Abstract
Granulomatous mastitis (GM) is an underdiagnosed and understudied benign inflammatory disease of the breast whose accurate diagnosis is confounded by mimicry of other breast pathologies (infectious mastitis and abscess, malignancy) and limited clinician knowledge of the disease. GM disproportionately affects minority women, furthering health disparities for a demographic already disadvantaged in the care of breast diseases. The first step in diagnosis is ultrasound followed by core needle biopsy yielding granulomatous inflammation. To far lesser degree, mammography, and MRI may play a role in narrowing the differential. A high index of clinical suspicion and multidisciplinary approach is required. The presence of Corynebacterium kroppensteddti may indicate one subtype of granulomatous mastitis called cystic neutrophilic granulomatous mastitis; disease stratification, and individualized therapy are on the horizon.
Keywords: Granulomatous mastitis, Disparities, Breast Imaging, Ultrasound, Corynebacterium, Cystic Neutrophilic Granulomatous Mastitis
Introduction
First described in 1972 [1] as a breast lesion mimicking carcinoma, granulomatous mastitis (GM) remains an evasive diagnosis. GM is estimated to occur in 2.4 per 100,000 women [2], however, this is almost certainly underestimated due to misdiagnosis. Although a benign process, GM may be mistaken for breast cancer on mammography [3], [4], [5]. It has a predilection toward pre-menopausal women with recent pregnancy and lactational history, and most prevalently affects Hispanic, Black, Arabic, and Asian women [2,6,7]. At the authors’ institution, the vast majority of patients are Latina women of childbearing age, with a minor percentage being Asian and Black childbearing women. The clinical presentation typically involves a painful peripheral breast mass which may involve concurrent inflammation, abscess, fistula, regional lymphadenopathy as well as nipple retraction. Thus, the clinical and radiologic features of GM overlap several malignant, infectious and autoimmune pathologies, contributing to misdiagnosis.
The first step in diagnosis is breast ultrasound which typically demonstrates a solid mass with or without concurrent abscess. This is followed by core needle biopsy demonstrating granuloma formation, multinucleated giant cells, epithelioid histiocytes, and plasma cells (Fig. 1) [5]. GM is a diagnosis of exclusion; thus, malignancy, infectious etiologies such as tuberculosis or histoplasmosis and autoimmune conditions such as granulomatosis with polyangiitis and sarcoidosis must be ruled out. Treatment interventions for GM include oral steroids and surgical excision, [8,9] however, given a paucity of data, there is no established treatment consensus. Unfortunately, misdiagnosis may lead to repeated but ineffective incision and drainage resulting in excess cost and morbidity to an already disadvantaged demographic. Mammogram and MRI are sometimes ordered to narrow the differential diagnosis.
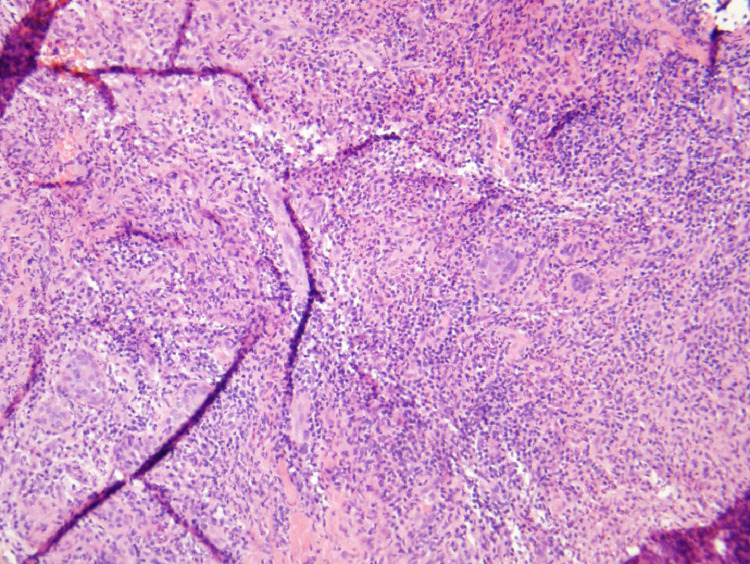
Fig. 1.
20x Hematoxylin and eosin stain demonstrates granuloma with inflammatory cells including neutrophils. Slide courtesy of Dr Siobhan O'Connor UNC department of pathology and laboratory medicine.
Here we present a case of a 23-year-old Latina woman who presented with asynchronous bilateral breast masses typical for granulomatous mastitis.
Case report
A 23-year-old, healthy, Spanish-speaking woman requiring interpreter was referred for evaluation of right breast mass discovered on self-exam approximately 2 months prior. She reported a right tender breast mass with overlying erythema which had begun to improve by the time she was able to be seen for her appointment. She did not have associated fever, chills, breast retraction, nipple inversion, breast swelling or discharge.
The patient's reproductive history was significant for G1P1, last pregnant at age 18. She reported onset of menarche at age 12. She had used oral contraceptive pills for the past 3 years. There was no personal or family history of breast cancer. A comprehensive exam of the breasts and chest wall was performed with the patient upright and supine in position. The right breast was mildly tender to palpation, with a 1.5 cm mass at the 9-o'clock position 3 cm from the nipple. Both breasts exhibited normal symmetry and contour without dimpling, skin changes, nipple inversion, nipple crusting or excoriation, and there was no palpable axillary lymphadenopathy.
Targeted right breast ultrasound demonstrated an irregular, poorly delineated, greater than 5 cm site of architectural distortion and serpiginous hypoechoic echogenicity at 8:00, with a parenchymal 3 cm parallel mass, and an adjacent but separate not parallel mass tracking to the posterior skin line (Fig. 2). This study was interpreted as BI-RADS Assessment Category 4A Suspicious, portending a 2%-9% risk of malignancy. A core needle biopsy was obtained (Fig. 3) yielding marked suppurative inflammation with granulation tissue, giant cells, and aggregates of histiocytes with at least 1 non–caseating granuloma. Adjacent to an abscess, inflammatory changes were present in a lobulocentric distribution. Given these right breast findings consistent with GM, she was prescribed a course of oral steroids but was lost to follow up for several months. She returned to clinic with recurrent symptoms in the ipsilateral breast 5 months later and was treated with an additional 7-month taper of oral steroids. This resulted in resolution of symptoms, with mass no longer clinically palpable. Notably, despite use of interpreter services, multiple instances of communication barriers were noted by clinicians related to the tapering instructions, resulting in prolongation of steroid treatment.
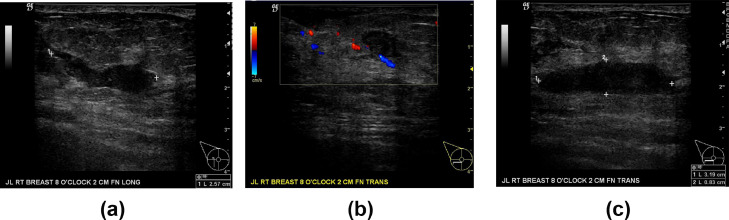
Fig. 2.
Targeted right breast ultrasound at the 8:00 position 2 cm from the nipple demonstrate an irregular, poorly delineated, site of architectural distortion, and serpiginous hypoechoic echogenicity. 2A, 2B, and 2C images show hypoechoic hypervascular inflammatory change.
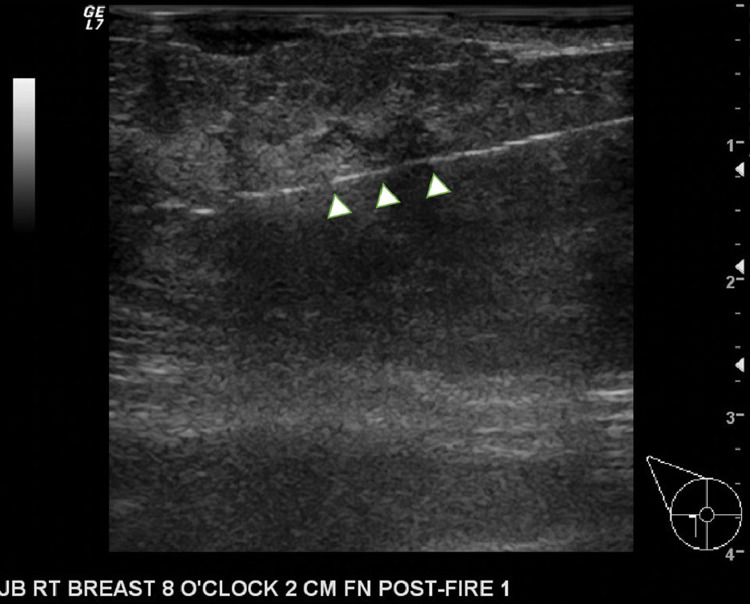
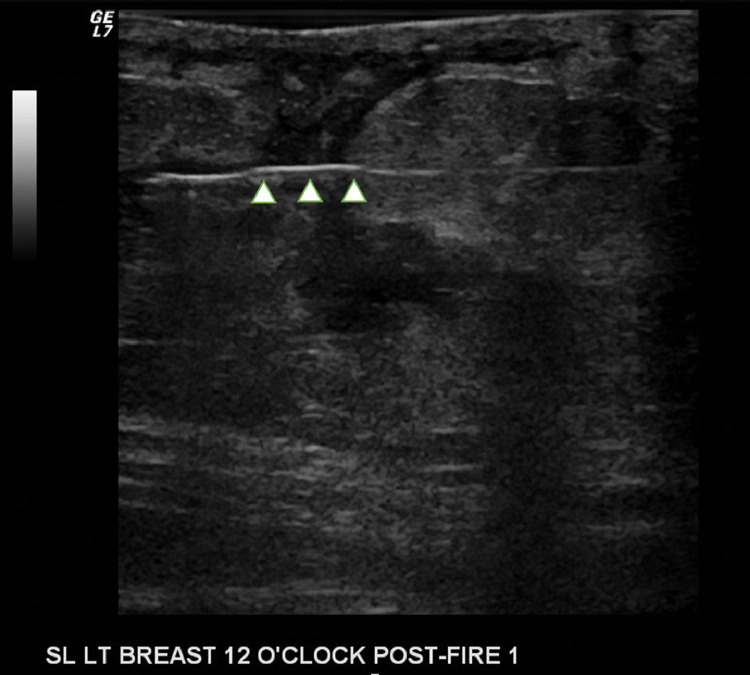
Fig. 3.
Targeted right breast ultrasound during core needle biopsy at the 8:00 position 2 cm from the nipple demonstrates the brightly echogenic biopsy needle (arrowheads) traversing one of the irregular hypoechoic portions of the mass.
Two months after resolution of her symptoms she presented to the emergency department for similar pain in the contralateral left breast along with erythema and fluctuance on exam. Targeted left breast ultrasound showed changes consistent with GM and concurrent abscess (Fig. 4A); this had increased in size 4 weeks later (Figs. 4B and C). A left breast core needle biopsy (Fig. 5) confirmed the diagnosis and cultures obtained were positive for Corynebacterium kroppenstedtii. The abscess was aspirated (Fig. 6) and she was treated successfully with a course of antibiotics for abscess combined with an oral steroid taper for GM. To date, the patient has not returned to clinic for recurrence of GM within the past 16 months.
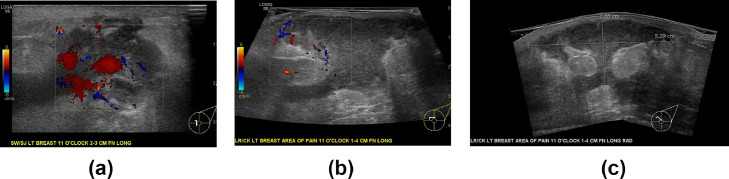
Fig. 4.
Targeted ultrasound of the left breast centered at 11:00 4 cm from the nipple demonstrates changes consistent with granulomatous mastitis, namely inflammatory phlegmon, a hypervascual irregular hypoechoic collection, and regional architectural distortion to include skin thickening in the left breast. The collection that increased in size over 4 weeks. Figures 4A in February 2020 shows marked hyperemia and acute inflammatory change. Figures 4B and 4C demonstrate abscess with internal scintillating debris measuring at least 5.3 × 2.3 cm. The collection involves the superficial and deep breast tissue with overlying skin thickening at the site of erythema.
Fig. 5.
Targeted left breast ultrasound during core needle biopsy at the 12:00 position demonstrates the brightly echogenic biopsy needle (arrowheads) traversing the irregular hypoechoic portions of the inflammatory mass.
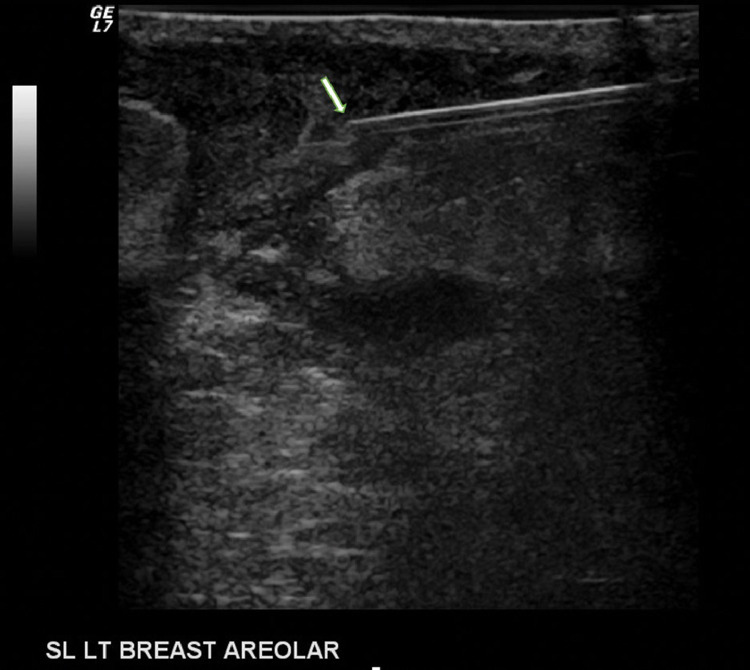
Fig. 6.
Targeted left breast ultrasound during aspiration demonstrates the brightly echogenic 18 g needle (arrow) in the left periareolar abscess. Culture yielded Corynebacterium kroppenstedtii, a gram positive aerobic bacillus.
Discussion
GM is an underdiagnosed and understudied chronic disease-causing significant morbidity and excess cost in minority populations already afflicted by disparaties of care and disease burden related to other breast pathologies [9], [10], [11], [12].
The pathogenesis of GM is not fully elucidated. Suggested mechanisms include autoimmune response to milk stasis; thus lactation and hyperprolactinemia with resultant ductal ectasia are risk factors. Reaction to trauma and infection with Corynebacterium kroppenstedtii have also been associated with GM [5]. As with all of GM, there is as yet no treatment consensus for Corynebacterium breast infection, a gram-positive aerobic bacilli bacteria. Established infections in lipophilic granulomata are often difficult for antibiotics to penetrate, and lipophilic antibiotics may be superior [13]. As in this patient, breast infection with Corynebacterium species is associated with a rare subtype of granulomatous mastitis called cystic neutrophilic granulomatous mastitis, [14], [15], [16] which seems to present one opportunity to stratify disease severity, and individualize therapy [17,18]. Alternative imaging modalities such as shear wave elastography, which may differentiate GM from other pathologies like malignancy, are also newly reported [19].
Despite these advances in knowledge and opportunities for GM patients’ personalized therapy, the most fundamental conundrum surrounding GM is how to minimize its underdiagnosis that in turn perpetuates disparities of breast care in minority women. A high index of suspicion and multidisciplinary approach between radiologists, emergency physicians, primary care physicians, and surgeons are necessary for diagnosis. As exemplified by this case, patients may face communication barriers, lack consistent follow-up, and may present to the emergency department late in disease course following complications. All of these factors serve to coalesce and contribute to diagnostic confusion and repeated core biopsies with attendant complications and financial toxicity. The phrase “you'll see it when you know it” [20] highlights the importance of increasing awareness and the responsibility physicians in multiple specialties bear in minimizing underdiagnosis and misdiagnosis of GM.
Patient consent
“Written patient consent was obtained for the use of medical imaging and case details for teaching publication purposes in a deidentified manner.”
Footnotes
Declaration of Competing Interest: None.
References
- 1.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. AM J Clin Pathol. 1972;58:642–646. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Idiopathic granulomatous mastitis in Hispanic women – Indiana, 2006-2008. MMWR Morb Mortal Wkly Rep. 2009;58:1317–1321. [PubMed] [Google Scholar]
- 3.Patel RA, Strickland P, Sankara IR, Pinkston G, Wickliffe M, Jr., Rodriguez M. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25(3):270–273. doi: 10.1007/s11606-009-1207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz E, Lebe B, Usal C. Mammographic and sonographic findings in the diagnosis of idiopathic granulomatous mastitis. Eur Radiol. 2001;11(11):2236–2240. doi: 10.1007/s003300100965. [DOI] [PubMed] [Google Scholar]
- 5.Aydin I, Kesicioglu T, Vural S. Idiopathic granulomatous lobular mastitis: an imitation of breast carcinoma. Cureus. 2021;13(5):e15206. doi: 10.7759/cureus.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfrum A, Kummel S, Theuerkauf I. Granulomatous mastitis: a therapeutic and diagnostic challenge. Breast Care. 2018;13:413–418. doi: 10.1159/000495146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreto DS, Sedgwick EL, Nagi CS. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. 2018;171(3):527–534. doi: 10.1007/s10549-018-4870-3. [DOI] [PubMed] [Google Scholar]
- 8.Lei X, Chen K, Zhu L, Song E, Su F, Li S. Treatments for idiopathic granulomatous mastitis: systematic review and meta-analysis. Breastfeed Med. 2017;12(7):415–421. doi: 10.1089/bfm.2017.0030. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, Queralt-Martin R, Granel-Villach L, Laguna-Sastre JM. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. 2019;25(6):1245–1250. doi: 10.1111/tbj.13446. [DOI] [PubMed] [Google Scholar]
- 10.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in african american women: a review. JAMA Surg. 2017;152(5):485–493. doi: 10.1001/jamasurg.2017.0005. [DOI] [PubMed] [Google Scholar]
- 11.Nahleh Z, Botrus G, Dwivedi A, Badri N, Otoukesh S, Diab N. Clinico-pathologic disparities of breast cancer in Hispanic/Latina women. Breast Dis. 2018;37(3):147–154. doi: 10.3233/BD-170309. [DOI] [PubMed] [Google Scholar]
- 12.Lopez R, Agullo P, Lakshmanaswamy R. Links between obesity, diabetes and ethnic disparities in breast cancer among Hispanic populations. Obes Rev. 2013;14(8):679–691. doi: 10.1111/obr.12030. [DOI] [PubMed] [Google Scholar]
- 13.Dobinson HC, Anderson TP, Chambers ST, Doogue MP, Seaward L, Werno AM. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species. J Clin Microbiol. 2015;53(9):2895–2899. doi: 10.1128/JCM.00760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;72(8):445–453. doi: 10.1136/jclinpath-2019-206180. [DOI] [PubMed] [Google Scholar]
- 15.Tauch A, Fernandez-Natal I, Soriano F. A microbiological and clinical review on Corynebacterium kroppenstedtii. Int J Infect Dis. 2016;48:33–39. doi: 10.1016/j.ijid.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Troxell ML, Gordon NT, Doggett JS. Cystic neutrophilic granulomatous mastitis: association with gram-positive bacilli and Corynebacterium. Am J Clin Pathol. 2016;145(5):635–645. doi: 10.1093/ajcp/aqw046. [DOI] [PubMed] [Google Scholar]
- 17.Li XQ, Wu HL, Yuan JP, Liu TG, Sun SR, Chen C. Bacteria associated with granulomatous lobular mastitis and the potential for personalized therapy. J Invest Surg. 2020:1–7. doi: 10.1080/08941939.2020.1833262. [DOI] [PubMed] [Google Scholar]
- 18.Bownson KE, Bertoni DM, Lannin DR, Cohen PJ, Pronovost MT. Granulomatous lobular mastitis-another paradigm shift in treatment. Breast J. 2019;25(4):790–791. doi: 10.1111/tbj.13338. [DOI] [PubMed] [Google Scholar]
- 19.Smith E, Moore DA, Jordan SG. You'll see it when you know it: granulomatous mastitis. Emerg Radiol. 2021 doi: 10.1007/s10140-021-01931-4. [DOI] [PubMed] [Google Scholar]
- 20.Makal GB, Guvenc I. The role of shear wave elastography in differentiating idiopathic granulomatous mastitis from breast cancer. Acad Radiol. 2021;28(3):339–344. doi: 10.1016/j.acra.2020.02.008. [DOI] [PubMed] [Google Scholar]