Abstract
Background
Atrial dilation is an important risk factor for atrial fibrillation (AF) and animal studies have found that acute atrial dilation shortens the atrial effective refractory period (AERP) and increases the risk of AF. Stretch activated ion channels (SACs) and calcium channels play a role in this. The expression profile and calcium dependent activation makes the small conductance calcium activated K+ channel (KCa2.x) a candidate for coupling stretch induced increases in intracellular calcium through K+-efflux and thereby shortening of atrial refractoriness.
Objectives
We hypothesized that KCa2.x channel inhibitors can prevent the stretch induced shortening of AERP and protect the heart from AF.
Methods
The effect of KCa2 channel inhibitor (N-(pyridin-2-yl)-4-(pyridin-2-yl)thiazol-2-amine (ICA) 1 µM) was investigated using the isolated perfused rabbit heart preparation. To stretch the left atrium (LA) a balloon was inserted and inflated. AERP and action potential duration (APD) were recorded before and after atrial stretch. AF was induced by burst pacing the LA at different degrees of atrial stretch.
Results
Stretching of the LA by increasing the balloon pressure from 0 to 20 mmHg shortened the AERP by 8.6 ± 1 ms. In comparison, the KCa2 inhibitor ICA significantly attenuated the stretch induced shortening of AERP to 2.5 ± 1.1 ms. Total AF duration increased linearly with atrial balloon pressure. This relationship was not found in the presence of ICA. ICA lowered the incidence of AF induction and total AF duration.
Conclusion
The KCa2 channel inhibitor ICA attenuates the acute stretch induced shortening of AERP and decreases stretch induced vulnerability to AF.
Keywords: Ion channel, Atrial fibrillation, SK channel, KCa2, Stretch, Pharmacology
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults with a prevalence of 2–4% [1]. Atrial dilation is an important risk factor for AF and can be both consequence and cause of AF (for review see [2]. Animal studies have found that acute atrial dilation shortens the atrial effective refractory period (AERP) and slows the electrical conduction [3], [4]. Similar effects have been observed in humans [5], [6]. This feedback where mechanical forces on the myocardium and eventually on the cell membrane cause changes in cardiac electrophysiology is known as mechanoelectrical feedback and stretch activated ion channels are key players in conveying the mechanosensitivity. (for review see [7], [8]. A role of stretch activated ion channels in the initiation of AF and as potential novel drug targets for preventing AF has been suggested [9], [10], [11]. Ion channels that change their open probability in response to cell stretch are divided into cation‐non‐selective (SACNs) and K+‐selective channels (SACK). One member of the SACK is the large conductance K+ channel (KCa1.1), belonging to the family of calcium activated K+ channels. The family also includes the intermediate conductance (KCa3.1) and small conductance K+ channel (KCa2.x). In the heart, KCa2.x predominantly exert its effects on the atria. In animal models and in human tissue, pharmacological inhibitors of the KCa2.x channel prolong the atrial action potential duration (APD) and refractory period with small effects on ventricular repolarization, and terminate and prevent the induction of AF [12], [13], [14], [15]. The open probability of the KCa2.x channel is not affected by stretch, but solely increased by rises in intracellular calcium. The expression profile and calcium dependent activation makes the KCa2.x channel a candidate ion channel for coupling stretch induced increases in intracellular calcium through SACNs or L-type calcium channels to K+ efflux and thereby shortening of atrial refractoriness. We hypothesized that KCa2.x channel inhibitors can prevent the stretch induced shortening of AERP and protect the heart from AF. To this end we utilized the isolated rabbit heart model, which is a well-known model for studying the acute effect of atrial stretching [3], and the KCa2 channel inhibitor ICA [15], [16].
2. Methods
All procedures were performed at the Department of Biomedical Sciences, University of Copenhagen, under licenses from the Danish Ministry of justice (licence no. 2017-15-0201-01296) and in accordance with the Danish guidelines for animal experiments according to the European Commission Directive 86/609/EEC. All animals received humane care and were housed in cages with free access to water and food at the animal house facility at room temperature (21 °C) and subjected to a 12 h light/dark cycle.
A total of 44 adult female white rabbits (Charles River, France) (2–3 kg) were included in the study. 9 experiments were excluded because of problems with instrumenting the heart, inability to maintain a constant perfusion pressure or loss of signals. The rabbits were anaesthetized with intramuscular S-ketamine-xylazine (Ketamin 35 mg/kg + xylazin 10 mg/kg I.M). When sedated, heparin (0.5 mL/kg Heparin 1000 IE/mL) and pentobarbital/lidocaine (200 mg/kg)/20 mg/kg were injected through the marginal ear vein. Following euthanasia, the heart was excised through a thoracotomy and immersed into ice-cold Krebs-Henseleit buffer. Then the aorta was cannulated with a metal cannula to a position just above the aortic valve to allow the perfusion fluid to enter through the coronary ostium. Sutures were tied around the aorta-cannula. Once cannulated, the hearts were perfused under a constant pressure of 80 mmHg and submerged in 37 °C Krebs-Henseleit buffer containing (mmol/L): 120 NaCl, 25 NaHCO3, 4 KCl, 0.6 MgSO4, 0.6 NaH2PO4, 2.5 CaCl2, 11 glucose. The buffer was continuously gassed with a mixture of 95% oxygen (O2) and 5% carbon dioxide (CO2) via a sintered glass gas distributor.
The Langendorff system was connected to a signal amplifier (Hugo Sachs Elektronik-Harvard Apparatus GmbH, Germany). All data were acquired at 2 KHz using the 16-channel PowerLab system (ADInstruments, Oxford, UK), and monitoried by LabChart 8 software (ADInstruments). Epicardial monophasic action potential (MAP) electrodes (Hugo Sachs Elektronik-Harvard Apparatus GmbH, March-Hugstetten, Germany) were placed on the LA and on both ventricles. A pacing electrode was placed on the LA, and LV.
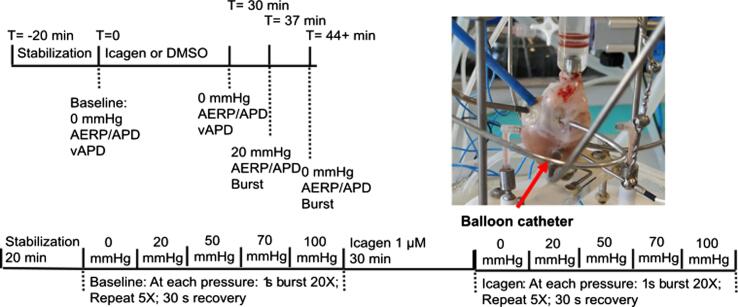
The heart was stabilized for 10 min before insertion of an intra-atrial balloon. A size ten (0.7 mL) latex balloon-tipped catheter coupled with a pressure transducer and microsyringe (Hugo Sachs Elektronik-Harvard Apparatus GmbH, Germany) was introduced through the pulmonary vein ostia into the left atrium and finally positioned predominantly in the left atrial appendage. This allowed for visual inspection of the location and dilation of the balloon.
The atrioventricular (AV) node was ablated by scarring the area known as the “triangle of Koch” of the right atrium using surgical scissors. During the ablation procedure, MAP recording were used to monitor the ventricular rate.
2.1. Experimental protocol
The AERP was measured by programmed electrical stimulation. The heart was paced at the left atrium for 1 min at 200 ms BCL in order to record atrial monophasic action potentials. At the end, ten S1 stimuli (2 ms) at 200 ms BCL (300 bpm) at 5 times rheobase was applied. An extra-stimuli S2 was imposed every 10th stimulus. The S1-S2 interval was increased until it induced an action potential. The longest S1-S2 interval failing to elicit an action potential was defined as the AERP. AF was induced by LA burst pacing (50 Hz). Runs of AF was defined as fast irregular atrial rhythm lasting more than 2 s. Sustained AF was defined as fast irregular atrial rhythm lasting more than 120 s. To record ventricular monophasic action potentials, the LV was paced at 200 ms BCL.
The study consisted of two separate parts. In part 1 (Effect of stretch on AERP) two experimental groups were compared (vehicle control vs ICA 1 µM). The experimental protocol consisted of four stages: Baseline, drug administration, stretch, and burst pacing (see Fig. 1). The heart was allowed to stabilize for minimally 20 min after the insertion of the balloon until at least three consecutive stable AERP (±3 ms) was measured. Hearts were randomly assigned to vehicle control or ICA group. ICA (1 µM) or DMSO was added to the perfusate and effects on AERP and APD were recorded after 30 min. Following drug perfusion the LA balloon pressure was increased to 20 mmHg and after 5 min of stretch AERP was recorded and AF was induced by burst pacing (50 Hz, 20 times burst pacing, 1 s each). The pressure was then reduced to 0 mmHg and AERP and after 5 min burst pacing was performed again.
Fig. 1.
Graphical representation of the protocols used for the two studies, and picture of the preparation. mmHg refers to LA balloon pressure. Please note that the atrial stretch was held for 5 min before AERP or burst pacing commenced. For study 1n = 22; study 2n = 13.
For part 2 (Effect of stretch on AF duration) (see Fig. 1) the hearts were instrumented and stabilized as in part 1. However, the remainder of the protocol differed. Following stabilization, the hearts were subjected to a burst pacing protocol consisting of 20 bursts (1 s, 50 Hz). This protocol was repeated 5 times with 30 s interval at increasing LA balloon pressures (0 mmHg, 20 mmHg, 50 mmHg, 70 mmHg and 100 mmHg). The atrium was stretched for 5 min before bursting commenced. After the highest level of atrial balloon pressure the balloon was deflated and 1 µM ICA was added. After 30 min the burst protocol was repeated in the presence of ICA, and data were compared to baseline.
2.2. Drugs and chemicals
N-(pyridin-2-yl)-4-(pyridin-2-yl)thiazol-2-amine (ICA) was synthezied at Syngene (India). A stock solution of 10 mM ICA in DMSO was used for the experiments and diluted in Krebs-Henseleit buffer to achieve a final concentration of 1 µM. All other chemicals were purchased from Sigma-Aldrich (Munich, Germany).
2.3. Data analysis
APD90 were analyzed in LabChart 8 (AD Instruments, Ltd., Dunedin, New Zealand) using the peak analysis plugin. Statistical data analysis was done by Graphpad Prism 9 (Graph Pad Software, La Jolla, CA, USA). In Figs. 2, 3, and 9 data are presented as the mean ± standard error of the mean. Data in Fig. 5, Fig. 6 are presented as median as they are not normally distributed, and non-parametric multiple comparison Friedman test was used to for statistical analyses. The frequency distribution of the individual AF durations was analyzed by binning the individual AF episodes in bins of 2 s. The relationship between total AF duration and atrial stretch was fitted by simple linear regression, and the slopes of the linear regression for before and after ICA were compared. Effects on AERP and APD90 within each group at different time points were analyzed for statistical differences by one-way ANOVA followed by Tukey’s post hoc test corrected for multiple comparisons. Student’s t-test was used to analyze differences in changes (Δ-values) between the two treatment groups. P < 0.05 was considered statistically significant.
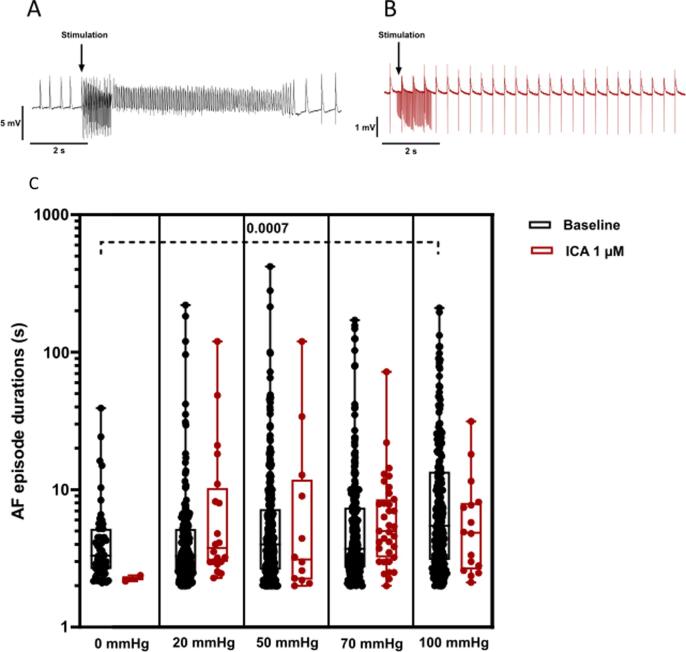
Fig. 5.
Individual AF durations. Individual AF durations (>2 s) induced by atrial burst pacing before (A, black) and after perfusion with ICA 1 µM (B, red) at 5 different stretch levels. Top panel shows example of atrial action potential recordings before, during and after burst. Data are presented as box and whiskers with median and 25–75 percentile, n = 13. Note that the Y-axis is logarithmic. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
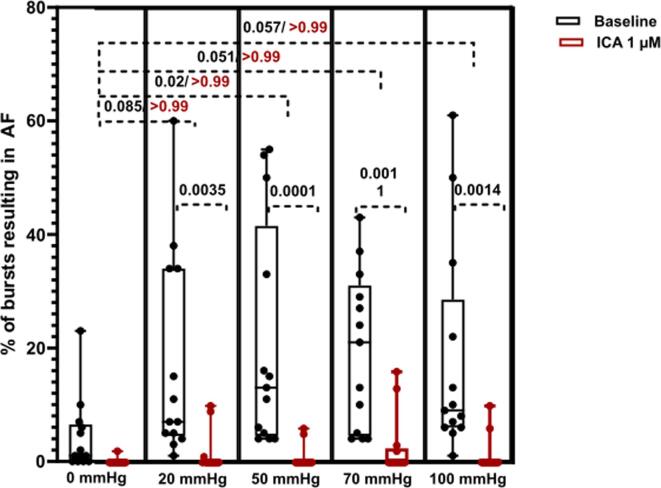
Fig. 6.
Incidence of AF. Incidence (%) of bursts resulting in AF > 2 s. Dots represents the incidence for each animal at each level of balloon pressure. A) before (black) and B) after perfusion with ICA 1 µM (red). Data are presented as box and whiskers with median and 25–75 percentile, n = 13. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. KCa2.x inhibitor attenuates atrial stretch induced shortening of AERP
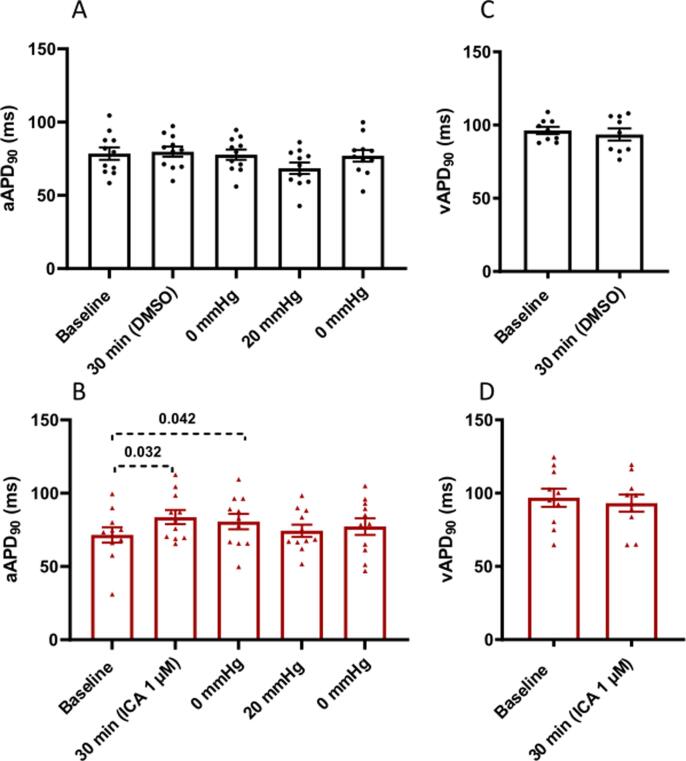
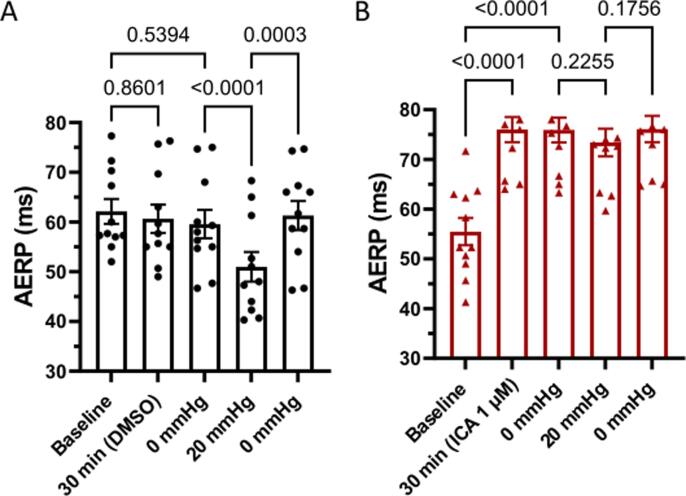
22 perfused rabbit hearts instrumented with a balloon to stretch the LA were used for part 1 of the study. AERP was recorded at baseline and after 30 min of perfusion with either vehicle (DMSO) or ICA (1 µM). ICA perfusion resulted in significant prolongation of AERP by 20.5 ± 2.2 ms (from 55.4 ms to 75.9 ms) and atrial APD90 by 9.1 ± 2.7 ms (from 71.5 ms to 80.6 ms) as compared to the changes observed in the vehicle group (see Table 1 and Fig. 3B). ICA had no effects on ventricular repolarization, when recorded during ventricular pacing at 200 ms (Table 1 and Fig. 3D). As expected, in the vehicle group inflation of the LA balloon caused a shortening of the AERP and LA APD90 by 8.6 ± 1 ms (from 59.6 ms to 51 ms) and 9.3 ± 4.1 ms (from 77.8 ms to 68.5 ms) respectively. This effect was reverted by deflating the balloon to 0 mmHg. In comparison, the KCa2.x inhibitor ICA significantly attenuated the stretch induced shortening of AERP as compared to the vehicle control group (Vehicle: from 59.6 ms to 51 ms and ICA: from 75.9 to 73.4) (Table 1 and Fig. 2).
Table 1.
Changes from baseline AERP, aAPD90, and vAPD90 for each group. P-value refers to the comparison of Δ-values between the vehicle control group and the treatment group (ICA 1 µM). mmHg refers to LA balloon pressure.
| Δ-values | Vehicle control | ICA | n; p-value |
|---|---|---|---|
| AERP, ms (0 min to 30 min) | −2.6 ± 1.6 | 20.5 ± 2.2 | 11; 0.000001 |
| aAPD90 , ms (0 min to 30 min) | −2.2 ± 2.1 | 9.1 ± 2.7 | 11; 0.048 |
| AERP, ms (Stretch 0–20 mmHg) | −8.6 ± 1.0 | −2.5 ± 1.1 | 11; 0.0038 |
| aAPD90, ms (Stretch 0–20 mmHg) | −9.3 ± 4.1 | −6.2 ± 4,3 | 11; 0.49 |
| vAPD90, ms (0 min to 30 min) | −2.7 ± 3.6 | −3.7 ± 2.9 | 11; 0.83 |
Fig. 3.
Effect of atrial stretch and ICA on action potential durations. (A,B) Effects of intervention on atrial APD90 (A,B), n = 11; and ventricular APD90 recorded at pacing basic cycle length of 200 ms (C,D) n = 9 and 10. Data are presented as mean ± SEM. Vehicle control group at top (black) and treatment group (ICA 1 µM) at bottom (red). Multiple comparisons tests were performed. For simplicity only the comparisons: baseline to 30 min; 0 mmHg to 20 mmHg; 20 mmHg to 0 mmHg are shown. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Effect of atrial stretch on atrial refractoriness. Time plot of the changes in AERP during the study before and after stretch in the vehicle control group (A; black) and treatment group (ICA 1 µM) (B; red). Data are presented as mean ± SEM, n = 11. Multiple comparisons tests were performed. For simplicity only the comparisons: baseline to 30 min; baseline to 0 mmHg, 0 mmHg to 20 mmHg; 20 mmHg to 0 mmHg are shown. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. KCa2.x inhibitor prevents induction of AF by burst pacing in the presence of atrial stretch
Dilation of the LA and AERP shortening increased the risk of burst induced AF. No spontaneous AF was detected upon stretch in our experiments, but to investigate if stretching increased the vulnerability of the heart to AF the LA was challenged with 20 times burst pace (50 Hz for 1 s) before and after stretch at 20 mmHg in the two treatment groups. At 0 mmHg 6 of 11 hearts in the control group had individual AF episodes > 2 s upon bursting, and in those 6 hearts the total AF duration had a range of 2.2–122.3 s with a median time of 15 s. During acute atrial dilation 7 of 11 hearts had individual AF episodes lasting more than 2 s upon burst, and of those 7 hearts the total AF duration of each experiment was from 24.3 s to 515.9 s with a median time of 57.8 s. In comparison, in the ICA treatment group two hearts had AF lasting more than 2 s at 0 mHg and three during stretch. Likewise, the total AF duration of each experiment was smaller in the ICA group with AF durations ranging from 7.9 s to 8.5 s with a median of 8.2 s and from 40.5 s to 92.6 s with a median time of 47.6 s for before and after stretch respectively.
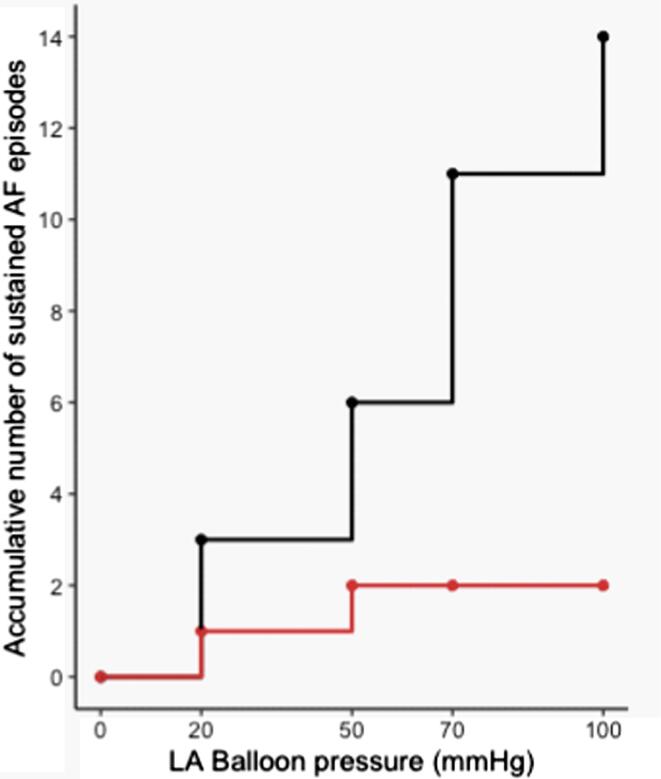
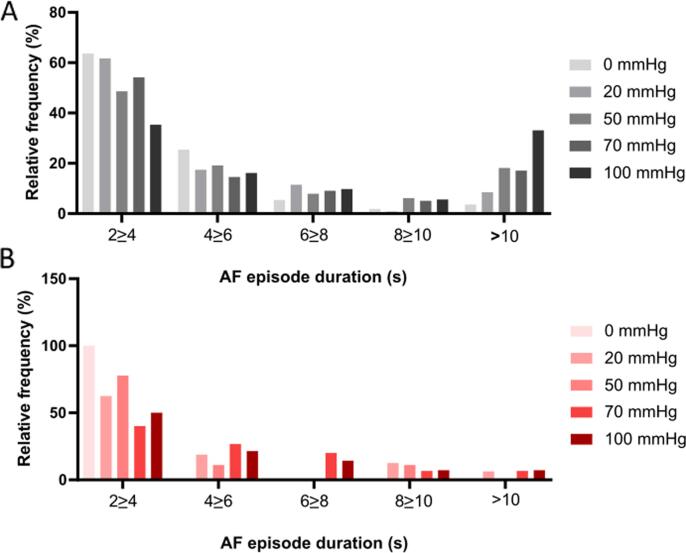
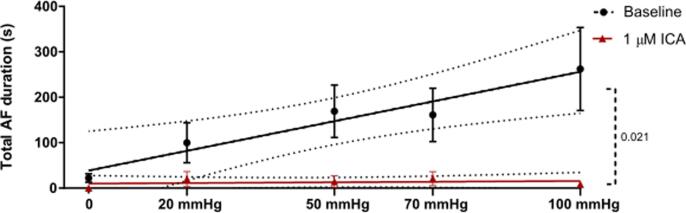
Encouraged by the data we performed additional experiments dedicated to investigate if KCa2.x channel inhibition could decrease the stretch induced vulnerability to AF. 13 isolated rabbit hearts were subjected to progressively increasing levels of atrial dilation and more forceful burst pacing protocol in order to induce sustained episodes of stretch associated AF. During perfusion with DMSO, a total of 14 episodes of sustained AF was induced in 5 of the 13 hearts (see Fig. 4). In comparison, during the ICA perfusion period, sustained AF only occurred in one rabbit (2 episodes). The median duration of the individual AF episodes was not different when comparing baseline to ICA perfusion (Fig. 5). However, the incidence of AF episodes and the % of bursts resulting in AF were significantly increased (Fig. 6). From the individual AF duration distribution (Fig. 7) it can be observed that increasing the balloon pressure shifts the frequency distribution towards longer AF episodes. Interestingly the presence of ICA appears to reduce the number of long lasting AF episodes. The total AF duration recorded at the different levels of atrial stretch induced by the 20 bursts (repeated 5 times) can be seen in Fig. 8. Here it can be observed that total AF duration increases linearly with atrial balloon pressure and that this is not the case in the presence of ICA, where the total AF duration is significantly reduced.
Fig. 4.
Number of sustained AF episodes. Accumulative number of sustained AF (AF > 2 min) episodes during the 5 atrial stretch levels before (black) and after (red) perfusion with ICA 1 µM. n = 13. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Frequency distribution of AF episode durations. Relative frequencies of the AF (>2 s) episodes before (A, black) and after ICA 1 µM (B, red). X-axis is divided in 2 s bins, except for the last bin which includes all AF episodes longer than 10 s. n = 13. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
AF duration increases with stretch. Total AF duration recorded at the different stretch levels before (black) and after ICA 1 µM (red). A positive linear correlation was found for the effect of stretching the left atrium and the AF duration (insert formula) at baseline. The two linear regressions (stretch and AF duration (baseline) vs stretch and AF duration (ICA) are significantly different. n = 13. mmHg refers to LA balloon pressure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Atrial dilation is an important risk factor for AF and studies have found that acute atrial dilation increases the risk of AF (for review see 2). The main finding of this study is that the KCa2 channel inhibitor ICA attenuates the acute stretch induced AERP shortening and decreases stretch induced vulnerability to AF.
Similar to other animal studies [3], [4] and observations in humans [5], [6], we found that acute atrial dilation shortens the AERP. Both voltage- and calcium-gated channels as well as stretch activated non-selective cation channels have been implicated in the stretch induced AF mechanism [9], [10], [17], [18]. In cardiomyocytes voltage-gated calcium channels co-localize with KCa2 channels via their interaction with the cytoskeletal protein α-actinin-2. This suggests that voltage-gated calcium channels regulate KCa2 channels by providing a local Ca2+ microdomain [19], [20]. Moreover, the voltage-dependent calcium channel inhibitor verapamil was found to prevent stretch induced AERP shortening in rabbits and humans [17], [18]. Based on this, we speculated if calcium influx via stretch activated cation channels and/or via voltage-dependent calcium channels could activate KCa2 channels and thereby contribute to the shortening of AERP that is observed upon acute atrial stretch. We therefore tested the hypothesis that KCa2 channel inhibition would prevent the atrial stretch induced shortening of AERP. To this end we used the KCa2 channel inhibitor ICA, which is a KCa2 channel inhibitor (KCa2.2 and KCa2.3 IC50 = 0.3–0.5 µM) with IC50 values > 20 µM on a panel of cardiac ion channels [15]. Similar to previous studies [15], [21] we found that ICA increased the atrial APD and AERP, with no effects on ventricular APD. Interestingly, the KCa2 channel inhibitor ICA attenuated the stretch induced shortening of atrial APD/AERP, suggesting a role for KCa2 channels in this phenomenon.
Acute atrial stretch has been found to increase the number of afterdepolarizations in animals [22], [23]. Genetic knock down of KCa2.2 in mice was found to increase the incidence of early afterdepolarizations (EAD) recorded in isolated cardiomyocytes [24]. An anti-arrhythmic importance of KCa2 channel activity has therefore been put forward. Here KCa2 channels are speculated to play a protective role in counteracting afterdepolarizations caused by calcium overload, because the excess calcium activates KCa2 channels, which helps to clamp the membrane potential and reduce the incidence of EADs. Contrary to this, many pharmacological large and small animal studies have demonstrated that KCa2 channel inhibitors and negative allosteric modulators terminate and protect the heart from AF [12], [13], [14], [25], [26], [27]. However, these studies did not address AF in the setting of stretch. In the present study we addressed if the KCa2 channel inhibitor ICA decreases stretch induced vulnerability to AF. In accordance with earlier findings we observed that stretching the atrium results in increased likelihood and total duration of burst induced AF. Without stretch the atrium can rarely be burst into AF, whereas upon stretch, which lowers the AERP, AF is more readily induced [3]. We observed a positive linear correlation between stretch and the total AF duration. The increase in total AF duration was a result of higher incidence of AF and longer individual episodes of AF as the atrial stretch was increased. Moreover, the occurrence of sustained AF upon bursting also increased with the level of stretch. ICA both prolonged the AERP and limited the incidence of AF, reduced the number of long lasting AF episodes and consequently removed the positive linear correlation between stretch and total AF duration. Interestingly, apart from the reduction in long lasting AF, the major effect of ICA appeared to be a reduction of AF incidence and not the individual AF episode durations, indicating that ICA primarily decreases the vulnerability to stretch induced AF by preventing its induction. Whether the protective effects of ICA translates to the setting of acute atrial stretch in human patients is unknown, but ICA has been found to prolong APDs recorded from human atrial tissue.
KCa2 channel expression appears to be down-regulated in atria of chronic/permanent AF patients [15], [28], [29] and upregulated in severely failing ventricles [28], [30], [31], [32], which could limit the clinical use of KCa2 channel inhibitors in these patient populations. Based on preclinical observations [13], [15] and the current study, targeting paroxysmal AF in non-valvular patients and valvular patients with enlarged atria could potentially be of interest. A phase II study evaluating the efficacy of a KCa2 channel inhibitor for cardioversion of recent onset AF is currently ongoing (Clinical Trial identifier: NCT04571385).
In conclusion, stretching of the left atrium was associated with shortening of the atrial ERP, and with increased vulnerability to AF. These effects were attenuated in the presence of KCa2 channel inhibitor ICA. This points to a role of KCa2 channels in the setting of atrial stretch.
5. Limitations
Occurrence of short runs of AF in isolated rabbit hearts does not directly translate into the clinical manifestation of AF in patients. Because AF does not readily occur in atria of rabbits under normal conditions, we defined AF as episodes > 2 s, this is much shorter than the long lasting episodes observed in patients with AF. Future studies in animals with larger atria exposed to experimental conditions mimicking human conditions with stretched atria (e.g. sleep apnea/negative airway pressure) will aid the translation to humans.
Sex differences in beta-adrenergic activation of ventricular KCa2 channels have been reported [33]. However, as female rabbits can be housed together, only female rabbits were used in the study, and therefore we do not know if similar effects of KCa2 channel inhibition would have been observed in male rabbits. The anti-arrhythmic effect of KCa2 channel inhibition upon atrial stretch was investigated in healthy rabbits. Whether, similar effects are observed in a setting of atrial fibrosis or atrial enlargement is unknown. Another KCa2 channel inhibitor has demonstrated anti-arrhythmic effects in an atrial tachypaced pig model [13], which shows fibrosis and increased left atrial volume [34].
We used a balloon inserted in the LA to induce acute stretching of the atrium, as has been done before [35]. The pressures do therefore not correspond directly to the pressures in the left atrium, but only reflects the pressure of the balloon. Therefore we cannot make a direct comparison to the atrial pressures observed in patients under acute stretch. To this end, future preclinical studies using ligation of vena cavae and pulmonales and precisely control of the atrial pressure would be valuable. We applied unphysiologically high intra-atrial pressures (>20 mmHg). These pressures are not clinically relevant but were used in this preclinical setting to investigate the correlation between stretch and AF.
From the conducted experiments it cannot be concluded whether KCa2 channels were activated by influx via voltage-dependent calcium channels and/or stretch activated cation channels. Moreover, it can be argued that the marked increase in AERP by ICA is responsible for the reduction in stretch-induced AERP shortening, and that no link to activation of KCa2 channels by stretch occurs. However, prolongation of AERP by K+ channel inhibition does not always result in reduction in stretch-induced AERP shortening, as illustrated by the effect of glibenclamide in rabbit atria [18]. Further experiments will be done to clarify this interesting aspect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was funded by a grant from Stock broker Henry Hansen & Karla Hansen, and an unrestricted grant to BHB from Acesion Pharma.
References
- 1.Benjamin E.J., Muntner P., Alonso A. Heart Disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein J., Verheule S., de Groot N.M., de Groot N., Allessie M., Schotten U. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog. Biophys. Mol. Biol. 2008;97(2–3):435–451. doi: 10.1016/j.pbiomolbio.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli F., Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96(5):1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 4.Huang J.-L., Tai C.-T., Chen J.-T. Effect of atrial dilatation on electrophysiologic properties and inducibility of atrial fibrillation. Basic Res. Cardiol. 2003;98(1):16–24. doi: 10.1007/s00395-003-0385-z. [DOI] [PubMed] [Google Scholar]
- 5.H. Calkins, R. el-Atassi, S. Kalbfleisch, J. Langberg, F. Morady, Effects of an acute increase in atrial pressure on atrial refractoriness in humans, Pacing Clin. Electrophysiol. 15(11 Pt 1) (1992) 1674–1680. [DOI] [PubMed]
- 6.Coronel R., Langerveld J., Boersma L.V.A. Left atrial pressure reduction for mitral stenosis reverses left atrial direction-dependent conduction abnormalities. Cardiovasc Res. 2010;85(4):711–718. doi: 10.1093/cvr/cvp374. [DOI] [PubMed] [Google Scholar]
- 7.Peyronnet R., Nerbonne J.M., Kohl P. Cardiac mechano-gated ion channels and arrhythmias. Circ Res. 2016;118(2):311–329. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izu L.T., Kohl P., Boyden P.A. Mechano-electric and mechano-chemo-transduction in cardiomyocytes. J. Physiol. 2020;598(7):1285–1305. doi: 10.1113/JP276494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode F., Katchman A., Woosley R.L., Franz M.R. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000;101(18):2200–2205. doi: 10.1161/01.cir.101.18.2200. [DOI] [PubMed] [Google Scholar]
- 10.Bode F., Sachs F., Franz M.R. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409(6816):35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 11.Franz M.R., Bode F. Mechano-electrical feedback underlying arrhythmias: the atrial fibrillation case. Prog. Biophys. Mol. Biol. 2003;82(1):163–174. doi: 10.1016/s0079-6107(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 12.Diness J.G., Sørensen U.S., Nissen J.D. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010;3(4):380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- 13.Diness J.G., Skibsbye L., Simó-Vicens R. Termination of vernakalant-resistant atrial fibrillation by inhibition of small-conductance Ca2+-activated K+ channels in pigs. Circ Arrhythm Electrophysiol. 2017;10(10) doi: 10.1161/CIRCEP.117.005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi X.-Y., Diness J.G., Brundel B.J.J.M. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129(4):430–440. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
- 15.Skibsbye L., Poulet C., Diness J.G. Small conductance calcium activated potassium (SK) channels contribute to action potential repolarisation in human atria. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- 16.Gentles R.G., Grant-Young K., Hu S. Initial SAR studies on apamin-displacing 2-aminothiazole blockers of calcium-activated small conductance potassium channels. Bioorg. Med. Chem. Lett. 2008;18(19):5316–5319. doi: 10.1016/j.bmcl.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Tse H.-F., Pelosi F., Oral H., Knight B.P., Strickberger S.A., Morady F. Effects of simultaneous atrioventricular pacing on atrial refractoriness and atrial fibrillation inducibility: role of atrial mechanoelectrical feedback. J. Cardiovasc. Electrophysiol. 2001;12(1):43–50. doi: 10.1046/j.1540-8167.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 18.Zarse M., Stellbrink C., Athanatou E., Robert J., Schotten U., Hanrath P. Verapamil prevents stretch-induced shortening of atrial effective refractory period in langendorff-perfused rabbit heart. J. Cardiovasc. Electrophysiol. 2001;12(1):85–92. doi: 10.1046/j.1540-8167.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- 19.Ling L., Qian Z., Valeriy T. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via α-actinin2. Circ. Res. 2007;100(1):112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X.-D., Coulibaly Z.A., Chen W.C. Coupling of SK channels, L-type Ca 2+ channels, and ryanodine receptors in cardiomyocytes. Sci. Rep. 2018;8(1):4670. doi: 10.1038/s41598-018-22843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skibsbye L., Wang X., Axelsen L.N. Antiarrhythmic mechanisms of SK channel inhibition in the rat atrium. J. Cardiovasc. Pharmacol. 2015;66(2):165–176. doi: 10.1097/FJC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 22.Nazir S.A., Lab M.J. Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovasc. Res. 1996;32(1):112–119. [PubMed] [Google Scholar]
- 23.Tavi P., Laine M., Weckström M. Effect of gadolinium on stretch-induced changes in contraction and intracellularly recorded action- and afterpotentials of rat isolated atrium. Br. J. Pharmacol. 1996;118(2):407–413. doi: 10.1111/j.1476-5381.1996.tb15417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N., Timofeyev V., Tuteja D. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J. Physiol. 2009;587(Pt 5):1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diness J.G., Skibsbye L., Jespersen T. Effects on atrial fibrillation in aged hypertensive rats by Ca(2+)-activated K(+) channel inhibition. Hypertension. 2011;57(6):1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613. [DOI] [PubMed] [Google Scholar]
- 26.Diness J.G., Kirchhoff J.E., Speerschneider T. The KCa2 channel inhibitor AP30663 selectively increases atrial refractoriness, converts vernakalant-resistant atrial fibrillation and prevents its reinduction in conscious pigs. Front Pharmacol. 2020;11:159. doi: 10.3389/fphar.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.L. Skibsbye, J.G. Diness, U.S. Sorensen, R.S. Hansen, M. Grunnet, The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+-activated K+ Channels, J. Cardiovascular Pharmacol. 2011;57(6):672–681. [DOI] [PubMed]
- 28.Darkow E., Nguyen T.T., Stolina M. Small conductance Ca2 +-activated K+ (SK) channel mRNA expression in human atrial and ventricular tissue: comparison between donor, atrial fibrillation and heart failure tissue. Front. Physiol. 2021;12:342. doi: 10.3389/fphys.2021.650964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu T., Deng C., Wu R. Decreased expression of small-conductance Ca2+-activated K+ channels SK1 and SK2 in human chronic atrial fibrillation. Life Sci. 2012;90:219–227. doi: 10.1016/j.lfs.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Chang P.-C., Turker I., Lopshire J.C. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J Am Heart Assoc. 2013;2(1) doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang P.-C., Hsieh Y.-C., Hsueh C.-H., Weiss J.N., Lin S.-F., Chen P.-S. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm. 2013;10(10):1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua S.-K., Chang P.-C., Maruyama M. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108(8):971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M., Yin D., Guo S. Sex-specific activation of SK current by isoproterenol facilitates action potential triangulation and arrhythmogenesis in rabbit ventricles. J. Physiol. 2018;596(18):4299–4322. doi: 10.1113/JP275681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Citerni C., Kirchhoff J., Olsen L.H. Characterization of atrial and ventricular structural remodeling in a porcine model of atrial fibrillation induced by atrial tachypacing. Front Vet Sci. 2020;7:179. doi: 10.3389/fvets.2020.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldkamp M.W., Geuzebroek G.S.C., Baartscheer A. Neurokinin-3 receptor activation selectively prolongs atrial refractoriness by inhibition of a background K+ channel. Nat. Commun. 2018;9(1):4357. doi: 10.1038/s41467-018-06530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]