Abstract
The main estrogens: estradiol, estrone, and their acyl-esters have been studied essentially related to their classical estrogenic and pharmacologic functions. However, their main effect in the body is probably the sustained control of core energy metabolism. Estrogen nuclear and membrane receptors show an extraordinary flexibility in the modulation of metabolic responses, and largely explain gender and age differences in energy metabolism: part of these mechanisms is already sufficiently known to justify both. With regard to energy, the estrogen molecular species act essentially through four key functions: (1) Facilitation of insulin secretion and control of glucose availability; (2) Modulation of energy partition, favoring the use of lipid as the main energy substrate when more available than carbohydrates; (3) Functional protection through antioxidant mechanisms; and (4) Central effects (largely through neural modulation) on whole body energy management. Analyzing the different actions of estrone, estradiol and their acyl esters, a tentative classification based on structure/effects has been postulated. Either separately or as a group, estrogens provide a comprehensive explanation that not all their quite diverse actions are related solely to specific molecules. As a group, they constitute a powerful synergic action complex. In consequence, estrogens may be considered wardens of energy homeostasis.
Keywords: Estrogens, Insulin, Estrogen receptors, Energy metabolism, Glucose, Antioxidants, Metabolic syndrome
Core Tip: Estrogens play a paramount and continued regulatory role, based on the synergy between the different forms of estrogen to maintain energy (and lipid/glucose) homeostasis. These functions include preventing: oxidative damage, lipid-induced inflammation, excess fat accrual and the complications of excess amino nitrogen. This short incomplete list is fairly close to a recipe for preventing the development of metabolic syndrome; abundant epidemiological and (partial) experimental data help support this assertion. We have to look more widely at estrogens (the different structural-functional types described in the text) to understand their extensive and powerful control of energy homeostasis.
INTRODUCTION
The complex and growing implication of steroid hormones in homeostasis
Steroid hormones are derived from sterols, which play a critical role in the structure of the (mainly) eukaryotic membrane[1]. Most steroid hormones regulate animal functions, especially in vertebrates. Only recently, plant steroid hormone analogs such as the brassinosterols[2] have been found to play a significant role in the metabolic regulation in the most evolved plants[3,4]. Other steroids, including estrogens have also been found to act as regulators in some plants, but the information is still relatively scarce[5,6]. In addition, a number of higher plants are able to synthesize animal steroid hormones, such as testosterone and estrogens[7], as well as structural analogs which interfere with physiological functions and vital cycles of some animals[8,9], including direct allopathic interference[10]. The steroid hormone ecdysone is critical for molting of insects and other animals[11], and plants synthesize the analogs ecdysteroids to limit insect development[12].
Nevertheless, the most studied types of steroid hormones (estrogen, androgen, corticosteroid, progesterone) are typical and characteristic of vertebrates. However, the variability in functions, regulation and even mechanism of action is considerable, since the degree of implication of these hormones in the fundamental aspects of life: reproduction, feeding-growth-metabolism, neural and metabolic regulation, fall squarely in their fine tuning of life cycles, survival and evolution. Their effects, largely gene expression modulation, are being continuously uncovered, in a way that deviates from the classical distribution of functions for human steroid hormones, often presented as mere sex-definition signals or regulators of mineral and glucose homeostasis. The implication of all of these hormones in the defense mechanisms (including a massive implication in the immune system control[13,14] and optimization of metabolic function[15,16]) has been growing in importance in parallel to their development along the evolution path[17] that brought humans to their amazing homeostatic resilience.
This linear review is focused on estrogens, one of the most important vertebrate steroid hormone types, due to their critical function on the control of core metabolic partition in addition to their fundamental immune system control and reproductive functions.
The estrogens are not only “sex hormones”
Most of the investigations of estrogen effects on metabolic regulation, irrespective of sex, are fairly recent and notably skewed (Box 1). So far we have only limited information on the major role played by different forms of physiological estrogens in the control of energy metabolism at the whole body level[16,18].
The initially intense development of research on estrogens came to a climax by the mid XXth century[19,20], and was essentially focused on their pharmacology, as part of the development of combined estrogen-progestogen preparations for safe birth control in humans[21]. The studies on steroid hormones were not limited to estrogens (and progestogens), but were extended to androgens[22] and, especially, to glucocorticoids[23] through the development of a large number of synthetic drugs, widely used and which their development continues[24]. In a way, this expansion in the pharmacology of steroid hormones also provided considerable information on their mechanisms of action[25] and metabolism[26], including an extensive analysis of some possible complications of their clinical use[27-29]. Nowadays, the natural human corticosteroids (cortisol and cortisone, but also corticosterone[30]) are seldom prescribed, despite showing often quite different effects and pharmacological profile than the myriad of synthetic corticosteroids in use[31]. The latter may bind to most of the natural receptors[32], but basically do not share the transporter proteins[33] or the inter-organ self-regulatory mechanisms of natural hormones (e.g. the hypothalamus-pituitary axis).
The common identification of “estrogen” with 17β-estradiol (E2) and “androgen” with testosterone (T) is an inadequate oversimplification that helps to dismiss the regulatory and fine-tuning interrelationships of the different molecular species of estrogens, both with themselves or with androgens and other steroid hormones.
The estrogens are ancient regulatory agents, remarkably preserved along evolution. The number and structure of relevant molecules remains small and unaltered in spite of the variety and complexity of the mechanisms modulating their actions, somehow reflecting the cumulative experience (and expansion of metabolic interventions) acquired during evolution. The versatility of the nuclear receptors’ modulation and signaling pathways allow the superposition of a dense web of signals, including fail-safe, duplicate, alternative and redundant mechanisms, which often make it difficult to find answers to the direct questions relevant to the clinicians.
The structures of estrogens
The principal distinguishing feature of animal estrogens is the phenolic nature of ring A, usually with a –OH in C3. No other type of steroid hormone contains a phenolic ring. The steroid nucleus of estrogens has 18 C, and lack a side chain. The main human functional estrogens are 3-hydroxy-17-keto-estrin (E1 estrone), 17β estradiol or 3, 17β-dihydroxy-estrin (E2 estradiol) and 3, 16α, 17β-trihydroxy-estrin (E3 estriol); during fetal development[34], another estrogen should be included: 3, 15α, 16α, 17β-tetrahydroxy-estrin (E4 estetrol). Compared with all other mammalian steroid hormones, they are highly lipophilic (E1 > E2 > E3). This peculiarity facilitates their transport by lipoproteins[35] and binding to membranes[36] (including their crossing). The interactions with lipophilic entourages have been credited as a main factor for their effects on membranes[37] and mitochondrial function[38]. It is often assumed that estrogens are carried in the blood bound to proteins, largely sex hormone-binding globulin (SHBG), but the higher affinity and metabolic response to energy changes[39] of T (competing with E2 for SHBG) favors a closer dependence of the globulin levels and/or structure/affinity[40,41]. The much lower levels of E2 than T in plasma (both in women and men)[33] suggest that this dual (if real) transport of hormones may be a consequence of modulation of the molecular affinity of SHBG, in part through modification of its molecular weight[39,40,42]. The key factor is that under physiological conditions SHBG (or a varied group of SHBG isoforms) binds essentially T[39] and estrogen (almost 90% of plasma E2, but practically no E1[33]). In addition, the in vitro estrogenicity of E1 is considerably lower than that of E2[43]. This fact, together with the abundance of E1 in men (despite being an estrogen), and its high lipophilia resulted in a limited pharmacological interest for this molecule and a consequent lack of literature on it, and its function as a free hormone remains obscure. E1 is the most abundant estrogen in the body (when its esterified forms are included)[44], since it is produced (and stored[44]) in large amounts in white adipose tissue (WAT)[45]. Probably because of its lipophilia, a large portion of E1 in plasma is found esterified as sulfate, much more soluble than the free hormone[46,47], which facilitates its transport and eventual excretion. However, E1 can be made even more lipophilic by esterification with a fatty acid on C3[48] yielding acyl-estrone (AE1). In this form it has been found in lipoproteins[49] and adipose tissue[44]. AE1 are synthesized by adipocytes and modulated by leptin and insulin[50].
E2 is also esterified with fatty acids (acyl-E2 or AE2), becoming more lipophilic than E2, and thus also found in blood lipoproteins[51,52]. However, AE2 is largely esterified in C17 and not in C3 as are the AE1 esters[53]. This peculiarity of AE2 has been attributed to its higher capability to protect the lipids which surround the hormone from oxidation thanks to the unaffected phenolic –OH[51]. In any case, the highly lipophilic estrogens (through esterification with long-chain fatty acids) are a common occurrence for which no definitive function has yet been fully agreed upon, and which shows that the usual molecular species of natural estrogens, their transport in the bloodstream including their binding and physiological functions are far from being fully known. The high concentration of these varied estrogen-acyl-ester molecules in tissues, such as WAT[54] suggests a possible role of reserve or storage of preformed estrogenic molecules[53], which has been explained in part by the easiness of their synthesis by acyl-transferases, widely present in lipoproteins and tissues[55].
Main gender differences in human estrogen function
The scant number of in-depth non-clinical or pharmacological studies may be in part a consequence of the bias against estrogens (and of their bad name, Box 1). The reasons usually presented to sustain negative opinions, (which in the end limit metabolic analyses, and the eventual therapeutic use) are based largely on two factors: their known role as promoters of some forms of cancer, mainly breast[56] and endometrial[57], and a number of risks derived from their use[58,59] other than their assumed role as “female-linked” hormones. The essentiality of estrogens has been proven (in both sexes) for many functions, such as those related with sex differentiation and reproduction, as well as bone health[60]; but the key factor is the increasing flow of data that establishes a direct implication of estrogens in the control of the basic core of energy metabolism[16,61]. This control is affected by age, sex and diet; thus, the simpler division of steroid hormones function using a strict sex-oriented focus is no longer applicable. We simply need to know more about the estrogens and their functions, in exactly the same way as any other hormone, keeping in mind the species-specificity in some of their functions when establishing comparisons with animal models (Box 2).
Women have higher circulating levels of E2 than men, from puberty to menopause, with notable variation between physiological situations[62]. Men, even at their maximal reproductive capacity age, also show fairly high blood levels of E2[63]. There are not enough data on AE2 levels and distribution to establish valid comparisons, but it is probable that the parallelism will be maintained. On the other side, seldom clear gender differences are found in E1, the most abundant estrogen (free or esterified) in human blood. E1-sulfate (SE1) is subjected to a regulative “solubility/excretion” cycle[46] comparable to that of dehydroepiandrosterone[64]. The ample abundance of AE1 in tissues (rat) shows a more marked dependence on the mass of WAT than on sex[65].
T and E2 differently influence brain development from its earliest stages, both in the setting of its functional structure and –later– its psychological orientation and focus[66,67]. The resilience of women against insulin resistance is higher than that of men[68,69], at least until menopause[69]. Estrogen protects bone from demineralization in women and men[70,71], a function in part shared by T (at a lower potency, however[72]). The accrual and maintenance of body protein falls largely on androgens, mainly T[73,74], acting in a synergic way with growth hormone[75] and insulin[76] and countering the proteolytic capability of glucocorticoids[77]. The contribution of free estrogens to the maintenance of body protein mass seems to be more limited[78].
Estradiol signaling: classical nuclear receptors. Estrogen receptors α and estrogen receptors β
Thanks to their lipophilic nature, E2 (and E1) can easily cross membranes and bind specific estrogen receptors (ER) within the cell[79]. After dimerization[80] they are brought to the nucleus, where the complex E2-ER binds to deoxyribonucleic acid (DNA)[81] or to specialized proteins[82,83], eventually eliciting the expression or repression of specific genes. The nuclear-type estrogen receptors are highly complex[79,84]. Estrogen signaling, up to its final manifestation is not a fast process such as that of nervous of rapid-signaling chemical regulating agents (Box 3).
Binding to ER is, essentially, specific for the physiological estrogens[75,85], but a wide number of plant secondary metabolism compounds, synthetic non-steroidal estrogenic drugs and even some toxic industrial waste also bind the ER[86]. In humans, there are two main types of nuclear estrogenic receptors: estrogen receptors α (ERα) and estrogen receptors β (ERβ)[87]. In fact, ERα and ERβ are two families of related receptors, which maintain the same overall structure but not their complete sequence, the ERs being adapted, adjusted or changed for best effectiveness, in different tissues or because of changing needs[84,88].
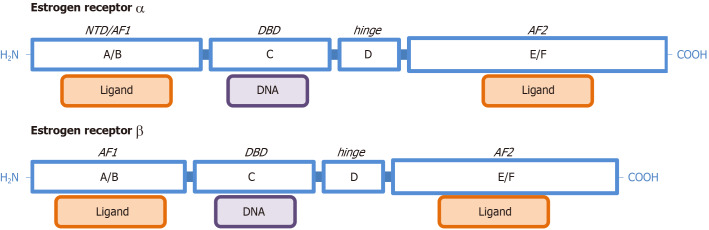
The structures of ERα and ERβ are shown in Figure 1. The main dominions are marked with letters (A to F), and correspond roughly (A/B) to a zinc finger and a binding site, activation function site 1 of the ERs (AF1); C is the place for binding estrogen-response elements (ERE) and then DNA[89]; D is a shorter sequence related to the binding of chaperone proteins and to the process of dimerization; and E/F is the ligand binding domain for estrogens and other factors, AF2[84]. The main ligands are the natural estrogens of mammals (E2, E1, E3 and E4), but some drugs, phytoestrogens[10], metals and diverse chemicals (xenoestrogens) can also bind the receptors[90]. Binding to the AF1 and AF2 may result in synergistic effects[91]. The length and distribution of ER parts may change within each receptor family depending on alternate sequences and splicing[84]. The affinity of ERα: is maximal for E2, followed by E1, and that of ERβ is also E2, followed by E3.
Figure 1.
Functional structure of estrogen receptors α and estrogen receptors β. Dominion names (A to F), and common denominations for the main functions are included: N-terminus dominion; DNA binding dominion, activation function site 1 of the estrogen receptors and activation function site 2 of the estrogen receptors. The graph is not drawn to scale and represents the complete (highest molecular weight) form for each of the two families of nuclear ERs. NTD: N-terminus dominion; DBD: DNA binding dominion; AF1: Activation function site 1 of the estrogen receptors; AF2: Activation function site 2 of the estrogen receptors.
The combination of affinities and the panoply of modulators and cell type-specific distribution of ERs results in an extended variety of possible effects, making widely variable the action of estrogens, in order to send specific signals to organs or groups of cells within a wide array of possibilities[92-95]. The ERs are, perhaps one of the best examples of receptor adjustment to the needs of tissues under varying conditions, attained through a considerable number of mechanisms. Both ERα and ERβ are dimeric and coded by different genes[96,97], with an additional abundance of polymorphisms[98,99]. Their distribution in the cells of different tissues and organs is independent for each receptor[100], as are their final gene expression effects[101,102]. They can show synergic[103] or antagonistic[104] effects, even for the same molecular species, and depend, largely, on the post-binding relationships of the E2-ER complex. This situation is further complicated by the interaction of the ER (or E2-ER) with a number of different mechanisms of modulation, such as selective estrogen receptor modulators (SERM)[105-107]; selective ER down-regulators (or degraders)[108]; and the specific ERE, which directly affect the function of ERs[109,110]. Other closely related (“orphan” up to recently) receptors participate in the regulation of critical pathways, but in many cases their relationship with the ERs remains unclear.
Membrane estrogen receptors
In addition to the canonic nuclear ERs, estrogens also bind cell surface ERs[111-113]. As usual with ERs, the terms used to define these receptors shift between the importance given to their location, partial signaling pathway, speed of action and other considerations: membrane ER[114], non-nuclear/non-transcriptional signaling ER[115,116] non-genomic signaling ER; membrane-linked ERα[117,118] or both ERα and ERβ[112]; caveolae- or lipid-related ER[119,120]; G-protein-coupled ER (GPER)[121,122]. This list adds to the existence of alternative or non-genomic “direct” or “rapid” effects of ER stimulation in some cells, eliciting immediate responses (a shift–or development–from the main advantage of the delayed steroid hormone signaling)[123,124].
All these effects suggest that, the ER structure is essentially that described above, with two main types (families) of complete/incomplete ERs: ERα and ERβ, which are found in the cytosol (and nuclei) of cells, including members of the ERα family linked to G-proteins (GPRA1/GPR30)[122,125] attached or close to the plasma membrane.
The proven relationship of ERα to membrane or fat droplet-related structures may be a consequence of the adaptability of ERα[120]; the existence of free and fatty acid-esterified estrogen in lipid-related cell structures[126], or both. In any case, the association of ERα to caveolin-1[119] and then complexed with G-proteins, helps explain the presence-binding of the ER[127], and then E2 in the membrane entourage[128]. In this case, the signal may be transferred to membrane-related structures, as is the case with increasing calcium release[115]. The G protein-ER complex, upon the binding of E2 may also induce nuclear effects via activation of tyrosine kinases[129] and the MAP/ERK or PI3α/Akt pathways[130]. The stimulated G protein-activated receptor may also signal through GPR30[131]. The activated system containing the ER also enhances adenylate cyclase activity[132] via phosphorylation of the cAMP response element[133]. However, these direct membrane-related mechanisms may coexist with also faster direct translational actions of conventional ERs somewhat linked to membranes[134] or with other mechanisms hinted at but not fully disclosed yet[135,136]. This includes the presence of ER receptors within cell structures, such as mitochondria (e.g. ERβ)[137].
It has been established that non-genomic effects of ER bound to E2 may belong to two confluent mechanism types: direct effects elicited from the membrane and effects developed through cytosol signaling cascades and actions. Both processes are probably coincident for different cell settings. Hypothalamic inhibition of guanylate cyclase[138] and LH secretion[139], as well as increased cell migration[140] and other brain effects[141] add to the widening array of non-genomic effects of ERs. Most of these effects have lately been attributed to the ERα irrespective of the place of binding with E2, but the implication of ERβ has also been described[142,143].
Notwithstanding, all these receptor-related mechanisms described cannot fully explain all the biochemical effects induced by estrogen signaling[144-148], leaving ample space for the assumption of direct, i.e. non ER-related, involvement (largely of E2 and its C17-fatty esters, AE2)[149,150]. However, these effects have been described only in lipoproteins, other lipid masses or lipid/protein interfaces[54,151]. The direct effects of estrogens on mitochondria have been related to specific mitochondria receptors[152,153], apparently containing ERα, ERβ[154] and other possible binding structures[155]. Their role on mitochondrial function, however, has been found to be significant[154,156], especially in the regulation of energy providing pathways[157,158]. The possibility of estrogen direct incrustation in the lipid layer of membranes has been proposed as a way to modify their functionality[159] and enhance the E2/AE2 anti-oxidative properties in a way similar to its postulated function in lipoproteins[160,161].
Estrone and AE1
E1 is a rather peculiar and resilient hormone (Box 4); we do not yet have a direct explanation for its massive synthesis and storage, since the lipophilic nature of E1 (but not that of SE1) limits its action in plasma, cell and interstitial space. Non-esterified E1 levels are related to those of E2, with E2/E1 ratios fairly stable for men (c. 1) and more variable for women (c. 1.5-2) up to menopause[162]. However, measurement of circulating estrogen is difficult, often showing poor correlations between instrumental and immunoassay results[163]. The relationship of E1 with E2 levels, in addition to sex (and age) is affected by diabetes/obesity[164]. Furthermore, analyses of SE1 seldom include other E1 esters nor free E1, which compartmentation (important in lipoproteins) skews E1 serum levels towards lower values. The obese show high plasma SE1 concentrations[165]. In any case, the whole-body AE1 content in rats is several orders of magnitude higher than free (and sulfate-esterified) E2[44], however, the AE1 content in obese rats is relatively lower than in normal-weight animals, despite AE1 being essentially stored in WAT[44].
The oral pharmacological administration of oleoyl-E1 to normal weight and obese rats[166,167], induces a marked decrease in fat depots[168], not dependent on the degree of obesity and diet[167,169]. The loss of fat runs parallel to the normalization of glycemia, blood lipids and other metabolic syndrome (MS) markers[170], without apparent effects of estrogenization, and irrespective of energy intake manipulation[171]. AE1 has been proposed as a ponderostat signal[170], since the excess fat is shed without accompanying metabolic disorders[170,172]. Its negative effects on humans are negligible (clinical studies, phase I, unpublished data), and the positive (i.e. loss of excess fat, lowered insulin resistance, absence of estrogenization) were outstanding in a single case published[172]. However, its development as a drug was abandoned because an ill-designed phase II failed to be conclusive. We have no hints as to the mechanism of AE1 signaling, other than it is synthesized in cultured adipocytes[50], and WAT stores these esters in large amounts[44,65]. AE1 treatment reduces the size of WAT lipid depots[173,174]. Natural AE1 is transported in the lipid fraction (lipoproteins) of blood[49,175]. Methodology is a critical factor for the analysis and tracing of acyl-estrogens, with disparate results; i.e., it has been reported that human plasma does not contain AE1 at all[176].
The main effects of AE1 are a consequence of the structural change on the whole ester, not through the release of E1[177]. When injected, marked estrogenic effects are observed, with increased E1 and E2 levels[178]. However, oral administration of AE1 does not elicit the same signs of estrogenization[171]. A highly critical analysis of oleoyl-E1 actions on rat body weight found no significant negative effects[179]. Body protein and N balance are preserved in AE1-treated (lean and obese) rats[166,167,174,180]. There is very little information available on AE1 mechanism of action. The structure of the orally administered ester seems to be modified, with low levels in blood plasma[49,181,182], but an unidentified derivative is present in large concentrations, maintaining the estrogen nucleus in a more hydrophilic form[181]. In liver, AE1 label can be found linked to DNA shortly after administration[181]; the effects of AE1 imply the stimulation of ERα[183,184]. Excess AE1 is essentially excreted as SE1[185].
There are sufficient elements to sustain the implication of AE1 in the regulation of body weight[170], but the lack of further complete studies on its mechanism of action has prevented both its clarification and its eventual therapeutic application. No other explanation has been put forward to justify the limited estrogenic potency of E1, despite its massive synthesis in the ovary and the brain[186], and, especially (in quantitative terms) in WAT[187], with a direct relationship of its total body content and circulating levels with WAT, lowered by obesity in rodents and humans[47]. The effect of the administration of free E1 to rats induce some estrogenic effects and slightly increases body weight, effects quite different to those of its acyl derivative[177].
Estrogens and the regulation of energy metabolism
Glucose is the main energy substrate, and the main simple nutrient of human diet. Glucose is also the primary inter-organ energy substrate carried by the blood to sustain the energy needs of body cells. Carbohydrates capable of yielding glucose (or other interconvertible hexoses) are a necessary part of our diet[188,189], and for many thousands of years they have constituted the main staple of our energy intake. This role has been already addressed in depth in a previous paper[189] in which we discussed the final fate of dietary carbohydrate, protein and lipids to yield two-and three-carbon metabolites (2C, 3C) and anaplerotic four-and five-carbon (4C, 5C) molecules from proteins (when excess N could be disposed of). The common shared groups of metabolites from dietary nutrients include 2C fragments (and a smaller amount of 3C from glycerol) from fats and, essentially 3C fragments from the six-carbon (6C) hexoses. The 3C could be largely used to maintain glycemia thanks to hepatic[190], renal[191] and intestinal[192] gluconeogenesis, or simply used (pyruvate) as a source of 2C (to yield acetyl-CoA), which is largely oxidized to CO2 in the mitochondria through the Krebs cycle. Most of the energy drawn from glucose is obtained from the pyruvate-lactate produced in the glycolytic pathway followed by the complete oxidation of pyruvate, as acetyl-CoA, in the Krebs cycle. The 3C fragments (essentially lactate, pyruvate, glycerol, alanine and serine) can substitute glucose as an energy substrate in many tissues, avoiding the strict control of glucose levels, and providing faster access to their energy when and if enough oxidative capability and oxygen are available[193,194]. Glucose isoforms often delay somehow the oxidation of glucose[195], and thus, the direct cell use of glucose-derived 3C fragments may speed up its catabolism. This C6→C3 massive conversion is one of the most important albeit less publicized functions of WAT[196]. The presence of excess lipids (and energy) in the diet often results in an excess of 2C fragments (mainly the result of catabolic oxidation of their polymers: fatty acids) that their oxidation becomes problematic, thus the excess of energy available facilitates their storage (often long term) as fats[189].
The inadequacy of diet composition, and especially the excess of energy from fats and carbohydrate results in the progressive metabolic disorders of MS[197] with the development of sustained hyperglycemia[198], often deriving into type 2 diabetes[199], obesity[200], altered blood lipids, with hyperlipidemia[201], deriving in endothelial inflammation[202,203] and increased cardiovascular risk[204], hepatic steatosis[205], depression[206], and increasingly functional alteration of the nervous system[207], bone[208] and practically all organ/cell systems, extended even to the microbiota[197]; and, essentially, the immune system[209,210]. The causes and effects of MS have been intensively and extensively studied, and a direct relationship has been found with diet composition and excess energy[211,212], but no effective solutions have been put forward. Medical treatment is commonly limited to increased energy expenditure and changes in type of food, and (decreased) energy intake[213-215], in most of the cases, without sufficient metabolic analyses[216]. This is complemented by the pharmacological treatment of the disorders included in the MS. The relative acceleration of the MS effects with age is more clearly observed in adult (and aging) men than in women[217,218]. This difference has been attributed to the obvious diet-driven inflammation of MS[219,220], compounded in men by the progressive decrease in the synthesis (and effects) of T, in part a consequence of aging but also by the hypoandrogenism that characterizes MS[221]. Women, from adolescence to the beginning of menopause maintain their high levels of E2 and functional hypothalamus-hypophysis-gonadal axis[62]. Menopause, aging and other causes break this equilibrium and the levels and protective effect of estrogens wane; The E1 vs E2 ratio of concentrations is maintained at E2 > E1 in premenopausal adult women, changing to E1 > E2 in post-menopausal women and in men (in which there is little change with age). In both cases, E2 levels were lower in men and post-menopausal women than in adult premenopausal women[62].
In aging men, especially those with MS, treatment with T reduces to some extent cardiovascular risk[222,223] and helps maintain glycemia[224,225], but possible dangers, insufficient knowledge and scant physiological analysis have limited the extension of this therapeutic avenue[226]. Similarly, for women, substitutive estrogenization is partly effective[227-229] at menopause, but its extension has been seriously limited by the fear of possible negative consequences, as discussed in Box 1. In addition, synthetic estrogens are the most used substitute drugs despite our very limited knowledge[230] of the intricacies of their action in such complex mechanisms as those described above for E2. The case of tamoxifen (agonist/antagonist) is a clear example[231]. This generalized (albeit undeclared) ban on sex hormones extends to the use of T in women, despite the fact that both E2 and T are needed for bone[70,232] health, and T for body protein maintenance[75]. Obviously there are problems to solve, but it seems that this line of study has not been sufficiently developed for reasons not based on contrasted arguments. In the case of AE1, a line of research developed by only one research group, obtained better results than those of any previous anti-obesity drug[170,179], but the development was discontinued largely for fear of “possible” future negative findings[179].
Estrogens, insulin and dietary nutrients handling
Most of the studies on the effects of estrogen on glucose metabolism have been done using E2 (and other ER ligands). There is a very limited amount of specific information on E1 direct effects; however, SE1 was found to induce hypoglycemia in genetically obese mice via glucose-6-phosphatase[233]. The effects of estrogens on glucose and energy handling are mediated through four coordinated actions: (1) Protection and facilitation of insulin secretion and function in the control of glucose availability to tissues; (2) Modulation of energy partition, favoring the use of lipid as the main energy substrate when their availability is higher than that of carbohydrates; (3) Functional protection through antioxidant mechanisms; and (4) Central effects on whole body energy metabolism and homeostasis maintenance.
Estrogens, insulin and glucose
E2 protects the functionality of the pancreatic β cells[234,235], preventing apoptosis[236], adapting their function to insulin resistance[237], and maintaining their insulin content[238]. ER stimulation inhibits lipogenesis in the β cells[158], which limits the negative effects of excess lipid in the cell. The loss of the ER (nuclear and/or membrane) impairs pancreatic insulin secretion[239], which is stimulated by estrogenic signaling[240]. The lack of E2 availability also increases hepatic insulin clearance[241].
Estrogens also prevent the development of diet-induced insulin resistance[242]. The gender-dependent effects of estrogen on high-fat diet-induced insulin resistance are largely dependent on the anti-inflammatory effects of the hormone[243]. E2 increases tissue insulin sensitivity[244], and lowers insulin resistance in peripheral tissues[245], with marked differences in the effects depending on gender[243]. In female mouse adipocytes, E2 lowers inflammation (and thus insulin resistance[246]), and enhances the effects of insulin on tissues[247]. The sole activation of ERα AF-1 is enough to prevent obesity, liver steatosis and insulin resistance in mice[248]. However, obesity and insulin resistance seems to require E2 in addition to ERα and AF-2, AF1 not being essential[249].
Estrogens induce a considerable number of actions in the brain, which is also able to synthesize them[250], playing an important role in its function[251,252] and behavior[253]. E2 also interacts with serotonin to affect insulin resistance[254]. Estrogenic deprivation induces mitochondrial dysfunctions in the brain which may induce the loss of cognitive functions[255]. More complex is the long saga of the relationship of estrogen in the peculiar placing of brain insulin resistance in Alzheimer’s disease[256,257]. The neuroprotective actions of estrogens[258], added to the inhibition by E2 of β-amyloid production[259] and the implication of E2 in the regulation of insulin degradation in the brain[260], suggest an overall beneficial effect of estrogen limiting the development of this disease. However, Alzheimer’s disease affects more women than men[261], and a number of caveats have been raised against the danger of natural estrogens being implicated in its development[262]. Right now the case is not solved, with studies showing a protective effect of ERβ[263] and others hinting at the implication of ERα in its pathology[264].
Estrogens, largely E2, facilitate the uptake of glucose from the intestine[265], and its extraction from the bloodstream by activation of transporters GLUT4[266] and, at least in the brain[267] GLUT1. E2 lowers liver glucose output with no changes in glycogen during mild exercise[268], a difference due in part to a modulable maintenance/ inhibition of gluconeogenesis[269]. E2 also regulates glycolysis in endothelial cells by non-genomic pathways[270], partly by increasing insulin signaling[271]. Glucose catabolism is affected by estrogens, which stimulate glycolysis via phosphofructokinase[272], and the pentose phosphate pathway via Akt[273]. In any case, the direct incidence of ER signaling on glucose handling is relatively limited and conducted via modulation of insulin[271]. Probably, the main effect of estrogen may be the utilization of lipids as alternative energy substrates. This is important for humans, because of the common occurrence of excess lipids (and energy) in Westernized diets, which leads to problems in dietary substrate partition[189] and the common development of MS.
Lipid handling and estrogens
Estradiol: Estrogens lower circulating triacylglycerols (TAG) favoring their transport with a higher expression of ApoA5[274], and protecting lipoproteins against oxidation[275]. However, the main effect of E2 on lipids is favoring the shift from lipid deposition (storage) to its oxidation as energy substrate. Perhaps this is the most critical effect of estrogens on energy partition.
Treatment with E2 decreases obesity[276], protects against hepatic steatosis[277], lowers the activity of cholesterol acyl-transferase[278] and limits fat deposition[279]. All these are –again– indirect actions aimed to decrease the storage of excess TAG, since E2 does not directly regulate lipolysis[280]. Nevertheless, estrogens decrease lipogenesis[281] in WAT; and adipogenesis is also inhibited through ERα activation[282].
Dietary composition directly affects the substrate partition and the regulation of substrate utilization to maintain both energy and nutrients homeostasis[283]; in rats, hyperlipidic diets induce increases in E2 levels, and are correlated with an increased use of fatty acids as energy substrate[284]. The decrease in lipogenesis/adipogenesis (and the relatively enhanced lipolysis) frees the use of excess glucose and glycolytic 3C towards 2C and its oxidation in mitochondria; thus, decreasing the synthesis and storage of fatty acids (and TAG).
The effects of E2 on lipid handling are coordinated with the actions of E2 on insulin[235,285], glycemia[286] and the use of glucose as the direct energy substrate[271] instead of using it to fuel the synthesis of fatty acids. E2 lowers insulin resistance and fat storage through the ERα and the FA2 binding site[249]. Estrogens also lower the insulin resistance induced by excess dietary lipids[245].
A key point of these E2-derived metabolic shifts lies on the mitochondria[287,288]. Estrogen controls mitochondrial biogenesis and function[289]. E2 deprivation induce mitochondrial dysfunction and insulin resistance, which may induce alterations in the cognitive ability of subjects[255]. E2 potentiates the oxidative capacity of mitochondria, through increases in cAMP and cytochrome C oxidase activity[290]. E2 also inhibits the synthesis of adenosine triphosphate (ATP) in the mitochondria[291], which may be related to the increase in oxygen consumption and energy expenditure elicited by E2[292], and its postulated role enhancing heat production and thermogenesis[293,294], which imply a higher overall substrate oxidative activity.
The pyruvate dehydrogenase complex (PDH) is a critical control node, which catalyzes the irreversible conversion of 3C pyruvate to 2C acetyl-CoA in the mitochondrion. The main mechanism of PDH control is phosphorylation, mainly by (inhibiting) PDH kinase 4 (PDHK4)[295]. Insulin inhibits the expression of PDHK4[296], which has an increased activity during starvation and diabetes[297]. The levels of PPARγ coactivator-1 α (PGC-1), an important cell energy regulator[298,299], which is also increased during diabetes and starvation, modulates the function of ERs[300]. PGC-1 increases hepatic gluconeogenesis through the expression of phospho-enolpyruvate-carboxykinase[301], and co-activates, with estrogen-related receptor (ERR), the expression of glucokinase[302]. PGC-1 increases the expression of PDHK4[303], essentially through the activation of ERR (mainly the ERRα and ERRγ isoforms)[304]. ERRs are homologous to the nuclear ERs, but they are orphan receptors, i.e. do not have specific ligands such as E2[305]. Recently, it has been suggested that PGC-1 could be considered, perhaps, their unique non-steroid ligand[306]. ERRs increase glycolysis and glucose uptake[307].
The activation of PDHK4 by ERRs and PGC-1 is inhibited by insulin[303]. However, E2 activates ERRs[308]. In sum: insulin activates PDH, which is inhibited by ERRs modulated by cell lipid energy conditions (PCG-1) in a way that facilitates a decrease in insulin resistance[309] and a steady flow of 3C to 2C into the mitochondria to fuel the Krebs cycle, since lipogenesis is inhibited[281,310] and cannot absorb the newly formed acetyl-CoA.
Further stimulation of mitochondrial oxidative capacity[153,311], and the availability of 4C and 5C derived from amino acids, further speeds up the oxidation of 2C by the mitochondria of liver, WAT and specific brain sites[312]. The accessibility of amino acid hydrocarbon skeletons depends on their increased oxidation (when in excess and limited capacity of the Krebs cycle[189]) via the alternative oxidation of amino groups to nitrogen gas[222,313]. The presence of these anaplerotic fragments and the higher oxidative capacity markedly increase the use of acetyl-CoA (from fatty acids or glucose) as the main energy substrate. The added relative inefficiency in the production of ATP[291] further helps the estrogen-controlled metabolism of adult women to dispose (albeit partially) of unwanted excess dietary energy. This effect may account in part for the resistance of women to develop the MS in its double facet of obesity and diabetes[314].
Estrone and acyl-estrone
E1 has not generated as much literature as E2, but this is probably a consequence of its limited direct effects on classical estrogenicity and energy metabolism. However, it has been found that SE1 also contributes to glucose homeostasis, inhibiting glucose-6 phosphatase under conditions of hyperglycemia[233]. SE1 also lowers the levels of lipoproteins in postmenopausal women[315]. And, obviously shows estrogenic effects when given in pharmacological doses, albeit less marked than those of E2.
The anti-obesity effects of oleoyl-estrone, an AE1 ester, were studied extensively for a short time[170], but ceased before the appearance of many key studies on estrogen function and mechanism of action cited above. Thus, these older studies have to be re-analyzed from the present-day perspective. The acyl moiety of AE1 comparatively affects only partially its slimming effects[48], thus, oleic acid (the most abundant in the rat body stores) was used as standard. The E1 moiety, surprisingly, is not essential either, since both AE2 (at pharmacological levels) and oleoyl-diethyl-stilbestrol show marked body fat slimming effects[48]; however, these compounds have not been studied further because of the marked estrogenic response (toxic at the pharmacological levels analyzed) they elicited in comparison with AE1 or even E1 alone.
AE1 is not estrogenic[171]. However, the injection of liposomes loaded with AE1 induces estrogenic effects in rats due to the large amount of E1 produced by its hydrolysis[178]. The oral administration of AE1 basically excludes most of the E1[316] formed in the intestinal hydrolysis of AE1. In any case, SE1 is, finally, the main catabolite of AE1[185] (and of E1), since the fatty moiety poses no problems to its complete oxidation. This way, the interference of estrogenic effects has been circumvented simply by oral (instead of i.v.) administration of AE1, allowing a more direct analysis of its effects on body lipids[167,169,173]. The administration of AE1 preserves body protein[167,169,317], but markedly decreases body fat through the maintenance of a negative energy balance[167,169,177,180,317]. The process is achieved by lower food intake and unchanged thermogenesis[180] (an effect partly shared by E2 but not by E1), as well as a shift in the management of dietary fat, from accrual to oxidation for energy[88,318], such as described for E2. The effects of additional reduction of food availability are comparable (and additive) to those due to decreased appetite elicited by AE1[317]. Circulating levels of AE1 are proportional to body fat[65,182]. However, obese rats have lower AE1 levels than their lean counterparts[44]. In sum, the main effect of AE1 (given at pharmacological levels) is to shed excess body fat, without additional metabolic interference[174]. AE1 circulating levels presumably act as an indicator of whole body fat reserves under normal (not MS) conditions[319]. The AE1-induced loss of body TAG implies the concordance (described for E2) of peripheral (especially WAT) lipolysis[320], decreased lipogenesis[321] and higher energy expenditure and lipid oxidation[318].
The effects of AE1 on glucose metabolism are comparable to those described for E2: regularize hyperglycemia[174], decrease insulin resistance[174] and an overall antidiabetic action[322]. However, these effects may be just the consequence of the normalization of energy homeostasis induced by pharmacological doses of AE1[170], with full activation of the estrogen shift explained above: increased mitochondrial oxidation of 2C (and excess 3C) instead of storage of excess 2C mainly in the form of TAG-fatty acids.
The similarities of AE1 with E2 are both quantitative and qualitative. The well-known summarization of E2 function as less estrogenic than neuroprotective[323] is not applicable to the comparison with AE1 because these esters do not show either of these functions. Nevertheless, injected AE1 label has been found in cell nuclei[181], and AE1 binds the ERα[183,184], but E2 cannot displace AE1 from its binding[171]. In addition, the pharmacological effects of E2 and AE1 are not superimposable[177]. This is compounded by the lack of full inhibition of AE1 actions on rodents by tamoxifen[324] and fulvestrant[184] (in fact, tamoxifen mimicked some of the effects of AE1[324]). These data help finally differentiate the effects of AE1 from both E1 and E2, and suggest that AE1 is, probably a SERM.
Functional protection through antioxidant mechanisms
E2 and E3 (but not E1) are considered effective antioxidants[325], since they help protect structural lipids from free radicals[326]. The polarized structure of estrogens makes them ideally suited to interact in interfaces between hydrophilic and lipophilic media[327], such as membranes, including mitochondria[160]. In this aspect, perhaps the AE2 esters may be the most effective, because in addition to E2, their most common acyl moiety, linoleic acid[328], is itself a main component of membranes[329], albeit being easily oxidized by free radicals[330]. The AE2 have been described as powerful antioxidants, more effective than free E2[331]. This role includes mitochondria, closely related to estrogen action for increased numbers, oxidative capacity, metabolic function and survival[161]. In this sense, both E2 and AE2 (and, probably to a lesser extent E1), control[332] and protect mitochondria in brain, liver and other tissues[156,161,333]. The antioxidant effects of estrogens seem unrelated to the classical estrogenic activity[334].
The AE2 antioxidant function is not limited to membranes, since their presence in lipoproteins helps protect them from scavenger radicals[275], maintaining their transport and signaling function. Since acylation on C17 of E2 results in a more effective antioxidant molecular type[55], and no other estrogens seem to specifically carry out this task, its uniqueness, and the importance of the function suggests that the AE2 may constitute, by themselves, a different specialized type of estrogens carrying out a critical and specific function for which they are best suited.
Whilst AE1 do not show significant estrogenicity[171], AE2 are markedly estrogenic[126,150,244], and maintain this estrogenicity longer than the 3-acyl-E2 esters[150], which suggests that they may–precisely-retain this property when packed in lipoproteins, such as low density lipoprotein (LDL)[126] or bound to plasma proteins[335]. When taken together, these properties suggest that the AE2 may, at least, fulfill the role of transport/storage of E2 in addition to an antioxidant function.
Central-mediated effects of estrogens on energy homeostasis
The main arguments for the postulated subdivision of estrogens in four separate classes, based on their structure and function is based on widely different availability of sources, but the marked differences observed suggest -at least- the existence of four groups, described in Table 1, which summarize most of the information provided in the present study.
Table 1.
Comparison of the effects/functions between the main functional types of estrogens1
|
Effect/ function/ action/ characteristic
|
E1
|
AE1
|
E2
|
AE2
|
| Bind the ERs at the hormone binding site | ↑↑2 | X | ↑↑2 | ~ |
| Bind the AF1 or AF2 sites of the ERs | X2 | ↑ | X2 | ~ |
| Bind to mitochondria (and some membranes) | ~ | ~ | ↑2 | ↑2 |
| Elicit a direct classic estrogenic response | ↑2 | X | ↑↑2 | ↑↑2 |
| Induces hypoglycemic effects | ~i | ↑↑2 | ↑2 | ~ |
| Is carried by lipoproteins | ↑2 | ↑↑2 | X | ↑2 |
| Show anti-oxidative effects | ~ | ~ | ↑2 | ↑↑2 |
| Activate the 3C→2C conversion (pyruvate dehydrogenase) | ~ | ↑2 | ↑↑2 | ~ |
| Increase mitochondrial oxidative activity | ~ | ~ | ↑ | ~ |
| Increase whole body thermogenesis | ~ | ↑2 | ↑2 | ~ |
| Show lipolytic effects | X | ↑ | ↔ | ~ |
| Show lipogenic effects | ↑ | ↓2 | ↓2 | ~ |
| Decrease WAT fat mass/ limits fat deposition | ↓ | ↑↑2 | ↑2 | ~ |
| Allow the activation of the alternative N disposal pathway | ~ | ~ | ↑ | ~ |
| Decrease body protein mass | ↔ | X2 | X2 | ~ |
Specific early development and pregnancy-related estrogen molecular species and functions not included.
Shows a coincidence of effect/function for different estrogen types in the same row.
↑: Exerts the effect described; ↓: Exerts an effect opposite to that described; ↔: Variable/not univocal responses; X: Does not exert the effect described; ~: Absent or insufficient information available; E1: Estrone; AE1: Acyl-estrone; E2: 17β-estradiol; AE2: Acyl-E2; ERs: Estrogen receptors; AF1: Activation function site 1 of the estrogen receptors; AF2: Activation function site 2 of the estrogen receptors; WAT: White adipose tissue.
E1:
Structural: Estrin nucleus, with only one phenolic-OH.
Functional: Mild estrogenic effect; a main precursor in the synthesis of E2; main catabolite in the excretion of estrogens as SE1; SE1 being probably the main signaling form of E1; increases growth during development; quantitatively the most abundant molecule with an estrogen nucleus in the body; possible “reserve” for rapid conversion to E2 or AE1.
Targets: (Generalized); WAT; reproductive-system organs.
AE1:
Structural: Estrin nucleus, with only the phenolic-OH esterified by a fatty acid.
Functional: No estrogenic effects; product of esterification (or interchange) of E1, probable active SERM for ERα, activates lipid catabolism, via lipolysis and oxidation of fatty acids; postulated as ponderostat signal, markedly lowers body fat: maintains glycemia.
Targets: WAT; brain; liver.
E2:
Structural: Estrin nucleus, with the phenolic–OH, and another-OH in C17.
Functional: Main estrogen; marked classical estrogenic effects, protects insulin and facilitates its secretion, maintains glycemia; indirectly activates lipolysis and inhibits lipogenesis; protects and favors the increase and oxidative function of mitochondria, lowers body fat, has antioxidant capability.
Targets: Brain, liver, mitochondria, reproductive-system organs, bone.
AE2:
Structural: Estrin nucleus, with only the phenolic–OH, esterified in C17 by a fatty acid, often polyunsaturated.
Functional: The most effective estrogen form of antioxidant; postulated as an element of transport or reserve of E2, protects lipoproteins, membranes and cell components, marked estrogenic action.
Targets: Mitochondria; plasma lipoproteins.
This partial (and incomplete) classification of the main estrogens is based on both structural and functional aspects. The estimated quantitative mass of these four types of estrogen (under standard conditions) in the whole body is: AE1 > E1 > E2 > AE2.
Estrogen is a fundamental modulator of female functions (including estrogenicity stricto sensu), which agrees with the high levels of E2 in adult pre-menopausal women. No sufficient data are available to show other gender differences in the postulated groups of estrogens, except for AE1, which pharmacological effects in obese rats are more intense in males than in females.
Estrogens, essentially E2, are responsible for the development of the sex-dependent structures (both physiological and psychological) related with mating and reproduction. Evidently this is achieved with the collaboration of other hormones, e.g. androgens, progestogens and peptidic hormones. It is unclear whether the other three groups of estrogens depicted in Table 1 play a specific significant role in the reproductive processes (with the exception, perhaps, of AE2).
The other key places for action of estrogens are characterized by containing ERs and are found throughout the whole body[336]; but WAT[337], liver[338], muscle[339], and, essentially the brain[340,341] are the main targets for their actions, and are, at least, the best studied. ERβ globally regulates lipid homeostasis[145], its activation in obesity increases whole body metabolism and mitochondrial biogenesis as a countermeasure to excess WAT lipid storage[312]. Oophorectomy alters WAT lipid metabolism[342], the plasma levels of E2 are affected by diet[284] and determine body fat deposition[343], probably through central (brain) control mechanisms.
In the hypothalamus, where E2 interaction with serotonin has been described[254]. E2 regulates sympathetic nervous control[344]. E2 and AE1 have marked anorectic effects[180,345]. The postulated ponderostat effect of AE1[181,319] depends on its action on brain, where blood-injected label has been found[181]. The effects of estrogens on glucose and body fat have been attributed to central actions on the brain[15,346]. This is a logical assumption, including also T[347], because the brain controls the body energy metabolism[16] and homeostasis[348], i.e. regulates the coordinated biological maintenance systems of the body[349,350].
General considerations and conclusions
The growing number of known actions of estrogens in metabolic control cannot be fully explained by only the analysis of the “common” estrogens, essentially E1, E2 and E3 (with their sulfates). Thus, acyl-estrogen derivatives have also been included: Despite being known for a long time, and used in pharmacology, they are seldom included in general analyses of estrogens. These compounds are quite diverse. However, in practice, the studies available are limited only to acyl esters of E1 (on C3) or E2 (largely on C17); their properties are quite different, starting with estrogenicity, and continuing on to antioxidant or lipid wasting effects (Table 1).
In any case, the differences in effects induced by E1 and E2 (those of E3 seem to be closer to E2), are considerable, both in their classical estrogenic power and in their implication in regulative mechanisms: E2 being more powerful than E1 in almost any aspect related to metabolic regulation, becoming the most representative estrogen. Nevertheless, a large proportion of E2 is synthesized from E1 by widely distributed 17 OH-steroid dehydrogenases[351]. The similarities between E1 and AE1 actions are small, the latter resembling more E2 in its metabolic effects, than E2 vs AE2, despite the strong relationships in the main functions of AE2: antioxidant and estrogenic.
Due to the crossed coincidences of effects between E1-E2 and their acyl esters, a loose classification based on functions and structure has been developed and presented here. Either separately or as a conjoint “estrogens” block they may provide a comprehensive explanation of most of the actions of estrogens, which could not be attributed in any way solely to either one or to all the usual non-esterified estrogens as a group. It is also remarkable that these four groups constitute, together, an extensive fully synergic unit of action: antioxidant effects protect mitochondria, membranes and lipids, which are actively used for energy, limiting insulin resistance. However, the protection extends to insulin (and the pancreatic β cells); insulin secretion is maintained to sustain a steady glycemic response. Glucose is converted to 2C only when it is in excess, whilst dietary lipids (at the root of inflammation and development of MS) are not accrued but oxidized. Protein is preserved by strong, effective and well established mechanisms; but excess energy (and glucose) does not prevent the utilization of amino acids for energy, and, especially for efficient operation of the Krebs cycle thanks to the supply of 4C and 5C fragments. The problem of excess N disposal[189], strictly overprotected via the urea cycle is compensated using the direct pathway to produce N2[189]. Lipoproteins remain functional thanks to the steroidal antioxidants, TAG transport is practically unaltered, but lipogenesis is maintained low, and lipolysis high to dispose of excess 2C energy. Thermogenesis is maintained and appetite (and food intake) are adjusted to the real needs of energy intake (plus the use of unnecessary fat reserves). A happy metabolic Arcadia under the rule of essentially one (multiple) hormonal factor: estrogens.
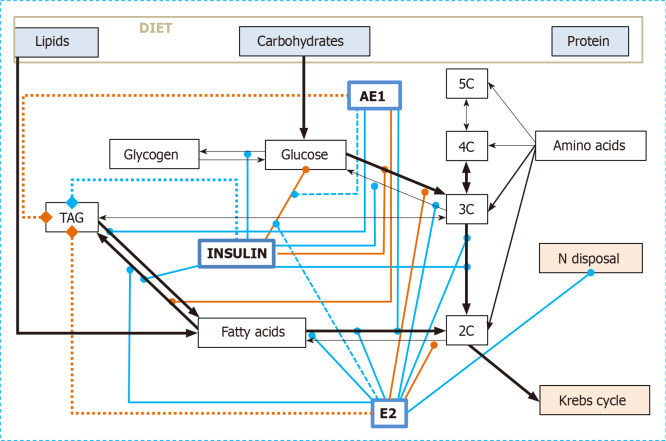
Figure 2 shows the main interactions of simple estrogens (essentially E2) and AE1 on the core of intermediate energy metabolism, the meeting point of carbohydrate, lipid and protein catabolism. The antioxidant effects of AE2 (and E2) have been omitted, since their main point of action lies, just from the critical step 3C→2C and the Krebs cycle, within the mitochondrion. The coordinated implication of estrogen types in the control of this segment of substrate handling is pervasive, both by direct implication and through its modulation of insulin action, and helps clarify that the implication of estrogens (as a whole) on glucose handling (and insulin control) is very high. The control node of energy metabolism lies in the mitochondria/cytoplasm interface, and-perhaps-critically on PDH. Around this point, directly linked to oxidative function of mitochondria and their generation of ATP, the implication of estrogens is high, as are the adjustment of the supply lines of 2C and 3C, the control of glycemia and the shift of amino acid catabolism under conditions of abundant energy (and glucose) availability[189].
Figure 2.
Combined regulatory effects on the utilization of dietary nutrients as energy substrates of insulin, 17β-estradiol and acyl-estrone. Black arrows: Metabolic relationships; solid green lines: activation/enhancement; solid red lines: deactivation/inhibition. Dashed green lines show activation effects on regulatory processes. Thicker dot lines with final diamond symbols represent the enhancement (green) or decrement (red) of TAG reserves. For the sake of simplicity, other regulatory agents are not shown, and neither are their interactions. AE1: Acyl-estrone; E2: 17β-estradiol and other E2-group estrogens
In addition to the need to consider the estrogens as a group of several molecular species sharing a common biochemical structure and origin, implied web-like in a large number of coordinated metabolic functions, the main conclusion of this review is, precisely, the paramount metabolic importance of the estrogens. This is based on the synergy between the different forms of estrogen to maintain energy (including, obviously, glucose homeostasis), whilst preventing oxidative damage, lipid-induced inflammation, excess fat accrual (and thus, obesity), and easing nitrogen excess normalization. This short (and incomplete) list is quite similar to a prescription for preventing the development of MS. Abundant epidemiological and limited experimental data support this assertion. It seems that we have to look more openly at estrogen forms to better understand their nature and properties, and to use them to fulfill their natural purpose as wardens of energy homeostasis (Box 5).
BOXES
Box 1 extended negative opinions on “sex hormones” hamper their investigative study and clinical development
In the case of “sex hormones” (i.e., estrogens and androgens), the intensive pharmacological development has not displaced from use the most representative natural hormones: E2 and T from the front line of pharmacotherapy. However, the abuse of synthetic drugs (anabolic steroids, for instance) for purposes not strictly medical[352,353] have clouded the relatively recent recovery of T as a critical hormone for energy-partition[354,355].
The widely extended negative opinion against “sex hormones” continues to seriously hinder the use of T in the treatment of aging- and MS-related hypogonadism in mature and old men[225,356]. The “opinion war” against estrogen is, currently, even harder to overcome, because of its direct implication on women’s sex; and because the social, political, and even religious arguments coalesced to raise questions (real or inflated[357]) against their use for any purpose outside a few restricted and socially-conditioned gynecologic disease applications[358-360]. The fact that the natural hormone E2 continues to be the main (and effective and cheap) estrogen standard drug only adds to the widely extended negative bias against estrogens[205,361].
Box 2 critical methodological questions - the differences between species
Most of the problems caused by pharmacological overdosing of androgenic anabolic drugs or estrogens are probably secondary to blocking the hypothalamus-hypophysis-gonadal axis, a possibility observed many years ago[362], but seldom taken into consideration in clinical practice (and even less when the use is not medically justified). Because of this problem, continuous administration of excess anabolic androgens, can result in the loss of reproductive function[363] in addition to the derangement of their regulative metabolic (or/and psychological) functions.
Most of the metabolic studies on estrogens have been carried out in rodents for obvious reasons, but there are clear differences in the estrogen (and androgen) functions in rodents from those in humans. This includes a number of aspects, starting with the most obvious: size, metabolic rate and lifespan. The duration of the reproductive cycle, “estrus” or “heat” of rats and mice is shorter than the human ovarian cycle (which incorporates parts of the estrus cycle[364]), but their extension, phasing and physiological structure are different. The estrus is observed in most mammalian species (not in humans and apes), and is marked by changes in body temperature and energy expenditure[365]. Size affects energy expenditure (allometry)[366] and lifespan[367]. The fact that women are usually uniparous and rats normally have a two-digit number of pups (requiring a much higher energy and nutrient supply effort at the expense of the dam) is also a quantitative difference that makes uncertain the direct comparison of hormone changes and their timing, and of substrate dynamics, between different species and reproductive cycle models. Another key difference, explained above, is the E2 (and T) transport in plasma. Humans carry E2 and T bound to SHBG in high proportions of total circulating hormone, but SHBG is absent in mature rodents.
Box 3 steroid hormones as medium-term signals focused to control gene expression
Steroid hormones have longer circulating-lives than most other hormones (or other signaling molecules), which are rapidly produced (or released), then act and are inactivated, all in a short time-span. The maintenance along time of rapid-response signals is kept thanks to repeated secretion-activation/inactivation-destruction processes, which allow for rapid regulation changes, again in a short period of time. Steroid hormones, however, are produced and secreted to last for much longer periods; their prime mechanism of action is, essentially, gene translation, a process which requires more intensity of signal and longer stimulation periods. The advantage of steroids is their unbeatable stability over time in comparison to short term-effect peptidic hormones, catecholamines, etc. The target tissue specific needs are adjusted through the expression of different receptors and signaling pathways for the same steroid hormone, with additional modulation of expression, or by the numbers or proportions of molecular species, allowing for further modification of their effects under changing conditions.
Box 4 estrogen structural resilience and the environment
Estrogenic signaling is very ancient, affecting quite a number of phyla as observed by the use of estrogen analogs in the context of co-evolution of plant allopathic defense against herbivores[10,368]. The estrin nucleus is highly resistant to environmental oxidation or bacterial catabolism[369]. The non-biological disposal of human waste induces the accumulation of estrogens in continental waters[370] and its sediments[371]; they are also found in sea sediments[372]. Persistence of estrogen in the natural medium has another negative aspect, the environmental effects of waste estrogen[373], affecting both invertebrates and vertebrates[8]. Human and domestic animal overpopulation extends the increase of estrogenic waste problem to become a serious health, ecologic and economic[8,374] problem that should be understood and adequately addressed.
Box 5 perspectives: the need for further advance in our knowledge of estrogens
A critical point for the continued use of estrogens in medicine is that of the extended use of molecules designed for specific clinical applications[375]. All these patented molecules do mimic some aspects of physiological estrogen actions, but not all of them[375]. The synthesis of estradiol analogs has been oriented to increase only some effects, sought for specific clinical applications[9,376]. However, most of these compounds were designed before our knowledge of estrogen function, mechanisms of action and metabolic effects were fully known[377]. In fact, right now, our knowledge of the full list of estrogen effects is incomplete. For instance, the role of estrogens in the control of brain organization[378,379], adjustment of the immune response[380,381], or even the estrogen function in core energy metabolism regulation[15].
The proof of our limited knowledge is the lack of a clear picture of all the physiological actions carried out by estrogens. Thus, how can we expect to extend this needed knowledge to drugs devised with specific (not global) objectives? How to test their effects on functions that so far have not even been uncovered or analyzed in the classical estrogens? In fact a similar caveat should be applied to all other steroid hormones, especially corticosteroids and androgens.
We are aware that the binding modulation of the effects of estrogenic drugs is unclear, especially as to which real estrogen (as a whole) actions are carried out by each of these compounds[382,383]. In any case, during the last 70-90 years, the use of synthetic estrogens has been slowly substituted by natural estrogens[383,384], with the practical abandonment of the wonder drug diethylstilbestrol. The case of anabolic androgens (and testosterone itself) is paradigmatic: the continued use of these drugs results at least in infertility[385] and cardiovascular damage[386]. The continued use of estrogens, in particular powerful analogs, may result in unexpected, possibly negative or unexplained effects[384,387,388], simply not detected because the protean nature of estrogenic action has not permeated yet to clinical (and, especially, pharmacological) practice.
We are uncovering the proverbial tip of the iceberg of a group of steroid hormones; we need a deep analysis of estrogen functions, both from the molecular and regulatory aspects, but never forgetting that steroids act on the whole body not only on specific organs and single isolated pathways. The actual role of hormone carriers in plasma (i.e. SHBG), the cyclic hypothalamic-hypophysis-gonadal axis function, and the possible disorders induced by estrogenic drug substitutes, must be studied and adapted to human physiology in order to be able to resolve endocrine disorders as a whole. First: do no harm; the effects of estrogens and analog drugs under clinical conditions must be known and fully evaluated because not all estrogens and related drugs act the same way[383,384].
Last, but not least, the real and realistic implication of estrogens (as well as androgens and corticosteroids) in the regulation of the metabolic hub of energy partition should be clarified. This is an essential step to limit the ravages of aging and to understand (and correct) disorders such as the widely extended MS.
CONCLUSION
The main conclusion of this review is, precisely, the paramount metabolic importance of the estrogens. This is based on the synergy between the different forms of estrogen to maintain energy (including, obviously, glucose homeostasis), whilst preventing oxidative damage, lipid-induced inflammation, excess fat accrual (and thus, obesity), and easing nitrogen excess normalization.
ACKNOWLEDGEMENTS
I wish to express my gratitude to the members of my former research group (Nitrogen-Obesity, Faculty of Biology, University of Barcelona) for their help, friendship, insight, support and patience.
Footnotes
Conflict-of-interest statement: The Author declares no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: January 22, 2021
First decision: March 1, 2021
Article in press: April 14, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kung WM, Navarro-González JF S-Editor: Zhang L L-Editor: Webster JR P-Editor: Wang LYT
References
- 1.Dufourc EJ. Sterols and membrane dynamics. J Chem Biol. 2008;1:63–77. doi: 10.1007/s12154-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J, Han Z, Chai J. Q&A: what are brassinosteroids and how do they act in plants? BMC Biol . 2016;14:113. doi: 10.1186/s12915-016-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heftmann E. Functions of steroids in plants. Phytochemistry. 1974;14:891–901. [Google Scholar]
- 4.Geuns JMC. Plant steroid hormones - what are they and what do they do? Trends Biochem Sci. 1982;7:7–9. [Google Scholar]
- 5.Hewitt S, Hillman JR, Knights BA. Steroidal oestrogens and plant growth and development. New Phytol . 1980;85:329–350. [Google Scholar]
- 6.Milanesi L, Boland R. Presence of estrogen receptor (ER)-like proteins and endogenous ligands for ER in solanaceae. Plant Sci. 2004;166:397–404. [Google Scholar]
- 7.Janeczko A, Skoczowski A. Mammalian sex hormones in plants. Folia Histochem Cytobiol. 2005;43:71–79. [PubMed] [Google Scholar]
- 8.Adeel M, Song X, Wang Y, Francis D, Yang Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ Int. 2017;99:107–119. doi: 10.1016/j.envint.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Shorr E, Robinson FH, Papanicolaou GN. A clinical study of the synthetic estrogen stilbestrol. JAMA . 1939;113:2312–2318. [Google Scholar]
- 10.Basu P, Maier C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother. 2018;107:1648–1666. doi: 10.1016/j.biopha.2018.08.100. [DOI] [PubMed] [Google Scholar]
- 11.Niwa YS, Niwa R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev Growth Differ. 2016;58:94–105. doi: 10.1111/dgd.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinan L, Harmatha J, Volodin V, Lafont R. Phytoecdysteroids: Diversity, biosynthesis and distribution. In: Smagghe G. Ecdysone: Structures and Functions. Springer Science & Business Media B.V.: Dordrecht, 2009: 3-45. [Google Scholar]
- 13.Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. doi: 10.3389/fimmu.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 15.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, López M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104–115. doi: 10.1016/j.molmet.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange IG, Hartel A, Meyer HH. Evolution of oestrogen functions in vertebrates. J Steroid Biochem Mol Biol. 2002;83:219–226. doi: 10.1016/s0960-0760(02)00225-x. [DOI] [PubMed] [Google Scholar]
- 18.Simpson E, Jones M, Misso M, Hewitt K, Hill R, Maffei L, Carani C, Boon WC. Estrogen, a fundamental player in energy homeostasis. J Steroid Biochem Mol Biol. 2005;95:3–8. doi: 10.1016/j.jsbmb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JD. The evolution of Endocrinology. Clin Endocrinol. 62:389–396. doi: 10.1111/j.1365-2265.2005.02209.x. [DOI] [PubMed] [Google Scholar]
- 20.O'Malley BW. 90 years of progesterone: Reminiscing on the origins of the field of progesterone and estrogen receptor action. J Mol Endocrinol. 2020;65:C1–C4. doi: 10.1530/JME-20-0042. [DOI] [PubMed] [Google Scholar]
- 21.Ball P. Carl Djerassi (1923-2015) Nature. 2015;519:34. doi: 10.1038/519034a. [DOI] [PubMed] [Google Scholar]
- 22.Nieschlag E, Nieschlag S. Endocrine History: The history of discovery, synthesis and development of testosterone for clinical use. Eur J Endocrinol. 2019;180:R201–R212. doi: 10.1530/EJE-19-0071. [DOI] [PubMed] [Google Scholar]
- 23.Benedek TG. History of the development of corticosteroid therapy. Clin Exp Rheumatol. 2011;29:S–5. [PubMed] [Google Scholar]
- 24.Hillier SG. Diamonds are forever: the cortisone legacy. J Endocrinol. 2007;195:1–6. doi: 10.1677/JOE-07-0309. [DOI] [PubMed] [Google Scholar]
- 25.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 26.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 27.Liehr JG, Avitts TA, Randerath E, Randerath K. Estrogen-induced endogenous DNA adduction: possible mechanism of hormonal cancer. Proc Natl Acad Sci U S A. 1986;83:5301–5305. doi: 10.1073/pnas.83.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire PJ. Estrogen replacement therapy and breast cancer. J Reprod Med. 1993;38:183–185. [PubMed] [Google Scholar]
- 29.Hierholzer K, Lichtenstein I, Siebe H. Does corticosteroid metabolism in target organs affect the cardiovascular system? J Auton Nerv Syst. 1996;57:188–192. doi: 10.1016/0165-1838(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 30.Hariharan M, Naga S, VanNoord T, Kindt EK. Assay of human plasma cortisone by liquid chromatography: normal plasma concentrations (between 8 and 10 a.m.) of cortisone and corticosterone. J Chromatogr. 1993;613:195–201. doi: 10.1016/0378-4347(93)80134-p. [DOI] [PubMed] [Google Scholar]
- 31.Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci. 2009;63:613–622. doi: 10.1111/j.1440-1819.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 32.Mayer M, Kaiser N, Milholland RJ, Rosen F. The binding of dexamethasone and triamcinolone acetonide to glucocorticoid receptors in rat skeletal muscle. J Biol Chem. 1974;249:5236–5240. [PubMed] [Google Scholar]
- 33.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 34.Coelingh Bennink HJ, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11 Suppl 1:47–58. doi: 10.1080/13697130802073425. [DOI] [PubMed] [Google Scholar]
- 35.Tang M, Abplanalp W, Subbiah MTR. Association of estrogens with human plasma lipoproteins: Studies using estradiol-17β and its hydrophobic derivative. J Lab Clin Med . 1997;129:447–452. doi: 10.1016/s0022-2143(97)90078-0. [DOI] [PubMed] [Google Scholar]
- 36.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsohn MK, Bauder S, Pine SR, Jacobsohn GM. Cholesterol limits estrogen uptake by liposomes and erythrocyte membranes. Biochim Biophys Acta. 1994;1195:131–140. doi: 10.1016/0005-2736(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 38.Arnold S, Victor MB, Beyer C. Estrogen and the regulation of mitochondrial structure and function in the brain. J Steroid Biochem Mol Biol. 2012;131:2–9. doi: 10.1016/j.jsbmb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Grasa MM, Gulfo J, Camps N, Alcalá R, Monserrat L, Moreno-Navarrete JM, Ortega FJ, Esteve M, Remesar X, Fernández-López JA, Fernández-Real JM, Alemany M. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur J Endocrinol. 2017;176:393–404. doi: 10.1530/EJE-16-0834. [DOI] [PubMed] [Google Scholar]
- 40.Parwanto MLE, Suweino S, Tjahjadi D, Senjaya H, Jaya Edy H, Pakpahan A. The effect of sex hormone-binding globulin (SHBG) protein polymorphism on the levels of SHBG, testosterone, and insulin in healthy Indonesian men. IJBMPH. 2016;5:799–806. [Google Scholar]
- 41.Vanbillemont G, Bogaert V, De Bacquer D, Lapauw B, Goemaere S, Toye K, Van Steen K, Taes Y, Kaufman JM. Polymorphisms of the SHBG gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin Endocrinol (Oxf) 2009;70:303–310. doi: 10.1111/j.1365-2265.2008.03365.x. [DOI] [PubMed] [Google Scholar]
- 42.Cousin P, Déchaud H, Grenot C, Lejeune H, Pugeat M. Human variant sex hormone-binding globulin (SHBG) with an additional carbohydrate chain has a reduced clearance rate in rabbit. J Clin Endocrinol Metab. 1998;83:235–240. doi: 10.1210/jcem.83.1.4515. [DOI] [PubMed] [Google Scholar]
- 43.van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. Comparative study on the in vitro/in vivo estrogenic potencies of 17beta-estradiol, estrone, 17alpha-ethynylestradiol and nonylphenol. Aquat Toxicol. 2004;66:183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Massanés RM, Grasa MM, López-Martí J, Díaz-Silva M, Fernández-López JA, Remesar X, Alemany M. Zucker obese rats store less acyl-estrone than lean controls. Int J Obes Relat Metab Disord. 2003;27:428–432. doi: 10.1038/sj.ijo.0802264. [DOI] [PubMed] [Google Scholar]
- 45.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 46.Ruder HJ, Loriaux L, Lipsett MB. Estrone sulfate: production rate and metabolism in man. J Clin Invest. 1972;51:1020–1033. doi: 10.1172/JCI106862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brind J, Strain G, Miller L, Zumoff B, Vogelman J, Orentreich N. Obese men have elevated plasma levels of estrone sulfate. Int J Obes. 1990;14:483–486. [PubMed] [Google Scholar]
- 48.Sanchis D, Balada F, Farrerons C, Virgili J, del Mar Grasa M, Adán C, Esteve M, Cabot C, Ardévol A, Vilà R, Fernández-López JA, Remesar X, Alemany M. Structural determinants of oleoyl-estrone slimming effects. Life Sci. 1998;62:1349–1359. doi: 10.1016/s0024-3205(98)00069-1. [DOI] [PubMed] [Google Scholar]
- 49.Virgili J, Casals I, Peinado-Onsurbe J, Esteve M, Julve-Gil J, Fernández-López JA, Remesar X, Alemany M. Distribution of oleoyl-estrone in rat plasma lipoproteins. Horm Metab Res. 1999;31:597–601. doi: 10.1055/s-2007-978803. [DOI] [PubMed] [Google Scholar]
- 50.Esteve M, Savall P, Virgilli J, Fernández-López JA, Remesar X, Alemany M. Modulation by leptin, insulin and corticosterone of oleoyl-estrone synthesis in cultured 3T3L1 cells. Biosci Rep. 2001;21:755–763. doi: 10.1023/a:1015580623325. [DOI] [PubMed] [Google Scholar]
- 51.Tikkanen MJ, Vihma V, Jauhiainen M, Höckerstedt A, Helisten H, Kaamanen M. Lipoprotein-associated estrogens. Cardiovasc Res. 2002;56:184–188. doi: 10.1016/s0008-6363(02)00535-7. [DOI] [PubMed] [Google Scholar]
- 52.Janocko L, Hochberg RB. Estradiol fatty acid esters occur naturally in human blood. Science. 1983;222:1334–1336. doi: 10.1126/science.6419346. [DOI] [PubMed] [Google Scholar]
- 53.Larner JM, MacLusky NJ, Hochberg RB. The naturally occurring C-17 fatty acid esters of estradiol are long-acting estrogens. J Steroid Biochem. 1985;22:407–413. doi: 10.1016/0022-4731(85)90446-7. [DOI] [PubMed] [Google Scholar]
- 54.Badeau M, Vihma V, Mikkola TS, Tiitinen A, Tikkanen MJ. Estradiol fatty acid esters in adipose tissue and serum of pregnant and pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:4327–4331. doi: 10.1210/jc.2007-1372. [DOI] [PubMed] [Google Scholar]
- 55.Vihma V, Tikkanen MJ. Fatty acid esters of steroids: synthesis and metabolism in lipoproteins and adipose tissue. J Steroid Biochem Mol Biol. 2011;124:65–76. doi: 10.1016/j.jsbmb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med . 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 57.Antunes CM, Strolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, Burnett L, Rutledge A, Pokempner M, Garcia R. Endometrial cancer and estrogen use. Report of a large case-control study. N Engl J Med. 1979;300:9–13. doi: 10.1056/NEJM197901043000103. [DOI] [PubMed] [Google Scholar]
- 58.Henderson BE, Ross RK, Paganini-Hill A, Mack TM. Estrogen use and cardiovascular disease. Am J Obstet Gynecol. 1986;154:1181–1186. doi: 10.1016/0002-9378(86)90696-4. [DOI] [PubMed] [Google Scholar]
- 59.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 60.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5:437–443. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 61.Samuel VT. The emerging role of oestrogen-related receptor γ as a regulator of energy metabolism. Diabetologia. 2014;57:2440–2443. doi: 10.1007/s00125-014-3377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, Andersen AN, Vestergaard ET, Schorring ME, Linneberg A, Main KM, Andersson AM, Juul A. Sex-specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto M, Hibi H, Katsuno S, Miyake K. Serum estradiol levels in normal men and men with idiopathic infertility. Int J Urol. 1995;2:44–46. [PubMed] [Google Scholar]
- 64.Yamaji T, Ibayashi H. Plasma dehydroepiandrosterone sulfate in normal and pathological conditions. J Clin Endocrinol Metab. 1969;29:273–278. doi: 10.1210/jcem-29-2-273. [DOI] [PubMed] [Google Scholar]
- 65.Fernández-Real JM, Sanchis D, Ricart W, Casamitjana R, Balada F, Remesar X, Alemany M. Plasma oestrone-fatty acid ester levels are correlated with body fat mass in humans. Clin Endocrinol (Oxf) 1999;50:253–260. doi: 10.1046/j.1365-2265.1999.00669.x. [DOI] [PubMed] [Google Scholar]
- 66.Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 2016;18:373–383. doi: 10.31887/DCNS.2016.18.4/jmarrocco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Goozen SH, Cohen-Kettenis PT, Gooren LJ, Frijda NH, Van de Poll NE. Gender differences in behaviour: activating effects of cross-sex hormones. Psychoneuroendocrinology. 1995;20:343–363. doi: 10.1016/0306-4530(94)00076-x. [DOI] [PubMed] [Google Scholar]
- 68.Høeg LD, Sjøberg KA, Jeppesen J, Jensen TE, Frøsig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, Richter EA, Kiens B. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lejsková M, Alušík S, Suchánek M, Zecová S, Pitha J. Menopause: clustering of metabolic syndrome components and population changes in insulin resistance. Climacteric. 2011;14:83–91. doi: 10.3109/13697131003692745. [DOI] [PubMed] [Google Scholar]
- 70.Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99:11–15. doi: 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Mohamad NV, Ima-Nirwana S, Chin KY. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? Endocr Metab Immune Disord Drug Targets. 2020;20:1478–1487. doi: 10.2174/1871530320666200604160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol (1985) 1989;66:498–503. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- 74.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol . 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 75.Birzniece V, Meinhardt UJ, Umpleby MA, Handelsman DJ, Ho KK. Interaction between testosterone and growth hormone on whole-body protein anabolism occurs in the liver. J Clin Endocrinol Metab. 2011;96:1060–1067. doi: 10.1210/jc.2010-2521. [DOI] [PubMed] [Google Scholar]
- 76.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin's anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab. 2006;291:E729–E736. doi: 10.1152/ajpendo.00003.2006. [DOI] [PubMed] [Google Scholar]
- 77.Ohtsuka A, Hayashi K, Noda T, Tomita Y. Reduction of corticosterone-induced muscle proteolysis and growth retardation by a combined treatment with insulin, testosterone and high-protein-high-fat diet in rats. J Nutr Sci Vitaminol (Tokyo) 1992;38:83–92. doi: 10.3177/jnsv.38.83. [DOI] [PubMed] [Google Scholar]
- 78.Mauras N. Estrogens do not affect whole-body protein metabolism in the prepubertal female. J Clin Endocrinol Metab . 1995;80:2842–2845. doi: 10.1210/jcem.80.10.7559861. [DOI] [PubMed] [Google Scholar]
- 79.Kofoed EM, Guerbadot M, Schaufele F. Structure, affinity, and availability of estrogen receptor complexes in the cellular environment. J Biol Chem. 2010;285:2428–2437. doi: 10.1074/jbc.M109.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horard B, Castet A, Bardet PL, Laudet V, Cavailles V, Vanacker JM. Dimerization is required for transactivation by estrogen-receptor-related (ERR) orphan receptors: evidence from amphioxus ERR. J Mol Endocrinol. 2004;33:493–509. doi: 10.1677/jme.1.01538. [DOI] [PubMed] [Google Scholar]
- 81.Schwabe JW, Chapman L, Finch JT, Rhodes D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell. 1993;75:567–578. doi: 10.1016/0092-8674(93)90390-c. [DOI] [PubMed] [Google Scholar]
- 82.Cheskis BJ, Karathanasis S, Lyttle CR. Estrogen receptor ligands modulate its interaction with DNA. J Biol Chem. 1997;272:11384–11391. doi: 10.1074/jbc.272.17.11384. [DOI] [PubMed] [Google Scholar]
- 83.Heldring N, Isaacs GD, Diehl AG, Sun M, Cheung E, Ranish JA, Kraus WL. Multiple sequence-specific DNA-binding proteins mediate estrogen receptor signaling through a tethering pathway. Mol Endocrinol. 2011;25:564–574. doi: 10.1210/me.2010-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller-Fahrnow A, Egner U. Ligand-binding domain of estrogen receptors. Curr Opin Biotechnol. 1999;10:550–556. doi: 10.1016/s0958-1669(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 86.Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- 87.Dechering K, Boersma C, Mosselman S. Estrogen receptors α and β: Two receptors of a kind? Curr Med Chem . 2000;7:561–576. doi: 10.2174/0929867003375010. [DOI] [PubMed] [Google Scholar]
- 88.Damdimopoulos AE, Spyrou G, Gustafsson JA. Ligands differentially modify the nuclear mobility of estrogen receptors alpha and beta. Endocrinology. 2008;149:339–345. doi: 10.1210/en.2007-0198. [DOI] [PubMed] [Google Scholar]
- 89.Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- 90.Farooq A. Structural and Functional diversity of estrogen receptor ligands. Curr Top Med Chem. 2015;15:1372–1384. doi: 10.2174/1568026615666150413154841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 92.Holm A, Nilsson BO. Identification and characterization of new mechanisms in vascular oestrogen signalling. Basic Clin Pharmacol Toxicol. 2013;113:287–293. doi: 10.1111/bcpt.12118. [DOI] [PubMed] [Google Scholar]
- 93.Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 96.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 97.Reid G, Denger S, Kos M, Gannon F. Human estrogen receptor-alpha: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59:821–831. doi: 10.1007/s00018-002-8470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sowers MR, Jannausch ML, McConnell DS, Kardia SR, Randolph JF Jr. Endogenous estradiol and its association with estrogen receptor gene polymorphisms. Am J Med. 2006;119:S16–S22. doi: 10.1016/j.amjmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Kjaergaard AD, Ellervik C, Tybjaerg-Hansen A, Axelsson CK, Grønholdt ML, Grande P, Jensen GB, Nordestgaard BG. Estrogen receptor alpha polymorphism and risk of cardiovascular disease, cancer, and hip fracture: cross-sectional, cohort, and case-control studies and a meta-analysis. Circulation. 2007;115:861–871. doi: 10.1161/CIRCULATIONAHA.106.615567. [DOI] [PubMed] [Google Scholar]
- 100.Porter JW, Barnas JL, Welly R, Spencer N, Pitt J, Vieira-Potter VJ, Kanaley JA. Age, sex, and depot-specific differences in adipose-tissue estrogen receptors in individuals with obesity. Obesity (Silver Spring) 2020;28:1698–1707. doi: 10.1002/oby.22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 102.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 103.Moggs JG, Orphanides G. Estrogen receptors: orchestrators of pleiotropic cellular responses. EMBO Rep. 2001;2:775–781. doi: 10.1093/embo-reports/kve185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shiau AK, Barstad D, Radek JT, Meyers MJ, Nettles KW, Katzenellenbogen BS, Katzenellenbogen JA, Agard DA, Greene GL. Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat Struct Biol. 2002;9:359–364. doi: 10.1038/nsb787. [DOI] [PubMed] [Google Scholar]
- 105.Xu B, Lovre D, Mauvais-Jarvis F. Effect of selective estrogen receptor modulators on metabolic homeostasis. Biochimie. 2016;124:92–97. doi: 10.1016/j.biochi.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Craig Jordan V, McDaniel R, Agboke F, Maximov PY. The evolution of nonsteroidal antiestrogens to become selective estrogen receptor modulators. Steroids. 2014;90:3–12. doi: 10.1016/j.steroids.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blizzard TA, Gude C, Morgan JD 2nd, Chan W, Birzin ET, Mojena M, Tudela C, Chen F, Knecht K, Su Q, Kraker B, Mosley RT, Holmes MA, Sharma N, Fitzgerald PM, Rohrer SP, Hammond ML. Androstenediol analogs as ER-beta-selective SERMs. Bioorg Med Chem Lett. 2006;16:834–838. doi: 10.1016/j.bmcl.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 108.McDonnell DP, Wardell SE, Norris JD. Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J Med Chem. 2015;58:4883–4887. doi: 10.1021/acs.jmedchem.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lisse TS, Hewison M, Adams JS. Hormone response element binding proteins: novel regulators of vitamin D and estrogen signaling. Steroids. 2011;76:331–339. doi: 10.1016/j.steroids.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bu H, Kashireddy P, Chang J, Zhu YT, Zhang Z, Zheng W, Rao SM, Zhu YJ. ERBP, a novel estrogen receptor binding protein enhancing the activity of estrogen receptor. Biochem Biophys Res Commun. 2004;317:54–59. doi: 10.1016/j.bbrc.2004.02.179. [DOI] [PubMed] [Google Scholar]
- 111.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286:14737–14743. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Jacob J, Sebastian KS, Devassy S, Priyadarsini L, Farook MF, Shameem A, Mathew D, Sreeja S, Thampan RV. Membrane estrogen receptors: genomic actions and post transcriptional regulation. Mol Cell Endocrinol. 2006;246:34–41. doi: 10.1016/j.mce.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 114.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 115.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 116.Sak K, Everaus H. Nongenomic effects of 17beta-estradiol--diversity of membrane binding sites. J Steroid Biochem Mol Biol. 2004;88:323–335. doi: 10.1016/j.jsbmb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Khbouz B, de Bournonville C, Court L, Taziaux M, Corona R, Arnal JF, Lenfant F, Cornil CA. Role for the membrane estrogen receptor alpha in the sexual differentiation of the brain. Eur J Neurosci. 2020;52:2627–2645. doi: 10.1111/ejn.14646. [DOI] [PubMed] [Google Scholar]
- 118.Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pastore MB, Landeros RV, Chen DB, Magness RR. Structural analysis of estrogen receptors: interaction between estrogen receptors and cav-1 within the caveolae. Biol Reprod. 2019;100:495–504. doi: 10.1093/biolre/ioy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Márquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 121.Ribeiro M, Sousa C, Rufino AT, Judas F, Mendes AF. Expression and function of the nonclassical estrogen receptor, GPR30, in human cartilage and chondrocytes. J Cell Physiol. 2020;235:8486–8494. doi: 10.1002/jcp.29691. [DOI] [PubMed] [Google Scholar]
- 122.Sharma G, Mauvais-Jarvis F, Prossnitz ER. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J Steroid Biochem Mol Biol. 2018;176:31–37. doi: 10.1016/j.jsbmb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 124.Sheppard PAS, Koss WA, Frick KM, Choleris E. Rapid actions of oestrogens and their receptors on memory acquisition and consolidation in females. J Neuroendocrinol. 2018;30 doi: 10.1111/jne.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinil. 2007;265:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Helisten H, Höckerstedt A, Wähälä K, Tiitinen A, Adlercreutz H, Jauhiainen M, Tikkanen MJ. Accumulation of high-density lipoprotein-derived estradiol-17beta fatty acid esters in low-density lipoprotein particles. J Clin Endocrinol Metab. 2001:86: 1294–1300. doi: 10.1210/jcem.86.3.7292. [DOI] [PubMed] [Google Scholar]
- 127.Ghaffari S, Naderi Nabi F, Sugiyama MG, Lee WL. Estrogen inhibits LDL (low-density lipoprotein) transcytosis by human coronary artery endothelial cells via GPER (G-protein-coupled estrogen receptor) and SR-BI (scavenger receptor class B type 1) Arterioscler Thromb Vasc Biol. 2018;38:2283–2294. doi: 10.1161/ATVBAHA.118.310792. [DOI] [PubMed] [Google Scholar]
- 128.Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J. 2010;57:101–107. doi: 10.1507/endocrj.k09e-332. [DOI] [PubMed] [Google Scholar]
- 129.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feldman RD, Limbird LE. GPER (GPR30): A Nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annu Rev Pharmacol Toxicol . 2017;57:567–584. doi: 10.1146/annurev-pharmtox-010716-104651. [DOI] [PubMed] [Google Scholar]
- 131.Lindsey SH, Chappell MC. Evidence that the G protein-coupled membrane receptor GPR30 contributes to the cardiovascular actions of estrogen. Gend Med. 2011;8:343–354. doi: 10.1016/j.genm.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roque C, Baltazar G. G protein-coupled estrogen receptor 1 (GPER) activation triggers different signaling pathways on neurons and astrocytes. Neural Regen Res. 2019;14:2069–2070. doi: 10.4103/1673-5374.262577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheong RY, Kwakowsky A, Barad Z, Porteous R, Herbison AE, Ábrahám IM. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology. 2012;153:3792–3803. doi: 10.1210/en.2012-1232. [DOI] [PubMed] [Google Scholar]
- 134.Kow LM, Pfaff DW. Rapid estrogen actions on ion channels: A survey in search for mechanisms. Steroids. 2016;111:46–53. doi: 10.1016/j.steroids.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu Q, Schnitzler GR, Ueda K, Iyer LK, Diomede OI, Andrade T, Karas RH. ER alpha rapid signaling is required for estrogen induced proliferation and migration of vascular endothelial cells. PLoS One. 2016;11:e0152807. doi: 10.1371/journal.pone.0152807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paletta P, Sheppard PAS, Matta R, Ervin KSJ, Choleris E. Rapid effects of estrogens on short-term memory: Possible mechanisms. Horm Behav. 2018;104:88–99. doi: 10.1016/j.yhbeh.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 137.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chu HP, Sarkar G, Etgen AM. Estradiol and progesterone modulate the nitric oxide/cyclic gmp pathway in the hypothalamus of female rats and in GT1-1 cells. Endocrine 2004; 24: 177-184. [DOI] [PubMed] [Google Scholar]
- 139.Otsuka M, Kadokawa H. GPR30 mediates estrone, estriol, and estradiol to suppress gonadotropin-releasing hormone-induced luteinizing hormone secretion in the anterior pituitary of heifers. J Reprod Dev. 2017;63:519–525. doi: 10.1262/jrd.2017-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y, Wang JP, Santen RJ, Kim TH, Park H, Fan P, Yue W. Estrogen stimulation of cell migration involves multiple signaling pathway interactions. Endocrinology. 2010;151:5146–5156. doi: 10.1210/en.2009-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kelly MJ, Qiu J, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2002;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- 142.Iorga A, Umar S, Ruffenach G, Aryan L, Li J, Sharma S, Motayagheni N, Nadadur RD, Bopassa JC, Eghbali M. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol Sex Differ. 2018;9:48. doi: 10.1186/s13293-018-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR. Selective estrogen receptor-alpha and estrogen receptor-beta agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1486–R1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Veen JE, Kammel LG, Bunda PC, Shum M, Reid MS, Massa MG, Arneson D, Park JW, Zhang Z, Joseph AM, Hrncir H, Liesa M, Arnold AP, Yang X, Correa SM. Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat Metab . 2020;2:351–363. doi: 10.1038/s42255-020-0189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Savva C, Korach-Andre M. Estrogen receptor β (ERβ) regulation of lipid homeostasis. Does sex matter? Metabolites. 2020;10:116. doi: 10.3390/metabo10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raut S, Kumar AV, Khambata K, Deshpande S, Balasinor NH. Genome-wide identification of estrogen receptor binding sites reveals novel estrogen-responsive pathways in adult male germ cells. Biochem J. 2020;477:2115–2131. doi: 10.1042/BCJ20190946. [DOI] [PubMed] [Google Scholar]
- 147.Piperigkou Z, Karamanos NK. Estrogen receptor-mediated targeting of the extracellular matrix network in cancer. Semin Cancer Biol. 2020;62:116–124. doi: 10.1016/j.semcancer.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 148.Hatcher KM, Royston SE, Mahoney MM. Modulation of circadian rhythms through estrogen receptor signaling. Eur J Neurosci. 2020;51:217–228. doi: 10.1111/ejn.14184. [DOI] [PubMed] [Google Scholar]
- 149.Janocko L, Larner JM, Hochberg RB. The interaction of C-17 esters of estradiol with the estrogen receptor. Endocrinology . 1984;114:1180–1186. doi: 10.1210/endo-114-4-1180. [DOI] [PubMed] [Google Scholar]
- 150.Vazquez-Alcantara MA, Menjivar M, Garcia GA, Díaz-Zagoya JC, Garza-Flores J. Long-acting estrogenic responses of estradiol fatty acid esters. J Steroid Biochem. 1989;33:1111–1118. doi: 10.1016/0022-4731(89)90417-2. [DOI] [PubMed] [Google Scholar]
- 151.Wang F, Vihma V, Soronen J, Turpeinen U, Hämäläinen E, Savolainen-Peltonen H, Mikkola TS, Naukkarinen J, Pietiläinen KH, Jauhiainen M, Yki-Järvinen H, Tikkanen MJ. 17β-Estradiol and estradiol fatty acyl esters and estrogen-converting enzyme expression in adipose tissue in obese men and women. J Clin Endocrinol Metab. 2013;98:4923–4931. doi: 10.1210/jc.2013-2605. [DOI] [PubMed] [Google Scholar]
- 152.Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Ventura-Clapier R, Piquereau J, Veksler V, Garnier A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front Endocrinol (Lausanne) 2019;10:557. doi: 10.3389/fendo.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Álvarez-Delgado C, Mendoza-Rodríguez CA, Picazo O, Cerbón M. Different expression of α and β mitochondrial estrogen receptors in the aging rat brain: Interaction with respiratory complex V. Exp Gerontol. 2010;45:580–585. doi: 10.1016/j.exger.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 155.Rangwala SM, Wang XM, Calvo JA, Lindsley L, Zhang YY, Deyneko G, Beaulieu V, Gao JP, Turner G, Markovits J. Estrogen-related receptor γ is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ponnusamy S, Tran QT, Harvey I, Smallwood HS, Thiyagarajan T, Banerjee S, Johnson DL, Dalton JT, Sullivan RD, Miller DD, Bridges D, Narayanan R. Pharmacologic activation of estrogen receptor β increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J. 2017;31:266–281. doi: 10.1096/fj.201600787RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou ZQ, Ribas V, Rajbhandari P, Drew BG, Moore TM, Fluitt AH, Reddish BR, Whitney KA, Georgia S, Vergnes L, Reue K, Liesa M, Shirihai O, van der Bliek AM, Chi NW, Mahata SK, Tiano JP, Hewitt SC, Tontonoz P, Korach KS, Mauvais-Jarvis F, Hevener AL. Estrogen receptor a protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J Biol Chem . 2018;293:4735–4751. doi: 10.1074/jbc.M117.805069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD. 17β-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metab. 2018;27:167–179.e7. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moor AN, Gottipati S, Mallet RT, Sun J, Giblin FJ, Roque R, Cammarata PR. A putative mitochondrial mechanism for antioxidative cytoprotection by 17beta-estradiol. Exp Eye Res. 2004;78:933–944. doi: 10.1016/j.exer.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 161.Borrás C, Gambini J, López-Grueso R, Pallardó FV, Viña J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta. 2010;1802:205–211. doi: 10.1016/j.bbadis.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 162.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 163.Pauwels S, Antonio L, Jans I, Lintermans A, Neven P, Claessens F, Decallonne B, Billen J, Vanderschueren D, Vermeersch P. Sensitive routine liquid chromatography-tandem mass spectrometry method for serum estradiol and estrone without derivatization. Anal Bioanal Chem. 2013;405:8569–8577. doi: 10.1007/s00216-013-7259-5. [DOI] [PubMed] [Google Scholar]
- 164.Jasuja GK, Travison TG, Davda M, Rose AJ, Zhang A, Kushnir MM, Rockwood AL, Meikle W, Coviello AD, D'Agostino R, Vasan RS, Bhasin S. Circulating estrone levels are associated prospectively with diabetes risk in men of the Framingham Heart Study. Diabetes Care. 2013;36:2591–2596. doi: 10.2337/dc12-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brind JL, Chervinsky K, Völgelman JH, Orentreich N. Radioimmunoassay of estrone sulfate in the serum of normal men after a non-chromatographic procedure that eliminates interference from dehydroepiandrosterone sulfate. Steroids. 1990;55:32–35. doi: 10.1016/0039-128x(90)90071-i. [DOI] [PubMed] [Google Scholar]
- 166.Sanchis D, Balada F, Grasa MM, Virgili J, Peinado J, Monserrat C, Fernández-López JA, Remesar X, Alemany M. Oleoyl-estrone induces the loss of body fat in rats. Int J Obes Relat Metab Disord . 1996;20:588–594. [PubMed] [Google Scholar]
- 167.López-Martí J, Díaz-Silva M, Salas A, Grasa MM, Fernández-López J, Remesar X, Alemany M. Oleoyl-estrone induces the massive loss of body weight in Zucker fa/fa rats fed a high-energy hyperlipidic diet. J Nutr Biochem. 2000;11:530–535. doi: 10.1016/s0955-2863(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 168.Remesar X, Fernández-López JA, Blay MT, Savall P, Salas A, Díaz-Silva M, Esteve M, Grasa MM, Alemany M. Effect of oral oleoyl-estrone on adipose tissue composition in male rats. Int J Obes Relat Metab Disord. 2002;26:1092–1102. doi: 10.1038/sj.ijo.0802056. [DOI] [PubMed] [Google Scholar]
- 169.Remesar X, Guijarro P, Torregrosa C, Grasa MM, López J, Fernández-López JA, Alemany M. Oral oleoyl-estrone induces the rapid loss of body fat in Zucker lean rats fed a hyperlipidic diet. Int J Obes Relat Metab Disord. 2000;24:1405–1412. doi: 10.1038/sj.ijo.0801393. [DOI] [PubMed] [Google Scholar]
- 170.Remesar X, Fernández-López JA, Alemany M. Oleoyl-estrone. Med Res Rev. 2012;32:1263–1291. doi: 10.1002/med.20240. [DOI] [PubMed] [Google Scholar]
- 171.Cabot C, Grasa MM, Massanés RM, de Matteis R, Cinti S, Fernández-López JA, Remesar X, Alemany M. Oleoyl-estrone does not have direct estrogenic effects on rats. Life Sci. 2001;69:749–761. doi: 10.1016/s0024-3205(01)01159-6. [DOI] [PubMed] [Google Scholar]
- 172.Alemany M, Fernández-López JA, Petrobelli A, Granada M, Foz M, Remesar X. [Weight loss in a patient with morbid obesity under treatment with oleoyl-estrone] Med Clin (Barc) 2003;121:496–499. doi: 10.1016/s0025-7753(03)74000-7. [DOI] [PubMed] [Google Scholar]
- 173.Cabot C, Grasa MM, Fernández-López JA, Alemany M. Oleoyl-estrone treatment reduces the volume of white adipose tissue cells in the rat. J Physiol Biochem. 2000;56:369–370. doi: 10.1007/BF03179805. [DOI] [PubMed] [Google Scholar]
- 174.Grasa MM, Cabot C, Esteve M, Yubero P, Masanés RM, Blay MT, Vilà R, López-Martí J, Fernández-López JA, Remesar X, Alemany M. Daily oral oleoyl-estrone gavage induces a dose-dependent loss of fat in Wistar rats. Obes Res. 2001;9:202–209. doi: 10.1038/oby.2001.22. [DOI] [PubMed] [Google Scholar]
- 175.Peinado-Onsurbe J, Blay M, Casadomé L, Fernández-López JA, Remesar X, Alemany M. Effect of 24-h food deprivation on lipoprotein composition and oleoyl-estrone content of lean and obese Zucker rats. Eur J Nutr. 2001;40:155–160. doi: 10.1007/s003940170003. [DOI] [PubMed] [Google Scholar]
- 176.Vihma V, Koskela A, Turpeinen U, Hämäläinen E, Tiitinen A, Wähälä K, Tikkanen MJ, Adlercreutz H. Are there endogenous estrone fatty acyl esters in human plasma or ovarian follicular fluid? J Steroid Biochem Mol Biol. 2011;127:390–395. doi: 10.1016/j.jsbmb.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 177.Ferrer-Lorente R, García-Peláez B, Gómez-Ollés S, Fernández-López JA, Remesar X, Alemany M. Effects of oral estrone on rat energy balance. Steroids. 2005;70:667–672. doi: 10.1016/j.steroids.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 178.Sanchis D, Balada F, Grasa MM, Virgili J, Monserrat C, Fernández-López JA, Remesar X, Alemany M. Short-term handling of the slimming agent oleoyl-estrone in liposomes (Merlin-2) by the rat. Mol Cell Biochem. 1997;177:153–157. doi: 10.1023/a:1006849128697. [DOI] [PubMed] [Google Scholar]
- 179.Strassburg S, Pfluger PT, Chaudhary N, Tso P, Tschöp MH, Anker SD, Nogueiras R, Pérez-Tilve D. Action profile of the antiobesity drug candidate oleoyl-estrone in rats. Obesity (Silver Spring) 2010;18:2260–2267. doi: 10.1038/oby.2010.53. [DOI] [PubMed] [Google Scholar]
- 180.Sanchis D, Balada F, Picó C, Grasa MM, Virgili J, Farrerons C, Palou A, Fernández-López JA, Remesar X, Alemany M. Rats receiving the slimming agent oleoyl-estrone in liposomes (Merlin-2) decrease food intake but maintain thermogenesis. Arch Physiol Biochem. 1997;105:663–672. doi: 10.1076/apab.105.7.663.11391. [DOI] [PubMed] [Google Scholar]
- 181.Vilà R, Cabot C, Villarreal L, Monegal A, Ayet E, Romero MM, Grasa MM, Esteve M, Fernández-López JA, Remesar X, Alemany M. Oleoyl-estrone is a precursor of an estrone-derived ponderostat signal. J Steroid Biochem Mol Biol 2011; 124: 99-111. [DOI] [PubMed] [Google Scholar]
- 182.Cabot C, Masanés R, Bulló M, García-Lorda P, Fernández-López JA, Salas-Salvadó J, Alemany M. Plasma acyl-estrone levels are altered in obese women. Endocr Res 2000; 26: 465-476. [DOI] [PubMed] [Google Scholar]
- 183.Bailly J, Raab S, Clerc R, Sebokova E, Krust A, Chambon P. The effect of oleoyl-estrone on body weight is mediated via the αestrogen receptor and not the beta estrogen receptor. Obes Rev . 2005;6:48. [Google Scholar]
- 184.Borràs M, Guerendain M, Cabo tC, Cederroth M, Esteve M, Remesar X, Grasa MM. The estrogen receptor alpha agonist ICI 182,780 partially blocked oleoyl-estrone slimming action in C57BL6 mice fed with cafeteria diet. Obes Rev. 2010;11:158. [Google Scholar]
- 185.Cabot C, González-Martínez D, Fernández-López JA, Alemany M. In the rat, estrone sulphate is the main serum metabolite of oral oleoyl-estrone. J Endocrinol Invest. 2007;30:376–381. doi: 10.1007/BF03346313. [DOI] [PubMed] [Google Scholar]
- 186.Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 187.Killinger DW, Strutt BJ, Roncari DA, Khalil MW. Estrone formation from dehydroepiandrosterone in cultured human breast adipose stromal cells. J Steroid Biochem Mol Biol. 1995;52:195–201. doi: 10.1016/0960-0760(94)00164-h. [DOI] [PubMed] [Google Scholar]
- 188.Westman EC. Is dietary carbohydrate essential for human nutrition? Am J Clin Nutr. 2002;75:951–3; author reply 953. doi: 10.1093/ajcn/75.5.951. [DOI] [PubMed] [Google Scholar]
- 189.Remesar X, Alemany M. Dietary energy partition: The central role of glucose. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21207729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ross BD, Hems R, Krebs HA. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967;102:942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Irias JJ, Greenberg RE. Relationship of renal glucconeogenesis to control of ammonia formation. Am J Physiol. 1972;223:750–755. doi: 10.1152/ajplegacy.1972.223.4.750. [DOI] [PubMed] [Google Scholar]
- 192.Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol. 2005;566:575–586. doi: 10.1113/jphysiol.2005.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arriarán S, Agnelli S, Sabater D, Remesar X, Fernández-López JA, Alemany M. Evidences of basal lactate production in the main white adipose tissue sites of rats. Effects of sex and a cafeteria diet. PLoS One. 2015;10:e0119572. doi: 10.1371/journal.pone.0119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ho-Palma AC, Rotondo F, Romero MM, Fernández-López JA, Remesar X, Alemany M. Use of 14C-glucose by primary cultures of mature rat epididymal adipocytes. Marked release of lactate and glycerol, but limited lipogenesis in the absence of external stimuli. Adipocyte. 2018;7:204–217. doi: 10.1080/21623945.2018.1460020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oliva L, Fernández-López JA, Remesar X, Alemany M. The anomeric nature of glucose and its implications on its analyses and the influence of diet: Are routine glycaemia measurements reliable enough? J Endocrinol Met . 2019;9:63–70. [Google Scholar]
- 196.Rotondo F, Ho-Palma AC, Remesar X, Fernández-López JA, Romero MM, Alemany M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep. 2017;7:8983. doi: 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Santos-Marcos JA, Pérez-Jiménez F, Camargo A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J Nutr Biochem. 2019;70:1–27. doi: 10.1016/j.jnutbio.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 198.Alexander CM, Landsman PB, Grundy SM. Metabolic syndrome and hyperglycemia: congruence and divergence. Am J Cardiol. 2006;98:982–985. doi: 10.1016/j.amjcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 199.Gallagher EJ, Leroith D, Karnieli E. The metabolic syndrome--from insulin resistance to obesity and diabetes. Med Clin North Am. 2011;95:855–873. doi: 10.1016/j.mcna.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 200.Shalitin S, Moreno LA. Obesity, Metabolic syndrome and nutrition. World Rev Nutr Diet. 2018;117:15–38. doi: 10.1159/000484498. [DOI] [PubMed] [Google Scholar]
- 201.Monnerie S, Comte B, Ziegler D, Morais JA, Pujos-Guillot E, Gaudreau P. Metabolomic and lipidomic signatures of metabolic syndrome and its physiological components in adults: A systematic review. Sci Rep. 2020;10:669. doi: 10.1038/s41598-019-56909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 203.Montecucco F, Mach F, Pende A. Inflammation is a key pathophysiological feature of metabolic syndrome. Mediators Inflamm. 2013;2013:135984. doi: 10.1155/2013/135984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem. 2007;44:232–263. doi: 10.1258/000456307780480963. [DOI] [PubMed] [Google Scholar]
- 205.Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87–92. doi: 10.1080/09513590.2016.1248932. [DOI] [PubMed] [Google Scholar]
- 206.Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome--causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 208.Ornstrup MJ, Kjær TN, Harsløf T, Stødkilde-Jørgensen H, Hougaard DM, Cohen A, Pedersen SB, Langdahl BL. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur J Endocrinol. 2015;172:205–216. doi: 10.1530/EJE-14-0792. [DOI] [PubMed] [Google Scholar]
- 209.Nguyen KD, Chawla A. Metabolic syndrome driven by macrophage interactions with the adipose tissue. Nat Med . 2011:17: 43–43. [Google Scholar]
- 210.Liu R, Nikolajczyk BS. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol. 2019;10:1587. doi: 10.3389/fimmu.2019.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Keane D, Kelly S, Healy NP, McArdle MA, Holohan K, Roche HM. Diet and metabolic syndrome: an overview. Curr Vasc Pharmacol. 2013;11:842–857. doi: 10.2174/15701611113116660173. [DOI] [PubMed] [Google Scholar]
- 212.Hosseini Z, Whiting SJ, Vatanparast H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr Res Rev. 2016;29:152–162. doi: 10.1017/S095442241600007X. [DOI] [PubMed] [Google Scholar]
- 213.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, Chen CS, Klag MJ, Whelton PK, He J. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161:309–318. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stange R, Pflugbeil C, Michalsen A, Uehleke B. Therapeutic fasting in patients with metabolic syndrome and impaired insulin resistance. Forsch Komplementmed. 2013;20:421–426. doi: 10.1159/000357875. [DOI] [PubMed] [Google Scholar]
- 215.Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, Giorgino F, Trimboli P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5–16. doi: 10.1007/s11154-019-09514-y. [DOI] [PubMed] [Google Scholar]
- 216.Watanabe M, Tuccinardi D, Ernesti I, Basciani S, Mariani S, Genco A, Manfrini S, Lubrano C, Gnessi L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes Rev. 2020;21:e13053. doi: 10.1111/obr.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nael R, Montgomery PS, Scott KJ, Blevins SM, Gardner AW. Gender differences in the prevalence and management of metabolic syndrome and its components in patients with peripheral artery disease. Angiology. 2011;62:657–661. doi: 10.1177/0003319711404025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laudisio A, Marzetti E, Antonica L, Pagano F, Vetrano DL, Bernabei R, Zuccalà G. Metabolic syndrome and quality of life in the elderly: age and gender differences. Eur J Nutr. 2013;52:307–316. doi: 10.1007/s00394-012-0337-1. [DOI] [PubMed] [Google Scholar]
- 219.Roche HM. Dietary modulation of energy homoeostasis and metabolic-inflammation. Proc Nutr Soc. 2019;78:313–318. doi: 10.1017/S0029665118002872. [DOI] [PubMed] [Google Scholar]
- 220.Ghorabi S, Esteghamati A, Azam K, Daneshzad E, Sadeghi O, Salari-Moghaddam A, Azadbakht L, Djafarian K. Association between dietary inflammatory index and components of metabolic syndrome. J Cardiovasc Thorac Res. 2020;12:27–34. doi: 10.34172/jcvtr.2020.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bianchi VE, Locatelli V. Testosterone a key factor in gender related metabolic syndrome. Obes Rev. 2018;19:557–575. doi: 10.1111/obr.12633. [DOI] [PubMed] [Google Scholar]
- 222.Haider A, Yassin A, Haider KS, Doros G, Saad F, Rosano GM. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–261. doi: 10.2147/VHRM.S108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: A real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–433. doi: 10.1177/1074248417691136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 225.Groti K, Žuran I, Antonič B, Foršnarič L, Pfeifer M. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169. doi: 10.1080/13685538.2018.1468429. [DOI] [PubMed] [Google Scholar]
- 226.Hwang K, Miner M. Controversies in testosterone replacement therapy: testosterone and cardiovascular disease. Asian J Androl. 2015;17:187–191. doi: 10.4103/1008-682X.146968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chalas E. Ovaries, estrogen, and longevity. Obstet Gynecol. 2013;121:701–702. doi: 10.1097/AOG.0b013e31828af732. [DOI] [PubMed] [Google Scholar]
- 228.Caruso S, Cianci S, Amore FF, Ventura B, Bambili E, Spadola S, Cianci A. Quality of life and sexual function of naturally postmenopausal women on an ultralow-concentration estriol vaginal gel. Menopause. 2016;23:47–54. doi: 10.1097/GME.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 229.Viña J, Sastre J, Pallardó FV, Gambini J, Borrás C. Modulation of longevity-associated genes by estrogens or phytoestrogens. Biol Chem. 2008;389:273–277. doi: 10.1515/BC.2008.027. [DOI] [PubMed] [Google Scholar]
- 230.vom Saal FS, Welshons WV. Endocrine disruptors: Manmade and natural oestrogens: opposite effects on assisted reproduction. Nat Rev Endocrinol. 2016;12:251–252. doi: 10.1038/nrendo.2016.38. [DOI] [PubMed] [Google Scholar]
- 231.Goodsell DS. The molecular perspective: tamoxifen and the estrogen receptor. Stem Cells. 2002;20:267–268. doi: 10.1634/stemcells.20-3-267. [DOI] [PubMed] [Google Scholar]
- 232.Zhang Z, Kang D, Li H. The effects of testosterone on bone health in males with testosterone deficiency: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20:33. doi: 10.1186/s12902-020-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Borthwick EB, Houston MP, Coughtrie MW, Burchell A. The antihyperglycemic effect of estrone sulfate in genetically obese-diabetic (ob/ob) mice is associated with reduced hepatic glucose-6-phosphatase. Horm Metab Res. 2001;33:721–726. doi: 10.1055/s-2001-19136. [DOI] [PubMed] [Google Scholar]
- 234.Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1:273–275. doi: 10.4161/isl.1.3.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bian C, Bai B, Gao Q, Li S, Zhao Y. 17β-Estradiol regulates glucose metabolism and insulin secretion in rat islet β cells through GPER and Akt/mTOR/GLUT2 pathway. Front Endocrinol (Lausanne) 2019;10:531. doi: 10.3389/fendo.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031–5037. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Allard C, Morford JJ, Xu B, Salwen B, Xu W, Desmoulins L, Zsombok A, Kim JK, Levin ER, Mauvais-Jarvis F. Loss of nuclear and membrane estrogen receptor-α differentially impairs insulin secretion and action in male and female mice. Diabetes. 2019;68:490–501. doi: 10.2337/db18-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48:2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 241.Santos RS, Batista TM, Camargo RL, Morato PN, Borck PC, Leite NC, Kurauti MA, Wanschel AC, Nadal Á, Clegg DJ, Carneiro EM. Lacking of estradiol reduces insulin exocytosis from pancreatic β-cells and increases hepatic insulin degradation. Steroids. 2016;114:16–24. doi: 10.1016/j.steroids.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 242.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 243.Camporez JP, Lyu K, Goldberg EL, Zhang D, Cline GW, Jurczak MJ, Dixit VD, Petersen KF, Shulman GI. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol. 2019;597:3885–3903. doi: 10.1113/JP277270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Inada A, Fujii NL, Inada O, Higaki Y, Furuichi Y, Nabeshima YI. Effects of 17β-estradiol and androgen on glucose metabolism in skeletal muscle. Endocrinology. 2016;157:4691–4705. doi: 10.1210/en.2016-1261. [DOI] [PubMed] [Google Scholar]
- 245.Jelenik T, Roden M. How estrogens prevent from lipid-induced insulin resistance. Endocrinology. 2013;154:989–992. doi: 10.1210/en.2013-1112. [DOI] [PubMed] [Google Scholar]
- 246.Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14:58–66. doi: 10.1111/j.1463-1326.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shen M, Kumar SP, Shi H. Estradiol regulates insulin signaling and inflammation in adipose tissue. Horm Mol Biol Clin Investig. 2014;17:99–107. doi: 10.1515/hmbci-2014-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Guillaume M, Handgraaf S, Fabre A, Raymond-Letron I, Riant E, Montagner A, Vinel A, Buscato M, Smirnova N, Fontaine C, Guillou H, Arnal JF, Gourdy P. Selective activation of estrogen receptor α activation function-1 is sufficient to prevent obesity, steatosis, and insulin resistance in mouse. Am J Pathol. 2017;187:1273–1287. doi: 10.1016/j.ajpath.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 249.Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Lière P, Krust A, Chambon P, Arnal JF, Gourdy P. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes. 2013;62:4098–4108. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li R, Shen Y. Estrogen and brain: synthesis, function and diseases. Front Biosci. 2005;10:257–267. doi: 10.2741/1525. [DOI] [PubMed] [Google Scholar]
- 251.Biegon A, Alia-Klein N, Alexoff DL, Fowler JS, Kim SW, Logan J, Pareto D, Preston-Campbell R, Wang GJ, Hildebrandt T. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc Natl Acad Sci U S A. 2020;117:22962–22966. doi: 10.1073/pnas.2006117117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sellers KJ, Denley MCS, Saito A, Foster EM, Salgarella I, Delogu A, Kamiya A, Srivastava DP. Brain-synthesized oestrogens regulate cortical migration in a sexually divergent manner. Eur J Neurosci. 2020;52:2646–2663. doi: 10.1111/ejn.14755. [DOI] [PubMed] [Google Scholar]
- 253.Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol. 2010;22:664–673. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ulhaq ZS. Estrogen ‒ serotonin interaction and its implication on insulin resistance. J Intern Med. 2019;55:76–81. [Google Scholar]
- 255.Pratchayasakul W, Sa-Nguanmoo P, Sivasinprasasn S, Pintana H, Tawinvisan R, Sripetchwandee J, Kumfu S, Chattipakorn N, Chattipakorn SC. Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm Behav. 2015;72:68–77. doi: 10.1016/j.yhbeh.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 256.Berlanga-Acosta J, Guillén-Nieto G, Rodríguez-Rodríguez N, Bringas-Vega ML, García-del-Barco-Herrera D, Berlanga-Sáez JO, García-Ojalvo A, Valdés-Sosa MJ, Valdés-Sosa PA. Insulin resistance at the crossroad of Alzheimer disease pathology: A review. Front Endocrinol (Lausanne) 2020;11:560375. doi: 10.3389/fendo.2020.560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. doi: 10.1016/S1474-4422(20)30231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Inestrosa NC, Marzolo MP, Bonnefont AB. Cellular and molecular basis of estrogen's neuroprotection. Potential relevance for Alzheimer's disease. Mol Neurobiol. 1998;17:73–86. doi: 10.1007/BF02802025. [DOI] [PubMed] [Google Scholar]
- 259.Greenfield JP, Leung LW, Cai D, Kaasik K, Gross RS, Rodriguez-Boulan E, Greengard P, Xu H. Estrogen lowers Alzheimer beta-amyloid generation by stimulating trans-Golgi network vesicle biogenesis. J Biol Chem. 2002;277:12128–12136. doi: 10.1074/jbc.M110009200. [DOI] [PubMed] [Google Scholar]
- 260.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ/PI3-K pathway in hippocampus: relevance to Alzheimer's prevention. Neurobiol Aging. 2011;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ma LH, Lin GZ, Wang M. Association between estrogen and female patients with Alzheimer's disease: a meta-analysis. Int J Clin Exp Med 2017; 10: 135-141. [Google Scholar]
- 262.Panidis DK, Matalliotakis IM, Rousso DH, Kourtis AI, Koumantakis EE. The role of estrogen replacement therapy in Alzheimer's disease. Eur J Obstet Gyn R B . 2001;95:86–91. doi: 10.1016/s0301-2115(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 263.Tian Z, Fan J, Zhao Y, Bi S, Si L, Liu Q. Estrogen receptor beta treats Alzheimer's disease. Neural Regen Res. 2013;8:420–426. doi: 10.3969/j.issn.1673-5374.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tang Y, Min Z, Xiang XJ, Liu L, Ma YL, Zhu BL, Song L, Tang J, Deng XJ, Yan Z, Chen GJ. Estrogen-related receptor alpha is involved in Alzheimer's disease-like pathology. Exp Neurol. 2018;305:89–96. doi: 10.1016/j.expneurol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 265.Kimura Y, Buddington KK, Buddington RK. The influence of estradiol and diet on small intestinal glucose transport in ovariectomized rats. Exp Biol Med (Maywood) 2004;229:227–234. doi: 10.1177/153537020422900302. [DOI] [PubMed] [Google Scholar]
- 266.Garrido P, Morán J, Alonso A, González S, González C. 17β-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology. 2013;154:1979–1989. doi: 10.1210/en.2012-1558. [DOI] [PubMed] [Google Scholar]
- 267.Shi J, Zhang YQ, Simpkins JW. Effects of 17beta-estradiol on glucose transporter 1 expression and endothelial cell survival following focal ischemia in the rats. Exp Brain Res. 1997;117:200–206. doi: 10.1007/s002210050216. [DOI] [PubMed] [Google Scholar]
- 268.Devries MC, Hamadeh MJ, Graham TE, Tarnopolsky MA. 17beta-estradiol supplementation decreases glucose rate of appearance and disappearance with no effect on glycogen utilization during moderate intensity exercise in men. J Clin Endocrinol Metab. 2005;90:6218–6225. doi: 10.1210/jc.2005-0926. [DOI] [PubMed] [Google Scholar]
- 269.Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y, Wu C, Allred K, Allred C, Sun Y, Guo S. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes. 2019;68:291–304. doi: 10.2337/db18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Boscaro C, Carotti M, Albiero M, Trenti A, Fadini GP, Trevisi L, Sandonà D, Cignarella A, Bolego C. Non-genomic mechanisms in the estrogen regulation of glycolytic protein levels in endothelial cells. FASEB J. 2020;34:12768–12784. doi: 10.1096/fj.202001130R. [DOI] [PubMed] [Google Scholar]
- 271.Narasimhan A, Sampath S, Jayaraman S, Karundevi B. Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocr Res. 2013;38:251–262. doi: 10.3109/07435800.2013.775148. [DOI] [PubMed] [Google Scholar]
- 272.Imbert-Fernandez Y, Clem BF, O'Neal J, Kerr DA, Spaulding R, Lanceta L, Clem AL, Telang S, Chesney J. Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3) J Biol Chem. 2014;289:9440–9448. doi: 10.1074/jbc.M113.529990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sun Y, Gu X, Zhang E, Park MA, Pereira AM, Wang S, Morrison T, Li C, Blenis J, Gerbaudo VH, Henske EP, Yu JJ. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death Dis. 2014;5:e1231. doi: 10.1038/cddis.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Luo F, Guo Y, Ruan GY, Peng R, Li XP. Estrogen lowers triglyceride via regulating hepatic APOA5 expression. Lipids Health Dis. 2017;16:72. doi: 10.1186/s12944-017-0463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Shwaery GT, Vita JA, Keaney JF Jr. Antioxidant protection of LDL by physiological concentrations of 17 beta-estradiol. Requirement for estradiol modification. Circulation. 1997;95:1378–1385. doi: 10.1161/01.cir.95.6.1378. [DOI] [PubMed] [Google Scholar]
- 276.Ting WJ, Huang CY, Jiang CH, Lin YM, Chung LC, Shen CY, Pai P, Lin KH, Viswanadha VP, Liao SC. Treatment with 17β-estradiol reduced body weight and the risk of cardiovascular disease in a high-fat diet-induced animal model of obesity. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tian GX, Sun Y, Pang CJ, Tan AH, Gao Y, Zhang HY, Yang XB, Li ZX, Mo ZN. Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes Rev. 2012;13:381–387. doi: 10.1111/j.1467-789X.2011.00978.x. [DOI] [PubMed] [Google Scholar]
- 278.Kavanagh K, Davis MA, Zhang L, Wilson MD, Register TC, Adams MR, Rudel LL, Wagner JD. Estrogen decreases atherosclerosis in part by reducing hepatic acyl-CoA:cholesterol acyltransferase 2 (ACAT2) in monkeys. Arterioscler Thromb Vasc Biol. 2009;29:1471–1477. doi: 10.1161/ATVBAHA.109.191825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, Enriori PJ. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology. 2014;155:4447–4460. doi: 10.1210/en.2014-1342. [DOI] [PubMed] [Google Scholar]
- 280.MacDonald TL, MacPherson R, Castellani L, Cervone D, Anderson E, Wright DC, Dyck DJ. Estradiol does not directly regulate adipose lipolysis. Adipocyte. 2017;6:76–86. doi: 10.1080/21623945.2017.1287638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lundholm L, Zang H, Hirschberg AL, Gustafsson JA, Arner P, Dahlman-Wright K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil Steril. 2008;90:44–48. doi: 10.1016/j.fertnstert.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 282.Ahluwalia A, Hoa N, Ge L, Blumberg B, Levin ER. Mechanisms by which membrane and nuclear ER alpha inhibit adipogenesis in cells isolated from female mice. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa175. [DOI] [PubMed] [Google Scholar]
- 283.Oliva L, Aranda T, Alemany M, Fernández-López JA, Remesar X. Unconnected body accrual of dietary lipid and protein in rats fed diets with different lipid and protein content. Mol Nutr Food Res. 2020;64:e2000265. doi: 10.1002/mnfr.202000265. [DOI] [PubMed] [Google Scholar]
- 284.Oliva L, Alemany M, Fernández-López JA, Remesar X. Estradiol determine liver lipid deposition in rats fed standard diets unbalanced with excess lipid or protein. Chemrxiv. 2020 doi: 10.1017/S0007114521004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17β-estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: Molecular mechanisms and islet functionality. Transplantation . 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 286.de Cleyn K, Buytaert P, Coppens M. Carbohydrate metabolism during hormonal substitution therapy. Maturitas. 1989;11:235–242. doi: 10.1016/0378-5122(89)90216-8. [DOI] [PubMed] [Google Scholar]
- 287.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 288.Viña J, Sastre J, Pallardó FV, Gambini J, Borrás C. Role of mitochondrial oxidative stress to explain the different longevity between genders: protective effect of estrogens. Free Radic Res. 2006;40:1359–1365. doi: 10.1080/10715760600952851. [DOI] [PubMed] [Google Scholar]
- 289.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105:1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Abelenda M, Puerta M. Dual control of cytochrome-C oxidase activity by female sex steroids. Eur J Endocrinol. 1999;141:630–636. doi: 10.1530/eje.0.1410630. [DOI] [PubMed] [Google Scholar]
- 291.Moreno AJM, Moreira PI, Custódio JBA, Santos MS. Mechanism of inhibition of mitochondrial ATP synthase by 17β-Estradiol. J Bioenerg Biomembr . 2013;45:261–270. doi: 10.1007/s10863-012-9497-1. [DOI] [PubMed] [Google Scholar]
- 292.Mamounis KJ, Hernandez MR, Margolies N, Yasrebi A, Roepke TA. Interaction of 17β-estradiol and dietary fatty acids on energy and glucose homeostasis in female mice. Nutr Neurosci. 2018;21:715–728. doi: 10.1080/1028415X.2017.1347374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Martínez de Morentin PB, González-García I, Martins L, Lage R, Fernández-Mallo D, Martínez-Sánchez N, Ruíz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Diéguez C, Nogueiras R, Rahmouni K, Tena-Sempere M, López M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sievers W, Rathner JA, Kettle C, Zacharias A, Irving HR, Green RA. The capacity for oestrogen to influence obesity through brown adipose tissue thermogenesis in animal models: A systematic review and meta-analysis. Obes Sci Pract. 2019;5:592–602. doi: 10.1002/osp4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wieland OH. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- 296.Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol. 2010;315:159–167. doi: 10.1016/j.mce.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998;329:197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 299.Fernández-Marcos P, Auwerx J. Regulation of PGC-1, a nodal regulator of mitochondrial biogenesis. An J Clin Nutr. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem. 2000;275:16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- 301.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan ML, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): Requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci U S A. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhu LL, Liu Y, Cui AF, Shao D, Liang JC, Liu XJ, Chen Y, Gupta N, Fang FD, Chang YS. PGC-1α coactivates estrogen-related receptor-α to induce the expression of glucokinase. Am J Physiol-cell Ph. 2010;298:E1210–E1218. doi: 10.1152/ajpendo.00633.2009. [DOI] [PubMed] [Google Scholar]
- 303.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 304.Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- 305.Horard B, Vanacker JM. Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31:349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 306.Hubbard WJ, Bland KI, Chaudry IH. The ERRor of our ways: Estrogen-related receptors are about energy, not hormones, and are potential new targets for trauma and shock. Shock. 2015;44:3–15. doi: 10.1097/SHK.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 307.Nie Y, Wong C. Suppressing the activity of ERR α in 3T3-L1 adipocytes reduces mitochondrial biogenesis but enhances glycolysis and basal glucose uptake. J Cell Mol Med. 2009;13:3051–3060. doi: 10.1111/j.1582-4934.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wiik A, Hellsten Y, Berthelson P, Lundholm L, Fischer H, Jansson E. Activation of estrogen response elements is mediated both via estrogen and muscle contractions in rat skeletal muscle myotubes. Am J Physiol Cell Physiol. 2009;296:C215–C220. doi: 10.1152/ajpcell.00148.2008. [DOI] [PubMed] [Google Scholar]
- 309.Wright LE, Brandon AE, Hoy AJ, Forsberg GB, Lelliott CJ, Reznick J, Löfgren L, Oscarsson J, Strömstedt M, Cooney GJ, Turner N. Amelioration of lipid-induced insulin resistance in rat skeletal muscle by overexpression of Pgc-1β involves reductions in long-chain acyl-CoA levels and oxidative stress. Diabetologia. 2011;54:1417–1426. doi: 10.1007/s00125-011-2068-x. [DOI] [PubMed] [Google Scholar]
- 310.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kemper MF, Stirone C, Krause DN, Duckles SP, Procaccio V. Genomic and non-genomic regulation of PGC1 isoforms by estrogen to increase cerebral vascular mitochondrial biogenesis and reactive oxygen species protection. Eur J Pharmacol. 2014;723:322–329. doi: 10.1016/j.ejphar.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gonzalez-Granillo M, Savva C, Li X, Fitch M, Pedrelli M, Hellerstein M, Parini P, Korach-Andre M, Gustafsson J-A. ERβ activation in obesity improves whole body metabolism via adipose tissue function and enhanced mitochondria biogenesis. Mol Cell Endocrinol . 2019;479:147–158. doi: 10.1016/j.mce.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 313.Esteve M, Rafecas I, Remesar X, Alemany M. Nitrogen balances of lean and obese Zucker rats subjected to a cafeteria diet. Int J Obes Relat Metab Disord. 1992;16:237–244. [PubMed] [Google Scholar]
- 314.Mauvais-Jarvis F. Epidemiology of gender differences in diabetes and obesity. Adv Exp Med Biol. 2017;1043:3–8. doi: 10.1007/978-3-319-70178-3_1. [DOI] [PubMed] [Google Scholar]
- 315.Luciano AA, Miller BE, Schoenenfeld MJ, Schaser RJ. Effects of estrone sulfate alone or with medroxyprogesterone acetate on serum lipoprotein levels in postmenopausal women. Obstet Gynecol. 2001;97:101–108. doi: 10.1016/s0029-7844(00)01081-4. [DOI] [PubMed] [Google Scholar]
- 316.Esteve M, Savall P, Blay MT, Fernández-López JA, Remesar X, Alemany M. Intestinal handling of an oral oleoyl-estrone gavage by the rat. Life Sci. 2001;69:763–777. doi: 10.1016/s0024-3205(01)01160-2. [DOI] [PubMed] [Google Scholar]
- 317.Romero MM, Esteve M, Alemany M. Combined effects of oral oleoyl-estrone and limited food intake on body composition of young overweight male rats. Int J Obes (Lond) 2006;30:1149–1156. doi: 10.1038/sj.ijo.0803224. [DOI] [PubMed] [Google Scholar]
- 318.Romero MM, Fernández-López JA, Alemany M, Esteve M. Gene expression modulation of liver energy metabolism by oleoyl-oestrone in overweight rats. Biosci Rep. 2009;30:81–89. doi: 10.1042/BSR20080182. [DOI] [PubMed] [Google Scholar]
- 319.Adán C, Cabot C, Vilà R, Grasa MM, Masanés RM, Esteve M, Estruch J, Fernández-López JA, Remesar X, Alemany M. Oleoyl-estrone treatment affects the ponderostat setting differently in lean and obese Zucker rats. Int J Obes Relat Metab Disord. 1999;23:366–373. doi: 10.1038/sj.ijo.0800828. [DOI] [PubMed] [Google Scholar]
- 320.Blay M, Peinado-Onsurbe J, Grasa MM, Díaz-Silva M, Fernandez-López JA, Remesar X, Alemany M. Effect of oral oleoyl-estrone treatment on plasma lipoproteins and tissue lipase activities of Zucker lean and obese female rats. Int J Obes Relat Metab Disord. 2002;26:618–626. doi: 10.1038/sj.ijo.0801985. [DOI] [PubMed] [Google Scholar]
- 321.Romero MM, Fernández-López JA, Esteve M, Alemany M. Oleoyl-oestrone inhibits lipogenic, but maintains thermogenic, gene expression of brown adipose tissue in overweight rats. Biosci Rep. 2009;29:237–243. doi: 10.1042/BSR20080089. [DOI] [PubMed] [Google Scholar]
- 322.Díaz M, Grasa MM, Fernández-López JA, Remesar X, Alemany M. Short-term effects of oleoyl-estrone on insulin sensitivity and glucose disposal in the rat. Int J Obes Relat Metab Disord . 2002;26:S204. [Google Scholar]
- 323.Moos WH, Dykens JA, Nohynek D, Rubinchik E, Howell N. Review of the effects of 17α estradiol in humans: A less feminizing estrogen with neuroprotective potential. Drug Develop Res. 2009;70:1–21. [Google Scholar]
- 324.Ferrer-Lorente R, García-Peláez B, Fernández-López JA, Remesar X, Alemany M. Tamoxifen does not prevent the mobilization of body lipids elicited by oleoyl-estrone. Steroids. 2004;69:661–665. doi: 10.1016/j.steroids.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 325.Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol. 1993;45:509–511. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 326.Shwaery GT, Vita JA, Keaney JF Jr. Antioxidant protection of LDL by physiologic concentrations of estrogens is specific for 17-beta-estradiol. Atherosclerosis. 1998;138:255–262. doi: 10.1016/s0021-9150(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 327.Badeau M, Adlercreutz H, Kaihovaara P, Tikkanen MJ. Estrogen A-ring structure and antioxidative effect on lipoproteins. J Steroid Biochem Mol Biol. 2005;96:271–278. doi: 10.1016/j.jsbmb.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 328.Larner JM, Pahuja SL, Shackleton CH, McMurray WJ, Giordano G, Hochberg RB. The isolation and characterization of estradiol-fatty acid esters in human ovarian follicular fluid. Identification of an endogenous long-lived and potent family of estrogens. J Biol Chem. 1993;268:13893–13899. [PubMed] [Google Scholar]
- 329.Carlson SE, Carver JD, House SG. High fat diets varying in ratios of polyunsaturated to saturated fatty acid and linoleic to linolenic acid: a comparison of rat neural and red cell membrane phospholipids. J Nutr. 1986;116:718–725. doi: 10.1093/jn/116.5.718. [DOI] [PubMed] [Google Scholar]
- 330.Raleigh JA, Kremers W, Gaboury B. Dose-rate and oxygen effects in models of lipid membranes: linoleic acid. Int J Radiat Biol Relat Stud Phys Chem Med. 1977;31:203–213. doi: 10.1080/09553007714550251. [DOI] [PubMed] [Google Scholar]
- 331.Kadoma Y, Fujisawa S. Radical-scavenging activity of estrogen and estrogen-like compounds using the induction period method. Int J Mol Sci. 2007:8: 295–303. [Google Scholar]
- 332.Klinge CM. Estrogens regulate life and death in mitochondria. J Bioenerg Biomembr. 2017;49:307–324. doi: 10.1007/s10863-017-9704-1. [DOI] [PubMed] [Google Scholar]
- 333.Sawada H, Shimohama S. Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav Rev. 2000;24:143–147. doi: 10.1016/s0149-7634(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 334.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Höckerstedt A, Jauhiainen M, Tikkanen MJ. Lecithin/cholesterol acyltransferase induces estradiol esterification in high-density lipoprotein, increasing its antioxidant potential. J Clin Endocrinol Metab. 2004;89:5088–5093. doi: 10.1210/jc.2004-0141. [DOI] [PubMed] [Google Scholar]
- 336.Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ. 2019;10:4. doi: 10.1186/s13293-019-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Dieudonné MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 338.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, hlman-Wright K, Nilsson S, Gustafsson JÅ, Efendic S, Khan A. Evidence that oestrogen receptor-a plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 339.Ikeda K, Horie-Inoue K, Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J Steroid Biochem Mol Biol. 2019;191:105375. doi: 10.1016/j.jsbmb.2019.105375. [DOI] [PubMed] [Google Scholar]
- 340.Morissette M, Le Saux M, d'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Méndez P, García-Segura LM, di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 341.Ali MH, Napit PR, Mahmood ASMH, Bheemanapally K, Alhamami HN, Uddin MM, Mandal SK, Ibrahim MMH, Briski KP. Hindbrain estrogen receptor regulation of ventromedial hypothalamic glycogen metabolism and glucoregulatory transmitter expression in the hypoglycemic male rat. Neuroscience. 2019;409:253–260. doi: 10.1016/j.neuroscience.2019.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Boldarine VT, Pedroso AP, Brandão-Teles C, loTurco EG, Nascimento CMO, Oyama LM, Bueno AA, Martins-de-Souza D, Ribeiro EB. Ovariectomy modifies lipid metabolism of retroperitoneal white fat in rats: a proteomic approach. Am J Physiol Endocrinol Metab. 2020;319:E427–E437. doi: 10.1152/ajpendo.00094.2020. [DOI] [PubMed] [Google Scholar]
- 343.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liu J, Bisschop PH, Eggels L, Foppen E, Ackermans MT, Zhou JN, Fliers E, Kalsbeek A. Intrahypothalamic estradiol regulates glucose metabolism via the sympathetic nervous system in female rats. Diabetes. 2013;62:435–443. doi: 10.2337/db12-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Shen L, Wang DQH, Xu M, Woods SC, Liu M. BDNF/TrkB signaling mediates the anorectic action of estradiol in the nucleus tractus solitarius. Oncotarget. 2017;8:84028–84038. doi: 10.18632/oncotarget.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kallen CB. Estrogen targets fat mass and glucose metabolism by acting in the brain. Am J Physiol Endocrinol Metab. 2012;303:E443–E444. doi: 10.1152/ajpendo.00277.2012. [DOI] [PubMed] [Google Scholar]
- 347.Comitato R, Saba A, Turrini A, Arganini C, Virgili F. Sex hormones and macronutrient metabolism. Crit Rev Food Sci Nutr. 2015;55:227–241. doi: 10.1080/10408398.2011.651177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;26:411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 349.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.van Pelt RE, Gavin KM, Kohrt WM. Regulation of body composition and bioenergetics by estrogens. Endocrinol Metab Clin North Am. 2015;44:663–676. doi: 10.1016/j.ecl.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Adamski J, Jakob FJ. A guide to 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 2001; 171: 1-4. [DOI] [PubMed] [Google Scholar]
- 352.Bonetti A, Tirelli F, Catapano A, d’Azzi D, dei Cas A, Solito F, Ceda G, Reverberi C, Monica C, Pipitone S, Elia G, Spattini M, Magnati G. Side effects of anabolic androgenic steroids abuse. Int J Sports Med. 2008;29:679–687. doi: 10.1055/s-2007-965808. [DOI] [PubMed] [Google Scholar]
- 353.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 354.Wilson C, Contreras-Ferrat A, Venegas N, Osorio-Fuentealba C, Pávez M, Montoya K, Durán J, Maass R, Lavandero S, Estrada M. Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J Cell Physiol. 2013;228:2399–2407. doi: 10.1002/jcp.24413. [DOI] [PubMed] [Google Scholar]
- 355.Kato Y, Shigehara K, Nakashima K, Iijima M, Kawagushi S, Nohara T, Izumi K, Kadono Y, Konaka H, Namiki M, Mizokami A. The five-year effects of testosterone replacement therapy on lipid profile and glucose tolerance among hypogonadal men in Japan: a case control study. Aging Male. 2020;23:23–28. doi: 10.1080/13685538.2018.1550060. [DOI] [PubMed] [Google Scholar]
- 356.Quang LM, Kalhan A. Cardiovascular benefits and risks of testosterone replacement therapy in hypogonadal men with type 2 diabetes mellitus and/or the metabolic syndrome: a systematic review. Brit J Nutr. 2018;18:141–146. [Google Scholar]
- 357.Wise P. Clearing estrogen's bad name. The Scientist. 2008;22:40–44. [Google Scholar]
- 358.Jayachandran M, Lahr BD, Bailey KR, Miller VM, Kantarci K. Menopausal hormone therapy, blood thrombogenicity, and development of white matter hyperintensities in women of the Kronos Early Estrogen Prevention Study. Menopause. 2020;27:305–310. doi: 10.1097/GME.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Styer AK. The impact of estrogen alone hormone therapy on breast cancer risk and health outcomes: reassurance for the treatment of climacteric symptoms in black women? Menopause. 2017;24:124–125. doi: 10.1097/GME.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 360.Miller EM. Hormone replacement therapy affects iron status more than endometrial bleeding in older US women: A role for estrogen in iron homeostasis? Maturitas. 2016;88:46–51. doi: 10.1016/j.maturitas.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 361.Mauvais-Jarvis F. Is estradiol a biomarker of type 2 diabetes risk in postmenopausal women? Diabetes. 2017;66:568–570. doi: 10.2337/dbi16-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Vermeulen A, Kaufman JM. Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res. 1995;43:25–28. doi: 10.1159/000184233. [DOI] [PubMed] [Google Scholar]
- 363.Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271–1279. doi: 10.1016/j.fertnstert.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 364.Gangestad SW, Thornhill R. Human oestrus. Proc Biol Sci. 2008;275:991–1000. doi: 10.1098/rspb.2007.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Anantharaman-Barr HG, Decombaz J. The effect of wheel running and the estrous cycle on energy expenditure in female rats. Physiol Behav. 1989;46:259–263. doi: 10.1016/0031-9384(89)90265-5. [DOI] [PubMed] [Google Scholar]
- 366.Smith RJ. Allometric scaling in comparative biology: problems of concept and method. Am J Physiol. 1984;246:R152–R160. doi: 10.1152/ajpregu.1984.246.2.R152. [DOI] [PubMed] [Google Scholar]
- 367.Calder WA 3rd. Body size, mortality, and longevity. J Theor Biol. 1983;102:135–144. doi: 10.1016/0022-5193(83)90266-7. [DOI] [PubMed] [Google Scholar]
- 368.Shutt DA. The effects of plant oestrogens on animal reproduction. Endeavour. 1976;35:110–113. doi: 10.1016/0160-9327(76)90004-1. [DOI] [PubMed] [Google Scholar]
- 369.Zang K, Kurisu F, Kasuga I, Furumai H, Yagi O. Analysis of the phylogenetic diversity of estrone-degrading bacteria in activated sewage sludge using microautoradiography-fluorescence in situ hybridization. Syst Appl Microbiol. 2008;31:206–214. doi: 10.1016/j.syapm.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 370.Sadílek J, Spálovská P, Vrana B, Vávrová M, Maršálek B, Šimek Z. Comparison of extraction techniques for isolation of steroid oestrogens in environmentally relevant concentrations from sediment. Int J Environ Anal Chem. 2016;96:1022–1037. [Google Scholar]
- 371.Peck M, Gibson RW, Kortenkamp A, Hill EM. Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol Chem. 2004;23:945–952. doi: 10.1897/03-41. [DOI] [PubMed] [Google Scholar]
- 372.Braga O, Smythe GA, Schäfer AI, Feitz AJ. Steroid estrogens in ocean sediments. Chemosphere. 2005;61:827–833. doi: 10.1016/j.chemosphere.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 373.Servos MR, Bennie DT, Burnison BK, Jurkovic A, McInnis R, Neheli T, Schnell A, Seto P, Smyth SA, Ternes TA. Distribution of estrogens, 17beta-estradiol and estrone, in Canadian municipal wastewater treatment plants. Sci Total Environ. 2005;336:155–170. doi: 10.1016/j.scitotenv.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 374.Encarnação T, Pais AA, Campos MG, Burrows HD. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci Prog. 2019;102:3–42. doi: 10.1177/0036850419826802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8 Suppl 1:3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 376.van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168–174. doi: 10.1016/j.contraception.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 377.RR. Effects of developmental exposure to diethylstilbestrol (DES) in rodents: clues for other environmental estrogens. APMIS. 2001;109:S261–S271. [Google Scholar]
- 378.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol (1985) 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 380.Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 381.Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2015;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Trémollieres F. Contraception orale estro-progestative: quelle différence entre éthinylestradiol et estradiol? Gynécologie Obstétrique et Fertilité. 2012;40:109–115. doi: 10.1016/j.gyobfe.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 383.Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47:269–275. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 384.Shoham Z, Kopernik G. Tools for making correct decisions regarding hormone therapy. Part I: Background and drugs. Fertil Steril. 2004;81:1447–1457. doi: 10.1016/j.fertnstert.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 385.Patel AS, Leong JY, Ramos L, Ramasamy R. Testosterone is a contraceptive and should not be used in men who desire fertility. World J Mens Health. 2019;37:45–54. doi: 10.5534/wjmh.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Liu JD, Wu YQ. Anabolic-androgenic steroids and cardiovascular risk. Chin Med J (Engl) 2019;132:2229–2236. doi: 10.1097/CM9.0000000000000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.O'Connell K, Davis AR, Kerns J. Oral contraceptives: side effects and depression in adolescent girls. Contraception. 2007;75:299–304. doi: 10.1016/j.contraception.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 388.Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]