Abstract
Egg turning during incubation plays important roles in achieving high hatching performance and gosling quality. The objective of this study was to improve embryonic and muscular developments so to achieve better gosling quality by wider egg turning angles during incubation, and to unravel the associated regulatory molecular mechanisms. In each of three consecutive incubations, 1,728 goose eggs were divided into 3 groups that were set in the same type of commercial incubators with turning angles adjusted differently to 50°, 60°, and 70°, respectively. On average of the 3 tests, incubation with wider 70° turning angle reduced the post-18-day embryo mortality, promoted embryonic growth and development, improved the hatchability and gosling quality. On embryonic day of 29, gene mRNA expression levels of the hypothalamic growth hormone-releasing hormone (GHRH), pituitary growth hormone (GH), and liver insulin-like growth factor 1 (IGF-1) were higher in the 70° turning group than in the 50° or 60° groups. Wider angle turning also increased mRNA expression levels of the muscle development regulatory genes such as MYF5, MyoD, Myogenin (MyoG), and MRF4. Changes in expression of the above genes, together with the upregulation of the Pax3 and Pax7 genes in leg muscles, well explained the enhancement of the muscular growth and development when eggs were incubated by wider turning angles. These results also extended our understanding of the impacts and mechanisms of egg turning during incubation on hatching performance and gosling quality.
Key words: goose, embryonic and muscular development, somatotropic axis, gene expression, egg turning angle
INTRODUCTION
In artificial hatching of fowl eggs, turning of the eggs is critical to achieving high hatching performance (Decuypere and Bruggeman, 2007; Deeming, 2009). Turning can prevent improper adhesions of the embryo to the inner shell membrane or of allantois to the yolk sac early in the embryonic development (Cutchin et al., 2009). Most importantly, it promotes embryonic development by stimulating the expansion of the area vasculosa of the yolk sac membrane, improving blood circulation and accelerating nutrient uptake from the yolk (Deeming, 1989a,b). Furthermore, at the later stages of incubation, turning can adjust the positioning of the embryo relative to the yolk and albumen sacs so to achieve the correct formation of the sero-amniotic connection, which is essential for delivery of proteins from albumen to amniotic cavity (Deeming, 1991). No turning or inadequate turning usually leads to malpositioning and mortality both in early and late stages of incubation. The dead embryos are characterized by small size, poor development of the chorioallantois membrane, and residual albumen in the bottom of the egg (Deeming, 2009). However, turning at angles greater than 45°, does not improve hatchability of chicken eggs. Funk and Forward (1960) investigated turning angles of 30, 45, 60, and 75° (either side from the vertical) and found that 45° produced the best results in chicken eggs. Turning eggs at 40° or 45° as compared to at 20° and 30° increased hatchability, with little difference being found between 40° and 45° angles (Funk and Forward, 1953). Over the years, the typical egg turning practices in incubating chicken eggs was set at 45° angle once an hour, with which most research have been conducted (Wilson, 1991).
Similarly, by increasing the vertical space between egg flats, the 45° angle chicken egg incubator can be used to hatch goose eggs, whose weights of approximately150 g are more than 2 times as heavy as chicken eggs’ 65 g. On average, the hatchability of fertile goose eggs is much lower than that of the chicken eggs and more variable. This could be partly attributed to the higher metabolic heat production of the developing goose embryo that could render ventilation more difficult. Besides, turning of the eggs is a more important factor, because eggs with thicker and richer albumen need to be turned more frequently (Deeming, 1991, 2009). To some extent, increasing the turning frequency has the same effect as increasing the turning angle (Elibol and Brake, 2006). In our previous study of improving goose egg hatching performance, increasing turning angle from the conventional 45° to 75° significantly increased the hatchability and improved gosling quality (Dai et al., 2017). Further, goslings hatched from incubation with wider turning angle were with higher body weight, more robust and energetic movement behaviors. The numerous studies that have been conducted thus far focused on hatchability, embryonic mortality (Cutchin et al., 2009), and physiological basis such as fluid transportation (Latter and Baggott, 2000; Baggott et al., 2010), membrane growth (Deeming, 1989a) and gas exchange (Tona et al., 2003a). Nothing is known about the pertinent molecular regulatory mechanisms affected by egg turning angle during incubation.
In recent years, studies have found that embryo growth and muscular development, together with chick quality could be improved by in-ovo photostimulation with monochromatic green light, which upregulated the somatotropic axis gene expressions (Zhang et al., 2012; Dishon et al., 2018). Further, IGF-1, through regulation of the expression of myogenic regulatory factors (MRFs) and Pax3/7 genes, induce myoblasts proliferation, and influence muscle development during secondary muscle development (Halevy et al, 2006; Liu et al., 2011). Therefore, this study aimed to tests the hypothesis that wider turning angle during incubation improves embryonic and muscle growth of goose embryos via similar molecular regulatory mechanisms. In the study, different turning angles were used during goose egg incubation, and the somatotropic axis gene expression, including GHRH, GH, and IGF-1, as well as the expression patterns of MRFs and Pax3/7 gene during embryo development of the gosling was analyzed.
MATERIALS AND METHODS
Eggs and Experiment Design
The experiment was approved by the Ethics Committee of Jiangsu Academy of Agricultural Sciences. It was replicated by 3 times as done by incubating the same number of Yangzhou goose eggs in each turning angle in 3 consecutive incubations that were conducted in Tianzhijiao Goose Breeding Farm in Quanjiao County, Anhui Province, China. Eggs were collected from geese at 36, 40, and 44 wk of age, respectively. These times were at the peak egg laying stages in lighting controlled breeding production (geese start egg laying at 30 wk of age and finished at 54 wk of age). Under well-environment control in goose house, the quality of eggs was relatively stable. According to the company, the average hatchability of fertile eggs was 84.12 ± 0.76% (with traditional 50° turning angle). Totally 5,184 eggs, with each batch 1,728 eggs were used. To eliminate the egg weight effect, they were pre-weighted and selected for an average weight of 145.0 g (range from 135.0 g to 155.0 g). Before hatching, eggs were stored for 3 d with a temperature of 20˚C and relative humidity of 50% in small-end-down position to improve early embryonic development (Shao et al., 2014). Then, 1,728 eggs were equally assigned to the same location of three commercial incubators (SY-7680 incubator, Sanyuan Incubation Ltd, Bengbu, China; capacity was 7,680) with 9 trays in each incubator and every 3 trays as a replicate. Turning angles of the 3 incubators were adjusted differently to 50°, 60°, and 70°, respectively. Variable temperature and humidity were used during incubation as standard practices (Table 1). Temperature was initially set at 38°C, dropping by about 0.2 to 0.3°C every 5 to 10 d. The corresponding relative humidity at each stage was 65, 60, 62, 65% and 70%, respectively. Machines were checked manually 2 times per day to avoid abnormal operation. Each incubation period was 28 d then the eggs were transferred into the hatcher with no further egg turning. The goslings were hatched on 29th to 30th days. So between each batch, 2 d were spent for cleaning, disinfection and drying of the incubators and the hatcher.
Table 1.
Temperature and relative humidity during different incubation phases.
| Incubation phase (d) | Temperature (°C) | Relative humidity (%) |
|---|---|---|
| 1-5 | 38 | 65 |
| 6-15 | 37.7 | 60 |
| 16-22 | 37.5 | 62 |
| 23-27 | 37.2 | 65 |
| 28-30 | 37.0 | 70 |
On the d 8 and 18 of each incubation, infertile eggs and dead embryos were picked out and counted following candling. These data were used to calculate fertility or motility rate. From the d 28 onward, hatched goslings were collected from the hatching trays every 12 h for analysis of hatching speed between the turning angles. According to actual production requirement, all hatched goslings were checked manually in order to assess their quality. The quality of hatched goslings was determined in accordance with the methodology described by Tona et al. (2003b), which included examinations of the activity, feathering, eyes, conformation of legs, aspect of navel area, yolk absorption, and so on. Goslings that were active, with dry and clean feather, bright eyes, healthy legs, and closed navel were considered to be of high quality.
Tissue Collection
On embryonic days of 15, 22, and 29 (referred to hereafter as E15, E22, and E29) of the third batch of incubation, 8 eggs were randomly selected from each turning angle group except on E29, when embryos that had just started pipping were selected. After being weighed, the eggs were opened from the air sac, the embryos were gently pulled out and weighed after the fluid was dried by absorbing with a piece of dry tissue. The embryo weight was used for relative embryo weight calculation (embryo weight/egg weight). Skin of the leg was removed and leg muscles were stripped from the bone. On E15, due to the bones were too soft and too small, they were not removed. Then leg muscles were weighed for relative leg muscle weight calculation (leg muscle weight/embryo weight). Further, a separate set of leg muscle samples were collected and immediately fixed in 4% paraformaldehyde for preparation of histological sectioning. In addition, tissues of the hypothalamus, pituitary, liver, and leg muscle were dissected and collected from each embryo. Immediately after collection, the tissues were stored separately in 1.5-mL RNase-free centrifuge tubes, snap frozen in liquid nitrogen and then kept at −80° C until analysis for gene mRNA expression.
Histological Assessments
After fixation in paraformaldehyde for 48 h, the leg muscle tissue pieces were dehydrated, cleared and embedded in paraffin. Sections of 5-mm thickness were cut under a microtome and mounted onto glass slides. For all the assays, sections were deparaffinized in xylene and rehydrated following a graded alcohol series, then processed by hematoxylin-eosin staining. The sections were observed under light microscope and the images were scanned with an electronic scanning camera. The cross-sectional areas (CSA) of approximately 3,000 myofibers in 8 different fields of each muscle sample were measured with Image J (version 1.8.0; National Institute of Health, Bethesda, MD). Data of myofiber CSA were sorted into distribution at step intervals of 20 mm2, and the distribution diagrams were plotted by the Excel 2007 software.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
Real-time quantitative polymerase chain reaction (qRT-PCR) was performed to quantify the relative abundance of GHRH mRNA in hypothalamus, GH mRNA in pituitary gland, GHR and IGF-1 mRNA in liver, and some genes in leg muscle, such as the Pax gene (Pax3, Pax7) and the MRFs, including MYF5, MyoD, MyoG, and MRF4. Total RNA was extracted from the TRIzol lysate (Invitrogen, Carlsbad, CA) and was reverse transcribed into cDNA using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan).
The qRT-PCR was carried out in a total volume of 20 mL (10 mL of 2xSYBR Premix ExTaq, 1 mL cDNA mix, 0.5 mL of each primer, and 8 mL of sterile distilled H2O). An ABI PRISM-7500 sequence detection system (Applied Biosystems; Foster City, CA) was used to detect the amplified products. The primers used were designed using Primer Premier 5.0 (Premier, Vancouver, Canada) and their sequences are listed in Table 2. RT-PCR was performed with SYBR Premix Ex TaqTM (Roche, Switzerland). All the RT-PCRs were performed in triplicate. The relative expression of target genes was determined using the 2−ΔΔCT method (Livak and Schmittgen, 2002) and were normalized to an internal housekeeping gene, β-actin.
Table 2.
Primer sequences for RT-PCR.
| Gene name | Forward primer (5’ to 3’) | Reversed primer (5’ to 3’) |
|---|---|---|
| β-actin | CTTCCTGGGCATGGAGTCC | GGCGCGATGATCTTGATCTTC |
| GHRH | GCAAGCGGCTCAGAAACAGT | CCAGGAAGTCCCTCAGTACCAT |
| GH | CACTGTGATCACCCTGGGAT | TTGGTGTGTCTCTGGTCCTC |
| GHR | TTACTTCAACACATCCTACACC | TCATAATCTCTTCCCATCTTCA |
| IGF-1 | TGAGGAGGCTGGAGATGTACTG | TTCCTTCTGTGCTTTTGGCATA |
| Pax3 | TTCACCTCAGGTAATGGGACTCTTG | TGTAGGCAGGCTGTGTAAACTCTTCA |
| Pax7 | GTTAGCAATGGCCTCTCTCCA | CAGGATGCTCATCACCTGATTGT |
| MYF5 | TGCAAAGCCTGCAAGAGAAAG | GTCTCAAACGCCTGGTTCAC |
| MyoD | CAGCAGTTCTTCAACCACCAC | GATGCCCATAGGCTTCTCAA |
| MyoG | CGCCGCCTGAAGAAGGTGAA | CCTGCTGGTTGAGGGTGCTGA |
| MRF4 | GGTTGTTCCTCGGGGTGTTTTC | CTCCTTCTCCCCGTCCAAGTAG |
Statistical Analysis
Data of hatching performance including the embryonic mortality, hatchability of fertile eggs, gosling quality, and hatching weight were analyzed using two-way analysis of variance (ANOVA) by SPSS version 18.0 (Statistical Package for Social Science, SPSS Inc.; Chicago, IL). For analysis of relative embryo weight, leg muscle weight and the gene mRNA expression, one-way ANOVA was used. Differences between means were examined using the Duncan test and differences were considered significant at P < 0.05.
RESULTS
Hatching Performance
Incubation batches had no effect on hatching performance. The effects of egg turning angle during incubation on embryonic mortality, hatchability of fertile eggs, gosling quality, and hatching weight are shown in Table 3. No effect of egg turning angle on embryonic mortality was observed between day 7 and 18 during the incubation. But during the late stage of incubation, that is, from E19 to E30, embryo mortality of wider angle groups, 60° and 70°groups, were significantly (P < 0.05) lower than that in the 50° angle turning group. Accordingly, the hatchability of fertile eggs of 60° and 70° angle groups were significantly (P < 0.01) higher. In addition, the proportion of high-quality goslings were significantly (P < 0.01) higher in 60° and 70° angle turnings compared with the 50° turning. Besides, hatched gosling live weight from 70° angle turning group was significantly (P < 0.01) higher than those in the 50° turning. That of 60° angle group was intermediate and not different from that of 50° angle group.
Table 3.
Effect of egg turning angle during embryogenesis on embryonic mortality, hatchability of fertile eggs, gosling quality and hatching weight.
| Items | Turning angle |
||
|---|---|---|---|
| 50° | 60° | 70° | |
| Hatchability of fertile eggs, % | 83.41 ± 0.56b | 87.23 ± 0.51a | 86.53 ± 0.89a |
| Goslings of high quality, % | 80.95 ± 0.98b | 91.28 ± 0.57a | 89.38 ± 0.65a |
| Mortality till 8th day, % | 4.54 ± 0.56 | 4.84 ± 0.39 | 4.34 ± 0.55 |
| Mortality from 9th to 18th day, % | 2.77 ± 0.43 | 2.71 ± 0.77 | 2.20 ± 0.43 |
| Mortality from 19th to 30th day, % | 10.11 ± 1.29a | 5.77 ± 0.97b | 7.50 ± 0.98b |
| Hatching weight, g (n = 50) | 98.31 ± 8.72b | 99.05 ± 9.81b | 105.52 ± 8.65a |
Values are presented as means ± SEM.
Different letters in each line indicates significant difference (P < 0.05).
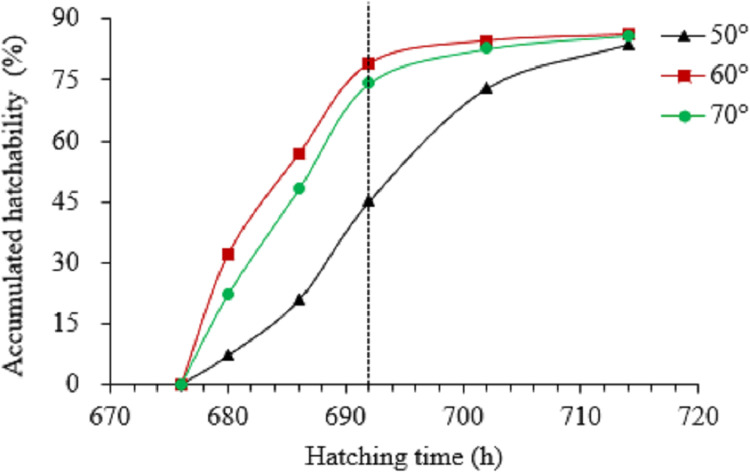
Eggs incubated under wider angle turnings hatched faster (Figure 1). Approximately 79 and 74% were hatched after eggs were set in incubators for 692 h for 60° and 70° groups, while for 50° group, it was only 45%. The calculated weighted average hatching time for the 70°, 60°, and 50° groups were 688, 686, and 693 h, respectively.
Figure 1.
Accumulated hatchability from 676 to 714 h of incubation time under different egg turning angles. Incubation time was calculated after eggs were placed in incubator. The vertical dotted line represents an incubation time of 692 h.
Embryonic and Muscle Growth
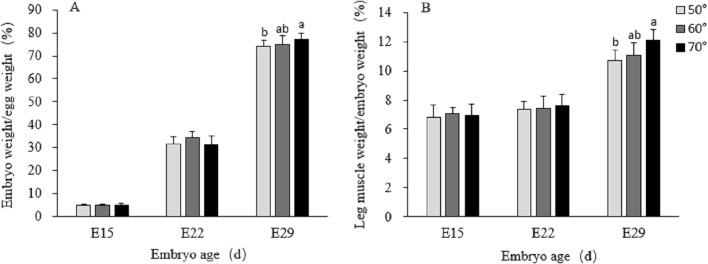
Turning angle during incubation had no effect (P > 0.05) on relative embryonic body weight and leg muscle weight on E15 and E22 (Figure 2). On E29, the relative embryonic body weight, leg muscle weight (Figure 2) and myofiber CSA (Figure 3) showed significant (P < 0.05) differences. 70° turning angle significantly (P < 0.05) enhanced embryonic and muscle growth. Compared with 50° group, embryonic body weight (Figure 2A) and leg muscle weight (Figure 2B), both expressed on a percentage basis, were significantly heavier under 70° angle on E29. The parameters of 60° turning group were intermediate and not statistically (P > 0.05) different from the other 2 groups.
Figure 2.
Relative embryonic body weight (A) and leg muscle weight (B) incubated under three different egg turning angles. Data were presented as means ± SEM (n = 8). Different letters above each bar indicate differences being significant (P < 0.05) at a given embryonic age. Leg muscle weight at E15 includes the leg bones.
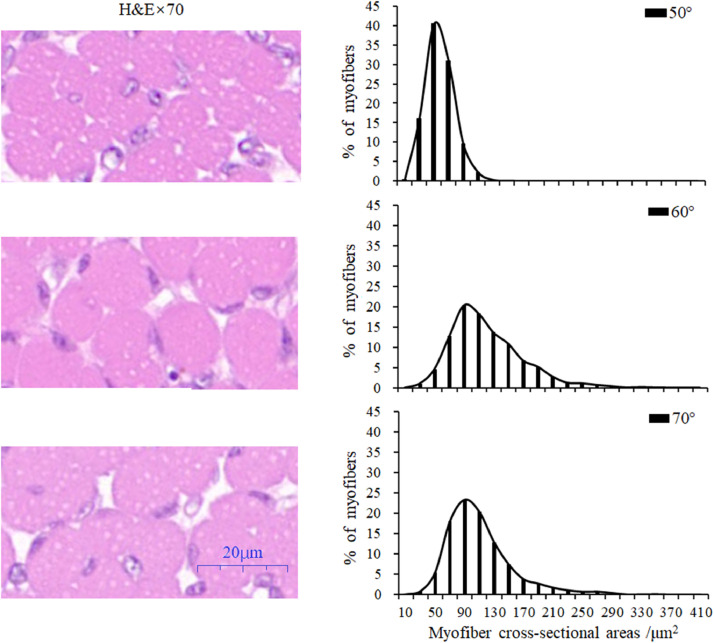
Figure 3.
Image of higher magnifications (magnified 70 times) of representative areas from the gastrocnemius muscle (left) and distribution of myofiber cross-sectional areas (right) under three different egg turning angles. Each graph on the right represents the composite of approximately 3,000 myofibers measurements in 8 different fields of each muscle sample from 8 embryos. Data of myofiber CSA were sorted into distribution at step intervals of 20 mm2.
Myofiber CSA of the three groups were in Gaussian distribution (Figure 3). There was a significant (P < 0.01) increase in myofiber CSA on E29 under wider turning angles. For 60° and 70° turning groups, 82.25 and 75.86% of total myofibers fell within the area range of 70 to 150 mm2, whereas in the 50° group, only 43.15% of total myofibers fell within this range. However, as was shown in Figure 3, myofiber CSA were more uniformed to 50 mm2 in the 50° group. There were more myofibers that had a larger CSA (from 170 to 390 mm2). For 60° and 70° group, the proportion was 14.70 and 8.24%, respectively, while for the 50° group, it was only 0.06%.
Gene mRNA Expressions
Somatotropic Axis Genes
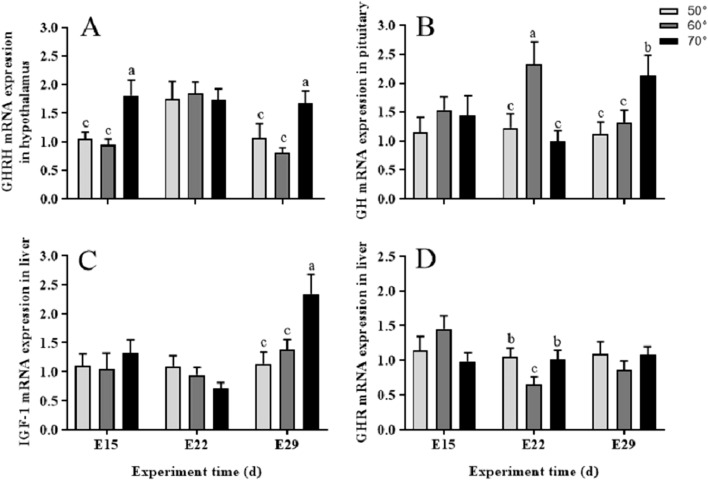
As was shown in Figure 4, turning angle during incubation influenced the relative expression of mRNA of the somatotropic axis genes. The hypothalamic GHRH mRNA (Figure 4A) was significantly (P < 0.01) higher in 70° group than that of the 50° group on E15 and E29. Also on E29, it was near 2 folds (P < 0.01) of GH (Figure 4B) and IGF-1 mRNA (Figure 4D) in the pituitary gland and the liver, respectively. Compared with 50° group, there was a significant (P < 0.01) increase of the pituitary gland GH mRNA in the 60° group on E22, while a significant (P < 0.05) decrease of the liver GHR mRNA (Figure 4C). Beyond that, no other differences were observed in somatotropic axis genes expression between 60° and 50° group.
Figure 4.
Relative expression of mRNA in somatotropic axis during incubation under 50°, 60°, and 70° egg turning angle, respectively. Panels (A–D) represent relative abundances of mRNA for GHRH, GH, IGF-1 and GHR, respectively. Each value represents the average data from eight embryos. Data are shown as mean values ± standard error of the mean. Different letters above the bars denote significant (a-b: P < 0.05, b-c: P < 0.05, a-c: P < 0.01) differences.
Muscle Development Related Genes
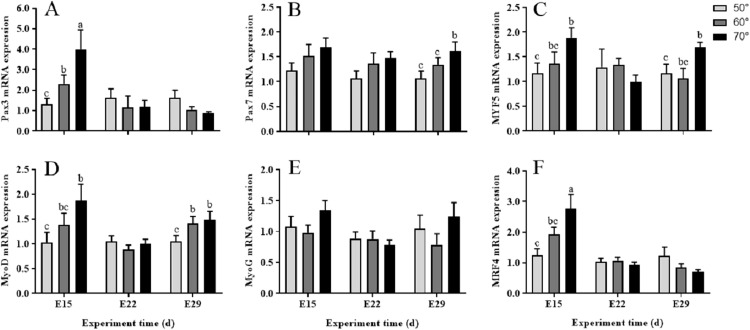
As were shown in Figure 5, muscle development related genes among the 3 turning angles showed different expression pattern at different incubation stages. Pax3 mRNA expression level of 70° group was higher (P < 0.01) than 50° group on E15, so were those of MYF5, MyoD (P < 0.05) and MRF4 (P < 0.01). However, the upregulation of Pax7 and MyoG mRNA expression were not significant (P > 0.05). No significant differences were observed of all the mRNA expression of these genes on E22. At the end of incubation (on E29), MYF5 and MyoD mRNA expression levels were significantly (P < 0.05) increased in 70° tuning group as compared with the 50° group.
Figure 5.
Relative expression of mRNA in leg muscles during incubation under 50°, 60°, and 70° egg turning angle, respectively. Panels (A and B) represent relative abundances of mRNA for Pax3 and pax7, respectively. Panels (C–F) represent relative abundances of mRNA for MYF5, MyoD, MyoG, and MRF4, respectively. Each value represents the average data from eight embryos. Data are shown as mean values ± standard error of the mean. Different letters above the bars denote significant (a-b: P < 0.05, b-c: P < 0.05, a-c: P < 0.01) differences.
DISCCUSION
Decades of research have shown that turning of eggs can reduce late embryonic mortality that can be brought about by embryo malpositioning or poor membrane extension (Tullett and Deeming, 1987; Wilson et al., 2003; Deeming, 2009). In this study, we found that wider egg turning angle during incubation significantly reduced late embryonic mortality, increased hatchability, and improved gosling quality. Together with improving hatchability, wider angle turning eggs can enhance embryonic development and accelerate hatching. It also enhanced muscular development which could be fulfilled by regulating expressions of the somatotropic axis and muscle development related genes.
There are 2 peaks of embryonic mortality during normal incubation (turned), one in the first few days, which is thought to be caused by the early adhesion of embryo to the inner shell membrane, and the other in the last week of incubation, which is caused by malpositioning of the embryos (Robertson, 1961; Schalkwyk et al., 2000). Correct egg turning has been proven to be an effective way to reduce embryonic mortality and to improve hatchability (Tullett and Deeming, 1987; Deeming, 2009; Dai et al., 2017). In this study, we sought to investigate whether enlarging egg turning angles can mitigate the poor hatching of goose eggs that could arise from the wider egg weight. No difference was observed in early embryonic mortality among turning angles from 50° through to 60° and 70°. However, at the later stage of incubation, the embryonic death rate in the 60° and 70° groups was significantly lower than that in the 50° group. According to Elibol and Brake (2006), this is because wider angle or appropriate turning can significantly reduce the incidence of embryos that died from pecking at the small end of eggs.
The hatching rate of fertile eggs increased significantly under wider angle turning due to reduced late embryonic mortality. Moreover, greater egg turning angle significantly promoted embryo development, shortened incubation time and improved gosling quality. Usually, the spread of hatching can affect the time of first feeding, thus affect post-hatch growth of poultry, whereas proper turning during incubation can shortened incubation duration, leads to low spread of hatching and improves day-old chick qualitative aspects (Decuypere and Bruggeman, 2007). Besides, sufficient turning could promote embryonic development, speed up embryo's metabolism, thus increasing the partial pressures of CO2 (pCO2) in the air cell during internal piping stage (Tona et al., 2003b; Tona et al., 2005). Higher pCO2 can stimulate the embryo to peck at the shell early and hatched early (Tona et al., 2007). Such principle can well explain the shortened incubation duration and better gosling quality of wider turning angles in the present study.
In addition, the higher BW and leg muscle weight of the embryos stimulated with wider turning angles, compared with that of the 50° turning angle, was evident on the hatching day. These findings are consistent with the results of previous studies, that is, the apparent effects of egg turning on embryo development are mainly reflected in the late incubation period (Deeming et al., 1987; Deeming, 1989b). Inadequate turning influences chorioallantois extension ability or speed underneath the inner surface of the inner shell membrane (Deeming, 1991). This will impede the area vasculosa growth rate (Deeming, 1989a) and will prevent the sero-amniotic connection from forming properly (Cutchin et al., 2009). The combination of these factors will cause residual albumen to remain unutilized in the bottom of the egg and inadequate absorption of nutrients (Tullett and Deeming, 1987; Deeming, 1991). On the contrary, wider angle turning of 70° would make full use of the albumen and produce heavier embryos and goslings as was observed in the present study. Thus, embryo body weight was higher under 70° turning as compared with in 50° angle turning. Others proposed that more frequent or wider angle turning should be practices when incubating fowl eggs with higher percentage of albumen content (Deeming, 2002; Elibol and Brake, 2006). Because the percentage of albumen content of goose eggs is higher than chicken eggs, which infers the traditional 45° turning angle suitable for incubation of chicken eggs is inadequate for goose eggs.
According to our present study, the related genes in somatotropic axis and muscles might be the most important contributor to embryo growth and development promoted by wider turning angle. In domestic fowls, the highest growth rate occurs during the late embryonic to juvenile stages of development (Kim, 2010). The somatotropic axis is regulated by GHRH, thyrotropin-releasing hormone (TRH) and somatostatin (SST), which regulate secretion of GH from the anterior pituitary gland and the hepatic IGF-I (Florini, 1996). IGF-1 can promote myoprotein synthesis and inhibits myoprotein degradation through the PI3K - Akt - mTOR - P70S6K signaling pathway (Ding et al., 1996; Liu, 2012). In our present study, we observed that the relative embryo leg muscle weight in 70° group was significantly higher than 50° group on E29. Moreover, using hematoxylin and eosin staining we observed that the CSA of myofibers in 70° group was substantially larger than in 50° group. This was accompanied by significant upregulation of the GHRH, GH, and IGF-1 mRNA expression. These results suggested that the enhanced muscle growth during late incubation stages by wider angle turning could be resulted from the regulation of myoprotein accretion following upregulated IGF-1 gene expression.
In addition to protein accretion, myofiber growth entails an increase in the number of myofiber nuclei that are derived from the myoblasts (Hawke and Geary, 2001; Halevy et al., 2004). IGF-1 promotes the proliferation of myoblasts, which provides the foundation for muscle growth and myoblasts fusion via increased amount of myoprotein accretion and myofilament numbers (Noguchi, 2005; Yu et al., 2016). This proposition was demonstrated by the previous study with duck embryos that IGF-1 upregulated expression of myogenic transcription factors and growth of muscle fibers in late embryonic period (Liu et al., 2011). Many genes are involved in this process, including the MRFs family and the Pax gene family (Schultz and Mccormick, 1994). First, Pax3 and Pax7 activate the expression of MYF5 and MyoD, which are responsible for myoblasts proliferation. Subsequently, myoblasts synthesize and secrete skeletal muscle-specific proteins and myoblasts adhesion molecules under the regulation of MyoG and MRF4. MyoG and MRF4 can promote the terminal differentiation of single free myoblasts and their fusion to form multinucleated myotubes (Tajbakhsh, 2003; Yu et al., 2016). In the present study, the relative expression of pax3, MyoD, MYF5, and MRF4 mRNA were significantly higher in 70° group on E15, surrounding the stage of myoblast formation and proliferation. This should result in large numbers of monocytes for muscle growth and proteins for myoblasts fusion.
During the late embryonic through to the juvenile stages, satellite-cell proliferation occurs actively, which becomes quiescent during the late growth phase and in adulthood (Schultz, 2010). Pax7 gene is considered as the indicator of the proliferation capacity of satellite cells (Buckingham, 2007). In this study, significantly higher mRNA expression level of Pax7 in 70°group on E29 indicated presence of a higher reservoir of myogenic progeny cells brought about by wider angle egg turning. Further, higher levels of MyoD and MYF5 mRNA expression on E29 in 70°group also indicated strong proliferation activity of satellite cells, because these genes are not expressed in quiescent satellite cells (Yablonka-Reuveni and Paterson, 2001). Changes in expressions of these muscle development regulation genes further explained the enhanced growth of leg muscle and bigger CSA of myofibers on E29 in the large angle egg turning group embryos.
In conclusion, we demonstrated that turning eggs at 70° angle during goose egg incubation fastened hatching speed and improved hatchability and gosling quality as compared to 50° turning angle. Wider angle turning of eggs also enhanced the growth and development of embryos and muscles by regulating the expression of genes in somatotropic axis and muscular development. Such theory can help guide development of new hatching technologies for poultry industry.
ACKNOWLEDGMENTS
This study was supported by the open project of Key Laboratory of Protected Agriculture Engineering in the Middle and Lower Reaches of Yangtze River, Ministry of Agriculture and Rural Affairs (grant number KF2018, KF2019), the China Agriculture Research System of MOF and MARA (grant number CARS-40-20), and Jiangsu Province Postdoctoral Science Foundation (grant number 2020Z396).
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Baggott G.K., Deeming D.C., Latter G.V. Electrolyte and water balance of the early avian embryo: effects of egg turning. Avian Poult. Biol. Rev. 2010;13:105–119. [Google Scholar]
- Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 2007;330:530–533. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Cutchin H.R., Wineland M.J., Christensen V.L., Davis S., Mann K.M. Embryonic development when eggs are turned different angles during incubation. J. Appl. Poult. Res. 2009;18:447–451. [Google Scholar]
- Decuypere E., Bruggeman V. The endocrine interface of environmental and egg factors affecting chick quality. Poult. 2007;86:1037–1042. doi: 10.1093/ps/86.5.1037. [DOI] [PubMed] [Google Scholar]
- Deeming D.C., Rowlett K., Simkiss K. Physical influences on embryo development. J. Exp. Zool. 1987;1:341–345. Supplement: published under auspices of the American Society of Zoologists and the Division of Comparative Physiology and Biochemistry /the Wistar Institute of Anatomy and Biology. [PubMed] [Google Scholar]
- Deeming D.C. Failure to turn eggs during incubation: development of the area vasculosa and embryonic growth. J. Morphol. 1989;201:179–186. doi: 10.1002/jmor.1052010207. [DOI] [PubMed] [Google Scholar]
- Deeming D.C. Characteristics of unturned eggs: critical period, retarded embryonic growth and poor albumen utilisation. Br. Poult. Sci. 1989;30:239–249. doi: 10.1080/00071668908417144. [DOI] [PubMed] [Google Scholar]
- Deeming D.C. In: Pages 307-323 in Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Deeming D.C., Ferguson M.W.J, editors. Cambridge University; Cambridgeshire, UK: 1991. Reasons for the dichotomy in egg turning in birds and reptiles. [Google Scholar]
- Deeming D.C. In: Pages 161-178 in Avian Incubation: Behaviour, Environment and Evolution. Deeming D.C., editor. Oxford University Press; Oxfordshire, UK: 2002. Patterns and significance of egg turning. [Google Scholar]
- Deeming D.C. The role of egg turning during incubation. Avian Biol. Res. 2009;2:67–71. [Google Scholar]
- Dai Z., Yao J., Ren Y., Shao X., Huang Y., Shi Z. Development of large egg turning angle hatching machine and its application in goose egg hatching. China Poult. 2017;39:63–66. (in Chinese) [Google Scholar]
- Dishon L., Avital-Cohen N., Zaguri S., Bartman J., Rozenboim I. In-ovo green light photostimulation during different embryonic stages affect somatotropic axis. Poult. Sci. 2018;97:1998–2004. doi: 10.3382/ps/pey078. [DOI] [PubMed] [Google Scholar]
- Ding H., Gao X.L., Hirschberg R., Vadgama J.V., Kopple J.D. Impaired actions of insulin-like growth factor 1 on protein Synthesis and degradation in skeletal muscle of rats with chronic renal failure. Evidence for a postreceptor defect. J. Clin. Invest. 1996;97:1064–1075. doi: 10.1172/JCI118499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elibol O., Brake J. Effect of egg turning angle and frequency during incubation on hatchability and incidence of unhatched broiler embryos with head in the small end of the egg. Poult. 2006;85:1433–1437. doi: 10.1093/ps/85.8.1433. [DOI] [PubMed] [Google Scholar]
- Florini J.R. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Funk E.M., Forward J. Effect of angle of turning eggs during incubation on hatchability. Missouri Agri. Exp. Sta. Bui. 1953:599. [Google Scholar]
- Funk E.M., Forward J. The relation of angle of turning and position of the egg to hatchability of chicken eggs. Poult. Sci. 1960;39:784–785. [Google Scholar]
- Halevy O., Piestun Y., Allouh M.Z., Rosser B.W.C., Rinkevich Y., Reshef R., Rozenboim I., Wleklinski-Lee M., Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Hawke T.J., Geary D.J. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Kim J.W. The endocrine regulation of chicken growth. Asian-Australas. J. Anim. Sci. 2010;23:1668–1676. [Google Scholar]
- Latter G.V., Baggott G.K. The effect of egg turning and fertility upon the potassium concentration of the albumen and yolk of the Japanese quail. Br. Poult. Sci. 2000;41:44–45. doi: 10.1080/00071669608417861. [DOI] [PubMed] [Google Scholar]
- Liu H.H., Wang W.J., Chen P.R., Zhang Y.H., Yu B.H. In ovo administration of rhIGF-1 to duck eggs affects the expression of myogenic transcription factors and muscle mass during late embryo development. J. Appl. Physiol. 2011;111:1789–1797. doi: 10.1152/japplphysiol.00551.2011. [DOI] [PubMed] [Google Scholar]
- Liu H.H. Sichuan Agri. Univ.; Ya'an, China: 2012. PhD Diss. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods. 2002;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Noguchi S. The biological function of insulin-like growth factor-I in myogenesis and its therapeutic effect on muscular dystrophy. Acta. Myol. 2005;24:115–118. [PubMed] [Google Scholar]
- Robertson I.S. The influence of turning on the hatchability of hens' eggs I. The effect of rate of turning on hatchability. J. Agric. Sci. 1961;57:49–56. [Google Scholar]
- Schalkwyk S.J.V., Cloete S.W.P., Brown C.R., Brand Z. Hatching success of ostrich eggs in relation to setting, turning and angle of rotation. Br. Poult. Sci. 2000;41:46–52. doi: 10.1080/00071660086394. [DOI] [PubMed] [Google Scholar]
- Schultz E., Mccormick K.M. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Schultz E. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J. Exp. Zool., Part A. 2010;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Shao X.B., Lei M.M., Chen X.P., Shi Z.D. Analysis of related factors affecting the hatching performance of out-of-season breeding geese. China Poult. 2014;36:52–54. (in Chinese) [Google Scholar]
- Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr. Opin. Genet. Dev. 2003;13:413–422. doi: 10.1016/s0959-437x(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., Ketelaere B.D., Decuypere E., Bruggeman V. Effects of turning duration during incubation on corticosterone and thyroid hormone levels, gas pressures in air cell, chick quality, and juvenile growth. Poult. Sci. 2003;82:1974–1979. doi: 10.1093/ps/82.12.1974. [DOI] [PubMed] [Google Scholar]
- Tona K., Bamelis F., Ketelaere B.D., Bruggeman V., Moraes V.M., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., Bruggeman V., Mertens K., Decuypere E. Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poult. Sci. 2005;84:315–320. doi: 10.1093/ps/84.2.315. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., Bruggeman V., Smit L.D., Figueiredo D., Decuypere E. Non-ventilation during early incubation in combination with dexamethasone administration during late incubation: 1. Effects on physiological hormone levels, incubation duration and hatching events. Domest. Anim. Endocrinol. 2007;33:32–46. doi: 10.1016/j.domaniend.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Tullett S.G., Deeming D.C. Failure to turn eggs during incubation: effects on embryo weight, development of the chorioallantois and absorption of albumen. Br. Poult. Sci. 1987;28:239–243. doi: 10.1080/00071668708416958. [DOI] [PubMed] [Google Scholar]
- Wilson H.R. In: Pages 145-156 in Avian Incubation. Tullett S.G., editor. Butterworth-Heinemann; London, UK: 1991. Chapter 9: Physiological requirements of the developing embryo: temperature and turning. [Google Scholar]
- Wilson H.R., Neuman S.L., Eldred A.R., Mather F.B. Embryonic malpositions in broiler chickens and bobwhite quail. J. Appl. Poult. Res. 2003;12:14–23. [Google Scholar]
- Yablonka-Reuveni Z., Paterson B.M. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J. Histochem. Cytochem. 2001;49:455–462. doi: 10.1177/002215540104900405. [DOI] [PubMed] [Google Scholar]
- Yu M., Wang H., Xu Y., Yu D., Li D., Liu X., Du W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2016;39:910–922. doi: 10.1002/cbin.10466. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Qiao X., Yue H.Y., Wu S.G., Yao J.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]