Abstract
This study aimed to investigate the age-related changes of hepatic metabolism and antioxidant capacity of laying hens at 3 different ages. A total of 192 Hy-line Brown laying hens were assigned into 3 groups: 1) 195-day-old (D195 group); 2) 340-day-old (D340 group); 3) 525-day-old (D525 group). Each group replicated 8 times with 8 hens at the same age. Higher activity of aspartate aminotransferase and lower contents of total protein and globulin were observed in the serum of 525-day-old hens in comparison with their 195-day-old counterparts (P < 0.05). The 525-day-old hens accumulated higher contents of total cholesterol and triglyceride in the liver than 195-day-old birds. Additionally, compared with hens from D195 or D340 group, 525-day-old birds exhibited a lower circulating estradiol level (P < 0.05). For antioxidant capacity, birds in the D525 group showed a higher malondialdehyde concentration in both serum and liver as compared with D195 or D340 group (P < 0.05). The 525-day-old hens also exhibited lower glutathione peroxidase activities in both serum and liver when compared with 195-day-old birds (P < 0.05). Simultaneously, there was a decline of hepatic superoxide dismutase activity in the D525 group in comparison with D195 group (P < 0.05). Compared with 195-day-old counterparts, 340-day-old birds upregulated the mRNA abundance of nuclear factor erythroid-2 related factor 2 and glutathione peroxidase 1 in the liver (P < 0.05). In contrast, hens from D525 group showed the downregulation of hepatic nuclear factor erythroid-2 related factor 2, NAD(P)H quinone dehydrogenase 1, and superoxide dismutase 1 when compared with D340 group (P < 0.05). These results indicated that increasing age can adversely affect liver metabolism and function of laying hens.
Key words: age, antioxidant capacity, laying hen, liver function, metabolism
INTRODUCTION
The laying hens undergo a temporary period of high egg production rate and then experience a gradual decline of productive performance after entering their peak laying stage (Tumova et al., 2017). This phenomenon occurs due to various reasons from age-related changes of digestive and reproductive systems (Peebles et al., 2006; Gu et al., 2021), internal metabolic pathways (Wang et al., 2019), intestinal microbial flora (Wang et al., 2020), etc. Of them, lipid metabolism constitutes an essential aspect in the process of egg formation, and it is also closely correlated with molecular actors and physiological characteristics of liver, the primary metabolic organ for laying hens (Gloux et al., 2019). A recent study has reported that the common structural changes naturally occurring in the aging hepatocytes are the declined cellular volume and accumulation of cytoplasmic lipofuscin, one kind of highly oxidized and cross-linked protein that can induce oxidative stress (Pinto et al., 2020). In accordance with an investigation performed on the mice with different ages, the aging liver of old mice (28-mo-old) is characterized by the activated endoplasmic reticulum unfolded protein response, and increased immunological stress and hepatic damage compared with their 6-mo-old counterparts (Pagliassotti et al., 2017). Simultaneously, the disorders of lipid metabolism and related metabolic diseases can be exacerbated by the hepatic stress and damage during the aging process (Xiong et al., 2014). In addition, a previous report has revealed that an age-specific pattern of genes and proteins involving in the hepatic lipid metabolism can account for the underlying mechanism of fatty liver, which has a high incidence in the aging individuals (Li et al., 2008).
Aging is a natural and irreversible physiological process that can progressively produce harmful reactive oxygen species (ROS) (Lee et al., 2004). As a result, when endogenous antioxidants are insufficient to neutralize the excessive free radicals and peroxides in the organisms, the disruption of redox homeostasis and oxidative stress would inevitably occur (Estevez, 2015). In a cell culture trial, in comparison with younger mice (2-mo-old), liver progenitor cells from aging mice (24-mo-old) have been observed to exhibit degenerated activating and proliferating capacity due to the higher ROS production from neutrophils, leading to the impaired regenerative ability on the injury (Cheng et al., 2017). Sverko et al. (2004) also demonstrated that aging can trigger the oxidative stress in the liver tissues of male mice, as evidenced by the increment of indicators of lipid peroxidation (thiobarbituric acid reactive substances) and antioxidant capacity (glutathione peroxidase [GSH-Px] and catalase [CAT] activities). Similarly, elevated biomarker levels of oxidative stress response, antioxidant defence, and chronic inflammation were observed in the senescence-accelerated rodent models (Bayram et al., 2013). Further, mRNA abundance of antioxidant-related genes has been proven to alter from prenatal stage to adulthood (60-day-old) of mice, suggesting that age-dependent pattern of antioxidant genes could exist in the liver (Wu et al., 2019), and some changes can also be predicted to occur when animals enter their senior ages. Besides, during aging, ROS inducer (i.e., hydrogen peroxide) can increase the lipid synthesis and accumulation, coupled with the upregulated mRNA levels of genes related to cholesterol synthesis and uptake in the hepatocytes, indicating the negative effects of age-related oxidative stress on the fat deposition (Seo et al., 2019).
For domestic laying hens, it has been identified that the decline of egg production rate is correlated with decreased levels of gonadotrophin hormones and associated neuroendocrine mRNA expression (Ciccone et al., 2005), as well as the hepatic yolk precursors (Liu et al., 2018). Based on the prerequisite that older laying birds could be more vulnerable against the internal and external stimulation than their younger counterparts, substantial published papers have concentrated on the ameliorative effects of bioactive additives on the productive performance and physiological function of aged laying hens (Jia et al., 2016; Jiang et al., 2020; Liu et al., 2020). In fact, however, little is known about the effects of aging on the organ function or metabolism of laying hens. This study was, therefore, conducted to evaluate and compare the liver metabolism and antioxidant capacity with increasing age after the peak laying stage of hens, providing a foundation for the application of additives in the poultry industry.
MATERIALS AND METHODS
Animals, Experimental Design, and Housing
The experiment was carried out under the guidelines issued by the Animal Care and Use Committee of Nanjing Agricultural University, and the management of birds complied with an ethics committee-approved protocol established by the Jiangsu Provincial Department of Science and Technology (SYXK (SU) 2017-0007).
A total of 192 Hy-line Brown laying hens with different ages were obtained from Tiancheng Group (Jiangsu Province, P. R. China) where these birds were reared in 3 different houses. Each age was composed of 8 replicates of 8 hens. All birds were from the same parental generation. The age of them was 195-day-old (D195 group), 340-day-old (D340 group), and 525-day-old (D525 group), and the egg production rate was 95.8, 90.3, and 81.5%, respectively. The data of egg quality could be found in our previous research (Gu et al., 2021). Each bird was provided with the same maize-soybean meal basal diet of around 110 g per day. After being transferred to the experimental sites, hens were kept together in one house for a 2-wk adaptation period. Every 4 birds were raised in one cage, which was 60 cm in length, 50 cm in width, and 40 cm in height, in a temperature-controlled chicken room with a lighting schedule of 16 h of light and 8 h of darkness. Throughout the entire trial, the mash diets and water were provided ad libitum, and average ambient temperature and relative humidity were kept at 18 to 25°C and 40 to 60%, respectively.
Sample Collection
After the 2-wk period, one bird from each replicate was selected for body weighing and sampling. The blood samples (around 5.0 mL each) were collected from the wing vein into sterile tubes to coagulate, and then centrifuged at 4,000 g for 15 min for serums. The serums were divided into 4 aliquots (around 0.5 mL each) and stored at −20°C for subsequent determination. Thereafter, the birds were euthanized and exsanguinated by cervical dislocation before the postmortem. The whole liver was then dissected immediately from other tissues and rinsed off with phosphate-buffered saline solution. After being dried by the filter paper, liver samples were weighed for the calculation of relative liver weight following the formula: relative liver weight (g/kg) = liver weight (g) / live body weight (kg). The right lobe of liver was then separated, collected, and frozen at −80°C until the further analysis.
Serum Biochemical Index and Hepatic Lipid Accumulation Analysis
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, glucose, high density lipoprotein cholesterol, low density lipoprotein cholesterol, total cholesterol, total protein, and triglyceride in the serum were determined by colorimetric commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing City, Jiangsu Province, P. R. China), as per the recommendations of the manufacturer. The serum globulin concentration was calculated as the difference between total protein and albumin contents.
Prior to the determination of lipid accumulation in the liver, around 0.5 g of thawy liver sample was homogenized with pre-cooled phosphate-buffered saline solution at a ratio of 1: 9 (wt/vol) using an Ultra-Turrax homogenizer (Tekmar Co., Cincinnati, OH). The supernatant, obtained through a centrifugation at 4000 g at 4°C for 15 min, was used for the measurement of total cholesterol and triglyceride with the same kits aforementioned. The results were normalized against the total protein concentration in the supernatant.
Measurement of Serum Reproductive Hormone Concentration
The contents of serum estradiol (E2), follicle stimulating hormone, luteinizing hormone, and progesterone were detected using commercial enzyme-linked immunosorbent kits (Nanjing Jiancheng Bioengineering Institute), strictly in accordance with protocols of manufacturer.
Redox Status Determination
As described above, the hepatic antioxidant capacity assay also required the preparation of supernatants from liver tissues. The levels of CAT, malondialdehyde (MDA), glutathione, GSH-Px, superoxide dismutase (SOD), and total antioxidant capacity in both serums and liver supernatants were assayed with the commercial reagent kits (Nanjing Jiancheng Bioengineering Institute). The total protein concentration in the liver supernatant was also measured for the normalization and comparison. All experimental procedures were totally in accordance with manufacture's manuals.
RNA Extraction and Qualification
The liver samples were homogenized in the TRIzol reagent (TaKaRa Biotechnology, Dalian City, Liaoning Province, P. R. China) for extracting the total RNA. The RNA concentration was quantified using a NanoDrop 1000 UV spectrophotometer (NanoDrop Technologies, Wilmington, DE), depending on the absorbance at the wavelength of 260/280 nm. Thereafter, RNA was adjusted to a concentration of 0.5 g/L, and 1.0 μg RNA was immediately reversely transcribed into complementary deoxyribonucleic acids using the PrimeScript RT reagent kits (TaKaRa Biotechnology). The primer sequences of nuclear factor-erythroid 2-related factor 2 (Nrf2), kelch-like epichlorohydrin-associated protein 1 (Keap1), heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), CAT, glutathione peroxidase 1 (GPX1), and β-actin were presented in Table 1. The β-actin was used as a housekeeping gene. Detection of amplified products was performed on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY). The reagents used for the PCR reaction were from the commercial TB Green Premix Ex Taq kits (TaKaRa Biotechnology). Subsequently, the quantitative real-time PCR was performed with the initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and then the annealing stage at 60°C for 30 s. The end of PCR reaction was a melting curve analysis starting at 1 cycle at 95°C for 10 s, followed by the temperature from 65 to 95°C with an increase of 0.5°C per second in the PCR plate. All reactions were performed in triplicate. The amplification efficiency of each gene was close to 100% in the exponential phase of reaction. The quantities of mRNA expression of target genes relative to β-actin were measured by using 2−ΔΔCT method (Livak and Schmittgen, 2001), and fold changes over the control group were presented as results.
Table 1.
Sequences for real-time PCR primers.
| Items | Gene bank ID | Primer sequence, forward/reverse |
|---|---|---|
| Nrf2 | NM_205117.1 | CGCTTTCTTCAGGGGTAGCA |
| AGTTCGGTGCAGAAGAGGTG | ||
| Keap1 | XM_025145847.1 | CCATCGACTGTTACAACCCCA |
| ATACTCACCTCTCACGCTGC | ||
| HO-1 | NM_205344.1 | GTCGTTGGCAAGAAGCATCC |
| GGGCCTTTTGGGCGATTTTC | ||
| NQO1 | NM_001277619.1 | GAGCGAAGTTCAGCCCAGTA |
| AGAGGTTTTCTCAGGGTGCG | ||
| SOD1 | NM_205064.1 | GAGCGGGCCAGTAAAGGTTA |
| CCCTTTGCAGTCACATTGCC | ||
| SOD2 | NM_204211.1 | TGGGGGTGGCTTGGGTATAA |
| CCCATACATCGATTCCCAGCA | ||
| CAT | NM_001031215.2 | GCCACATGGTGACTACCCTC |
| TGTTGCTAGGGTCATACGCC | ||
| GPX1 | NM_001277853.2 | AGTACATCATCTGGTCGCCG |
| CTCGATGTCGTCCTGCAGTT | ||
| β-actin | NM_205518.1 | TGCTGTGTTCCCATCTATCG |
| TTGGTGACAATACCGTGTTCA |
Abbreviations: CAT, catalase; GPX1, glutathione peroxidase 1; HO-1, heme oxygenase-1; Nrf2, nuclear factor-erythroid 2-related factor 2; NQO1, NAD(P)H quinone dehydrogenase 1; Keap1, kelch-like epichlorohydrin-associated protein 1; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
Statistical Analysis
The data were analyzed by the one-way analysis of variance (ANOVA) under a completely randomized design using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL). The experimental unit was defined as the individual bird from each replicate for measured parameters. The means of all variables among 3 ages were compared with Tukey's multiple range test. The probability (P) values less than 0.05 were considered statistically significant. Results were presented as means with pooled standard errors.
RESULTS
Liver Weight, Serum Biochemical Parameters, and Hepatic Lipid Accumulation
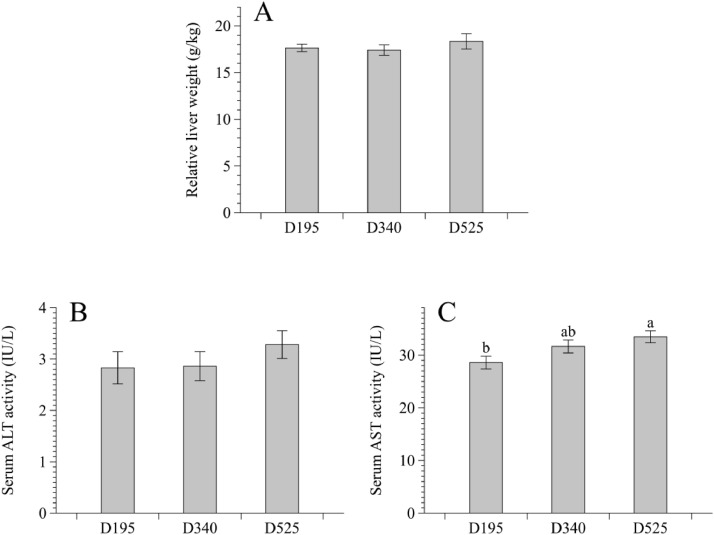
As shown in Figure 1, 525-day-old hens exhibited a higher AST activity in the serum in comparison with their 195-day-old counterparts (P < 0.05). However, age did not affect serum ALT activity and relative liver weight of birds, although there was a numerical increasing trend of these 2 indices in the D525 group compared with other 2 groups (P > 0.05).
Figure 1.
Age-related changes in relative liver weight (A), and serum ALT (B) and AST (C) activities of laying hens. Data are presented as mean with the pooled standard error of each experimental group (8 replicates). abDifferent letters above the error bar indicate significant difference (P < 0.05). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens.
Compared with birds in the D195 group, lower contents of total protein and globulin were observed in the serum of 525-day-old hens (Table 2, P < 0.05), whereas other serum parameters did not differ among 3 groups (P > 0.05). In the liver, triglyceride, and total cholesterol contents were both higher in the D525 group than D195 group (P < 0.05), with the level of D340 group being intermediate among 3 groups (P > 0.05).
Table 2.
Age-related changes in serum biochemical parameters and hepatic lipid accumulation of laying hens.
| Treatments |
|||||
|---|---|---|---|---|---|
| Items | D195 | D340 | D525 | SEM | P-value |
| Serum | |||||
| Albumin (g/L) | 26.44 | 26.00 | 26.51 | 0.291 | 0.756 |
| Globulin (g/L) | 36.74a | 35.56ab | 31.99b | 0.789 | 0.030 |
| Glucose (mmol/L) | 13.03 | 13.11 | 12.81 | 0.347 | 0.943 |
| HDL-C (mmol/L) | 0.78 | 0.77 | 0.66 | 0.056 | 0.657 |
| LDL-C (mmol/L) | 0.20 | 0.21 | 0.24 | 0.020 | 0.794 |
| Total cholesterol (mmol/L) | 2.79 | 3.01 | 3.64 | 0.170 | 0.101 |
| Total protein (g/L) | 63.17a | 61.56ab | 58.49b | 0.806 | 0.047 |
| Triglyceride (mmol/L) | 9.41 | 9.55 | 10.58 | 0.428 | 0.501 |
| Liver | |||||
| Total cholesterol (mmol/g protein) | 0.190b | 0.215ab | 0.242a | 0.008 | 0.021 |
| Triglyceride (mmol/g protein) | 0.398b | 0.424ab | 0.451a | 0.008 | 0.027 |
Abbreviations: D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Mean values within a row with different superscripts letters are significantly different at P < 0.05.
Circulating Hormone Contents
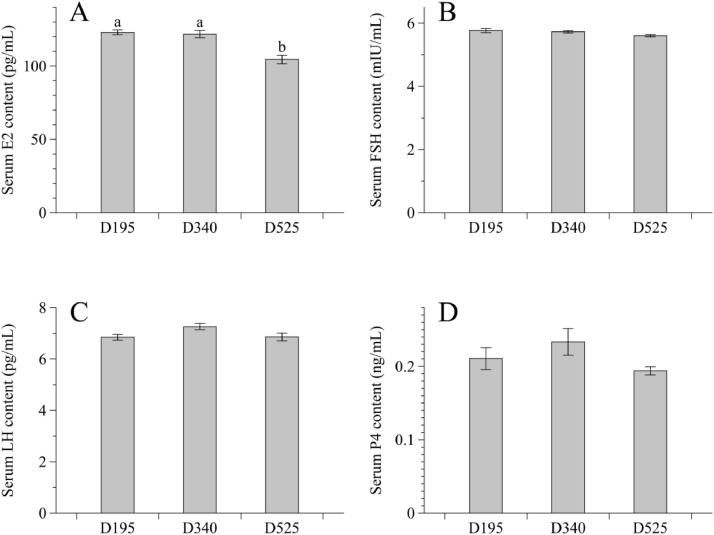
Compared with D195 or D340 group, hens in the D525 group showed a lower E2 concentration in the serum (Figure 2, P < 0.05), whereas no significant difference was observed in the levels of other serum reproductive hormones (follicle stimulating hormone, luteinizing hormone, and progesterone) among 3 ages (P > 0.05).
Figure 2.
Age-related changes in serum hormone concentrations of E2 (A), FSH (B), LH (C), and P4 (D) of laying hens. Data are presented as mean with the pooled standard error of each experimental group (8 replicates). abDifferent letters above the error bar indicate significant difference (P < 0.05). Abbreviations: D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; P4, progesterone.
Serum and Hepatic Antioxidant Capacity
The serum MDA concentration of birds kept constant from D195 to D340 group (Table 3, P > 0.05), whereas it increased significantly in the D525 group (P < 0.05). The 525-day-old hens exhibited a lower serum GSH-Px activity than their 195-day-old counterparts (P < 0.05), with the value of D340 group being intermediate among groups (P > 0.05). Also, there was a trend of numerical decrease in serum total antioxidant capacity from 195-day- to 340-day- or 525-day-old birds (P = 0.055). No significant difference or clear trends were observed in other serum antioxidant-related indices (P > 0.05).
Table 3.
Age-related changes in serum antioxidant capacity of laying hens.
| Treatments |
|||||
|---|---|---|---|---|---|
| Items | D195 | D340 | D525 | SEM | P-value |
| CAT (U/mL) | 1.41 | 1.56 | 1.33 | 0.064 | 0.321 |
| MDA (nmol/mL) | 3.59b | 3.66b | 4.99a | 0.233 | 0.014 |
| GSH (mg/L) | 6.15 | 4.38 | 4.41 | 0.431 | 0.162 |
| GSH-Px (U/mL) | 234.42a | 196.90ab | 178.66b | 7.688 | 0.005 |
| SOD (U/mL) | 253.55 | 260.00 | 244.15 | 6.012 | 0.578 |
| T-AOC (U/mL) | 2.22 | 1.72 | 1.70 | 0.102 | 0.055 |
Abbreviations: CAT, catalase; D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens; GSH, glutathione; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Mean values within a row with different superscripts letters are significantly different at P < 0.05.
For hepatic antioxidant capacity, the birds from D525 group showed a higher MDA level in the liver tissues in comparison with other 2 groups (Table 4, P < 0.05). The oldest laying hens exhibited the lower activities of GSH-Px and SOD in the liver than those birds from D195 group (P < 0.05). However, other antioxidant-related data in the liver were similar among 3 ages (P > 0.05).
Table 4.
Age-related changes in hepatic antioxidant capacity of laying hens.
| Treatments |
|||||
|---|---|---|---|---|---|
| Items | D195 | D340 | D525 | SEM | P-value |
| CAT (U/mg protein) | 22.97 | 22.66 | 22.22 | 0.428 | 0.787 |
| MDA (nmol/mg protein) | 2.00a | 2.25a | 2.39b | 0.057 | 0.012 |
| GSH (mg/g protein) | 26.09 | 24.57 | 24.50 | 0.380 | 0.156 |
| GSH-Px (U/mg protein) | 64.21a | 61.31ab | 59.02b | 0.769 | 0.014 |
| SOD (U/mg protein) | 266.41a | 267.52ab | 256.80b | 1.934 | 0.037 |
| T-AOC (U/mg protein) | 3.56 | 3.36 | 3.33 | 0.057 | 0.200 |
Abbreviations: CAT, catalase; D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens; GSH, glutathione; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; .
Mean values within a row with different superscripts letters are significantly different at P < 0.05.
Hepatic mRNA Abundance
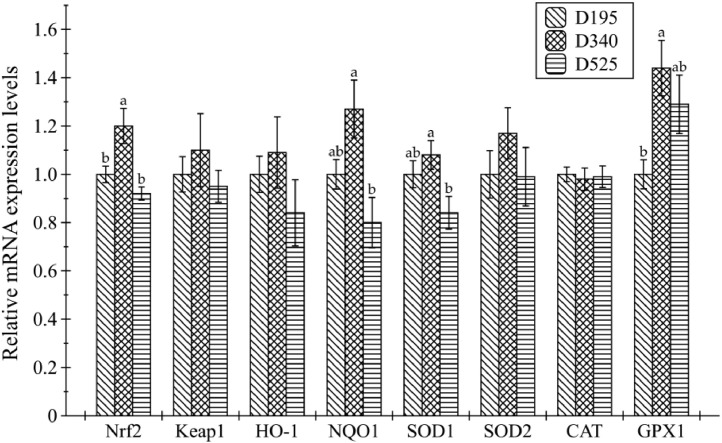
Compared with the youngest group, the mRNA abundance of hepatic Nrf2 and GPX1 increased in the D340 group (Figure 3, P < 0.05). Conversely, birds showed the downregulation of Nrf2, NQO1, and SOD1 mRNA expression in the liver as the age increased from 340-day- to 525-day-old (P < 0.05). However, no significant statistical difference was observed in the expression of other 4 genes, although there was a numerical increase of Keap1, HO-1, and SOD2 mRNA levels in the D340 group when compared with other groups (P > 0.05).
Figure 3.
Age-related changes in mRNA expression levels of genes related to the hepatic antioxidant capacity of laying hens. Data are presented as mean with the pooled standard error of each experimental group (8 replicates). abDifferent letters above the error bar indicate significant difference (P < 0.05). Abbreviations: CAT, catalase; D195, 195-day-old laying hens; D340, 340-day-old laying hens; D525, 525-day-old laying hens; GPX1, glutathione peroxidase 1; HO-1, heme oxygenase-1; Keap1, kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor-erythroid 2-related factor 2; NQO1, NAD(P)H quinone dehydrogenase 1; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
DISCUSSION
In cell and mice models, it has been widely proven that age has a close relationship with metabolism and organ function in accordance with in vitro and in vivo researches (Bonomini et al., 2015; Gong et al., 2017; Allaire and Gilgenkrantz, 2020). In this study, in comparison with younger counterparts, 525-day-old hens exhibited the higher AST activity but lower protein (total protein and globulin) levels in the serum, indicating that aging liver might have been injured (Hanley et al., 2004), and have poor protein synthesis efficiency (Papet et al., 2003). As liver is the primary metabolic organ for laying hens (Li et al., 2015a), these changes may influence the material synthesis and transfer through multiple pathways. Subsequently, we found that aged birds had higher concentrations of triglyceride and cholesterol deposited in their livers. Similarly, a report has demonstrated that aging could promote lipid accumulation in the liver and white adipose tissues via increasing plasma insulin concentrations in senescence-accelerated rodent models (Honma et al., 2011). In accordance with substantial data, the excessive fat in the liver is responsible for the metabolic syndrome and diseases, thereby affecting the growth and productive performance of animals (Kuhla et al., 2011; Moradi et al., 2013; Shini et al., 2019). In addition, the present study indicated that aged hens exhibited a lower concentration of circulating E2, which is a main category of estrogen and secreted from follicles in the ovary of laying hens. This alteration may imply the degenerative status of reproductive organs in older birds. In consistent with our finding, a similar result has been observed by Liu et al. (2018), who also demonstrated that E2 could influence lipid precursor formation by binding to the specific receptors in the liver (Liu et al., 2018). Meanwhile, a report has revealed that E2 can upregulate the expression of long noncoding RNAs, which are associated with hepatic lipid metabolism, especially for triglyceride biosynthesis and transport (Li et al., 2018). Besides, circulating E2 can also act as regulators of antioxidant-related gene expression and redox biology, and the decrease of it might lead to the degenerated antioxidant system of older individuals (Pajovic and Saicic, 2008; Bellanti et al., 2013).
Oxidative stress has been considered as a key factor contributing to the lipid accumulation and liver injury through complex signal transduction pathways (Czaja, 2007; Morita et al., 2012; Li et al., 2015b; Zhao et al., 2020), hence, the age-related antioxidant capacity has also been measured for evaluating the hepatic function of birds in the current study. In the antioxidant defence system, SOD is regarded as the first-line to detoxify superoxide radicals to hydrogen peroxide, which is then converted to water by CAT or to nontoxic hydroxyl compounds by GSH-Px by using glutathione as a redundant (He et al., 2017). Accumulating evidence has suggested that aging can induce lipid peroxidation and protein oxidation, and lead to the delayed production and recovery of antioxidants, suppressing the hepatocyte proliferation, and liver tissue repair (Hanada et al., 2012; Cheng et al., 2017; Tanimizu et al., 2020). In line with these data, the present study also witnessed a decline of several antioxidant enzyme (SOD and GSH-Px) activities with increasing age in both serum and liver tissues. These findings implied that aging layers have regressive antioxidant capacity in response to stress conditions, rendering them more susceptible to the attack from reactive oxygen and free radicals, probably because of the inactive adaptive regulation of redox system (Surai and Kochish, 2019). Also, MDA, a final product of lipid peroxidation, was observed to increase in the serum and liver of birds in the D525 group, further indicating the adverse consequences of aging on the redox biology of laying hens. It was in accordance with a previous research of Coban et al. (2014), who have demonstrated that MDA, diene conjugate, and protein carbonyl all increase in the liver of aged Wistar rats (20-mo-old) compared with young rats (3-mo-old). Besides, as aforementioned, the elevated hepatic lipid deposition observed in this study can also be a possible explanation for the degenerated antioxidant capacity of aged birds (Aikawa et al., 2002). To further verify the effects of age on hepatic redox status in laying hens, the mRNA abundance of associated genes in the liver were also detected in this study. The Nrf2-Keap1 signaling pathway provides an efficient and vital protection against harmful oxidants (Huang et al., 2015). Upon activating the code gene Nrf2, the coordinated genes related to antioxidation and detoxication, such as NQO1, HO-1, SOD1, SOD2, CAT, GPX1, etc., are triggered and transcribed to resist against the oxidative stress (Huang et al., 2002; Jung and Kwak, 2010). In the present study, the relatively lower levels of Nrf2, NQO1, and SOD1 were observed in the D525 group compared with D340 group, and it was parallel with the changes of the associated antioxidant enzymes in the serum and liver, providing a more convincing interpretation for the compromised antioxidant defence system in the aging liver. However, we also saw a rise in the expression of Nrf2 and GPX1 from D195 to D340 group, although there was no difference in the protein levels of antioxidant enzymes between these 2 groups. The upregulated expression of antioxidant genes can be explained by the fact that more severe redox imbalance has occurred with increasing age at an adult stage, and thus more members in the gene reservoir need to be mobilized for counterbalancing the adverse consequences (Wu et al., 2019). With respect to the discrepancy in antioxidant enzymes, it might have something to do with the increase of circulating ROS and protein carbonyl levels at the middle age compared with the younger age, resulting in a higher consumption of antioxidants (Luceri et al., 2018). Additionally, for older (525-day-old) laying hens, our results indicated that they might have lacked the ability of upregulating the associated antioxidant genes, and a similar result has been reported in a previous research (Collins et al., 2009).
In conclusion, the results of this study suggested that age can affect the metabolic process and lead to the impairment of liver function of laying hens, including the increased lipid deposition and degenerated antioxidant capacity from the peak at young age to the late laying stage. These findings will provide a guideline for ameliorating feeding program and management of older laying hens to improve their productive performance.
ACKNOWLEDGMENTS
This study was funded by the earmarked fund for Jiangsu Agricultural Industry Technology System (JATS2020414).
DISCLOSURES
The authors declare no conflict of interest with respect to the authorship and publication of this article.
REFERENCES
- Aikawa M., Sugiyama S., Hill C.C., Voglic S.J., Rabkin E., Fukumoto Y., Schoen F.J., Witztum J.L., Libby P. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- Allaire M., Gilgenkrantz H. The aged liver: beyond cellular senescence. Clin. Res. Hepatol. Gas. 2020;44:6–11. doi: 10.1016/j.clinre.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Bayram B., Nikolai S., Huebbe P., Ozcelik B., Grimm S., Grune T., Frank J., Rimbach G. Biomarkers of oxidative stress, antioxidant defence and inflammation are altered in the senescence-accelerated mouse prone 8. Age. 2013;35:1205–1217. doi: 10.1007/s11357-012-9448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanti F., Matteo M., Rollo T., De Rosario F., Greco P., Vendemiale G., Serviddio G. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol. 2013;1:340–346. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.J., Wang X., Wang B., Zhou H., Dang S.P., Shi Y.F., Hao L., Luo Q.Q., Jin M., Zhou Q.J., Zhang Y.Y. Aging-associated oxidative stress inhibits liver progenitor cell activation in mice. Aging-US. 2017;9:1359–1374. doi: 10.18632/aging.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone N.A., Sharp P.J., Wilson P.W., Dunn I.C. Changes in reproductive neuroendocrine mRNAs with decreasing ovarian function in ageing hens. Gen. Comp. Endocr. 2005;144:20–27. doi: 10.1016/j.ygcen.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Coban J., Oztezcan S., Dogru-Abbasoglu S., Bingul I., Yesil-Mizrak K., Uysal M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr. Gerontol. Int. 2014;14:996–1002. doi: 10.1111/ggi.12192. [DOI] [PubMed] [Google Scholar]
- Collins A.R., Lyon C.J., Xia X.F., Liu J.Z., Tangirala R.K., Yin F., Boyadjian R., Bikineyeva A., Pratico D., Harrison D.G., Hsueh W.A. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ. Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- Czaja M.J. Cell signaling in oxidative stress-induced liver injury. Semin. Liver Dis. 2007;27:378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- Estevez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Gloux A., Duclos M.J., Brionne A., Bourin M., Nys Y., Rehault-Godbert S. Integrative analysis of transcriptomic data related to the liver of laying hens: from physiological basics to newly identified functions. BMC Genomics. 2019;20:821. doi: 10.1186/s12864-019-6185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z.W., Tas E., Yakar S., Muzumdar R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol. Cell Endocrinol. 2017;455:115–130. doi: 10.1016/j.mce.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Gu Y.F., Chen Y.P., Jin R., Wang C., Wen C., Zhou Y.M. A comparison of intestinal integrity, digestive function, and egg quality in laying hens with different ages. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada S., Harada M., Abe M., Akiba J., Sakata M., Kwan R., Taniguchi E., Kawaguchi T., Koga H., Nagata E., Ueno T., Sata M. Aging modulates susceptibility to mouse liver Mallory-Denk body formation. J. Histochem. Cytochem. 2012;60:475–483. doi: 10.1369/0022155412441478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley A.J.G., Williams K., Festa A., Wagenknecht L.E., D'Agostino R.B., Kempf J., Zinman B., Haffner S.M. Elevations in markers of liver injury and risk of type 2 diabetes - the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L.B., Liu T.Y., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Honma T., Yanaka M., Tsuduki T., Ikeda I. Increased lipid accumulation in liver and white adipose tissue in aging in the SAMP10 mouse. J. Nutr. Sci. Vitaminol. 2011;57:123–129. doi: 10.3177/jnsv.57.123. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li W.J., Su Z.Y., Kong A.N.T. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Jia Y.X., Yang M.H., Zhu K.F., Wang L., Song Y.K., Wang J., Qin W.X., Xu Z.Y., Chen Y., Liu G.S. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep.-UK. 2016;6:39799. doi: 10.1038/srep39799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.L., Qi L.N., Dai H.J., Hu C.H., Lv Z.P., Wei Q.W., Shi F.X. Dietary stevioside supplementation improves laying performance and eggshell quality through increasing estrogen synthesis, calcium level and antioxidant capacity of reproductive organs in aged breeder hens. Anim. Feed Sci. Tech. 2020;269 [Google Scholar]
- Jung K.A., Kwak M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla A., Blei T., Jaster R., Vollmar B. Aging is associated with a shift of fatty metabolism toward lipogenesis. J. Gerontol. A-Biol. 2011;66:1192–1200. doi: 10.1093/gerona/glr124. [DOI] [PubMed] [Google Scholar]
- Lee J., Koo N., Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. F. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Li H., Gu Z.Z., Yang L.Y., Tian Y.D., Kang X.T., Liu X.J. Transcriptome profile analysis reveals an estrogen induced lncRNA associated with lipid metabolism and carcass traits in chickens (gallus gallus) Cell. Physiol. Biochem. 2018;50:1638–1658. doi: 10.1159/000494785. [DOI] [PubMed] [Google Scholar]
- Li Y.F., Sugiyama E., Yokoyama S., Jiang L.L., Tanaka N., Aoyama T. Molecular mechanism of age-specific hepatic lipid accumulation in PPAR alpha (+/-): LDLR (+/-) mice, an obese mouse model. Lipids. 2008;43:301–312. doi: 10.1007/s11745-008-3161-x. [DOI] [PubMed] [Google Scholar]
- Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L.X., Wong C.W., Feng Y.B. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang T.A., Xu C.L., Wang D.D., Ren J.X., Li Y.M., Tian Y.D., Wang Y.B., Jiao Y.P., Kang X.T., Liu X.J. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. BMC Genomics. 2015;16:763. doi: 10.1186/s12864-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.T., Lin X., Mi Y.L., Zeng W.D., Zhang C.Q. Age-related changes of yolk precursor formation in the liver of laying hens. J. Zhejiang Univ. Sci. B. 2018;19:390–399. doi: 10.1631/jzus.B1700054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tang J., Feng F.Q. Medium-chain alpha-monoglycerides improves productive performance and egg quality in aged hens associated with gut microbiota modulation. Poult. Sci. 2020;99:7122–7132. doi: 10.1016/j.psj.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luceri C., Bigagli E., Femia A.P., Caderni G., Giovannelli L., Lodovici M. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicol. Rep. 2018;5:141–145. doi: 10.1016/j.toxrep.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi S., Zaghari M., Shivazad M., Osfoori R., Mardi M. The effect of increasing feeding frequency on performance, plasma hormones and metabolites, and hepatic lipid metabolism of broiler breeder hens. Poult. Sci. 2013;92:1227–1237. doi: 10.3382/ps.2012-02483. [DOI] [PubMed] [Google Scholar]
- Morita M., Ishida N., Uchiyama K., Yamaguchi K., Itoh Y., Shichiri M., Yoshida Y., Hagihara Y., Naito Y., Yoshikawa T., Niki E. Fatty liver induced by free radicals and lipid peroxidation. Free Radical Res. 2012;46:758–765. doi: 10.3109/10715762.2012.677840. [DOI] [PubMed] [Google Scholar]
- Pagliassotti M.J., Estrada A.L., Hudson W.M., Wei Y.R., Wang D., Seals D.R., Zigler M.L., LaRocca T.J. Trehalose supplementation reduces hepatic endoplasmic reticulum stress and inflammatory signaling in old mice. J. Nutr. Biochem. 2017;45:15–23. doi: 10.1016/j.jnutbio.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajovic S.B., Saicic Z.S. Modulation of antioxidant enzyme activities by sexual steroid hormones. Physiol. Res. 2008;57:801–811. doi: 10.33549/physiolres.931377. [DOI] [PubMed] [Google Scholar]
- Papet I., Dardevet D., Sornet C., Bechereau F., Prugnaud J., Pouyet C., Obled C. Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J. Nutr. 2003;133:215–219. doi: 10.1093/jn/133.1.215. [DOI] [PubMed] [Google Scholar]
- Peebles E.D., Baseako E.Y., Branton S.L., Whitmarsh S.K., Gerard P.D. Effects of S6-strain Mycoplasma gallisepticum inoculation at 10, 22, or 45 weeks of age on the digestive and reproductive organ characteristics of commercial egg-laying hens. Poult. Sci. 2006;85:825–830. doi: 10.1093/ps/85.5.825. [DOI] [PubMed] [Google Scholar]
- Pinto C., Ninfole E., Gaggiano L., Benedetti A., Marzioni M., Maroni L. Aging and the biological response to liver injury. Semin. Liver Dis. 2020;40:225–232. doi: 10.1055/s-0039-3402033. [DOI] [PubMed] [Google Scholar]
- Seo E., Kang H., Choi H., Choi W., S H. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell. 2019;18:e12895. doi: 10.1111/acel.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I. Nutritional modulation of the antioxidant capacities in poultry: the case of selenium. Poult. Sci. 2019;98:4231–4239. doi: 10.3382/ps/pey406. [DOI] [PubMed] [Google Scholar]
- Sverko V., Sobocanec S., Balog T., Marotti T. Age and gender differences in antioxidant enzyme activity: potential relationship to liver carcinogenesis in male mice. Biogerontology. 2004;5:235–242. doi: 10.1023/B:BGEN.0000038024.58911.6e. [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Ichinohe N., Suzuki H., Mitaka T. Prolonged oxidative stress and delayed tissue repair exacerbate acetaminophen-induced liver injury in aged mice. Aging-US. 2020;12:18907–18927. doi: 10.18632/aging.103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova L., Uhlirova L., Tuma R., Chodova D., Machal L. Age related changes in laying pattern and egg weight of different laying hen genotypes. Anim. Reprod. Sci. 2017;183:21–26. doi: 10.1016/j.anireprosci.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Wang W.W., Wang J., Zhang H.J., Wu S.G., Qi G.H. Transcriptome analysis reveals mechanism underlying the differential intestinal functionality of laying hens in the late phase and peak phase of production. BMC Genomics. 2019;20:970. doi: 10.1186/s12864-019-6320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.B., Xu L.P., Sun X.L., Wan X.H., Sun G.R., Jiang R.R., Li W.T., Tian Y.D., Liu X.J., Kang X.T. Characteristics of the fecal microbiota of high- and low-yield hens and effects of fecal microbiota transplantation on egg production performance. Res. Vet. Sci. 2020;129:164–173. doi: 10.1016/j.rvsc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Wu K.C., Cui J.Y., Liu J., Lu H., Zhong X.B., Klaassen C.D. RNA-Seq provides new insights on the relative mRNA abundance of antioxidant components during mouse liver development. Free Radical Bio. Med. 2019;134:335–342. doi: 10.1016/j.freeradbiomed.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X.L., Wang X.L., Lu Y., Wang E., Zhang Z.J., Yang J., Zhang H.J., Li X.Y. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J. Hepatol. 2014;60:847–854. doi: 10.1016/j.jhep.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Zhao T., Wu K., Hogstrand R.E., Xu Y.H., Chen G.H., Wei C.C., Luo Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPAR gamma pathways. Cell. Mol. Life Sci. 2020;77:1987–2003. doi: 10.1007/s00018-019-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]