Abstract
In 2 broiler trials, the effects of chestnut tannins on performance and meat quality (trial 1), and digestion (trial 2) were evaluated. In both trials, Ross 308 broilers received one of 2 basal diets: one basal diet contained corn and soy as main feed ingredients, while the challenge basal diet contained wheat, palm oil, and rapeseed meal. The composition of the basal diets was chosen to assess the interaction between chestnut tannins and diet composition. To both basal diets, chestnut tannins were added at 3 doses: 0 mg/kg (T−), 500 mg/kg (T+), or 2,000 mg/kg (T++), resulting in a total of 6 treatments. In trial 1, both basal diets containing 2,000 mg/kg chestnut tannins lowered broiler performance in grower and finisher phases. A tannin dose of 500 mg/kg had no effect on performance in either basal diet. Corn-based diets resulted in lower meat pH compared to wheat diets. Further, addition of chestnut tannins resulted in increased meat pH, and caused proportionally a lower meat drip loss and shear force for both basal diets. During the digestibility study (trial 2), blood was also collected. None of the treatments affected digestibility or blood parameters (glucose, non-esterified fatty acids, and triacylglycerols). Malondialdehyde (MDA) was measured in plasma to assess antioxidative properties of chestnut tannins. In wheat diets, chestnut tannins significantly lowered plasma MDA demonstrating its antioxidative nature. Regarding gut health, crypt depth decreased proportionally with the dosage of chestnut tannins in both basal diets with significantly shallower crypts for the wheat diets compared to the corn diets. Relative intestinal growth was stimulated in the wheat diets proportionally to the tannin dose based on the larger relative gut length. In conclusion, chestnut tannins did not influence digestive metabolism, yet they lowered performance at higher doses regardless of feed ingredients used in the diet. Tannins positively affected meat quality and when added to wheat diets, intestinal growth was stimulated and the antioxidative status of the broilers improved.
Key words: broiler, chestnut wood tannin, performance, meat quality, digestion
INTRODUCTION
The poultry sector is increasingly important in world meat production since broiler physiology lends itself to efficient conversion of feed into animal protein. In the early days, antibiotic growth promotors (AGP) were used to support broiler growth and health. However, since 2006 these AGP are banned in the European Union, and since then in many other parts of the world. Therefore, there is an increasing need for sustainable alternatives (Huyghebaert et al., 2011).
Polyphenols are present in many plant species and consist of a wide array of organic molecules having a phenol ring in common. Plant polyphenols in general and the subclass of the tannins in particular, have great potential as feed additives (Huyghebaert et al., 2011). Tannins are secondary metabolites produced by plants as a nonspecific defence mechanism. These molecules are antioxidative, scavenge metal ions, and stimulate the immune system (Fraga et al., 2010), all properties that could be used to improve chicken health. In chestnut wood (Castanea sativa L.) hydrolysable tannins such as castalin, castalagin, vescalin, and vescalagin are present, with gallic acid and ellagic acid as main functional units (Khanbabaee and van Ree, 2001; Sanz et al., 2010). Traditionally, tannins have been described as antinutritional factors, and consequently as inhibitors of performance (Treviño et al., 1992; Jansman, 1993; Smulikowska et al., 2001). Literature is scarce on the effects of hydrolysable tannins, which presumably have less tendency to behave as antinutritional factors in the diet compared to condensed tannins. However, when condensed tannins were dosed precisely in Muscovy ducks and laying hens, no adverse effects could be registered (Castillo et al., 2020; Marzoni et al., 2020).
Chestnut tannin extract has been used with success in broiler diets. Studies indicated that chestnut tannins could improve litter quality and footpad lesion scores (Jamroz et al., 2009; Hooge et al., 2012), which presumably implies better gut health. Nevertheless, doses that have been recommended under commercial circumstances were hardly able to improve performance (Schiavone et al., 2007, 2008; Jamroz et al., 2009; Rezar and Salobir, 2014; Starčević et al., 2015). Furthermore, little effect on digestibility has been noted (Schiavone et al., 2008; Rezar and Salobir, 2014). However, chestnut wood extracts do have an influence on gut morphology (Jamroz et al., 2009), as does one of the breakdown products, gallic acid (Samuel et al., 2017). The latter study also noted an improvement in the breast muscle to carcass ratio. The antioxidative traits of tannins in broiler diets have been demonstrated by Starčević et al. (2015) and Samuel et al. (2017). The latter reported lowered plasma and tissue malondialdehyde (MDA), an increased total plasma antioxidant capacity and plasma superoxide dismutase activities. Despite some promising results for the application of chestnut tannins in broiler feeds, their specific effects on performance and digestive physiology are not yet fully elucidated (Rezar and Salobir, 2014).
The goal of this study was to assess the effect of different doses of chestnut tannins on broiler performance, digestibility, metabolism and meat quality. Testing a highly digestible corn-soy diet and a more nutritionally challenging wheat-rapeseed-palm oil diet can elucidate the possible interactions of tannins and digestion. Comparing the effects of tannins in different diets may reveal possible modes of action of these molecules, altering the nature of the raw feed ingredients which impacts digestibility and metabolism.
MATERIALS AND METHODS
All experimental procedures in this study were in compliance with the European guidelines for the care and use of animals in research (Directive 2010/63/EU) and were approved by the Ethical Committee of the Research Institute for Agriculture, Fisheries and Food (ILVO), Merelbeke, Belgium under authorization number 2018/350.
Trial 1 – Performance and Meat Quality
Experimental Design
A total of 2,160 ROSS 308-day-old male broilers were obtained from a commercial hatchery (Belgabroed, Merksplas, Belgium), and were randomly allocated to 72 floor pens in an alternating block design, each block containing one replicate. Each treatment was distributed among 12 pens and each pen contained 30 chicks. Broilers were housed on a solid floor with wood shavings (3 kg/m²) and were kept in 18L6D schedule from d 7 till d 41. The last day and first week a 23L1D cycle was used. In the first week, the poultry house temperature was 32°C. Thereafter it was gradually decreased by 4°C each week until 22°C. This temperature was then kept for the rest of the trial. All broilers were vaccinated against Newcastle disease on d 0 (Nobilis ND C2, Intervet International BV, Boxmeer, the Netherlands) and d 17 (Nobilis ND Clone 30, Intervet International BV, Boxmeer, the Netherlands).
Two basal diets (Table 1) were formulated: a corn-soy based diet and a wheat-rapeseed-palm oil based challenge diet. The goal was to achieve 2 diets differing in starch, protein, and fat sources to assess the impact of diet composition on the effect of chestnut tannins. The challenge diet contained more antinutritional factors, a different protein composition and had a fat source that contained a higher percentage of saturated fatty acids. Diets were formulated to meet the birds’ requirements, and both diets were isocaloric and isonitrogenous. Tannins extracted from chestnut wood (Tanno-SAN, Sanluc International NV, Belgium) were added to both diets at different levels: 0 mg/kg (T−), 500 mg/kg (T+), and 2,000 mg/kg (T++), resulting in 6 different dietary treatments (Corn T−; Corn T+; Corn T++; Wheat T−; Wheat T+;Wheat T++). The diets were supplemented with equal amounts of non–starch-polysaccharide enzymes (100 mg/kg, Ronozyme Multigrain, DSM, Heerlen, the Netherlands), phytase (200 ppm, Ronozyme HiPhos, DSM, Heerlen, Netherlands), and diclazuril (500 ppm, Coxiril, Huvepharma, Sofia, Bulgaria). Broilers were reared using a 3-phase schedule with a starter (d 0 to d 10), grower (d 11 to d 29), and finisher (d 30 to d 42). The starter diet was a mash feed, while the grower and finisher diets were pelleted. Feed and drinking water were given ad libitum.
Table 1.
Diet composition and calculated nutrient composition of the corn-soy based diet and of the wheat-palm oil-rapeseed based diet for the different periods.
| Starter |
Grower |
Finisher |
||||
|---|---|---|---|---|---|---|
| Ingredient (%) | Corn | Wheat | Corn | Wheat | Corn | Wheat |
| Corn | 59.8 | - | 63.5 | - | 66.5 | - |
| Wheat | - | 60.7 | - | 64.6 | - | 67.4 |
| Wheat bran | 3.00 | - | 1.86 | - | 1.78 | - |
| Soybean meal (48% CP) | 30.0 | 15.8 | 28.1 | 14.0 | 25.4 | 11.0 |
| Full fat soybeans | - | 7.50 | - | 7.50 | - | 8.50 |
| Rapeseed meal | - | 8.00 | - | 6.50 | - | 6.00 |
| Lard | 1.12 | 1.50 | 1.50 | 1.50 | 1.45 | 1.50 |
| Soy oil | 2.00 | - | 1.58 | - | 1.66 | - |
| Palm oil | - | 2.50 | - | 2.40 | - | 2.40 |
| Vitamin & mineral premix1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CaCO3 | 0.670 | 0.590 | 0.620 | 0.560 | 0.540 | 0.490 |
| Di-Ca-phosphate | 1.04 | 0.960 | 0.720 | 0.650 | 0.480 | 0.400 |
| NaCl | 0.150 | 0.140 | 0.190 | 0.150 | 0.150 | 0.080 |
| Na2CO3 | 0.220 | 0.290 | 0.220 | 0.330 | 0.300 | 0.430 |
| L-Lysine HCl | 0.390 | 0.460 | 0.261 | 0.310 | 0.280 | 0.320 |
| DL-methionine | 0.380 | 0.310 | 0.295 | 0.340 | 0.310 | 0.300 |
| L-threonine | 0.190 | 0.190 | 0.090 | 0.100 | 0.100 | 0.100 |
| Calculated nutrient composition | ||||||
| Crude protein (g/kg) | 205 | 205 | 195 | 194 | 185 | 185 |
| Crude fat (g/kg) | 69.5 | 71.4 | 70.0 | 70.1 | 71.0 | 71.7 |
| Crude fibre (g/kg) | 33.7 | 37.6 | 32.8 | 35.8 | 32.0 | 34.7 |
| Metabolizable energy (Kcal/kg) | 2 796 | 2 772 | 2 844 | 2 820 | 2 892 | 2 868 |
| Dig. Lysine (g/kg) | 11.50 | 11.90 | 10.00 | 10.10 | 9.50 | 9.70 |
| Dig. Methionine+Cysteine (g/kg) | 8.80 | 8.85 | 7.79 | 8.85 | 7.67 | 8.24 |
| Dig. threonine (g/kg) | 7.70 | 7.75 | 6.50 | 6.50 | 6.20 | 6.20 |
| Dig. valine (g/kg) | 7.60 | 7.78 | 7.28 | 7.40 | 6.84 | 7.02 |
| Ca (g/kg) | 8.50 | 8.50 | 7.50 | 7.50 | 6.50 | 6.50 |
| Available P (g/kg) | 4.00 | 4.00 | 3.50 | 3.50 | 3.10 | 3.10 |
| Na+Cl-K (meq/kg) | 230 | 222 | 225 | 223 | 220 | 223 |
| C18:2 (g/kg) | 28.3 | 18.8 | 26.9 | 18.6 | 27.6 | 19.5 |
| C18:1 (g/kg) | 17.0 | 22.4 | 18.0 | 21.8 | 18.2 | 22.1 |
| C16:0 (g/kg) | 8.53 | 17.7 | 9.11 | 17.3 | 9.13 | 17.5 |
Vitamin and mineral premix composed of vitamin A/retinyl acetate 3a672a (10,00,000 IU/kg); vitamin D3 E671 (299,999.4 IU/kg); vitamin E 3a700 (all-rac-alpha-tocopheryl acetate) (5,000 IU/kg); vitamin K3 3a710 (250 mg/kg); vitamin B1/thiamine mononitrate 3a821 (200 mg/kg); vitamin B2/riboflavin (500 mg/kg); calcium D-pantothenate 3a841 (1,500 mg/kg); vitamin B6/pyridoxine hydrochloride 3a831 (400 mg/kg); vitamin B12/cyanocobalamin (2.5 mg/kg); niacinamide 3a315 (3,000 mg/kg); folic acid 3a316 (100 mg/kg); biotin/D-(+)-biotin 3a880 (15 mg/kg); choline chloride 3a890 (68,965.5 mg/kg); iron(II)sulphate (monohydrate) - iron E1 (4,920 mg/kg); copper(II)sulphate (pentahydrate) - copper E4 (2,000 mg/kg); zinc oxide 3b603 (6,000 mg/kg); manganese(II)oxide - manganese E5 (9,590.2 mg/kg); calcium iodate (anhydrous) - iodine 3b202 (120 mg/kg); sodium selenite - selenium E8 (36 mg/kg); sepiolite E562 (700 mg/kg); propyl gallate E310 (200 mg/kg); BHT E321(300 mg/kg); citric acid E330
Performance
Mortality was registered daily to correct performance parameters. Animal live weight and feed leftovers were recorded at d 0, 10, 29, and 42 to assess average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). At d 29 and 42, litter was visually scored on pen level for its consistency using the scoring system of the welfare quality protocol (Welfare Quality®, 2009). At the same time, litter was also sampled at 3 different places per pen and pooled to determine dry matter content. Furthermore, hock and footpad lesions were scored at d 29 and 42 (8 ad random birds per pen) with a scoring system based on the Welfare quality assessment protocols (Welfare Quality®, 2009).
Meat Quality
At the end of the rearing period, 5 ad random birds per pen were slaughtered in a commercial slaughterhouse and the cleaned eviscerated carcasses were recovered. They were stored at 4°C until the following day, weighed and carcass yield was calculated as eviscerated carcass weight relative to live weight before slaughtering. All carcasses were cut up (fillets, thighs, drumsticks, wings, and waste) and all parts were weighed to determine carcass composition. Temperature, pH (Type HI98163 pH meter, electrode FC2323, Hannah Instruments, Temse, Belgium) and color (Hunterlab Miniscan 45/0, AMETEK Hunterlab, Reston, VA) were measured at 3 different places on the right breast fillet. Meat color was recorded with CIE L*a*b* where L*, a* and b* values represent the axes on a 3-dimensional color scheme. L* represents lightness with a higher number representing a lighter color. The a* axis represents the red-green scale with the more negative value toward green an a more positive value toward red. Similarly, b* represents the blue-yellow axis with negative values toward blue and yellow represented by positive values. Then, drip loss was assessed on the right breast fillet using the EZ-drip loss method (Rasmussen and Andersson, 1996). Finally, all fillets were weighed, vacuum packed and frozen (−20°C) for further analysis.
Thawing loss was assessed by letting the vacuum-packed left breast fillets thaw at room temperature for 16 h, after which the fillet was dried using a paper towel and weighed. The thawing loss was calculated by subtracting the weight before freezing and after thawing. Next, each fillet was packed in a sealed cooking bag and placed in a warm water bath for 45 min at 80°C. Breast fillets were then taken out and let cool down to room temperature. Breast fillets were then dried with a paper towel, weighed again to calculate cooking loss. Afterward, the fillets were placed in a refrigerator (4°C) for 12 h. From each cooked fillet 10 cylindrical samples were taken (1.27 cm Ø) parallel to muscle fibres to measure maximal shear force (TA500 Lloyd Texture Analyzer fitted with a triangular Warner-Bratzler shear, Lloyd instruments, Bognor Regis, UK), and calculate a mean maximal shear force after eliminating the highest and lowest shear force values per fillet.
Trial 2 – Nutrient Digestibility and Digestive Physiology
Digestibility Parameters
In this trial, 216 broilers (3 ad random birds per pen of trial 1) were relocated at d 17 to digestibility units. Each digestibility unit housed 3 birds and was considered as one replicate. The balance period in which feces were collected and feed intake was registered lasted 5 d according to the European reference method and started after a 4-d adaptation period (Bourdillon et al., 1990). Feed intake and amount of excreta produced were recorded each 2 d to assess the feces to feed ratio. Total excreta were collected and stored at −18°C in closed containers during the balance period to prevent evaporation. The excreta of each unit were pooled at the end of the balance period and thoroughly mixed to obtain a representative sample. Hereafter, all excreta samples were freeze dried, ground to 1 mm and stored at ambient temperature for further analysis. Dry matter (152/2009/EG), crude protein (ISO 5983-2), crude fat (ISO 6492), and gross energy (ISO 9831) were analysed using ISO standards from which apparent digestibility coefficients were calculated using the feces to feed ratio. Apparent crude protein digestibility was corrected for the amount of uric acid (Marquardt, 1983) found in the excreta. Apparent metabolizable energy corrected for nitrogen (AMEn) was calculated as the digested energy corrected for the energy content of the retained nitrogen.
Blood Parameters
At the end of the balance period all birds were euthanized and blood was collected by exsanguination in plasma tubes and glucose tubes (respectively, BD vacutainer K2E EDTA 10 mL and BD vacutainer Fluoride 6 mL, BD, Franklin Lakes, NJ). Blood was centrifuged (RCF 1500, 10 min, 4°C), plasma aliquots (2 mL) were prepared in Eppendorf tubes and frozen at −20°C until further analysis. Plasma triglycerides (TG), glucose (GLU), and non-esterified fatty acids (NEFA) were determined spectrophotometrically using a commercial colorimetric diagnostic kit (TR210 for TG, GL2623 for GLU and FA115 for NEFA, Randox Laboratories Ltd., Crumlin, UK). MDA was determined using a commercial diagnostic kit (KB03016, Bioquochem, Asturias, Spain).
Viscosity
Total gut content was collected from the gizzard to Meckel's diverticulum (proximal small intestine, PSI) and from Meckel's diverticulum to the ileo-ceco-colic junction (distal small intestines, DSI) to determine gut content viscosity (n = 12/treatment). Immediately after collection, gut content was centrifuged (1,200 RCF, 5 min, 21°C) and 1.5 mL of supernatant was collected, and stored for 72 h at 4°C. Viscosity was tested using a DVT2-LV viscometer (AMETEK Brookfield, Middleborough, MA) fitted with a CP40 spindle. Analysis for the PSI samples were done under the following conditions: 100 RPM; temperature: 39.0°C, one time point measurement after 30 s, whereas for DSI samples the conditions were: 75 RPM; temperature: 39.0°C, one time point measurement after 30 s. Viscosity was recorded in centipoises (cP).
Intestinal Morphometry
At d 25, the end of the digestibility trial, the duodenum loop was sampled for histological analysis (n = 12/treatment) and placed in a 4% buffered formalin solution for 24 h, after which the formalin was replaced with distilled water. These tissue sections were then dehydrated with xylene, embedded in paraffin, and cut in 4-µm sections with a microtome (Microm; Prosan, Merelbeke, Belgium). The sections were automatically (Shandon Varistain-Gemini, Thermofisher Scientific, Waltham, MA) deparaffinized in xylene and rehydrated in isopropylene (5 min), 95% ethanol (5 min), and 50 % ethanol (5 min) and stained with hematoxylin and eosin. Villus length and crypt depth were randomly measured in 10 villi per section, whereas the width of the tunica muscularis was assessed in 10 different points per section, using a light microscope (Leica DM LB2 Digital, Leica Microsystems, Wetzlar, Germany) and a computer-based image analysis program (Leica Application Suite V4.1, Leica microsystems).
At d 28 one broiler per pen was euthanized (n = 12) to measure body weight and weight/length of 3 gut segments: the duodenum (the origin from the gizzard to the insertion of the pancreatic duct and bile duct), the jejunum (the end of the duodenum to Meckel's diverticulum), and the ileum (the end of the jejunum to the ileo-ceco-colic junction).
Statistical Analysis
Statistical analysis was performed in R Studio for Windows (version 1.1.456). For the performance trial, the pen was considered as the experimental unit, whereas for the digestibility trial, the digestibility unit was the experimental unit. Data was analysed using least-squares linear regression with tannin content and feed type as independent variables and the tested parameter as dependent variable. All data were checked for outliers and checked for a normal distribution of the residuals. Lesion scores (footpad lesions and hock burns) and litter quality were analysed using Chi-square test. The effect of tannins on intestinal morphometry was assessed with unpaired t tests and significance was confirmed with least-squares linear regression analysis. Differences were considered statistically significant at P < 0.05.
RESULTS
Performance and Litter Quality
No significant interactions were observed between the diet type and tannin addition, but there was a significant main effect of diet type and tannin dosage on performance (Table 2). Animals fed wheat-based diets achieved a lower body weight, and ADG at the end of the trial, with a higher FCR compared to animals fed the corn-soy based diets. This confirms that the wheat-palm oil-rapeseed diet successfully created a nutritional challenge to the broilers. Effect of diet was mainly observed in the grower and finisher period, while no significant effects were seen during the starter period.
Table 2.
Effect of different doses of chestnut wood tannins (T−: 0 mg/kg, T+: 500 mg/kg and T++: 2,000 mg/kg) added to a corn-soy based diet or wheat-palm oil-rapeseed based diet on mean body weight (BW, g) of broiler chickens and average daily gain (ADG, g/bird/day), average feed intake (ADFI, g/bird/day) and feed conversion ratio (FCR) during the starter period (d 0–10), grower period (d 11–29), finisher period (d 30–42), and the whole rearing period (overall) (n = 12).
| Corn |
Wheat |
P1 |
SEM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T- | T+ | T++ | T- | T+ | T++ | D | T | D*T | ||
| BW | ||||||||||
| D 10 | 255 | 257 | 249 | 254 | 254 | 250 | 0.691 | 0.052 | 0.681 | 1 |
| D 29 | 1,639a | 1,661a | 1,605b | 1,524a | 1,525a | 1,481b | <0.001 | 0.002 | 0.783 | 12 |
| D 42 | 3,145a | 3,150a | 3,056b | 2,943a | 2,903a | 2,828b | <0.001 | <0.001 | 0.674 | 18 |
| ADG | ||||||||||
| Starter | 21.07 | 21.31 | 20.48 | 20.95 | 21.01 | 20.67 | 0.718 | 0.064 | 0.647 | 0.22 |
| Grower | 72.83a | 73.87a | 71.38b | 66.88a | 66.89a | 64.76b | <0.001 | 0.004 | 0.774 | 0.60 |
| Finisher | 115.91a | 114.50ab | 111.64b | 109.11a | 105.99ab | 103.66b | <0.001 | <0.001 | 0.770 | 1.00 |
| Overall | 73.84a | 73.95a | 71.72b | 69.02a | 68.07a | 66.30b | <0.001 | <0.001 | 0.672 | 0.50 |
| ADFI | ||||||||||
| Starter | 26.29 | 26.88 | 25.97 | 26.32 | 26.88 | 26.80 | 0.165 | 0.050 | 0.175 | 0.11 |
| Grower | 99.90ab | 101.22a | 98.76b | 95.87ab | 98.01a | 94.93b | <0.001 | 0.050 | 0.935 | 0.93 |
| Finisher | 183.31 | 183.71 | 181.42 | 182.32 | 180.55 | 182.89 | 0.580 | 0.914 | 0.484 | 0.76 |
| Overall | 108.19 | 109.08 | 107.01 | 106.07 | 106.62 | 105.93 | 0.013 | 0.342 | 0.745 | 0.39 |
| FCR | ||||||||||
| Starter | 1.25 | 1.26 | 1.26 | 1.26 | 1.28 | 1.30 | 0.110 | 0.118 | 0.768 | 0.01 |
| Grower | 1.37 | 1.37 | 1.38 | 1.43 | 1.47 | 1.47 | <0.001 | 0.072 | 0.215 | 0.01 |
| Finisher | 1.58b | 1.61a | 1.63a | 1.67b | 1.71a | 1.74a | <0.001 | <0.001 | 0.682 | 0.01 |
| Overall | 1.47b | 1.48a | 1.49a | 1.54b | 1.57a | 1.60a | <0.001 | <0.001 | 0.282 | 0.01 |
Values for a specific parameter within a diet with no letters or sharing a letter are not significantly different (P > 0.05).
D, Diet; T, tannin treatment.
There was a trend toward lower performance in broilers given the high dose tannins during the starter period. Moreover, during the grower and finisher period, BW and ADG were significantly lower for animals fed the high dose of chestnut tannins (2,000 mg/kg) compared to the control group, and the low dose of chestnut tannins (500 mg/kg). Feed intake tended to be higher for broilers fed 500 mg/kg tannins for each diet type in the starter period and grower period. FCR was highest for broilers fed 2,000 mg/kg and lowest for control fed broilers in the finisher period. The same was true for the FCR of the overall experimental period. Mortality rate was not affected by the treatments (P = 0.820).
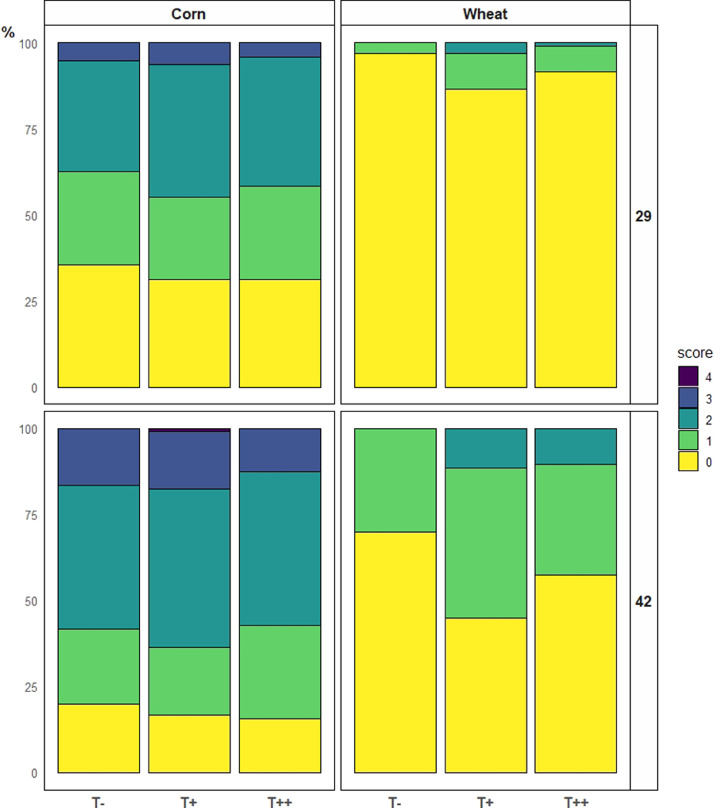
Although animals fed corn diets showed more footpad lesions and hock burns than wheat fed animals, there were no significant differences in the incidence of the scores between groups (Figure 1). The incidence of footpad lesions increased with the addition of both doses of tannins in animals fed wheat diets, both at d 29 (P = 0.024) and d 42 (P < 0.001). Dry matter content of the litter was significantly higher (P< 0.001) when animals were fed the wheat diets and the highest dose of chestnut tannins compared to the control and 500 mg/kg fed groups, both at d 29 and 42 (Table 3).
Figure 1.
Footpad lesion scores of broilers fed either corn-soy based diets or wheat-palm oil-rapeseed based diets, added or not with chestnut wood tannins at two different doses (T−: 0 mg/kg, T+: 500 mg/kg, and T++: 2,000 mg/kg) at 29 and 42 d of age (n = 96) with score 0: no footpad lesions and score 4: as the worst grade of lesions.
Table 3.
Effect of different doses of chestnut wood tannins (T−: 0 mg/kg, T+: 500 mg/kg and T++: 2,000 mg/kg) added to a wheat-palm oil-rapeseed based diet or corn soy based diet on litter dry matter content (DM, %) at d 29 and 42 of age. Broilers were housed at a maximum density of 14 birds/m² (n = 12).
| Corn |
Wheat |
P1 |
SEM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T− | T+ | T++ | T− | T+ | T++ | D | T | D*T | ||
| DM | ||||||||||
| D 29 | 65.93ab | 62.54b | 65.96a | 70.02ab | 70.00b | 71.71a | <0.001 | 0.022 | 0.178 | 0.54 |
| D 42 | 56.51ab | 56.01b | 58.94a | 63.48ab | 62.46b | 66.40a | <0.001 | 0.009 | 0.915 | 0.97 |
Values for a specific parameter within a diet with no letters or sharing a letter are not significantly different (P > 0.05).
D, Diet; T, tannin treatment.
Meat Quality
No significant differences were seen on the yield of the different carcass sections. Addition of chestnut tannins only influenced meat pH in the corn diet, with the highest tannin dose resulting in a higher meat pH compared to the control group (Table 4). Meat color parameters changed when adding tannins to the wheat diet. This resulted in lower a* values, that represent the green (negative values) to red (positive values) scale, compared to the respective control group. Animals fed the corn diet produced meat with significantly higher a* and b* values compared with wheat diets. Carcass yield was not affected by the addition of tannins, but was affected by the type of basal diet used (Table 4), with corn fed broilers having a higher carcass yield than wheat fed broilers. Furthermore, the addition of chestnut tannins to both diets resulted in lower shear force and drip loss in a dose-dependent manner. Thawing and cooking losses were not affected by the addition of tannins(Table 4).
Table 4.
Effect of different doses of chestnut wood tannins (T−: 0 mg/kg, T+: 500 mg/kg and T++: 2,000 mg/kg) added to a corn-soy based diet or wheat-palm oil-rapeseed based diet on meat pH, color (L*, a*, b*), carcass yield (%) and meat quality (drip, thawing and cooking loss (%), and shear force (N)) of broilers slaughtered at 42 d of age (n = 60).
| Corn |
Wheat |
P1 |
SEM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T− | T+ | T++ | T− | T+ | T++ | D | T | D*T | ||
| pH | 6.15c | 6.24b | 6.29b | 6.38a | 6.35a | 6.39a | <0.001 | <0.001 | <0.001 | 0.01 |
| Meat color2 | ||||||||||
| L* | 56.70 | 57.12 | 56.78 | 57.36 | 57.39 | 57.47 | 0.094 | 0.848 | 0.837 | 0.16 |
| a* | 5.99a | 6.14a | 5.92ab | 5.44bc | 4.85d | 4.96cd | 0.001 | 0.421 | 0.010 | 0.06 |
| b* | 16.97 | 17.27 | 17.13 | 14.65 | 14.12 | 14.31 | <0.001 | 0.520 | 0.088 | 0.11 |
| Carcass yield | 67.51 | 67.53 | 67.40 | 66.84 | 66.63 | 66.19 | <0.001 | 0.291 | 0.557 | 0.11 |
| Meat quality | ||||||||||
| Drip loss | 1.75a | 1.60ab | 1.35b | 1.80a | 1.28ab | 1.12b | 0.285 | 0.021 | 0.607 | 0.08 |
| Thawing loss | 5.96 | 5.52 | 5.63 | 6.21 | 5.76 | 5.51 | 0.660 | 0.273 | 0.833 | 0.14 |
| Cooking loss | 16.45 | 15.07 | 15.37 | 18.24 | 19.21 | 19.29 | <0.001 | 0.935 | 0.125 | 0.28 |
| Shear force | 9.35a | 8.53ab | 8.66b | 9.00a | 8.98ab | 8.54b | 0.954 | 0.041 | 0.219 | 0.10 |
Values for a specific parameter within a diet with no letters or sharing a letter are not significantly different (P > 0.05).
D, Diet; T, tannin treatment.
L*: lightness (0 = black, 100 = white); a* green-red axis (green = negative values, red = positive values); b* blue-yellow axis (blue = negative values, yellow = positive values).
Digestibility and Blood Parameters
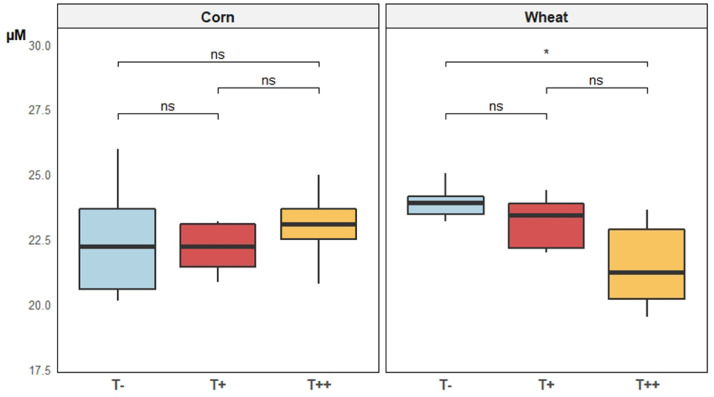
The two supplementation levels of tannins had no effect on the apparent digestibility of crude fat (aDCCF) and crude protein corrected for uric acid (aDCCP-UA). In wheat diets, 2,000 mg/kg tannins resulted in a slightly higher digestibility of gross energy (aDCGE) and caused a change in the AMEn in comparison with the group that received 500 mg/kg. Nevertheless, for these parameters there was no difference with the control group (Table 5). Similarly, tannins did not affect plasma glucose, NEFA and triglyceride levels. In contrast, plasma MDA levels in wheat diets were significantly lower with increasing tannin content (Figure 2). This effect was not observed for corn diets.
Table 5.
Effect of different doses of chestnut tannins (T−: 0 mg/kg, T+: 500 mg/kg and T++: 2,000 mg/kg) added to a corn-soy based diet or wheat-palm oil-rapeseed based diet on apparent fecal digestibility coefficients (%) of gross energy (aDCGE), crude fat (aDCCF), crude protein corrected for uric acid excretion (aDCCP-UA), on apparent metabolizable energy corrected for nitrogen retention (AMEn, Kcal/kg), glucose (mg/dL), non-esterified fatty acids (NEFA, mg/dL), and triglycerides (TG, mg/dL) of broiler chickens at 25 d of age (n = 12).
| Corn |
Wheat |
P1 |
SEM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T− | T+ | T++ | T− | T+ | T++ | D | T | D*T | ||
| digestibility | ||||||||||
| aDCGE | 78.44a | 77.71a | 78.37a | 66.90bc | 65.13c | 69.81b | <0.001 | 0.743 | 0.021 | 0.78 |
| aDCCF | 84.94 | 84.26 | 86.41 | 54.95 | 54.37 | 58.85 | <0.001 | 0.081 | 0.677 | 1.64 |
| aDCCP-UA | 80.18 | 79.24 | 79.22 | 78.16 | 79.40 | 79.65 | 0.346 | 0.927 | 0.103 | 0.52 |
| AMEn | 3,437a | 3,401a | 3 430a | 2,918bc | 2,834c | 2,980b | <0.001 | 0.742 | 0.020 | 0.15 |
| blood | ||||||||||
| Glucose | 251.92 | 261.04 | 266.81 | 258.27 | 254.73 | 268.37 | 0.927 | 0.298 | 0.760 | 5.21 |
| NEFA | 0.76 | 0.73 | 0.72 | 0.37 | 0.35 | 0.38 | <0.001 | 0.884 | 0.778 | 0.03 |
| TG | 166.27 | 167.69 | 161.68 | 112.77 | 89.27 | 117.10 | <0.001 | 0.435 | 0.224 | 8.28 |
Values for a specific parameter within a diet with no letters or sharing a letter are not significantly different (P > 0.05).
D, Diet; T, tannin treatment.
Figure 2.
Mean plasma malondialdehyde of broilers fed either corn-soy based diets or wheat-palm oil-rapeseed based diets, added or not with chestnut wood tannins at two different doses (T−: 0 mg/kg, T+: 500 mg/kg, and T++: 2,000 mg/kg) at 25 d of age (n = 12). Pairwise comparison tests (t test): P < 0.001 = ***; 0.001 < P < 0.01 = **; 0.01 < P < 0.05 = *; P > 0.1 = ns.
Type of diet resulted in significant effects regarding nutrient digestibility. Gross energy and crude fat were better digested in broilers fed corn diets, with higher AMEn than for broilers fed wheat diets. No significant difference was found for protein digestion, despite the differences in protein source. Type of diet affected plasma NEFA and TG levels, with higher levels in broilers fed corn diets than in broilers fed wheat diets. No effect could be observed on blood glucose (Table 5).
Viscosity
Addition of chestnut tannins to both basal diets had no impact on gut content viscosity for both the PSI and DSI at 25 d of age, whereas wheat diets had a significantly higher viscosity in the proximal part of the intestines (PSI: P < 0.001) compared to corn diets. In the distal part, viscosity was only numerically different (DSI: P = 0.094) (results not shown).
Intestinal Morphometry
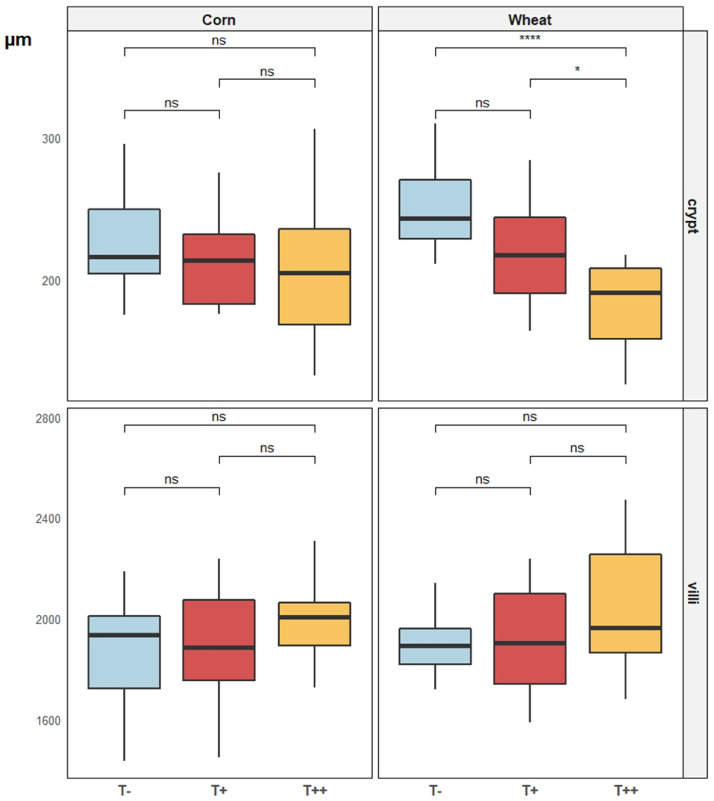
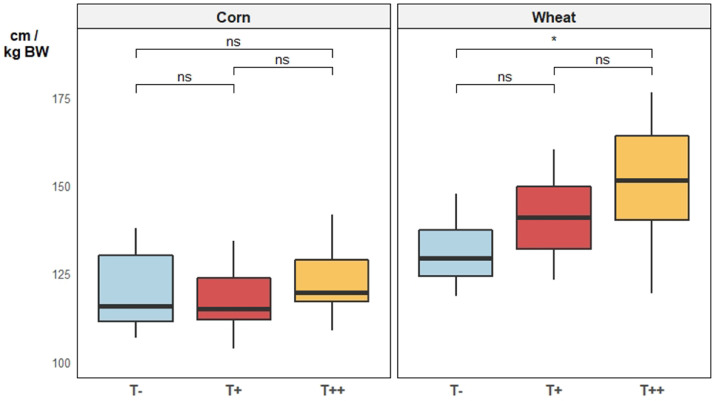
Addition of chestnut tannins had no significant impact on gut histology (Figure 3) or gut length (Figure 4) for animals fed corn diets. In contrast, in broilers fed wheat diets, addition of tannins resulted in a significant decrease in crypt depth (Figure 3), with the lowest values for the highest dose of tannins. Villus height was not affected by the presence of tannins for both basal diets (Figure 3). Yet, the total gut length was significantly increased by the presence of tannins when animals were fed the wheat diet (Figure 4). The total gut length increased with the amount of chestnut tannin added to the diet for the animals fed the wheat diet.
Figure 3.
Mean villus height and crypt depth (µm) of broilers fed either corn-soy based diets or wheat-palm oil-rapeseed based diets, added or not with chestnut wood tannins at two different doses (T−: 0 mg/kg, T+: 500 mg/kg, and T++: 2,000 mg/kg) at 25 d of age (n = 12). Pairwise comparison tests (t test): P < 0.001 = ***; 0.001 < P < 0.01 = **; 0.01 < P < 0.05 = *; P > 0.1 = ns.
Figure 4.
Mean relative total gut length (cm) of broilers fed either corn-soy based diets or wheat-palm oil-rapeseed based diets, added or not with chestnut wood tannins at two different doses (T−: 0 mg/kg, T+: 500 mg/kg, and T++: 2,000 mg/kg) at 25 d of age (n = 12). Pairwise comparison tests (t test): P-value < 0.001 = ***; 0.001 < P < 0.01 = **; 0.01 < P < 0.05 = *; P > 0.1 = ns.
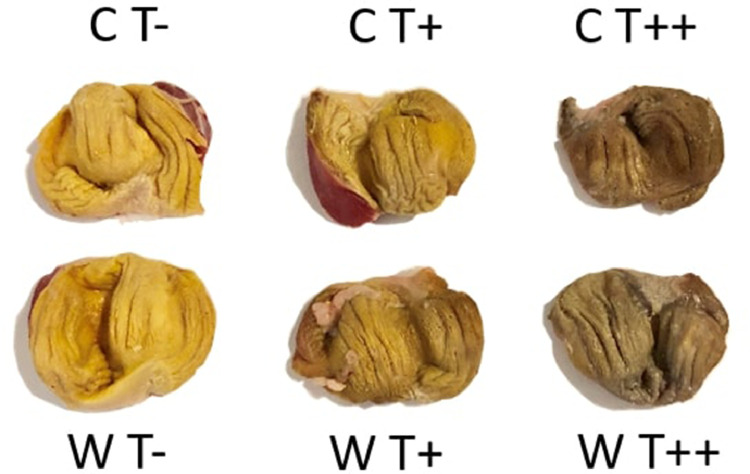
A brown discoloration of the feces was observed while sampling. This anomaly led to a further investigation of the internal layer of the gut wall. Indeed, a consistent change in color of the koilin layer of the gizzard was observed, with more intense staining with increasing dose of chestnut tannins (Figure 5).
Figure 5.
Staining of the koilin layer in the gizzard of broilers fed either corn-soy based diets (C) or wheat-palm oil-rapeseed based diets (W), added or not with chestnut wood tannins at two different doses (T−: 0 mg/kg, T+: 500 mg/kg, and T++: 2,000 mg/kg).
DISCUSSION
The low dosage of chestnut tannins (500 mg/kg) had little effect on the overall broiler performance for both basal diets. The negative effect of the high supplementation of chestnut tannins (2,000 mg/kg) was most pronounced in the grower and finisher period, although a numerical difference was apparent in the starter period as well. The lack of interaction between diet and tannin dosage on performance suggests that the effect of tannin supplementation is independent of the diet composition. The negative effect of the high tannin dose in the present study is in contrast with the study of Schiavone et al. (2008) and Hooge at al. (2012) where 2,000 mg/kg and 1,000 mg/kg tannins, respectively resulted in better performance. Nevertheless, the latter study was performed in a challenging environment with built-up litter and a live coccidia vaccine, whereas in this study such challenges were not present. In another study (Ahmed et al., 1991), broilers aged 14 to 42 d had decreased growth with increasing amount of high doses of tannins (13,500 mg/kg to 50,000 mg/kg) with a concomitant higher nitrogen excretion and thus lower apparent crude protein digestibility. In all other studies available in literature which use lower doses, little effects of tannin supplementation on broiler performance have been reported (Jamroz et al., 2009; Rezar and Salobir, 2014; Starčević et al., 2015). This is in line with the present study.
A lower ADG paired with an almost equal ADFI in high tannin supplemented diets, would lead to think that broilers were not able to use the dietary nutrients efficiently. One would expect a decreased nutrient apparent digestibility coefficient, but this was not the case in the present study. Similar effects of chestnut tannins on digestibility were obtained in other studies using similar dietary chestnut tannin concentrations (Schiavone et al., 2008; Rezar and Salobir, 2014). Moreover, the difference in gross energy digestibility in wheat diets suggests a different effect of tannins depending on feed composition. Yet no differences were noted in other digestibility parameters that could be linked to this feed composition dependency these effects. If a lower performance would have been paired with a lower nutrient digestibility, one could also expect that broilers would mobilise reserves more intensively and plasma NEFA levels would be increased, but this was not the case either. In theory, broilers could have compensated any reduced digestibility with increased nutrient absorption, but for instance plasma TG concentrations failed to show changes due to chestnut tannin supplementation. These results were similar to the study of Samuel et al. (2017) where other blood biochemical parameters also remained unaffected.
It is widely known that tannins can bind various nutrients such as proteins and carbohydrates, acting as an antinutritional factor by rendering these nutrients unavailable for digestion or absorption. Indeed, high doses as well as certain types of tannins can inhibit digestion and subsequently decrease performance (Ahmed et al., 1991; Smulikowska et al., 2001). It is then intriguing that animals fed the higher chestnut tannin dose digested the feed equally well as the respective control group but grew at a lower rate.
The observation of the tannin-induced dark staining of the gizzard koilin layer demonstrates the strong ability of chestnut tannins to interact with the proteins, as observed by Bittner (2006). A similar effect of chestnut tannins was also seen in pig stomachs, together with increased mucus production (Galassi et al., 2019). Interaction of tannins with the intestinal mucus layer was also described by Ahmed et al. (1991), while observing a lowered membrane enzyme activity as well. Additionally, studies with rats showed a higher intestinal mucus production when rats were fed 3% tannic acid (Mitjavila et al., 1977), suggesting that tannins can interact with intestinal mucus production. In the present study, we hypothesise that the intestines of growing broilers adapted their morphometry to assure a guaranteed nutrient absorption rate into the body. The longer intestinal tract of broilers on the wheat diets compared with the corn diets already shows how the chickens maximized transit time and digestion and hence nutrient absorption rate, instead of further elongating the villi to increase nutrient absorption. This clearly indicates that chickens might have different adaptation strategies regarding nutrient digestion and nutrient absorption. Next to the effect of basal diet on gut length, chestnut tannin dosage also affected gut length in broilers fed wheat diets, with higher gut lengths with increasing tannin dose. The elongation of the gut in the presence of tannins could be explained by the binding of tannins to nutrients, making it less accessible for digestion, therefore, stimulating intestinal growth to increase absorption capacity. In addition, the breakdown products of chestnut tannins, gallic and ellagic acid, could protect the intestinal cells against oxidative damage from unsaturated fats for example. This protective role can be suggested from the lower plasma MDA levels found in broilers fed wheat diets, where palm oil was present in this study. The latter has also been reported when gallic acid was supplemented to broiler diets (Samuel et al., 2017).
Shallower crypts and equally long villi in the duodenum of the chestnut tannin groups, particularly when combined with wheat diets, suggests mature enterocytes to live longer in the gut. This can reflect the possible protective effect of tannins or their breakdown products on the intestinal lining. Furthermore, villi were not shortened, proving the nontoxic nature of the extract. Similar effects were also seen by Jamroz et al. (2009) and Samuel et al. (2017), but when using pure gallic acid and not tannin extract as in the present study, highlighting the importance of breakdown products besides the tannins themselves. Gut morphology was not affected by chestnut tannins in broilers fed corn diets demonstrating the importance of nutrient composition.
Longer intestines could also have drawbacks. While it helps processing more challenging diets, the longer gut itself will require more energy and nutrients for maintenance leaving fewer nutrients available for growth. This matches with the observation of similar nutrient digestibility but reduced performance with increasing tannin dosage.
The abovementioned actions of the chestnut tannins may also have resulted in altered water exchange in the intestine, explaining the 3% dryer excreta of broilers fed the high tannin dose, a phenomenon also observed by Jamroz et al. (2009) using gallic acid, and Marzoni et al. (2020) using quebracho tannins. The lower moisture content in the litter of the wheat diet groups compared with the corn diet groups is typically considered as a logical explanation for the lower prevalence of footpad dermatitis. The difference in moisture content between the two diets could possibly be attributed to the longer intestines which could stimulate water resorption from the intestinal chyme. Yet, the dryer excreta for the high tannin dose groups coincided with a higher incidence of footpad dermatitis. Other mechanisms than just wet litter should therefore have been responsible for the observed footpad lesions and need further attention (Dunlop et al., 2016).
The relative longer intestines of broilers fed the wheat diet, could explain the slightly lower relative carcass yield. The tannin-induced reduction in the redness of the meat is mainly linked to the amount of myoglobin present and pH of muscle tissue (Mir et al., 2017). This decrease in meat redness could be explained by the binding of tannins to heme iron, inhibiting absorption or metabolism of iron (McDonald et al., 1996; South and Miller, 1998). However, the light scattering (L*) was not significantly different suggesting undetectable differences in heme pigments to the naked eye (Mir et al., 2017). This decrease in meat redness was not noticed upon visual inspection of the meat in our study. It can be concluded that polyphenols in lower dosages do not significantly affect meat iron concentration, also described by Starčević et al. (2015). In the latter study no antioxidative parameters were studied, but these parameters were ameliorated in rabbit meat when fed chestnut tannins (Mancini et al., 2019).
Basal diet and addition of chestnut tannins affected meat pH. The pH differences could not be correlated to meat color. Another effect of tannins in both diets is the decrease in drip loss and shear force. This could be related to meat pH (Barbut, 1993), but no correlations were found. These results suggest that tannins could lead to tender meat that would be slightly juicier judging from the lower drip loss observed in the meat of broilers fed tannins.
CONCLUSIONS
Despite the observed binding of chestnut tannins to the gut wall and altered gut morphology, reduced nutrient digestibility was not observed in broiler chickens supplemented with chestnut tannins. The interaction with the intestinal wall is the likely reason for the reduced performance in broilers fed the high chestnut tannin dose (2,000 mg/kg). Despite the feed source, the same dose of tannins induced similar significant effects in broilers. Although addition of chestnut tannin during the entire growth period of broiler chickens had no major advantage for performance or nutrient digestibility in this study, the effects on intestinal development and health may open a window of opportunity for the use of chestnut tannins to modulate absorptive capacity.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to acknowledge Jolien Vander Linden and the animal care takers (ILVO, Melle, Belgium) for their technical support during this study. Authors wish also to acknowledge fellow colleagues for their help during sampling and for their skilled technical assistance. This study received the financial support of VLAIO (Flemish Innovation & Entrepreneurship) through the O&O project HBC 2018.0294.
DISCLOSURES
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
REFERENCES
- Ahmed A.E., Smithard R., Ellis M. Activities of enzymes of the pancreas, and the lumen and mucosa of the small intestine in growing broiler cockerels fed on tannin-containing diets. Br. J. Nutr. 1991;65:189–197. doi: 10.1079/bjn19910080. [DOI] [PubMed] [Google Scholar]
- Barbut S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in turkey meat. Food Res. Int. 1993;26:39–43. [Google Scholar]
- Bittner S. When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids. 2006;30:205–224. doi: 10.1007/s00726-005-0298-2. [DOI] [PubMed] [Google Scholar]
- Bourdillon A., Carré B., Conan L., Duperray J., Huyghebaert G., Leclercq B., Lessire M., McNab J., Wiseman J. European reference method for the in vivo determination of metabolisable energy with adult cockerels: reproducibility, effect of food intake and comparison with individual laboratory methods. Br. Poult. Sci. 1990;31:557–565. doi: 10.1080/00071669008417287. [DOI] [PubMed] [Google Scholar]
- Castillo A., Schiavone A., Cappai M.G., Nery J., Gariglio M., Sartore S., Franzoni A., Marzoni M. Performance of slow-growing male muscovy ducks exposed to different dietary levels of quebracho tannin. Animals. 2020;10:979. doi: 10.3390/ani10060979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M.W., Moss A.F., Groves P.J., Wilkinson S.J., Stuetz R.M., Selle P.H. The multidimensional causal factors of ‘wet litter’ in chicken-meat production. Sci. Total Environ. 2016;562:766–776. doi: 10.1016/j.scitotenv.2016.03.147. [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Galassi G., Mason F., Rapetti L., Crovetto G.M., Spanghero M. Digestibility and metabolic utilisation of diets containing chestnut tannins and their effects on growth and slaughter traits of heavy pigs. Ital. J. Anim. Sci. 2019;18:746–753. doi: 10.2527/jas.2013-6507. [DOI] [PubMed] [Google Scholar]
- Hooge D.M., Mathis G.F., Lumpkins B., Ponebsek J., Moran D. Dose-responses of broiler chicks, given live coccidia vaccine on day of hatch, to diets supplemented with various levels of Farmatan® (Sweet Chestnut Wood Tannins) or BMD®/Stafac® in a 42-day pen trial on built-up litter. Int. J. Poult. Sci. 2012;11:474–481. [Google Scholar]
- Huyghebaert G., Ducatelle R., Immerseel F.V. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Skorupińska J., Orda J., Kuryszko J., Tschirch H. Effect of sweet chestnut tannin (SCT) on the performance, microbial status of intestine and histological characteristics of intestine wall in chickens. Br. Poult. Sci. 2009;50:687–699. doi: 10.1080/00071660903191059. [DOI] [PubMed] [Google Scholar]
- Jansman A.J.M. Tannins in feedstuffs for simple-stomached animals. Nutr. Res. Rev. 1993;6:209–236. doi: 10.1079/NRR19930013. [DOI] [PubMed] [Google Scholar]
- Khanbabaee K., van Ree T. Tannins: classification and definition. Nat. Prod. Rep. 2001;18:641–649. doi: 10.1039/b101061l. [DOI] [PubMed] [Google Scholar]
- Mancini S., Minieri S., Buccioni A., di Cossato M.M.F., Russo C., Paci G. The influence of dietary chestnut and quebracho tannins mix on rabbit meat quality. Anim. Sci. J. 2019;90:680–689. doi: 10.1111/asj.13194. [DOI] [PubMed] [Google Scholar]
- Marquardt R.R. A simple spectrophotometric method for the direct determination of uric acid in avian excreta. Poult. Sci. 1983;62:2106–2108. doi: 10.3382/ps.0622106. [DOI] [PubMed] [Google Scholar]
- Marzoni M., Castillo A., Franzoni A., Nery J., Fortina R., Romboli I., Schiavone A. Effects of dietary quebracho tannin on performance traits and parasite load in an italian slow-growing chicken (White Livorno Breed) Animals. 2020;10:684. doi: 10.3390/ani10040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M., Mila I., Scalbert A. Precipitation of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J. Agric. Food Chem. 1996;44:599–606. [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjavila S., Lacombe C., Carrera G., Derache R. Tannic acid and oxidized tannic acid on the functional state of rat intestinal epithelium. J. Nutr. 1977;107:2113–2121. doi: 10.1093/jn/107.12.2113. [DOI] [PubMed] [Google Scholar]
- Rasmussen A.J., Andersson M. New method for determination of drip loss in pork muscles. Page 286287 in Proceedings of the 42nd International Congress of Meat Science and Technology; Lillehammer, Norway; Elsevier Applied Science; 1996. [Google Scholar]
- Rezar V., Salobir J. Effects of tannin-rich sweet chestnut (Castanea sativa mill.) wood extract supplementation on nutrient utilisation and excreta dry matter content in broiler chickens. Eur. Poult. Sci. 2014;78:1–10. [Google Scholar]
- Samuel K.G., Wang J., Yue H.Y., Wu S.G., Zhang H.J., Duan Z.Y., Qi G.H. Effects of dietary gallic acid supplementation on performance, antioxidant status, and jejunum intestinal morphology in broiler chicks. Poult. Sci. 2017;96:2768–2775. doi: 10.3382/ps/pex091. [DOI] [PubMed] [Google Scholar]
- Sanz M., Cadahía E., Esteruelas E., Muñoz A.M., Fernández de Simón B., Hernández T., Estrella I. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010;58:9631–9640. doi: 10.1021/jf102718t. [DOI] [PubMed] [Google Scholar]
- Schiavone A., Guo K., Tassone S., Gasco L., Hernandez E., Denti R., Zoccarato I. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult. Sci. 2008;87:521–527. doi: 10.3382/ps.2007-00113. [DOI] [PubMed] [Google Scholar]
- Schiavone A., Guo K., Tassone S., Gasco L., Malfatto V., Zoccarato I. Use of natural extract of chestnut (Silvafeed ENC®) in broiler feeding: effect on growth performance. Ital. J. Anim. Sci. 2007;6:731–733. [Google Scholar]
- Smulikowska S., Pastuszewska B., Święch E., Ochtabińska A., Mieczkowska A., Nguyen V.C., Buraczewska L. Tannin content affects negatively nutritive value of pea for monogastrics. J. Anim. Feed Sci. 2001;10:511–523. [Google Scholar]
- South P.K., Miller D.D. Iron binding by tannic acid: effects of selected ligands. Food Chem. 1998;63:167–172. [Google Scholar]
- Starčević K., Krstulović L., Brozić D., Maurić M., Stojević Z., Mikulec Ž., Bajić M., Mašek T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015;95:1172–1178. doi: 10.1002/jsfa.6805. [DOI] [PubMed] [Google Scholar]
- Treviño J., Ortiz L., Centeno C. Effect of tannins from faba beans (Vicia faba) on the digestion of starch by growing chicks. Anim. Feed Sci. Technol. 1992;37:345–349. [Google Scholar]
- Welfare quality® . Welfare Quality® Consortium; Lelystad, the Netherlands: 2009. Welfare Quality® Assessment Protocol for Poultry (Broilers, Laying Hens) [Google Scholar]