Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections have posed a problematic healthcare situation worldwide since December 2019. Diabetes mellitus is associated with an increased risk and severity of coronavirus disease 2019 (COVID-19). While interacting with various other risk factors, high blood sugar was found to reduce immunity and increase the replication of SARS-CoV-2. Oxidative stress and the release of pro-inflammatory cytokines are greater in diabetic individuals than in healthy people, worsening the outcome of SARS-CoV-2 infection in diabetics. Increased expression of furin and angiotensin converting enzyme 2 (ACE-2) receptor in the hyperglycemic environment may promote the entry of SARS-CoV-2 in the host cell. COVID-19 infection primarily modulates immune and inflammatory responses, and may cause a cytokine storm, resulting in possible lethal outcomes in diabetics. An experimental report suggests that ACE expressed in the pancreas and the SARS-CoV-2 virus invariably destroy β-cells which contain ACE-2 receptors and results in acute diabetes. Moreover, COVID-19 also causes hyperglycemia in an individual with diabetes which may be related to insulin resistance and destruction of β-cells during SARS-CoV-2 infection. Early observations also suggest a correlation between oral hypoglycemic agents and the risk of COVID-19. This review focused on the possible cause and mechanism involved in SARS-CoV-2 infection in diabetics and the role of antidiabetic drugs in COVID-19.
Keywords: Diabetes mellitus, SARS-CoV-2, COVID-19, Angiotensin converting enzyme 2, Antidiabetic drug, Cytokine storm
Core Tip: This review highlights the significant correlation between diabetes mellitus and coronavirus disease 2019 (COVID-19) in light of available published information. COVID-19 is responsible for increased risk and disease severity in diabetic patients. Cytokine storm, destruction of beta cells and the expression of angiotensin converting enzyme 2 are some of the fundamental mechanisms discussed. This review also summarizes the range of investigations that have been undertaken across a large set of published papers on COVID-19 and diabetes mellitus.
INTRODUCTION
Coronaviruses (CoVs) (family: Coronaviridae, order: Nidovirales) are recognized as a large family of single-stranded RNA viruses responsible for mild to severe respiratory infections. The presence of distinct spikes with rounded tips on the surface of the virus provides the appearance of having crowns; hence the virus named coronavirus (in Latin ‘corona’ means crown)[1-3]. The first case of coronavirus was reported in 1960 as a cold[4]. CoVs belong to four genera, namely α-CoV, β-CoV, γ-CoV and δ-CoV. Mammals can be infected by α-CoV, β-CoV and δ-CoV, while γ-CoV and δ-CoV can infect avian species. In the 21st century, before coronavirus disease 2019 (COVID-19), two CoVs of the genera β-CoV, namely severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), emerged as significant public health concerns[1,2]. In 2002, SARS-CoV was first found in China, and in 2012 the first case of MERS-CoV was reported in Jordan[3]. In December 2019, COVID-19 was first reported in Wuhan, China and soon emerged as the most dangerous infectious disease of the century. Angiotensin-converting enzyme 2 (ACE-2) is considered the dominant host receptor of COVID-19 expressed in different organs in humans, and COVID-19 uses the ACE-2 receptor to enter host cells[4,5]. A total of 110749023 confirmed cases, including 2455131 deaths due to COVID-19, have been reported globally as of 21st February 2021 [as per WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/].
Diabetes mellitus (DM) is characterized by a spectrum of metabolic disorders and has become one of the most significant global public health concerns in the 21st century. The prevalence of DM is increasing rapidly worldwide among all groups of people without any barrier and is also responsible for the burden on socio-economic development[6,7]. The International Diabetes Federation (IDF), in its report (IDF Diabetes Atlas 2019), estimated that 463 million people (20-79 years age group) live with DM globally, with a projected increase to 700 million by 2045. The IDF Diabetes Atlas also predicted that almost half of these people (232 million) were unaware of their diabetic condition, and approximately 374 million adults are at risk of developing type 2 DM[8]. DM is considered one of the top 10 causes of death, and in 2019, the IDF predicted 4.2 million deaths due to DM globally[7,8]. People with DM are at increased risk (2-3-fold) of all-cause mortality. DM is responsible for various complications and the link between increased risk of infections and DM is well established. People with DM have a high risk of various infections[7,9].
The overall burden of DM and its complications is increasing significantly, and COVID-19 poses a problematic situation for those with DM.
RESPIRATORY COMPLICATIONS OF DM
Diabetic patients are susceptible to a sequence of acute or chronic complications such as microvascular and macrovascular complications, infection and are at risk of premature death[9]. Defective pulmonary function is well documented in diabetic patients. A decrease in lung function and forced vital capacity, obstruction of the peripheral airway, and decreased capacity for carbon monoxide (CO) pulmonary diffusion have been observed in diabetic patients. In diabetic individuals, sympathetic and parasympathetic neuropathy is responsible for a series of pulmonary function deficits[10]. Pulmonary autonomic neuropathy is responsible for reducing mucociliary clearance and thus enhancing the risk of lung infections. Defective muscle metabolism and neuropathy of the phrenic nerve decrease respiratory muscle strength in diabetic individuals. It is well established that hyperresponsiveness increases through the Rho-associated protein kinase (Rock) pathway in diabetics[10]. Hyperglycemia is also responsible for oxidative stress by enhancing the generation of reactive oxygen species (ROS), reactive nitrogen species and advanced glycation end products (AGEs)[9]. Increased blood glucose level has been found to promote lung fibrosis, chronic inflammation, and inflammatory cytokine release. Higher glucose concentrations promote susceptibility to pulmonary infections[10]. Alterations in the levels of inflammatory cytokines have been reported in diabetics. Glycation was found to decrease class I major histocompatibility complex (MHC) expression on the surface of myeloid cells responsible for impaired cell immunity in diabetic patients[11]. Impaired neutrophil and macrophage function including chemotaxis, adherence, free radical induced destruction of microorganisms, phagocytosis, and respiratory burst have been well established in diabetics[10,11]. In DM, a decreased complement system C4 and humoral immunity linked with a reduced number and response of T cells enhances susceptibility to infection. Increased blood sugar and insulin resistance in type 2 diabetic patients weaken collective surfactant D-mediated host defenses of the lung. In diabetic individuals, loose junctions between epithelial cells in the airway enhance the transepithelial glucose gradient and an upsurge in the concentration of glucose in airway surface liquid, reducing the defense mechanism in the airway against infection[10]. Immunoglobulin glycation usually occurs in diabetic patients in proportion with the upsurge in hemoglobin (Hb)A1c, which may damage the antibody biological activity[11]. The risk of lung infection due to different microorganisms such as Streptococcus pneumonia, Staphylococcus aureus, Klebsiella pneumonia, Pseudomonas aeruginosa, influenza virus, fungus, i.e., Mucorales and Aspergillus species is increased in diabetic people, and such infections may increase morbidity and mortality in diabetics[10-12].
PREVALENCE OF SARS-COV-2 INFECTION IN DIABETICS
Singh et al[13] reviewed various research papers (13 articles from China, two from Italy and two from the United States) to understand the risk of COVID-19 in diabetics. Evolving data showed that DM was associated with 5.3%-58.0% of patients with COVID-19. The papers also showed that COVID-19 patients with DM were more frequently associated with severe or critical disease conditions varying from 14% to 32% in areas[13]. In the study by Abdi et al[14] who reviewed 18 papers, it was concluded that the cumulative prevalence of DM in COVID-19 individuals was 14.5%. They also reported that those with DM were more prone to developing severe COVID-19 and increased mortality[14]. Increased blood glucose level is considered an independent factor that can increase the severity of COVID-19 and mortality[15]. Faghir-Gangi et al[16], in their report, concluded that the pooled prevalence of DM in individuals with COVID-19 was 14%, based on the analysis of 23 articles. Another research group analyzed various studies up to April 2020 and reported 451.9 cases of DM/1000 patients with MERS-CoV, 90.38 cases/1000 patients with SARS-CoV-1, and 100.42 cases/1000 patients with SARS-CoV-2. The mortality rate was found to be 10%, 6% and 36% for SARS-CoV-2, SARS-CoV-1, and MERS-CoV, respectively. Research also pointed towards fewer studies on determining the exact situation concerning SARS-CoV-2 and DM[17]. Kumar et al[18] analyzed 33 articles and concluded that DM in individuals with COVID-19 was associated with a two-fold increase in mortality and severity of SARS-CoV-2 compared to non-diabetic people. A meta-analysis reported that the pooled prevalence of obesity and diabetes in SARS-Cov-2 infected patients was 29% and 22%, respectively. The study also indicated that the severity of COVID-19 in diabetic patients was greater and may require hospitalization compared to non-diabetic people[19]. Shang et al[20] analyzed 76 studies involving 31,067 COVID-19 patients and concluded that diabetics with COVID-19 had a more severe infection (21.4% vs 10.6%) and higher case-mortality rates (28.5% vs 13.3%) as compared to non-diabetics. COVID-19 patients with DM had a considerably higher risk of severe infection and mortality[20]. Chen et al[21] analyzed the impact of COVID-19 on blood glucose. They found that severe COVID-19 infection was linked with higher blood sugar, and the level of HbA1c was slightly higher in individuals with severe COVID-19 than in patients with mild COVID-19. Another meta-analysis of 65 observational studies that included 15794 participants found that the overall prevalence of diabetes was 12%, and in the case of severe COVID-19, the prevalence of DM was 18%[22]. Mantovani et al[23] also reported a similar phenomenon after analyzing 83 papers that included 78,874 hospitalized COVID-19 patients and summarized that the pooled prevalence of DM was 14.34%. They also reported that the prevalence of DM in COVID-19 patients was higher in non-Asian vs Asian countries and in the older age group (> 60 years). Preexisting DM showed an approximately 2-fold higher risk of having severe/critical COVID-19 illness and an approximately 3-fold enhanced risk of in-hospital mortality[23]. Current evidence indicates that people with DM are more prone to COVID-19 infection, and the risk of severity in diabetics is greater than that in non-diabetics.
LINK BETWEEN DM AND COVID-19
The spread and severity of SARS-CoV-2 infection among individuals can be linked to health status and exposure. Several reasons for this have been highlighted by researchers around the world and may be responsible for the extent and severity of COVID-19 during the last year. It is well established that the extent and severity of COVID-19 is linked to diabetic status. DM is considered one of the worse risk factors for COVID-19 and COVID-19 was found to increase the risk of mortality in diabetics. Some of the possible mechanisms explaining this situation are discussed here. Figure 1 summarizes the possible associations between DM and COVID-19.
Figure 1.
Possible association between diabetes mellitus and coronavirus disease 2019. ACE-2: Angiotensin converting enzyme 2; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Altered glucose metabolism
Elevated glucose level in diabetic patients is considered an independent risk factor in the early effect of COVID-19[24]. Elevated glucose level associated with severe pancreatic problems can lead to worsening of the pancreatic condition. It was reported by different investigators that there was a substantial difference between infection caused by SARS-CoV-2 in diabetic and non-diabetic individuals. A recent analysis showed that diabetic patients had a higher risk of 79% compared to non-diabetics[25,26]. In addition, fluctuations in biochemical parameters (i.e., alanine aminotransferase; alkaline phosphatase (ALP); blood urea nitrogen (BUN); C-reactive protein (CRP); lactate dehydrogenase (LDH); D-dimer) were also reported in a diabetic patient and were considered important factors associated with the high level of blood glucose in diabetic patients[27]. Poor glycemic control in diabetic patients enhances the risk of COVID-19 and death. Another study concluded that HbA1c (> 86 mmol/mol) may be considered a mortality factor in diabetic patients (type 1 DM and type 2 DM) with COVID-19[28-30]. A retrospective study also mentioned that due to hyperglycemic conditions, the mortality rate was high, and was found to be 41.7%. Poor glycemic control or hyperglycemia increased the rate of death of hospitalized COVID-19 patients[31]. Diabetic ketoacidosis or hyperglycemia has such an impact that it can quickly cause the death of patients with severe COVID-19. When patients stop taking glucose-lowering agents, there is a strong chance of SARS-CoV-2 binding to the ACE-2 receptors and spreading throughout different organs, effecting β-cell function and survival directly and causing deterioration of the metabolic syndrome[31,32].
It was found that increased blood glucose directly enhanced replication of SARS-CoV-2, and glycolysis sustains replication of SARS-CoV-2 through the generation of ROS in mitochondria and hypoxia-inducible factor 1α activation[7]. Natural killer (NK) cell activity was found to be reduced in those with impaired glucose tolerance, and DM might be one reason that makes diabetic people more susceptible to COVID-19[7].
Altered immune response
DM is responsible for the slow destruction of the immune response within the patient’s physiological system. DM-induced imbalance of the immune response can enhance the chance of dysregulation of immune modulators. Immunological dysregulation in diabetic patients is also considered a risk factor for SARS-CoV-2 infection and is also responsible for disease severity[7,33]. The diabetic condition causes a decrease in CD3+ T cell count, which may alter adaptive immunity and chronic inflammation. Low levels of lymphocyte and T-lymphocyte subtypes, including CD3, CD4, and CD8, were found in diabetics compared with non-diabetics. Thus, high blood sugar may affect lymphocytes and subset numbers in COVID-19[34]. Antigen presentation and immunity against different pathogens, including CoV via the production of interferon gamma (IFN-γ), is controlled by CD4+ T helper (Th1) cells. SARS-CoV-2 was found to destroy circulating immune cells and enhance apoptosis of CD3, CD4, and CD8 T-cells, which is responsible for lymphocytopenia[35]. DM reduces neutrophil phagocytosis, chemotaxis and intracellular destruction of microbes. Deficiencies in adaptive immunity categorized as an initial delay of Th1 cell-mediated immunity activation and a delayed hyperinflammatory response are frequently witnessed in people with DM[35]. Individuals with DM also have altered primary immune cell function. In addition, reduced activity of NK cells, alteration in the activity and number of neutrophils and macrophages, and abnormal differentiation of T-cells have been reported in diabetic patients[36].
Cytokine storm
The expression of ACE-2 is observed in different locations such as the upper respiratory tract and lungs (type I and II alveolar epithelial cells), pancreas, heart, endothelium, renal tubular and intestinal epithelium. A conformational change in S-glycoprotein on the surface of SARS-CoV-2 occurs due to its binding with ACE-2. This process triggers proteolytic digestion by proteases (TMPRSS2 and furin) of the host cell and ultimately causes virion internalization. The entry of SARS-CoV-2 induces an inflammatory reaction with T helper cells and interferon γ produced. This process results in the generation of other inflammatory cells and causes the 'cytokine storm'[13]. It was reported that COVID-19 patients admitted to the ICU had increased plasma levels of interleukin (IL)-2, IL-6, IL-7, IL-10, granulocyte colony stimulating factor (GCSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1A (MIP-1A), and tumor necrosis factor-α (TNF-α), indicating the cytokine storm and disease severity. The substantial rise in the level of IL-6 may be linked to mortality due to hyperinflammation in COVID-19 patients. Individuals with COVID-19 and hyperglycemia have increased cytokine release and immune responses[37,38]. Thus, SARS-CoV-2 infection is responsible for a rise in the levels of IL-1β, IL-4, and IL-10, IFN-γ, IP-10, and MCP-1[38]. It was shown that people with DM have an impaired adaptive immune response. Due to the deficiency of an immunostimulant, DM causes an increased pro-inflammatory cytokine response indicated by the enhanced release of IL-1, IL-6, IL-8 and TNF-α. An increased basal level of cytokine might also be attributed to AGEs. Diabetic individuals have a constant low-grade inflammation facilitating the occurrence of a cytokine storm, which in turn is directly related to the severity of COVID-19 pneumonia and to subsequent death[38].
Expression of ACE, protease and other specific proteins
In DM, enhanced ACE-2, furin, and IL-6 expression and reduced T-cell function are linked to the risk and severity of SARS-CoV-2 infection. If the expression of ACE-2 increases in pulmonary cells, heart muscle, kidney, and pancreas, it increases the binding of SARS-CoV-2. In an experimental diabetic animal model, enhanced expression of ACE-2 in those tissues was recorded[13,39]. It was also reported that insulin may downregulate ACE-2 expression, while other hypoglycemic drugs such as glucagon-like peptide-1 (GLP-1) agonists and pioglitazone, may enhance ACE-2 expression. The binding of SARS-CoV-2 to ACE-2 can damage islets resulting in acute diabetes[39].
The relationship between ACE-2 receptors and diabetes is contentious. Through the envelope spike glycoprotein (S-protein), SARS-CoV-2 enters the host cell via ACE-2, and tissue expressing ACE-2 becomes the prime target and the lung is the worst affected organ[40]. ACE-2 has been reported to be expressed in the pancreas, especially in β-cells that produce insulin and overexpression of ACE-2 prevents β-cell dysfunction. Deletion of ACE-2 promotes oxidative stress in mice, followed by renin-angiotensin system (RAS) dysfunction and decreased glucose tolerance and insulin expression[41]. Specific tropism of SARS-CoV-2 occurs on the ACE-2 receptor in COVID-19, the expression of ACE-2 increases with an increase in pro-inflammatory cytokines TNFα, IL-1β, and IFN-γ at the initial stage of infection in patients without a previous clinical history of DM[42]. On entering the host cells, SARS-CoV-2 invariably destroys β-cells that contain the ACE-2 receptors, which may be the reason behind new-onset diabetes in individuals without preexisting diabetes[43]. Although new-onset diabetes in COVID-19 patients does not persist, these patients should be monitored for a longer time to assess hyperglycemia, which was observed three years after SARS infection due to transient damaged β-cells. The SARS virus also shares the same receptor in humans as SARS-CoV-2 virus, i.e., ACE-2, and binding to this receptor is reported to damage pancreatic islets and results in acute diabetes as ACE-2 expression is found in the pancreas[44]. Destruction of ACE-2 also occurs in the lung when infected by SARS-CoV-2. Although SARS-CoV-2 enters the cell via ACE-2, type II alveolar cells that express ACE-2 are predominantly destroyed following entry of the virus. ACE-2 in the lungs is expressed on the airway epithelium apical surface, where type II alveolar cells are responsible for lung surfactant that protects the lung[45]. It was also reported that SARS-CoV-2 has an approximately 4-fold greater affinity for ACE-2 compared to SAR-CoV-1[46]. It can be seen from the above findings that the presence of ACE-2 is essential for proper functioning of the body, and is essential due to its homoeostatic role in the RAAS mechanism as well as in organs such as the kidneys, pancreas, and lungs. Nevertheless, to contain SARS-CoV-2 infection, inhibition of ACE-2 is vital. However, inhibition may impair various physiological mechanisms in the body.
Balance in the RAS system maintained by an ACE-1 and ACE-2 collaboration is important for local vasoconstrictor/proliferative (ACE-1/Ang-II/AT1-axis) and vasodilator/antiproliferative (ACE-2/Ang1-7/MA). Enhanced imbalance of ACE-1/ACE-2 may trigger RAS-driven injury that results in hyper-inflammation. Therefore, gene polymorphisms in ACE-1 and ACE-2 may alter their expression level that can cause enhanced capillary permeability, fibrosis, and apoptosis in lung cells, quickening lung damage and pulmonary shut-down triggered/worsened in COVID-19[46,47].
Furin, a type-1 membrane-bound protease, is involved in the cleavage of cell surface proteins that consequently release the spike fusion peptide; thus, the virus enters the cell through an endosomal pathway. Therefore, an increased level of furin increases the capability of the virus to enter the host cell. Diabetes linked with an increased level of furin might facilitate replication of the virus[13,35,39].
Successive cleavage of viral S-protein via transmembrane serine protease 2 (TMPRSS2) and furin can initiate viral entry to release viral genome into host cell ACE-2, A disintegrin and metalloproteinase 17 (ADAM17) and TMPRSS2/furin is located in the diabetic pancreatic β-cell membrane. A rise in glycosylated ACE-2, ADAM17 and TMPRSS2 expression in pancreatic islets and glycated SARS-CoV-2 S-protein in an individual with DM were observed. Thus, it is predicted that increased TMPRSS2 expression is linked to entry of the virus in humans[39,48]. The role of interferon-induced transmembrane proteins and ADAM17 was also investigated, and a possible association of their expression with SARS-CoV-2 infection and severity was suggested[39,48].
Vitamin D deficiency
It has been established that vitamin D works against viral disease and plays a crucial role in protecting organs from damage. In diabetics, there is a high chance of reduced vitamin D level. Vitamin D deficiency may enhance the risk of COVID-19. The severity of COVID-19 arises due to damage in different organs by SARS-CoV-2. In contrast, a high level of vitamin D has a preventive effect on viral infection in diabetics[49].
Comorbidity and multi-morbidity in diabetic patients
DM is responsible for various complications such as hypertension, hyperlipidemia, obesity, cardiovascular diseases etc. Diabetic patients with comorbidities are very prone to COVID-19. The severity and mortality of COVID-19 is very high in diabetic patients with comorbidities[28]. Earlier studies revealed that diabetic patients with COVID-19 are at high risk for cardiovascular diseases (20.9%), hypertension (56.9%), and cerebrovascular diseases (7.8%) compared to non-diabetics. Long-term diabetics are at risk of various other comorbidities such as nervous system diseases, chronic kidney diseases, and recent studies have indicated that SARS-CoV-2 damages the kidney via the ACE-2 pathways[50,51]. It was found that SARS-CoV-2 can cause damage to β-cells directly, and insulin resistance accompanied by hypokalemia, cytokine and fetuin-A levels can also deteriorate in diabetic patients with COVID-19. Cardiovascular disease, collective comorbidity towards endocrine disease comprising DM, is a noteworthy contributor to COVID-19 morbidity[51-53].
ANTIDIABETIC MEDICATION AND COVID-19
DM is one of the most common comorbidities in COVID-19. Clinical management and treatment of DM in patients with COVID-19 or in diabetic patients who are more susceptible to SARS-CoV-2 infection is complex as the selection of antidiabetic medication in such cases is important in light of current evidence. Some of the antidiabetic drugs that enhance the expression of ACE-2 act as a double-edged sword. When prescribing antidiabetic drugs to diabetic patients with COVID-19 or to patients with new-onset diabetes, one should be very careful as most of these drugs increase the level of ACE-2 in various organs, including the lungs and pancreas, which may further enhance the infection. Patients infected with SARS-CoV-2 may develop acute lung injury (ALI) in the severe stage leading to acute respiratory distress syndrome (ARDS)[54,55].
Metformin is reported to increase the expression of ACE-2[56] as well as play an important role in the microvascular repair mechanism through AMP-activated protein kinase (AMPK) activation during ALI[57]. A recent retrospective study reported that metformin, a safe and inexpensive drug, decreased mortality in type-2 diabetic patients, mainly women, admitted to hospital with COVID-19. Apart from reducing pro-inflammatory cytokines such as TNFα and IL-6, increasing anti-inflammatory cytokine IL-10, stabilizing mast cells, and improving endothelial function, modulation of ACE-2 was found to be a major mechanism of metformin in reducing the severity of SARS-CoV-2 infection[58]. However, metformin discontinuation has been advised in the event of COVID-19 in diabetic patients as it is reported to cause lactic acidosis[59]. SARS-CoV-2 infection activates the NLRP3 inflammasome, leading to the production of a number of pro-inflammatory cytokines such as IL-1β, IL-6, and TNFα[60]. Glyburide, a sulfonylurea, is reported to inhibit the activation of NLRP3 inflammation by blocking the ATP-sensitive K+ channels (KATP)[61]. However, it is suggested that sulfonylureas such as glipizide, glibenclamide, tolbutamide, gliclazide, chlorpropamide, etc., should be stopped during COVID-19 infection to prevent hypoglycemia[59]. Diabetic patients and patients with hypertension are treated with ACE inhibitors, and angiotensin II type I receptor blockers (ARBs), which increase the expression of ACE-2[62], similar to the thiazolidinedione class of drugs. TNF-α converting enzyme (TACE) cleaves ACE-2 into the soluble form sACE2, which circulates in the blood and is present in the extracellular spaces. As SARS-CoV-2 enters the cell via ACE-2, drug-induced modulation or increased expression of ACE-2 may allow entry of the virus, increase the viral load in diabetics and may lead to fatal consequences, which has been a concern among scientists. The formation of sACE2 may decrease the viral load in the body as it binds to SARS-CoV-2 but blocks its association with the host cell[63], thereby neutralizing it. ACE inhibitors, ARBs, and GLP-1 agonists are hypothesized to increase the level of sACE2 in the extracellular tissues, and may project sACE2 as a decoy receptor to neutralize the virus[64].
As reports of drugs augmenting the expression of ACE-2 in COVID-19 have received a mixed response around the globe, physicians have attempted to identify safer alternatives for diabetic patients with COVID-19. Of all the mentioned drugs, insulin has been reported as a safer alternative in critically ill or new-onset diabetic patients suffering from COVID-19 as it is reported to decrease the level of disintegrin, pro-inflammatory cytokines, and metalloprotease (ADAM-17), thereby inhibiting the cytokine storm[15]. However, the administration of insulin also has certain disadvantages. Patients need to undergo continuous glucose monitoring to avoid hypoglycemia, and this may pose a possible health risk for the healthcare provider as this practice may increase exposure to COVID-19 patients. However, self-monitoring of glucose by patients or infusion using an insulin pump may be performed[65]. ACE-2 is thought to be the main SARS-CoV-2 receptor for entry into host cells; however, it was later found that the MERS-CoV receptor dipeptidyl peptidase-4 (DPP4) is also a potential target receptor for the spike receptor-binding domain of SARS-CoV-2, and this discovery paves the way for various therapeutic manipulations[54]. Interestingly, the DPP4 class of drugs viz., linagliptin, sitagliptin, saxagliptin, vildagliptin, and alogliptin are potent hypoglycemic drugs and are reportedly used specifically in obese diabetics and patients with improper renal function for a longer duration[66,67]. In certain African people, polymorphism of the DPP4 protein was related to a reduced incidence of MERS-CoV infection, which further suggests that DPP4 plays a protective role during MERS-type infection. However, it was found in an in-vitro setup that vildagliptin, sitagliptin, or saxagliptin were ineffective in blocking entry of coronavirus into host cells[15]. In a recently reported study of 1531 patients with COVID-19, it was observed that DPP4-inhibitors produced neither harmful nor beneficial effects and did not support the discontinuation of this class of drugs[68]. Thus, it is premature to predict the effect of DPP4-inhibitors in COVID-19 patients with DM as most of the published reports were carried out using a small set of patients showing only clinical outcomes; however, molecular aspects should also be verified. Recently, human recombinant soluble ACE-2 (hrsACE2) has been proved to be a new and promising therapy for diabetic patients with severe COVID-19. When hrsACE2 (0.4 mg/kg) was administered for seven days to a 45-year-old woman with non-pharmacologically controlled type-2 diabetes, she recovered gradually without showing any adverse effects, which demonstrated the beneficial effects of hrsACE2 which neutralized the SARS-CoV-2 viral load and protected organs expressing ACE-2 receptors[69].
Pioglitazone, another antidiabetic drug, may upregulate ACE-2 expression and might be linked theoretically with possible augmented susceptibility to SARS-CoV-2 infection. Pioglitazone was found to exhibit anti-inflammatory and antifibrotic effects, and reduced the release of different pro-inflammatory cytokines in monocytes and macrophages. Thus, some researchers suggested that the drug could be continued in diabetic individuals with moderate COVID-19 as it may be helpful in preventing the cytokine storm[70,71]. The use of SGLT2 inhibitors in diabetic patients with COVID-19 was not beyond criticism as they were found to enhance the expression of ACE-2 in the kidney. A study also recommended avoiding SGLT2 inhibitors in such cases as they enhanced the risk of dehydration and euglycemic diabetic ketoacidosis. Although, an SGLT2 inhibitor, i.e., dapagliflozin, was found to reduce lactic acidosis during hypoxia and reverse acid-base balance inside the cells, preclinical studies indicated that SGLT2 inhibitors could be helpful in averting the cytokine storm[70,71]. Insulin is preferred in diabetic patients with serious COVID-19. Insulin was found to exhibit an important role in the anti-inflammatory effect and decrease inflammatory markers in critically ill patients[70].
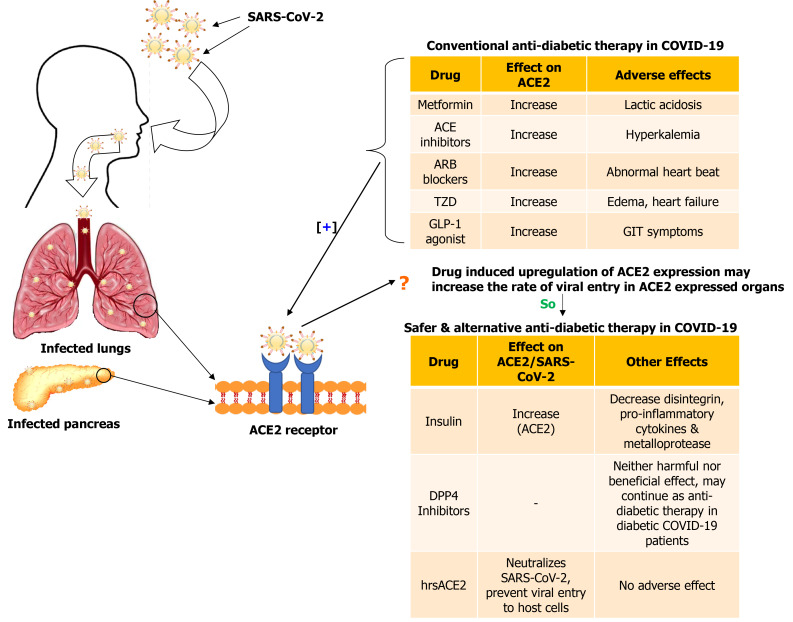
From the above-mentioned reports, it was observed that most antidiabetic drugs play a contentious role in diabetic patients with COVID-19 (Figure 2). Therefore, recommending an appropriate drug is not feasible. An in-depth retrospective study is warranted to determine the exact efficacy of these drugs against SARS-CoV-2 infection in diabetic patients.
Figure 2.
Effect of conventional and alternative drugs in diabetic patients with coronavirus disease 2019. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ACE: Angiotensin converting enzyme; ARB: Angiotensin II receptor blocker; TZD: Thiazolidinediones; GLP-1: Glucagon-like peptide 1; DPP4: Dipeptidyl peptidase 4; hrsACE2: Human recombinant soluble angiotensin converting enzyme 2.
CONCLUSION
COVID-19 is superimposing on the diabetes epidemic. Individuals with diabetes are more prone to SARS-CoV-2 infection, and severity of the disease is on the rise. Pathogenic links between DM and COVID-19 include altered glucose homeostasis, increased release of cytokinin that leads to the cytokine storm and enhanced oxidative stress. Alteration of the immune system, increased expression of ACE-2, and other enzymes such as furin are also crucial in diabetics, enhancing the risk of infection. Preexisting pathological pathways in hyperglycemic individuals elevate the risk of infectivity and are accountable for increased tissue injury and mortality. Antidiabetic drug-induced expression of ACE-2 may enhance the rate of viral entry. However, many of these drugs can also exert beneficial effects. Therefore, caution is necessary when prescribing such drugs. However, the exact underlying mechanism for the differential effect in individuals with and without diabetes requires further study.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Indian Science Congress Association, No. L24015; Indian Pharmaceutical Association, No. ASS/GUWA/LM/0050; Association of Pharmaceutical Teachers of India, No. AS/LM-025; National Academy of Biological Sciences, No. LM-028-2014.
Peer-review started: March 6, 2021
First decision: March 30, 2021
Article in press: July 29, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Şehirli AÖ, Wang QY S-Editor: Gao CC L-Editor: Webster JR P-Editor: Xing YX
Contributor Information
Saikat Sen, Faculty of Pharmaceutical Science, Assam down town University, Guwahati 781026, Assam, India. saikat.pharm@rediffmail.com.
Raja Chakraborty, Department of Pharmaceutical Technology, School of Medical Sciences, ADAMAS University, Kolkata 700 126, West Bengal, India.
Pratap Kalita, Department of Pharmacy, Pratiksha Institute of Pharmaceutical Sciences, Guwahati 781026, Assam, India.
Manash Pratim Pathak, Faculty of Pharmaceutical Science, Assam down town University, Guwahati 781026, Assam, India.
References
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar D, Malviya R, Sharma PK. Coronavirus: a review of COVID-19. EJMO. 2020;4:8–25. [Google Scholar]
- 5.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen S, Chakraborty R, De B, Devanna N. Trends in diabetes epidemiology in Indian population in spite of regional disparities: a systemic review. Int J Diabetes Dev Ctries. 2015;35:264–279. [Google Scholar]
- 7.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. IDF Diabetes Atlas, 9th ed. 2019. [cited 2 Feb 2021]. In: International Diabetes Federation [Internet]. Available from: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf .
- 9.Sen S, Chakraborty R, De B. Diabetes mellitus in 21st century. Springer, 2016. [Google Scholar]
- 10.Kolahian S, Leiss V, Nürnberg B. Diabetic lung disease: fact or fiction? Rev Endocr Metab Disord. 2019;20:303–319. doi: 10.1007/s11154-019-09516-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klekotka RB, Mizgała E, Król W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83:401–408. doi: 10.5603/PiAP.2015.0065. [DOI] [PubMed] [Google Scholar]
- 13.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdi A, Jalilian M, Sarbarzeh PA, Vlaisavljevic Z. Diabetes and COVID-19: A systematic review on the current evidences. Diabetes Res Clin Pract. 2020;166:108347. doi: 10.1016/j.diabres.2020.108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faghir-Gangi M, Moameri H, Abdolmohamadi N, Nematollahi S. The prevalence of type 2 diabetes in patients with COVID-19 : a systematic review and meta-analysis. Clin Diabetol. 2020;9:271–278. [Google Scholar]
- 17.Pinedo-Torres I, Flores-Fernández M, Yovera-Aldana M, Gutierrez-Ortiz C, Zegarra-Lizana P, Intimayta-Escalante C, Moran-Mariños C, Alva-Diaz C, Pacheco-Barrios K. Prevalence of Diabetes Mellitus and Its Associated Unfavorable Outcomes in Patients With Acute Respiratory Syndromes Due to Coronaviruses Infection: A Systematic Review and Meta-Analysis. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420962495. doi: 10.1177/1179551420962495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moazzami B, Chaichian S, Kasaeian A, Djalalinia S, Akhlaghdoust M, Eslami M, Broumand B. Metabolic risk factors and risk of Covid-19: A systematic review and meta-analysis. PLoS One. 2020;15:e0243600. doi: 10.1371/journal.pone.0243600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang L, Shao M, Guo Q, Shi J, Zhao Y, Xiaokereti J, Tang B. Diabetes Mellitus is Associated with Severe Infection and Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis. Arch Med Res. 2020;51:700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Wu C, Wang X, Yu J, Sun Z. The Impact of COVID-19 on Blood Glucose: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:574541. doi: 10.3389/fendo.2020.574541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrera FJ, Shekhar S, Wurth R, Moreno-Pena PJ, Ponce OJ, Hajdenberg M, Alvarez-Villalobos NA, Hall JE, Schiffrin EL, Eisenhofer G, Porter F, Brito JP, Bornstein SR, Stratakis CA, González-González JG, Rodíguez-Gutiérrez R, Hannah-Shmouni F. Prevalence of Diabetes and Hypertension and Their Associated Risks for Poor Outcomes in Covid-19 Patients. J Endocr Soc. 2020;4:bvaa102. doi: 10.1210/jendso/bvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Wang M, Wang Y, Li T, Zeng J, Wang L, Li C, Gong Y. Risk factors associated with the progression of COVID-19 in elderly diabetes patients. Diabetes Res Clin Pract. 2021;171:108550. doi: 10.1016/j.diabres.2020.108550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JK, Jin JM, Liu S, Bai P, He W, Wu F, Liu XF, Han DM. Blood glucose is a representative of the clustered indicators of multi-organ injury for predicting mortality of COVID-19 in Wuhan, China. SSRN. 2020:28. [Google Scholar]
- 27.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, Qiu K, Zhang J, Zeng T, Chen L, Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22:1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayman G, Lumb A, Kennon B, Cottrell C, Nagi D, Page E, Voigt D, Courtney H, Atkins H, Platts J, Higgins K, Dhatariya K, Patel M, Narendran P, Kar P, Newland-Jones P, Stewart R, Burr O, Thomas S. Guidance on the management of Diabetic Ketoacidosis in the exceptional circumstances of the COVID-19 pandemic. Diabet Med. 2020;37:1214–1216. doi: 10.1111/dme.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute Hyperglycemic Crises with Coronavirus Disease-19: Case Reports. Diabetes Metab J. 2020;44:349–353. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Yue L, Wang Z, Zhang J, Xiang G. Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J Diabetes Complications. 2021;35:107809. doi: 10.1016/j.jdiacomp.2020.107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng M, Wang X, Guo H, Fan Y, Song Z, Lu Z, Wang J, Zheng C, Dong L, Ma Y, Zhu Y, Fang H, Ye S. The Cytokine Profiles and Immune Response Are Increased in COVID-19 Patients with Type 2 Diabetes Mellitus. J Diabetes Res. 2021;2021:9526701. doi: 10.1155/2021/9526701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azar WS, Njeim R, Fares AH, Azar NS, Azar ST, El Sayed M, Eid AA. COVID-19 and diabetes mellitus: how one pandemic worsens the other. Rev Endocr Metab Disord. 2020;21:451–463. doi: 10.1007/s11154-020-09573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Farha M, Al-Mulla F, Thanaraj TA, Kavalakatt S, Ali H, Abdul Ghani M, Abubaker J. Impact of Diabetes in Patients Diagnosed With COVID-19. Front Immunol. 2020;11:576818. doi: 10.3389/fimmu.2020.576818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan T, Xiao R, Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 2020;34:6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roca-Ho H, Palau V, Gimeno J, Pascual J, Soler MJ, Riera M. Angiotensin-converting enzyme 2 influences pancreatic and renal function in diabetic mice. Lab Invest. 2020;100:1169–1183. doi: 10.1038/s41374-020-0440-5. [DOI] [PubMed] [Google Scholar]
- 42.Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P, Mathieu C, Eizirik DL, Sebastiani G, Dotta F. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) Is Expressed in Human Pancreatic β-Cells and in the Human Pancreas Microvasculature. Front Endocrinol (Lausanne) 2020;11:596898. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14:2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirastschijski U, Dembinski R, Maedler K. Lung Surfactant for Pulmonary Barrier Restoration in Patients With COVID-19 Pneumonia. Front Med (Lausanne) 2020;7:254. doi: 10.3389/fmed.2020.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177:4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int J Mol Sci. 2020;21 doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao YH, Zheng JQ, Zheng CM, Lu KC, Chao YC. Novel Molecular Evidence Related to COVID-19 in Patients with Diabetes Mellitus. J Clin Med. 2020;9 doi: 10.3390/jcm9123962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh SK, Jain R, Singh S. Vitamin D deficiency in patients with diabetes and COVID- 19 infection. Diabetes Metab Syndr. 2020;14:1033–1035. doi: 10.1016/j.dsx.2020.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Q, Zhang X, Jiang F, Hu N, Bimu C, Feng J, Yan S, Guan Y, Xu D, He G, Chen C, Xiong X, Liu L, Li H, Tao J, Peng Z, Wang W. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 51.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, Amadou C, Arnault G, Baudoux F, Bauduceau B, Borot S, Bourgeon-Ghittori M, Bourron O, Boutoille D, Cazenave-Roblot F, Chaumeil C, Cosson E, Coudol S, Darmon P, Disse E, Ducet-Boiffard A, Gaborit B, Joubert M, Kerlan V, Laviolle B, Marchand L, Meyer L, Potier L, Prevost G, Riveline JP, Robert R, Saulnier PJ, Sultan A, Thébaut JF, Thivolet C, Tramunt B, Vatier C, Roussel R, Gautier JF, Gourdy P CORONADO investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, Lu J. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. iScience. 2020;23:101160. doi: 10.1016/j.isci.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L, Huang Q, Wang DC, Ingbar DH, Wang X. Acute lung injury in patients with COVID-19 infection. Clin Transl Med. 2020;10:20–27. doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra A, Hepokoski M, McCowen KC, Y-J Shyy J. ACE2, Metformin, and COVID-19. iScience. 2020;23:101425. doi: 10.1016/j.isci.2020.101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J, McNeil C, Feng R, Guzman G, Abdelwahab N, King S, Tamariz L, Meehan T, Pendleton KM, Benson B, Vojta D, Tignanelli CJ. Metformin and risk of mortality in patients hospitalized with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NAS London. Sick day rules: how to manage Type 2 diabetes if you become unwell with coronavirus and what to do with your medication. [cited 5 May 2021]. In: NAS London [Internet]. Available from: https://www.england.nhs.uk/London/wp-content/uploads/sites/8/2020/04/3.-COVID-19-Type-2-Sick-Day-Rules-Crib-Sheet-06042020.pdf .
- 60.Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218 doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao L, Sakagami H, Miwa N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses. 2020;12 doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang J, Liu M, Ling W, Jin T. Friend or foe? Obes Med. 2021;22:100312. doi: 10.1016/j.obmed.2020.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta R, Hussain A, Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74:864–870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine. 2014;46:406–419. doi: 10.1007/s12020-014-0179-0. [DOI] [PubMed] [Google Scholar]
- 67.Röhrborn D, Wronkowitz N, Eckel J. DPP4 in Diabetes. Front Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kow CS, Hasan SS. A meta-analysis on the preadmission use of DPP-4 inhibitors and risk of a fatal or severe course of illness in patients with COVID-19. Therapie. 2020 doi: 10.1016/j.therap.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, Grieb A, Pawelka E, Laferl H, Wenisch C, Neuhold S, Haider D, Stiasny K, Bergthaler A, Puchhammer-Stoeckl E, Mirazimi A, Montserrat N, Zhang H, Slutsky AS, Penninger JM. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirabelli M, Chiefari E, Puccio L, Foti DP, Brunetti A. Potential Benefits and Harms of Novel Antidiabetic Drugs During COVID-19 Crisis. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun B, Huang S, Zhou J. Perspectives of Antidiabetic Drugs in Diabetes With Coronavirus Infections. Front Pharmacol. 2020;11:592439. doi: 10.3389/fphar.2020.592439. [DOI] [PMC free article] [PubMed] [Google Scholar]