Abstract
Coronary artery disease (CAD) and osteoporosis both cause significant morbidity and mortality. Recent interest in inflammation and the bone-vascular axis suggests a mechanistic link between the two conditions. This review and meta-analysis was conducted to examine the potential association between low bone mineral density (BMD) and CAD in adults. Two authors searched for studies that examined the association between low BMD and CAD. Risk of bias assessment was conducted using the modified Newcastle Ottawa score. Ten studies were selected from the 2258 unique records identified. Pooled analysis showed a significant association between low BMD and CAD (OR 1.65, 95%CI 1.37–2.39, p < 0.01). Subgroup analysis investigating males and females separately was not significant. The subgroup analyses looking for any differences across geographic locations and differences between coronary imaging modalities were also negative. Studies with adjusted ORs (n = 4) were also pooled (OR 3.01, 95%CI 0.91–9.99, p = 0.07). Low BMD is associated with CAD; however, it is unclear whether this result is confounded by common risk factors given the heterogeneity between study populations and methodologies. Further large-scale epidemiological studies are required.
Keywords: Bone mineral density, Coronary artery disease, Osteoporosis
1. Introduction
Coronary Artery Disease (CAD) and osteoporosis are responsible for significant morbidity and mortality. Cardiovascular diseases are the leading cause of death globally, representing 31% of all deaths [1]. Approximately half of these deaths are attributable to CAD [2]. Similarly, osteoporosis has high prevalence with the lifetime risk for a wrist, hip or vertebral fragility fracture estimated to be 30–40% [3]. Both conditions often occur concurrently and are associated with shared risk factors such as age, hypertension, smoking, and low physical activity. A number of observational studies have reported increased carotid atherosclerotic plaque in individuals with low bone mineral density (BMD) [4], [5], [6]. There are also a number of prospective studies that show increased risk of cardiovascular events in those with low BMD [7], [8]. This suggests that these diseases may share common pathophysiologic mechanisms [9]. For instance, secretory proteins such as klotho and osteoprotegerin (OPG) have been implicated in the bone-vascular axis [10], [11], [12]. Low circulating levels of klotho are associated with both bone and atherosclerotic disease [10]. However, the evidence for OPG is mixed [11], [12]. Lastly caspase-dependent inflammatory cytokines may contribute to both disease processes [13]. While previous observational studies have examined this potential association, the results have been mixed without unequivocal correlation being established. Thus, the purpose of this article is to conduct a meta-analysis comprehensively including the published data to-date to examine whether low BMD is significantly associated with CAD.
2. Methods
Two authors (CK and KV) independently searched MEDLINE, EMBASE and CENTRAL (Cochrane Central Register of Controlled Trials) to identify relevant studies for review. Grey literature was searched via OpenGrey. The search strategy can be viewed in Supplementary Material 1. Reference lists of relevant literature reviews were also used to capture further studies.
Studies were eligible for selection if they reported an effect size for the association between low BMD and CAD. Case-control, cohort and cross-sectional studies were all considered. Only full articles were considered for the review. Stringent definitions were used for eligibility criteria. Low BMD was either defined as a T score less than −1, or a quantitative computed tomography (QCT) measurement < 120 mg/cm3 [14]. CAD was defined as having at least 50% stenosis in at least one major coronary artery [15]. There were no date limitations. Results were limited to English.
Two authors (CK and KV) screened titles and abstracts to identify potential studies. Full text reviews were then conducted to identify studies to be included in the review. A third author (SP) was to be consulted if there was any disagreement.
All data were extracted by one author (CK). The primary end point was to examine any association between low BMD and CAD. If a study did not report an odds ratio but included sufficient detail for an odds ratio to be calculated, it was still eligible for the review.
One author (CK) assessed methodologic quality of studies included in this review. The modified Newcastle Ottawa Score [16] as described elsewhere [17] was used to objectively assess bias in observational studies selected for review. A second author (SP) was to be consulted if there was any indecision.
The primary endpoint was pooled from included studies using a random effects model given the anticipated heterogeneity. An a-priori decision was made to analyse unadjusted odds ratios (ORs) and adjusted ORs from included studies separately. Three subgroup analyses were conducted. The first subgroup analysis was to investigate any difference between sexes. The second subgroup analysis was to investigate any difference across the coronary imaging modalities. The third subgroup analysis was to investigate any regional differences by geographic location. ORs were used as the summary effect, along with 95% confidence intervals (CIs). If ORs were calculated using study data available, significance was determined using Pearson’s Chi-Square test. If any cell in a derived 2x2 frequency table had a small frequency (n < 5), Fischer’s exact Test was used instead. If any cell had a zero-value, a Haldane-Anscombe correction was applied [18]. Two-sided p-values were determined and considered significant if p < 0.05. Statistical heterogeneity was assessed using the Cochrane Q test and Higgin’s I2 statistic. Publication bias was assessed by funnel plot asymmetry using Egger’s regression test. A post-hoc analysis to determine potential sources of heterogeneity was conducted by analysing the influence of each trial to the Cochrane Q-test for heterogeneity and overall treatment effect as described elsewhere [19].
All analyses were performed by using Review Manager (RevMan version 5.3; Cochrane Collaboration, Oxford, United Kingdom) and R (R: A Language and Environment for Statistical computing, Vienna, Austria).
3. Results
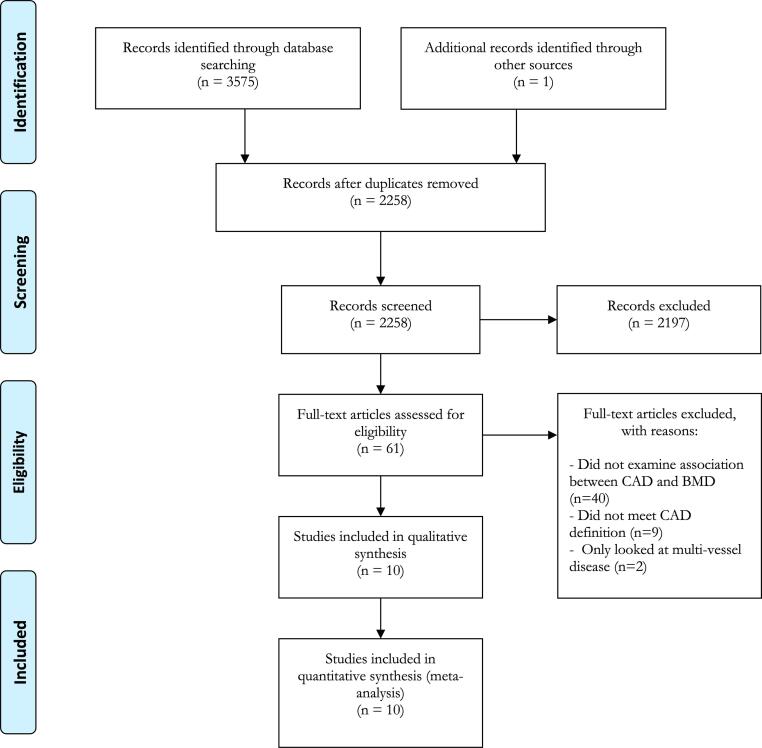
A total of 2258 unique records were identified though initial searching. Of these, 2197 records were excluded by screening their titles and abstracts. A full text review was conducted of the remaining 61 records, of which 40 were removed as they did not examine any association between CAD and low BMD. Nine records were then removed as they did not meet the pre-specified definition of CAD. Two further records were removed as they only compared multi-vessel disease against single vessel disease. Thus, 10 full articles [20], [21], [22], [23], [24], [25], [26], [27], [28], [29] were selected for inclusion in the review and meta-analysis. This is summarised in the accompanying PRIMSA flowchart (see Fig. 1).
Fig. 1.
Flow diagram of study selection as per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis).
The characteristics of included studies are summarised in Table 1. There were a total of 4156 patients across 10 studies of which 59% were female. The mean age of the total cohort was 61 years with a standard deviation (SD) of 10 years. The prevalence of CAD in study cohorts ranged from 9 to 90%, whilst the prevalence of low BMD ranged from 21 to 75%. There was a notable clinical heterogeneity in baseline clinical characteristics across all the included studies. Almost all studies measured BMD using Dual Energy X-ray Absorption (DEXA) scanning (n = 11), in which the lumbar spine was the most common site. Invasive coronary angiography was the most common modality to detect CAD.
Table 1.
Baseline characteristics of included studies.
| Author (Year) | Study Design | Country | Age (SD) | Population (% female) | Smoking Hx | DM | HTN | LDL (SD) | CAD test | CAD | Low BMD | BMD method | BMD location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marcovitz (2005) | Cross-sectional | USA | 67(11) | n = 209 (88) | 10%^ | 23% | 75% | – | CA | 56% | 75% | DEXA | Lumbar spine, femur, radius |
| Erbelin (2007) | Cross-sectional | Turkey | 64(9) | n = 47 (0) | 34%^ | 34% | 28% | 95 (33) | CA | 68% | 40% | DEXA | Lumbar spine, femur, radius |
| Tekin (2008) | Cross-sectional | Turkey | 60(9) | n = 216 (100) | 16%^ | 27% | 74% | – | CA | 50% | 77% | DEXA | Lumbar spine |
| Varma (2008) | Cross-sectional | USA | 66(6) | n = 198 (74) | 26%^ | 26% | 72% | – | CA | 63% | 67% | DEXA | Lumbar spine, left hip |
| Beer (2010) | Cross sectional | Austria | 64(11) | n-623 (0) | 71% | 27% | 80% | 125(40) | CA | 65% | 44% | DEXA | Lumbar spine, hip |
| Hajsadeghi (2011) | Cross-sectional | Iran | 59(8) | n = 119 (48) | 81% | 31% | 57% | 100(32) | CA | 33% | 79% | DEXA | Lumbar spine, femur |
| Iranpour (2014) | Cross-sectional | Iran | 56(10) | n = 216 (47) | 48%^ | 25% | 27% | – | CA | 54% | 69% | DEXA | Lumbar spine*, femur |
| Alissa (2015) | Case-control | Saudia Arabia | 63(8) | n-178 (100) | 7% | 41% | 57% | 96(31) | CA | 49% | 42% | DEXA | Femoral neck |
| Lee (2016) | Cross-sectional | South Korea | 65(8) | n = 863 (100) | 3%^ | 15% | 42% | 126(38) | CTCA | 9% | 69% | DEXA | Lumbar spine, Femoral neck |
| Therkildsen (2019) | Cross-sectional | Denmark | 57(9) | n = 1487 (47) | 52% | 5% | 35% | – | CTCA | 23% | 53% | QCT | Lumbar spine |
Abbreviations: CA = Coronary Angiography, CAD = Coronary Artery Disease, CT = Computed Tomography Coronary Angiogram, DEXA = Dual energy X-ray Absorption, QCT = Quantitative Computed Tomography, SD = Standard Deviation. Units: Age (years), LDL (mg/dl).
Data in our meta-analysis was only used for lumbar spine BMD measurements as this dataset was complete for both males and females.
Data only available for current smokers.
The risk of bias assessment can be viewed in Supplementary Material 2. There was a variable range of quality in studies included in this review and meta-analysis. The median modified Newcastle Ottowa Score was 7.5 with an inter-quartile range from 5 to 9.
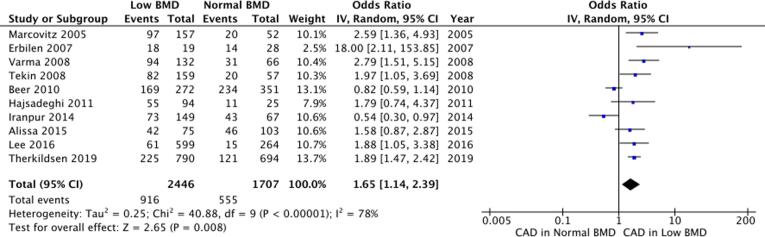
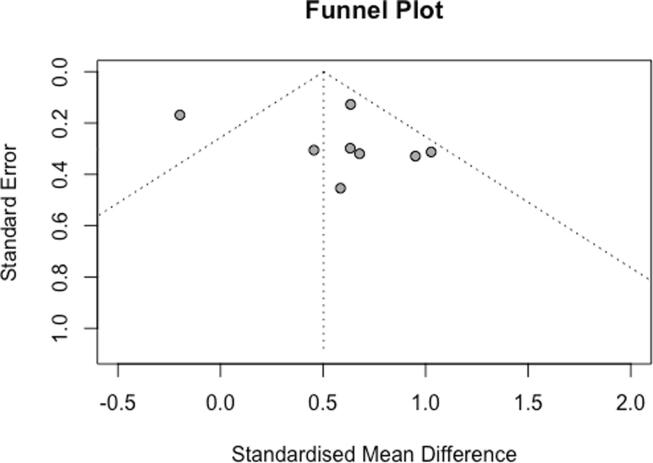
All 10 studies were pooled together in a meta-analysis as shown in Fig. 2. There was a significant positive association between low BMD and CAD (OR 1.65, 95%CI 1.37–2.39, p < 0.01). There was significant statistical heterogeneity evidenced by Cochrane Q test (χ2 = 40.88, df = 9, p < 0.01) and Higgin’s I2 test (I2 = 78%). A funnel plot examining all included studies is illustrated in Fig. 3. Egger’s regression analysis did not show funnel plot asymmetry (Β0 = 1.233, p = 0.44).
Fig. 2.
Pooled meta-analysis of studies examining a univariate association between BMD and CAD. BMD = Bone mineral density, CAD = Coronary artery disease, CI = Confidence interval, IV = Inverse-variance.
Fig. 3.
A funnel-plot of all included studies in this meta-analysis.
Subgroup analyses may be viewed in Supplementary Material 3. A subgroup analysis examining males and females separately was not statistically significant in either group (Males: OR 1.53, 95%CI 0.62–3.77, p = 0.35; Females: OR 1.46, 95%CI 0.91–2.34, p = 0.13). There was significant statistical heterogeneity in both subgroups respectively (Males: χ2 = 11.67, df = 3, p < 0.01, I2 = 74%; Females: χ2 = 13, df = 4, p = 0.01. I2 = 68%). A second subgroup analysis examined studies which used invasive coronary angiography and non-invasive computer tomography coronary angiography (CTCA) separately. Both imaging modalities favoured an associated between low BMD and CAD (CTCA: OR 1.89, 95%CI 1.50–2.37, p < 0.01; Coronary Angiogram: OR 1.64, 95%CI 0.99–2.71, p = 0.06). There was no statistically significant difference across these two groups (p = 0.62). There was no significant heterogeneity across the two studies in the CTCA subgroup (χ2 = 0.00, df = 1, p = 1, I2 = 0%). A third subgroup analysis examined for geographic regional variation amongst the included studies. All included regions favoured a positive association between low BMD and CAD but were not statistically significant. There was no statistical difference between subgroups (p = 0.34).
A post-hoc analysis was conducted to assess which studies were contributing most to the identified heterogeneity, using methods described elsewhere [19]. The Baujat plot in Fig. 4 illustrates that heterogeneity was mostly contributed by two studies (Beer et al. and Iranpour et al.) [24], [26]. A repeat meta-analysis excluding these two studies showed a greater effect size (OR 2.00, 95%CI 1.67–2.40, p < 0.01) with no statistically significant heterogeneity (χ2 = 6.70, df = 7, p = 0.46, I2 = 0%).
Fig. 4.
Baujat Plot showing the influence of studies on effect size and heterogeneity.
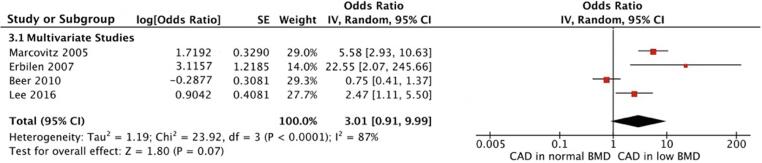
A total of 4 studies also reported adjusted odds ratios, accounting for several risk factors (Supplementary Material 4). These risk factors differed between studies. A pooled analysis (Fig. 5) of these studies did not show a statistically significant association between low BMD and CAD (OR 3.01, 95%CI 0.91–9.99, p = 0.07). There was significant statistical heterogeneity with this result (χ2 = 23.92, df = 3, p < 0.01, I2 = 87%).
Fig. 5.
A pooled meta-analysis of all studies with adjusted odds ratios. BMD = Bone mineral density, CAD = Coronary artery disease, CI = Confidence Interval, IV = Inverse-variance, SE = Standard Error.
4. Discussion
Our results suggest that there is a statistically significant positive association between low BMD and CAD. There was also significant statistical heterogeneity, which was not explained by the three subgroup analyses performed (sex, coronary imaging modality, and geographic variation). The source of heterogeneity appeared to be derived from the inclusion of two studies (Iranpour et al. and Beer et al.) [24], [26]. It is unclear why these two studies contributed such significant heterogeneity. Whilst Beer et al. [24] surveyed a male-only population, this was not the only study to do so. Baseline characteristics were comparable to other studies, although Iranpour et al. [26] and Beer et al. [24] had the lowest and highest proportion of patients with hypertension respectively. There were no major methodological differences in these two studies. There is likely underlying clinical heterogeneity that is difficult to explore given the lack of individual level patient data. Reassuringly, the direction and magnitude of effect size after the exclusion of these two studies from the meta-analysis was similar. An appropriate random-effects model was used in the context of this significant heterogeneity.
After pooling studies with adjusted multivariate analyses only, there was a positive association between low BMD and CAD; however, this result was marginally insignificant (p = 0.07). Hence there may be merit in conducting further studies which account for multiple confounders to explore whether our pooled result may represent a type II error. The heterogeneity of this analysis (Fig. 5) was very high (I2 = 87%), thus it is difficult to interpret the generalisability of this result. This heterogeneity can be partly explained by the range of differing confounders present across the 4 studies.
In order to explain this observed univariate association between low BMD and CAD, there has been interest in the role of secretory proteins in the bone-vascular axis [30]. OPG has been postulated to have a central role in the dysregulation of the bone-vascular axis, however the evidence is mixed. Initial studies in mice showed that lack of OPG led to early osteoporosis and vascular calcifications [11]. However other in-vitro studies suggest OPG contributes to both systemic and vascular-specific inflammation by increasing macrophage infiltration and promoting vascular medial fibrosis [31], [32], [33], [34]. A recent meta-analyses [12] showed that elevated circulating OPG in patients at high risk for cardiovascular disease was associated with higher risk of cardiovascular events. Klotho is another mediator implicated in the bone-vascular axis; a co-factor for fibroblast growth factor receptor shown to reduce both osteogenic capacity and osteoclastogenesis [35]. Klotho-deficient mice are shown to exhibit severe osteoporosis and progressive atherosclerosis [36]. Furthermore, low circulating levels of klotho were recently shown to predict macrovascular outcomes in patients with type 2 diabetes [10].
There has also been recent interest in the role of vascular smooth muscle cells (VSMCs) in arterial calcification. These calls are known to display diverse plasticity, and have been shown to transdifferentiate into osteoblastic cells in response to inflammation [37]. Inflammatory cytokines derived from M1-macrophages such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) have all been shown to drive this osteogenic differentiation of VSMCs [38], [39]. TNF-α has been shown to activate the osteogenic program of VSMCs via Msx2-Wnt signalling in animal studies [40]. IL-1β has also been shown to increase VSMC driven calcification in-vitro [41]. Interestingly, inhibition of the NLP3 inflammasome complex, known to be key in IL-1β production, was shown to inhibition vascular calcification [41]. Kurozumi et al. [42] showed that VSMCs differentiation in-vitro into osteoporosis-blast like cells was also regulated by IL-6.
Moreover, higher levels of caspase-dependent inflammatory cytokines IL-1β, IL-6 and IL-18 are key mediators in plaque development and de-stabilisation [43]. These cytokines are shown to be predictive of CAD, independent of traditional risk factors [43], [44]. Pro-inflammatory cytokines such as IL-6 and TNF-α have also been shown as potent stimulators of bone resorption [45], [46]. Hence the association between bone disease and CAD may be explained by a heightened inflammatory state. This is already seen in inflammatory conditions such as systemic lupus erythematosus and rheumatoid arthritis [45].
The pattern of calcification may also play a role in susceptibility to acute coronary syndromes [39]. Menini et al. [47] showed a higher expression of interferon γ and TNF-α in unstable plaques versus stable plaques, in which micro-calcifications were more prevalent compared to macro-calcification. This phenomenon has been reflected in more recent imaging studies, with the presence of micro-calcifications signalling vulnerable plaque. High resolution CT imaging of fibrous cap have shown areas increased local stress to correlate with micro-calcific deposits [48]. Moreover, intravascular optical computed tomography-based studies have showed spotty calcification to be an independent predictor of plaque rupture [49], [50]. VSMCs are thought to drive this development of these micro-calcific deposits in the intimal wall, thus weakening its integrity and increasing the risk of rupture [51].
Similar to the results of our meta-analysis, a large study (n = 1824) based in China [52] examined the potential relationship between osteoporosis and CAD and showed mixed results. The univariate analysis showed a statistically significant positive association (OR 1.65, p = 0.002). The multivariate analysis, however, adjusting for age, smoking, alcohol intake, education, and medical therapy history did not show a statistically significant association (OR 1.39, p = 0.08). This study was excluded from the meta-analysis due to its wide ranging definition of CAD, which included any of the following; (i) a history of angina/myocardial infarction; (ii) a history of coronary artery revascularization procedures or a history of documented 50% stenosis is ≥1 major coronary arteries on coronary angiography; or (iii) regional wall-motion abnormalities on rest echocardiography.
There is also mixed data regarding the role of statin therapy on the risk of osteoporosis. Mechanistic in-vitro studies and animal models have demonstrated that statins augment osteoblastic activity by stimulating production of bone morphogenic protein-2 (BMP-2) [53]. Statins have also been shown to inhibit osteoclastic activity by reducing mevalonate synthesis, and down-regulating receptor activator of nuclear factor kappa-Β ligand (RANKL) (an OPG ligand) expression; which in turn up-regulates OPG expression [54], [55]. However, statins also suppress hepatic cholesterol synthesis, which can inhibit sex hormone (estradiol and testosterone) production, and subsequently reduce BMD [42]. Several observational and cohort studies have indicated a lower fracture risk and higher BMD in statin users, but post-hoc analyses of large randomised trials have failed to affirm these findings [55]. A large population-based European cohort study [56] showed that osteoporosis is underrepresented at lower doses (OR 0.39–0.70, p < 0.01) and overrepresented at higher doses (OR 1.64–2.04, p < 0.01) of statins; suggesting a dose-dependent effect on bone metabolism.
There is mixed evidence when comparing the effects of low BMD on the severity of CAD. Xu et al. showed that T-scores at the femoral neck were independent predictors of Gensini scores on CT angiography in their multiple regression model; which also included age, body mass index (BMI), hypertension and diabetes [57]. Guan et al. showed more significant multi-vessel CAD (OR 4.34, 95%CI 2.05–9.20, p < 0.01) in those with osteoporosis at the femoral neck in their multivariate model; accounting for age, gender, BMI, smoking, hypertension, diabetes, hypercholesterolemia and serum creatinine levels [58]. However, there was no significant association when looking at the BMD of the lumbar spine. It is unclear why there is an observed difference between the two sites of BMD testing. Bagger et al. hypothesised that unilateral blood supply of the proximal femur was more vulnerable to atherosclerotic disease compared to the bilateral blood supply of vertebrae [59]. Both studies were excluded from our meta-analysis as they only compared multi-vessel against simple coronary disease. Neither study had control arms comprised of patients free of coronary disease.
Calcium and vitamin D supplementation are commonly prescribed in the initial management of low BMD. Whilst the cardiovascular safety of vitamin D is well-established [60], the safety of calcium supplementation has been a point of scientific controversy. A 2016 meta-analysis [61] of 4 randomized controlled trials formed the basis of the recommendation by the National Osteoporosis Foundation that calcium with or without vitamin D had no relationship to the risk of cardiovascular disease [62]. However, a more recent meta-analysis [63] pooling 12 randomized controlled trials suggested that calcium supplementation increased the risk of CAD by 8% (RR 1.08 95%CI 1.02–1.22 I2 = 0%). Sub-analyses revealed a greater association when calcium was supplemented alone (RR 1.20 95%CI 1.08–1.33), and a lack of association when calcium and vitamin D were supplemented together (RR 1.02 95%CI 0.95–1.10). A ten-year follow up of the MESA study [64] suggested a slight increase in risk of coronary artery calcification with calcium supplementation (RR 1.12 95%CI 1.00–1.26, p = 0.047). There is little evidence to suggest that dietary calcium is similarly associated with cardiovascular risk [65].
One of the biggest limitations of this meta-analysis is the significant degree of clinical and methodological heterogeneity across all included studies. Statistical heterogeneity was high at 78% (Fig. 2). Subgroup analysis by sex, coronary imaging modality, and geographic variation could not account for this heterogeneity. Furthermore, given the observational nature of included studies, we cannot determine the underlying cause of this association between low BMD and CAD. Adjustment for co-variates was inconclusive, given the differing confounders across the 4 studies and the subsequent large statistical heterogeneity. Finally, the risk of bias assessment was conducted by only one author (CK), which may serve as a source of bias for this review.
Unlike previous meta-analyses [66], [67], [68], we used strict definitions of CAD (≥50% stenosis in ≥1 vessel) and low BMD (T-score ≤ −1 OR QCT ≤ 120 mg/cm3). Using 50% as a threshold for potentially haemodynamically significant stenosis has been a historical clinical standard for publications. Furthermore, most of the literature has used the binary cut-off of 50% stenosis and above in validating CTCA against invasive coronary angiograms. Hence using a more stringent definition for coronary artery stenosis is likely to correspond to more clinically significant coronary artery disease. Other meta-analyses have a more ambiguous definition of coronary disease [66], [67], [68]. These strict definitions also reduce heterogeneity amongst the included studies compared to other systematic reviews. A previous review by Zhang et al. [66] was trending towards but did not show a statistical significant association between low BMD and CAD (OR 1.50, 95%CI 0.99–2.52, p = 0.56). This may reflect a Type II error, given our more contemporary review has a larger sample size with a statistically significant result. Meta-analyses by Ye et al. [67] and Veronese et al. [68] also showed statistically significant results, although these studies had a broader scope than coronary artery disease. The former showed an association between low BMD and atherosclerotic disease (ie. including carotid and peripheral vascular disease). Veronese et al. [68] showed an association between low BMD and cardiovascular disease, without necessarily requiring objective imaging evidence of coronary artery disease.
In conclusion, low BMD is associated with CAD; however, it is unclear whether this result is confounded by common risk factors given the heterogeneity between the study populations and methodologies. Inflammation is postulated to be the common pathobiological basis driving these two processes. Further large-scale epidemiological studies are required to better address this question.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100891.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.World Health Organization, Cardiovascular diseases (CVDs) [Fact Sheet], 2017. Retrieved from https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- 2.Allener S., Peto V., Scarborough P., Kaur A., Rayner M. University of Oxford; 2008. Coronary Heart Disease Statistics 2008 Edition: British Heart Foundation Promotion Research Group. [Google Scholar]
- 3.World Health Organization, WHO Scientific Group on the assessment of Osteoporosis at Primary Health Care Level [Report], 2007. Retrieved from https://www.who.int/chp/topics/Osteoporosis.pdf.
- 4.Frysz M., Deere K., Lawlor D.A., Benfield L., Tobias J.H., Gregson C.L. Bone mineral density is positively related to carotid intima-media thickness: findings from a population-based study in adolescents and premenopausal women. J. Bone Miner. Res. 2016;31(12):2139–2148. doi: 10.1002/jbmr.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.N., Lee H.S., Nam H.S., Lee H.R., Kim J.M., Han S.W. Carotid intima-media thickness is inversely related to bone density in female but not in male patients with acute stroke. J. Neuroimaging. 2016;26(1):83–88. doi: 10.1111/jon.12284. [DOI] [PubMed] [Google Scholar]
- 6.Liu D., Chen L., Dong S., Peng Z., Yang H., Chen Y. Bone mass density and bone metabolism marker are associated with progression of carotid and cardiac calcified plaque in Chinese elderly population. Osteoporos. Int. 2019;30(9):1807–1815. doi: 10.1007/s00198-019-05031-5. [DOI] [PubMed] [Google Scholar]
- 7.Szulc P., Samelson E.J., Kiel D.P., Delmas P.D. Increased bone resorption is associated with increased risk of cardiovascular events in men: the MINOS study. J. Bone Miner. Res. 2009;24(12):2023–2031. doi: 10.1359/JBMR.090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tankó L.B., Christiansen C., Cox D.A., Geiger M.J., McNabb M.A., Cummings S.R. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner. Res. 2005;20(11):1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 9.West S.L., O’Donnell E. Cardiovascular disease and bone loss—new research in identifying common disease pathophysiologies and predictors. AME Med. J. 2018;3(3) [Google Scholar]
- 10.Pan H.-C., Chou K.-M., Lee C.-C., Yang N.-I., Sun C.-Y. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90. doi: 10.1016/j.atherosclerosis.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Bucay N., Sarosi I., Dunstan C.R., Morony S., Tarpley J., Capparelli C. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Gene Dev. 1998;12(9):1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschiderer L., Klingenschmid G., Nagrani R., Willeit J., Laukkanen Jari A., Schett G. Osteoprotegerin and cardiovascular events in high-risk populations: meta-analysis of 19 prospective studies involving 27 450 participants. J. Am. Heart Assoc. 2018;7(16):e009012. doi: 10.1161/JAHA.118.009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessueille L., Magne D. Inflammation: a culprit for vascular calcification in atherosclerosis and diabetes. Cell. Mol. Life Sci. 2015;72(13):2475–2489. doi: 10.1007/s00018-015-1876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Radiology, ACR-SPR-SSR Practice parameter for the performance of musculoskeletal quantitative computed tomography (QCT) ACR Practice Parameters and Techincal Standards, 2018.
- 15.Ryan T.J., Faxon D.P., Gunnar R.M., Kennedy J.W., King S.B., 3rd, Loop F.D. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78(2):486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- 16.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M. University of Ottawa; Ottawa: 2012. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Stidoes in Meta-analyses. [Google Scholar]
- 17.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruxton G.D., Neuhäuser M. Review of alternative approaches to calculation of a confidence interval for the odds ratio of a 2 × 2 contingency table. Methods Ecol. Evol. 2013;4(1):9–13. [Google Scholar]
- 19.Baujat B., Mahe C., Pignon J., Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat. Med. 2002;21(18) doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 20.Marcovitz P.A., Tran H.H., Franklin B.A., O'Neill W.W., Yerkey M., Boura J., Kleerekoper M., Dickinson C.Z. Usefulness of bone mineral density to predict significant coronary artery disease. Am. J. Cardiol. 2005;96:1059–1063. doi: 10.1016/j.amjcard.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Erbilen E., Yazici S., Ozhan H., Bulur S., Ordu S., Yazici M. Relationship between angiographically documented coronary artery disease and low bone mass in men. Circ. J. 2007;71:1095–1098. doi: 10.1253/circj.71.1095. [DOI] [PubMed] [Google Scholar]
- 22.Tekin G.O., Kekilli E., Yagmur J., Uckan A., Yagmur C., Aksoy Y., Turhan H., Yetkin E. Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography. Int. J. Cardiol. 2008;131:66–69. doi: 10.1016/j.ijcard.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Varma R., Aronow W.S., Basis Y., Singh T., Kalapatapu K., Weiss M.B., Pucillo A.L., Monsen C.E. Relation of bone mineral density to frequency of coronary heart disease. Am. J. Cardiol. 2008;101:1103–1104. doi: 10.1016/j.amjcard.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Beer S., Saely C.H., Hoefle G., Rein P., Vonbank A., Breuss J., Gaensbacher B., Muendlein A., Drexel H. Low bone mineral density is not associated with angiographically determined 4coronary atherosclerosis in men. Osteoporos. Int. 2010;21:1695–1701. doi: 10.1007/s00198-009-1103-y. [DOI] [PubMed] [Google Scholar]
- 25.Hajsadeghi S., Khamseh M.E., Larijani B., Abedin B., Vakili-Zarch A., Meysamie A.P., Yazdanpanah F. Bone mineral density and coronary atherosclerosis. J Saudi Heart Assoc. 2011;23:143–146. doi: 10.1016/j.jsha.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iranpour D., Pourbehi M., Afroozandeh M., Davoodi N., Bidel-Khoshbakht S., Nabipour I., Javadi H., Seyedabadi M., Assadi M. Bone mineral density is not related to angiographically diagnosed coronary artery disease. Hell J Nucl Med. 2014;17:111–115. doi: 10.1967/s002449910138. [DOI] [PubMed] [Google Scholar]
- 27.Alissa E.M., Alnahdi W.A., Alama N., Ferns G.A. Bone mineral density and cardiovascular risk factors in postmenopausal women with coronary artery disease. Bonekey Rep. 2015;4:758. doi: 10.1038/bonekey.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.N., Cho J.Y., Eun Y.M., Song S.W., Moon K.W. Associations between osteoporosis and coronary artery disease in postmenopausal women. Climacteric. 2016;19:458–462. doi: 10.1080/13697137.2016.1200550. [DOI] [PubMed] [Google Scholar]
- 29.Therkildsen J., Winther S., Nissen L., Jørgensen H.S., Thygesen J., Ivarsen P., Frost L., Isaksen C., Langdahl B.L., Hauge E.M., Böttcher M. Sex differences in the association between bone mineral density and coronary artery disease in patients referred for cardiac computed tomography. J. Clin. Densitom. 2021;24:55–66. doi: 10.1016/j.jocd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Fadini G.P., Rattazzi M., Matsumoto T., Asahara T., Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation. 2012;125(22):2772–2781. doi: 10.1161/CIRCULATIONAHA.112.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernardi S., Fabris B., Thomas M., Toffoli B., Tikellis C., Candido R. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol. Cell. Endocrinol. 2014;394(1):13–20. doi: 10.1016/j.mce.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Heymann M.F., Herisson F., Davaine J.M., Charrier C., Battaglia S., Passuti N. Role of the OPG/RANK/RANKL triad in calcifications of the atheromatous plaques: comparison between carotid and femoral beds. Cytokine. 2012;58(2):300–306. doi: 10.1016/j.cyto.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Toffoli B., Pickering R.J., Tsorotes D., Wang B., Bernardi S., Kantharidis P. Osteoprotegerin promotes vascular fibrosis via a TGF-β1 autocrine loop. Atherosclerosis. 2011;218(1):61–68. doi: 10.1016/j.atherosclerosis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Zauli G., Corallini F., Bossi F., Fischetti F., Durigutto P., Celeghini C. Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood. 2007;110(2):536–543. doi: 10.1182/blood-2007-01-068395. [DOI] [PubMed] [Google Scholar]
- 35.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P. Suppression of aging in mice by the Hormone Klotho. Science. 2005;309(5742):1829. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 37.Tintut Y., Patel J., Parhami F., Demer L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.J., Lee I.-K., Jeon J.-H. Vascular calcification-new insights into its mechanism. Int. J. Mol. Sci. 2020;21(8):2685. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H.Y., Lim S., Park S. Role of inflammation in arterial calcification. Korean Circ J. 2021;51(2):114–125. doi: 10.4070/kcj.2020.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Aly Z., Shao J.S., Lai C.F., Huang E., Cai J., Behrmann A. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 2007;27(12):2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 41.Wen C., Yang X., Yan Z., Zhao M., Yue X., Cheng X. Nalp3 inflammasome is activated and required for vascular smooth muscle cell calcification. Int. J. Cardiol. 2013;168(3):2242–2247. doi: 10.1016/j.ijcard.2013.01.211. [DOI] [PubMed] [Google Scholar]
- 42.Kurozumi A., Nakano K., Yamagata K., Okada Y., Nakayamada S., Tanaka Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. 2019;124:53–61. doi: 10.1016/j.bone.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Martinez G., Robertson S., Barraclough J., Xia Q., Mallat Z., Bursill C. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luc G., Bard J.M., Juhan-Vague I., Ferrieres J., Evans A., Amouyel P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler. Thromb. Vas. 2003;23(7):1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 45.Laroche M., Pecourneau V., Blain H., Breuil V., Chapurlat R., Cortet B. Osteoporosis and ischemic cardiovascular disease. Joint Bone Spine. 2017;84(4):427–432. doi: 10.1016/j.jbspin.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Walsh N.C., Gravallese E.M. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr. Opin. Rheumatol. 2004;16(4):419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 47.Menini S., Iacobini C., Ricci C., Blasetti Fantauzzi C., Salvi L., Pesce C.M. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc. Res. 2013;100(3):472–480. doi: 10.1093/cvr/cvt206. [DOI] [PubMed] [Google Scholar]
- 48.Vengrenyuk Y., Cardoso L., Weinbaum S. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps. Mol. Cell. Biomech. 2008;5(1):37–47. [PubMed] [Google Scholar]
- 49.Sakaguchi M., Hasegawa T., Ehara S., Matsumoto K., Mizutani K., Iguchi T. New insights into spotty calcification and plaque rupture in acute coronary syndrome: an optical coherence tomography study. Heart Vessels. 2016;31(12):1915–1922. doi: 10.1007/s00380-016-0820-3. [DOI] [PubMed] [Google Scholar]
- 50.Ehara S., Kobayashi Y., Yoshiyama M., Shimada K., Shimada Y., Fukuda D. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 51.Durham A.L., Speer M.Y., Scatena M., Giachelli C.M., Shanahan C.M. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018;114(4):590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu N., Chen J., Zhang K., Tang Z. A community-based study of the relationship between coronary artery disease and osteoporosis in Chinese postmenopausal women. Coron. Artery Dis. 2016;27(1):59–64. doi: 10.1097/MCA.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 53.Sharif P.S., Abdollahi M. A systematic review on the relation between use of statins and osteoporosis. Int. J. Pharmacol. 2011;7:180–188. [Google Scholar]
- 54.Jadhav S.B., Jain G.K. Statins and osteoporosis: new role for old drugs. J. Pharm. Pharmacol. 2006;58(1):3–18. doi: 10.1211/jpp.58.1.0002. [DOI] [PubMed] [Google Scholar]
- 55.Lin T.K., Chou P., Lin C.H., Hung Y.J., Jong G.P. Long-term effect of statins on the risk of new-onset osteoporosis: a nationwide population-based cohort study. PLoS ONE. 2018;13(5):e0196713. doi: 10.1371/journal.pone.0196713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leutner M., Matzhold C., Bellach L., Deischinger C., Harreiter J., Thurner S. Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann. Rheum. Dis. 2019;78(12):1706. doi: 10.1136/annrheumdis-2019-215714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu R., Cheng X.C., Zhang Y., Lai H.M., Yang H.N. Association of severity of coronary lesions with bone mineral density in postmenopausal women. Arq. Bras. Cardiol. 2018;110(3):211–216. doi: 10.5935/abc.20180035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan X., Xue Y., Wang J., Ma J., Li Y., Zheng C. Low bone mineral density is associated with global coronary atherosclerotic plaque burden in stable angina patients. Clin. Interv. Aging. 2018;13:1475–1483. doi: 10.2147/CIA.S168445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bagger Y.Z., Rasmussen H.B., Alexandersen P., Werge T., Christiansen C., Tankó L.B. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos. Int. 2007;18(4):505–512. doi: 10.1007/s00198-006-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbarawi M., Kheiri B., Zayed Y., Barbarawi O., Dhillon H., Swaid B. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4(8):765–776. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung M., Tang A.M., Fu Z., Wang D.D., Newberry S.J. Calcium intake and cardiovascular disease risk: an updated systematic review and meta-analysis. Ann. Intern. Med. 2016;165(12):856–866. doi: 10.7326/M16-1165. [DOI] [PubMed] [Google Scholar]
- 62.Kopecky S.L., Bauer D.C., Gulati M., Nieves J.W., Singer A.J., Toth P.P. Lack of evidence linking calcium with or without Vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the national osteoporosis foundation and the American society for preventive cardiology. Ann. Intern. Med. 2016;165(12):867–868. doi: 10.7326/M16-1743. [DOI] [PubMed] [Google Scholar]
- 63.Yang C., Shi X., Xia H., Yang X., Liu H., Pan D. The evidence and controversy between dietary calcium intake and calcium supplementation and the risk of cardiovascular disease: a systematic review and meta-analysis of cohort studies and randomized controlled trials. J. Am. Coll. Nutr. 2020;39(4):352–370. doi: 10.1080/07315724.2019.1649219. [DOI] [PubMed] [Google Scholar]
- 64.Anderson J.J., Kruszka B., Delaney J.A., He K., Burke G.L., Alonso A. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA) J. Am. Heart Assoc. 2016;5(10) doi: 10.1161/JAHA.116.003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolland M., Grey A., Ried I. Calcium and cardiovascular risks. Aust. Prescr. 2013;36:148–149. [Google Scholar]
- 66.Zhang Y., He B., Wang H., Shi J., Liang H. Associations between bone mineral density and coronary artery disease: a meta-analysis of cross-sectional studies. Arch. Osteoporos. 2020;15(1):24. doi: 10.1007/s11657-020-0691-1. [DOI] [PubMed] [Google Scholar]
- 67.Ye C., Xu M., Wang S., Jiang S., Chen X., Zhou X. Decreased bone mineral density is an independent predictor for the development of atherosclerosis: a systematic review and meta-analysis. PLoS ONE. 2016;11(5):e0154740. doi: 10.1371/journal.pone.0154740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veronese N., Stubbs B., Crepaldi G., Solmi M., Cooper C., Harvey N.C., Reginster J.Y., Rizzoli R., Civitelli R., Schofield P., Maggi S., Lamb S.E. Relationship between low bone mineral density and fractures with incident cardiovascular disease: a systematic review and meta-analysis. J. Bone Miner. Res. 2017;32:1126–1135. doi: 10.1002/jbmr.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.