Abstract
Adiponectin (ADPN) is related to fatty acid synthesis and oxidation in mammals. In chickens, the lipid metabolism, structure and sequence of ADPN are different from that in mammals. The aim of this study was to determine the role of ADPN in broilers lipid metabolism by investigating the temporal and spatial expression profiles of ADPN and its receptors, as well as their response to feed restriction. The results showed that the abdominal fat has the highest expression level, followed by the duodenum, glandular stomach, heart, hypothalamus, liver, and skeletal muscle. Broilers have high energy mobilization during their early stage of growth, in which the fat demand in the liver and muscles is high, thus the expression of ADPN and its receptor are also increased. To study the effects of feed restriction on ADPN and lipid metabolism, broilers were fasted for 12 h and refeed for 2 h. The results showed that fasting decreased the concentration of triglyceride (TG) (P < 0.05) and total cholesterol (TCHO) (P < 0.05) in plasma. The mRNA expression of ADPN in the liver (P < 0.05), breast (P < 0.05) and thigh (P < 0.05), and the mRNA expression of ADPNR1 in the liver (P < 0.05) and duodenum (P < 0.05) were significantly increased in the Fasted group. All above phenomena were recovered after refeeding, suggesting that feed restriction may promote the utilization of fatty acids in active metabolism tissues through ADPN, to guarantee the energy homeostasis of the body. However, the AMP-activated protein kinase (AMPK) signaling pathway and hepatic lipid metabolism were not necessary to cause the above changes under this experimental condition.
Key words: broiler, lipid metabolism, adiponectin, fasting
INTRODUCTION
Genetic improvement in growth rate and feed efficiency has allowed modern broilers to reach market weight in a shorter period of time, which fully reflects the characteristics of fast growth and large weight of broilers, with huge economic benefits for farmers. However, selection for these economically important traits has been accompanied by an increase in the number of problems encountered during production, including obesity, ascites, sudden death syndrome, and leg abnormalities (Alrubaye, 2013). The increased fat deposition of broilers will reduce feed conversion efficiency and the body's resistance to disease, impact on carcass quality, which will reduce meat production and causing economic losses to processors and consumers. Previous studies showed that feed restriction could decrease the fat deposition and improve the carcass quality of broilers (Santoso, 2001; Nielsen et al., 2003), since it is accompanied by a decrease in growth rate. The negative impact on broiler growth and carcass weight could be counterbalanced partly by appropriate intensity of feed restriction and compensatory growth (Yagoub and Babiker, 2008). Similar findings have been reported as well in mammals (Keogh et al., 2015).
Adiponectin (ADPN) is a protein produced and released primarily by adipocytes into the peripheral blood stream (Sreenivasa et al., 2005). It plays a dominant role in lipid metabolism in mammals (Lee and Shao, 2012; Sena et al., 2017). In chickens, ADPN genes has been isolated, cloned and sequenced (Sreenivasa et al., 2005). Compared with mammals, Sreenivasa et al. (2005) reported that there are 50 to 65% similarities of ADPN cDNA sequences between chickens and mammals. In mammalian adipocytes, the ADPN monomers are organized as oligomers and multimers, they are released in 3 isoforms with distinct molecular weights, denoted as low, medium and heavy isoforms (Tsao et al., 2002, 2003; Waki et al., 2003). Unlike the mammalian ADPN, chicken ADPN expressed in adipose tissue or in plasma is predominantly a heavy molecular weight multimeric (Hendricks et al., 2009). Adiponectin receptor1 (ADPNR1) and adiponectin receptor2 (ADPNR2) are 2 main receptors of ADPN (Yamauchi et al., 2003), they are more conserved in mammals (Ramachandran et al., 2007).
The lipid metabolism in chickens is different from mammals. Modern broilers have the characteristics of high fat deposition and high feed conversion efficiency (Halevy et al., 2000), suggesting chickens have a more refractory insulin cascade and lipid metabolism in skeletal muscle tissues, the study of the chicken model would be beneficial to our understanding of ADPN. Previous studies reported that chicken ADPN mRNA is expressed in various tissues (Sreenivasa et al., 2005; Yuan et al., 2006; Zhang et al., 2017). Overfeeding and obesity decreases blood ADPN concentrations, whereas calorie restriction results in increased ADPN levels and insulin sensitization in rats (Min et al., 2004). Ventricular injection of ADPN can regulate energy metabolism and body weight in mice (Yong et al., 2004). Recent research has suggested that calorie restriction may increase circulating ADPN levels (Rusinov et al., 2018). It's been revealed that nutritional deficiency can trigger increased ATP consumption, resulting in activation of the AMP-activated protein kinase (AMPK) signaling pathway (Choi and Lee, 2013; Wierman et al., 2017). This connotes that AMPK plays a crucial role in the regulation of feed intake during calorie restriction. Several studies have demonstrated the significance of AMPK in the control of glucose and fatty acid metabolism in birds, pointing to its essential role in regulating whole body energy utilization (Andersson et al., 2004; Arsenault et al., 2013, Hu et al., 2015). Also, studies in chickens have found that ADPN regulates fatty acid-induced mitochondrial biosynthesis through AMPK/acetyl CoA carboxylase (ACC) pathway (Gan et al., 2015). Therefore, in the present study, we investigated the role of ADPN in broilers lipid metabolism by examining the temporal and spatial expression profiles of ADPN and its receptors, as well as their response to feed restriction. The involvement of AMPK pathway was further highlighted.
MATERIALS AND METHODS
Animals
One-day-old healthy male broiler chicks (Arbor Acres, Gallus gallus domesticus) were obtained from a local hatchery (Dabao Breeding Technology Co., Ltd., Taian, China). The chicks were managed in environmentally controlled chambers. The brooding temperature was maintained at 32°C for the first 5 d and then gradually reduced 1°C every 3 d until the final temperature was 25°C and this was maintained throughout the experiment. The humidity was maintained at 60 to 70% for the first 2 wk of brooding and then at 50 to 60%. Chicks were fed starter diet (apparent metabolizable energy: 2,897 Kcal/kg; 21% crude protein) until 21 d of age; thereafter, they received a grower diet (apparent metabolizable energy: 3,002 Kcal/kg; 18% crude protein) until the end of the experiment.
The experimental design and setup was reviewed and approved by the Institutional Animal Care and Use Committee of Shandong Agricultural University (No. 2001002) and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China).
Experiment 1: Spatial and Temporal Expression Profiles of ADPN and Its Receptors
The Spatial Expression Profiles of ADPN and Its Receptors
Eight, 42-day-old broilers with similar body weight (BW, 2.42 ± 0.09 kg) were sacrificed by cervical dislocation, followed by exsanguination (Close et al., 1997). The abdominal fat, liver, breast muscle, thigh muscle, heart, glandular stomach, duodenum, and hypothalamus were sampled, snap frozen in liquid nitrogen, and then stored at −80°C for further analysis.
The Temporal Expression Profiles of ADPN and Its Receptors
-
•
Embryonic age: The hatching broilers’ embryos were collected at the 11th, 13th, 15th, 17th, 19th day of embryonic age. Six chicken embryos were collected every time. The fertile eggs were purchased from the same hatchery as the broiler chicks (Dabao Breeding Technology Co., Ltd., Taian, China) and incubated at 37°C under a relative humidity of 60 to 70%. On the sampling day, all live embryos were euthanized. The liver, breast muscle, and thigh muscle were sampled, snap frozen in liquid nitrogen and then stored at −80°C for further analysis.
-
•
Before and after hatch: Ten chickens were selected per week (18E, 0W, 1W, 2W, 3W, 4W, 5W, and 6W) and they were sacrificed by cervical dislocation, followed by exsanguination (Close et al., 1997). The abdominal fat, liver, breast muscle, thigh muscle, heart, glandular stomach, duodenum, and hypothalamus were sampled, snap frozen in liquid nitrogen and then stored at −80°C for further analysis.
Experiment 2: Effect of Feed Restriction on ADPN Expression and Lipid Metabolism
One hundred twenty six, 41-day-old broilers were randomly divided into 3 groups: fed ad libitum as control (Feed), fasted for 12 h (Fast), 2 h re-fed after 10 h feed withdrawal (Refeed), with 6 replicates and 7 chickens each. Chickens had free access to water during the experimental period. Before being sacrificed, a blood sample was drawn from a wing vein and collected in blood vessel of heparin sodium. Plasma samples were obtained after centrifugation at 4,000 rpm for 10 min at 4°C and were stored at −20°C for further analysis. Tissue samples were obtained by the same method as Experiment 1.
Blood Metabolites and ADPN Measurement
Plasma concentration of glucose (GLU, CH0101101, Maccura Biotechnology Co., Sichuan, China), triglycerides (TG, CH0101151, Maccura Biotechnology Co.), and total cholesterol (TCHO, CH010140, Maccura Biotechnology Co.) were measured with automatic biochemistry analyzer (Maccura Biotechnology Co.). Plasma concentration of very low density lipoprotein (VLDL) was measured according to Whitehead and Griffin (1982). The concentration of plasma ADPN was measured with a commercial diagnostic ELISA kit (H179, Jiancheng Bioengineering Institute, Nanjing, China).
Tissue TG, TCHO, and CPT-1 Enzyme Activity Measurement
TG (F001) and TCHO (F002) were measured with commercial diagnostic kits (Jiancheng Bioengineering Institute). The enzyme activity of carnitine palmitoyl transferase-1 (CPT-1) was measured with a commercial diagnostic ELISA kit (H230, Jiancheng Bioengineering Institute).
RNA Isolation and Analysis
The mRNA expression of ADPN and its receptors were determined by real-time PCR. Total RNA of the collected tissues was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA). The quantity and quality of the isolated RNA were determined using a bio-photometer (Eppendorf, Hamburg, Germany) and agarose-gelelectrophoresis, respectively. RT-qPCR analysis was conducted using 20 μL mix consisting of 2 μL the cDNA template (diluted 5 or 10 times), 0.8 μL of each primer, 0.4 μL ROX Reference Dye (50X) and 10 μL TB Green Premix Ex Taq II (Tli RNaseH Plus) (2X) (TaKaRa, Dalian, China) on ABI 7500 Real-Time PCR System (Q5, Applied Biosystems Inc., Carlsbad, CA) using the following parameters: 95°C for 30 s of predenaturation, followed by 40 cycles consisting of denaturation at 95°C for 5 s, annealing and extension at 60°C for 34 s. The expression levels of target genes were normalized with the expression of chicken GAPDH mRNA. The relative amount of mRNA of target gene was calculated according to the 2−ΔΔCT method (Livak and Schmittgen, 2001). Therefore, all gene transcription results are reported as the n-fold difference relative to the calibrator. All of the samples were run in duplicate, the primers were designed to span an intron to avoid genomic DNA contamination. Primers used in this study were designed using Primer 5.0 software and synthesized by Sangon Biotech (Shanghai, P. R. China, Table 1). The specificity of the amplification product was verified by the standard curve and dissolution curve.
Table 1.
Primers for the targeted and reference transcripts.
| Gene | Primer sequence | Accession NO. | Product size (bp) |
|---|---|---|---|
| GAPDH | F:ACATGGCATCCAAGGAGTGAG R:GGGGAGACAGAAGGGAACAGA |
NM-204305.1 | 244 |
| ADPN | F:ACCCAGACACAGATGACCGTT R:GAGCAAGAGCAGAGGTAGGAGT |
NM-206991 | 238 |
| ADPNR1 | F:GGAGAAGGTTGTGTTTGGGATGT R:TGGAGAGGTAGATGAGTCTTGGC |
NM-001031027 | 218 |
| ADPNR2 | F:ACACACAGAGACTGGCAACATC R:CCCAAGAAGAACAATCCAACAACC |
NM-001007854 | 144 |
Abbreviation: ADPN, adiponectin.
Western Blotting Analysis
Tissue samples (0.1 g) were homogenized using 1 mL lysis buffer (Beyotime, Shanghai, China), centrifuged at 4°C, 12,000 g for 10 min. The supernatant (500 uL aliquot) was collected, the protein content was determined using the BCA protein assay kit (Beyotime) according to the manufacturer's protocol. Protein samples (18 µg) were separated using 7.5 to 10% SDS polyacrylamide gels (Bio-Rad, Richmond, CA), transferred onto polyvinylidene fluoride membrane (Millipore, Bilrica, MA) at 200 mA for 2 h. After western transfer, the membranes were incubated with western blocking buffer (Beyotime) for 1 h at room temperature, thereafter membranes were incubated with the primary antibodies at 4°C, with gentle shaking overnight. The primary antibodies used were anti-AMPKα (Cell Signaling Technology, Danvers, MA), anti-CPT-1 (Cell Signaling Technology) and anti-β-actin (Beyotime). Membrane was washed with Tris-buffered saline/Tween buffer for 3 times at 10-min interval, and then incubated with secondary antibodies (HRP-conjugated anti-rabbit or anti-mouse IgG, 1:1,000; Beyotime) for 4 h at 4°C. Afterward, washing was carried out and membranes were visualized by exposing to Hyperfilm ECL (Beyotime). Western blots were developed and quantified using BioSpectrum 810 with VisionWorks LS 7.1 software (UVP LLC, Upland, CA). The band intensity was normalized to the β-actin band within the same sample.
Statistical Analysis
All the values were expressed as the means ± standard error (SEM). All statistical analyses were performed by one-way ANOVA using SAS statistical software (SAS version 8e, SAS Institute, Cary, NC). When the main effect of the treatment was significant, the differences between means were assessed by Student's t test. P < 0.05 was considered as statistically significant. GraphPad Prism software (version 6, GraphPad Software Inc., San Diego, CA) was used for regression analysis between plasma ADPN concentration and abdominal fat rate of broilers.
RESULTS
Spatial Expression Profiles of ADPN and Its Receptors in Broilers
ADPN and its receptors mRNA were highly expressed in the abdominal fat in broilers, followed by the glandular stomach, duodenum, heart, liver, hypothalamus, and skeletal muscle (P < 0.0001; Figure 1).
Figure 1.
The mRNA expression levels of target genes in different tissues of broilers at 42 d. (A) Adiponectin (ADPN), (B) adiponectin receptor 1 (ADPNR1), (C) adiponectin receptor 2 (ADPNR2). Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
Temporal Expression Profiles of ADPN and Its Receptors and Lipid Metabolites in Broilers
With the growth of broilers, plasma ADPN concentration was significantly increased (P < 0.0001; Figure 2A); plasma VLDL concentration was significantly declined (P < 0.0001; Figure 2B); TG concentration was at an unstable state, there were significant difference (P < 0.01) between 3 W and 4 W, while other time point were no difference (P > 0.05; Figure 2C). Abdominal fat rate was significantly increased (P < 0.0001; Figure 2D). Linear regression analysis showed that there was a positive correlation between plasma ADPN and abdominal fat rate in broilers (r = 0.5799; P < 0.0001; Figure 2E).
Figure 2.
The blood metabolities and abdominal fat rate during the development of broilers. The contents of (A) adiponectin (ADPN, mg/L), (B) very low density lipoprotein (VLDL, O. D.), and (C) triglyceride (TG, m mol/L), (D) abdominal fat rate (%). Data were presented as mean ± SEM (n = 10). Different letters mean significant difference, P < 0.05. (E) The relationship between abdominal fat rate (%) and plasma concentration of ADPN (n = 50).
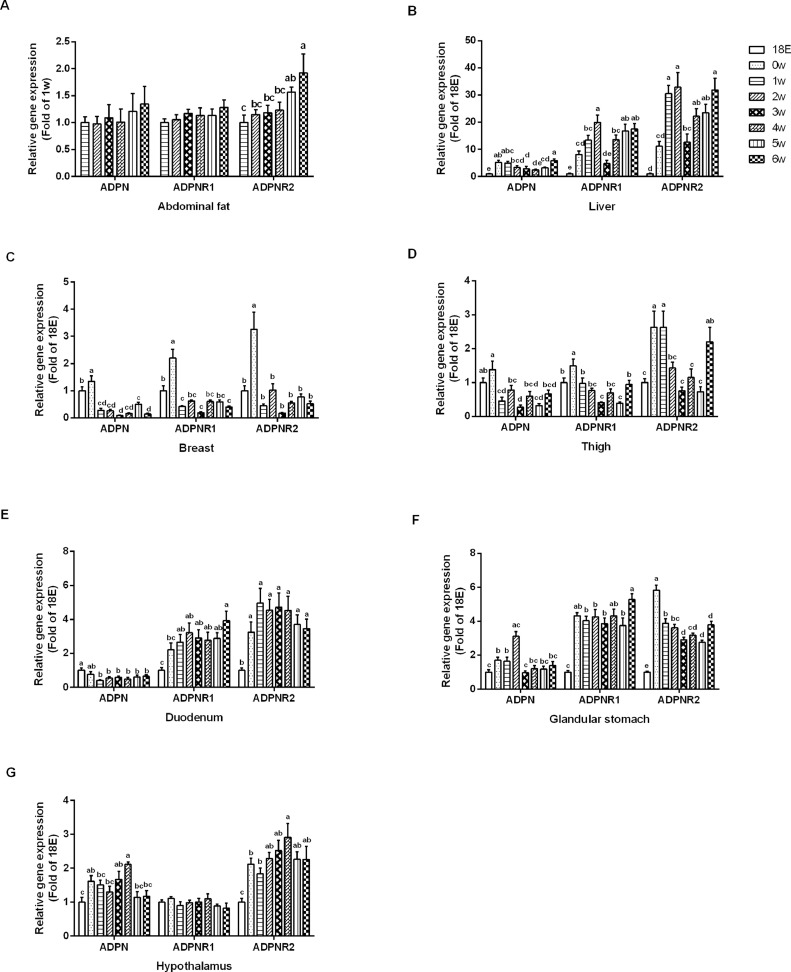
The target genes expressions were evaluated before and after hatch. In the abdominal fat, the mRNAs expression of ADPN and ADPNR1 were not different (P > 0.05), but ADPNR2 was significantly increased (P < 0.01), with the growth of broilers (Figure 3A). The expression levels of ADPN, ADPNR1, and ADPNR2 were unstable (P < 0.0001) in the liver (Figure 3B). These genes increased before hatch, whereas, they decreased after hatch in the skeletal muscles (P < 0.0001; Figures 3C and 3D). In the duodenum, the mRNA expression of ADPN was significantly higher (P < 0.01) before 1 W, it was stable thereafter; The ADPNR1 (P < 0.001) and ADPNR2 (P < 0.001) were lower until hatch and showed no significant difference (P > 0.05) after 1 W (Figure 3E). In the glandular stomach, ADPN expression level was highest at 2 W (P < 0.0001), ADPNR1 (P < 0.0001) and ADPNR2 (P < 0.0001) were significantly increased at 0 W, ADPNR1 was stable after hatch, and ADPNR2 was gradually reduced with the growth of broilers (Figure 3F). In the hypothalamus, the mRNAs expression of ADPN and ADPNR2 were highest at 4 W, it was lowest before hatch (P < 0.01) and there were no differences (P > 0.05) at other age; ADPNR1 did not altered (P > 0.05) with the growth of broilers (Figure 3G).
Figure 3.
The mRNA expression levels of adiponectin (ADPN) and adiponectin receptors (ADPNR1 and ADPNR2) during the development of broilers. (A) Abdominal fat, (B) liver, (C) breast, (D) thigh, (E) duodenum, (F) glandular stomach, and (G) hypothalamus. Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
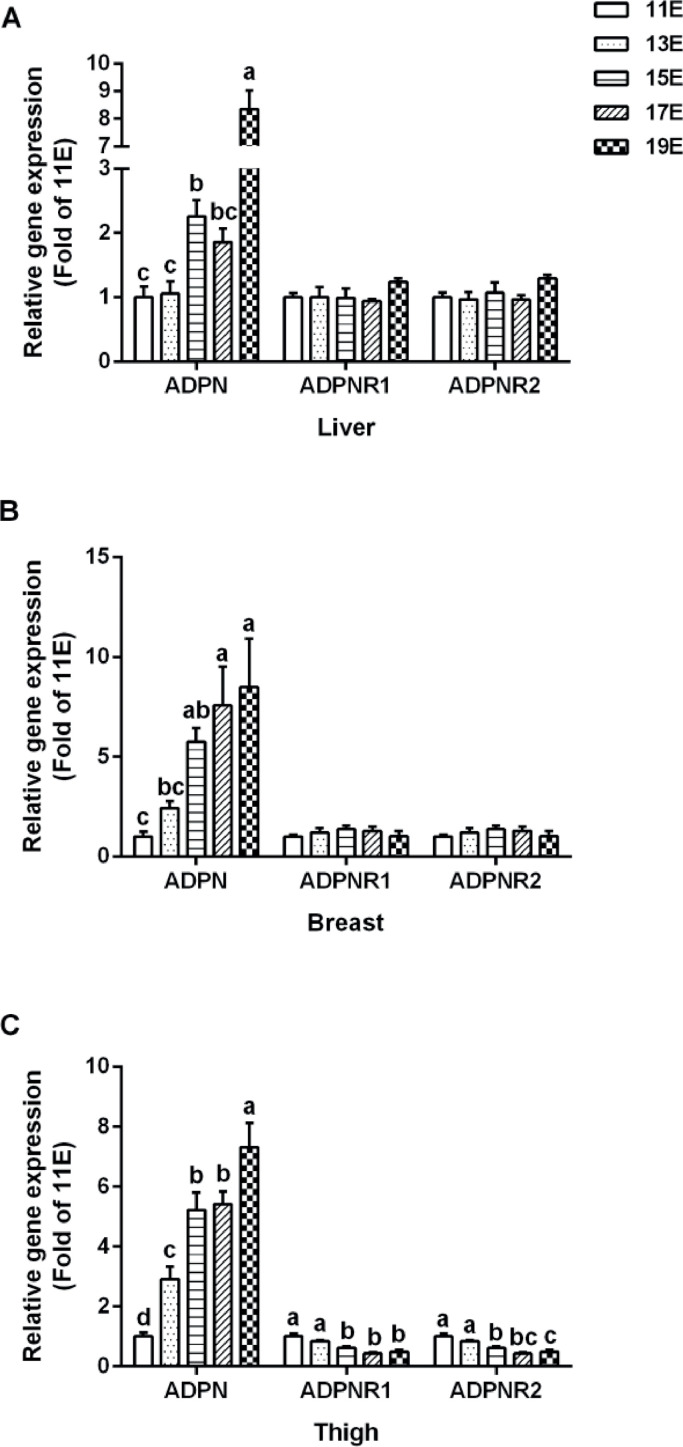
The mRNA expression of ADPN, ADPNR1, and ADPNR2 were determined in liver and skeletal muscle during embryonic age. The expression of ADPN was significantly increased in the liver (P < 0.0001), breast (P < 0.01), and thigh (P < 0.0001) along with the development (Figures 4A–4C). The expression of ADPNR1 and ADPNR2 were not significantly different (P > 0.05) in the liver and breast muscle (Figures 4A and 4B), however, the levels of ADPNR1 (P < 0.0001) and ADPNR2 (P < 0.0001) were significantly decreased along with the development of thigh muscle (Figure 4C).
Figure 4.
The mRNA expression levels of adiponectin (ADPN) and adiponectin receptors (ADPNR1 and ADPNR2) during the development of broilers’ embryo. (A) Liver, (B) breast, and (C) thigh. Data were presented as mean ± SEM (n = 6). Different letters mean significant difference, P < 0.05.
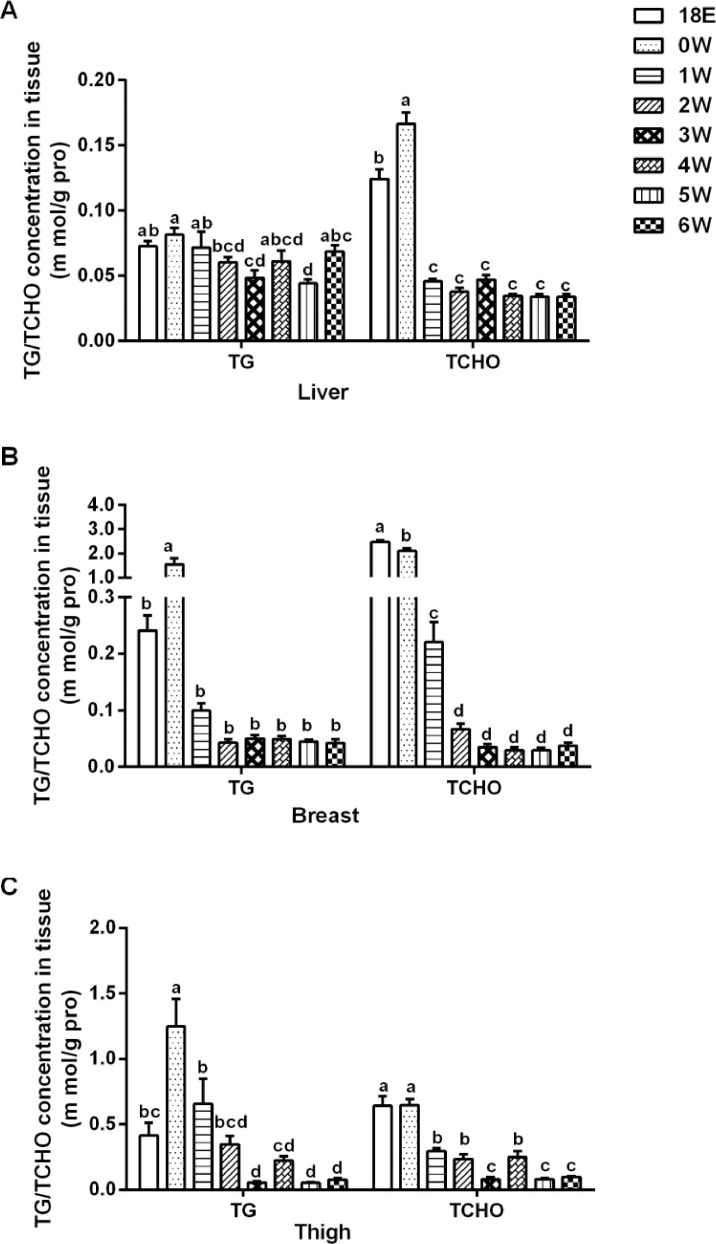
We further measured the content of TG and TCHO in the liver, breast, and thigh muscle before and after hatch. TG and TCHO content in liver were highest at 0W (P < 0.01) and showed a downward trend overall after hatch (Figure 5A). In breast muscle, the concentration of TG was highest (P < 0.0001) at 0 W, there was no significant difference (P > 0.05) after 1 W; the concentration of TCHO was significantly decreased (P < 0.0001) with age, it had no significant difference (P > 0.05) after 2 W (Figure 5B). In thigh muscle, the concentration of TG was significantly higher at 0 W, it was decreased (P < 0.0001) with the growth of broilers afterward (Figure 5C); the concentration of TCHO was significantly higher (P < 0.0001) until 0 W, it was gradually declined afterward (Figure 5C).
Figure 5.
The concentration of triglyceride (TG, m mol/g protein) and total cholesterol (TCHO, m mol/g protein) in tissues during the development of broiler. (A) Liver, (B) breast, and (C) thigh. Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
The Effect of Feed Restriction on ADPN and Its Receptors in Broilers
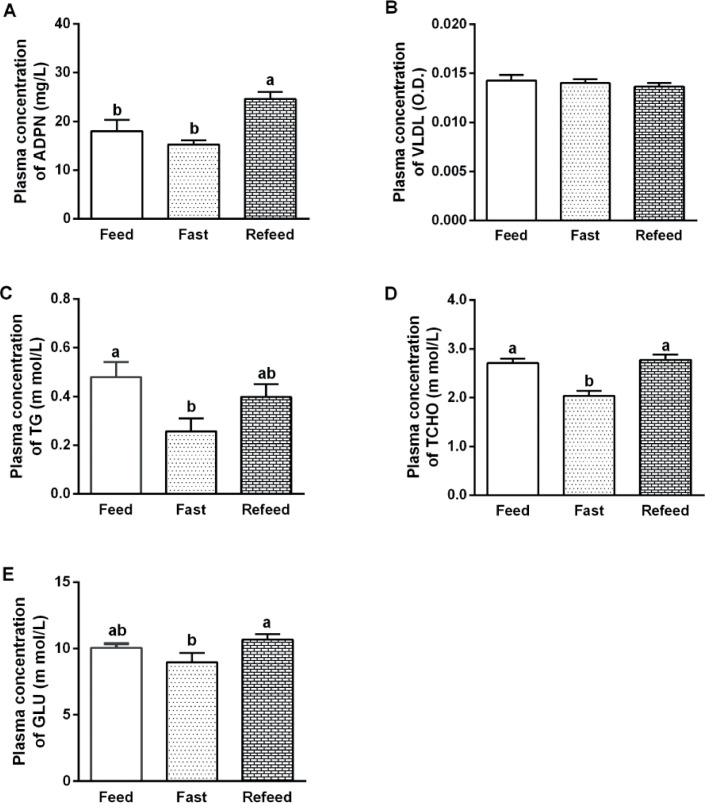
We first investigated the influence of feed restriction on plasma metabolites in broilers. ADPN concentration of Refeed group was significantly higher (P < 0.01) than other groups (Figure 6A). Feed restriction had no effect (P > 0.05) on plasma VLDL content (Figure 6B). Plasma concentration of TG (P < 0.05) and TCHO (P < 0.0001) were decreased after fasting for 12 h and they were recovered after refeeding (Figures 6C and 6D). GLU concentration of Refeed group was higher (P < 0.05) than Fast group, but not changed compared with Feed group (P > 0.05; Figure 6E).
Figure 6.
The effects of feed restriction on blood metabolites. The contents of (A) adiponectin (ADPN, mg/L), (B) very low density lipoprotein (VLDL, O.D.), (C) triglyceride (TG, m mol/L), (D) total cholesterol (TCHO, m mol/L), and (E) glucose (GLU, m mol/L). Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
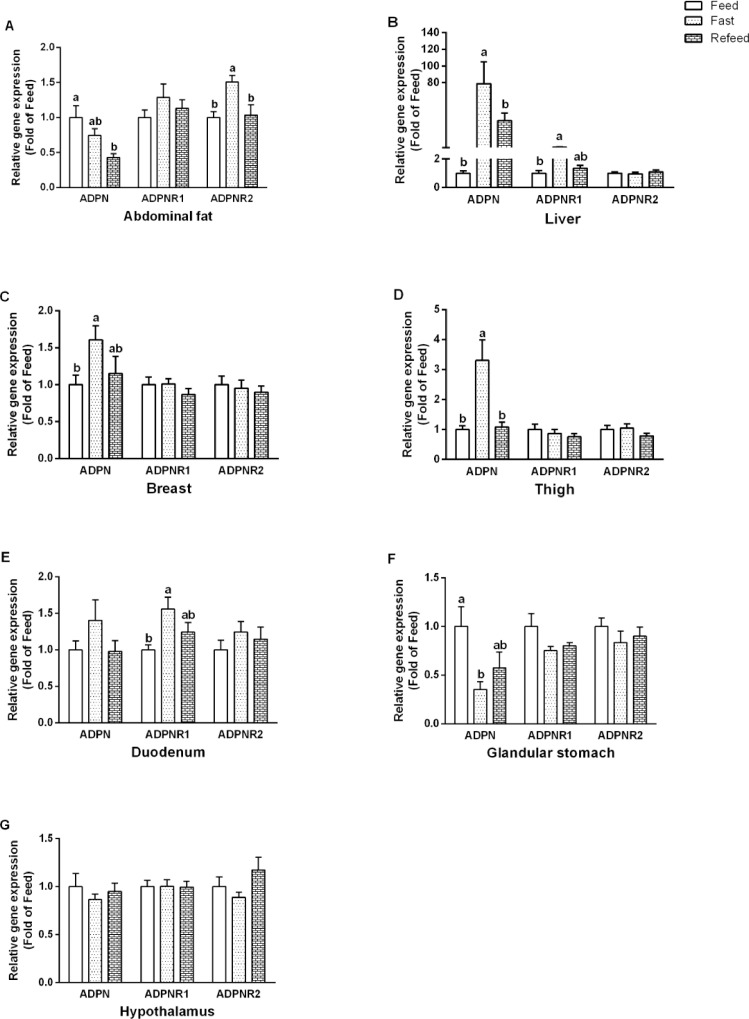
We thereafter examined the effect of feed restriction on mRNA expression of ADPN, ADPNR1, and ADPNR2 in different tissues. In the abdominal fat, the ADPN level was decreased (P < 0.01) in the Refeed group, ADPNR2 level was highest (P < 0.01) in the Fast group (Figure 7A). Fasted chickens had significantly higher (P < 0.01) ADPN mRNA level in the liver. The ADPNR1 levels were significantly upregulated (P < 0.05) in the Fast group compared to the Feed group in the liver and duodenum (Figures 7B and 7E). ADPN expressions in the Fast group were significantly increased (P < 0.05) in muscle tissues (Figures 7C and 7D). In the glandular stomach, ADPN level was significantly decreased (P < 0.05) at the Fast group (Figure 7F). Feed restriction had no effect (P > 0.05) on above 3 genes expression in the hypothalamus (Figure 7G), neither on the mRNAs expressions of ADPNR1 and ADPNR2 in the skeletal muscle and glandular stomach.
Figure 7.
The effects of feed restriction on the mRNA expression levels of adiponectin (ADPN) and adiponectin receptors (ADPNR1 and ADPNR2) in broilers’ tissues. (A) Abdominal fat, (B) liver, (C) breast, (D) thigh, (E) duodenum, (F) glandular stomach, and (G) hypothalamus. Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
The Effect of Feed Restriction on AMPK Signaling Pathway
As Figure 8 A shown, among the seven tissue indexes examined, only liver index decreased significantly (P < 0.05) caused by fasting. The duodenal index also shows a decrease, which largely due to intestinal emptying, however. Therefore, we focused on the liver and further explored the effect of feed restriction on lipid metabolism as well as the involvement AMPK pathway. We found that fasting and refeeding did not cause significant changes (P > 0.05) in TG concentration in the liver (Figure 8B), neither in the enzyme activity and protein of CPT-1 (Figures 8C and 8E). Compared with feed group, the phosphorylation level of AMPK in fasting group increased by 40.65%, but the difference was not significant (P > 0.05; Figure 8D). The absence of significant difference indicated that 12-h feed restriction did not cause significant changes in AMPK signaling pathways.
Figure 8.
The effects of feed restriction on (A) organ indexes of tissues, (B) TG concentration in liver, (C) CPT-1 activity in liver, the protein levels of (D) AMPK and (E) CPT-1 in liver. Data were presented as mean ± SEM (n = 8). Different letters mean significant difference, P < 0.05.
Discussion
ADPN Secretion is Positively Correlated With Fat Deposition in Broilers
ADPN is mainly secreted from adipose tissue in mammals (Hu et al., 1996; Maeda et al., 1996; Yasuko et al., 1996). In the present study in chickens, the mRNAs level of ADPN and its receptors were highest in the abdominal fat, followed by the duodenum and glandular stomach, they were also expressed at the liver and skeletal muscle, as previously reported in chickens (Zhang et al., 2017) and in mammals (Sreenivasa et al., 2005). These results clearly indicated that the ADPN mRNA in broilers was mainly secreted by adipose tissue, acting on other tissues and playing its biological role.
It's well known that fat deposition mainly depends on the available VLDL transported TG (Hermier, 1997; Nielsen et al., 2003; Musa et al., 2006). TG is the major form of stored fat and a principal energy source for vertebrates. When the body has excess lipid content, the lipid will be transformed into TG by a series of enzymes and stored in the adipose tissue (Yang et al., 2009). The blood VLDL concentration was found to be associated with an increased hepatic lipogenesis (Hermier, 1997; Rezaei and Hajati, 2010). The balance between VLDL synthesis and VLDL secretion may be the determinant to regulate fattening in broilers. In the present study, with the growth of broilers, plasma VLDL concentration was significantly decreased and TG concentration was at an unstable state, while the plasma ADPN concentration significantly increased. In accordance with the previous reports (Li et al., 2015), linear regression analysis showed that there was a positive correlation between ADPN in plasma and abdominal fat rate from 3 to 6 wk old in broilers (r = 0.5799; P < 0.0001), but it was oppositely correlated with VLDL. Thus, plasma ADPN is positively related to the fat deposition of broilers and is involved in body lipid metabolism. Adipocytes are divided into preadipocytes and mature adipocytes, the preadipocytes can transform into mature adipocytes (Gregoire et al., 1998). It can be seen that the rate of abdominal fat deposition significantly increased from 1 to 6 wk of age as shown in Figure 2D, indicating that the precursor fat cells differentiate into mature adipocytes. It might be possible that the fat deposition inhibit ADPN by impairing adipocyte differentiation when it reached a certain extent, and after, ADPN mRNA expression and plasma ADPN concentration may decrease. Previous studies showed that ADPN gene may be associated with the initiation and growth processes of adipose tissue deposition in chickens (Hendricks et al., 2009; Tahmoorespur et al., 2010). But Li et al. (2015) showed that ADPN mRNA level was negatively correlated with abdominal fat deposition from 7 to 8 wk old. Zhang et al. (2017) showed ADPN mRNA was decreased in adipose tissue after 81 d in Tibetan chicken. Combined with the above results, we speculated that the secretion of ADPN might be related to age. However in this study, we didn't measure the abdominal fat after 6 wk of age, so more studies are needed to confirm this hypothesis in future. In mammals, abdominal fat rate was negatively correlated with plasma ADPN levels, possibly because they did cross-sectional studies and all experimental animals were under the same growth state (Yong et al., 2004; Vendrell et al., 2012; Mohammadpour et al., 2020). We conducted a longitudinal study on the relationship of ADPN and abdominal fat rate with growing.
ADPN Plays a More Important Role in Regulating Lipid Metabolism at the Early Stage of Broilers
In the present study, ADPNR2 expression in abdominal fat increased with age, while in other tissues, mRNAs expressions of ADPN and its receptors were higher at the early stage after birth. We then examined the expressions of ADPN and its receptors in tissues with high fat metabolism (liver and muscles) during the embryonic period and found that ADPN expression was increased with the growth of the embryo. These results were in line with previous work (Li et al., 2010), which showed that intramuscular fat deposition was fastest around hatch. We thus speculate that the expressions of ADPN and its receptors were gradually increased during the embryonic stage; they were significantly higher at the early growth stages after hatching. During the late embryonic age and early birth age, energy mobilization, and consumption increase and fatty acid oxidation enhances gradually, so ADPN may play an important role in promoting fatty acid oxidation.
Fatty acids are mainly formed in adipose tissue in mammals; however, the main site of fatty acid synthesis is the liver in chickens (Bedu et al., 2002). In the present study, the concentrations of TG and TCHO in the liver were significantly higher around hatch, and there were no differences after 2 W. In tissues with active lipid metabolism including breast and thigh muscles, TG, and TCHO patterns were largely consistent with liver. Previous work of mammals showed that adipocytes in the skeletal muscle of cattle began to proliferate in the middle of embryonic age, and completed until the early stage of growth (Du and Dreyfus, 2010). The changes in intramuscular fat during embryonic age to early stage of growth have great influence on the deposition ability of intramuscular fat in later stage (Du and Dreyfus, 2010). Some studies have found that intramuscular fat is highest at 1 d of age in poultry ducks (Chartrin and Baéza, 2007), which is in line with the present study. Also, we found that the expressions of ADPN and its receptors in the liver and skeletal muscle were largely consistent with the TG and TCHO content in these tissues. The results suggested that at the early growth stage of broilers, the body has a higher energy mobilization; the liver and skeletal muscle have a greater demand for fat, which requires higher expression levels of ADPN and its receptors. ADPN can not only promote fatty acid oxidation, but also inhibit fat synthesis (Lee and Shao, 2012). Previous studies showed that during chickens’ embryonic period, lipid serves as the primary energy source, whereas at hatch, the main metabolic adaptations is carbohydrate utilization over the first 2 wk post-hatch (Noy and Sklan, 1999; Sklan and Noy, 2000; Zhang and Hillgartner, 2004). Thus, ANPN and its receptors will promote energy homeostasis and they will mobilize or deposit as needed.
Feed Restriction Affects ADPN mRNA Expression and Lipid Metabolism
Changing birds’ diet by altering the energy levels or feeding time can modify the endocrine and blood metabolite patterns (Hornick et al., 2000). Fasting is usually employed to limit fat deposition in broilers and to improve feed conversion (Savory et al., 2006; Boostani et al., 2010; Rezaeipor et al., 2010). Herein, we investigated the changes in energy and lipid metabolites of birds’ plasma subjected to 12-h feed restriction followed by 2-h refeeding. Results showed that VLDL concentration was not changed by feed restriction. However, the content of TG, TCHO, and GLU were decreased after fasting, and restored after refeeding, which were in accordance with the results of previous studies (Song et al., 2012; Hu et al., 2015; Song et al., 2018). This can be attributed to the acute feed restriction enhanced lipid oxidation in an attempt to meet energy needs, eliciting negative effects on the carcass fat content (Jahanpour et al., 2013). In the present study, the plasma concentration of ADPN showed no significant change after fasting for 12 h. Similar findings had also been reported in mammals, which showed that acute food deprivation for 48 h had no effect on serum concentration of ADPN in rats (Zhang et al., 2002).
Consistent with the studies in rats fasted for 24 h (Bertile and Raclot, 2004) and in laying hens fasted for 48 h (Sreenivasa et al., 2005), we observed that the ADPN mRNA expression was downregulated by feed restriction in the abdominal fat of broilers. However, ADPN in the liver and skeletal muscle, and ADPNR1 in liver and duodenum were all upregulated after fasting, indicating that 12-h fasting resulted in energy deficiency, which caused the response of ADPN. Moreover, the above genes were restored after refeeding. In line with our findings, it has been reported that circulating ADPN was increased after 60% calorie restriction for 4 mo in mice, and the fat will be reduced to maintain the body's energy balance during calorie restriction (Berg et al., 2001). Contrasting with our findings, it has been reported that ADPN mRNA expression decreased after 48-h fasting in the adipose tissue, liver, and the anterior pituitary gland but not in the diencephalon (Sreenivasa et al., 2005). The decrease of ADPN mRNA expression in the white adipose tissue caused by short-term fasting and the increase of serum ADPN with long-term calorie restriction may be due to the duration and severity of the dietary restriction. The energy utilization during fasting is mainly divided into 3 stages, first, the depletion of carbohydrate reserves; second, mobilization of lipids; finally, breakdown of protein (Cuendet et al., 1975; Goodman et al., 1980). The 12-h fasting in the present study maybe at the second stage of energy utilization, lipids are mobilized to provide the energy needed for body, ADPN and its receptors are involved in this stage, fatty acid oxidation function was enhanced in the internal organs (liver, muscle, and duodenum) leading to increase the ADPN mRNA expression. In the second stage, the digestive function of the glandular stomach is low, which resulted in a decline in ADPN mRNA expression. After refeeding, the glandular stomach began to accelerate digestion and tissue oxidation, which may lead to increase of ADPN mRNA expression. It's not obvious that there is any impact of fasting on the abdominal fat ADPN mRNA expression, maybe because fatty acid oxidation in the second stage mainly occurs in visceral fat than abdominal fat. It is also possible that a decrease in ADPN mRNA expression need not necessarily mean a decrease in ADPN protein concentration in abdominal fat or other tissues. Previously, such an unparalleled gene expression and protein expression level have been reported for leptin (Ranganathan et al., 1998). Further research is needed to understand whether ADPN protein has changed in chickens by fasting and refeeding.
Among the seven tissue indexes examined in Experiment 2, only liver index decreased significantly caused by fasting. The duodenal index also shows a decrease, which largely due to intestinal emptying, however. As the main site of fatty acid synthesis in poultry, liver plays an important role in lipid metabolism and energy regulation of body (Bedu et al., 2002). Therefore, we focused on the liver and further explored the effect of feed restriction on lipid metabolism as well as the involvement AMPK pathway. The results showed that the TG concentration in liver did not change significantly with feed restriction, and although AMPK phosphorylation increased by 40.65% in the Fast group compared with Feed chickens, there was no statistical difference in protein phosphorylation of AMPK and CPT-1, or in CPT-1 activity. These results indicated that fasting did not obviously affect AMPK signaling pathway. Likewise, in others reports, fasting for 24 h and calorie restriction for 4 mo did not activate AMPK pathway in the heart, liver, and muscle in rats (Gonzalez et al., 2004). This results indicated that activation of AMPK is not a necessary pathway for the liver to respond to the complex metabolic caused by 12-h fasting. The most likely explanation for this result is the reduction of GLU, TG, TCHO induced by 12-h fasting was not sufficient to cause a decrease in intracellular ATP/AMP ratios and initiate an increase in AMPK activity.
In conclusion, ADPN is closely related to lipid metabolism and energy regulation of broilers. Twelve-hour feed restriction causes energy deficiency in the body, leading to the upregulation of ADPN and its receptors expression in liver, muscle, and gut. AMPK is not involved in this short-time feed restriction.
ACKNOWLEDGMENTS
This research was funded by the National Natural Science Foundation of China, grant number 31672441, the National Key Research and Development Program of China, grant number 2018YFE0128200, the Key Technology Research and Development Program of Shandong province, grant number 2019JZZY020602, the Taishan Scholars Program, grant number 201511023, and the Funds of Shandong “Double Tops” Program.
DISCLOSURES
The authors have declared no conflicts of interest.
REFERENCES
- Alrubaye, A. 2013. Molecular Diagnosis of Metabolic Fast Growth Related Diseases in Broiler. PhD Diss. Univ. Arkansas, Fayetteville, AR.
- Andersson U., Filipsson K., Abbott C.R., Woods A., Smith K., Bloom S.R., Carling D., Small C.J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Napper S., Kogut M.H. Salmonella enterica Typhimurium infection causes metabolic changes in chicken muscle involving AMPK, fatty acid and insulin/mTOR signaling. Vet. Res. 2013;44:35–50. doi: 10.1186/1297-9716-44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedu E., Chainier F., Sibille B., Meister R., Dalleve G., Garin D., Duchamp C. Increased lipogenesis in isolated hepatocytes from cold-acclimated ducklings. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1245–R1253. doi: 10.1152/ajpregu.00681.2001. [DOI] [PubMed] [Google Scholar]
- Berg A.H., Combs T.P., Du X., Brownl Ee M., Scherer P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bertile F., Raclot T. Differences in mRNA expression of adipocyte-derived factors in response to fasting, refeeding and leptin. Biophys. Acta Mol. Cell Biol. Lipids. 2004;1683:101–109. doi: 10.1016/j.bbalip.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Boostani A., Ashayerizadeh A., Fard H.R., Kamalzadeh A. Comparison of the effects of several feed restriction periods to control ascites on performance, carcass characteristics and hematological indices of broiler chickens. Braz. J. Poult. Sci. 2010;12:170–177. [Google Scholar]
- Chartrin P., Baéza E. Effects of age on lipid deposition in breast muscle of mule ducks. J. Anim. Sci. 2007;342:134–140. doi: 10.1017/S1751731107658029. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Lee C.K. Maintenance of cellular ATP level by caloric restriction correlates chronological survival of budding yeast. Biochem. Biophys. Res. Commun. 2013;439:126–131. doi: 10.1016/j.bbrc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Close B., Banister K., Baumans V., Bernoth E.M., Bromage N., Bunyan J., Erhardt W., Flecknell P., Gregory N., Hackbarth H., Morton D., Warwick C. Recommendations for euthanasia of experimental animals: part 2. DGXT of the European Commission. Lab Anim. 1997;31:293–316. doi: 10.1258/002367797780600297. [DOI] [PubMed] [Google Scholar]
- Cuendet G.S., Loten E.G., Cameron D.P., Renold A.E., Marliss E.B. Hormone substrate responses to total fasting in lean and obese mice. Am. J. Physiol. 1975;228:276–283. doi: 10.1152/ajplegacy.1975.228.1.276. [DOI] [PubMed] [Google Scholar]
- Du Y., Dreyfus C.F. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 2010;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- Gan L., Yan J., Liu Z., Feng M., Sun C. Adiponectin prevents reduction of lipid-induced mitochondrial biogenesis via AMPK/ACC2 pathway in chicken adipocyte. J. Cell. Biochem. 2015;116:1090–1100. doi: 10.1002/jcb.25064. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.A, Kumar R., Mulligan J.D., Davis A.J., Saupe K.W. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Goodman M.N., Larsen P.R., Kaplan M.M., Aoki T.T., Young V.R., Ruderman N.B. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am. J. Physiol. 1980;239:E277–E286. doi: 10.1152/ajpendo.1980.239.4.E277. [DOI] [PubMed] [Google Scholar]
- Gregoire F.M., Smas C.M., Sook S.H. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Halevy O., Geyra A., Barak M., Uni Z., Sklan D. Early posthatch starvation decreases statellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- Hendricks G.L., Hadley J.A., Krzysik-Walker S.M., Sandeep P.K., Regina V.Y., Ramesh R. Unique profile of chicken adiponectin, a predominantly heavy molecular weight multimer, and relationship to visceral adiposity. Endocrinology. 2009;150:3092–3100. doi: 10.1210/en.2008-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermier D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997;127:805S–808S. doi: 10.1093/jn/127.5.805S. [DOI] [PubMed] [Google Scholar]
- Hornick J.L., Eenaeme C.V., Gérardx O., Dufrasne I., Istasse L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000;19:121–132. doi: 10.1016/s0739-7240(00)00072-2. [DOI] [PubMed] [Google Scholar]
- Hu E., Liang P., Spieglman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hu X., Liu L., Song Z., Sheikhahmadi A., Wang Y., Buyse J. Effects of feed deprivation on the AMPK signaling pathway in skeletal muscle of broiler chickens. Physiol. Part B Biochem. Mol. Biol. 2015;191:146–154. doi: 10.1016/j.cbpb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Jahanpour H., Seidavi A., Qotbi A.A.A., Carreira R.P. Effects of two levels of quantitative feed restriction for a 7- or 14- days period on broilers blood parameters. Acta Sci. Vet. 2013;41:24. [Google Scholar]
- Keogh K., Water S.M., Kelly A.K., Kenny D.A. Feed restriction and realimentation in Holstein–Friesian bulls: II. Effect on blood pressure and systemic concentrations of metabolites and metabolic hormones. J. Anim. Sci. 2015;93:3590–3601. doi: 10.2527/jas.2014-8471. [DOI] [PubMed] [Google Scholar]
- Lee B., Shao J. Adiponectin and lipid metabolism in skeletal muscle. Acta Pharm. Sin. B. 2012;2:335–340. [Google Scholar]
- Li H., Gilbert E.R., Zhang Y., Crasta O., Emmerson D., Jr K., Wong E.A. Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages. Anim. Genet. 2010;39:407–424. doi: 10.1111/j.1365-2052.2008.01744.x. [DOI] [PubMed] [Google Scholar]
- Li S., Wang H., Cong L. AA rouji zhiliansu jiyin baoda yu pixia zhifang he fuqiangnei zhifang hanliang de guanxi. [Expression of adiponectin gene in relation to subcutaneous fat and intraperitoneal fat content in AA broilers] Siliao Yanjiu. 2015;2015:6–8. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Min Z., Miura J., Lu L.X., Bernier M., Decabo R., Lane M.A., Roth G.S., Ingram D.K. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp. Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Mohammadpour F., Darmani-Kuhi H., Mohit A., Sohani M.M. Obesity, insulin resistance, adiponectin and PPAR-γ gene expression in broiler chicks fed diets supplemented with fat and green tea (Camellia sinensis) extract. Domest. Anim. Endocrinol. 2020;20 doi: 10.1016/j.domaniend.2020.106440. [DOI] [PubMed] [Google Scholar]
- Musa H.H., Chen G.H., Wang K.H., Li B.C., Mekki D., Shu J.T., Ju H.P. Relation between serum cholesterol level, lipoprotein concentration and carcass characteristics in genetically lean and fat chicken breeds. J. Biol. Sci. 2006;6:616–620. [Google Scholar]
- Nielsen B.L., Litherland M., Flemming N. Effects of qualitative and quantitative feed restriction on the activity of broiler chickens. Appl. Anim. Behav. Sci. 2003;83:309–323. [Google Scholar]
- Noy Y., Sklan D. Energy utilization in newly hatched chicks. Poult. Sci. 1999;78:1750–1756. doi: 10.1093/ps/78.12.1750. [DOI] [PubMed] [Google Scholar]
- Ramachandran R., Ocon-Grove O., Metzger S.L. Molecular cloning and tissue expression of chicken AdipoR1 and AdipoR2 complementary deoxyribonucleic acids. Domest. Anim. Endocrinol. 2007;33:19–31. doi: 10.1016/j.domaniend.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Ranganathan S., Maffei M., Kern P.A. Adipose tissue ob mRNA expression in humans: discordance with plasma leptin and relationship with adipose TNFα expression. J. Lipid. Res. 1998;39:724–730. [PubMed] [Google Scholar]
- Rezaei M., Hajati H. Effect of diet dilution at early age on performance, carcass characteristics and blood parameters of broiler chicks. Ital. J. Anim. Sci. 2010;9:93–100. [Google Scholar]
- Rezaeipor V., Palangi M., Irani M. Effect of early feed restriction with or without enzyme supplementation on performance, nutrients digestibility and blood biochemical parameters of broiler chickens. J. Anim. Vet. Adv. 2010;11:622–626. [Google Scholar]
- Rusinov I.S., Ershova A.S., Karyaginax A.S., Spirin S.A., Alexeevski A.V. Avoidance of recognition sites of restriction-modification systems is a widespread but not universal anti-restriction strategy of prokaryotic viruses. BMC Genomics. 2018;19:885–896. doi: 10.1186/s12864-018-5324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso U. Effects of early feed restriction on growth, fat accumulation and meat composition in unsexed broiler chickens. Asian-Australas. J. Anim. Sci. 2001;14:1585–1591. [Google Scholar]
- Savory C.J., Kostal L., Nevison I.M. Circadian variation in heart rate, blood pressure, body temperature and EEG of immature broiler breeder chickens in restricted-fed and ad libitum-fed states. Br. Poult. Sci. 2006;47:599–606. doi: 10.1080/00071660600939719. [DOI] [PubMed] [Google Scholar]
- Sena C.M., Pereira A., Fernandes R., Letra L. Adi-ponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br. J. Pharmacol. 2017;174:3514–3526. doi: 10.1111/bph.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan D., Noy Y. Hydrolysis and absorption in the small intestines of posthatch chicks. Poult. Sci. 2000;79:1306–1310. doi: 10.1093/ps/79.9.1306. [DOI] [PubMed] [Google Scholar]
- Song X., Jiao H., Zhao J., Wang X., Lin H. Ghrelin serves as a signal of energy utilization and is involved in maintaining energy homeostasis in broilers. Gen. Comp. Endocrinol. 2018;268:14–21. doi: 10.1016/j.ygcen.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Song Z., Liu L., Yue Y., Jiao H., Lin H., Sheikhahmadi A., Everaert N., Decuypere E., Buyse J. Fasting alters protein expression of AMP-activated protein kinase in the hypothalamus of broiler chicks (Gallus gallus domesticus) Gen. Comp. Endocrinol. 2012;178:546–555. doi: 10.1016/j.ygcen.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Sreenivasa M., Shana M., Olga O.n., Gilbert H., Ramesh R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–4256. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- Tahmoorespur M., Ghazanfari S., Nobari K. Evaluation of adiponectin gene expression in the abdominal adipose tissue of broiler chickens: feed restriction, dietary energy, and protein influences adiponectin messenger ribonucleic acid expression. Poult. Sci. 2010;89:2092–2100. doi: 10.3382/ps.2010-00772. [DOI] [PubMed] [Google Scholar]
- Tsao T.S., Murrey H.E., Hug C., Lee D.H., Lodish H.F. Oligomerization state-dependent activation of nf-kb signaling pathway by adipocyte complement-related protein of 30 kda (acrp30) J. Biol. Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- Tsao T.S., Tomas E., Murrey H.E., Hug C., Lee D.H., Ruderma N.B., Heuser J.E., Lodish H.F. Role of disulfide bonds in acrp30/adiponectin structure and signaling specificity: different oligomers activate different signal transduction pathways. J. Biol. Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- Vendrell J., Broch M., Vilarrasa N., Molina A., Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes. Res. 2012;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- Waki H., Yamauchi T., Kamon J., Ito Y., Uchida S., Kita S., Hara K., Hada Y., Vasseur F., Froguel P., Kimura S., Nagai R., Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Griffin H.D. Plasma lipoprotein concentration as an indicator of fatness in broilers: effect of age and diet. Br. Poult. Sci. 1982;23:299–305. doi: 10.1080/00071688208447961. [DOI] [PubMed] [Google Scholar]
- Wierman M.B., Maqani N., Strickler E., Li M., Smith J.S. Caloric restriction extends yeast chronological lifespan by optimizing the Snf1 (AMPK) signaling pathway. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00562-16. E00562-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoub Y.M., Babiker S.A. Effect of compensatory growth on the performance and carcass characteristics of the broiler chicks. Pak. J. Nutr. 2008;7:497–499. [Google Scholar]
- Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yang Y.X., Guo J., Yoon S.Y., Jin Z., Choi J.Y., Piao X.S., Kim B.W., Ohh S.J., Wang M.H., Chae B.J. Early energy and protein reduction: effects on growth, blood profiles and expression of genes related to protein and fat metabolism in broilers. Br. Poult. Sci. 2009;50:218–227. doi: 10.1080/00071660902736706. [DOI] [PubMed] [Google Scholar]
- Yasuko N., Takashi T., Nam-Ho C.M., Toshio M., Motowo T. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- Yong Q., Takahashi N., Hileman S.M., Patel H.R., Berg A.H., Pajvani U.B., Scherer P.E., Ahima R.S. Adiponectin acts in the brain to decrease weight. Nat. Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Yuan J., Liu W., Liu Z.L., Li N. cDNA cloning, genomic structure, chromosomal mapping and expression analysis of ADIPOQ (adiponectin) in chicken. Cytogenet. Genome. Res. 2006;112:148–151. doi: 10.1159/000087527. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hillgartner F.B. Starvation and feeding a high-carbohydrate, low-fat diet regulate the expression sterol regulatory element-binding protein-1 in chickens. J. Nutr. 2004;134:2205–2210. doi: 10.1093/jn/134.9.2205. [DOI] [PubMed] [Google Scholar]
- Zhang R., Lin Y., Zhi L., Liao H., Zuo L., Li Z., Xu Y.O. Expression profiles and associations of adiponectin and adiponectin receptors with intramuscular fat in Tibetan chicken. Br. Poult. Sci. 2017;58:151–157. doi: 10.1080/00071668.2016.1268252. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Matheny M., Zolotukhin S., Tumer N., Scarpace P.J. Regulation of adiponectin and leptin gene expression in white and brown adipose tissues: influence of beta 3-adrenergic agonists, retinoic acid, leptin and fasting. Biochim. Biophys. Acta. 2002;1584:115–122. doi: 10.1016/s1388-1981(02)00298-6. [DOI] [PubMed] [Google Scholar]