Abstract
Cyanobacteria can utilize CO2 or even N2 to produce a variety of high value-added products efficiently. Plastoquinone (PQ) is an important electron carrier in both of the photosynthetic and respiratory electron transport chain. Although the content of PQ, as well as their redox state, have an important effect on physiology and metabolism, there are relatively few studies on the synthesis of PQ and its related metabolic regulation mechanism in photosynthetic microorganisms. In this study, the strategies of overexpression of Geranyl diphosphate: 4-hydroxybenzoate geranyltransferase (lepgt) and addition of 4-hydroxybenzoate (4-HB) as the quinone ring precursor were adopted to regulate the biosynthesis of PQ in Synechocystis PCC 6803. Combined with the analysis the photosystem activity, respiration rate and metabolic components, we found the changes of intracellular PQ reprogrammed the metabolism of Synechocystis PCC 6803. The results showed that the overexpression of lepgt reduced PQ content dramatically, by 22.18%. Interestingly, both of the photosynthesis and respiration rate were enhanced. In addition, the intracellular lipid and protein contents were significantly increased. Whereas, the addition of low concentrations of 4-HB enhanced the biosynthesis of PQ, and the intracellular PQ contents were increased by 14.76%–70.86% in different conditions. Addition of 4-HB can regulate the photosystem efficiency and respiration and reprogram the metabolism of Synechocystis PCC 6803 efficiently. In a word, regulating the PQ biosynthesis provided a novel idea for promoting the reprogramming the physiology and metabolism of Synechocystis.
Keywords: Cyanobacteria, Plastoquinone, LePGT, 4-HB, Metabolic rewiring
1. Introduction
Cyanobacteria is a photosynthetic microorganism, which can use CO2 even N2 to efficiently synthesize high value-added compounds (e.g. ethanol, fatty acids, isoprene) [1]. It has broad application prospects in the fields of food, bioenergy and environments. A variety of strategies, such as genetic engineering or stress tolerance, have been applied to increasing the level of target products of Cyanobacteria. For example, both nitrogen stress conditions [2] and overexpression of key enzymes [3] can be used to increase lipids accumulation. Reducing power and energy play a crucial role in the growth and metabolism of algal cells. The change and consumption rate of NADPH and ATP in algae can regulate the intracellular physiological metabolic rate and biomass productivity [[4], [5], [6]].
Synechocystis is one of the most widely used prokaryotic model cyanobacteria nowadays [7]. Synechocystis sp. PCC 6803 is a model cyanobacteria which can synthesize glycogen, fatty acids and polyhydroxybutyrate. Photosynthetic and respiratory electron transport chain plays a vital role in photosynthetic microorganisms. Plastoquinone (PQ) is an important electron transfer intermediate, which mediates the transfer of reducing power and energy in Synechocystis [8]. In Synechocystis, the quinone ring precursor of PQ is 4-hydroxybenzoic acid (4-HB), which is different from eukaryotic microalgae [9]. The synthesis pathway of 4-HB in Synechocystis is similar to that of Escherichia coli which synthesized from chorismite [10]. The isoprene side chain precursor of PQ is solanesyl diphosphate (SPP) or dipropyl diphosphate (DPS), which is synthesized through the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in Synechocystis [11]. In the second synthesis stage, 4-HB and SPP undergo a series of decarboxylation, hydroxylation, methylation, and other steps to synthesize PQ-9. However, the genes and metabolic pathways of the PQ biosynthetic pathway in Synechocystis are not completely understood yet.
The content and the redox status of PQ are important to the growth and metabolism of cyanobacteria. Firstly, PQ acts as a carrier of electrons in the photosynthetic electron transport chain. PQ is an intermediate of acyclic electron transport chain, which transmits electrons from PSII to cytochrome b6f (Cyt b6f). Besides, PQ is also an important part of the cyclic electron transport chain. In this cycle, electrons can be transmitted from PQ to PSI through Cyt b6f, then back to PQ through reduced ferredoxin or NADPH [12]. What's more, PQ also plays an important role in respiratory electron transport chain in Synechocystis. There are many studies on the PQ-mediated photosynthetic electron transport chain, while relatively few exploration on the PQ-mediated respiratory electron transport chain of Synechocystis. It is known that the electrons generated by the substrate on the respiratory electron transport chain are transferred to PQ through NDH-1 or SDH, and then transferred to electron carriers such as cyt b6f, and finally transferred to terminal oxidase [13]. Studies have shown that electrons generated from respiratory can participate in the photosynthetic electron transport chain too [14]. The respiratory chain can provide or remove electrons in the photosynthetic electron transport chain to prevent excessive oxidation or reduction of PQ [15]. The energy and reducing power generated by the PQ-mediated photosynthetic and respiratory electron transport chain can participate in the synthesis of various important chemical compounds in Synechocystis.
Furthermore, PQ is involved in the anabolism of various compounds in microalgae and cyanobacteria. For example, it is involved in the synthesis of carotenoids [16]. It plays an important role in the formation of the transmembrane proton gradient required for ATP synthesis in the chloroplast [17]. Finally, PQ can participate in a variety of biological behaviors, including acting as redox sensor [18], responding to heavy metal ion concentration changes [19], responding to biological pressure [20], and mediating programmed death [21].
As a carrier of electrons for photosynthesis and respiration, changes in PQ content will have a vital impact on the growth and metabolism of photosynthetic organisms [22]. Previous studies have found that complete lack of PQ would result corn to turn yellow and was prone to death in the seedling stage [23]. The complete lack of PQ inhibits the photosynthetic electron transfer in the chloroplast and results in overexcitation of PSII and PSI [24]. Excessive excitation of PSII and PSI will produce a large amount of reactive oxygen species (ROS), cause severe photo-oxidation damage, and seriously affect chloroplast metabolism [25]. The complete lack of PQ in the mesophyll cell (MC) results in the accumulation of large starch granules in the chloroplasts, but no starch accumulation in bundle sheath cells (BSC) chloroplasts of maize [26]. The difference in the degradation rate of ROS between MC and BSC chloroplasts indicates that there are differences in the mechanism of physiological metabolism and synthesis between the two organelle types. Therefore, the variation of PQ content in different photosynthetic organisms may lead to different phenotypes.
Geranyl diphosphate: 4-hydroxybenzoate geranyltransferase (lepgt) from Lithospermum erythrorhizon is a key catalytic enzyme in shikonin biosynthesis pathway. LePGT synthesizes isoprene 4-hydroxybenzoate (G-4HB) by using 4-HB and GPP [27,28]. Interestingly, 4-HB and GPP are the same substrates for ubiquinone biosynthesis [29]. We previously studied the expression of lepgt in E. coli, and found that ubiquinone (UQ) content was reduced and led to the accumulation of lactic acid, which was approximately the theoretical yield [30]. It was proved that small changes in UQ content in E. coli could achieve precise regulation of metabolism. In addition, green microalgae, such as Chlamydomonas reinhardtii, can synthesize UQ by adding 4-HB [31].
Here, we attempted to regulate the intracellular PQ content through the strategies of overexpressing lepgt or adding 4-HB to Synechocystis. The metabolic rules like photosystem efficiency and respiration rate, as well as intracellular metabolite production mode were studied. To the best of our knowledge, this is the first attempt to discuss the relationship between PQ content and intracellular metabolites in photoautotrophic microorganism.
2. Materials and methods
2.1. The culture condition of Synechocystis PCC 6803
All Synechocystis strains used in this study were cultured with BG11 medium at 28 °C, 120 rpm and continuous illumination of 50 μmol photons/m2/s. The logarithmic phase of Synechocystis strains were seeded into fresh BG11 medium with initial concentration of OD730 = 0.1. 4-HB was dissolved in ethanol and diluted down in the growth medium to a final concentration of 1% ethanol, and the final concentration in the experimental group was 0 mM, 1 mM, 2 mM. The group of 0 mM was wild type Synechocystis which 1% ethanol was added.
2.2. Construction of plasmids and transformation of Synechocystis
The sequence of lepgt was optimized according to the codon preference of the Synechocystis and synthesized by GenScript China. The primer sequences were shown in Table S1. Plasmid pUC-lepgt was constructed by inserting promoter psbAII, lepgt, and terminator TrbcL.
The transformants in this study were obtained by the natural transformation of Synechocystis and the expression cassette of lepgt was integrated into the slr0168 site of the Synechocystis chromosome by homologous recombination. The single colonies, which were isolated form plate resistance screen several times, were identified at DNA and RNA levels. The single colonies that had been demonstrated successful transcription were selected as transformants (named “LePGT”).
2.3. Optical density and cytochrome content assays
The optical density (OD) of Synechocystis was calculated according to the absorbance of spectrophotometer at 730 nm. During the cultivation of Synechocystis, 1 mL culture was centrifuged every 48 h (12000 g, 5 min) and the pellet was resuspended in 1 mL N, N-dimethylformamide. The solution was mixed before dark treatment 5min for extraction. The mixture was collected by centrifugation (12000 g, 5 min), and the absorbance of the supernatant was used to calculated cytochrome content. The cytochrome content calculating was done based on previous study [32].
2.4. Measurements of photosystem efficiency and respiration rate
Cells growing in the middle of the exponential phase (cultured 7 days in Fig. 1e), were centrifuged, and concentrated to a chlorophyll concentration of 20 μg/mL. Taken 3 mL of the algae solution concentrated to a chlorophyll concentration of 20 μg/mL, and leaved it in the dark for 15 min. The chlorophyll fluorescence parameters of PSII were measured by AquaPen FP110 (FluorCam, Czech Republic).
Fig. 1.
Destabilization of plastoquinone content in Synechocystis PCC 6803 with two strategies. (a): Construction of transformant; (b): Identification of transformants pUC-lepgt at DNA levels; (c): Identification of transformants pUC-lepgt at cDNA levels; (d): PQ content in different treatments; (e): Growth comparison. The group of 0 mM was wild type Synechocystis which 1% ethanol was added. We verified the upstream homology arms, the target gene and the downstream homology arm by using primers Up-F and psbAII-R, primers LePGT-F and LePGT-R, Trbcl-F and Down-R, respectively. RNA was extracted and reverse transcribed into cDNA. The primers LePGT-F and LePGT-R were used to screen the successfully transcribed strains. Standard errors was calculated from three biological independent experiments; asterisks represent significant differences (*p < 0.05, **p < 0.01).
Cells in the middle of the exponential phase were centrifuged, and concentrated to a chlorophyll concentration of 100 μg/mL. Taken 2 mL of the algae solution concentrated to a chlorophyll concentration of 100 μg/mL, and leaved it in the dark for 1 min. The P700 and P700+ parameters of PSI were measured by Dual-PAM-100 (Walz, Germany).
Cells in the middle of the exponential phase were centrifuged, and concentrated to a chlorophyll concentration of 10 μg/mL. Taken 10 mL of the algae solution concentrated to a chlorophyll concentration of 100 μg/mL, and leaved it in the dark for 5 min. The respiration rate of Synechocystis was measured by FireStingGO2 oxygen electrode (Pyro Science Sensor Technology, Germany) under dark conditions [33].
2.5. Plastoquinone content assays
The extraction and analysis method of PQ referred to the method of PQ extraction and HPLC separation from Chlamydomonas reinhardtii with slight modifications [34]. The Synechocystis under mid-log phase was collected by centrifugation (12000 g, 2 min, 4 °C) and quickly resuspended in methanol. The supernatant was collected by centrifugation (12000 g, 2 min, 4 °C) and quickly entered HPLC system for analysis. The analysis method of PQ-9 was as follows: C18 reversed-phase column (Hypersil ODS2 5 μm, 4.6 mM × 250 mM) with mobile phase of methanol: n-hexane (340/40, v/v). The column temperature was 40 °C. The flow rate was 1.6 mL/min and the absorbance detection wavelength was 255 nm. The concentrations of PQ-9 in the extract were evaluated by comparing with the PQ-9 standard and normalized based on OD730 value of the Synechocystis sample.
2.6. Intracellular metabolites assays
For fatty acid, cells were collected at the stable phase by centrifugation (6500 g, 8 min), freeze-dried for 48 h, and ground into algal powder. 10 mg algae powder was used to extract the crude oil according to the method of Folch et al. [35]. Adding 1% H2SO4-CH3OH (2 mL), vertexing, and incubating at 70 °C for 30 min. Then adding cyclohexane containing 0.133 g/L methyl heptadecanoate (5 mL) to mixture and vertexing. Water was added to mixture, vortexed and then stratified. The Agilent GC-6890 N equipped with 5975 inert mass selective detector was used to analyze the profiles and contents of fatty acids. The GC-MS analysis program was kept at 120 °C for 1 min. The temperature was increased to 170 °C at a temperature increase of 10 °C/min and retained for 1 min. The temperature was raised to 260 °C at the rate of 10 °C/min until the analysis was completed.
The Synechocystis on stable phase was extracted and calculated by glycogen extraction kit (Suzhou Keming Company, China). For protein production assay, the NaOH solution (0.5 mol/L, 10 mL) was added into a centrifuge tube containing 5 mg algal powder. The cells were crushed by ultrasonication and incubated at 75 °C for 30 min. The supernatant was collected by rapid centrifugation (12000 g, 10 min, 4 °C). The protein concentration was determined by BCA method.
For neutral lipid content assay, Synechocystis on the stable phase was centrifuged and resuspended to OD730 = 0.5 with BG11 medium. 10 μL Nile red was added into the mixture. The mixture was vortexed and incubated for 10 min in the dark. The excitation wavelength was at 530 nm and the emission wavelength was at 575 nm in the multifunctional enzyme marker. The neutral lipid content was calculated according to fluorescence intensity.
2.7. Statistical analysis
The mean, error bars, and p-values in this study were calculated from three independent experiments and three biological replicates in each experiment using t-test in SPSS. p < 0.05 represents a significant difference.
3. Results and discussion
3.1. Two strategies of regulating the PQ biosynthesis in Synechocystis
To reduce the content of PQ, we constructed the plasmid pUC-lepgt and integrated the expression cassette of lepgt into the Synechocystis chromosome through natural transformation and homologous recombination (Fig. 1a). The single colony, after multiple purification, was boiling to quickly extract the genome. We verified the upstream homology arms, the target gene and the downstream homology arm by using primers Up-F, psbAII-R, primers LePGT-F and LePGT-R and Trbcl-F and down-R, respectively (Table S1 and Fig. 1b). The successfully integrated single algal colonies were selected and cultured to the middle of logarithmic growth phase. RNA was extracted and reverse transcribed into cDNA (Fig. 1c). We confirmed that the lepgt in the selected transformants was successfully transcribed by identifying the DNA and RNA of the transformants. Phenotypes of transformants and wild type strains were measured. The content of PQ in transformant was significantly reduced by 22.18% (p < 0.05) compared with the wild-type (Fig. 1d).
In another strategy, different concentrations (0 mM, 1 mM, 2 mM) of 4-hydroxybenzoic acid (4-HB) were added to promote the synthesis of PQ in Synechocystis. The experimental group of 0 mM was wild type Synechocystis with 1% (v/v) ethanol. After addition of 1 mM 4-HB, the intracellular PQ content of the experimental group increased by 14.76% than that of the control. In addition, when Synechocystis cultured in the medium containing 2 mM 4-HB, the intracellular PQ content increased to 596.16 nmol/OD and significantly increased by 70.86% than that of the control one (p < 0.01) (Fig. 1d).
Different from eukaryotic microalgae, the quinone ring precursor of PQ in Synechocystis is 4-HB and the precursor of isoprene side chain is GPP. Therefore, the overexpression of lepgt in Synechocystis can drain the precursors of PQ, 4-HB and GPP, to reduce PQ content. The increasing of PQ content with 4-HB treatment confirmed that Synechocystis could synthesize PQ with exogenous 4-HB. The changes of PQ content will affect electron transfer efficiency of the photosynthetic and respiratory, and further affect the efficiency of photosynthetic and respiratory system. It ultimately influences production of reducing power and energy. Hence, small changes in PQ content will probably reprogram the intracellular metabolic flux.
3.2. Changes in PQ content had little effect on the growth
The growth curves of the wild type and the transformant showed no significant difference (Fig. 1e). The results indicated that the small amount of reduced PQ content might have no significant effect on the growth of Synechocystis. Meanwhile, the effect of the addition of 4-HB on the growth rate of Synechocystis were also studied. As shown in Fig. 1e, compared to the control group, the addition of 1 mM 4-HB and 2 mM 4-HB had little effect on the growth of Synechocystis. However, higher concentrations (3 mM) inhibited the growth of Synechocystis (50% at 240 h), and the growth was completely inhibited with 5 mM addition (data not shown). In order to further study the effects on metabolism of Synechocystis, we measured the efficiency of the photosystem, respiration rate, and the concentrations of intracellular metabolites in Synechocystis.
3.3. The effect of changes in PQ content on photosystem II
3.3.1. The effect of reduced PQ content on photosystem II
In order to further study the effect of PQ content reduction on photosynthesis, the chlorophyll fluorescence parameters of the wild type and transformant in the exponential phase were measured and analyzed according to previous method [36]. The fluorescence values at step O, J, and I (Fo, Fj, Fi) and the maximum fluorescence value (Fm) of the transformant were all higher than those of the wild type (Table S2). The basic shape of the OJIP fast fluorescence kinetic curve was consistent with that of the wild type (Fig. 2a and c). The initial slope of the fluorescence curve (Mo) and the fluorescence level of step J (Vj) decreased by 7.95% and 7.55%, respectively. This suggested that overexpression of lepgt promoted the electron transport chain between Qa and Qb in Synechocystis [37]. The values of parameter Sm and Ψ0 in transformant were both higher than those of the wild type (Table S2). It was indicated that the transfer yield of per captured electron in transformant was higher [38]. Though the values of Fv and Fm in transformant were increased by 9.32% and 17.62%, respectively, the maximum electron transfer efficiency (Fv/Fm) of the photosystem II (PSII) of transformant was slightly increased by 0.24%.
Fig. 2.
Chlorophyll a fluorescence transients of Synechocystis PCC 6803.
(a): Chlorophyll a
fluorescence kinetics curve of wild-type and transformant; (b): Chlorophyll a fluorescence kinetics curve with different concentrations of 4-HB; (c): The spider-plot presentation of selected parameters quantifying the behavior of PS II of wild-type and transformant; (d): Spider-plot presentation of selected parameters quantifying the behavior of PS II with different concentrations of 4-HB. The group of 0 mM was wild type Synechocystis which 1% ethanol was added.
PIABS, based on the “vitality” index or survival index of light quantum flux absorption, was the overall expression of photosynthetic activity. The PIABS value of the transformant increased by 22.54% which proved that the overexpression of lepgt increased the “vitality” of PSII in Synechocystis. The values of φPo and φEo of the transformant increased by 12.94% and 9.21% than the wild-type, respectively. The overexpression of lepgt enhanced the capture efficiency of absorbed energy and the transfer the absorbed energy to the photosynthetic electron transport chain.
When we considered the changes in the specific energy fluxes used for energy absorption (ABS/RC), capture (TRo/RC), transport (ETo/RC), and consumption (DIo/RC), the data showed that the values of absorption and capture efficiency were slightly reduced and the electron transfer efficiency was increased by 11.55% compared to the wild type. This indicated that the overexpression of lepgt might increase the electron transfer efficiency and reduce the energy dissipation efficiency in Synechocystis. We found that the PIABS value of the transformant increased due to the increasing in the efficiency of electron transport in the photosynthetic pathway and other pathways flowing into the photosynthetic electron transport chain [39]. qPQ represented the non-photochemical quenching of PSII and the value of qPQ in transformant significantly increased by 180.70% (p < 0.05). It was proved that the energy absorption in the photosynthetic electron transport chain of the transformant was much higher than the energy utilization efficiency and the over-excitation of PSII was prevented by non-photochemical quenching.
3.3.2. The effect of different increments of PQ on photosystem II
We measured the effect on the chlorophyll fluorescence parameters of Synechocystis with low-concentration 4-HB addition. The results showed that the initial slope of the fluorescence curve (Mo) and the fluorescence level of step J (Vj) gradually increased with the increasing of 4-HB concentration (Fig. 2b and d). Compared with the control group, the values of Mo, Vi and Vj amounted to 149.20%, 193.65%, and 152.16% of the control in the Synechocystis treated with 2 mM 4-HB. The addition of low-concentration 4-HB inhibited electron transfer from Qa to Qb in Synechocystis [40]. The basic shape of the OJIP fast fluorescence kinetic curve of Synechocystis with 1 mM 4-HB addition was consistent with that of the control group, but it changed with 2 mM 4-HB addition.
Though the values of the parameter Fv and Fm of the experimental group treated with 1 mM 4-HB were diminished by 11.30% and 9.26%, respectively, the maximum electron transfer efficiency (Fv/Fm) of PSII slightly reduced and had no significant change compared with the control group. The values of the Fv and Fm at 2 mM of 4-HB were dropped by 70.48% and 70.09%, respectively. The value of Fv/Fm at 2 mM of 4-HB was only 0.04 and significantly decreased by 83.82% (p < 0.05). 4-HB diminished the Fv/Fm value of Synechocystis in a concentration-dependent manner [41].
We found that the PIABS value of Synechocystis gradually decreased with the increasing of 4-HB concentration. The PIABS value with 2 mM 4-HB addition was only 0.015. This result showed that the addition of 2 mM 4-HB could significantly decrease “vitality” of photosynthesis of Synechocystis. N is the reduction turnover amount of PQ. The N value of Synechocystis with 2 mM 4-HB improved by 494.36% which proved that 4-HB improved turnover numbers of PQ.
The values of parameter ETo/RC, ETo/ABS and ETo/TRo in Synechocystis treated with 2 mM 4-HB dropped by 33.86%, 20.83% and 9.60%, respectively, and the DIO/RC value increased by 974.86% compared with the control group. This indicated that the addition of 2 mM 4-HB could reduce the transfer efficiency of absorbed energy and captured energy and the electron transfer of per unit reaction center. It also increased the efficiency of energy dissipation in Synechocystis. In summary, by measuring the chlorophyll fluorescence parameters, we found that the PSII efficiency with 1 mM 4-HB addition was slightly reduced, whereas was significantly reduced with 2 mM 4-HB addition. The decrease of PIABS value in Synechocystis with 4-HB addition was diminished, because of the reducing of electron transfer rate in the electron transport chain and the increasing of energy dissipation efficiency [40].
3.4. The effect of changes in PQ content on photosystem I
3.4.1. The effect of reduced PQ content on photosystem I
In the photosynthesis of Synechocystis, Photosystem I (PSI) could transfer electrons from phycocyanin or cytochrome to ferredoxin. PSI is the most efficient photoelectric device in nature which every photon captured could be used for electron displacement [42]. Photosynthetic cyclic electron transport is the photosynthetic electron transport around PSI which starts and ends with PSI. It only produces ATP and does not produce NADPH which could regulate the ratio of ATP/NADPH [43]. As a carrier of electrons for photosynthesis and respiration, changes of PQ content will have a vital impact on the photosynthesis and respiration efficiency of photosynthetic organisms [44]. Under the stimulation of far red light and white light, the total photosystem efficiency of PSI in Synechocystis was measured by Dual-PAM-100. The parameter of P700 represents the photosynthetic system efficiency. The parameter of P700+ represents the efficiency of the linear electron transfer chain and the amount of PSI [45]. We found that the total photosystem efficiency of transformant was higher than the wild type (Fig. 3a and b). Our results indicated that the expression of lepgt increased the total photosystem efficiency of Synechocystis. DCMU could inhibit the electron transport from PSII to PQ, thus blocking the linear electron transport chain. With the addition of DCMU and far red light, we measured the efficiency of cyclic electron transport (P700+) in Synechocystis and found the photosystem I efficiency of the transformant in transformant was increased by 40.51% compared with the wild type (Fig. 3b). Taken together, the overexpression of lepgt increased the amount of PSI in the transformants. In conclusion, the overexpression of lepgt in Synechocystis diminished the PQ content, promoted the linear electron transport and the total photosystem efficiency of photosynthesis, and had little effect on the efficiency of cyclic electron transport chain. It would benefit to the NADPH and ATP produced by photosynthesis and regulated the NADPH/ATP ratio produced by photosynthesis.
Fig. 3.
Phenotypes variation of photosystem I and respiration of Synechocystis PCC 6803. (a): Effect of the overexpressed lepgt and exogenous 4-HB on the intital rate of 700 dark reduction; (b): Effect of the overexpression of lepgt and exogenous addition of 4-HB on the intital rate of P700+ dark reduction; (c): Respiration rate of Synechocystis PCC 6803. The group of 0 mM was wild type Synechocystis which 1% ethanol was added. The value obtained for WT was considered as 100. Standard errors was calculated from three biological independent experiments; asterisks represent significant differences (*p < 0.05, **p < 0.01).
3.4.2. The effect of different increments of PQ on photosystem I
The total photosystem efficiency in Synechocystis with low-concentration 4-HB was measured by Dual-PAM-100 with the stimulation of far red light and white light. The results showed that 4-HB can effectively improve the total photosystem efficiency (P700) at 1 mM addition and it can significantly inhibit photosystem at 2 mM addition (Fig. 3a). Then, we measured the photosystem I efficiency with the addition of DCMU and far red light (Fig. 3b) [45]. The results showed that 4-HB slightly increased the PSI efficiency of Synechocystis at 1 mM addition. However, with higher concentrations (2 mM) 4-HB showed inhibition effect. Furthermore, the number of PSI with 1 mM 4-HB addition slightly increased by 13.87% while the number of PSI treated with 2 mM 4-HB dropped by 50.82% in cyanobacteria.
In conclusion, 4-HB could slightly decrease the efficiency of PSII and increase the efficiency of cycle electron transport and the total photosystem at 1 mM. We assumed that the addition of 1 mM 4-HB slightly decreased the NADPH content produced by the linear electron transport chain, increased the ATP content produced by the cycle electron transport chain and adjusted the ratio of NADPH/ATP produced by the photosystem in Synechocystis. However, higher concentrations (2 mM) significantly restrain the efficiency of photosystem and decrease the production of both NADPH and ATP. Detailed multi-omics analysis is need to be conducted to further elucidate the mechanisms beyond the PQ regulation.
3.5. The effect of changes in PQ content on respiration rate
Respiration in cyanobacteria maintains a transmembrane proton gradient in the dark to promote the production of ATP and water, thus provides energy for some active transport [46]. In addition, PQ mediated respiration and photosynthesis are both on the thylakoid membrane. Therefore, the data of photosynthesis and respiratory system should be combined to analyze the impact of PQ content fluctuation on Synechocystis. We used FirestingGO2 oxygen electrode to measure the respiration rates of Synechocystis in the logarithmic period under dark conditions. As shown in Fig. 3c, the respiration rate of the transformant increased by 27.35% compared to the wild-type. In order to prevent the photosystem from overreduction, the metabolic cooperation between photosynthetic and respiratory is needed to supply more ATP [47]. From these results, we could infer that the overexpression of lepgt in Synechocystis could significantly reduce PQ content and significantly increase photochemical quenching efficiency of the photosystem. Therefore, the respiration rate was enhanced to promote the production of ATP.
As shown in Fig. 3c, the respiration rate of control group amounted to 20.20 μmol O2/mg Chl/h and increased by 20.01% compared with WT. It was an interesting phenomenon that the addition of 1% ethanol decreased the efficiency of total photosystem and improved the respiration rate. The respiration rate of cells with 1 mM 4-HB addition increased by 20.08%, and the respiration rate of cells with 2 mM 4-HB addition reduced by 54.90% (p < 0.01).
Combined with the analysis of chlorophyll fluorescence parameters, we found that the addition of 1 mM 4-HB had almost no significant effect on the PSII efficiency and increased the total photosystem efficiency and respiration rate. However, with the addition of 2 mM 4-HB, both photosystem efficiency and respiratory rate reduced significantly. Although the addition of two different concentrations of 4-HB both can lead to the increase of PQ content, it will have completely different effects on photosynthesis and respiration. This indicated that PQ not only acts as redox intermediate, but also affects the physiological state of Synechocystis in other ways. Thus the changes of PQ content will have a vital impact on the photosynthesis and respiration of Cyanobacteria.
3.6. The effect of changes in PQ content on intracellular metabolites
3.6.1. The effect of reduced PQ content on intracellular metabolites
Lipid and glycogen are the main intracellular reduction products of Synechocystis. Glycogen is the main carbohydrates store in cyanobacteria and has a wide range of biological functions. For example, it can be converted to bioethanol by yeast fermentation or be converted to methane by anaerobic fermentation in biogas plants [48]. Glycogen production in cyanobacteria can be significantly promoted under nitrogen stress. However, the nutritional stress significantly reduced the growth [49]. The overexpression of lepgt decreased the content of PQ and promoted the efficiency of photosynthesis and respiration in Synechocystis. In order to study the regulation mechanism of reduced PQ content on intracellular metabolism, the changes of fatty acids, glycogen, protein and other metabolites were analyzed (Table 1).
Table 1.
Physiological metabolism parameters of Synechocystis PCC 6803 with different treatments.
| WT | LePGT | Control | 1 mM 4-HB | 2 mM 4-HB | |
|---|---|---|---|---|---|
| Caretoind (μg/mL) | 2.27 ± 0.31 | 2.38 ± 0.050 | 2.03 ± 0.085 | 1.91 ± 0.015 | 1.86 ± 0.025 |
| PQ (nmol/OD730) | 337.78 ± 11.64 | 262.86* ± 6.47 | 348.91 ± 16.34 | 400.40 ± 15.81 | 596.16## ± 21.43 |
| Glycogen (mg/DCW) | 170.15 ± 15.73 | 173.76 ± 14.21 | 155.89 ± 3.93 | 205.21## ± 15.30 | 177.61# ± 0.92 |
| Protein (mg/DCW) | 221.36 ± 18.35 | 262.02 ± 35.89 | 220.99 ± 18.58 | 230.70 ± 33.02 | 253.03 ± 29.00 |
| Total fatty acid (mg/DCW) | 34.68 ± 0.34 | 42.19 ± 1.92 | 46.85 ± 0.83 | 37.87# ± 1.16 | 34.90## ± 1.84 |
The values shown are the averages of three biological replicates and three measurement replicates.
*Difference between transformants and wild types,*p < 0.05,**p < 0.01.
#Difference between the experimental group and the control group with addition of 4-HB,#p < 0.05,##p < 0.01.
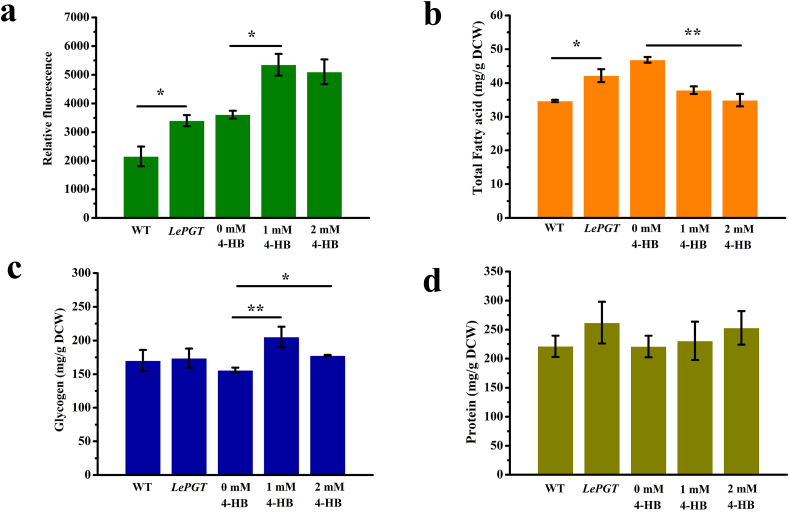
The level of intracellular neutral lipids in transformant significantly increased by 58.18% (p < 0.05) (Fig. 4a). We found each fatty acid composition increased in transformant (Table 2). The total fatty acid content in transformant was 42.18 mg/g DCW, which significantly increased by 10.56% compared with the wild type (p < 0.01) (Fig. 4b). In addition, the protein and glycogen content of transformant was increased by 8.16% and 18.37% respectively (Fig. 4c and d). Based on the above results, we can speculate that the lepgt overexpression could transfer more carbon sources to the synthesis pathway of lipid and protein in Synechocystis.
Fig. 4.
Intracellular metabolite contents of Synechocystis PCC 6803. (a): the fluorescence intensity of the neutral lipid; (b): the production of total fatty acid; (c): the production of glycogen; (d): the production of protein. The group of 0 mM was wild type Synechocystis which 1% ethanol was added. Standard errors was calculated from three biological independent experiments; asterisks represent significant differences (*p < 0.05, **p < 0.01).
Table 2.
Fatty acid profiles of Synechocystis PCC 6803 with different treatments.
| Fatty acid type |
WT | LePGT | Control | 1 mM 4-HB | 2 mM 4-HB |
|---|---|---|---|---|---|
| (mg/g DCW) | |||||
| C16:0 | 23.40 ± 2.38 | 25.81 ± 1.18 | 32.78 ± 1.74 | 27.32# ± 3.31 | 22.27# ± 2.71 |
| C16:1 | 1.00 ± 0.2 | 1.01 ± 0.118 | 1.54 ± 0.39 | 2.16 ± 0.33 | 3.21# ± 0.32 |
| C18:0 | 1.46 ± 0.26 | 1.76 ± 0.343 | 1.14 ± 0.62 | 0.43 ± 0.077 | 0.55 ± 0.13 |
| C18:1 | 3.47 ± 0.868 | 4.13 ± 0.487 | 3.26 ± 1.79 | 0.42## ± 0.052 | 0.88## ± 0.12 |
| C18:2 | 3.96 ± 0.521 | 4.14 ± 0.472 | 3.35 ± 0.75 | 4.29# ± 0.44 | 3.33 ± 0.54 |
| C18:3 | 4.90 ± 0.90 | 5.33 ± 0.319 | 6.23 ± 0.30 | 5.90 ± 0.60 | 4.66 ± 0.81 |
The values shown are the averages of three biological replicates and three measurement replicates.
# Difference between the experimental group and the control group with addition of 4-HB, #p < 0.05, ##p < 0.01.
3.6.2. The effect of different increments of PQ on intracellular metabolites
4-HB addition dramatically increased the neutral lipid content by 54.82% at 1 mM and by 41.35% at 2 mM (Fig. 4a). As shown in Fig. 4b and Table 2, the intracellular fatty acid content of Synechocystis was decreased by the addition of exogenous 4-HB. Compared to the control group, the total fatty acids content was decreased by 20.34% with 1 mM 4-HB addition and decreased by 26.59% with 2 mM 4-HB addition. Significant decreases of C16:0 and C18:1 were observed with 1 mM 4-HB addition. The yield of C16:1 and C18:2 enhanced with 1 mM 4-HB addition. In addition, 4-HB dropped the levels of C18:0 and C18:0 at 1 mM. The content of C16:1 was significantly increased, but the content of other fatty acid decreased after a 2 mM 4-HB treatment (Table 2).
Glycogen content was increased in Synechocystis with 4-HB addition (1 mM, 34.92%; 2 mM 16.78%) (Fig. 4c). Compared with the control group, the level of protein was slightly decreased by 5.98% at 1 mM, but increased by 15.80% at 2 mM (Fig. 4d). According to the results, we can infer that the addition of 4-HB could regulate carbon flow, and its impact on carbon flow varies with concentration (Fig. 5).
Fig. 5.
Scheme illustrating metabolic regulation of Synechocystis PCC 6803 upon photosynthetic and respiratory electron transport chain mediated by plastoquinone. Red: Respiratory electron transport chain; Green: Photosynthetic electron transport chain; Thick line means the pathway is enhanced. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Due to the different physiological metabolic pathways, the phenotype changes of microalgae caused by the addition of compounds are different. For example, when Chlamydomonas reinhardtii was treated with exogenous low-concentration 4-HB, the growth was significantly stimulated and the lipid peroxidation significantly inhibited [50]. Low-concentration 4-HB treatment can promote the growth of Chlorella and the synthesis of intracellular nucleic acids, cytochromes and soluble proteins [51]. In this work, it was worth noting that the addition of 1% ethanol could significantly promote the accumulation of neutral lipid and fatty acid, as ethanol may affect the stability and fluidity of cell membrane of Synechocystis [52]. In addition, 4-HB had little effect on growth, intracellular protein and cytochrome content in Synechocystis at concentrations of both 1 mM and 2 mM.
The addition of a range of chemicals, such as 4-HB, salicylic acid, traumatic acid, can promote cell growth and metabolism in green algae (Chlamydomonas reinhardtii, Chlorella spp.). While adding higher concentrations of such compounds could have a significant inhibitory effect on cell growth and intracellular anabolic pathways. There is not a linear relationship between the changes of microalgae phenotype and the concentration of the compounds [32,51]. The phenotypic changes, such as intracellular fatty acid content, do not show a linear change in Synechocystis treated with different concentration of exogenous ethylene [53]. In this study, 4-HB enhanced the contents of intracellular glycogen and the neutral lipid at 1 mM which was higher than those at 2 mM 4-HB.
In summary, the overexpression of lepgt in Synechocystis could competitively drain 4-HB and GPP, significantly reduce the content of PQ and promote the synthesis of intracellular fatty acids, neutral lipids, and glycogen. By the addition of exogenous 4-HB, we found that different increment of intracellular PQ could regulate the metabolism of Synechocystis, showing different contents of intracellular fatty acid, glycogen, and neutral lipid. The results showed that the small changes of PQ content in Synechocystis can achieve precise regulation of physiology and metabolism. The further experiment can be combined with metabolomics to further explore how the changes of PQ content cause different effects on intracellular metabolic pathways. The strategies of genetic engineering and compound addition in this work provide a new strategy for promoting the synthesis of active substances in Cyanobacteria.
4. Conclusion
PQ is an important redox cofactor in Cyanobacterium. In this study, the synthesis of PQ in Synechocystis were regulated by two strategies: genetic engineering and the addition of 4-HB. The overexpression of lepgt and the addition of low-concentration 4-HB had little effect on the growth and promoted the synthesis of neutral lipid. Small changes in PQ content would lead to more changes of metabolic flux and result in different phenotypes. This study explored the effect of PQ content changes on the metabolic regulation of prokaryotic photoautotrophs. The strategies of genetic engineering and compound addition in this work provide a new method for regulating the anabolism of active substances in cyanobacteria.
CRediT authorship contribution statement
Jianhua Fan: Conceptualization, Supervision, Investigation, Formal analysis, Writing – review & editing. Dongqing Zhou: Investigation, Conceptualization, Formal analysis, Writing – original draft. Cheng Chen: Formal analysis, Investigation, Formal analysis. Ju Wu: Formal analysis, Investigation, Formal analysis. Hui Wu: Conceptualization, Supervision, Investigation, Formal analysis, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank for Professor Jerzy Kruk from Jagiellonian University in Poland for providing the PQ-9 standard. We also thank for Professor Hualing Mi from CAS Center for Excellence in Molecular Plant Sciences/Institute of Plant Physiology and Ecology in China for enthusiastic introducing the theoretical knowledge of the photosystem in cyanobacteria. This work was sponsored by National Key Research and Development Project of China 2019YFA0906300 and 2020YFA0907304, National Natural Science Foundation of China 21776083, the Fok Ying Tong Education Foundation 161017, Natural Science Foundation of Shandong Province ZR2019ZD17, Natural Science Foundation of Shanghai 21ZR1416400 and 19ZR1472700, the Fundamental Research Funds for the Central Universities 22221818014, Funding Project of the State Key Laboratory of Bioreactor Engineering.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2021.10.004.
Author contributions
J.F., D.Z., H.W. designed the experiments. J.F., D.Z., C.C., J.W. performed the experiments. J.F., D.Z., H.W. analyzed the data. J.F., D.Z., H.W. wrote the paper.
Declaration of competing interest
The authors declare that they have no competing interests.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Liu D., Liberton M., Hendry J.I., Aminian-Dehkordi J., Maranas C.D., Pakrasi H.B. Engineering biology approaches for food and nutrient production by cyanobacteria. Curr Opin Biotechnol. 2021;67:1–6. doi: 10.1016/j.copbio.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Neag E., Török A.I., Cadar O., Băbălău-Fuss V., Roman C. Enhancing lipid production of Synechocystis PCC 6803 for biofuels production, through environmental stress exposure. Renew Energy. 2019;143:243–251. [Google Scholar]
- 3.Sivaramakrishnan R., Incharoensakdi A. Enhancement of lipid production in Synechocystis sp. PCC 6803 overexpressing glycerol kinase under oxidative stress with glycerol supplementation. Bioresour Technol. 2018;267:532–540. doi: 10.1016/j.biortech.2018.07.058. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y.-N., Park J.M. Enhancing biomass and ethanol production by increasing NADPH production in Synechocystis sp. PCC 6803. Bioresour Technol. 2016;213:54–57. doi: 10.1016/j.biortech.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Zhang F., Meng H., Zhang Y., Li Y. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria. Metab Eng. 2016;38:217–227. doi: 10.1016/j.ymben.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Mustila H., Kugler A., Stensjö K. Isobutene production in Synechocystis sp. PCC 6803 by introducing α-ketoisocaproate dioxygenase from Rattus norvegicus. Metab Eng Com. 2021;12 doi: 10.1016/j.mec.2021.e00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagemann M., Hess W.R. Systems and synthetic biology for the biotechnological application of cyanobacteria. Curr Opin Biotechnol. 2018;49:94–99. doi: 10.1016/j.copbio.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Du W., Liang F., Duan Y., Tan X., Lu X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab Eng. 2013;19:17–25. doi: 10.1016/j.ymben.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Pfaff C., Glindemann N., Gruber J., Frentzen M., Sadre R. Chorismate pyruvate-lyase and 4-hydroxy-3-solanesylbenzoate decarboxylase are required for plastoquinone biosynthesis in the Cyanobacterium Synechocystis sp. PCC6803. J Biochem. 2014;289:2675–2686. doi: 10.1074/jbc.M113.511709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dähnhardt D., Falk J., Appel J., van der Kooij T.A.W., Schulz-Friedrich R., Krupinska K. The hydroxyphenylpyruvate dioxygenase from Synechocystis sp. PCC 6803 is not required for plastoquinone biosynthesis. FEBS (Fed Eur Biochem Soc) Lett. 2002;523:177–181. doi: 10.1016/s0014-5793(02)02978-2. [DOI] [PubMed] [Google Scholar]
- 11.Liu M.M., SF Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci. 2016;7:1898. doi: 10.3389/fpls.2016.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munekage Y., Hashimoto M., Miyake C., Tomizawa K.-I., Endo T., Tasaka M., Shikanai T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature. 2004;429:579–582. doi: 10.1038/nature02598. [DOI] [PubMed] [Google Scholar]
- 13.Ho M.-Y., Soulier N.T., Canniffe D.P., Shen G., Bryant D.A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr Opin Plant Biol. 2017;37:24–33. doi: 10.1016/j.pbi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Mullineaux C.W. Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim Biophys Acta. 2014;1837:503–511. doi: 10.1016/j.bbabio.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Peschek G.A., Obinger C., Paumann M. The respiratory chain of blue-green algae (cyanobacteria) Physiol Plantarum. 2004;120:358–369. doi: 10.1111/j.1399-3054.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- 16.Mullineaux C.W. Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim Biophys Acta. 2014;1837:503–511. doi: 10.1016/j.bbabio.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Steccanella V., Hansson M., Jensen P.E. Linking chlorophyll biosynthesis to a dynamic plastoquinone pool. Plant Physiol Biochem. 2015;97:207–216. doi: 10.1016/j.plaphy.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Piller L.E., Besagni C., Ksas B., Rumeau D., Brehelin C., Glauser G., Kessler F., Havaux M. Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K1 accumulation. Proc Natl Acad Sci U S A. 2011;108:14354–14359. doi: 10.1073/pnas.1104790108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruk J., Szymańska R., Nowicka B., Dłużewska J. Function of isoprenoid quinones and chromanols during oxidative stress in plants. N Biotech. 2016;33:636–643. doi: 10.1016/j.nbt.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Nowicka B., Pluciński B., Kuczyńska P., Kruk J. Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions. Ecotoxicol Environ Saf. 2016;130:133–145. doi: 10.1016/j.ecoenv.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Nowicka B., Pluciński B., Kuczyńska P., Kruk J. Prenyllipid antioxidants participate in response to acute stress induced by heavy metals in green microalga Chlamydomonas reinhardtii. Ecotoxicol Environ Saf. 2016;123:98–107. [Google Scholar]
- 22.Muhlenbock P., Szechynska-Hebda M., Plaszczyca M., Baudo M., Mateo A., Mullineaux P.M., Parker J.E., Karpinska B., Karpinski S. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in arabidopsis. Plant Cell Rep. 2008;20:2339–2356. doi: 10.1105/tpc.108.059618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajda A., Konopka-Postupolska D., Krzymowska M., Hennig J., Swiezewska E. Role of polyisoprenoids in tobacco resistance against biotic stresses. Physiol Plantarum. 2010;135:351–364. doi: 10.1111/j.1399-3054.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 24.Hunter C.T., Saunders J.W., Magallanes-Lundback M., Christensen S.A., Koch K.E. Maize w3 disrupts homogentisate solanesyl transferase (ZmHst) and reveals a plastoquinone-9 independent path for phytoene desaturation and tocopherol accumulation in kernels. Plant J. 2018;93:799–813. doi: 10.1111/tpj.13821. [DOI] [PubMed] [Google Scholar]
- 25.Knox J.P.D., D A. Singlet oxygen and plants. Phytochemistry. 1985;24:889–896. [Google Scholar]
- 26.Ksas B., Becuwe N., Chevalier A., Havaux M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci Rep. 2015;5 doi: 10.1038/srep10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise R.R.C., B W. Development of ultrastructural damage to chloroplasts in a plastoquinone-deficient mutant of maize. Environ Exp Bot. 1998;40:221–228. [Google Scholar]
- 28.Kazuaki O., Kyoko Y., Masafumi H., Kanako S., Kazufumi Y. Functional characterization of OsPPT1, which encodes p-hydroxybenzoate polyprenyltransferase involved in ubiquinone biosynthesis in Oryza sativa. Plant Cell Physiol. 2006;47:581–590. doi: 10.1093/pcp/pcj025. [DOI] [PubMed] [Google Scholar]
- 29.Ohara K., Muroya A., Fukushima N., Yazaki K. Functional characterization of LePGT1, a membrane-bound prenyltransferase involved in the geranylation of p-hydroxybenzoic acid. Biochem J. 2009;421:231. doi: 10.1042/BJ20081968. [DOI] [PubMed] [Google Scholar]
- 30.Yazaki K., Kunihisa M., Fujisaki T., Sato F. Geranyl diphosphate:4-hydroxybenzoate geranyltransferase from Lithospermum erythrorhizon. Cloning and characterization of a ket enzyme in shikonin biosynthesis. J Biochem. 2002;277:6240–6246. doi: 10.1074/jbc.M106387200. [DOI] [PubMed] [Google Scholar]
- 31.Wu H., Tuli L., Bennett G.N., San K.Y. Metabolic transistor strategy for controlling electron transfer chain activity in Escherichia coli. Metab Eng. 2015;28:159–168. doi: 10.1016/j.ymben.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awad N.S.V.-E.G. Salicylic acid and aspirin stimulate growth of Chlamydomonas and inhibit lipoxygenase and chloroplast desaturase pathways. PPB (Plant Physiol Biochem) 2020;149:256–265. doi: 10.1016/j.plaphy.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Sun T., Gao X., Shi M., Wu L., Chen L., Zhang W. Biosynthesis of platform chemical 3-hydroxypropionic acid (3-HP) directly from CO2 in cyanobacterium Synechocystis sp. PCC 6803. Metab Eng. 2016;34:60–70. doi: 10.1016/j.ymben.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki R.I., T An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J Oceanogr Soc Jpn. 1990;46:190–194. [Google Scholar]
- 35.Liang F., Lindblad P. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium Synechocystis PCC 6803. Metab Eng. 2016;38:56–64. doi: 10.1016/j.ymben.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Nowicka B., Fe Senko T., Walczak J., Kruk J. The inhibitor-evoked shortage of tocopherol and plastoquinol is compensated by other antioxidant mechanisms in Chlamydomonas reinhardtii exposed to toxic concentrations of cadmium and chromium ions. Ecotoxicol Environ Saf. 2020;191 doi: 10.1016/j.ecoenv.2020.110241. [DOI] [PubMed] [Google Scholar]
- 37.J F. A simple method for the isolation and purification of total lipides from animal tissues. J Bid Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 38.Aksmann A., Tukaj Z. Intact anthracene inhibits photosynthesis in algal cells: a fluorescence induction study on Chlamydomonas reinhardtii cw92 strain. Chemosphere. 2009;74:26–32. doi: 10.1016/j.chemosphere.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D., Singh H., Raj S., Soni V.J.B., Reports B. Chlorophyll a fluorescence kinetics of mung bean (Vigna radiata L.) grown under artificial continuous light. Biochim Biophys Acta. 2020;24 doi: 10.1016/j.bbrep.2020.100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tóth S., Schansker G., Strasser R.J. In intact leaves, the maximum fluorescence level (F(M)) is independent of the redox state of the plastoquinone pool: a DCMU-inhibition study. Biochim Biophys Acta. 2005;1708:275–282. doi: 10.1016/j.bbabio.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Strasser R.J., Srivastava A., Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Prob Photosynth Mechan Regulat Adapt. 2000;25:445–483. [Google Scholar]
- 42.Bendall D.S., Manasse R.S. Cyclic photophosphorylation and electron transport. Biochim Biophys Acta. 1995;1229:23–38. [Google Scholar]
- 43.Soo R.M., Hemp J., Parks D.H., Fischer W.W., Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science. 2017;355:1436–1440. doi: 10.1126/science.aal3794. [DOI] [PubMed] [Google Scholar]
- 44.Hays S.G., Ducat D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth Res. 2015;123:285–295. doi: 10.1007/s11120-014-9980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishna P.S., Styring S., Mamedov F. Photosystems ratio imbalance promotes direct sustainable H2 production in Chlamydomonas reinhardti. Green Chem. 2019;21:4683–4690. [Google Scholar]
- 46.Noreña-Caro D., Benton M. Cyanobacteria as photoautotrophic biofactories of high-value chemicals. J CO2 Util. 2018;28:335–366. [Google Scholar]
- 47.Hagemann M., Hess W.R. Systems and synthetic biology for the biotechnological application of cyanobacteria. Curr Opin Biotechnol. 2018;49:94–99. doi: 10.1016/j.copbio.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Grundel M., Scheunemann R., Lockau W., Zilliges Y. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology. 2012;158:3032. doi: 10.1099/mic.0.062950-0. [DOI] [PubMed] [Google Scholar]
- 49.Bajguz A., Czerpak R., Piotrowska A., Polecka M. Effect of isomers of hydroxybenzoic acid on the growth and metabolism of Chlorella vulgaris Beijerinck (Chlorophyceae) Acta Soc Bot Pol. 2001;70:253–259. [Google Scholar]
- 50.Bernal P., Segura A., Ramos J.-L. Compensatory role of the cis-trans-isomerase and cardiolipin synthase in the membrane fluidity of Pseudomonas putida DOT-T1E. Environ Microbiol. 2007;9:1658–1664. doi: 10.1111/j.1462-2920.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J., Yang F., Zhang F.L., Meng H.K., Zhang Y.P., Li Y. Impairing photorespiration increases photosynthetic conversion of CO2 to isoprene in engineered cyanobacteria. Biores Bioprocess. 2021;8:42. doi: 10.1186/s40643-021-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pietryczuk A., Piotrowska A., Czerpak R.J.O., Studies H. The influence of traumatic acid on the growth and metabolite content of the green alga Chlorella vulgaris Beijerinck. Oceanol Hydrobiol Stud. 2008;37:3–15. [Google Scholar]
- 53.Le Henry M., Charton M., Alignan M., Maury P., Luniov A., Pelletier I., Pontalier P.-Y., Binder B.M., Vaca-Garcia C., Chervin C. Ethylene stimulates growth and affects fatty acid content of Synechocystis sp. PCC 6803. Algal Res. 2017;26:234–239. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.