Abstract
Schizophrenia is a mental health disorder that occurs worldwide, cutting across cultures, socioeconomic groups, and geographical barriers. Understanding the details of the neurochemical basis of schizophrenia, factors that contribute to it and possible measures for intervention are areas of ongoing research. However, what has become more evident is the fact that in targeting the neurochemical imbalances that may underlie schizophrenia, the type of response seen with currently available phamacotherapeutic agents does not provide all the answers that are needed. Therefore, the possible contribution of non-pharmacological approaches to schizophrenia management is worthy of consideration. In recent times, research is beginning to show nutrition may play a possibly significant role in schizophrenia, affecting its development, progression and management; however, while attempts had been made to examine this possible relationship from different angles, articles addressing it from a holistic point of view are not common. In this review, we examine existing scientific literature dealing with the possible relationship between nutrition and schizophrenia, with a view to elucidating the impact of diet, nutritional deficiencies and excesses on the aetiology, progression, management and outcome of schizophrenia. Secondly, the effect of nutritional supplements in prevention, as sole therapy, or adjuncts in schizophrenia management are examined.
Keywords: Diet, Brain, Mental health, Nutritional psychiatry, Psychosis, Schizophrenia spectrum disorders
Core Tip: Schizophrenia is a mental health disorder with a significant socioeconomic burden and a complex pathophysiology. As the relationships that potentially link nutrition to the expression of symptoms and the progression of schizophrenia are becoming more apparent, the role of nutrition in schizophrenia management is becoming more important than previously thought. However, despite our current knowledge of the possible links between nutrition and schizophrenia, and some tentative pathways for this relationship, the larger challenge remains how to get to the point where precision dietary manipulation becomes a widely-accepted approach to schizophrenia prevention and a day-to-day management strategy.
INTRODUCTION
Globally, the societal and economic burden of mental health disorders have continued to increase, with available data currently showing an increasing prevalence of a number of these psychiatric disorders in high- and upper-middle income economies[1]. In 2016, mental health and addictive disorders were reported to have affected over 1 billion people worldwide, accounting for about 7% of the global burden of disease measured in disability adjusted life years and approximately 19% of years lived with disability[1]. However, there have been suggestions that these data (despite being high) are still grossly underestimated[2].
Schizophrenia (which is a component of the schizophrenia spectrum disorders) is a chronic, debilitating and complex mental health disorder characterised by impairments in cognition, mood, perception of reality and interpersonal relationships[3]. Worldwide, the case prevalence of schizophrenia has been reported to have risen from 13.1 million in the 1990s to about 20.9 million in 2016[4]. Despite schizophrenia having a low prevalence (lifetime prevalence of about 1%), the burden of schizophrenia is mainly disability-related[4]. It is associated with significantly higher suicide rates, premature deaths and inability to sustain gainful employment[5-8]. The increasing burden of neuropsychiatric disorders (schizophrenia included) has been attributed partly to public health neglect (linked to stigmatisation) and the absence of effective and affordable treatment options[1]. Hence, in the last few decades, there has been a renewed drive to develop less toxic/more effective therapies and novel strategies for the management of schizophrenia and other mental health disorders.
Recently, there have been reports that nutrition, nutritional deficiencies and excesses are important determinants of mental health[9-12]. Over the years, nutritional interventions have been considered as possible preventive and therapeutic options first in high-prevalence mental health disorders like depression and anxiety and more recently in low prevalence disorders such as schizophrenia[10,13,14]. Nutritional interventions are also crucial to combatting the physical health inequalities and decrements in life-expectancy associated with the psychotic-spectrum[15]. Therefore, there is a growing body of knowledge examining the impact of nutrition, nutritional deficiencies/excesses and nutritional interventions on aetiology, progression and management of schizophrenia. In this article, we examine the existing scientific literature dealing with the possible relationship between nutrition and schizophrenia. Secondly, the effect of nutritional supplements in prevention, as sole therapy, or adjuncts in schizophrenia management are examined.
NUTRITION IN MENTAL HEALTH AND DISEASE
Mental health disorders have at present become a growing societal concern, with recent data continuing to show that the economic, social and health burden of psychiatric disorders (including depression, anxiety and schizophrenia) is rising globally[9,16,17]. However, in recent years, the inadequacy of conventional therapeutic options and the need for more effective preventive or therapeutic strategies are directing the focus of research towards examining the possible relationship between nutrition, brain function and the risk of mental health disorders[9,16].
The inkling or proof of the concept that nutrition is important in the maintenance of mental health arose from the descriptions of diseases such as pellagra, beriberi, scurvy and phenylketonuria (which are some of the earliest known human diseases with psychiatric presentations). The pathogenesis of these diseases was linked to nutritional deficiencies and was corrected by nutritional supplementation[18]. Since this period, studies have continued to demonstrate that deficiencies of a number of nutrients, including vitamins (B1, B6, B9, B12) could be linked to the development of psychiatric symptoms or increased risk of developing mental health disorders in adulthood[19-21].
In the last decade or more, the understanding of the crucial role played by environmental factors as determinants of psychiatric disorder risks has increased tremendously. Also, data demonstrating the impact of lifestyle modifications on several non-communicable diseases such as diabetes mellitus and cardiovascular disease are increasing advocacy that lifestyle modification could also be beneficial in addressing mental health disorders[22,23]. Although information is still evolving, the fact that several environmental factors are adaptable has led to suggestions that nutrition or nutritional supplementation could be beneficial in the maintenance of mental well-being or the management of mental health disorders[22].
Normal development of brain structure and function is dependent to a large extent on the availability of amino acids, lipids, minerals and vitamins, which are mainly sourced from dietary intake[17,24]. Hence, there have been suggestions that diet, being a modifiable determinant, can be targeted for the maintenance of mental health and possibly the management of neuropsychiatric disease[25].
The emerging, yet rapidly evolving field of nutritional psychiatry supports the clinical consideration of dietary modifications or the use of nutritional supplementation in the prevention and/or management of psychiatric disorders[26]. There have been reports demonstrating the relationship between the adoption of healthy dietary practices (diets rich in vegetables, whole grains, fruit, nuts, and fish) and the risk of developing mental health disorders like depression was inversely proportional[27-30]. While the possible mechanisms through which dietary strategies and/or interventions could be beneficial in mental health are still being studied, there have been reports suggesting dietary interventions or nutritional supplementation act by modulating biological pathways (oxidative stress, inflammation, neurogenesis, the gut–brain axis)[16]. There have also been reports suggesting that nutrition and dietary composition could directly impact determinants of mental health risk including the gut microbiome, endogenous hormones of the gastrointestinal tract, gut neurotransmitters and neuropeptides[17,31].
Pathophysiology of schizophrenia
Our understanding of the pathophysiology of schizophrenia had in times past been dependent on the concept that schizophrenia was for a large part a hyperdopaminergic state[32,33]. Proposed in the 1960s following the discovery of the antipsychotic benefits of chlorpromazine, the original dopamine hypothesis was a successful heuristic approach to understanding the symptomatology of schizophrenia and response to management[3,33]. The increasing individual and economic burden of schizophrenia has swayed research in the direction of searching for ways to understand better the aetiology of schizophrenia towards the discovery of more effective and efficient treatment and preventive options. Currently, schizophrenia is being described as a complex disorder whose pathophysiology has been attributed to the dysregulation of multiple pathways, including glutamatergic, dopaminergic and γ-amino butyric acid-ergic neurotransmitter systems[34]. Deficits in acetylcholine muscarinic receptors have also been described. Other factors include inflammation, oxidative stress, autoimmunity and genetics[34].
The modified dopamine hypothesis has attributed the hyperdopaminergic state observed in most patients with schizophrenia to an increased presynaptic striatal dopaminergic activity, which is a final common pathway through which a number of the other factors (numerous genetic and/or environmental) can result in the development of schizophrenia[35,36]. The broadening of the dopamine hypothesis has allowed aetiological factors to be classified as either upstream or downstream[35]. While current treatment options are directed towards addressing the downstream factors responsible for neurotransmitter dysfunction. There have been suggestions that more effective treatments could be achieved if drugs and/or other interventions are directed at manipulating the upstream factors[35].
Diet and nutritional factors in schizophrenia pathogenesis
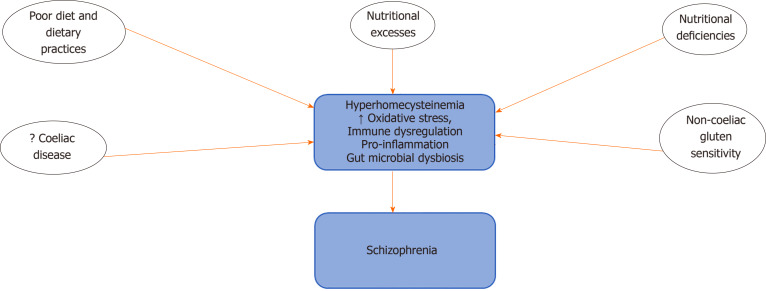
Current evidence from studies on the impact of diet and dietary intake (Figure 1) in schizophrenia shows that compared to normal controls, persons with schizophrenia have poor dietary practices characterised by increased intake of sodium, cholesterol and higher saturated fats, with low fibre content[37-40]. Increased consumption of sugars and processed foods have also been reported[41-43]. These poor dietary choices have been suggested as possible causes of obesity, metabolic syndrome and high mortality figures, which have been associated with schizophrenia[14,43]. There have also been reports that diets low in omega-3 fatty acids or D-vitamins could also increase risk of developing psychosis[40,44]. The unhealthy dietary practices of persons with schizophrenia have been suggested to result from dysregulation of the reward circuitry, mediated by increased dopamine activity in the mesolimbic pathway and brain regions responsible for the control of cognition[45,46]. The development of obesity, eating disorders, food cravings and addictive behaviours observed in persons with schizophrenia have also been linked to this pathway[47-49].
Figure 1.
Diet/nutritional factors and potential mechanisms in schizophrenia pathogenesis.
There have been reports suggesting that the incidence of coeliac disease and non-coeliac gluten sensitivity is higher in patients with schizophrenia compared to the general population[50,51]. Coeliac disease is an autoimmune disorder characterised by inflammation-induced intestinal injury arising from the consumption of gluten-rich diet such as barley, wheat, rye and bulgur. A few studies have reported the triggering of psychotic symptoms by gluten in persons with gluten intolerance[52]. Food sensitivities that are characterised by the elevation of immunoglobulin G antibodies to wheat glutens and bovine milk caseins have also been observed in persons with schizophrenia[53].
Nutritional deficiencies that occur as a result of inadequate intake or the poor absorption of nutrients that are critical to the sustenance of human health have also been recognised as possible risk factors for the development of psychiatric disorders including schizophrenia[13]. Also, nutrition and nutritional composition of foods have also been implicated in mental health. Earlier reports had shown that increased intake of foods deficient in critical compounds such as essential fatty acids[54,55] or rich in substances that can elicit an autoimmune reaction such as gluten could be linked to the development of brain disease and more recently mental illness including schizophrenia[13,22,28,56].
Schizophrenia has also been associated with the development of nutritional deficiencies and metabolic disorders. The results of clinical studies and systematic reviews have demonstrated a higher incidence of metabolic syndrome in patients with schizophrenia compared to the general population; this has been attributed in part to poor eating habits and possibly side-effects of pharmacotherapy[57,58]. Results of biochemical tests had also reported high homocysteine levels as well as low levels of vitamins B9, B12, C and E in the blood of newly diagnosed patients and patients with long-term schizophrenia. Decreased brain levels of vitamin B12 have also been reported in schizophrenia[59]. Deficiencies in vitamin D have also been implicated in schizophrenia, and developmental deficiency of D3 has been associated with an increased risk of developing schizophrenia in adulthood[13,60,61]. There have also been suggestions that low maternal vitamin D levels do not constitute a continuous risk factor for the development of schizophrenia; however, below a critical threshold, it could be associated with an increased risk of developing schizophrenia[13,62].Deficiencies or excesses of essential trace elements including calcium, zinc, selenium, copper and manganese have also been observed in persons with schizophrenia[63-66].
Further buttressing the importance of vitamins and minerals in schizophrenia are results of clinical studies that had also shown that supplementation with a number of these vitamins was associated with reduction in symptoms and the amelioration of neurological deficits associated with schizophrenia[13,55].
Mechanisms through which nutrition and nutritional factors influence schizophrenia pathophysiology
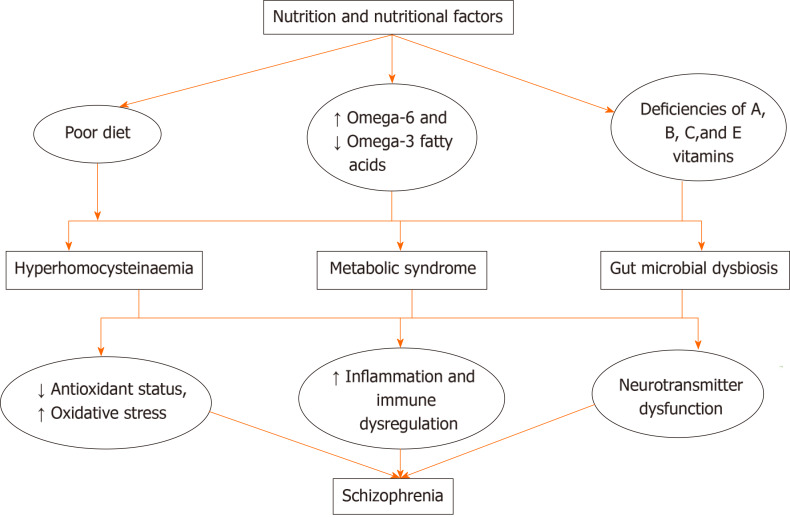
The mechanisms through which diet, nutrient and/or nutritional deficiencies cause schizophrenia or worsen its severity are still being studied. However, a number of modalities (Figure 2) have been suggested. These include factors like poor diet, poor dietary practices and/or nutritional deficiencies that have been linked with the development of hyperhomocysteinaemia, derangement of oxidant-antioxidant status, immune dysregulation and alterations in the levels of pro-inflammatory markers. The importance of oxidative stress and inflammation in schizophrenia had been described in the neuroprogressive hypothesis, which considered alterations in oxidative stress and immune-inflammatory markers as possible mechanisms in the pathophysiology of schizophrenia[67-69].
Figure 2.
Mechanisms through which nutrition and nutritional factors influence schizophrenia pathophysiology.
The gut microbiota, which plays a strategic role in food digestion, metabolism and storage of fats/absorption of monosaccharides, has also been reported to be influenced significantly by dietary choices. Several studies have reported that dietary composition and nutritional status are very important modifiable factors in maintaining gut microbiome composition and gut microbial diversity[31,70,71].
There is also growing interest in the relationship that exists between diet and the gut microbiota, and how this relationship impacts schizophrenia pathophysiology and management. However, it is worthy of note that while diet is an important factor determining the composition of the gut microbiota, other factors such as stress and sleep can also alter it. Also, interindividual and time-defined variations in their composition make it harder to define accurately gut microbiota dysbiosis. In recent times, there has been increasing evidence of the importance of the multidimensional relationship that exists between the gut microbiota and the brain, especially its ability to influence the brain through the utilisation of immunologic, endocrine and neurocrine signalling pathways[72]. Alterations in the composition of the gut microbiota and microbial metabolite have been reported to influence the integrity of the gut and bodily immune responses. Gut dysbiosis has been reported in persons with schizophrenia, with reports that increase in the Succinivibrio and Corynebacterium bacterial genera exacerbated schizophrenia symptom severity[73]. Gut dysbiosis also increases susceptibility to infections and inflammation[74]. However, direct proof that diet-induced change in gut microbiota may be a direct cause of schizophrenia symptoms is harder to obtain. Also, dietary manipulations have not been shown to be consistently successful in alleviating schizophrenia symptoms. So far, the link between diet and schizophrenia has been suggested to involve the induction of neuroinflammation and modulation of the gut microbiota; these in turn have been associated with worsening symptoms of schizophrenia in some cases.
Gut-dwelling microbes such as bacteria have the potential to alter neurotransmitter equilibrium through production and/or consumption of a wide range of mammalian neurotransmitters such as dopamine, noradrenaline, serotonin, or γ-amino butyric acid; a number of which are closely-linked to the pathogenesis of schizophrenia[75]. Already, evidence from animal studies (and even some human studies) suggest that gut bacteria can be used to manipulate neurotransmitter equilibrium to impact host physiology and that microbiota-based interventions can alter neurotransmitter levels[75]. However, how this knowledge can be used to develop a robust preventive and curative strategy for schizophrenia is still a challenge.
Studies have reported that diets that are low in anti-inflammatory or rich in pro-inflammatory factors (omega-6 fatty acids are pro-inflammatory in contrast to omega-3 fatty acids that are anti-inflammatory) activate or worsen neuroinflammation, and if left uncontrolled, can induce pathologic changes of schizophrenia and exacerbate schizophrenia symptom severity[74,76]. Exposure to diets rich in gluten has been associated with an increase in the expression of human leukocyte antigen markers, which increase cells susceptible to attacks by T-cells, allowing the release of pro-inflammatory cytokines. Also, there have been reports suggesting that a number of other immune pathways such as the complement protein (C1q) are activated in persons with schizophrenia[53].
A number of the B vitamins, including B2, B6, B9 and B12, are the sources of coenzymes and cofactors needed in one carbon metabolism[77]. One-carbon metabolism is crucial to cell proliferation and survival; therefore, deficiencies in these B vitamins (particularly B9 and B12) have been associated with severe alterations in normal cell biology. There is alteration of S-adenosyl methionine production, impairment of nucleotide synthesis, reduction in cell proliferation and a global hypomethylation of deoxyribonucleic acid[78]. More recently, alterations in one-carbon metabolism resulting in elevation in the level of homocysteine have been considered an independent risk factor for schizophrenia[79]. Homocysteine is a non-protein neurotoxic amino acid that can alter brain structure and function, especially during early phases of brain development[79-81]. The interaction of homocysteine with glutamatergic transmission has also been suggested as one of the possible links between homocysteinaemia and schizophrenia[82]. There have also been suggestions that the activity of homocysteine on other neuromodulators such dopamine, serotonin and acetylcholine could be linked to the development of schizophrenia[82-84].
Deficiencies or low levels of antioxidant vitamins such as C, E and beta carotene have been reported to cause oxidative stress[85]. There is overwhelming evidence demonstrating the crucial role played by oxidative stress in the pathophysiology of schizophrenia[86,87]. Higher lipid peroxidation levels, alterations in plasma and brain levels of antioxidants and/or antioxidant enzyme activity have also been observed in persons with schizophrenia[87-90]. Oxidative stress has also been described as a possible link between vitamins deficiencies and schizophrenia[86]. Studies have also shown that antioxidant vitamins such as C and E offer protection against cellular damage due to either inflammation or highly reactive oxygen-species[85].
NUTRITION IN SCHIZOPHRENIA THERAPY
In the last few decades, the crucial role played by nutrition in the maintenance of mental health has continued to be emphasised. The dynamic relationship between adequate intake of dietary nutrients and optimal mental health has also been reported[91,92]. The need to use nutritional interventions to achieve general health and well-being was the beginning of orthomolecular medicine. Orthomolecular medicine is defined as the restoration and maintenance of health through the administration of adequate amounts of substances normally occurring in the body. It allows the use of endogenous and natural compounds as either supplement in conditions of nutritional deficiencies or in the provision of additional requirements in conditions of increased demand[93]. Although, in the past, it had been regarded as a non-scientific approach to healing, the availability of increasing scientific evidence supporting its benefits in aging-related disease[94] and, more recently, in the management of a number of neuropsychiatric disorders (including depression and schizophrenia) is redefining its importance in clinical medicine[94,95].
Evolving knowledge regarding the impact of derangements in one-carbon metabolism, oxidative stress, autoimmunity, inflammation and the gut microbiome on the neuropathophysiology of schizophrenia[56,96,97] is leading scientists to suggest potential mechanisms that allow for the use of diet, nutrition and nutritional supplements as sole or adjunctive therapies in the management of psychiatric disorders such as schizophrenia[36].
Dietary intervention as a therapeutic target in schizophrenia
In the last half century, modifying dietary behaviours for the prevention and management of chronic disease has been a crucial area of research[98-102]. There is now overwhelming evidence that modifying important dietary habits, and dietary composition such as the consumption of vegetables/fruits, and increasing intake of fibre, polyunsaturated fats, and omega-3 fatty acid (in contrast to omega-6) have significant and sustained general health benefits[103,104].
More recently, the benefits of dietary choices and nutritional factors in sustaining mental health[105,106] especially in preventing the development of medical and metabolic complications are generally being recognised in psychiatry. Also, the use of dietary interventions particularly in the management of schizophrenia is slowly evolving. Gradually, emphasis is being placed on the need to include dietary interventions as sole (in the case of psychosis due to nutritional deficiencies) or adjunctive therapies in the management of positive and/or negative symptoms in schizophrenia[92,107].
Healthy diets such as the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet are lifestyle changes or dietary modifications have been shown to be beneficial in the prevention, risk factor reduction and management of chronic diseases. Several studies have demonstrated that strict adherence to these dietary choices have rewarding effects on diabetes, cardiovascular diseases, obstructive sleep apnoea, chronic kidney disease, arthritis, cancer and metabolic diseases[104,108-110]; with significant impact in reducing total mortality[104,111].
The Mediterranean diet, which reflects the eating habits of the Mediterranean or Middle East countries, is based on the consumption of fresh vegetables, cereals, olive oil and plants; with low consumption of meat[104]. While the DASH diet, which was designed by National Institute of Health funded researchers in the 1990s to aid in the prevention and treatment of high blood pressure, was also shown to be effective in lowering blood cholesterol levels. It is a diet rich in vegetables, fruits, low-fat dairy products, whole grains, nuts, grains, fish, meat and poultry[110]. Dietary interventions have proven to be successful in reducing the risk factors for a number of chronic diseases including diabetes mellitus, cardiovascular disease, stroke, metabolic syndrome and in some mental health disorders including depression and anxiety[112].
The benefits of good nutrition in schizophrenia have been self-reported[113]; and while the details of its benefits are still being studied scientifically, proponents of these alternative treatment approach have suggested that the beneficial effects of good nutrition and or dietary interventions could be multipronged. Good nutrition and healthy diets can impact schizophrenia symptom severity, duration of episodes, and the incidence of negative symptoms[113]. Also, there have been reports that persons with schizophrenia are more likely to have co-morbid health problems, including obesity, chronic inflammation, metabolic syndrome, autoimmune disease and cardiovascular disease[40,113], all of which when they occur in the general population benefit from nutritional intervention. This has led to suggestions that these nutritional interventions could help in reducing schizophrenia mortality, especially when it is a sequela of these co-morbid conditions[114,115]. In a few instances, there have also been suggestions that dietary interventions could be beneficial in schizophrenia, especially if there is a causal relationship between diet and schizophrenia[114].
The earliest instances of dietary intervention in schizophrenia were from suggestions that gluten-free diets could be successful in mitigating schizophrenia or militating against its severity[116,117]. However, while a few studies (Table 1) have demonstrated the ability of diet free of gluten to improve functioning in schizophrenia or decrease symptoms severity[118-121], there have also been several studies that have reported no effect[122,123].
Table 1.
Diet and Nutritional supplements as therapeutic targets
|
Item
|
Subject
|
Model
|
Outcome
|
Ref.
|
| DASH diet | Human | Randomised controlled study | No difference in the prevalence of metabolic syndrome between groups | [131] |
| Interventional study | Reduced sodium load and caloric intake compared to baseline | [14] | ||
| Gluten-free diet | Human | Double-blind gluten-free vs gluten-rich diet study | Improved functioning or decreased symptoms severity | [118-121] |
| Clinical study | Improvements in symptoms of schizophrenia and extrapyramidal side-effects | [124] | ||
| Clinical study | no significant effect of gluten-free diet | [122,123] | ||
| Ketogenic diet | Human | Case report | Complete amelioration of schizophrenia symptom | [130] |
| Omega-3 fatty acid | Rodents | Ketamine model of schizophrenia | Was protective against the development of behavioural changes | [134] |
| Protective against decreased inhibition of the startle reflex, lipid peroxidation, and decreased antioxidant status in different brain regions | [139] | |||
| Human | Clinical study | Decrease in the rate of transition to full-threshold psychosis and a decrease in the positive and negative symptoms, compared to those administered placebo | [95] | |
| Randomised, double-blind, placebo-controlled clinical trials | Omega-3 fatty acid PUFA supplementation was effective in reducing clinical symptoms in persons with prodrome and/or first episode schizophrenia; mixed results were observed in persons with chronic schizophrenia | [140] | ||
| Clinical study amongst hospitalised persons with acute violent schizophrenia | No significant effect of adjunctive omega-3 fatty acid supplementation on symptoms, when compared to conventional therapy alone | [141] | ||
| Eicosapentaenoic acid (EPA) | Human | Clinical study | Improvements in symptoms were observed in persons with early schizophrenia | [136,142] |
| No benefits were observed in persons with chronic forms of schizophrenia | [143,144] | |||
| Zinc | Rodent | Ketamine model of schizophrenia | Reversal of schizophrenia-like behaviours | [107] |
| Age-related decrease in ketamine-induced alterations in behaviours (open field memory and anxiety) acetylcholinesterase activity, and oxidative stress parameters | [92] | |||
| Melatonin | Rodent | Ketamine model of schizophrenia | Reversal of schizophrenia-like symptoms, with benefits comparable to standard medications | [29] |
DASH: Dietary approaches to stop hypertension; PUFA: Polyunsaturated fatty acid.
In a double-blind gluten-free vs gluten-rich diet study, Vlissides et al[120] reported significant improvement in symptoms measured on a psychosis in-patient’s profile. Jackson et al[124] tested the feasibility and efficacy of administering gluten-free diet in 2 patients with schizophrenia who were either positive for anti-tissue transglutaminase/anti-endomysial antibodies (coeliac disease) or antibodies to gliadin (indicative of gluten sensitivity). They reported that gluten-free diet was well-tolerated in patients with schizophrenia, resulting in no adverse effects; also, in both patients (irrespective of the antibodies), improvements in schizophrenia symptoms and extrapyramidal side-effects were observed[124]. Porkins et al[122] and Storms et al[123] in separate studies that compared the effects of a gluten-free diet or added gluten in patients with schizophrenia reported no significant effect of gluten-free diet on tests and rating scales. However, in those who received gluten–rich diet, a significant improvement was observed in tension-anxiety and anger-hostility[123]. The potential adverse effect of gluten-free diet has also been reported. De Palma et al[125] reported that the administration of gluten-free diet to persons who do not have coeliac disease or non-coeliac gluten sensitivity was associated with alteration of the gut microbial flora and an increase in host immune activation.
Ketogenic diets are high in fat but low in carbohydrate and have been shown to be successful in weight loss and/or control, reduction of cardiovascular risks and the management of diabetes mellitus[126,127]. While it has also been shown to be beneficial in the management of Alzheimer’s disease and seizure disorders[128,129], there is however a dearth of scientific information on its benefits in schizophrenia. Kraft and Westman[130] published a case report that demonstrated the complete amelioration of schizophrenia symptoms in a 70-year-old Caucasian woman with long-standing schizophrenia following the commencement of a ketogenic diet.
Currently available literature points to the fact that the beneficial effects of a gluten-free diet is limited to a small subset of persons with schizophrenia (those with coeliac disease or non-coeliac gluten sensitivity). So, there have been suggestions that alternative dietary modifications such as diets high in fibre, the DASH diet or Mediterranean diet, which are associated with minimal side-effects, could become beneficial as adjunctive therapies in improving immune, metabolic and cardiovascular events linked to the premature mortality observed in schizophrenia. Sorić et al[131] conducted a 3-mo randomised controlled study to examine the benefits of the DASH diet in reducing metabolic syndrome parameters in hospitalised schizophrenic patients and observed no significant difference in the prevalence of metabolic syndrome between the intervention group and control after 3 mo of dietary intervention[131]. However, the result of another interventional study carried out in a sample of young persons with first episode schizophrenia showed reduced sodium load and caloric intake compared to baseline[14]. The Mediterranean diet has also been suggested to be beneficial in schizophrenia, largely due to its ability to reduce pro-inflammatory markers, cardiovascular disease risk and immune markers; while improving the ratio of omega-3 to omega-6 fatty acids[131-133].
Overall, while there have been several suggestions of the possible benefits of dietary modifications and interventions in the management of schizophrenia, the dearth of scientific information in this regard limits its use.
Nutritional supplements as therapeutic targets or adjuncts in schizophrenia
There is a great deal of interest directed towards the use of nutritional supplements such as vitamins and trace elements either as therapeutic targets or adjuncts in the management of schizophrenia (Table 1). While this is an evolving area of medicine, evidence of the strong correlation amongst nutritional deficiencies, mineral excess/deficiencies and the awareness of the impact of oxidative stress, neuroinflammation and immune mediated responses in the pathophysiology of schizophrenia are increasing research into the benefits of nutritional interventions and nutritional supplements in schizophrenia management. Also, more recently, there is increasing consideration of diet and nutrition as important modifiable factors in mental health disorders; with a growing body of evidence showing that nutritional supplementation with vitamins, essential fatty acids, minerals and trace elements not only provide additional physiological benefits but could also act as adjuncts to pharmacologic therapy[16,25].
The earliest suggestions that vitamins could provide therapeutic benefits in schizophrenia possibly arose from reports that associated schizophrenia with vitamin deficiencies[85]. These benefits have been attributed to their ability to act by using mechanisms that are separate from those employed by the current pharmacologic agents. The result of clinical studies had reported that supplementation with folic acid, pyridoxine and vitamins B12 and C were beneficial in ameliorating schizophrenia symptoms when used alone or as adjuncts with conventional therapy[85]. Also, the results of preclinical[69,92,134,135] and clinical studies[95,136-138] have continued to provide evidence in support of the beneficial effects of other nutritional supplements in schizophrenia; although there have also been a few dissenting reports. Gama et al[134] reported that the administration of omega-3 fatty acid polyunsaturated fatty acid (PUFA) supplements to adolescent rats was protective against the development of behavioural changes in a ketamine model of schizophrenia, features that are synonymous to positive, negative and cognitive symptoms of schizophrenia[134]. In another study, Zugno et al[139] observed that pre-treatment of adolescent rats with omega-3 fatty acid was also protective against decreased inhibition of the startle reflex, lipid peroxidation and decreased antioxidant status in different brain regions (prefrontal cortex, hippocampus, striatum). In humans, Amminger et al[95] examined the ability of omega-3 fatty acid supplementation to protect against the development of first-episode psychosis in adolescents and young adults (13 to 25 years of age) with subthreshold psychosis. They observed that in the group administered omega-3 PUFA, there was a decrease in the rate of transition to full-threshold psychosis and a decrease in the positive and negative symptoms, compared to those administered placebo[95]. There have also been reports of non-consistent effects of omega-3 fatty acids supplementation in schizophrenia[140,141]. Chen et al[140] following a metanalysis of randomised, double-blind, placebo-controlled clinical trials reported that while omega-3 fatty acid PUFA supplementation was effective in reducing clinical symptoms in persons with prodrome and/or first episode schizophrenia, mixed results were observed in persons with chronic schizophrenia[140]; suggesting that it may not be consistently beneficial in this subset. While examining the effect of omega-3 fatty acids on the development of hostility and psychopathology amongst hospitalised persons with acute violent schizophrenia, Qiao et al[141] observed no significant effect of the adjunctive use of omega-3 fatty acids supplementation on symptoms, when compared to conventional therapy alone. Similarly, improvements in symptoms had been reported for eicosapentaenoic acid supplementation in persons with early schizophrenia[136,142], while there have also been reports of no benefits of eicosapentaenoic acid in persons with chronic forms of schizophrenia[143,144].
A number of other preclinical studies had also examined the effects of other dietary supplements such as zinc or melatonin as therapeutic targets and/or adjuncts in mouse models of schizophrenia[29,92,107]. Onaolapo et al[107] reported that zinc administered (either alone or with standard antipsychotics) was associated with a reversal of ketamine-induced alteration in open-field behaviours, working memory, social interaction, antioxidant status and lipid peroxidation. Onaolapo et al[92] also examined the ability of dietary zinc supplementation to mitigate the development of ketamine-induced schizophrenia-like behaviours in prepubertal and aged mice. Results showed age-related decrease in ketamine-induced alterations in behaviours (open field memory and anxiety) acetylcholinesterase activity and oxidative stress parameters[92]. Dietary supplementation with melatonin has also been shown to reverse schizophrenia-like symptoms in mice, with benefits comparable to standard medications[29].
The benefits of dietary supplements in mitigating the pro-oxidant and other drug-related side-effects of antipsychotics like haloperidol have also been reported in mice[135] and in humans[145]. Sivrioglu et al[145] examined the effects of combining antioxidant supplements (omega-3 fatty acid, vitamins E and C) in schizophrenia patients with ongoing haloperidol therapy over a 4 mo period. Results showed an improvement in clinical symptoms and a decrease in the severity of side-effects induced by haloperidol[145]. Dietary supplementation with zinc had also been shown to reverse haloperidol-induced changes in open field behaviour and spatial working memory, while potentiating haloperidol induced anxiolysis; suggesting that co-administration of haloperidol with zinc can reduce some side-effects that are known to be associated with haloperidol therapy[135].
As research continues to reveal the relationships that link nutrition to the expression of symptoms, and the progression of schizophrenia, it is becoming apparent that the roles of nutrition might emerge to be larger than previously-thought. The pro- or anti-inflammatory effects of some food components are already known. However, while the impact of gut bacteria on neurotransmitter levels is now being understood; the indirect route from consumed food to possible changes in neurotransmitter balance appears more difficult to navigate.
CONCLUSION
Overall, despite our current knowledge of the possible links between nutrition and schizophrenia, and some tentative pathways for this relationship, the larger challenge remains how to get to the point where precision dietary manipulation becomes a widely-accepted approach to schizophrenia prevention and a day-to-day management strategy.
Footnotes
Conflict-of-interest statement: The authors declare that there is no any conflicts of interest.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Society for Neuroscience, No. 100035408.
Peer-review started: February 28, 2021
First decision: April 20, 2021
Article in press: August 10, 2021
Specialty type: Neurosciences
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kotzalidis GD, Li CP S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Olakunle James Onaolapo, Behavioural Neuroscience/Neuropharmacology Unit, Department of Pharmacology, Ladoke Akintola University of Technology, Osun State 234, Nigeria.
Adejoke Yetunde Onaolapo, Department of Anatomy, Ladoke Akintola University of Technology, Osun State 234, Nigeria. adegbayibiy@yahoo.com.
References
- 1.Rehm J, Shield KD. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep. 2019;21:10. doi: 10.1007/s11920-019-0997-0. [DOI] [PubMed] [Google Scholar]
- 2.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–178. doi: 10.1016/S2215-0366(15)00505-2. [DOI] [PubMed] [Google Scholar]
- 3.Onaolapo AY, Onaolapo OJ. Schizophrenia aetiology and drug therapy: a tale of progressive demystification and strides in management. Adv Pharmacol Pharmacy. 2018;6:19–42. [Google Scholar]
- 4.Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44:1195–1203. doi: 10.1093/schbul/sby058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon L. What It Will Take to Make Coordinated Specialty Care Available to Anyone Experiencing Early Schizophrenia: Getting Over the Hump. JAMA Psychiatry. 2017;74:7–8. doi: 10.1001/jamapsychiatry.2016.2665. [DOI] [PubMed] [Google Scholar]
- 6.Galletly CA. Premature death in schizophrenia: bridging the gap. Lancet Psychiatry. 2017;4:263–265. doi: 10.1016/S2215-0366(17)30079-2. [DOI] [PubMed] [Google Scholar]
- 7.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. doi: 10.1016/S2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan SKW, Chan SWY, Pang HH, Yan KK, Hui CLM, Chang WC, Lee EHM, Chen EYH. Association of an Early Intervention Service for Psychosis With Suicide Rate Among Patients With First-Episode Schizophrenia-Spectrum Disorders. JAMA Psychiatry. 2018;75:458–464. doi: 10.1001/jamapsychiatry.2018.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan AC, Jacka FN. Nutritional psychiatry research: an emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J Physiol Anthropol. 2014;33:22. doi: 10.1186/1880-6805-33-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, Stubbs B, Schuch FB, Carvalho AF, Jacka F, Sarris J. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom Med. 2019;81:265–280. doi: 10.1097/PSY.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teasdale S, Mörkl S, Müller-Stierlin AS. Nutritional psychiatry in the treatment of psychotic disorders: current hypotheses and research challenge. Brain Behav Immun Health . 2020;5:10070. doi: 10.1016/j.bbih.2020.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeman MV. The gut microbiome and antipsychotic treatment response. Behav Brain Res. 2021;396:112886. doi: 10.1016/j.bbr.2020.112886. [DOI] [PubMed] [Google Scholar]
- 13.Firth J, Carney R, Stubbs B, Teasdale SB, Vancampfort D, Ward PB, Berk M, Sarris J. Nutritional Deficiencies and Clinical Correlates in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2018;44:1275–1292. doi: 10.1093/schbul/sbx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teasdale SB, Ward PB, Rosenbaum S, Watkins A, Curtis J, Kalucy M, Samaras K. A nutrition intervention is effective in improving dietary components linked to cardiometabolic risk in youth with first-episode psychosis. Br J Nutr. 2016;115:1987–1993. doi: 10.1017/S0007114516001033. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organisation (WHO) Management of physical health conditions in Adults with severe mental disorders, WHO guidelines WHO. Geneva, 2018. [PubMed] [Google Scholar]
- 16.Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. 2017;76:427–436. doi: 10.1017/S0029665117002026. [DOI] [PubMed] [Google Scholar]
- 17.Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, Schellekens H, Dickson SL. Nutritional psychiatry: Towards improving mental health by what you eat. Eur Neuropsychopharmacol. 2019;29:1321–1332. doi: 10.1016/j.euroneuro.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Raju MSVK. Medical nutrition in mental health and disorders. Indian J Psychiatry. 2017;59:143–148. doi: 10.4103/psychiatry.IndianJPsychiatry_193_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enderami A, Zarghami M, Darvishi-Khezri H. The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review. Neurol Sci. 2018;39:1667–1675. doi: 10.1007/s10072-018-3473-4. [DOI] [PubMed] [Google Scholar]
- 20.Giannunzio V, Degortes D, Tenconi E, Collantoni E, Solmi M, Santonastaso P, Favaro A. Decision-making impairment in anorexia nervosa: New insights into the role of age and decision-making style. Eur Eat Disord Rev. 2018;26:302–314. doi: 10.1002/erv.2595. [DOI] [PubMed] [Google Scholar]
- 21.Smith AD, Warren MJ, Refsum H. Vitamin B12. Adv Food Nutr Res. 2018;83:215–279. doi: 10.1016/bs.afnr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, Hibbeln J, Matsuoka Y, Mischoulon D, Mizoue T, Nanri A, Nishi D, Ramsey D, Rucklidge JJ, Sanchez-Villegas A, Scholey A, Su KP, Jacka FN International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization and Calouste Gulbenkian Foundation. Social determinants of mental health. Geneva, World Health Organization, 2014. [Google Scholar]
- 24.Castro AI, Gomez-Arbelaez D, Crujeiras AB, Granero R, Aguera Z, Jimenez-Murcia S, Sajoux I, Lopez-Jaramillo P, Fernandez-Aranda F, Casanueva FF. Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese Patients. Nutrients. 2018;10 doi: 10.3390/nu10101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacka FN. Nutritional Psychiatry: Where to Next? EBioMedicine. 2017;17:24–29. doi: 10.1016/j.ebiom.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarris J. Nutritional Psychiatry: From Concept to the Clinic. Drugs. 2019;79:929–934. doi: 10.1007/s40265-019-01134-9. [DOI] [PubMed] [Google Scholar]
- 27.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99:181–197. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- 28.O'Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, Berk M, Jacka FN. Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health. 2014;104:e31–e42. doi: 10.2105/AJPH.2014.302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emerson SD, Carbert NS. An apple a day: Protective associations between nutrition and the mental health of immigrants in Canada. Soc Psychiatry Psychiatr Epidemiol. 2019;54:567–578. doi: 10.1007/s00127-018-1616-9. [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Agostino D, Caballero FF, Martín-María N, Tyrovolas S, López-García P, Rodríguez-Artalejo F, Haro JM, Ayuso-Mateos JL, Miret M. Mediterranean diet and wellbeing: evidence from a nationwide survey. Psychol Health. 2019;34:321–335. doi: 10.1080/08870446.2018.1525492. [DOI] [PubMed] [Google Scholar]
- 31.Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gründer G, Cumming P. The Dopamine Hypothesis of Schizophrenia: Current status. In: Abel, T, Nickl-Jockschat, T. The Neurobiology of Schizophrenia. San Diego (CA): Elsevier, 2016: 109-124. [Google Scholar]
- 34.Deng C, Dean B. Mapping the pathophysiology of schizophrenia: interactions between multiple cellular pathways. Front Cell Neurosci. 2013;7:238. doi: 10.3389/fncel.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arroll MA, Wilder L, Neil J. Nutritional interventions for the adjunctive treatment of schizophrenia: a brief review. Nutr J. 2014;13:91. doi: 10.1186/1475-2891-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- 38.Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29:393–397. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- 39.Nunes D, Eskinazi B, Camboim Rockett F, Delgado VB, Schweigert Perry ID. Nutritional status, food intake and cardiovascular disease risk in individuals with schizophrenia in southern Brazil: a case-control study. Rev Psiquiatr Salud Ment. 2014;7:72–79. doi: 10.1016/j.rpsm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Joseph J, Depp C, Shih PB, Cadenhead KS, Schmid-Schönbein G. Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front Neurosci. 2017;11:155. doi: 10.3389/fnins.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stokes C, Peet M. Dietary sugar and polyunsaturated fatty acid consumption as predictors of severity of schizophrenia symptoms. Nutr Neurosci. 2004;7:247–249. doi: 10.1080/10284150400010012. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara N, Yasui-Furukori N, Sato Y, Saito M, Furukori H, Nakagami T, Ishioka M, Kaneko S. Dietary patterns are associated with obesity in Japanese patients with schizophrenia. BMC Psychiatry. 2014;14:184. doi: 10.1186/1471-244X-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito H, Kumagai T, Kimura M, Koike S, Shimizu T. Dietary Intake in Body Mass Index Differences in Community-Based Japanese Patients with Schizophrenia. Iran J Public Health. 2015;44:639–645. [PMC free article] [PubMed] [Google Scholar]
- 44.Dealberto MJ. Why are immigrants at increased risk for psychosis? Med Hypotheses. 2007;68:259–267. doi: 10.1016/j.mehy.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 45.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- 47.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 48.Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des. 2011;17:1158–1167. doi: 10.2174/138161211795656819. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 50.Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37:94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okusaga O, Yolken RH, Langenberg P, Sleemi A, Kelly DL, Vaswani D, Giegling I, Hartmann AM, Konte B, Friedl M, Mohyuddin F, Groer MW, Rujescu D, Postolache TT. Elevated gliadin antibody levels in individuals with schizophrenia. World J Biol Psychiatry. 2013;14:509–515. doi: 10.3109/15622975.2012.747699. [DOI] [PubMed] [Google Scholar]
- 52.Lionetti E, Leonardi S, Franzonello C, Mancardi M, Ruggieri M, Catassi C. Gluten Psychosis: Confirmation of a New Clinical Entity. Nutrients. 2015;7:5532–5539. doi: 10.3390/nu7075235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Alaedini A, Dupont D, Dickerson FB, Yolken RH. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis. 2012;48:447–453. doi: 10.1016/j.nbd.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 55.Lakhan SE, Vieira KF. Nutritional therapies for mental disorders. Nutr J. 2008;7:2. doi: 10.1186/1475-2891-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemperman RF, Veurink M, van der Wal T, Knegtering H, Bruggeman R, Fokkema MR, Kema IP, Korf J, Muskiet FA. Low essential fatty acid and B-vitamin status in a subgroup of patients with schizophrenia and its response to dietary supplementation. Prostaglandins Leukot Essent Fatty Acids. 2006;74:75–85. doi: 10.1016/j.plefa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47:197–207. doi: 10.1016/j.jpsychires.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Bly MJ, Taylor SF, Dalack G, Pop-Busui R, Burghardt KJ, Evans SJ, McInnis MI, Grove TB, Brook RD, Zöllner SK, Ellingrod VL. Metabolic syndrome in bipolar disorder and schizophrenia: dietary and lifestyle factors compared to the general population. Bipolar Disord. 2014;16:277–288. doi: 10.1111/bdi.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Hodgson NW, Trivedi MS, Abdolmaleky HM, Fournier M, Cuenod M, Do KQ, Deth RC. Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PLoS One. 2016;11:e0146797. doi: 10.1371/journal.pone.0146797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouaziz N, Ayedi I, Sidhom O, Kallel A, Rafrafi R, Jomaa R, Melki W, Feki M, Kaabechi N, El Hechmi Z. Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 2010;179:24–29. doi: 10.1016/j.psychres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 61.García-Miss Mdel R, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, Gurubel-Maldonado J, Arankowsky-Sandoval G. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44:441–446. doi: 10.1016/j.jpsychires.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 62.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63:73–78. doi: 10.1016/s0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 63.Rahman A, Azad MA, Hossain I, Qusar MM, Bari W, Begum F, Huq SM, Hasnat A. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol Trace Elem Res. 2009;127:102–108. doi: 10.1007/s12011-008-8230-8. [DOI] [PubMed] [Google Scholar]
- 64.Sharma SK, Sharma A, Sood S, Gupta I. Study of serum selenium levels in schizophrenic patients. Ind Med Gaz. 2014;148:403–407. [Google Scholar]
- 65.Cai L, Chen T, Yang J, Zhou K, Yan X, Chen W, Sun L, Li L, Qin S, Wang P, Yang P, Cui D, Burmeister M, He L, Jia W, Wan C. Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Sci Rep. 2015;5:15013. doi: 10.1038/srep15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu T, Lu QB, Yan L, Guo J, Feng F, Qiu J, Wang J. Comparative Study on Serum Levels of 10 Trace Elements in Schizophrenia. PLoS One. 2015;10:e0133622. doi: 10.1371/journal.pone.0133622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson G, Berk M, Dodd S, Bechter K, Altamura AC, Dell'osso B, Kanba S, Monji A, Fatemi SH, Buckley P, Debnath M, Das UN, Meyer U, Müller N, Kanchanatawan B, Maes M. Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:1–4. doi: 10.1016/j.pnpbp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 69.Onaolapo AY, Aina OA, Onaolapo OJ. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed Pharmacother. 2017;92:373–383. doi: 10.1016/j.biopha.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 70.Kovatcheva-Datchary P, Arora T. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2013;27:59–72. doi: 10.1016/j.bpg.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Li T, Beasley DE, Heděnec P, Xiao Z, Zhang S, Li J, Lin Q, Li X. Diet Diversity Is Associated with Beta but not Alpha Diversity of Pika Gut Microbiota. Front Microbiol. 2016;7:1169. doi: 10.3389/fmicb.2016.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onaolapo OJ, Onaolapo AY, Olowe AO. The neurobehavioral implications of the brain and microbiota interaction. Front Biosci (Landmark Ed) 2020;25:363–397. doi: 10.2741/4810. [DOI] [PubMed] [Google Scholar]
- 73.Li S, Zhuo M, Huang X, Huang Y, Zhou J, Xiong D, Li J, Liu Y, Pan Z, Li H, Chen J, Li X, Xiang Z, Wu F, Wu K. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi: 10.7717/peerj.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cha HY, Yang SJ. Anti-Inflammatory Diets and Schizophrenia. Clin Nutr Res. 2020;9:241–257. doi: 10.7762/cnr.2020.9.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jahrami H, Faris MA, Ghazzawi HA, Saif Z, Habib L, Shivappa N, Hébert JR. Increased Dietary Inflammatory Index Is Associated with Schizophrenia: Results of a Case-Control Study from Bahrain. Nutrients. 2019;11 doi: 10.3390/nu11081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 78.Lyon P, Strippoli V, Fang B, Cimmino L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients. 2020;12 doi: 10.3390/nu12092867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AS, Bottiglieri T, Schaefer CA, Quesenberry CP Jr, Liu L, Bresnahan M, Susser ES. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 80.Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:143–149. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- 81.Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, Tomotake M, Ohi K, Hashimoto R, Imoto I, Takeda M, Ohmori T. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in schizophrenia. Schizophr Bull. 2014;40:1154–1163. doi: 10.1093/schbul/sbt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front Behav Neurosci. 2014;8:343. doi: 10.3389/fnbeh.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen CS, Kuo YT, Tsai HY, Li CW, Lee CC, Yen CF, Lin HF, Ko CH, Juo SH, Yeh YC, Liu GC. Brain biochemical correlates of the plasma homocysteine level: a proton magnetic resonance spectroscopy study in the elderly subjects. Am J Geriatr Psychiatry. 2011;19:618–626. doi: 10.1097/JGP.0b013e318209ddf1. [DOI] [PubMed] [Google Scholar]
- 84.Gao L, Zeng XN, Guo HM, Wu XM, Chen HJ, Di RK, Wu Y. Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol Sci. 2012;33:39–43. doi: 10.1007/s10072-011-0645-x. [DOI] [PubMed] [Google Scholar]
- 85.Brown HE, Roffman JL. Vitamin supplementation in the treatment of schizophrenia. CNS Drugs. 2014;28:611–622. doi: 10.1007/s40263-014-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D'Souza B, D'Souza V. Oxidative injury and antioxidant vitamins E and C in Schizophrenia. Indian J Clin Biochem. 2003;18:87–90. doi: 10.1007/BF02867671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madireddy S, Madireddy S. Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia. Brain Sci. 2020;10 doi: 10.3390/brainsci10100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L, Maynard TM, Meyer U, Morishita H, O'Donnell P, Puhl M, Cuenod M, Do KQ. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22:936–943. doi: 10.1038/mp.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An H, Du X, Huang X, Qi L, Jia Q, Yin G, Xiao C, Huang XF, Ning Y, Cassidy RM, Wang L, Soares JC, Zhang XY. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl Psychiatry. 2018;8:258. doi: 10.1038/s41398-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2019;45:742–751. doi: 10.1093/schbul/sby125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grønli O, Kvamme JM, Friborg O, Wynn R. Zinc deficiency is common in several psychiatric disorders. PLoS One. 2013;8:e82793. doi: 10.1371/journal.pone.0082793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onaolapo OJ, Jegede OR, Adegoke O, Ayinde MO, Akeredolu OM, Onaolapo AY. Dietary zinc supplement militates against ketamine-induced behaviours by age-dependent modulation of oxidative stress and acetylcholinesterase activity in mice. Pharmacol Rep. 2020;72:55–66. doi: 10.1007/s43440-019-00003-2. [DOI] [PubMed] [Google Scholar]
- 93.Zell M, Grundmann O. An orthomolecular approach to the prevention and treatment of psychiatric disorders. Adv Mind Body Med. 2012;26:14–28. [PubMed] [Google Scholar]
- 94.Janson M. Orthomolecular medicine: the therapeutic use of dietary supplements for anti-aging. Clin Interv Aging. 2006;1:261–265. doi: 10.2147/ciia.2006.1.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 96.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 97.Das UN. Polyunsaturated fatty acids and their metabolites in the pathobiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:122–134. doi: 10.1016/j.pnpbp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 98.O'Loughlin J, Paradis G, Meshefedjian G. Evaluation of two strategies for heart health promotion by direct mail in a low-income urban community. Prev Med. 1997;26:745–753. doi: 10.1006/pmed.1997.0214. [DOI] [PubMed] [Google Scholar]
- 99.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 100.Bowen DJ, Beresford SA. Dietary interventions to prevent disease. Annu Rev Public Health. 2002;23:255–286. doi: 10.1146/annurev.publhealth.23.100901.140555. [DOI] [PubMed] [Google Scholar]
- 101.Onaolapo AY, Onaolapo OJ. Nutraceuticals and Diet-based Phytochemicals in Type 2 Diabetes Mellitus: From Whole Food to Components with Defined Roles and Mechanisms. Curr Diabetes Rev. 2019;16:12–25. doi: 10.2174/1573399814666181031103930. [DOI] [PubMed] [Google Scholar]
- 102.Onaolapo AY, Onaolapo OJ. Circadian dysrhythmia-linked diabetes mellitus: Examining melatonin's roles in prophylaxis and management. World J Diabetes. 2018;9:99–114. doi: 10.4239/wjd.v9.i7.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed. December 2020. Available from: http://health.gov/dietaryguidelines . [Google Scholar]
- 104.Tsigalou C, Konstantinidis T, Paraschaki A, Stavropoulou E, Voidarou C, Bezirtzoglou E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines. 2020;8 doi: 10.3390/biomedicines8070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Onaolapo OJ, Onaolapo AY. Melatonin in drug addiction and addiction management: Exploring an evolving multidimensional relationship. World J Psychiatry. 2018;8:64–74. doi: 10.5498/wjp.v8.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Onaolapo OJ, Onaolapo AY. Nutrition in autism spectrum disorders: A review of evidences for an emerging central role in aetiology, expression, and management. AIMS Med Sci . 2018;5:122–144. [Google Scholar]
- 107.Onaolapo OJ, Ademakinwa OQ, Olalekan TO, Onaolapo AY. Ketamine-induced behavioural and brain oxidative changes in mice: an assessment of possible beneficial effects of zinc as mono- or adjunct therapy. Psychopharmacology (Berl) 2017;234:2707–2725. doi: 10.1007/s00213-017-4666-x. [DOI] [PubMed] [Google Scholar]
- 108.Winkvist A, Bärebring L, Gjertsson I, Ellegård L, Lindqvist HM. A randomized controlled cross-over trial investigating the effect of anti-inflammatory diet on disease activity and quality of life in rheumatoid arthritis: the Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA) study protocol. Nutr J. 2018;17:44. doi: 10.1186/s12937-018-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hidalgo-Mora JJ, García-Vigara A, Sánchez-Sánchez ML, García-Pérez MÁ, Tarín J, Cano A. The Mediterranean diet: A historical perspective on food for health. Maturitas. 2020;132:65–69. doi: 10.1016/j.maturitas.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Gallieni M, Cupisti A. DASH and Mediterranean Diets as Nutritional Interventions for CKD Patients. Am J Kidney Dis. 2016;68:828–830. doi: 10.1053/j.ajkd.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 112.Aucoin M, LaChance L, Cooley K, Kidd S. Diet and Psychosis: A Scoping Review. Neuropsychobiology. 2020;79:20–42. doi: 10.1159/000493399. [DOI] [PubMed] [Google Scholar]
- 113.Royal B. Schizophrenia: Nutrition and Alternative Treatment Approaches. Schizophr Bull. 2016;42:1083–1085. doi: 10.1093/schbul/sbu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robst J. Addressing the gastrointestinal health associated with schizophrenia: The argument for a new nutrition-based intervention. J Schizophrenia Dis Therapy. 2016;1:7–18. [Google Scholar]
- 115.Kim EJ, Lim SY, Lee HJ, Lee JY, Choi S, Kim SY, Kim JM, Shin IS, Yoon JS, Yang SJ, Kim SW. Low dietary intake of n-3 fatty acids, niacin, folate, and vitamin C in Korean patients with schizophrenia and the development of dietary guidelines for schizophrenia. Nutr Res. 2017;45:10–18. doi: 10.1016/j.nutres.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Dohan FC, Grasberger JC, Lowell FM, Johnston HT Jr, Arbegast AW. Relapsed schizophrenics: more rapid improvement on a milk- and cereal-free diet. Br J Psychiatry. 1969;115:595–596. doi: 10.1192/bjp.115.522.595. [DOI] [PubMed] [Google Scholar]
- 117.Dohan FC, Grasberger JC. Relapsed schizophrenics: earlier discharge from the hospital after cereal-free, milk-free diet. Am J Psychiatry. 1973;130:685–688. doi: 10.1176/ajp.130.6.685. [DOI] [PubMed] [Google Scholar]
- 118.De Santis A, Addolorato G, Romito A, Caputo S, Giordano A, Gambassi G, Taranto C, Manna R, Gasbarrini G. Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J Intern Med. 1997;242:421–423. doi: 10.1046/j.1365-2796.1997.00200.x. [DOI] [PubMed] [Google Scholar]
- 119.Kelly DL, Demyanovich HK, Rodriguez KM, Ciháková D, Talor MV, McMahon RP, Richardson CM, Vyas G, Adams HA, August SM, Fasano A, Cascella NG, Feldman SM, Liu F, Sayer MA, Powell MM, Wehring HJ, Buchanan RW, Gold JM, Carpenter WT, Eaton WW. Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): a pilot feasibility study J Psychiatry Neurosci 2019; 44: 269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vlissides DN, Venulet A, Jenner FA. A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry. 1986;148:447–452. doi: 10.1192/bjp.148.4.447. [DOI] [PubMed] [Google Scholar]
- 121.Levinta A, Mukovozov I, Tsoutsoulas C. Use of a Gluten-Free Diet in Schizophrenia: A Systematic Review. Adv Nutr. 2018;9:824–832. doi: 10.1093/advances/nmy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Potkin SG, Weinberger D, Kleinman J, Nasrallah H, Luchins D, Bigelow L, Linnoila M, Fischer SH, Bjornsson TD, Carman J, Gillin JC, Wyatt RJ. Wheat gluten challenge in schizophrenic patients. Am J Psychiatry. 1981;138:1208–1211. doi: 10.1176/ajp.138.9.1208. [DOI] [PubMed] [Google Scholar]
- 123.Storms LH, Clopton JM, Wright C. Effects of gluten on schizophrenics. Arch Gen Psychiatry. 1982;39:323–327. doi: 10.1001/archpsyc.1982.04290030055010. [DOI] [PubMed] [Google Scholar]
- 124.Jackson J, Eaton W, Cascella N, Fasano A, Warfel D, Feldman S, Richardson C, Vyas G, Linthicum J, Santora D, Warren KR, Carpenter WT Jr, Kelly DL. A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophr Res. 2012;140:262–263. doi: 10.1016/j.schres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102:1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 126.Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health. 2014;11:2092–2107. doi: 10.3390/ijerph110202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Westman EC, Tondt J, Maguire E, Yancy WS Jr. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13:263–272. doi: 10.1080/17446651.2018.1523713. [DOI] [PubMed] [Google Scholar]
- 128.Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic Diet in Alzheimer's Disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20163892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic Diet and Epilepsy. Nutrients. 2019;11 doi: 10.3390/nu11102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (Lond) 2009;6:10. doi: 10.1186/1743-7075-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sorić T, Mavar M, Rumbak I. The Effects of the Dietary Approaches to Stop Hypertension (DASH) Diet on Metabolic Syndrome in Hospitalized Schizophrenic Patients: A Randomized Controlled Trial. Nutrients. 2019;11 doi: 10.3390/nu11122950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, Christou A, Chatzigeorgiou M, Skoumas I, Tousoulis D, Stefanadis C ATTICA Study Group. Adherence to Mediterranean diet and 10-year incidence (2002-2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32:73–81. doi: 10.1002/dmrr.2672. [DOI] [PubMed] [Google Scholar]
- 133.Neale EP, Batterham MJ, Tapsell LC. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res. 2016;36:391–401. doi: 10.1016/j.nutres.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 134.Gama CS, Canever L, Panizzutti B, Gubert C, Stertz L, Massuda R, Pedrini M, de Lucena DF, Luca RD, Fraga DB, Heylmann AS, Deroza PF, Zugno AI. Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia. Schizophr Res. 2012;141:162–167. doi: 10.1016/j.schres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Onaolapo OJ, Ayanwale T, Agoi O, Adetimehin C, Onaolapo AY. Zinc Tempers Haloperidol-induced Behavioural Changes in Healthy Mice. Int J Neurosci Biobehav Sci . 2016;4:21–31. [Google Scholar]
- 136.Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:1867–1875. doi: 10.4088/jcp.v68n1206. [DOI] [PubMed] [Google Scholar]
- 137.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 138.Farokhnia M, Azarkolah A, Adinehfar F, Khodaie-Ardakani MR, Hosseini SM, Yekehtaz H, Tabrizi M, Rezaei F, Salehi B, Sadeghi SM, Moghadam M, Gharibi F, Mirshafiee O, Akhondzadeh S. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2013;36:185–192. doi: 10.1097/WNF.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 139.Zugno AI, Chipindo HL, Volpato AM, Budni J, Steckert AV, de Oliveira MB, Heylmann AS, da Rosa Silveira F, Mastella GA, Maravai SG, Wessler PG, Binatti AR, Panizzutti B, Schuck PF, Quevedo J, Gama CS. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience. 2014;259:223–231. doi: 10.1016/j.neuroscience.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 140.Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann Clin Psychiatry. 2015;27:289–296. [PubMed] [Google Scholar]
- 141.Qiao Y, Liu CP, Han HQ, Liu FJ, Shao Y, Xie B. No Impact of Omega-3 Fatty Acid Supplementation on Symptoms or Hostility Among Patients With Schizophrenia. Front Psychiatry. 2020;11:312. doi: 10.3389/fpsyt.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- 143.Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, Jackson GD, Velakoulis D, Pantelis C, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- 144.Fusar-Poli P, Berger G. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol. 2012;32:179–185. doi: 10.1097/JCP.0b013e318248b7bb. [DOI] [PubMed] [Google Scholar]
- 145.Sivrioglu EY, Kirli S, Sipahioglu D, Gursoy B, Sarandöl E. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499. doi: 10.1016/j.pnpbp.2007.07.004. [DOI] [PubMed] [Google Scholar]