Abstract
Certain cancer therapy has been shown to induce immunogenic cell death in cancer cells and may promote tumor progression instead. The external stress or stimuli may induce cell death and contribute toward the secretion of pro inflammatory molecules. The release of damage-associated molecular patterns (DAMPs) upon induction of therapy or cell death has been shown to induce an inflammatory response. Nevertheless, the mechanism as to how the DAMPs are released and engage in such activity needs further in-depth investigation. Interestingly, some studies have shown that DAMPs can be released through extracellular vesicles (EVs) and can bind to receptors such as toll-like receptors (TCRs). Ample pre-clinical studies have shown that cancer-derived EVs are able to modulate immune responses within the tumor microenvironment. However, the information on the presence of such DAMPs within EVs is still elusive. Therefore, this mini-review attempts to summarize and appraise studies that have shown the presence of DAMPs within cancer-EVs and how it affects the downstream cellular process.
Keywords: exosome, TLR, PRR, tumor microenvironment, cancer
Introduction
Cancer has emerged as a significant issue globally, and it is now one of the main causes of mortality (1). The tumor microenvironment is heterogeneous consisting of cancer cells, stromal tissue, and the extracellular matrix (2, 3). Over the last few decades, the complex interaction between cancer cells and the host immune response has been extensively studied. The immune system plays a critical role in the tumor microenvironment, such as affecting cancer development and progression. One of the ways of eliminating cancer cells is by undergoing therapy such as chemotherapy, radiotherapy, or targeted therapy. Although some of these modalities have been proven effective, the after-effects of therapy may cause immunogenic cell death and eventually inflammation (4–7).
Cells undergoing cell death will secrete certain molecules into the environment that are immune-stimulating and may induce further inflammation (8–11). To survive, cells have a detection system that can sense possible danger and threats in their environment. In 1994, Matzinger (12) proposed the “danger” theory that cells can recognize and destruct danger when it is presented upon them without the need to distinguish self and non-self-threats (12). During an insult or intrusion, cells will release these endogenous molecules from within their compartment that is called damage-associated molecular patterns or DAMPs to alert the immune system (10, 13, 14). To note, PAMPs or known as the microbial pathogen-associated molecular patterns, such as formyl peptides or bacterial DNA, that are expressed by pathogenic microbes will also alert and activate the immune system (14). Likewise, dying cells also possess these “patterns” that act in a similar manner (10, 14, 15). These patterns coined, DAMPs can have different forms and be derived from various sources (14, 16). They can be expressed on the plasma membrane, be excreted extracellularly, or even be the breakdown products of certain pathways, and more recently, it can be found in extracellular vesicles (14, 17, 18). As such, this mini-review attempts to uncover some of the reported DAMPs derived from cancer-derived vesicles and how the downstream effects.
DAMPs
DAMPs are molecules that are produced endogenously by cells in response to stress (19, 20). In cancers, high tumor apoptosis exerts stress and inflammatory signal that triggers the secretion of DAMPs leading to immunogenic cell death (ICD) of cancer cells (6). Unlike apoptosis, ICD is pro-inflammatory and requires the involvement of phagocytic immune cells such as dendritic cells (DC) and macrophages (4). It was found that the combination of apoptosis DAMPs secretion and ICD in response to specific anti-cancer agents such as chemo- and radiotherapy drugs could bring into play a potent and effective anti- tumor immunity, thus significantly becoming the target for cancer therapy (7, 21, 22). Krysko et al. defined DAMPs as molecules that perform non-inflammatory functions in living cells and acquire immune-modulatory properties when released on the cell surface (23). Work done by Apetoh et al. showed direct interaction of High molecular Group Box-1 (HMBG1), a well-known DAMP with Toll-like receptor-4 (TLR-4) on DCs that affect their antigenic presentation in breast cancer patients (24). On top of that, it was found that another type of DAMPs such as adenosine triphosphate (ATP) also plays a critical role in the degree of successful DC priming with cytotoxic T cells through NLRP3-dependent caspase-1 activation complex (25). As cancers are extremely adaptive, the failure of DAMPs to exert complete and effective anti-tumor response could also generate opposite mechanisms whereupon DAMPs are exploited by cancers to promote cancer growth and survival (26). As DAMPs could deliver effects on both anti-tumor and pro-tumor activities, the effort to decipher the molecular mechanism behind this is crucial.
Extracellular Vesicles
Extracellular vesicles (EVs) are a class of membrane-encapsulated vesicles that are released by cells into the secretory system. Several types of EVs have been discovered depending on the biogenesis, function and size (27–29). According to the revised guidelines from International Society for Extracellular Vesicles (ISEV) committee, exosomes, or also known based on its size as small extracellular vesicles (sEVs), are a class of EVs derived from the formation of intraluminal vesicles that are usually sized between 30-100nm. Microvesicles, on the other hand, are a class of EVs that are released via the fusion of the cellular membrane and are usually larger than exosomes. Another class of EVs, called apoptotic bodies, are vesicles that are released by dying cells (27, 30). The heterogeneity of EVs has been one of the main limiting factors in understanding the role of EVs in cancer progression. Nevertheless, it has been shown by multiple studies that cancer-derived EVs can modulate the immune response through the regulation of immune cells such as CD8+ T cells, CD4+ T cells or natural killer cells (31). Upon response to therapy, cancer cells have shown to release a higher level of EVs (32). These EVs were shown to induce an immune response and may carry pro-tumorigenic cargo (32). Recently, DAMPs have been reported to be present within EVs and may affect the inflammatory balance within tumor sites. More importantly, some studies have shown that tumor-derived EVs are able to mediate toll-like receptor (TLR) signaling (33, 34). Since studies on DAMPs within EVs are limited, we will include all types of EVs including exosomes, small extracellular vesicles and microvesicles.
DAMPs and EVs
HMGB1 and EVs
Several immunostimulating molecules are discharged when cells die including HMGB1, uric acid, ATP and heat shock proteins (HSP) (14, 16, 35–37). HMGB1 is the most frequently encountered DAMP in cells undergoing stress (14). This protein, initially found in the 1970s, is a nuclear protein and binds to chromatin (5, 38). HMGB1 is highly conserved and is involved in various cellular processes such as DNA repair, gene expression and replication (39–42). Upon inflammation or cellular stress, HMGB1 binds to immune cell receptors such as Toll-like receptor 2 (TLR2), Toll-like receptor 4 (TLR4), and Receptor for advanced glycation end products (RAGE) (14, 43, 44). Apart from being released by cells infected with pathogens, dying cells or cells undergoing necrosis are also able to secrete HMGB1 (45). The release of HMGB1 may induce inflammation upon binding to different immune receptors through the release of cytokines such as tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) (45). In cancer, the role of HMGB1 needs further understanding as dual roles of HMGB1 have been reported (42, 46). Pro-tumorigenic roles of HMGB1 include initiation of inflammation (47), enhance tumor cell proliferation (48), promotes tumor invasion and metastasis (49), enforces angiogenesis (50), involves in chemoresistance (51) and promotes antitumor immunity (52). Nevertheless, there have been reports that HMGB1 may also play a role as a tumor suppressor (53, 54). The paradoxical roles of HMGB1 in cancer have been of interest to many researchers over the years. What is more interesting, HMGB1 has been reported to be present in EVs as well. A study by Deng et al. showed that hepatocytes release HMGB1 via vesicles after being stimulated by lipopolysaccharide (LPS) (55). A follow-up study by the authors showed that HMGB1 was indeed packaged in exosomes and released extracellularly (56). The authors showed that HMGB1 was released in exosomes via the TLR4 pathway (56). In a different study, it was discovered that large burn injuries (LBI) were able to secrete plasma microvesicles enriched with HMGB1 (57). The study found that the released HMGB1 formed complexes with pro-interleukin-1-beta (pro-IL-1β) in both human and mouse plasma, and this heterocomplexes were able to induce immune dysfunction in LBI (57).
According to vesiclepedia (58), a database for protein/mRNA enriched in extracellular vesicles, HMGB1 is present in EVs such as exosomes and microvesicles from various sources. For instance, HMGB1 protein was reported present in EVs derived from breast cancer cells (59), bronchial epithelial cells (60), chronic lymphocytic leukemia cells (61), colorectal cancer cells (62, 63), glioblastoma cells (64), among others. These studies show that while HMGB1 is secreted as free HMGB1, this protein can also be secreted and packaged in vesicles as well. However, the type of EVs that contain HMGB1 has not been reported exclusively for one type of EV. The abovementioned studies show that HMGB1 can be present in exosomes, general extracellular vesicles as well as microvesicles. The exact mechanism as to how HMGB1 is sorted into these vesicles is still lacking information. The method of isolation of different types of EVs may also influence the presence of HMGB1 in these EVs. HMGB1 that is present within EVs has been shown to affect other surrounding cells as well. Functionally, several reports have also shown that EV-derived HMGB1 can participate in the carcinogenesis process. A study by Li et al. suggested that exosomal HMGB1 derived from esophageal squamous cell carcinoma managed to differentiate monocytes into the pro-tumorigenic Programmed cell death-bearing-tumor-associated macrophages (PD1+ TAMs) phenotype (65). A different study by Ye et al. showed that exosome-derived HMGB1 in hepatocellular carcinoma can activate B cells (66). This subsequently leads to the enhanced proliferation of TIM-1+ regulatory B cells by the TLR2/4 and Mitogen-Activated Protein Kinase (MAPK) pathways (66). Additionally, it was also shown that exosomal HMGB1 play a role in platelet-driven cancer malignancy. It was reported that treatment with anti-platelet drug, dipyridamole and aspirin inhibited tumor progression in Lewis lung carcinoma (LLC) cell lines and reduced the exosomal HMGB1 content. Similar finding was displayed in a tumor-bearing mouse model where combined treatment of dipyridamole and exosome-release inhibitor, GW4869 significantly mitigated tumor growth (67). Exosomal HMGB1 was also found to be involved in angiogenesis. A recent study by Gao et al. showed that hypoxic bone marrow mesenchymal cells were able to release exosomal HMGB1 that further enhanced angiogenesis via c-Jun N-terminal Kinase JNK/Hypoxia-inducible factor (JNK/HIF) pathway (68). It is interesting to note that the role of HMGB1 may differ depending on the form it is released. For instance, a study by Ma et al. showed that extracellular HMGB1 had opposing effects towards the expression of SAM and SH3 domain containing protein 1 (SASH1) as compared to exosomal HMGB1 (69). Although it is well known that extracellular HMGB1 is able to activate the inflammatory pathway via the TLR/RAGE receptors, information on EV-derived HMGB1 is still lacking and this calls for the need of further research. Since HMGB1 is a nuclear protein, it can be assumed that HMGB1 is packaged within EVs and may not be present on the surface, but further verification is needed. Therefore, the mechanism by which HMGB1 is able to stimulate immune response upon internalization of EVs still needs to be determined.
HSP and EVs
Besides HMGB1, HSPs are commonly categorized as DAMPs as well (70). HSPs act as chaperones to ensure the proper folding of proteins (70, 71). These proteins are typically released when cells are under stress and are usually overexpressed in tumor cells due to the demand for cellular energy and the unstable environment (72). It was shown that certain HSPs trigger a pro-inflammatory response in mouse macrophage and human monocytes (73). Upon encountering HSPs, T regulatory cells (Tregs), T cytotoxic cells, natural killer (NK) cells, macrophages and DCs are activated (74). Nevertheless, the roles of HSP as DAMPs are still debatable. However, for the purpose of this review, we will consider HSPs as DAMPs and discuss the presence of HSPs in EVs. The presence of HSP-containing EVs released from cancer samples has been reported by several groups (75–80). For instance, a report by Gastpar et al. showed that HSP70 was present on the membrane of tumor-derived exosomes from pancreatic and colon cancer cell lines (81). The authors also showed that these exosomes were able to stimulate migration and HSP70 reactivity in NK cells (81). HSP70 has been reported to be released by tumor cells upon external stress such as radio or chemotherapy (77, 82). Therefore, it is presumed that under stressful conditions, the expression of HSP70 on exosomes is also increased. Lv et al. showed that there was indeed, a difference in the expression of HSP60, HSP70 and HSP90 in exosomes derived from HepG2 cells after treatment with chemotherapeutic drugs (83). Similar to the previous study, these HSP-containing exosomes were able to increase NK cell cytotoxic ability (83). A similar study by Elsner et al. suggested that HSP70-positive exosomes from melanoma cells were able to enhance NK cells cytolytic activity against YAC-1 cells (84). The increase of HSP70 in exosomes has also been shown upon induction by heat stress in murine models (85). Cho et al. showed that these heat-induced exosomes containing HSP70 elicit a stronger T helper type 1 (Th1) immune response (85). The presence of HSP70 in tumor-derived EVs has also been reported elsewhere. A study by Xie et al. showed that exosomes containing HSP70 stimulate anti-tumor immunity by enhancing the maturation of DCs and Th1 cells (86). A recent pilot study by Chanteloup et al. reported that exosomal HSP70 can be used to detect and monitor metastatic solid tumors such as breast and ovarian cancer (75, 76). HSP60 has also been shown to be released by tumor-derived exosomes (87, 88). A study by Wyciszkiewicz et al. showed that certain HSPs such as AlphaB-crystallin and HSP22 are present in exosomes from gynecological cancers (89). The authors showed that although these HSPs were present in both exosomes and serum, there is no correlation between the two sources (89). Similar to HMGB1, extracellular HSPs are not representative of exosomal/EV-derived HSPs in terms of abundance and function. According to vesiclepedia (58), HSPs, such as HSP90 were reported in cancer-derived EVs such as bladder cancer cells (90) and breast cancer (59). Almost all the reported studies show that HSPs are present within exosomes and not in other types of EVs. However, these studies report different techniques of isolation and characterization of exosomes and may not be conclusive enough to state that HSPs are exclusively found in exosomes. Nevertheless, though the presence of HSPs has been reported in EVs, the exact mechanism as to how these proteins induce an inflammatory/immune response is still elusive. The localization of HSPs as to whether it is present internally or on the surface of EVs warrants more studies (91). A study by Tang et al. showed that HSP90α is present on the surface of tumor-derived exosomes and is able to mediate communication with other cells (92). However, an earlier study by Clayton et al. showed that HSPs are also present in the lumen of exosomes and may not interact with target cells through cell surface receptors (93). Therefore, more in-depth studies are needed to determine whether HSPs are able to act as DAMPs and activate inflammation through certain receptors.
S100 and EVs
S100 are a class of proteins known to bind to calcium and regulate intracellular and extracellular processes (94, 95). There are around 24 types of S100 protein members that can be divided into three main subclasses depending on their function (96). S100 proteins have long been recognized as DAMPs due to their ability to elicit an inflammatory response (97, 98). In cancer, the S100 proteins have been reported to be involved in carcinogenesis. In a study done by Hiratsuka et al., S100A8 and S100A9 proteins are found to be involved in lung cancer invasion and myeloid cell recruitment (99). S100 proteins have been reported to be present in EVs as well. A study by Prieto et al. showed that in chronic lymphocytic leukemia (CLL), S100A9 protein was present in the plasma exosomes (100). The authors showed that the exosomes containing S100A9 were able to activate the nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB) pathway in leukemic cells (100). Not only that, in a different study by Li et al., the authors demonstrated that the S100A9 protein was also present in exosomes derived from follicular fluid of polycystic ovary syndrome patients (101). These exosomes were also able to promote inflammation via the NF-κB pathway (101). Although the molecular mechanism of the activation was still unmapped, this study, however, displayed an interesting finding in which the levels of NF-κB pro-inflammatory cytokines were increased upon incubation with S100A9-enriched exosomes (101). According to vesiclepedia (58), the presence of members from the S100 family was reported to be present in EVs from various sources. For instance, the S100A7A protein was found in EVs from colorectal cancer cell lines (62) and T cells (102). S100A5 protein was also found in colorectal cancer cell lines (103), and S100A12 protein was found in EVs from brain cancer cells, colorectal cancer cells, melanoma cells, kidney cancer cells and more (59). Similar to other DAMPs, the presence of S100 proteins is also not exclusive to one type of EV. Although the presence of S100 proteins has been reported in EVs, the actual function of S100 as DAMPs within EVs remains to be elucidated. Generally, free or extracellular S100 proteins are able to act as DAMPs by binding to receptors such as RAGE or TLR, but the mechanism of S100 within EVs still needs to be investigated. The presence of S100 proteins in EVs and how this affects the pathway leading to inflammation is still unknown.
Micro RNA (miRNA)
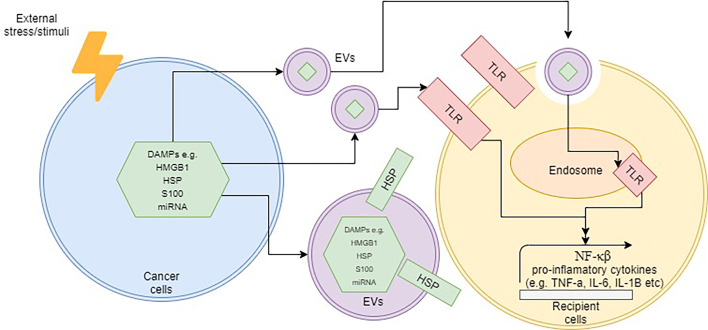
Besides the abovementioned molecules, other components within EVs that are also able to elicit an immune response is nucleic acid. It is well-established that microRNAs (miRNAs), short-lengthed nucleic aids, can be encapsulated within EVs. Some studies have shown that these EV-bound miRNAs were able to induce an immune response via the intracellular TLR pathway in several diseases (104–107). In rheumatoid arthritis, for instance, exosome-containing let-7b was able to differentiate macrophages into the M1 phenotype via TLR7 (108). A different study was able to show that miR-21 encapsulated in EVs was able to induce neurotoxicity through TLR7 signaling as well (109). In cancer, a study by Fabbri et al. demonstrated that exosome-derived miRNAs from lung cancer cells were able to bind to TLR8 on macrophages and activate the NF-κB pathway (110). This, in turn, led to the release of pro-inflammatory cytokines such as TNF-α and interleukin-6 (IL-6) (110). Although the presence of miRNA in EVs such as exosomes and microvesicles is well-established, there are still limited studies on whether these encapsulated miRNAs are able to stimulate TLR pathway, and subsequently activate inflammation. Additionally, most of the reported studies had purified EVs from sources that did not undergo any cellular stress such as chemotherapy or radiation, and thus the role of miRNA-EVs as DAMPs needs to be further determined. Figure 1 demonstrates the overall schematic representation of how DAMPs are released within EVs and subsequently interact with target cells.
Figure 1.
Schematic representation of how DAMP-containing EVs operate upon cellular stress. The localization of DAMPs can be internally or on the surface of EVs. Upon contact with target cells, the TLR pathway may be activated by DAMPs via the surface or endosomal route and subsequently trigger inflammation.
Future Recommendations and Conclusion
Upon cellular stress or cell death, cancer cells will release a variety of molecules in response to the stimuli. Extracellular vesicles containing DAMPs have been hypothesized to induce an inflammatory response via the TLR/NF-κB pathway but are still in need of further verification. Table 1 summarizes some of the reports that have shown the presence of DAMPs within EVs. However, most of these studies collect EV from samples that were not subjected to any treatment-induced stress. As such, we are not able to establish whether these DAMPs are significantly released upon stress or not. It has been well known that EVs released from cancer cells are able to modulate immune responses (31). Nevertheless, whether these modulations are induced through the regulation of DAMPs contained within the EVs remains to be elucidated. Additionally, little is known on whether DAMPs present in the EVs may induce the same response as free/extracellular DAMPs, and whether EVs provide more physiological benefits such as higher stability or longer half-lives. Additionally, the heterogeneity of EVs also plays a role in further understanding the role of EVs-DAMPs in inflammation. For instance, we are still unsure as to whether a certain subpopulation of EVs may carry certain DAMPs over other types of EVs. Most of the reported studies report either exosomes, extracellular vesicles and microvesicles as the source, which strengthens the fact that further studies are needed to determine whether DAMPs are secreted selectively. More importantly, the techniques used to isolate and characterize EVs such as exosomes vary from one study to another. It is imperative that studies pertaining to EVs adhere to the recommendation of the International Society of Extracellular Vesicles (ISEV) to ensure reproducible outcomes (111). Apart from that, the terminology used to describe EVs must follow the standards recommended by ISEV (111). Furthermore, the information on the localization of DAMPs within the EVs is also critical as this determines on which TLR or receptor is stimulated. Also, whether certain stimuli/therapy may induce the release of certain EV-derived DAMPs differently than the free DAMPs is still unknown. Most of the reported studies suggested that EV-derived DAMPs promote the pro-tumor environment. Nevertheless, the balance between pro- and anti- inflammatory and tumor responses regarding the release of DAMPs still needs further understanding. There are still some important questions that need to be answered in terms of the role of DAMPs within EVs, especially on how these molecules affect the tumor microenvironment and eventually cancer progression.
Table 1.
A list of some of the reported studies that have shown the presence of DAMPs within EVs.
| DAMPs | Type of EVs | Disease | Source | Reference | ||
|---|---|---|---|---|---|---|
| HMGB1 | Exosome | Esophageal squamous cell carcinoma cell lines | Cell culture medium | (65) | ||
| Exosome | Hepatocellular carcinoma cell lines | Cell culture medium | (66) | |||
| Exosome | Lewis lung carcinoma (LLC) cell lines and mice model | Cell culture medium and blood | (67) | |||
| Exosome | Glioma cells | Cell culture medium | (69) | |||
| HSP60, HSP70 and HSP90 | Exosome | HepG2 hepatocellular carcinoma cells | Cell culture medium | (83) | ||
| HSP70 | Exosome | Melanoma | (84) | |||
| HSP60 and HSP70 | Exosome | NCI-H292 (human mucoepidermoid bronchial carcinoma), A549 (human lung adenocarcinoma) and K562 (human erythroleukemia) cell lines | Cell culture medium | (88) | ||
| HSP90 | Extracellular vesicles | Bladder cancer cell lines | Cell culture medium | (90) | ||
| HSP90α | Exosome | Breast cancer cell lines | Cell culture medium | (92) | ||
| Alpha crystalline and HSP22 | Exosome | Gynecological cancers | Serum | (89) | ||
| S100A9 | Exosome | Chronic lymphocytic leukemia | Plasma | (100) | ||
| S100A9 | Exosome | Polycystic ovary syndrome | Follicular fluid | (101) | ||
| S100A7A | Exosome | LIM1863 colon carcinoma cell-derived organoids | Cell culture medium | (62) | ||
| S100A5 | Microvesicle | Colorectal cancer cells | Cell culture medium | (103) | ||
| S100A12 | Extracellular Vesicles | Brain cancer cells, colorectal cancer cells, melanoma cells, kidney cancer cells | Cell culture medium | (59) | ||
| S100A4 | Extracellular vesicles | Bladder cancer cell lines and bladder cancer patients | Cell culture medium and urine | (90) | ||
Author Contributions
NA conceived the idea. NA, NR, and SN contributed towards the writing of the manuscript. NA provided critical review and input. All authors contributed to the article and approved the submitted version.
Funding
NAARB was funded by FRGS/1/2019/SKK08/UKM/01/2.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Ma X, Yu H. Global Burden of Cancer. Yale J Bio Med (2006) 79(3-4):85–94. [PMC free article] [PubMed] [Google Scholar]
- 2. Chew V, Toh HC, Abastado J-P. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. J Oncol (2012) 2012:608406. doi: 10.1155/2012/608406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lei X, Lei Y, Li J-K, Du W-X, Li R-G, Yang J, et al. Immune Cells Within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 4. Ahmed A, Tait SWG. Targeting Immunogenic Cell Death in Cancer. Mol Oncol (2020) 14(12):2994–3006. doi: 10.1002/1878-0261.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic Cell Death, Damps and Anticancer Therapeutics: An Emerging Amalgamation. Biochim Biophys Acta (2010) 1805:19. doi: 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 6. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic Cell Death and Damps in Cancer Therapy. Nat Rev Cancer (2012) 12(12):860–75. doi: 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 7. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic Cell Death in Cancer Therapy: Present and Emerging Inducers. J Cell Mol Med (2019) 23(8):4854–65. doi: 10.1111/jcmm.14356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallach D, Kovalenko A, Kang T-B. ‘Necrosome’-Induced Inflammation: Must Cells Die for It? Trends Immunol (2011) 32(11):5. doi: 10.1016/j.it.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 9. Piccinini AM, S.Midwood K. Dampening Inflammation by Modulating TLR Signalling. Mediators Inflammation (2010) 2010:21. doi: 10.1155/2010/672395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubartelli A, Lotze MT. Inside, Outside, Upside Down: Damage-Associated Molecular-Pattern Molecules (Damps) and Redox. Trends Immunol (2007) 28(10):8. doi: 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 11. McCall K. Genetic Control of Necrosis—Another Type of Programmed Cell Death. Curr Opin Cell Biol (2010) 22:7. doi: 10.1016/j.ceb.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matzinger P. Tolerance, Danger, and the Extended Family. Annu Rev Immunol (1994) 12:55. doi: 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- 13. West AP, Shadel GS, Ghosh S. Mitochondria in Innate Immune Responses. Nat Rev Immunol (2011) 11:14. doi: 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srikrishna G, Freeze HH. Endogenous Damage-Associated Molecular Pattern Molecules at the Crossroads of Inflammation and Cancer. Neoplasia (2009) 11(7):14. doi: 10.1593/neo.09284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manfredi AA, Rovere-Querini P. The Mitochondrion — A Trojan Horse That Kicks Off Inflammation? N Engl J Med (2010) 362(22):3. doi: 10.1056/NEJMcibr1003521 [DOI] [PubMed] [Google Scholar]
- 16. Chen GY, Nuñez G. Sterile Inflammation: Sensing and Reacting to Damage. Nat Rev Immunol (2010) 10:12. doi: 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging Role of Damage-Associated Molecular Patterns Derived From Mitochondria in Inflammation. Trends Immunol (2011) 32(4):8. doi: 10.1016/j.it.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 18. Murao A, Brenner M, Aziz M, Wang P. Exosomes in Sepsis. Front Immunol (2020) 11:2140. doi: 10.3389/fimmu.2020.02140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franklin TC, Xu C, Duman RS. Depression and Sterile Inflammation: Essential Role of Danger Associated Molecular Patterns. Brain Behav Immun (2018) 72:2–13. doi: 10.1016/j.bbi.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 20. Venereau E, Ceriotti C, Bianchi ME. Damps From Cell Death to New Life. Front Immunol (2015) 6:422. doi: 10.3389/fimmu.2015.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernandez C, Huebener P, Schwabe RF. Damage-Associated Molecular Patterns in Cancer: A Double-Edged Sword. Oncogene (2016) 35(46):5931–41. doi: 10.1038/onc.2016.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of Action of Conventional and Targeted Anticancer Therapies: Reinstating Immunosurveillance. Immunity (2013) 39(1):74–88. doi: 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 23. Krysko O, Aaes TL, Kagan VE, D'Herde K, Bachert C, Leybaert L, et al. Necroptotic Cell Death in Anti-Cancer Therapy. Immunol Rev (2017) 280(1):207–19. doi: 10.1111/imr.12583 [DOI] [PubMed] [Google Scholar]
- 24. Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The Interaction Between HMGB1 and TLR4 Dictates the Outcome of Anticancer Chemotherapy and Radiotherapy. Immunol Rev (2007) 220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 25. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 Inflammasome in Dendritic Cells Induces IL-1beta-Dependent Adaptive Immunity Against Tumors. Nat Med (2009) 15(10):1170–8. doi: 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 26. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 27. Kang T, Atukorala I, Mathivanan S. Biogenesis of Extracellular Vesicles. Sub-Cellular Biochem (2021) 97:19–43. doi: 10.1007/978-3-030-67171-6_2 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine (2020) 15:6917–34. doi: 10.2147/IJN.S264498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience (2015) 65(8):783–97. doi: 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol (2018) 9:738. doi: 10.3389/fimmu.2018.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Othman N, Jamal R, Abu N. Cancer-Derived Exosomes as Effectors of Key Inflammation-Related Players. Front Immunol (2019) 10:2103. doi: 10.3389/fimmu.2019.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ab Razak NS, Ab Mutalib NS, Mohtar MA, Abu N. Impact of Chemotherapy on Extracellular Vesicles: Understanding the Chemo-Evs. Front Oncol (2019) 9:1113. doi: 10.3389/fonc.2019.01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, et al. Melanoma Extracellular Vesicles Generate Immunosuppressive Myeloid Cells by Upregulating PD-L1 via TLR4 Signaling. Cancer Res (2019) 79: (18):4715–371. doi: 10.1158/0008-5472.CAN-19-0053 [DOI] [PubMed] [Google Scholar]
- 34. Guo H-Y, Cheng A-C, Wang M-S, Yin Z-Q, Jia R-Y. Exosomes: Potential Therapies for Disease via Regulating Tlrs. Mediators Inflammation (2020) 2020:2319616. doi: 10.1155/2020/2319616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zong W-X, Thompson CB. Necrotic Death as a Cell Fate. Genes Dev (2006) 20:15. doi: 10.1101/gad.1376506 [DOI] [PubMed] [Google Scholar]
- 36. Lotze MT, Deisseroth A, Rubartelli A. Damage Associated Molecular Pattern Molecules. Clin Immunol (2007) 124:4. doi: 10.1016/j.clim.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moskowitz MA, Lo EH, Iadecola C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron (2010) 67:18. doi: 10.1016/j.neuron.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodwin GH, Sanders C, Johns EW. A New Group of Chromatin-Associated Proteins With a High Content of Acidic and Basic Amino Acids. Eur J Biochem (1973) 38(1):14–9. doi: 10.1111/j.1432-1033.1973.tb03026.x [DOI] [PubMed] [Google Scholar]
- 39. Hubert P, Roncarati P, Demoulin S, Pilard C, Ancion M, Reynders C, et al. Extracellular HMGB1 Blockade Inhibits Tumor Growth Through Profoundly Remodeling Immune Microenvironment and Enhances Checkpoint Inhibitor-Based Immunotherapy. J ImmunoTherapy Cancer (2021) 9(3):e001966. doi: 10.1136/jitc-2020-001966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magna M, Pisetsky DS. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol Med (2014) 20(1):138–46. doi: 10.2119/molmed.2013.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, et al. High Mobility Group Box Chromosomal Protein 1, a DNA Binding Cytokine, Induces Arthritis. Arthritis Rheumatism (2003) 48(6):1693–700. doi: 10.1002/art.11028 [DOI] [PubMed] [Google Scholar]
- 42. Tripathi A, Shrinet K, Kumar A. HMGB1 Protein as a Novel Target for Cancer. Toxicol Rep (2019) 6:253–61. doi: 10.1016/j.toxrep.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korbelik M, Zhang W, Merchant S. Involvement of Damage-Associated Molecular Patterns in Tumor Response to Photodynamic Therapy: Surface Expression of Calreticulin and High Mobility Group Box-1 Release. Cancer Immunol Immunotherapy (2011) 60:7. doi: 10.1007/s00262-011-1047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demaria S, Pikarsky E, Karin M, Coussens LM, Chen Y-C, El-Omar EM, et al. Cancer and Inflammation: Promise for Biological Therapy. J Immunotherapy (2010) 33(4):33. doi: 10.1097/CJI.0b013e3181d32e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in Inflammation and Cancer. Annu Rev Immunol (2010) 28(1):367–88. doi: 10.1146/annurev.immunol.021908.132603 [DOI] [PubMed] [Google Scholar]
- 46. Wang S, Zhang Y. HMGB1 in Inflammation and Cancer. J Hematol Oncol (2020) 13(1):116. doi: 10.1186/s13045-020-00950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-Mediated Skin Carcinogenesis Is Dependent on Immune and Radioresistant Cells. EMBO J (2010) 29(13):2242–52. doi: 10.1038/emboj.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He S, Cheng J, Sun L, Wang Y, Wang C, Liu X, et al. HMGB1 Released by Irradiated Tumor Cell Promotes Living Tumor Cell Proliferation via Paracrine Effect. Cell Death Dis (2018) 9(6):648. doi: 10.1038/s41419-018-0626-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, et al. High-Mobility Group Box 1 Activates Caspase-1 and Promotes Hepatocellular Carcinoma Invasiveness and Metastases. Hepatol (Baltimore Md) (2012) 55(6):1863–75. doi: 10.1002/hep.25572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor Angiogenesis Is Enforced by Autocrine Regulation of High-Mobility Group Box 1. Oncogene (2013) 32(3):363–74. doi: 10.1038/onc.2012.49 [DOI] [PubMed] [Google Scholar]
- 51. Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, et al. HMGB1 Promotes Drug Resistance in Osteosarcoma. Cancer Res (2012) 72(1):230–8. doi: 10.1158/0008-5472.CAN-11-2001 [DOI] [PubMed] [Google Scholar]
- 52. Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of Immunological Tolerance by Apoptotic Cells Requires Caspase-Dependent Oxidation of High-Mobility Group Box-1 Protein. Immunity (2008) 29(1):21–32. doi: 10.1016/j.immuni.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cebrián MJG, Bauden M, Andersson R, Holdenrieder S, Ansari D. Paradoxical Role of HMGB1 in Pancreatic Cancer: Tumor Suppressor or Tumor Promoter? Anticancer Res (2016) 36(9):4381–9. doi: 10.21873/anticanres.10981 [DOI] [PubMed] [Google Scholar]
- 54. Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, et al. Intracellular HMGB1 as a Novel Tumor Suppressor of Pancreatic Cancer. Cell Res (2017) 27(7):916–32. doi: 10.1038/cr.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity (2018) 49(4):740–53.e7. doi: 10.1016/j.immuni.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li W, Deng M, Loughran PA, Yang M, Lin M, Yang C, et al. LPS Induces Active HMGB1 Release From Hepatocytes Into Exosomes Through the Coordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front Immunol (2020) 11:229. doi: 10.3389/fimmu.2020.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coleman LG, Jr., Maile R, Jones SW, Cairns BA, Crews FT. Hmgb1/Il-1β Complexes in Plasma Microvesicles Modulate Immune Responses to Burn Injury. PloS One (2018) 13(3):e0195335. doi: 10.1371/journal.pone.0195335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: A Compendium for Extracellular Vesicles With Continuous Community Annotation. PloS Biol (2012) 10(12):e1001450. doi: 10.1371/journal.pbio.1001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG., Jr. Proteomic Profiling of NCI-60 Extracellular Vesicles Uncovers Common Protein Cargo and Cancer Type-Specific Biomarkers. Oncotarget (2016) 7(52):86999–7015. doi: 10.18632/oncotarget.13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, et al. Ultrafiltration Combined With Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles From Cell Culture Media for Compositional and Functional Studies. Sci Rep (2017) 7(1):15297. doi: 10.1038/s41598-017-15717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes Released by Chronic Lymphocytic Leukemia Cells Induce the Transition of Stromal Cells Into Cancer-Associated Fibroblasts. Blood (2015) 126(9):1106–17. doi: 10.1182/blood-2014-12-618025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two Distinct Populations of Exosomes Are Released From LIM1863 Colon Carcinoma Cell-Derived Organoids. Mol Cell Proteomics MCP (2013) 12(3):587–98. doi: 10.1074/mcp.M112.021303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liem M, Ang CS, Mathivanan S. Insulin Mediated Activation of PI3K/Akt Signalling Pathway Modifies the Proteomic Cargo of Extracellular Vesicles. Proteomics (2017) 17:23–24. doi: 10.1002/pmic.201600371 [DOI] [PubMed] [Google Scholar]
- 64. Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, et al. Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma via Intercellular Transfer of Splicing Factors. Cancer Cell (2018) 34(1):119–35.e10. doi: 10.1016/j.ccell.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li B, Song T-N, Wang F-R, Yin C, Li Z, Lin J-P, et al. Tumor-Derived Exosomal HMGB1 Promotes Esophageal Squamous Cell Carcinoma Progression Through Inducing PD1+ TAM Expansion. Oncogenesis (2019) 8(3):17. doi: 10.1038/s41389-019-0126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, et al. Tumor-Derived Exosomal HMGB1 Fosters Hepatocellular Carcinoma Immune Evasion by Promoting TIM-1+ Regulatory B Cell Expansion. J ImmunoTherapy Cancer (2018) 6(1):145. doi: 10.1186/s40425-018-0451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang J-D, Wang Y-Y, Lin S-Y, Chang C-Y, Li J-R, Huang S-W, et al. Exosomal Hmgb1 Promoted Cancer Malignancy. Cancers (2021) 13(4):877. doi: 10.3390/cancers13040877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gao W, He R, Ren J, Zhang W, Wang K, Zhu L, et al. Exosomal HMGB1 Derived From Hypoxia- Conditioned Bone Marrow Mesenchymal Stem Cells Increases Angiogenesis via the JNK/HIF-1α Pathway. FEBS Open Bio (2021) 11(5):1364–73. doi: 10.1002/2211-5463.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma C, Chen H, Zhang S, Yan Y, Wu R, Wang Y, et al. Exosomal and Extracellular HMGB1 Have Opposite Effects on SASH1 Expression in Rat Astrocytes and Glioma C6 Cells. Biochem Biophys Res Commun (2019) 518(2):325–30. doi: 10.1016/j.bbrc.2019.08.057 [DOI] [PubMed] [Google Scholar]
- 70. Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Molecular Characteristics of Immunogenic Cancer Cell Death. Cell Death Differentiation (2008) 15:10. doi: 10.1038/sj.cdd.4402269 [DOI] [PubMed] [Google Scholar]
- 71. Rosin DL, Okusa MD. Dangers Within: DAMP Responses to Damage and Cell Death in Kidney Disease. J Am Soc Nephrol (2011) 22:10. doi: 10.1681/ASN.2010040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, et al. Immunogenic Cancer Cell Death: A Key-Lock Paradigm. Curr Opin Immunol (2008) 20:8. doi: 10.1016/j.coi.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 73. Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-Kda Heat-Shock Protein: A Danger Signal to the Innate Immune System. J Immunol (1999) 162(6):3212–9. [PubMed] [Google Scholar]
- 74. Foell D, Wittkowski H, Roth J. Mechanisms of Disease: A 'DAMP' View of Inflammatory Arthritis. Nat Clin Pract Rheumatol (2007) 3(7):8. doi: 10.1038/ncprheum0531 [DOI] [PubMed] [Google Scholar]
- 75. Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Hervieu A, Hennequin A, et al. Monitoring HSP70 Exosomes in Cancer Patients’ Follow Up: A Clinical Prospective Pilot Study. J Extracellular Vesicles (2020) 9(1):1766192. doi: 10.1080/20013078.2020.1766192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Marcion G, Garrido C, et al. Membrane-Bound Exosomal HSP70 as a Biomarker for Detection and Monitoring of Malignant Solid Tumours: A Pilot Study. Pilot Feasibility Stud (2020) 6(1):35. doi: 10.1186/s40814-020-00577-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ostheimer C, Gunther S, Bache M, Vordermark D, Multhoff G. Dynamics of Heat Shock Protein 70 Serum Levels as a Predictor of Clinical Response in Non-Small-Cell Lung Cancer and Correlation With the Hypoxia-Related Marker Osteopontin. Front Immunol (2017) 8:1305. doi: 10.3389/fimmu.2017.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stope MB, Klinkmann G, Diesing K, Koensgen D, Burchardt M, Mustea A. Heat Shock Protein HSP27 Secretion by Ovarian Cancer Cells Is Linked to Intracellular Expression Levels, Occurs Independently of the Endoplasmic Reticulum Pathway and HSP27’s Phosphorylation Status, and Is Mediated by Exosome Liberation. Dis Markers (2017) 2017:1575374. doi: 10.1155/2017/1575374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seclì L, Fusella F, Avalle L, Brancaccio M. The Dark-Side of the Outside: How Extracellular Heat Shock Proteins Promote Cancer. Cell Mol Life Sci (2021) 78(9):4069–83. doi: 10.1007/s00018-021-03764-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Taha EA, Ono K, Eguchi T. Roles of Extracellular Hsps as Biomarkers in Immune Surveillance and Immune Evasion. Int J Mol Sci (2019) 20(18):4588. doi: 10.3390/ijms20184588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res (2005) 65(12):5238–47. doi: 10.1158/0008-5472.CAN-04-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, et al. A Stress-Inducible 72-Kda Heat-Shock Protein (HSP72) Is Expressed on the Surface of Human Tumor Cells, But Not on Normal Cells. Int J Cancer (1995) 61(2):272–9. doi: 10.1002/ijc.2910610222 [DOI] [PubMed] [Google Scholar]
- 83. Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, et al. Anticancer Drugs Cause Release of Exosomes With Heat Shock Proteins From Human Hepatocellular Carcinoma Cells That Elicit Effective Natural Killer Cell Antitumor Responses. J Biol Chem (2012) 287(19):15874–85. doi: 10.1074/jbc.M112.340588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeböller H, et al. The Heat Shock Protein HSP70 Promotes Mouse NK Cell Activity Against Tumors That Express Inducible NKG2D Ligands. J Immunol (2007) 179(8):5523–33. doi: 10.4049/jimmunol.179.8.5523 [DOI] [PubMed] [Google Scholar]
- 85. Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC Independent Anti-Tumor Immune Responses Induced by Hsp70-Enriched Exosomes Generate Tumor Regression in Murine Models. Cancer Lett (2009) 275(2):256–65. doi: 10.1016/j.canlet.2008.10.021 [DOI] [PubMed] [Google Scholar]
- 86. Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al. Membrane-Bound HSP70-Engineered Myeloma Cell-Derived Exosomes Stimulate More Efficient CD8(+) CTL- and NK-Mediated Antitumour Immunity Than Exosomes Released From Heat-Shocked Tumour Cells Expressing Cytoplasmic HSP70. J Cell Mol Med (2010) 14(11):2655–66. doi: 10.1111/j.1582-4934.2009.00851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DFV, et al. The Odyssey of Hsp60 From Tumor Cells to Other Destinations Includes Plasma Membrane-Associated Stages and Golgi and Exosomal Protein-Trafficking Modalities. PloS One (2012) 7(7):e42008. doi: 10.1371/journal.pone.0042008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Merendino AM, Bucchieri F, Campanella C, Marcianò V, Ribbene A, David S, et al. Hsp60 Is Actively Secreted by Human Tumor Cells. PloS One (2010) 5(2):e9247. doi: 10.1371/journal.pone.0009247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wyciszkiewicz A, Kalinowska-Łyszczarz A, Nowakowski B, Kaźmierczak K, Osztynowicz K, Michalak, et al. Expression of Small Heat Shock Proteins in Exosomes From Patients With Gynecologic Cancers. Sci Rep (2019) 9(1):9817. doi: 10.1038/s41598-019-46221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Silvers CR, Miyamoto H, Messing EM, Netto GJ, Lee YF. Characterization of Urinary Extracellular Vesicle Proteins in Muscle-Invasive Bladder Cancer. Oncotarget (2017) 8(53):91199–208. doi: 10.18632/oncotarget.20043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular Small Heat Shock Proteins: Exosomal Biogenesis and Function. Cell Stress Chaperones (2018) 23(3):441–54. doi: 10.1007/s12192-017-0856-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tang X, Chang C, Guo J, Lincoln V, Liang C, Chen M, et al. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour - Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci Rep (2019) 9(1):15108. doi: 10.1038/s41598-019-51704-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of Heat Shock Proteins in B-Cell Exosomes. J Cell Sci (2005) 118(Pt 16):3631–8. doi: 10.1242/jcs.02494 [DOI] [PubMed] [Google Scholar]
- 94. Bresnick AR, Weber DJ, Zimmer DB. S100 Proteins in Cancer. Nat Rev Cancer (2015) 15(2):96–109. doi: 10.1038/nrc3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xia C, Braunstein Z, Toomey AC, Zhong J, Rao X. S100 Proteins as an Important Regulator of Macrophage Inflammation. Front Immunol (2018) 8:1908. doi: 10.3389/fimmu.2017.01908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 Proteins. Curr Mol Med (2013) 13(1):24–57. doi: 10.2174/156652413804486214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Foell D, Wittkowski H, Vogl T, Roth J. S100 Proteins Expressed in Phagocytes: A Novel Group of Damage-Associated Molecular Pattern Molecules. J Leukoc Biol (2007) 81(1):28–37. doi: 10.1189/jlb.0306170 [DOI] [PubMed] [Google Scholar]
- 98. Kligman D, Hilt DC. The S100 Protein Family. Trends Biochem Sci (1988) 13(11):437–43. doi: 10.1016/0968-0004(88)90218-6 [DOI] [PubMed] [Google Scholar]
- 99. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-Mediated Upregulation of Chemoattractants and Recruitment of Myeloid Cells Predetermines Lung Metastasis. Nat Cell Biol (2006) 8(12):1369–75. doi: 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- 100. Prieto D, Sotelo N, Seija N, Sernbo S, Abreu C, Durán R, et al. S100-A9 Protein in Exosomes From Chronic Lymphocytic Leukemia Cells Promotes NF-κb Activity During Disease Progression. Blood (2017) 130(6):777–88. doi: 10.1182/blood-2017-02-769851 [DOI] [PubMed] [Google Scholar]
- 101. Li H, Huang X, Chang X, Yao J, He Q, Shen Z, et al. S100-A9 Protein in Exosomes Derived From Follicular Fluid Promotes Inflammation via Activation of NF-κb Pathway in Polycystic Ovary Syndrome. J Cell Mol Med (2020) 24(1):114–25. doi: 10.1111/jcmm.14642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, et al. The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries Toward Exosomes. J Biol Chem (2013) 288(17):11649–61. doi: 10.1074/jbc.M112.445304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, et al. Colorectal Cancer Cell-Derived Microvesicles Are Enriched in Cell Cycle-Related mRNAs That Promote Proliferation of Endothelial Cells. BMC Genomics (2009) 10:556. doi: 10.1186/1471-2164-10-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bosch S, Young NA, Mignot G, Bach J-M. Epigenetic Mechanisms in Immune Disease: The Significance of Toll-Like Receptor-Binding Extracellular Vesicle-Encapsulated MicroRNA. Front Genet (2020) 30(11):578335. doi: 10.3389/fgene.2020.578335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liang H, Kidder K, Liu Y. Extracellular MicroRNAs Initiate Immunostimulation via Activating Toll- Like Receptor Signaling Pathways. ExRNA (2019) 1(1):9. doi: 10.1186/s41544-019-0009-x [DOI] [Google Scholar]
- 106. Bayraktar R, Bertilaccio MTS, Calin GA. The Interaction Between Two Worlds: MicroRNAs and Toll-Like Receptors. Front Immunol (2019) 10:1053. doi: 10.3389/fimmu.2019.01053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fabbri M, Paone A, Calore F, Galli R, Croce CM. A New Role for MicroRNAs, as Ligands of Toll-Like Receptors. RNA Biol (2013) 10(2):169–74. doi: 10.4161/rna.23144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim SJ, Chen Z, Essani AB, Elshabrawy HA, Volin MV, Volkov S, et al. Identification of a Novel Toll-Like Receptor 7 Endogenous Ligand in Rheumatoid Arthritis Synovial Fluid That can Provoke Arthritic Joint Inflammation. Arthritis Rheumatol (Hoboken NJ) (2016) 68(5):1099–110. doi: 10.1002/art.39544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, et al. MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PloS Pathog (2015) 11(7):e1005032. doi: 10.1371/journal.ppat.1005032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs Bind to Toll-Like Receptors to Induce Prometastatic Inflammatory Response. Proc Natl Acad Sci USA (2012) 109(31):E2110–6. doi: 10.1073/pnas.1209414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Théry C, Witwer KW. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Ves (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]