Abstract
This study investigated the effects of trans-anethole (TA) supplementation on the carcass characteristics, meat quality, fatty acid, and amino acid profiles of breast muscle in broilers. A total of 40 one-day-old male broiler chicks (Arbor Acres) were randomly allocated to 5 treatments, respectively, fed a corn-soybean basal diet supplemented with 0 (control), 200, 400, 600, and 800 mg TA/kg diet for 42 d. 600 mg/kg of TA supplementation decreased (P < 0.05) serum triglycerides (TG) on d 21 and d 42, and high density lipoprotein cholesterol (HDL-C) concentration on d 21, but increased (P < 0.01) serum HDL-C concentration on d 42. Dietary supplementation of TA increased (P < 0.01) the half chamber rate (HCR) and eviscerated rate (ER) of broilers. The drip loss (storing 24 and 48 h) and cooking loss of breast muscle in 600 mg/kg TA groups were lower (P < 0.05) than those in control group. The concentration of palmitoleic acid, daturic acid, oleic acid, linoleic acid, α-Linolenic acid, eicostrienoic acid, and pentosapentanoic acid (EPA), MUFA, and PUFA in the breast muscle were higher (P < 0.05) in the 600 mg/kg of TA group compared with other groups. Dietary inclusion of 600 mg/kg of TA also increased (P < 0.05) the concentration of Met, Thr, Asp, Ser, and Glu in breast muscle, tended to increase (P = 0.069) the Lys concentration. In conclusion, results indicated that TA inclusion improved the lipid metabolism, meat quality, fatty acid composition, and amino acid profile of breast muscle in broilers.
Key words: trans-anethole, broiler, carcass characteristics, meat quality, fatty acid and amino acid profiles
INTRODUCTION
In the recent years, the high-density intensive feeding mode has been rapidly developed to meet the high demands of broiler meats by consumers. However, in this case, birds are more susceptible to stresses such as oxidative stress and pathogenic diseases. The occurrence of stress resulted in an inferior effect on meat quality (Yang et al., 2019). With the consumer demands for safer, healthier, and tastier meat, the flavor and quality of meat has gained more and more attention (Cheng et al., 2017). The breast muscle of broilers is the preferred parts of consumers. The chemical composition of chicken meat, including the amino acid and fatty acid profiles, is of great concern to consumers, and becomes the focus of research due to their direct association with cardiovascular diseases in humans (Waheed et al., 2018). Polyunsaturated fatty acids (PUFA) are considered as flavor and functional ingredients to prevent cardiovascular disease (Simopoulos, 2001). The use of natural plant essential oils in improving meat quality with antioxidant and antimicrobial activities has been a worldwide research hotspot. Previous study showed that botanical extracts significantly increased the concentration of amino acids, and unsaturated fatty acids in broiler meat, indicating its role on improving meat quality (Waheed et al., 2018). Cheng et al. (2017) demonstrated that dietary oregano essential oil enhanced the sensor attributes by improving ω-3 PUFA proportion in pork meat.
Trans-anethole (TA) is a volatile anise flavoring constituent of many essential oils of medicinal aromatic plants of more than 20 species (e.g., fennel, anise, and star anise). Its antimicrobial and antioxidant activities have been widely reported (Senatore et al., 2013; Hançer Aydemir et al., 2018; Sá et al., 2018; Wieczyńska and Cavoski, 2018). TA has been generally recognized as safe by the FDA and widely used as an odorant in foods, cosmetics, alcoholic beverage, and perfumes (Sheikh et al., 2015; Aprotosoaie et al., 2016). It was reported that TA greatly improved the overall freshness, odor, and quality of active packaging for organic ready-to-eat iceberg lettuce (Wieczyńska and Cavoski, 2018). However, to the best of our knowledge, no research has been conducted on the investigation of the effects of long-term TA supplementation on chicken flavor and quality. The objective of the present study was to investigate the effects of TA supplementation on lipid metabolism, carcass characteristics, meat quality, fatty acid, and amino acid profiles of breast muscle in broilers.
MATERIALS AND METHODS
Preparation of TA
TA was purchased from Nanjing Dilger Medical Technology Co., Ltd (Nanjing, China) (purity, 98.35%). The TA was stored in glass bottles in the dark and stored at 4°C until use.
Animals, Experimental Design and Diets
All the procedures carried out in the study were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China). A total of 40 one-day-old male broiler chicks (Arbor Acres) with similar initial body weight (BW) of 39.75 ± 0.47 g were obtained from a commercial hatchery (Yantai Land Animal Husbandry Co., Ltd, Yantai, China). All of the birds were randomly allocated to 1 of 5 treatment groups based on initial BW with 8 replicates of 1 bird per replicate. The treatment groups were basal diet supplemented with 0 (control group), 200, 400, 600, and 800 mg of TA/kg of diet. The experimental period lasted for 42 d. The experimental diets were based on corn-expanded soybean meal and formulated to meet nutrient requirements recommended by Feeding Standard of chicken of the People's Republic of China (NY/T 33-2004) for the starter (1–21 d) and grower (22–42 d) birds. Ingredients and nutrient contents of the basal diet were presented in Table 1. The TA was firstly mixed with soybean oil and then mixed with other ingredients. All birds were kept in an environmentally controlled room and provided with ad libitum access to feed and water. The temperature was kept at 35°C at the first week and was reduced linearly by 0.5°C per day to a temperature of approximately between 21°C and 26°C.
Table 1.
Ingredients and nutrient composition of the basal diets1 (%, as-fed basis).
| Ingredients | Starter (1–21 d) | Grower (22–42 d) |
|---|---|---|
| Corn | 55.60 | 54.40 |
| Expanded soybean meal (46% CP) | 29.00 | 24.15 |
| Cottonseed meal | 2.50 | 3.00 |
| Wheat flour | 4.00 | 4.00 |
| Hydrolyzed feather meal | 1.50 | 1.50 |
| Soybean oil | 2.00 | 7.25 |
| Dicalcium phosphate | 0.90 | 0.80 |
| Limestone | 1.50 | 1.50 |
| Bentonite | 1.00 | 1.00 |
| Premix1 | 2.00 | 2.00 |
| Titanium dioxide | 0.00 | 0.40 |
| Nutrient levels2 | ||
| ME, MJ/kg | 12.11 | 13.44 |
| CP | 21.50 | 19.51 |
| Calcium | 0.96 | 0.84 |
| Total phosphorus | 0.66 | 0.55 |
| Lys | 1.45 | 1.40 |
| Met | 0.54 | 0.50 |
| Thr | 0.91 | 0.80 |
The experimental diet was the same basal diet supplemented with 200, 400, 600, 800 mg of TA/kg of the basal diet.
Supplied per kilogram of diet: vitamin A, 11,500 IU; cholecalciferol, 3,500 IU; vitamin E, 30 mg; vitamin K3, 5 mg; thiamin, 3.38 mg; riboflavin, 9.0 mg; pyridoxine, 8.96 mg; vitamin B12, 0.025 mg; choline chloride, 800 mg; calcium pantothenate, 13 mg; niacin, 45 mg; biotin, 0.15 mg; folic acid, 1.20 mg; Mn, 60 mg; Fe, 66.5 mg; Zn, 88 mg; Cu, 8.8 mg; I, 0.70 mg; Se, 0.288 mg.
Sampling
On d 21 and d 42, five mL of blood was collected into coagulation-promoting tube from the wing vein of birds (8 birds per treatment). The serum was obtained by centrifuging the blood at 3,000 × g for 10 min. The serum supernatants were subsequently transferred into 1.5 mL centrifuge tubes and stored at −20°C until lipid metabolism parameters analysis.
On d 42, eight birds per treatment were electrically stunned, weighted, and sacrificed by cervical dislocation. The half chamber rate (HCR) was determined by removed organs except the hearts, livers, stomachs, lungs, and kidneys. Then the heads, feet, neck, and organs except lungs and kidneys were all discarded and weighted to calculate the eviscerated rate (ER) following Ding et al. (2020). Then, the left breast muscle were collected and used for the physical parameters analysis. Meanwhile, 15 g of right breast muscle in the same position of individual bird was removed and rapidly frozen in liquid nitrogen, later stored at −80°C for fatty acid composition, and amino acid profile analyses.
Serum Lipid Metabolism Parameters
The concentration of triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) in the serum were determined by using Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China) combined with a UV Spectrophotometer (Shimadzu Co., Ltd, Japan) following the manufacturer's instructions.
Carcass Characteristics
The carcass parameters were measured according to the Agricultural Industry Standard of People's Republic of China (NY/T 823-2004) and calculated by the following equation.
Physical Parameters
The left fresh breast muscle samples were used in the following order: 1) 3.0-cm-thick chop used for pH measurement after stored at 4°C for 45 min and 24 h; 2) 3.0-cm-thick chop used for objective fleshcolor measurement (L*, a*, and b*) after stored at 4°C for 24 h; 3) 4.0-cm-thick chop used for drip loss measurement; 4) 4.0-cm-thick chop used for cooking loss measurement after stored at 4°C for 24 h.
All the physical parameters determination of the breast muscle was performed with the method as described by Cheng et al. (2017). Measurements of pH were performed using a portable pH meter (HI9125, HanNa Instruments, Italy), and the reading of pH was conducted after pH meter stabilizing for 15 s. The electrode was flushed using specified buffer solution when measuring different samples. Objective color measurements (L*, a*, b*) were performed from a mean of 3 random position in the same muscle sample with a chromameter (NR60CP, 3nh Technology Co., Ltd, Shenzhen, China) under normal light conditions. Shortly, a rectangular piece of muscle (4 × 2 × 1 cm3) was weighted and recorded as W1, subsequently placed in a plastic container on a grid parallel with a paper clip to the fiber direction (Honikel, 1998). The weight loss percentages after 24 h and 48 h of storage at 4°C were calculated for drip loss analysis. A fresh slice of breast muscle (4 × 3 × 1 cm3) from each sample was weighed, placed in a self-sealing bag, and cooked to an internal temperature of 70°C for 15 min in a 80°C water bath (Honikel, 1998). Then water cooling, blotted dry, and weighed. The drip loss and cooking loss was calculated as (initial weight − final weight) / initial weight × 100.
Analysis of the Fatty Acid Composition
Lipids were extracted from breast muscle samples with chloroform-methanol according to the method of Folch et al. (1957). NaOH/methanol was used to prepare fatty acid methyl esters for gas chromatography determination. Fatty acid compositions were determined with a 7890A Gas Chromatograph (Agilent Technologies Co., Ltd, Palo Alto, CA) equipped with a flame ionization detector, and a SP-2380 Capillary Chromatographic Column (Anpel Laboratory Technologies Inc. Shanghai, China) for fatty acid methyl esters (100 m × 0.25 mm × 0.20 μm). The fatty acids were identified by comparing the retention times of the peaks with those of known standard substance (Anpel Laboratory Technologies Inc. Shanghai, China). The calculation of fatty acid compositions were performed by internal standard method as described by Luo et al. (2009).
Analysis of the Amino Acid Profile
Shortly, 100 mg of fresh breast muscle samples were hydrolyzed in the ampoule bottle using 6 N hydrochloric acid at 110°C for 24 h. The ampoule bottle was sealed under an atmosphere of N to prevent amino acid oxidation. The amino acid profile of the meat samples was determined according to the method of Adeola et al. (2008) using an HPLC-based automatic amino acid analyzer (LA8080, Hitachi high-technologies Co. Ltd, Japan). Post-drying samples were dissolved in citrate buffer (2.2 pH) solution followed by filtration with 0.22-μm filter membrane (aqueous phase) to obtain a clear solution of the free amino acids for post-column derivation of amino acids.
Statistical Analysis
All data were analyzed by one-way ANOVA using the GLM procedure of SAS (version 9.2; SAS Institute, Cary, NC). Each bird was considered as the experimental unit. Differences among means were analyzed by Tukey's HSD test method. The data was presented as the means with the standard error of the mean (SEM). The significance for all analyses was indicated as P < 0.05.
RESULTS
Serum Lipid Metabolism Parameters
Compared to control group, 600 mg/kg of TA supplementation decreased (P < 0.05) serum TG on d 21 and d 42, and HDL-C concentration on d 21, but increased (P < 0.01) serum HDL-C concentration on d 42 (Table 2). The serum TC and LDL-C were not affected (P > 0.05) by TA inclusion.
Table 2.
Effects of dietary TA supplementation on the serum lipid metabolism parameters of broilers.
| Item1 | Dietary TA concentration, mg/kg |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| 21 d | |||||||
| TG, mmol/L | 0.70ab | 0.69ab | 0.79a | 0.54b | 0.62ab | 0.026 | 0.041 |
| TC, mmol/L | 2.64 | 2.48 | 2.46 | 2.39 | 2.65 | 0.063 | 0.592 |
| HDL-C,mmol/L | 0.46a | 0.28ab | 0.11b | 0.20b | 0.17b | 0.036 | 0.035 |
| LDL-C,mmol/L | 0.10 | 0.09 | 0.09 | 0.13 | 0.14 | 0.011 | 0.467 |
| 42 d | |||||||
| TG, mmol/L | 1.46a | 1.34ab | 1.43a | 1.14b | 1.38ab | 0.060 | 0.038 |
| TC, mmol/L | 2.27 | 2.60 | 2.59 | 2.20 | 2.27 | 0.078 | 0.226 |
| HDL-C,mmol/L | 0.21b | 0.23b | 0.35ab | 0.53a | 0.54a | 0.040 | 0.009 |
| LDL-C,mmol/L | 0.20 | 0.25 | 0.25 | 0.15 | 0.21 | 0.022 | 0.489 |
Means in the same row with different letters differ significantly (P < 0.05). Data are means for 8 birds per treatment.
Abbreviations: HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol.
Carcass Characteristics
The effects of TA supplementation on carcass characteristics are shown in Table 3. Dietary supplementation of TA increased (P < 0.01) the HCR and ER, but had no effect on PCY, BMR, and TMR (P > 0.05).
Table 3.
Effect of dietary TA supplementation on the carcass characteristics of broilers.
| Item1 | Dietary TA concentration, mg/kg |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| PCY | 91.51 | 91.24 | 90.66 | 91.61 | 91.71 | 0.153 | 0.217 |
| HCR | 77.68b | 78.92ab | 79.82ab | 80.72a | 79.57ab | 0.249 | 0.008 |
| ER | 66.89b | 68.76ab | 70.16a | 70.01a | 71.08a | 0.310 | 0.002 |
| BMR | 25.60 | 26.21 | 26.48 | 27.44 | 26.87 | 0.411 | 0.687 |
| TMR | 10.12 | 10.71 | 10.76 | 11.50 | 10.44 | 0.174 | 0.167 |
Means in the same row with different letters differ significantly (P < 0.05). Data are means for 8 birds per treatment.
Abbreviations: BMR, breast muscle rate; ER, eviscerated rate; HCR, half chamber rate; PCY, percentage of carcass yield; TMR, thigh muscle rate.
Physical Parameters of Meat Quality
The pH values, and color indices (objective color measurements) are shown in Table 4. Compared with control group, 600 mg/kg of TA addition tended (P = 0.056) to increase pH45min. No effects of TA were detected on pH values (24 h), and color indices (24 h).
Table 4.
Effect of dietary TA supplementation on the physical parameters of breast muscle in broilers.
| Item | Dietary TA concentration, mg/kg |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| pH, 45 min | 6.34ab | 6.23b | 6.29ab | 6.38a | 6.34ab | 0.016 | 0.056 |
| pH, 24 h | 5.93 | 5.88 | 5.86 | 5.82 | 5.80 | 0.015 | 0.108 |
| Color indices, 24 h | |||||||
| Lightness, L* | 51.00 | 52.00 | 51.53 | 49.40 | 50.98 | 0.430 | 0.403 |
| Redness, a* | 4.68 | 4.28 | 4.02 | 5.23 | 4.75 | 0.196 | 0.360 |
| Yellowness, b* | 10.46 | 10.10 | 10.36 | 9.82 | 10.13 | 0.207 | 0.883 |
Means in the same row with different letters differ significantly (P < 0.05). Data are means for 8 birds per treatment.
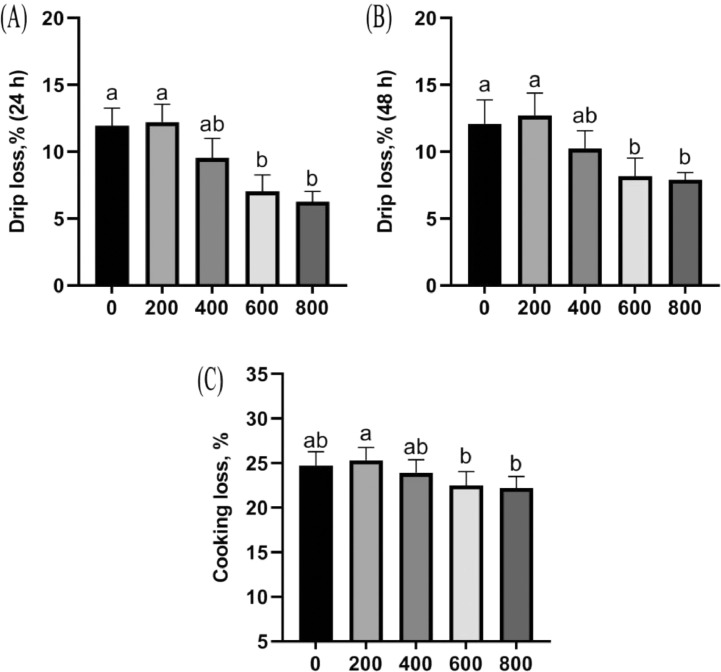
The changes of drip loss and cooking loss of broiler breast muscle in connection with dietary TA concentration are presented in Figure 1. Six hundred mg/kg of TA significantly decreased drip loss (storing 24 and 48 h) and cooking loss of breast muscle compared with nonsupplemented group.
Figure 1.
Breast muscle drip loss and cooking loss. (A) Drip loss (storing 24 h), P < 0.001; (B) drip loss (storing 48 h), P = 0.001; (C) cooking loss, P = 0.049. Values are means (n = 8), with their standard errors represented by vertical bars.
Fatty Acid Composition
As shown in Table 5, the concentration of palmitoleic acid, daturic acid, oleic acid, linoleic acid, α-linolenic acid, eicostrienoic acid, and pentosapentanoic acid (EPA) in breast muscle of broilers were higher (P < 0.05) in the 600 mg/kg of TA group compared with other groups. Furthermore, broilers fed with 600 mg/kg of TA had higher (P < 0.05) percentages of MUFA and PUFA in breast muscle compared with other groups. No significant difference in docosahexaenoic acid (DHA) and total SFA of breast muscle were detected among treatment groups.
Table 5.
Effect of dietary TA supplementation on fatty acid composition (%) of breast muscle in broilers.
| Item1 | Dietary TA concentration, mg/kg |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| C14:1n-9c | 6.70 | 6.73 | 6.10 | 6.34 | 5.18 | 0.253 | 0.402 |
| C15:0 | 44.09 | 38.39 | 37.53 | 40.10 | 40.19 | 0.702 | 0.064 |
| C16:0 | 13.75 | 14.89 | 14.89 | 15.16 | 15.07 | 0.210 | 0.215 |
| C16:1n-9c | 0.37ab | 0.25b | 0.23b | 0.42a | 0.17b | 0.020 | 0.010 |
| C17:0 | 0.24b | 0.21b | 0.80a | 0.82a | 0.39b | 0.062 | 0.015 |
| C18:0 | 10.56 | 10.58 | 10.25 | 10.30 | 8.96 | 0.235 | 0.289 |
| C18:1n-9t | 7.15b | 7.18b | 7.75ab | 10.65a | 7.63ab | 0.225 | 0.001 |
| C18:1n-9c | 1.45b | 1.38b | 1.93a | 2.16a | 1.49b | 0.082 | 0.023 |
| C18:2n- 9,12t | 0.15b | 0.41ab | 0.24b | 1.25a | 0.50ab | 0.037 | <0.001 |
| C18:2n-9,12c | 10.87b | 11.93b | 13.50a | 13.90a | 13.11ab | 0.321 | 0.034 |
| C18:3n-9,12,15c | 0.21b | 0.24b | 0.23b | 0.42a | 0.37ab | 0.023 | 0.040 |
| C20:1n-11c | 0.44 | 0.26 | 0.41 | 0.32 | 0.28 | 0.021 | 0.154 |
| C20:2n-11,14c | 0.91 | 0.75 | 0.77 | 0.98 | 0.91 | 0.093 | 0.924 |
| C20:3n-8,11,14c | 0.28b | 0.54ab | 0.64ab | 0.90a | 0.71ab | 0.045 | 0.013 |
| C20:5n-5,8,11,14,17c | 0.23b | 0.22b | 0.43b | 1.40a | 0.14b | 0.039 | <0.001 |
| C22:0 | 1.12 | 1.30 | 1.39 | 1.44 | 1.18 | 0.159 | 0.148 |
| C22:2n-13,16c | 0.25 | 0.24 | 0.33 | 0.32 | 0.30 | 0.018 | 0.431 |
| C22:6n-4,7,10,13,16,19c | 0.67 | 0.81 | 0.91 | 0.88 | 0.71 | 0.059 | 0.656 |
| SFA3 | 69.76 | 65.37 | 64.86 | 67.82 | 65.79 | 0.525 | 0.114 |
| MUFA4 | 16.11ab | 15.8ab | 16.42ab | 19.89a | 14.75b | 0.309 | 0.005 |
| PUFA5 | 13.57b | 15.14b | 17.05ab | 20.05a | 16.75ab | 0.354 | 0.002 |
Means in the same row with different letters differ significantly (P < 0.05). The fatty acid results were presented as g/100 g fatty acids (wt%). Data are means for 8 birds per treatment.
C14:1n-9c, Myristic acid; C15:0, Pentadecanoic acid; C16:0, Palmitic acid; C16:1n-9c, Palmitoleic acid; C17:0, Daturic acid; C18:0, Stearic acid; C18:1n-9t, Trans-oleic acid; C18:1n-9c, Oleic acid; C18:2n-9,12t, Trans-linoleic acid; C18:2n-9,12c, Cis-linoleic acid; C18:3n-9,12,15c, α-Linolenic acid; C20:1n-11c, Eicosenoic acid; C20:2n-11,14c, Eicosadienoic acid; C20:3n-8,11,14c, Eicostrienoic acid; C20:5n-5,8,11,14,17c, Pentosapentanoic acid (EPA); C22:0, Docosanoic acid; C22:2n-13,16c, Docosaedienoic acid; C22:6n-4,7,10,13,16,19c, Docosahexaenoic acid (DHA); SFA, Saturated fatty acids; MUFA, Monounsaturated fatty acids; PUFA, Polyunsaturated fatty acids.
SEM, standard error of the means.
SFA percentage is the sum of 15:0, 16:0, 17:0, 18:0 and 22:0.
MUFA percentage was calculated as the sum of C14:1n-9c, C16:1n-9c, C18:1n-9t, C18:1n-9c and C20:1n-11c.
PUFA percentage was calculated as the sum of C18:2n- 9,12t, C18:2n-9,12c, C18:3n-9,12,15c, C20:2n-11,14c, C20:3n-8,11,14c, C20:5n-5,8,11,14,17c, C22:2n-13,16c, and C22:6n-4,7,10,13,16,19c.
Amino Acid Profile
Amino acid profile in breast muscle of broilers is shown in Tables 6 and 7. Results indicated that dietary inclusion of 600 mg/kg of TA increased (P < 0.05) the concentration of Met, Thr, Asp, Ser, and Glu in breast muscle compared with other groups. In addition, the Lys concentration tended to be increased (P = 0.069) by 600 mg/kg of TA inclusion. There was no significant difference in the level of Ile, Leu, Val, His, Arg, Phe, Gly, Ala, Cys, and Tyr among treatment groups.
Table 6.
Effect of dietary TA supplementation on essential amino acid profile (%) of breast muscle in broilers.
| Item | Dietary TA concentration, mg/kg |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| Lys | 1.33 | 1.32 | 1.32 | 1.47 | 1.44 | 0.019 | 0.069 |
| Met | 0.32b | 0.39ab | 0.35ab | 0.44a | 0.42ab | 0.012 | 0.026 |
| Ile | 0.61 | 0.62 | 0.61 | 0.69 | 0.66 | 0.008 | 0.130 |
| Leu | 1.19 | 1.20 | 1.19 | 1.33 | 1.29 | 0.016 | 0.137 |
| Thr | 0.67b | 0.70ab | 0.67b | 0.74a | 0.71ab | 0.005 | 0.002 |
| Val | 0.67 | 0.67 | 0.67 | 0.74 | 0.71 | 0.008 | 0.158 |
| His | 0.60 | 0.59 | 0.60 | 0.62 | 0.62 | 0.011 | 0.831 |
| Arg | 1.01 | 1.00 | 1.01 | 1.12 | 1.09 | 0.014 | 0.140 |
| Phe | 0.56 | 0.57 | 0.56 | 0.62 | 0.62 | 0.008 | 0.158 |
Means in the same row with different letters differ significantly (P < 0.05). Data are means for 8 birds per treatment.
Table 7.
Effect of dietary TA supplementation on nonessential amino acid profile (%) of breast meat in broilers.
| Item | Dietary TA concentration, mg/kg |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | |||
| Asp | 1.36b | 1.45ab | 1.37b | 1.52a | 1.45ab | 0.010 | 0.002 |
| Ser | 0.60ab | 0.59ab | 0.58b | 0.65a | 0.62ab | 0.007 | 0.044 |
| Glu | 2.28b | 2.37ab | 2.27b | 2.54a | 2.42ab | 0.020 | 0.004 |
| Pro | 0.29b | 0.35ab | 0.37ab | 0.40a | 0.38ab | 0.010 | 0.025 |
| Gly | 0.57 | 0.58 | 0.62 | 0.62 | 0.60 | 0.009 | 0.353 |
| Ala | 0.83 | 0.83 | 0.85 | 0.92 | 0.88 | 0.010 | 0.165 |
| Cys | 0.15 | 0.15 | 0.15 | 0.17 | 0.15 | 0.016 | 0.349 |
| Tyr | 0.51 | 0.51 | 0.51 | 0.57 | 0.55 | 0.007 | 0.120 |
Means in the same row with different letters differ significantly (P < 0.05). Data are means for 8 birds per treatment.
DISCUSSION
The star anise fruits have been used in traditional Chinese medicine and food industry for many years (Li et al., 2013). Biological activities of star anise oil (SAO), including antimicrobial, antioxidant, and growth promoting, have been widely reported (Huang et al., 2018; da Rocha Neto et al., 2019). In our previous studies, we found that inclusion of SAO enhanced yolk antioxidant status of laying hens, suggesting that SAO may extend shelf life of eggs (Yu et al., 2018). It was reported that star anise extract had good antioxidant and antibacterial activities in chilled pork patties (Yin et al., 2014). Moreover, SAO could increase the flavor of breast muscle by increasing the concentration of inosinic acid (Ding et al., 2020). These encouraging findings indicated that SAO may be used for improving the flavor and quality of chicken meat. However, as the main active constituent of SAO, there is limited data regarding the effects of TA on meat quality of broilers. Based on that, this study was conducted to investigate the effects of TA (the main active constituent of SAO) on serum lipid metabolism parameters, carcass characteristics, meat quality, fatty acid composition, and amino acid profile of breast muscle in broilers, and identify whether the beneficial effects of SAO on meat quality is due to the TA. It should be noted that we investigated the effects of TA on growth performance in broilers in a previous study, and found that TA administration had no remarkable effect on growth performance, but significantly increased the average daily feed intake (ADFI) when supplemented at 400 and 600 mg/kg in the grower phase. To the best of our knowledge, SAO with aromatic anise flavor could stimulate appetite thus increase the ADFI of broilers (Wang et al., 2011). Consisted with that, we found that laying hens consumed SAO containing diets tended to have a higher ADFI of laying hens (Yu et al., 2018). However, it was reported that the growth performance of broilers was not affected by inclusion of essential oils consisting of menthol and anethole (Hafeez et al., 2016). Taken together, the effects of TA on the growth performance of broilers may be concerned with the experimental conditions, hygiene, and animal age (Goel et al., 2008). Further research on effects of TA supplementation on feed intake and growth performance of broilers needed to be investigated.
In this study, the results showed that serum concentration of TG was significantly decreased, whereas HDL-C on d 42 was increased by 600 mg/kg of TA supplementation. It is well known that serum HDL-C level is inversely associated with the incidence of coronary heart disease (CHD) in human and animals (Curb et al., 2004). The increased serum HDL-C concentration suggested that TA may have lipid-lowing and anti-atherosclerosis activities. In addition, the role of TA on lipid metabolism has been demonstrated in previous studies (Kang et al., 2018; Rhee et al., 2018; Song et al., 2020). It was demonstrated that TA may have therapeutic implications for ameliorating obesity by inducing the white fat browning, activating brown adipocytes, and promoting lipid catabolism (Kang et al., 2018). Rhee et al. (2018) reported that TA suppressed the adipogenic differentiation of human mesenchymal stem cells (hMSCs), indicating that TA may inhibit lipid generation. These supporting results suggested that TA could regulate lipid metabolism in broilers, which may contribute to decreasing percentage of abdominal fat of broilers.
To the best of our knowledge, broilers consuming SAO diets showed a higher carcass yield by increasing organ weight (Simsek et al., 2007). However, in the study conducted by Ding et al. (2020), the author observed no effects of SAO on the carcass traits, including PCY, ER, and rates of breast and thigh muscle in birds. These results were similar with the results of this study that TA significantly increased the HCR and ER, but had no influence on rates of breast and thigh muscle. The increased HCR and ER may be due to the beneficial effects of TA on nutrient digestibility, and intestinal barrier integrity (Amad et al., 2011; Yi et al., 2021). Additionally, the drip loss and cooking loss of breast muscle were significantly decreased by 600 mg/kg of TA supplementation. They are important parameters reflecting the juiciness and tenderness of meat (Yang et al., 2010). The lower drip loss and cooking loss indicated a higher water-holding capacity and juiciness of chicken caused by TA. In agreement with our results, it was reported that star anise extract had no effect on pH of chilled pork patties (Yin et al., 2014). Ding et al. (2020) also observed no changes on pH and meat color, but lower drip loss of breast muscle in broilers.
There is limited data concerning the beneficial effects of TA on the fatty acid composition, and amino acid profile of chicken meat. ω-3 PUFA has positive effects on preventing insulin resistance, cardiovascular disease and ameliorating non-alcoholic fatty liver disease (Capanni et al., 2006; Fedor and Kelley, 2009). ω-6 PUFA, such as linoleic acid, has positive effects on downregulating serum cholesterol concentration and preventing cardiovascular disease (Troisi et al., 1992; Simopoulos, 2002). In this study, inclusion of 600 mg/kg of TA in broiler diets led to a remarkable increase in the levels of unsaturated fatty acids and a numerical decrease in saturated fats of breast muscle. The concentration of α-linolenic acid, EPA, oleic acid, and linoleic acid in the breast muscle of broilers were significantly increased by 600 mg/kg of TA compared to nonsupplemented TA group. These noteworthy results indicated that TA could increase the ω-3 and ω-6 PUFA proportion in chicken meat. Further research requires to be carried out to clarify the mechanism of the changes. Furthermore, free amino acid profile, especially Ala, Leu, Ser, Val, and Glu, was reported to contribute to the flavor of meat by maillard reaction (Rabie et al., 2009). The results showed that 600 mg/kg of TA significantly increased the concentration of Met, Thr, Asp, Ser, Glu, and Pro in the breast muscle, indicating that TA supplementation may improve the flavor of chicken meat by increasing the concentration of flavor amino acids. This promising result may be explained by the digestive function of TA (Yu et al., 2019; Yu et al., 2020). In addition, it was demonstrated that dietary supplementation of natural antioxidants could enhance the quality of meat by preventing lipid peroxidation and protein denaturation, eventually improving the fatty acid and amino acid profile of meat (Upton and Edens, 2009). Based on the widely reported antioxidant and antimicrobial properties of TA, further research of TA on the preservation and shelf life extension of chicken meat needs to be characterized.
An interesting finding of this study was that the efficiency of 600 mg/kg of TA on improving meat quality was better than 800 mg/kg. It was reported that the inclusion concentration of plant essential oils could affect broiler response (Mountzouris et al., 2011; Reyer et al., 2017). The stimulating odors of essential oils may result in inferior sensory effects on animals, thereby influence gut motility and ingestion when be used at a high concentration (Laugerette et al., 2005; Huang et al., 2018). Taken together, highly pungent anise flavor of TA when supplemented at a high concentration may influence the diet's taste and broilers’ appetite, thereby lead to stress response of broilers. Hereunder, we can conclude that the quality of chicken meat may response to TA administration in a dose dependent manner.
CONCLUSIONS
Supplementation of TA at the level of 600 mg/kg of broiler diets promoted lipid metabolism, and improved meat quality. In addition, long-term supplementation with 600 mg/kg of TA has the optimum potential to change the fatty acid composition toward a higher percentage of PUFA, and increase concentration of flavor amino acids in the breast muscle.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No.2018YFD0501101).
DISCLOSURES
The authors declare that there is no conflict of interest.
REFERENCES
- Adeola O., Shafer D.J., Nyachoti C.M. Nutrient and energy utilization in enzyme-supplemented starter and grower diets for white pekin ducks. Poult. Sci. 2008;87:255–263. doi: 10.3382/ps.2007-00155. [DOI] [PubMed] [Google Scholar]
- Amad A.A., Manner K., Wendler K.R., Neumann K., Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Aprotosoaie A.C., Costache I.I., Miron A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;929:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Capanni M., Calella F., Biagini M.R., Genise S., Raimondi L., Bedogni G., Svegliati-Baroni G., Sofi F., Milani S., Abbate R., Surrenti C., Casini A. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol. Ther. 2006;23:1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- Cheng C.S., Liu Z.H., Zhou Y.F., Wei H.K., Zhang X.M., Xia M., Deng Z., Zou Y., Jiang S.W., Peng J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017;133:103–109. doi: 10.1016/j.meatsci.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Curb J.D., Abbott R.D., Rodriguez B.L., Masaki K., Chen R., Sharp D.S., Tall A.R. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J. Lipid Res. 2004;45:948–953. doi: 10.1194/jlr.M300520-JLR200. [DOI] [PubMed] [Google Scholar]
- da Rocha Neto A.C., Navarro B.B., Canton L., Maraschin M., Di Piero R.M. Antifungal activity of palmarosa (Cymbopogon martinii), tea tree (Melaleuca alternifolia) and star anise (Illicium verum) essential oils against penicillium expansum and their mechanisms of action. LWT/Food Sci. Technol. 2019;105:385–392. [Google Scholar]
- Ding X., Yang C.W., Wang P.P., Yang Z.B., Ren X.J. Effects of star anise (Illicium verum Hook.f) and its extractions on carcass traits, relative organ weight, intestinal development, and meat quality of broiler chickens. Poult. Sci. 2020;99:5673–5680. doi: 10.1016/j.psj.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor D., Kelley D.S. Prevention of insulin resistance by n-3 poly-unsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Goel G., Makkar H.P., Becker K. Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J. Appl. Microbiol. 2008;105:770–777. doi: 10.1111/j.1365-2672.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- Hafeez A., Männer K., Schieder C., Zentek J. Effect of supplementation of phytogenic feed additives (powdered vs. encapsulated) on performance and nutrient digestibility in broiler chickens. Poult. Sci. 2016;95:622–629. doi: 10.3382/ps/pev368. [DOI] [PubMed] [Google Scholar]
- Hançer Aydemir D., Çifci G., Aviyente V., Boşgelmez-Tinaz G. Quorum-sensing inhibitor potential of trans-anethole against Pseudomonas aeruginosa. J. Appl. Microbiol. 2018;125:731–739. doi: 10.1111/jam.13892. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Huang Z., Liu X., Jia S., Zhang L., Luo Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018;266:52–59. doi: 10.1016/j.ijfoodmicro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Kang N.H., Mukherjee S., Min T., Kang S.C., Yun J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie. 2018;151:1–13. doi: 10.1016/j.biochi.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J.P., Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.G., Li M.Y., Huang Y.Z., Hua R.M., Lin H.F., He Y.J., Wei L.L., Liu Z.Q. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-trans-ferase activities in adult sitophilus zeamais. J. Pest Sci. 2013;86:677–683. [Google Scholar]
- Luo H.F., Wei H.K., Huang F.R., Zheng Z., Jiang S.W., Jian P. The effect of linseed on intramuscular fat content and adipogenesis related genes in skeletal muscle of pigs. Lipids. 2009;44:999–1010. doi: 10.1007/s11745-009-3346-y. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Paraskevas V., Tsirtsikos P., Palamidi I., Steiner T., Schatzmayr G., Fegeros K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed Sci. Technol. 2011;168:223–231. [Google Scholar]
- Rabie M., Simon-Sarkadi L., Siliha H., El-seedy S., El-badawy A. Changes in free amino acids and biogenic amines of Egyptian salted-fermented fish (Feseekh) during ripening and storage. Food Chem. 2009;115:635–638. [Google Scholar]
- Reyer H., Zentek J., Männer K., Youssef I.M.I., Aumiller T., Weghuber J., Wimmers K., Mueller A.S. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J Agric. Food Chem. 2017;65:6821–6830. doi: 10.1021/acs.jafc.7b01925. [DOI] [PubMed] [Google Scholar]
- Rhee Y-H., Moon J.H., Mo J-H., Pham T., Chung P-S. mTOR and ROS regulation by anethole on adipogenic differentiation in human mesenchymal stem cells. BMC Cell Biol. 2018;19:12. doi: 10.1186/s12860-018-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá N.A.R., Bruno J.B., Guerreiro D.D., Cadenas J., Alves B.G., Cibin F.W.S., Leal-Cardoso J.H., Gastal E.L., Figueiredo J.R. Anethole reduces oxidative stress and improves in vitro survival and activation of primordial follicles. Braz. J. Med. Biol. Res. 2018;51:e7129. doi: 10.1590/1414-431X20187129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore F., Oliviero F., Scandolera E., Taglialatela-Scafati O., Roscigno G., Zaccardelli M., De Falco E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell] Fitoterapia. 2013;90:214–219. doi: 10.1016/j.fitote.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Sheikh B.A., Pari L., Rathinam A., Chandramohan R. Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochim. 2015;112:57–65. doi: 10.1016/j.biochi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. N-3 fatty acids and human health: defining strategies for public policy. Lipids. 2001;36:83–89. doi: 10.1007/s11745-001-0687-7. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Simsek U.G., Ciftci M., Dalkilic B., Guler T., Ertas O.N. The effects of dietary antibiotic and anise oil supplementation on body weight, carcass characteristics and organoleptic analysis of meat in broilers. Rev. Med. Vet. 2007;158:514–518. [Google Scholar]
- Song A., Park Y., Kim B., Lee S.G. Modulation of lipid metabolism by trans-anethole in hepatocytes. Molecules. 2020;25:4946. doi: 10.3390/molecules25214946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R., Willett W.C., Weiss S.T. Trans-fatty acid intake in relation to serum lipid concentrations in adult men. Am. J. Clin. Nutr. 1992;56:1019–1024. doi: 10.1093/ajcn/56.6.1019. [DOI] [PubMed] [Google Scholar]
- Upton J.R., Edens F.W. The effects of dietary oxidized fat and selenium source on performance, glutathione peroxidase, and glutathione reductase activity in broiler chickens. J. Appl. Poult. Res. 2009;18:193–202. [Google Scholar]
- Waheed S., Hasnain A., Ahmad A., Tarar O.M., Yaqeen Z., Ali T.M. Effect of botanical extracts on amino acid and fatty acid profile of broiler meat. Braz. J. Poult. Sci. 2018;20:507–516. [Google Scholar]
- Wang G.W., Hu W.T., Huang B.K., Qin L.P. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011;136:10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- Wieczyńska J., Cavoski I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food Chem. 2018;259:251–260. doi: 10.1016/j.foodchem.2018.03.137. [DOI] [PubMed] [Google Scholar]
- Yang X., Zhang B., Guo Y., Jiao P., Long F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 2010;89:254–260. doi: 10.3382/ps.2009-00234. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu G., Zhu X., Luo Y., Shang Y., Gu X.L. The anti-inflammatory and antioxidant effects of leonurine hydro-chloride after lipopolysaccharide challenge in broiler chicks. Poult. Sci. 2019;98:1648–1657. doi: 10.3382/ps/pey532. [DOI] [PubMed] [Google Scholar]
- Yin Y., Zhang W.G., Zhou G.H. Antioxidative and antibacterial activities of star anise extract in chilled pork patties. J. Nanjing Agr. Univer. 2014;37:89–96. [Google Scholar]
- Yi Q.Y., Liu J.X., Zhang Y.F., Qiao H.Z., Chen F., Zhang S.H., Guan W.T. Anethole attenuates enterotoxigenic Escherichia coli-induced intestinal barrier disruption and intestinal inflammation via modification of TLR signaling and intestinal microbiota. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.Y., Wei J.D., Yang C.W., Yang Z.B., Yang W.R., Jiang S.Z. Effects of star anise (Illicium verum Hook.f.) essential oil on laying performance and antioxidant status of laying hens. Poult. Sci. 2018;97:3957–3966. doi: 10.3382/ps/pey263. [DOI] [PubMed] [Google Scholar]
- Yu C.Y., Guo Y.X., Yang Z.B., Yang C.W., Jiang S.J. Effects of star anise (Illicium verum Hook.f.) essential oil on nutrient and energy utilization of laying hens. Anim. Sci. J. 2019;90:880–886. doi: 10.1111/asj.13221. [DOI] [PubMed] [Google Scholar]
- Yu C.Y., Yang W.R., Jiang S.Z., Wang T., Yang Z.B. Effects of star anise (Illicium verum Hook.f.) essential oil administration under three different dietary energy levels on growth performance, nutrient and energy utilization in broilers. Anim. Sci. J. 2020;92:e13496. doi: 10.1111/asj.13496. [DOI] [PubMed] [Google Scholar]