e.g., Alderman et al., 2015; Clawson et al., 2013) and the color-word Stroop task (e.g., Holmes & Pizzagalli, 2008). Thus, the current pattern of results would indicate that individuals with MDD display overall decrements in attention categorization (P300) but not with selective attention or discerning of conditions (N200) in the MID paradigm. The CNV also did not demonstrate differences across group or condition, indicating that in these data attentional preparation was not influenced by opportunities for loss or gain nor diagnostic group.
The lack of condition modulation in the group P300 effects in combination with previous literature regarding P300 blunting in individuals with MDD indicates that the attentional decrements associated with MDD are not specific to reward anticipation context. Consistent with the present results, a recent meta-analysis of fMRI and EEG effects in reward tasks indicated that MDD-related differences in anticipation may not be specific to reward versus loss contexts (Keren et al., 2018). The current findings extend this literature, as the authors of the meta-analysis noted a relative dearth of research examining EEG reactivity during anticipation (Keren et al., 2018). It is notable that the present analysis did not examine target elicited ERPs or reward consumption elicited due to conceptual focus on the anticipation period as well as task design considerations and limitations noted below and that the current pattern of results do not preclude reward-specific differences in MDD relative to HC in the consummatory phase of reward processing noted in other studies (e.g., Keren et al., 2018).
Longitudinal data indicated pre-treatment P300 amplitude was also higher for MDD participants who went on to complete therapy relative to those who did not. This finding extends previous evidence that the P300 distinguishes individuals with MDD from HC participant to differentiating individuals who go on to complete behavioral treatments from those who do not. Notably, MDD completers and non-completers did not differ on frequency of depressive episodes or symptom severity. Findings indicate that individuals with higher attentional engagement with cue stimuli were more likely to complete behavior therapy (EXP and BA), information that may be useful in informing treatment decisions. Specifically, research has demonstrated that brief (e.g., Funderburk et al., 2020) and even single session (e.g., Gawrysiak et al., 2009) behavior therapies are beneficial for treating MDD or comorbid anxiety conditions. Thus, P300 reactivity may be helpful in directing individuals to treatments that they are more likely to complete. Although the current study did not include a medication treatment, previous work has demonstrated that baseline P300 latency predicts response to sertraline treatment (Isintas et al., 2012) and that resting-state EEG robustly predicted response to sertraline in a placebo controlled trial (Wu et al., 2020). Thus current results add to burgeoning evidence of the utility of EEG metrics in informing treatment decisions for MDD.
The current study possesses several limitations. First, the MID task was initially designed for fMRI data collection, not for measuring ERP responding to target cues. Thus, the design included variability in target presentation timing and duration that led to offset potential and motor confounds in the ERP data during target response. Furthermore, feedback is related to performance and reward consumption, leading to discrepancies in the number of trials available for analyzing feedback potentials(i.e., RewP, FRN) and conceptual differences with reward anticipation which are beyond the scope of the current study. However, these limitations did not influence ERP responding to cue stimuli during the anticipation phase, which is an understudied area within this literature (Keren et al., 2018). Second, this study does not distinguish between EXP or BA in terms of treatment completion due to lack of power to examine differences between treatmants; thus this research cannot distinguish between potential nuances in effects between treatment modalities. Third, although MDD is a highly heterogeneous condition, the present analyses do not account for differential presentations of the disorder, including the presence of current versus past MDE; however examination of the cross-sectional effects can be found in the supplemental material and both groups showed at least a trending effect in the same direction for P300 amplitude results. It is notable that depression-anxiety comorbidity and symptom severity did not influence primary effects observed. Fourth, although fMRI data were collected simultaneously with EEG data for this task, the present report does not integrate fMRI and ERP data. As our primary research questions focused on the degree of neural resources allocated to stimulus processing in MDD, ERP metrics possessing resolution on the order of milliseconds were ideal to address these particular questions. Finally, sex and education were not equivalent across groups in the cross sectional analysis. Follow-up LMEs including sex and education as predictors are included in supplement. Notably these analyses are underpowered to rigorously characterize the effects. Furthermore, sex and education did not differ between the completer and non-completer groups examined in the longitudinal analyses. Future integration of EEG and fMRI data will be useful for targeting spatial resolution of neural processing alterations in MDD.
Despite these limitations, the current investigation benefits from the testing of well-characterized ERP components and longitudinal therapy data to show that blunted resource allocation (P300 amplitude) to stimulus attention present in MDD when compared to HC is also linked to future non-completion of behavioral therapy for MDD patients. P300 metrics may show utility in a precision-medicine approach to treatment selection for individuals with MDD, directing patients to treatment options which they are more likely to complete. Therefore, these results extend a growing body of literature indicating the utility of EEG measures in predicting treatment considerations for depression (e.g. Isintas et al., 2012; Wu ert al., 2020).
Supplementary Material
Figure 2.
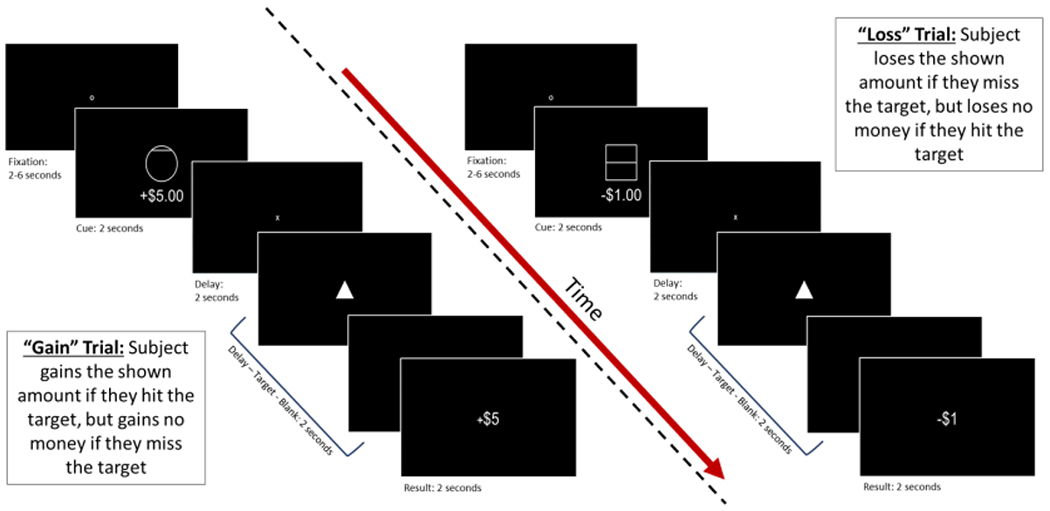
MID task design. This figure provides an overview of stimulus type and duration for gain trials (left) and loss (right) trials. Analyses for the present study focus on event related potentials during presentation of each cue stimulus.
Highlights.
P300 amplitude is smaller for individuals with major depressive disorder compared to healthy controls
Other cue elicited event-related potentials during the monetary incentive delay task do not differ between individuals with depression and healthy individuals
Among individuals with depression, P300 amplitude is predictive of behavioral treatment completion
Acknowledgements
This work has been supported in part by The William K. Warren Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author statement:
Evan J. White Ph.D. receives funding support from the National Institue on Minority Health and Health Disparaties (NIMHD) under award number: K99MD015736. Rayus Kuplicki, Ph.D. Jennifer L. Stewart, Ph.D., Namik Kirlic, Ph.D., Robin Aupperle Ph.D., and Martin Paulus M.D. receive funding from the National Institute of General Medical Sciences (NIGMS) center grant P20GM121312; Robin Aupperle Ph.D. has additional grant funding from NIMH (K23MH108707; R01MH123691); and Martin Paulus, M.D. has additional grant funding from the National Institute of Drug Abuse (U01DA041089). Timothy McDermott, M.A., received support from the National Institute of Mental Health under Award Number F31MH122090. All authors have approved the final manuscript for submission.
Financial Disclosures:
Evan J. White Ph.D. receives funding support from the National Institue on Minority Health and Health Disparaties (NIMHD) under award number: K99MD015736. Rayus Kuplicki, Ph.D. Jennifer L. Stewart, Ph.D., Namik Kirlic, Ph.D., Robin Aupperle Ph.D., and Martin Paulus M.D. receive funding from the National Institute of General Medical Sciences (NIGMS) center grant P20GM121312; Robin Aupperle Ph.D. has additional grant funding from NIMH (K23MH108707; R01MH123691); and Martin Paulus, M.D. has additional grant funding from the National Institute of Drug Abuse (U01DA041089) and Dr. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate. Timothy McDermott, M.A., received support from the National Institute of Mental Health under Award Number F31MH122090.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
Dr. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate. Authors have no other conflicts of interest to disclose.
References
- Alderman BL, Olson RL, Bates ME, Selby EA, Buckman JF, Brush CJ, … & Shors TJ (2015). Rumination in major depressive disorder is associated with impaired neural activation during conflict monitoring. Frontiers in Human Neuroscience, 9, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. American Psychiatric Association; 2013. [Google Scholar]
- Angus DJ, Latham AJ, Harmon-Jones E, Deliano M, Balleine B, & Braddon-Mitchell D (2017). Electrocortical components of anticipation and consumption in a monetary incentive delay task. Psychophysiology, 54, 1686–1705. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98, 227–239. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown G (1996). Beck depression inventory-II. Psychological Assessment. [Google Scholar]
- Bennett MP, Kiiski H, Cao Z, Farina FR, Knight R, Sweeny A, Roddy D, Kelly C, & Whelan R (2019). Hyperactive/impulsive and inattention symptoms are associated with reduced ERP activity during different reward processing stages: Evidence from the electrophysiological Monetary Incentive Delat Task in adult ADHD. bioRxiv. 10.1101/817973 [DOI] [Google Scholar]
- Borsini A, Wallis ASJ, Zunszain P, Pariante CM, & Kempton MJ (2020). Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognitive, Affective & Behavioral Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, & Sonuga-Barke EJ (2012). An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. Journal of Neuroscience Methods, 209, 40–49. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, & Tenke CE (2012). Event-related brain potentials in depression: Clinical, cognitive, and neurophysiological implications. In Luck SJ & Kappenman ES (Eds.), Oxford library of psychology. The Oxford handbook of event-related potential components (p. 563–592). Oxford University Press. [Google Scholar]
- Brush CJ, Foti D, Bocchine AJ, Muniz KM, Gooden MJ, Spaeth AM, … & Alderman BL (2020). Aerobic exercise enhances positive emotional reactivity in individuals with depressive symptoms: Evidence from neural responses to reward and emotional content. Mental Health and Physical Activity, 19, 100339. 10.1016/i.mhpa.2020.100339 [DOI] [Google Scholar]
- Burkhouse KL, Gorka SM, Klumpp H, Kennedy AE, Karich S, Francis J, … & Flajcak G (2018). Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and selective serotonin reuptake inhibitor treatment. Journal of Clinical Psychiatry, 79, 17m11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, & Klumpp H (2016). Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depression and Anxiety, 33, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- Celia D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, … & Cook K (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson A, Clayson PE, & Larson MJ (2013). Cognitive control adjustments and conflict adaptation in major depressive disorder. Psychophysiology, 50, 711–721. [DOI] [PubMed] [Google Scholar]
- Debener S, Strobel A, Sorger B, Peters J, Kranczioch C, Engel AK, & Goebel R (2007). Improved quality of auditory event-related potentials recorded simultaneously with 3-T fMRI: removal of the ballistocardiogram artefact. Neuroimage, 34, 587–597. 10.1016/j.neuroimage.2006.09.031 [DOI] [PubMed] [Google Scholar]
- Diner BC, Holcomb PJ, & Dykman RA (1985). P300 in major depressive disorder. Psychiatry Research, 15, 175–184. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, & Gilbody S (2008). A meta-analysis of randomized trials of behavioural treatment of depression. Psychological Medicine, 38, 611–623. [DOI] [PubMed] [Google Scholar]
- Field A, Miles J, & Field Z (2012). Discovering statistics using R. Sage publications. [Google Scholar]
- Flores A, Munte TF, & Donamayor N (2015). Event-related EEG responses to anticipation and delivery of monetary and social reward. Biological Psychology, 109, 10–19. [DOI] [PubMed] [Google Scholar]
- Funderburk JS, Pigeon WR, Shepardson RL, & Maisto SA (2020). Brief behavioral activation intervention for depressive symptoms: Patient satisfaction, acceptability, engagement, and treatment response. Psychological Services, 17, 443–451. [DOI] [PubMed] [Google Scholar]
- Gawrysiak M, Nicholas C, & Hopko DR (2009). Behavioral Activation for Moderately Depressed University Students: Randomized Controlled Trial. Journal of Counseling Psychology, 56, 468–475. [Google Scholar]
- Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, & Nusslock R (2018). Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International Journal of Psychophysiology, 132, 184–202. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, & Kessler RC (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry, 76, 155–162. [DOI] [PubMed] [Google Scholar]
- Gu R, Jiang Y, Kiser S, Black CL, Broster LS, Luo YJ, & Kelly TH (2017). Impulsive personality dimensions are associated with altered behavioral performance and neural responses in the monetary incentive delay task. Neuropsychologia, 103, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Klawohn J, & Meyer A (2019). The utility of event-related potentials in clinical psychology. Annual Review of Clinical Psychology, 15, 71–95. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, & Pizzagalli DA (2008). Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia, 46(12), 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D (2012). ERPs and the study of emotion. In Luck SJ & Kappenman ES (Eds.), Oxford library of psychology. The Oxford handbook of event-related potential components (p.441–472). Oxford University Press. [Google Scholar]
- Iancu SC, Wong YM, Rhebergen D, van Balkom AJ, & Batelaan NM (2020). Long-term disability in major depressive disorder: a 6-year follow-up study. Psychological Medicine, 50, 1644–1652. [DOI] [PubMed] [Google Scholar]
- Ilardi SS, Atchley RA, Enloe A, Kwasny K, & Garratt G (2007). Disentangling attentional biases and attentional deficits in depression: An event-related potential P300 analysis. Cognitive Therapy and Research, 31, 175–187. [Google Scholar]
- Isintas M, Ak M, Erdem M, Oz O, & Ozgen F (2012). Event-related potentials in major depressive disorder: the relationship between P300 and treatment response. Turk Psikiyatri Derg, 23, 9–33. [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, & Williams LM (2008). Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectrums, 13, 1066–1086. [DOI] [PubMed] [Google Scholar]
- Klawohn J, Burani K, Bruchnak A, Santopetro N, & Hajcak G (2020). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychological Medicine, 1–9. 10.1017/s0033291719003659 [DOI] [PubMed] [Google Scholar]
- Klawohn J, Santopetro NJ, Meyer A, & Hajcak G (2020). Reduced P300 in depression: Evidence from a flanker task and impact on ERN, CRN, and Pe. Psychophysiology, 57, e13520. [DOI] [PubMed] [Google Scholar]
- Landes I, Bakos S, Kohls G, Bartling J, Schulte-Korne G, & Greimel E (2018). Altered neural processing of reward and punishment in adolescents with major depressive disorder. Journal of Affective Disorders, 232, 23–33. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Crane NA, Jenkins LM, Phan KL, & Klumpp H (2018). Pathways to neuroprediction: Opportunities and challenges to prediction of treatment response in depression. Current Behavioral Neuroscience Reports, 5, 48–60. [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An Introduction to the Event-Related Potential Technique, second edition. MIT Press. [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: An event-related potential study. Cognitive Therapy and Research, 40, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsen J, Perrone-McGovern K, & Marmarosh C (2020). Using event-related potentials to explore processes of change in counseling psychology. Journal of Counseling Psychology, 67, 500–508. [DOI] [PubMed] [Google Scholar]
- Mayeli A, Al Zoubi O, Henry K, Wong CK, White E, Luo Q, Zotev V, Refai H, & Bodurka JA (2021). Automated Pipeline for EEG Artifact Reduction (APPEAR) Recorded during fMRI. Journal of Neural Engineering. 10.1088/1741-2552/ac1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, & Poulton R (2007). Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry, 64, 651–660. [DOI] [PubMed] [Google Scholar]
- Nagy GA, Cernasov P, Pisoni A, Walsh E, Dichter GS, & Smoski MJ (2020). Reward network modulation as a mechanism of change in behavioral activation. Behavior Modification, 44, 186–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan C, Wang G, Wang H, Wang X, Liu Z, Xiao L, … & Wu S (2018). The P300 component decreases in a bimodal oddball task in individuals with depression: an event-related potentials study. Clinical Neurophysiology, 129, 2525–2533. [DOI] [PubMed] [Google Scholar]
- Novak KD, & Foti D (2015). Teasing apart the anticipatory and consummatory processing of monetary incentives: An event-related potential study of reward dynamics. Psychophysiology, 52, 1470–1482. [DOI] [PubMed] [Google Scholar]
- Novak BK, Novak KD, Lynam DR, & Foti D (2016). Individual differences in the time course of reward processing: stage-specific links with depression and impulsivity. Biological Psychology, 119, 79–90. [DOI] [PubMed] [Google Scholar]
- Olbrich S, & Arns M (2013). EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. International Review of Psychiatry, 25, 604–618. [DOI] [PubMed] [Google Scholar]
- Oumeziane BA, Schryer-Praga J, & Foti D (2017). “Why don’t they ‘like’ me more?”: Comparing the time courses of social and monetary reward processing. Neuropsychologia, 107, 48–59. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, & Klein DN (2015). Depression and event-related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology, 4, 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santopetro NJ, Kallen AM, Threadgill AH, & Hajcak G (2020). Reduced flanker P300 prospectively predicts increases in depression in female adolescents. Biological Psychology, 156, 107967. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y. The Mini International Neuropsychiatric Interview Version 6.0. MINI 6.0. Medical Outcomes Systems Inc.; 2010. [Google Scholar]
- Sheehan D, Janavs J, Baker R, Sheehan KH, Knapp E, Sheehan M. MINI International Neuropsychiatric Interview-Version 7.0. (MINI 7.0). Medical Outcomes Systems Inc.; 2015. [Google Scholar]
- Sherbourne CD, & Wells KB (1997). Course of depression in patients with comorbid anxiety disorders. Journal of Affective Disorders, 43, 245–250. [DOI] [PubMed] [Google Scholar]
- Stange JP, MacNamara A, Kennedy AE, Hajcak G, Phan KL, & Klumpp H (2017). Brain-behavioral adaptability predicts response to cognitive behavioral therapy for emotional disorders: A person-centered event-related potential study. Neuropsychologia, 145, 106408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, MacNamara A, Barnas O, Kennedy AE, Hajcak G, Phan KL, & Klumpp H (2017). Neural markers of attention to aversive pictures predict response to cognitive behavioral therapy in anxiety and depression. Biological Psychology, 123, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2007). Using multivariate statistics (5th ed.). Boston, MA: Allyn & Bacon/Pearson Education. [Google Scholar]
- Vanderhasselt MA, De Raedt R, De Paepe A, Aarts K, Otte G, Van Dorpe J, & Pourtois G (2014). Abnormal proactive and reactive cognitive control during conflict processing in major depression. Journal of Abnormal Psychology, 123, 68. [DOI] [PubMed] [Google Scholar]
- Wade EC, & Iosifescu DV (2016). Using electroencephalography for treatment guidance in major depressive disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1, 411–422. [DOI] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, Bondy E, Stanton CH, Appleman L, & Pizzagalli DA (in press). Reward-related neural predictors and mechanisms of symptom change in cognitive behavioral therapy for depressed adolescent girls. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, Wright R, Toll RT, Trivedi HM, Monuszko K, Caudle TL, Sarhadi K, Jha MK, Trombello JM, Deckersbach T, Adams P, McGrath PJ, Weissman MM, Fava M, Pizzagalli DA, Arns M, Trivedi MH, & Etkin A (2020). An electroencephalographic signature predicts antidepressant response in major depression. Nature Biotechnology, 38, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Jiao X, Tang Y, Chen S, Tong S, Wang J, & Sun J (2019). Temporal characteristics of attentional disengagement from emotional facial cues in depression. Neurophysiologie Clinique, 49, 235–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


