Abstract
BACKGROUND
Extracellular matrix (ECM) remodeling and stiffening, which are correlated with tumor malignancy, drives tumor development. However, the relationship between ECM remodeling and rat experimental model of 1,2-dimethylhyrazine (DMH)-induced colorectal cancer (CRC) imposed by cold and capsaicin exposure remains unclear.
AIM
To explore the effects of cold exposure and capsaicin on ECM remodeling and ECM enzymes in DMH-induced CRC.
METHODS
For histopathological analysis, the sections of colon tissues were stained with hematoxylin and eosin, Masson’s trichrome, Picrosirius red, and Weigert’s Resorcin-Fuchsin to observe the remodeling of collagen and elastin. Additionally, the protein expression level of type I collagen (COL I), type 3 collagen (COL III0, elastin, matrix metalloproteinase (MMP) 1, MMP2, MMP9, and tissue-specific matrix metalloproteinase 1 (TIMP1) was assessed by immunohistochemistry. The messenger RNA (mRNA) levels of COL I, COL III, elastin, and lysyl oxidase-like-2 (LOXL2) in the colon tissues of rats was measured by reverse-transcriptase quantitative polymerase chain reaction.
RESULTS
Although no differences were observed in the proportion of adenomas, a trend towards the increase of invasive tumors was observed in the cold and capsaicin group. The cold exposure group had a metastasis rate compared with the other groups. Additionally, abnormal accumulation of both collagen and elastin was observed in the cold exposure and capsaicin group. Specifically, collagen quantitative analysis showed increased length, width, angle, and straightness compared with the DMH group. Collagen deposition and straightness were significantly increased in the cold exposure group compared with the capsaicin group. Cold exposure and capsaicin significantly increased the protein levels of COL I, elastin, and LOXL2 along with increases in their mRNA levels in the colon tissues compared with the DMH group, while COL III did not show a significant difference. Furthermore, in immunohistochemical evaluations, MMP1, MMP2, MMP9, and TIMP1 staining increased in the cold exposure and capsaicin group compared with the DMH group.
CONCLUSION
These results suggest that chronic cold and capsaicin exposure further increased the deposition of collagen and elastin in the colonic tissue. Increased COL I and elastin mRNA and protein levels expression may account for the enhanced ECM remodel and stiffness variations of colon tissue. The upregulated expression of the LOXL2 and physiological imbalance between MMP/TIMP activation and deactivation could contribute to the progression of the CRC resulting from cold and capsaicin exposure.
Keywords: Colon cancer, Cold exposure, Capsaicin, Extracellular matrix remodeling, Extracellular matrix enzymes
Core Tip: In this study, we discovered that remodeling of extracellular matrix (ECM) plays an important role in the progression of colorectal cancer (CRC). These results suggest that increased stiffness of colonic tissue and the remodeling of ECM mediated by ECM enzymes resulting from cold and capsaicin exposure predisposes an environment suitable for CRC development and progression. To target ECM in CRC tumor tissue could represent a novel potential therapeutic strategy.
INTRODUCTION
Colorectal cancer (CRC), a common cause of cancer deaths in the world, is a multifactorial disease driven by genetic predisposition, epigenetic alterations, and environmental factors[1]. Only a minority of CRC is caused by the accumulation of genetic epigenetic alterations, while the majority is linked to environmental factors such as dietary intake, alcohol consumption, and ambient environment[2,3]. Increasing epidemiological data have indicated that cold weather might be associated with an increased occurrence of cancer[4]. Additionally, the consumption of chili-pepper and cancer incidence have a positive correlation[5,6]. However, the mechanisms underlying the effects of cold exposure and capsaicin in 1,2-dimethylhyrazine (DMH)-induced CRC tumorigenesis and progression remain poorly understood. Extracellular matrix (ECM) is a non-cellular structure that is essential for the maintenance of normal tissue and organ function and disease pathophysiology[7]. Collagen is a major component of the ECM. It provides cellular components with physical support and is an important contributor to tumor growth and progression[8]. Tumor progression is accompanied by the dysregulation of collagen structure and deposition. Tumor associated-collagen is usually compacted to thick collagen bundles and the anisotropic arrangement of relatively straight in the matrix of malignancies compared with healthy tissues[9,10]. In clinical samples of breast tumors, collagen deposition increased, and linearization and thickening of collagen occurred; these processes can be linked to poor prognosis and high risk of mortality[11,12].
Elastin is another important fibrous ECM protein that provides elastic recoil to tissue. Importantly, excessive accumulation of ECM, particularly collagen and elastin, gradually leads to progressive organ fibrosis[13]. The fibrosis results in tissue stiffness and can predispose tissue to malignancy. Several human studies indicated that patients with liver fibrosis and stiffness are positively correlated with the risk hepatocellular carcinoma[14]. ECM remodeling is mainly orchestrated by ECM modifying enzymes such as lysyl oxidase-like-2 (LOXL2), matrix metalloproteinases (MMP), and tissue-specific matrix metalloproteinase inhibitors (TIMPs)[15]. LOXL2 is a key factor in ECM remodeling and is a copper-dependent amine oxidase that catalyzes the cross-linking of collagen or elastin in the ECM and thus regulates the tensile strength of tissues. LOXL2 causes disorganization and composition of ECM, resulting in many pathological conditions, including fibrosis and cancer[16]. Active LOXL2 is involved in stiffness-associated cancer progression, whereas inhibition of LOXL2 result in less collagen cross-linking and impeded cancer progression[17]. Moreover, LOXL2 expression is overexpressed in many types of tumors and is associated with poor prognosis[18-20].
MMP has been implicated in cancer development, progression, invasiveness, and dissemination by promoting a protumorigenic microenvironment and modulating the ECM and intercellular junctions[21]. MMPs and TIMPs are the main enzymes involved in the regulation of ECM remodeling and collagen degradation process[22]. Their expression and activity are upregulated in almost all human cancers with disparate changes, and this phenomenon is associated with advanced tumor stage, poor prognosis, and decreased overall survival rate[23,24]. Increased MMP expression/ activity or decreased TIMPs could lead to MMP/TIMP imbalance, resulting in various pathological conditions including fibrosis and cancers[25].
Limited information, however, is available about ECM remolding and ECM enzyme activity in the progression of experimental colorectal malignancy. We have previously shown that cold exposure and long-term administration of capsaicin at a low dose further promote the development and progression of CRC[26]. However, the specific mechanisms underlying cold and capsaicin exposure tumor promotion remained unknown. This study aimed to investigate the effects of cold exposure and capsaicin on ECM remodeling and ECM enzymes in DMH-induced CRC. Moreover, we determined whether excessive ECM deposition, particular whether collagen and elastin and dysregulation of ECM enzymes expression and/or secretion in rat treatment with cold exposure, could further stiffen the colon tissues and disrupt the intestinal morphogenesis to exacerbate the experimental colorectal malignancy.
MATERIALS AND METHODS
Experimental design in adult male rats
Wistar rats weighing 200-250 g (6-wk-old) were obtained from Experimental Animal Center in Guangzhou University of Chinese Medicine. Animals were housed in plastic cages under a controlled environment (24 ± 2 °C, 50% ± 5% humidity, 12 h/12 h light-dark cycle) with ad libitum food and water access. All the experimental protocols were approved by the Institutional Animal Ethics Committee of the Guangzhou University of Chinese Medicine (No. 20130001). Briefly, after 3 d of acclimation, the animals were randomly assigned into four groups (n = 10). Rats in group A received no treatment and served as control. Five weeks later, rats in groups B-D received subcutaneous injection of DMH (25 mg/kg) once a week for 12 wk. In addition to DMH, Group C rats received cold distilled water (10 mg/kg) until the end of 38 wk. Group D rats were given capsaicin (0.9 mg/mL) every day throughout the experiment. By the end of the week, 10 rats from each group were sacrificed. For macroscopic evaluation of the incidence of polyps at the end of the experimental period, rats were sacrificed and colons were incised and washed with physiological saline. Then cleaned colons were cut opened longitudinally and the total number of polyps/tumors was carefully counted and later verified with histopathological examination. The counting and histopathological analysis of gross macroscopic neoplastic lesions was carried out by two investigators from this study. If the histopathological types of these two investigators were different, then tumor histology was classified as adenomas and adenocarcinomas by one pathologist under blinded conditions from the Pathology Department in Guangzhou University of Chinese Medicine. Microscope findings were classified as adenomas and adenocarcinomas according to previous criteria described by Jikihara et al[27]. Tumor incidence is the percentage of rat bearing the indicated type of tumor.
Histopathological staining
All specimens were fixed in 4% paraformaldehyde solution for 24 h and embedded in paraffin and processed by standard histological processing techniques. Serial issue sections (8-µm thick) were obtained from each sample with the microtome and then were stained with hematoxylin and eosin (HE), picrosirius red, Masson’s trichrome (MT), and Weigert’s Resorcin-Fuschin (WRF). For Picrosirius red staining, sections were stained in picrosirius red solution 0.1) (Sirius red F3B; Sigma-Aldrich Co., St Louis, MO, United States) in a saturated aqueous solution of picric acid for 1 h at room temperature for collagen bundle staining. Images were subsequently analyzed using ImageJ to calculate the fiber density, which was measured as image % area coverage. MT was performed according to the manufacture’s protocol including Weigert’s Iron Hematoxylin Solution, Ponceau acid fuchsin, and Aniline Blue as reagents. The collagen volume fraction was measured by ImageJ software and calculated as the proportion of blue positive areas in the total section areas. The process of WRF used reagents and kits from Solarbio (Beijing, China). Sections were then mounted for observation under polarized light microscopy (NIKON Eclipse Ci, Tokyo, Japan) and light microscopy, respectively. Three microphotographs of the reticular dermis were taken with a 400 × magnification with light microscopy and polarized microscopy, respectively. Digitized images of histological sections obtained under final magnification of × 400 were analyzed using the Image-Pro Plus 4.5 software.
Collagen fiber analysis
CT-FIRE, an open-resource software (http://Loci.wisc.edu/software/cifire), was used as previously described to quantify automatically collagen fibers[28]. The quantitative parameters included alignment of collagen fibers as well as individual length, straightness, and width. These features of collagen fibers are widely used to investigate collagen organization in a various of cancer[29]. All the picrosirius red images were converted to 8-bit images and threshold values between 10-255 to eliminate background noise using FIJI ImageJ[30]. These images were uploaded to CT-FIRE; collagen fiber extraction parameters were set to default parameters.
Real-time quantitative reverse-transcriptase polymerase chain reaction
Total RNA extracts of colon tissues were prepared using TRIZOL reagent (TaKaRa, Kusatsu, Japan). RNA (1 µg) was reverse transcribed in a 20 µL reaction mixture using Prime Script RT Master Mix (TaKaRa). The purity of total RNA was evaluated by measuring the concentration and OD260:280 values with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). The mRNA levels of collagen type 1, alpha1 (COL1A1), collagen type 3, alpha1 (COL3A1), LOXL2, and elastin in colon mucosa were assessed using a Step One Plus real-time polymerase chain reaction system (CFX384TM Real-time System; Thermo Fisher Scientific). The relative levels of gene expression were enumerated using the comparative formula 2-ΔΔCt. The primer sequences used in this polymerase chain reaction amplification were as follows: 5’-AGCCATGTACGTAGCCATCC-3’/3’-ACCCTCATAGATGGGCACAG-5’ for β-actin; 5’-AGGCATAAAGGGTCATCGTGGCTT-3’/3’-AGTCCATCTTTGCCAGGAGAACCA-5’ for COL1a1; 5’-GGTTTGGAGAATCTATGAATGGTGG-3’/3’-GCTGGAAAGAAGTCTGAGGAAGG-5’ for Col3a1; 5’-AGCCTATAAGCCGGAGCAAC-3’/3’-GTCCCACTTGTCATCGCAGA-5 ’for LOXL2; 5’-CGCCTGTAATGCCTCCAATC-3’/3’-AGCAGCTAAAGCAGCGAAGT-5’ for elastin.
Immunohistochemistry
Colonic tissue sections were deparaffinized and rehydrated through a series of xylene and ethanol/water. The sections were placed in a 95 °C antigen retrieval solution (citrate buffer; PH 6.0) for 15 min. After cooling in retrieval solutions for 20 min at room temperature, the slides were treated with hydrogen peroxide for 10 min to block endogenous peroxidase activity. Primary rabbit anti-histone polyclonal antibodies were applied for 14 h at 4 °C overnight at the following dilutions: Type I collagen (COL I) (1:500; ab34710; Abcam, Cambridge, United Kingdom), type III collagen (COL III) (1:200, ab7778; Abcam), LOXL2 (1:400), elastin (1:600; ab217356; Abcam), MMP1 (1:500; 10371-2-AP; Proteintech, Rosemont, IL, United States), MMP2 (1:200; ab86607; Abcam), MMP9 (1:800; ab38898; Abcam), and TIMP1 (1:600, ab61224; Abcam). The next day, biotin-conjugated secondary antibody and streptavidin-biotin peroxidase were applied each for 20 min. 3,3’-Diaminobenzidine tetrahydrochloride (0.05%) was used as the substrate, and nuclear contrast was performed using hematoxylin counterstaining. Each section was analyzed in three different fields using Image Pro Plus software. The density of yellow reflects the expression levels of target proteins. Integral optical density sum / area SUM was applied to quantify the relative expression of COL I, COL III, LOXL2, elastin, MMP1, MMP2, MMP9, and TIMP1.
Statistical analysis
All the data are summarized as mean ± SD, and data were analyzed using SPSS 23.0 statistical software. We performed the data with a one-way analysis of variance with the post-hoc comparison by the L.S.D. method and Fisher’s exact test was employed to compare tumor incidence. Differences with values of P < 0.05 were considered statistically significant.
RESULTS
Macroscopic and pathological observation study of colon tumor
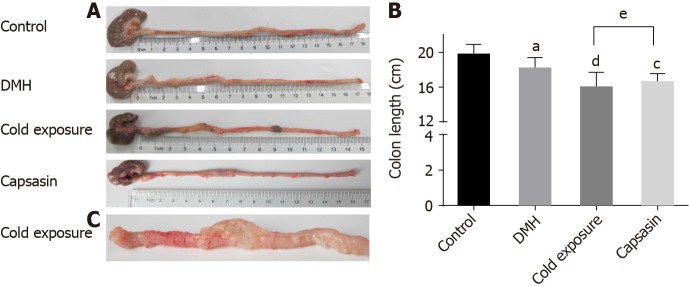
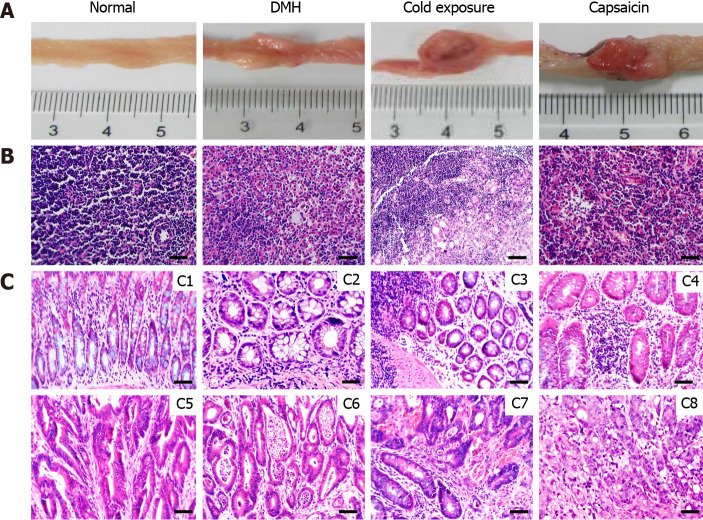
No visible colon tumor was found in the normal control. We observed findings such as colonic mucosal thickening, stiffness, and not tiled completely on filter paper in the majority of rats of the cold exposure group (Figure 1C). As shown in Figure 1B, the length of colon in the DMH and normal group was not significantly different. However, the length of the colon in the cold exposure and capsaicin group was shorter than that of DMH group. The pathological classification of colonic tumors in each group is shown in Table 1. No difference was observed in the proportion of adenomas among groups. In the DMH-induced group of animals, most tumors had well-differentiated tubular adenocarcinomas. Invasive tumors increased in the cold and capsaicin group. Histopathological analysis showed in the cold exposure group that an evident malignant transformation occurred in the colon with the features of poor-differentiated mucinous adenocarcinomas, and some of the glands were filled with mucinous material (Figure 2C). In addition, the mesenteric lymph nodes of rats in each group were stained with hematoxylin and eosin, and the lymphatic metastasis was observed under light microscope (Figure 2B). No lymphatic metastasis was observed in the DMH and capsaicin group, while a mesocolic lymph node was totally replaced by metastatic cancer tissue in the cold exposure group; the lymph node metastasis rate was 20.0% (Table 1, Figures 1 and 2).
Figure 1.
Colonic morphology of rats in different groups. A and B: Changes in colon length and colonic morphology; C: Stiff colonic tissues in cold exposure group. aP < 0.05, control compared with 1,2-dimethylhyrazine (DMH); cP < 0.05, dP < 0.01, DMH compared with cold exposure and capsaicin-treated group; eP < 0.05, cold exposure compared with capsaicin-treated group.
Table 1.
Incidence of various tumors induced in different treatment groups
|
Group
|
Total number of tumors
|
Adenoma incidence, %
|
Adenocarcinoma incidence, %
|
With lymphytic metastasis (%)
|
|||||
|
Mild
|
Moderate
|
Severe
|
Well-differentiated
|
Moderate-differentiated
|
Poor-differentiated
|
Mucinous
|
Metastasis rate
|
||
| Control | - | - | - | - | - | - | - | - | - |
| DMH | 23 | 2/23 (8.7) | 2/23 (8.7) | 3/23 (13) | 12/23 (52.2) | 4/23 (17.4) | 3/23 (13.0) | - | - |
| Cold exposure | 38 | 1/38 (2.6) | 2/38 (5.3) | 3/38 (7.8) | 5/38 (15.8)b | 8/38 (21.1) | 15/38 (36.8)a | 4/38 (10.5) | 20.0 |
| Capsaicin | 34 | 2/31 (5.9) | 1/34 (2.9) | 4/31 (12.9) | 7/31(22.6)a | 12/31 (38.7) | 7/31(22.6) | 1/31 (3.2) | - |
Values are expressed as the proportion of lesions-bearing rats. n = 10 rats/group. Incidence data was analyzed by using chi-square or Fisher’s exact test.
P < 0.05.
P < 0.01.
1,2-dimethylhyrazine compared with cold exposure and capsaicin-treated group. DMH: 1,2-Dimethylhyrazine.
Figure 2.
Pathological observation and lymph node metastases of different groups. A: Macroscopic image of the colonic tumors; B: Representative sections stained with hematoxylin and eosin (HE) showing the histopathology of the mesocolic lymph node in the different groups; C: Representative sections stained with HE showing the histopathology of the colonic mucosa in the different groups. Normal architecture of colon was observed in the control groups (C1), adenoma with mild dysplasia with massive infiltration of inflammatory cells (C2). Histology of adenoma with moderate dysplasia in cold exposure groups (C3). Histology of adenoma with severe dysplasia (C4). Histology of well-differentiated tubular adenocarcinomas (C5). Histology of Moderately differentiated adenocarcinomas (C6). Histology of Poorly differentiated adenocarcinomas (C7). Histology of Mucinous adenocarcinoma with signet ring cells (C8) (HE staining, × 400, scalar bar 20 μm). DMH: 1,2-Dimethylhyrazine.
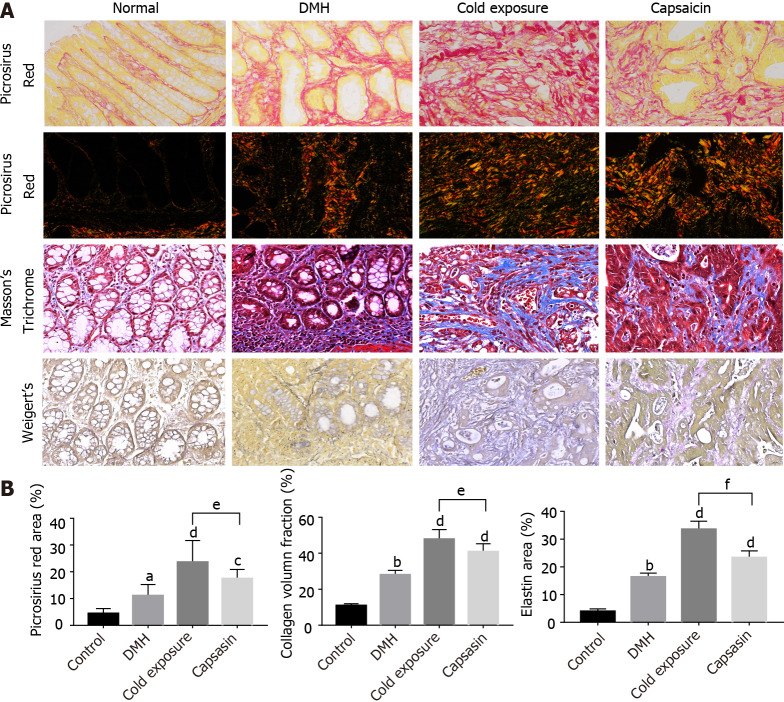
Alterations in collagens after cold exposure and capsaicin
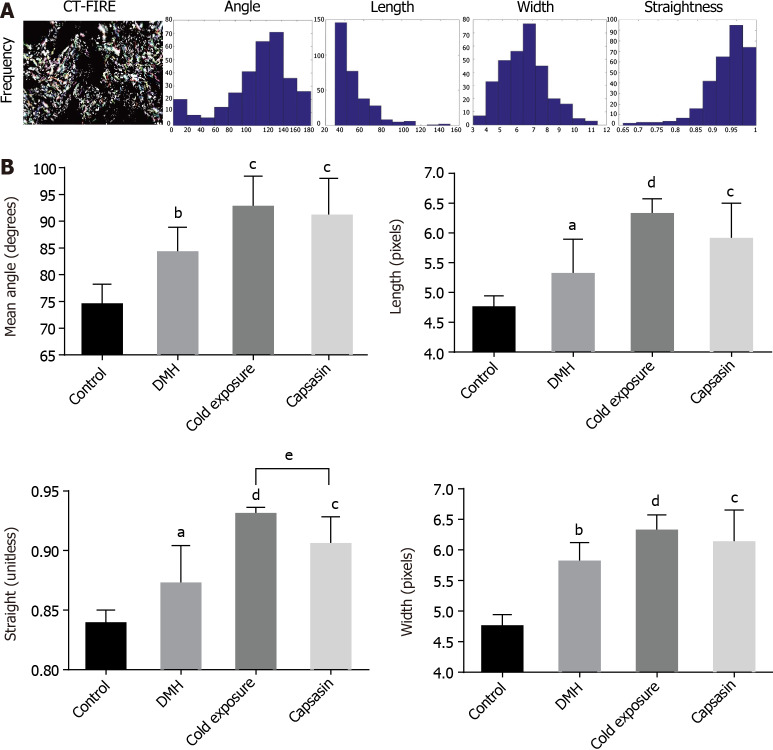
Colon tissue sections were stained by MT and picrosirius red to identify the total collagen in the colon mucosa. As shown in Figure 3A, there were few collagen fibers in normal colonic mucosa. After DMH treatment, wave shape collagens stained blue were markedly increased around the glands, and this was increased further in the cold exposure and capsaicin treatment group. The collagen density was quantified using ImageJ, and it was significantly increased in the colonic tissue of cold exposure and capsaicin group. This excessive collagen deposition was further confirmed by picrosirius red staining. As shown in Figure 3B, picrosirius red staining revealed in normal tissue the collagen fibers with sparse deposition composed of thin collagen fibers. The collagen fibers in the DMH group were denser than that in normal collagen fibers. In the capsaicin treatment group, collagen fibers showed an evident increase and were crosslinked into bundles. On the other hand, the cold exposure group apparently displayed an increased amount of collagen fibers with heterogeneous thickness and alignment. The collagen in the cold exposure and capsaicin group exhibited a predominant reddish or yellow-orange. The structure and organization of collagen fibers were evaluated in colon tissue sections by quantifying the polarization microscopy images. As shown in Figure 4A, visualized collagen fibers were extracted and analyzed for fiber width, angle, length, and straightness using CT-FIRE software. As shown in Figure 4B, compared with the DMH group, collagen fibers in the cold exposure and capsaicin group showed a significant increase in angle, length, width, and straightness. These results revealed that cold exposure and capsaicin induced a progressive increase in the content and orientation of collagen fibers in CRC as a function of malignancy.
Figure 3.
Changes in extracellular matrix components (collagen fibers and elastin) in colonic mucosa of different treatment groups. A: Representative photographs of colonic tissues in rats of normal, 1,2-dimethylhyrazine (DMH), cold exposure and capsaicin groups using Masson’s trichrome: collagen (blue), nuclei and cytoplasm (red); picrosirius red in bright-field: collagen (red); polarized light: collagen (yellow-orange to green birefringence) and Weigert’s Resorcin-Fuschin: elastin (blue-black), myofibers (yellow). Magnification, × 400, scalar bar 20 μm; B: Quantitative analysis of picrosirius red staining, trichrome and Weigert’s staining as a measure of collagen and elastin density.
Figure 4.
Collagen fibers were automatically extracted for analysis using open-source software CT-FIRE. A: Histograms were generated to show the distribution of various parameters in each polarized light microscopy imaging; B: Quantitative analysis of collagen fibers from polarized light microscopy imaging in the colonic mucosa of different treatment groups. Data are mean ± SE of three images per tissues region. aP < 0.05, bP < 0.01, control compared with 1,2-dimethylhyrazine (DMH); cP < 0.05, dP < 0.01, DMH compared with cold exposure and capsaicin-treated group; eP < 0.05, Cold exposure compared with capsaicin-treated group.
Alterations in elastin after cold exposure and capsaicin
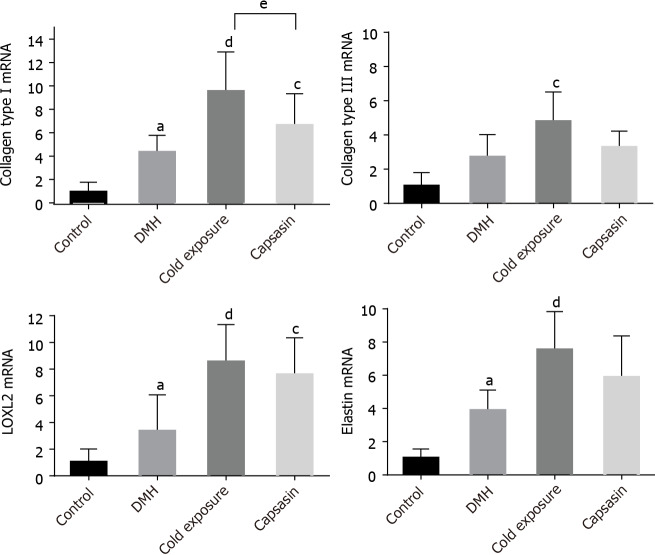
Treatment WRF was used to identify the elastin fibers, which were stained black. As shown in Figure 3A, elastin was hardly expressed in the colonic mucosa of the normal rats. After treatment with DMH, the elastin fibers aligned surrounding the epithelium and stroma. After cold exposure and capsaicin treatment, the amount of elastin fibers increased, and thick elastic fibers was found highly disorganized between the gland compared with their respective control and DMH groups. Alterations in the mRNA levels of COL I, COL III, LOXL2, and elastin. The expression levels of COL I, COL III, LOXL2, and elastin mRNAs in colonic tissue are shown in Figure 5. The COL I, LOXL2, and elastin mRNA levels were higher in the DMH-induced cancer group than in the control group. In comparison with the DMH group, a significant increase was detected both in the cold exposure and capsaicin treatment group, but the mRNA levels of COL III were not significantly different between DMH and capsaicin exposure group.
Figure 5.
The mRNA expression levels of collagen type I, III, elastin and lysyl oxidase-like-2 in the colon tissues of rats in different groups. Data were presented as the mean ± SD form six independent experiments. aP < 0.05, control compared with 1,2-dimethylhyrazine (DMH); cP < 0.05, dP < 0.01, DMH compared with cold exposure and capsaicin-treated group; eP < 0.05, cold exposure compared with capsaicin-treated group. LOXL2: Lysyl oxidase-like-2.
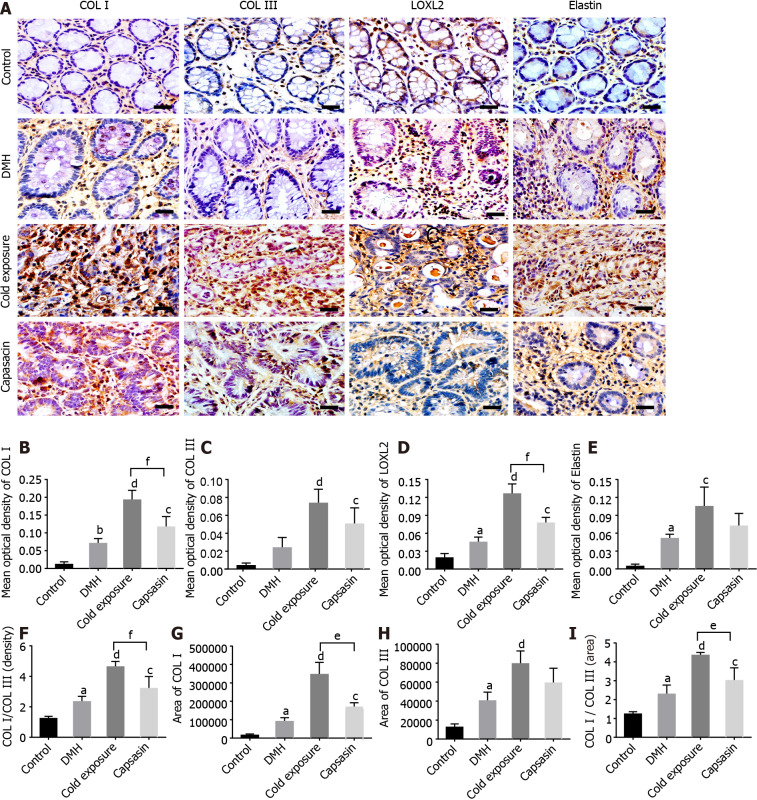
Alterations in the protein levels of COL I, COL III, LOXL2, and elastin
The expression levels of COL I, COL III, LOXL2, and elastin in colonic tissue are shown in Figure 6A and B. The protein expression levels of collagen type I, III, LOXL2, and elastin were significantly elevated in colonic tissue from DMH-treated rats in comparison to the control group. The expression level of proteins in the cold exposure and capsaicin treatment group increased. COL I and LOXL2 levels were significantly higher in the cold exposure group, but no statistical difference was observed in the change of COL III and elastin between cold exposure and capsaicin group.
Figure 6.
Changes in collagen, elastin and lysyl oxidase-like-2 proteins in the colonic tissues of different treatment groups. A: Protein expressions of type I collagen (COL I), type III collagen (COL III), lysyl oxidase-like-2 (LOXL2), and elastin in the colonic tissues via immunohistochemical staining. Magnification, × 400, scalar bar 20 μm; B-I: Densitometric analysis of COL I (B), COL III (C), LOXL2 (D), elastin(E), COL I/COL III (F), COL I area (G), COL III area (H), and COL I area/COL III area (I) during immunohistochemical staining. aP < 0.05, bP < 0.01, control compared with 1,2-dimethylhyrazine (DMH); cP < 0.05, dP < 0.01, DMH compared with cold exposure and capsaicin-treated group; eP < 0.05, fP < 0.01, cold exposure compared with capsaicin-treated group.
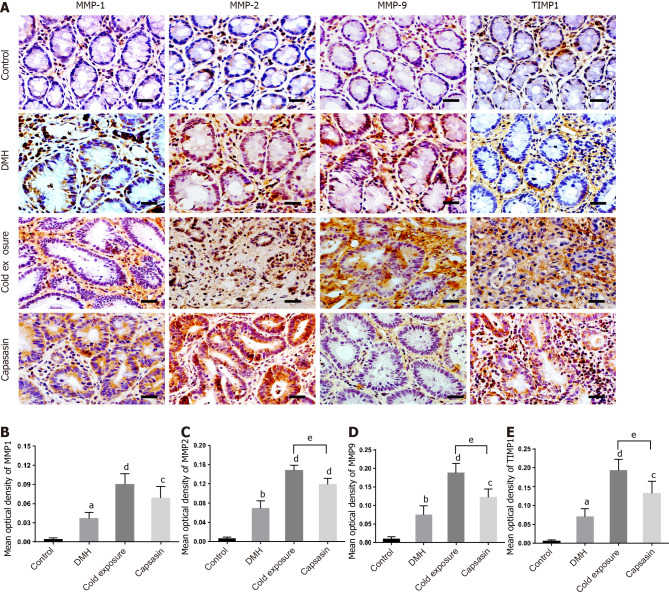
Alterations in the protein levels of MMP1, MMP2, MMP9, and TIMP1
The expression levels of MMP1, MMP2, MMP9, and TIMP1 in colonic tissue are shown in Figure 7. Significantly elevated MMP1, MMP2, MMP9, and TIMP1 immunoreactivity was observed in DMH-treated rats compared with the control group. The expression levels of proteins in the cold and capsaicin group increased compared with the DMH group. In comparison with the capsaicin group, the expression of MMP2, MMP9, and TIMP1 increased in the cold exposure group.
Figure 7.
Changes in matrix metalloproteinase 1, matrix metalloproteinase 2, matrix metalloproteinase 9, and tissue-specific matrix metalloproteinase 1 proteins in the colonic tissues of different treatment groups. A: Protein expressions of matrix metalloproteinase (MMP) 1, MMP2, MMP9 and tissue-specific matrix metalloproteinase 1 (TIMP1) in the colonic tissues via immunohistochemical staining. Magnification, × 400, scalar bar 20 μm; B-E: Densitometric analysis of MMP1 (B), MMP2 (C), MMP9 (D) and TIMP1 (E) during immunohistochemical staining. aP < 0.05, bP < 0.01, control compared with 1,2-dimethylhyrazine (DMH); cP < 0.05, dP < 0.01, DMH compared with cold exposure and capsaicin-treated group; eP < 0.05, cold exposure compared with capsaicin-treated group.
DISCUSSION
ECM has been increasingly considered as an important regulator at diverse aspects of tumor initiation, promotion, neoplastic transformation, invasion, and metastasis[31]. Furthermore, ECM remodeling is a consequence of or increases risk for malignant transformation of colonic, hepatic, pulmonary, and pancreatic cells[32,33]. Collagen and elastin are the major components of ECM, and their excessive deposition has been implicated in a number of diseases, particularly fibrosis and cancer. However, the morphology and structure of collagen and elastin fibers in the animal models of CRC remains unclear. In the present study, we analyzed the morphology and structure of collagen and elastin fibers in rat experimental model of DMH-induced CRC imposed by cold and capsaicin exposure. Results showed an association between collagen expression or ECM modifying enzymes and CRC development, thus supporting ECM remodeling is highly relevant to CRC cancer progression. Tumor tissue often exhibits fibrosis, and this fibrotic state is characterized by the excessive deposition of collagen and elastin[34].
Fibrosis can develop in nearly any organ, and it is an important driver of tissue stiffness and increases the risk of malignancy[35]. In fibrotic kidney biopsy specimens or multiple experimental kidney fibrosis rodent models, the accumulation of elastin can be observed in renal tissue[36]. In human fibrosis of the liver, kidney, and pancreas, the ECM on average becomes stiffer than normal. Our previous study indicated in human CRC that the collagen development features numerous changes in composition and organization compared with normal colonic tissues[37]. In the present study, we found that collagen components were quantitatively and qualitatively changed in the rat experimental model of CRC. By using picrosirius red, MT, and WRF staining, we revealed a marked increase in collagen and elastin deposition in rats exposed to cold and capsaicin treatment. Furthermore, they were more orderly organized based on the collagen fibers being more aligned with each other, longer, wider, and slightly straighter.
The structure, orientation, and physical properties of collagen regulate the aggressive behavior of cancer. For example, in glioblastoma, Pointer et al[38] showed that patients with more organized glioblastoma multiforme collagen survive longer than patients with less organized glioblastoma multiforme collagen. Zhou et al[39] also demonstrated that the increased density, length, or width of collagen negatively affects patients with gastric cancer prognosis. The stromal tissue in CRC has also shown that an increase in the collagen content of the ECM increases mechanical stiffness, which predisposes to aggressive CRC[40]. In human breast cancer, linear organization and relatively straight collagen that facilitates migration of tumor cells indicate poor cancer outcome[41]. Similarly, our data indicated that cold and capsaicin exposure increased collagen and elastin deposition, thus triggering alterations in the ECM architecture and organization in the DMH-induced CRC for further tumor development and progression. Furthermore, all parameters of collagen fibers (e.g., density, angle, length, width, and straightness) significantly increased in the cold exposure group and could accurately explain the cold-induced CRC more seriously.
Recently, the relationship between ECM remodeling and malignant transformation of cancer has attracted much attention[42]. COL I is the most abundant protein present in the body. COL I, a major component of collagen, was significantly up-regulated in CRC tissues and showed enhanced CRC migratory capabilities through the overexpression of WNT/planar cell polarity signaling pathway[43]. Moreover, the elevated expression of type I collagen in CRC tissues is correlated to patients with high metastasis that was due to activation of phosphatidylinositol-3-kinase/AKT signaling[44]. Bode et al[45] also showed that the expression of COL was increased in malignant colon tissue compared with COL III. Moreover, the elevated expression of COL I has been linked to the invasive and aggressive behavior of CRC[46]. In the present study, we also evaluated the expression of COL I in cold exposure and capsaicin treatment CRC colonic tissue. The increase in expression levels of COL I in our study is consistent with other reports in CRC. However, the mRNA level of COL III was not significantly different between the DMH and capsaicin group. In addition, compared with other groups, the COL I/ COL III in cold exposure group was significantly increased. With the increase in collagen expression, distribution area, and collagen ratio, the degree of fibrosis in ECM pathological characteristics was aggravated[47,48]. Therefore, the colonic tissue stiffness was significantly higher than that of the other groups, and the degree of ECM fibrosis in CRC with cold exposure was more serious than that in other groups. ECM remodeling is regulated by ECM enzymes such as LOXL2, MMPs, and TIMPs. ECM-crosslinking enzyme LOXL2 has been implicated in stiffness-associated tumor progression[49]. The LOXL2-mediated collagen cross-linking, both in vitro and in vivo models of CRC, results in increased tissue stiffness and activation of the focal adhesion kinase/SRC signaling[50].
MMP1, MMP-2, and MMP-9 play a fundamental role in many pathophysiological processes such as cell migration, angiogenesis, and the invasion and metastasis of malignant tumors[51]. TIMPs are the most important physiological inhibitors of MMP, and they are also commonly expressed in tumor sites[52]. The expression of both MMP-2 MMP9 and TIMP-2 is higher in invasive tumors and is strongly associated with angiogenesis in DMH-induced CRC[24]. A previous study indicated that the cross-linking of collagen is known to activate enzymes involved in matrix remodeling, such as LOXL2, MMPs, and TIMPs[53,54]. MMPs are responsible for the degradation of ECM; LOXL2 mediate ECM cross-linking and stiffening[55]. However, recent studies indicated that LOXL2 activity promotes breast cancer metastasis by regulating the expression of MMPs and TIMPs involved in matrix remodeling[56]. LOXL2, TIMP1, and MMP9 are co-expressed during mammary metastasis, suggesting that they function together in glandular remodeling. Our previous study also found that expression levels of LOXL2, MMP1, MMP2, and MMP9 are positively correlated in CRC tissues, and they play synergistic roles in ECM remodeling of human CRC[37].
In the present study, the LOXL2, MMP1, MMP2, MMP9, and TIMP parameters were analyzed, and the results indicated a significant increase in the expression of these proteins in the cold exposure and capsaicin group accompanied by the enhancement of collagen and elastin deposition. Therefore, they may act together in regulating ECM remodeling. Growing insights from experimental studies on the roles of the ECM in CRC suggest that the quantitative and qualitative changes in ECM mediated by specific enzymes promote numerous cellular functions that steer cancer progression and metastasis[57,58]. In the present study, the ECM remodeling in the colonic tissue under cold and capsaicin exposure was more serious, thus increasing the exacerbation severity of CRC. Therefore, environmental factors, such as diet, will affect the internal and external constitutions of organism, causing different manifestations and disease progression in the organism. In the cold exposure and capsaicin treatment group, remodeling of the ECM and stromal stiffness is associated with increased propensity for progression to invasive CRC. Therefore, the levels of ECM remodeling can distinguish different organism characteristics and evaluate the CRC progression successfully, thus providing a novel pathological direction of analysis for clinicians. Furthermore, nanoscale mechanical imaging can be used to observe patients with heterogenous features of ECM, who would benefit most from ECM target therapies.
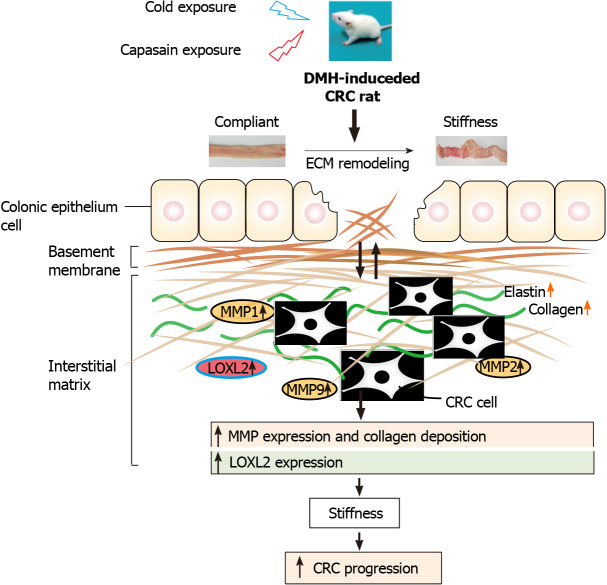
CONCLUSION
In summary, as shown in Figure 8, the present study revealed profound remodeling of the ECM in cold exposure and long-term administration of capsaicin at a low dose in rats. Collagen signatures including angle, length, width, and straightness have a great impact on CRC progression. Additionally, our results show that higher colonic tissue stiffness might result from ECM enzymes-mediated ECM crosslinking and excessive deposition of collagen and elastin, and such changes are strongly associated with the tumor progression of cold and capsaicin exposure CRC. A better understanding of the role of ECM remodeling and ECM enzymes on the pathogenic mechanisms of colon cancer may help in determining the molecular mechanism of CRC progression and could afford a novel therapeutic intervention in the treatment of this disease.
Figure 8.
Schematic diagram depicting the role of extracellular matrix and extracellular matrix enzymes in promoting colorectal cancer pathogenesis. Comparison with the normal rats, rats exposed to cold and capsaicin with profound remodeling of extracellular matrix (ECM) in the colonic tissue, which was mediated by ECM enzymes. These results implicate a crucial role of ECM remodeling on cold and capsaicin exposure colorectal cancer development and progression. CRC: Colorectal cancer; DMH: 1,2-Dimethylhyrazine; MMP: Matrix metalloproteinase.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer (CRC) is a cancer with high prevalence and mortality in the world. Extracellular matrix (ECM) is a dynamic compartment that regulates tissue development and homeostasis, and its remodeling contributes to neoplastic progression. The cancerous ECM can change cell phenotype and has profound influence on the colonization of metastatic cancer cells. However, the relationship between ECM remodeling and progression and aggression CRC from imposed by cold and capsaicin exposure remains unclear.
Research motivation
To identify the effect of cold exposure and capsaicin on ECM remodeling, ECM enzymes, and the underlying mechanism.
Research objectives
To explore the role of ECM remodeling and ECM enzymes in the 1,2-dimethylhydrazine (DMH)-induced CRC progression and the underlying mechanism.
Research methods
The CRC rat model was conducted by adding DMH and examining the role of ECM remodeling and ECM enzymes on DMH-induced CRC in the model. We investigated the morphology and structure of collagen and elastin using Masson’s trichrome, Picrosirius red, and Weigert’s Resorcin-Fuchsin stains. Additionally, we evaluated the protein expression level of type I collagen (COL I), type III collagen (COL III), elastin, lysyl oxidase-like 2 (LOXL2), matrix metalloproteinase (MMP) 1, MMP2, MMP9, and tissue-specific matrix metalloproteinase 1 by immunohistochemistry and observed the expression of COL I, COL III, elastin, and LOXL2 in the colon tissues of rats by reverse-transcriptase quantitative polymerase chain reaction.
Research results
We found that although there were no differences in the proportion of adenomas, a trend towards the increase of invasive tumors was observed in the cold and capsaicin group. Cold exposure group had a metastasis rate comparative with the other groups. Additionally, abnormal accumulation of both collagen and elastin was observed in the cold exposure and capsaicin group. Specifically, collagen quantitative analysis showed increased length, width, angle, and straightness compared with the DMH group. Collagen deposition and straightness were significantly increased in the cold exposure group compared with the capsaicin group. Cold exposure and capsaicin significantly increased the protein levels of COL I, elastin, and LOXL2 along with increases in their messenger RNA levels in the colon tissues compared with the DMH group, while COL III did not show a significant difference. Furthermore, in immunohistochemical evaluations, MMP1, MMP2, MMP9, and tissue-specific matrix metalloproteinase 1 staining increased in the cold exposure and capsaicin group compared with the DMH group.
Research conclusions
Increased stiffness of colonic tissue and the remodeling of ECM mediated by ECM enzymes resulted from cold and capsaicin exposure, predisposing an environment suitable for CRC development and progression.
Research perspectives
To target ECM in CRC tumor tissue could represent a novel potential therapeutic strategy.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Xiao XX for skillful technical assistance.
Footnotes
Institutional animal care and use committee statement: The study was reviewed and approved by the Institutional Animal Ethics Committee of the Guangzhou University of Chinese Medicine (approval No. 20130001).
Conflict-of-interest statement: We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Cold exposure and Capsaicin promote 1,2-dimethylhydrazine-induced colon carcinogenesis in rats correlates with extracellular matrix remodeling”.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: April 1, 2021
First decision: June 24, 2021
Article in press: July 30, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dahiya DS S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Jing-Chun Qin, Institute of Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangdong 530001, Guangdong Province, China; Liuzhou People’s Hospital, Guangxi, 545006, Guangxi Province China.
Wei-Tao Yu, Traditional Chinese Medicine Department, The Second People’s Hospital of Lianyungang, Lianyungang 222000, Jiangsu Province, China.
Hui-Xuan Li, National Center for Respiratory Medicine, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, State Key Laboratory of Respiratory Disease and National Clinical Research Center for Respiratory Disease, Guangdong 510000, Guangdong Province, China.
Yu-Qi Liang, Institute of Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangdong 530001, Guangdong Province, China.
Fei-Fei Nong, Institute of Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangdong 530001, Guangdong Province, China.
Bin Wen, Institute of Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangdong 530001, Guangdong Province, China. wenbin@gzucm.edu.cn.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at wenbin@gzucm.edu.cn. Participants gave informed consent for data sharing.
References
- 1.Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149:1204–1225.e12. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionigi G, Bianchi V, Rovera F, Boni L, Annoni M, Castano P, Villa F, Dionigi R. Genetic alteration in hereditary colorectal cancer. Surg Oncol. 2007;16 Suppl 1:S11–S15. doi: 10.1016/j.suronc.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, Gunter MJ, Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspects Med. 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer S, Rosenzweig KE. Cold Climate Is a Risk Factor for Thyroid Cancer. Clin Thyroidol. 2014;26:273–276. doi: 10.1089/ct.2014;26.273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Carrillo L, López-Cervantes M, Robles-Díaz G, Ramírez-Espitia A, Mohar-Betancourt A, Meneses-García A, López-Vidal Y, Blair A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer. 2003;106:277–282. doi: 10.1002/ijc.11195. [DOI] [PubMed] [Google Scholar]
- 6.Serra I, Yamamoto M, Calvo A, Cavada G, Báez S, Endoh K, Watanabe H, Tajima K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer. 2002;102:407–411. doi: 10.1002/ijc.10716. [DOI] [PubMed] [Google Scholar]
- 7.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen NI, Karsdal M, Willumsen N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J Exp Clin Cancer Res. 2019;38:115. doi: 10.1186/s13046-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33:230–236. doi: 10.1016/j.tibtech.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drifka CR, Tod J, Loeffler AG, Liu Y, Thomas GJ, Eliceiri KW, Kao WJ. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod Pathol. 2015;28:1470–1480. doi: 10.1038/modpathol.2015.97. [DOI] [PubMed] [Google Scholar]
- 11.Esbona K, Yi Y, Saha S, Yu M, Van Doorn RR, Conklin MW, Graham DS, Wisinski KB, Ponik SM, Eliceiri KW, Wilke LG, Keely PJ. The Presence of Cyclooxygenase 2, Tumor-Associated Macrophages, and Collagen Alignment as Prognostic Markers for Invasive Breast Carcinoma Patients. Am J Pathol. 2018;188:559–573. doi: 10.1016/j.ajpath.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest. 2018;128:45–53. doi: 10.1172/JCI93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall TJ, Dolman GE, Duff CM, Paish EC, Zaitoun A, Irving W, Fallowfield JA, Guha IN. Hepatic elastin content is predictive of adverse outcome in advanced fibrotic liver disease. Histopathology. 2018;73:90–100. doi: 10.1111/his.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmelzer CEH, Heinz A, Troilo H, Lockhart-Cairns MP, Jowitt TA, Marchand MF, Bidault L, Bignon M, Hedtke T, Barret A, McConnell JC, Sherratt MJ, Germain S, Hulmes DJS, Baldock C, Muller L. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019;33:5468–5481. doi: 10.1096/fj.201801860RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TH, Hsia SM, Shieh TM. Lysyl Oxidase and the Tumor Microenvironment. Int J Mol Sci. 2016;18 doi: 10.3390/ijms18010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachman-Tzemah C, Zaffryar-Eilot S, Grossman M, Ribero D, Timaner M, Mäki JM, Myllyharju J, Bertolini F, Hershkovitz D, Sagi I, Hasson P, Shaked Y. Blocking Surgically Induced Lysyl Oxidase Activity Reduces the Risk of Lung Metastases. Cell Rep. 2017;19:774–784. doi: 10.1016/j.celrep.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui X, Wang G, Shen W, Huang Z, He H, Cui L. Lysyl oxidase-like 2 is highly expressed in colorectal cancer cells and promotes the development of colorectal cancer. Oncol Rep. 2018;40:932–942. doi: 10.3892/or.2018.6452. [DOI] [PubMed] [Google Scholar]
- 19.Zhan P, Lv XJ, Ji YN, Xie H, Yu LK. Increased lysyl oxidase-like 2 associates with a poor prognosis in non-small cell lung cancer. Clin Respir J. 2018;12:712–720. doi: 10.1111/crj.12584. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Chung T, Rhee H, Kim YJ, Jeon Y, Yoo JE, Noh S, Han DH, Park YN. Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis. Gut Liver. 2019;13:83–92. doi: 10.5009/gnl17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li BH, Zhao P, Liu SZ, Yu YM, Han M, Wen JK. Matrix metalloproteinase-2 and tissue inhibitor of metallo-proteinase-2 in colorectal carcinoma invasion and metastasis. World J Gastroenterol. 2005;11:3046–3050. doi: 10.3748/wjg.v11.i20.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gungor H, Ilhan N, Eroksuz H. The effectiveness of cyclooxygenase-2 inhibitors and evaluation of angiogenesis in the model of experimental colorectal cancer. Biomed Pharmacother. 2018;102:221–229. doi: 10.1016/j.biopha.2018.03.066. [DOI] [PubMed] [Google Scholar]
- 25.Xu GF, Li PT, Wang XY, Jia X, Tian DL, Jiang LD, Yang JX. Dynamic changes in the expression of matrix metalloproteinases and their inhibitors, TIMPs, during hepatic fibrosis induced by alcohol in rats. World J Gastroenterol. 2004;10:3621–3627. doi: 10.3748/wjg.v10.i24.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li HX, Tan JQ, Lv ZH, Liang YQ, Wen B. Colorectal Cancer Modeling and Difference Analysis in Cold and Heat Conditions. Zhongguo Shiyan Fangji Xue Zazhi. 2020;26:109–117. [Google Scholar]
- 27.Jikihara H, Qi G, Nozoe K, Hirokawa M, Sato H, Sugihara Y, Shimamoto F. Aged garlic extract inhibits 1,2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol Rep. 2015;33:1131–1140. doi: 10.3892/or.2014.3705. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Keikhosravi A, Mehta GS, Drifka CR, Eliceiri KW. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol Biol. 2017;1627:429–451. doi: 10.1007/978-1-4939-7113-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keikhosravi A, Bredfeldt JS, Sagar AK, Eliceiri KW. Second-harmonic generation imaging of cancer. Methods Cell Biol. 2014;123:531–546. doi: 10.1016/B978-0-12-420138-5.00028-8. [DOI] [PubMed] [Google Scholar]
- 30.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker C, Mojares E, Del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotti S, Piccoli M, Rizzolio F, Giordano A, Nitti D, Agostini M. Extracellular Matrix and Colorectal Cancer: How Surrounding Microenvironment Affects Cancer Cell Behavior? J Cell Physiol. 2017;232:967–975. doi: 10.1002/jcp.25658. [DOI] [PubMed] [Google Scholar]
- 33.Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200. doi: 10.1016/j.semcancer.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cernaro V, Lacquaniti A, Donato V, Fazio MR, Buemi A, Buemi M. Fibrosis, regeneration and cancer: what is the link? Nephrol Dial Transplant. 2012;27:21–27. doi: 10.1093/ndt/gfr567. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Baues M, Klinkhammer BM, Ehling J, Djudjaj S, Drude NI, Daniel C, Amann K, Kramann R, Kim H, Saez-Rodriguez J, Weiskirchen R, Onthank DC, Botnar RM, Kiessling F, Floege J, Lammers T, Boor P. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y, Lv Z, Huang G, Qin J, Li H, Nong F, Wen B. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol Rep. 2020;44:1671–1685. doi: 10.3892/or.2020.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pointer KB, Clark PA, Schroeder AB, Salamat MS, Eliceiri KW, Kuo JS. Association of collagen architecture with glioblastoma patient survival. J Neurosurg. 2017;126:1812–1821. doi: 10.3171/2016.6.JNS152797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou ZH, Ji CD, Xiao HL, Zhao HB, Cui YH, Bian XW. Reorganized Collagen in the Tumor Microenvironment of Gastric Cancer and Its Association with Prognosis. J Cancer. 2017;8:1466–1476. doi: 10.7150/jca.18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nebuloni M, Albarello L, Andolfo A, Magagnotti C, Genovese L, Locatelli I, Tonon G, Longhi E, Zerbi P, Allevi R, Podestà A, Puricelli L, Milani P, Soldarini A, Salonia A, Alfano M. Insight On Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci Rep. 2016;6:22522. doi: 10.1038/srep22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Wang Y, Zhang J, Zhong J, Yang R. COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway. Mol Med Rep. 2018;17:5037–5042. doi: 10.3892/mmr.2018.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Cai J, Zuo Z, Li J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother. 2019;114:108708. doi: 10.1016/j.biopha.2019.108708. [DOI] [PubMed] [Google Scholar]
- 45.Bode MK, Karttunen TJ, Mäkelä J, Risteli L, Risteli J. Type I and III collagens in human colon cancer and diverticulosis. Scand J Gastroenterol. 2000;35:747–752. doi: 10.1080/003655200750023435. [DOI] [PubMed] [Google Scholar]
- 46.Wei B, Zhou X, Liang C, Zheng X, Lei P, Fang J, Han X, Wang L, Qi C, Wei H. Human colorectal cancer progression correlates with LOX-induced ECM stiffening. Int J Biol Sci. 2017;13:1450–1457. doi: 10.7150/ijbs.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beam J, Botta A, Ye J, Soliman H, Matier BJ, Forrest M, MacLeod KM, Ghosh S. Excess Linoleic Acid Increases Collagen I/III Ratio and "Stiffens" the Heart Muscle Following High Fat Diets. J Biol Chem. 2015;290:23371–23384. doi: 10.1074/jbc.M115.682195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piersma B, Bank RA. Collagen cross-linking mediated by lysyl hydroxylase 2: an enzymatic battlefield to combat fibrosis. Essays Biochem. 2019;63:377–387. doi: 10.1042/EBC20180051. [DOI] [PubMed] [Google Scholar]
- 50.Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 51.Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 53.Dittmore A, Silver J, Sarkar SK, Marmer B, Goldberg GI, Neuman KC. Internal strain drives spontaneous periodic buckling in collagen and regulates remodeling. Proc Natl Acad Sci USA. 2016;113:8436–8441. doi: 10.1073/pnas.1523228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afratis NA, Klepfish M, Karamanos NK, Sagi I. The apparent competitive action of ECM proteases and cross-linking enzymes during fibrosis: Applications to drug discovery. Adv Drug Deliv Rev. 2018;129:4–15. doi: 10.1016/j.addr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brauchle E, Kasper J, Daum R, Schierbaum N, Falch C, Kirschniak A, Schäffer TE, Schenke-Layland K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018;68-69:180–193. doi: 10.1016/j.matbio.2018.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at wenbin@gzucm.edu.cn. Participants gave informed consent for data sharing.