Abstract
Background
The trauma of surgery is a neglected area of research. Our aim was to examine the differential expression of genes of stress, metabolism and inflammation in the major organs of a rat following a laparotomy.
Materials and methods
Anaesthetised Sprague-Dawley rats were randomised into baseline, 6-hr and 3-day groups (n = 6 each), catheterised and laparotomy performed. Animals were sacrificed at each timepoint and tissues collected for gene and protein analysis. Blood stress hormones, cytokines, endothelial injury markers and coagulation were measured.
Results
Stress hormone corticosterone significantly increased and was accompanied by significant increases in inflammatory cytokines, endothelial markers, increased neutrophils (6-hr), higher lactate (3-days), and coagulopathy. In brain, there were significant increases in M1 muscarinic (31-fold) and α-1A-adrenergic (39-fold) receptor expression. Cortical expression of metabolic genes increased ∼3-fold, and IL-1β by 6-fold at 3-days. Cardiac β-1-adrenergic receptor expression increased up to 8.4-fold, and M2 and M1 muscarinic receptors by 2 to 4-fold (6-hr). At 3-days, cardiac mitochondrial gene expression (Tfam, Mtco3) and inflammation (IL-1α, IL-4, IL-6, MIP-1α, MCP-1) were significantly elevated. Haemodynamics remained stable. In liver, there was a dramatic suppression of adrenergic and muscarinic receptor expression (up to 90%) and increased inflammation. Gut also underwent autonomic suppression with 140-fold increase in IL-1β expression (3-days).
Conclusions
A single laparotomy led to a surgical-induced proinflammatory phenotype involving neuroendocrine stress, cortical excitability, immune activation, metabolic changes and coagulopathy. The pervasive nature of systemic and tissue inflammation was noteworthy. There is an urgent need for new therapies to prevent hyper-inflammation and restore homeostasis following major surgery.
Keywords: Trauma, Surgery, Inflammation, Laparotomy, Stress response, Gene expression
Graphical abstract
Highlights
-
•
A single laparotomy activated a surgical-induced stress response.
-
•
This was accompanied by inflammation, immune activation, hypermetabolism and coagulopathy.
-
•
A standout result was the pervasive nature of systemic and tissue inflammation.
-
•
Our study highlights the dynamic nature of genetic changes underpinning the body's early response to major surgery.
-
•
There is an urgent need for new therapies to prevent surgical stress and secondary injury and restore homeostatic balance.
1. Introduction
Globally, a staggering 310 million major surgeries are performed each year, and it is estimated that 1–4% of patients will die in the first 30-days, up to 15% will have serious postoperative morbidity, and 5–15% will be readmitted within 30-days [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. A key unanswered question that remains is; how does the trauma of surgery itself contribute to postoperative mortality and morbidity? A century ago, neurosurgeon George Crile (1864–1943) was among the first to show that major surgery elicits a stress response [9]. Surgical stress is triggered by neural and molecular signals sent to the central nervous system (CNS) and body organs that begins with anaesthesia and the first incision [2,[9], [10], [11], [12], [13]]. Crile recognised that general anaesthesia by itself was insufficient to reduce the patient's stress response, from which he proposed the word anoci-association (harmful stimuli) to describe the ‘stressors’ of surgery [9].
Today, these ‘harmful stimuli’ include damage-associated molecular patterns (DAMPs), circulating cytokines, and multiple neural signals from injury afferents, that collectively can lead to stress-related neuroendocrine discharge, systemic inflammation, coagulopathy, immune activation, reduced tissue oxygenation, and increased susceptibility to infection [9,12]. Following on from Crile's astute observations, it is not widely appreciated that, despite anaesthesia, the brain and other organs of the body continue to receive these ‘harmful stimuli’ during and following the surgical procedure [9]. The aim of our study was to investigate the effect of surgical trauma on the differential expression of key neural, inflammatory, and metabolic genes and proteins in brain, heart, liver and gut following a laparotomy in rats. We hypothesised that a single transverse laparotomy would lead to a systemic surgical-induced inflammatory phenotype comprising neuroendocrine stress, acute coagulopathy and metabolic changes lasting at least 3-days.
2. Materials and Methods
2.1. Animals and ethics
Adult male Sprague-Dawley rats (406 ± 41g; n = 18) were bred and housed in individually ventilated cages in a non-specific pathogen-free facility, and acclimated in a 14-10hr light-dark cycle with free access to food and water ad libitum [14,15]. The rat was chosen over the mouse because the mouse can enter a metabolic-lowering state called torpor when subjected to traumatic stress [16], whereas rats and humans do not share this ability. The study was approved by the Institutional Animal Ethics Committee (IACUC:A2296/A2386) and US Animal Care and Review Use Office (ACURO), and complies with the Guide for Care and Use of Laboratory Animals (2011). Animals were assessed twice daily using the IACUC- and ACURO-approved monitoring sheet and humane endpoints of Morton [17], Toth [18], and Nemzek [19] as per previous studies [20]. The study was conducted and is reported according to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (Supplementary Content 3).
2.2. Surgical protocol
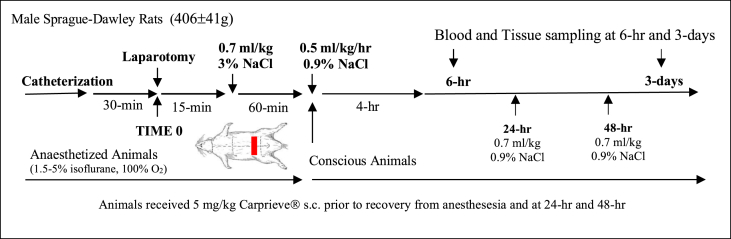
Animals were anaesthetised with isoflurane 1.5–5% (in 100% oxygen). Anaesthetised animals had sterile chronic catheters (Access Technologies, Skokie, IL, USA) implanted in the left femoral artery and vein for mean arterial pressure (MAP) monitoring and fluid infusions, respectively [20]. Lead-II electrocardiogram was subcutaneously implanted for heart rate (HR) monitoring (BioAMP coupled to Powerlab; ADInstruments). Catheterisations involved 1 cm incisions in the groin and back of neck for access [20]. Following 30-min stabilisation, a 3 cm transverse laparotomy was performed 2 cm below the xiphoid process [21] (Fig. 1). The liver was isolated and briefly eventrated and returned, and the muscle/fascia and skin closed in two layers with running braided suture. Any animal that exhibited arrhythmias, a sustained fall in MAP<80 mmHg, or abnormal blood parameters, prior to laparotomy was excluded. Nonsteroidal analgesic drug Carprieve® (5 mg/kg s.c.) was administered prior to recovery from anaesthesia, and again at 24-hr and 48-hr. Carprieve® was preferred over widely used buprenorphine because of the latter's emerging adverse effects in animal research [22].
Fig. 1.
Schematic of the study protocol. Animals were randomised into baseline, 6-hr and 3-day groups and a 3 cm transverse laparotomy was performed with liver manipulation. Blood was collected for stress hormones, cytokines, endothelial injury and coagulation. Tissues were freeze-clamped after 6-hrs and 3-days for gene expression and inflammatory mediators. An additional six animals were euthanized to collect baseline tissue samples and blood samples (see Materials and Methods).
2.3. Fluid therapy
Fifteen minutes after laparotomy, animals received a 0.7 ml/kg intravenous (IV) fluid bolus of 3% NaCl as per previous resuscitation studies (Fig. 1) [23]. The bolus equates to 0.28ml/26 ml total blood volume x 513 mM = 5.5 mM NaCl added to blood versus 1.54 mM if 0.9% NaCl bolus was used. There was no change in plasma Na+ or Cl− at 6-hr compared to baseline (unpublished data). After 60-min, animals were recovered from anaesthesia and received a 4-hr drip infusion of 0.5 ml/kg/h 0.9% NaCl. A further 0.7 ml/kg IV fluid bolus of 0.9% NaCl was administered at 24-hr and 48-hr (Fig. 1). We did not investigate limited versus higher volumes of fluid therapy [24].
2.4. Blood and tissue sampling
Arterial blood samples were collected at baseline, 6-hr and 3-days. Lactate was measured using ABL800 analyser (Radiometer, Mt Waverley, Australia) and hematology using VetScan HM5 (REM Systems, Macquarie Park, Australia). Animals were randomised using a random number generator to be sacrificed at 6-hr post-laparotomy (n = 6) or 3-days (n = 6) to collect blood and brain cortex, left ventricle, left lateral lobe, and small intestine samples. Euthanasia was performed in isoflurane-anaesthetised animals by thoracotomy and transection of the great vessels to induce rapid exsanguination. An additional n = 6 healthy male Sprague-Dawley rats were euthanized to collect baseline tissue and blood samples for coagulation measurements. Tissue samples were snap frozen in liquid nitrogen immediately upon collection to preserve RNA integrity and stored at −80 °C.
2.5. Coagulation
Coagulation was measured at sacrifice in both the 6-hr and 3-day animal groups. Prothrombin time (PT, sec), activated partial thromboplastin time (aPTT, sec), and fibrinogen (g/dL), were measured in citrated plasma on STA Compact (Diagnostica Stago, Doncaster, Australia). For rotational thromboelastometry (ROTEM®; Tem International, Munich, Germany), three 60-min assays were performed: EXTEM (extrinsically-activated test using tissue factor), INTEM (intrinsically-activated test using ellagic acid), and FIBTEM (fibrin-based EXTEM-activated test with 50 μg/ml cytochalasin D, to inhibit platelet contribution to clot formation) [25]. Platelet function was assessed in platelet-rich plasma (PRP) using ADP as an agonist (200 μM; Helena Laboratories, Mount Waverley, Australia) [26].
2.6. Inflammatory analysis
Milliplex® Rat Cytokine/Chemokine Magnetic Bead Panel (RECYTMAG-65K; Abacus, Meadowbrook, Australia) was used to measure plasma, heart, gut, and liver levels of inflammatory mediators (Supplementary Table 1). Plasma levels of cardiac troponin I were quantitated using Abcam Rat Cardiac Troponin I ELISA Kit (ab246529). Assays were performed according to manufacturer's instructions.
2.7. Vascular injury and hormone analysis
Plasma levels of soluble intercellular adhesion molecule (sICAM)-1, soluble E-selectin, von Willebrand Factor (vWF), adiponectin, adrenocorticotropic hormone (ACTH), and corticosterone were measured using Milliplex® Rat Vascular Injury Magnetic Bead Panel 2 (RV2MAG-26K; Abacus) and Rat Stress Hormone Magnetic Bead Panel (RSHMAG-69K; Abacus) with Magpix® (Supplementary Table 1).
2.8. Gene expression
Quantitative real-time reverse transcription PCR (qPCR) assays were performed to assess relative expression of key adrenergic/muscarinic receptors, inflammation and metabolic genes in heart, brain, gut, and liver at 6-hr and 3-days (Table 1). Total RNA was extracted using RNeasy Mini Kit (Qiagen) according to manufacturer's instructions. QuantiTect SYBR Green RT-PCR Kit was used in combination with QuantiTect Primer Assays (Supplementary Table 2) with 20 ng of total RNA as template. Relative gene expression was calculated using the concentration-Ct-standard curve method and normalised using the average expression of the ribosomal protein S13 (Rps13) gene for each sample (Rotor-Gene Q 2.0.24). Rps13 was chosen as the “housekeeping” gene since analyses showed its expression to be similar in all tissues assessed [20]. All reactions were independently performed in duplicate and the mean of the two raw values for each sample was used for analyses.
Table 1.
Genes measured in brain, heart, liver and gut 6-hr and 3-days following a laparotomy.
| Gene | Name | Function |
|---|---|---|
| Autonomic Regulation and Stress | ||
| Adrb1 | β-1 adrenergic receptor (β-1 AR) | In brain appears to play a role in synaptic plasticity and memory. Abundant in heart where it regulates HR and contractility during exercise, fight-or-flight and stress response. Increases liver lipid storage. Decreases intestinal motility. |
| Adra1a | α-1A adrenergic receptor (α-1A AR) | Abundant in brain and has a key role in neurotransmission, synaptic efficiency, plasticity, learning, memory and anxiety. Excitation is mediated by glutamate or Ach. Target for neurological disease. Less abundant in heart but highly cardioprotective. Potent vasoconstrictor. Involved in regulation of hepatocyte proliferation and liver regeneration after injury. Stimulates intestinal relaxation. |
| Chrm1 | muscarinic Ach receptor (mAChR) M1 | Abundant excitatory subtype in brain. High levels in cortical regions (layers III and V/VI). Involved in neuronal excitability, synaptic plasticity, learning and memory and development. Increases HR, contractility and vascular tone. Inhibits oxidative-stress sensors in hepatocytes after injury. Promotes intestinal contraction. |
| Chrm2 | mAChR M2 | Mostly inhibitory in brain (↓Ach). Increases cortical sensitivity. Nearly one-third of cortical GABAergic neurons express M2 receptors. Opposes cAMP effects. Predominate subtype in heart. Modulates HR, contractility, sympathetic heart activation, and vasodilation. Protects liver from injury. Promotes intestinal contraction. |
| Energy Metabolism and Mitochondrial Function | ||
| Ampk | 5′ AMP-activated protein kinase | Cellular energy homeostasis. Fuel gauge sensor of the ATP/ADP Pi ratio and master regulator of metabolism. |
| Ppargc1a | peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | Regulator and sensor of mitochondrial biogenesis and metabolism. Primary regulator of liver gluconeogenesis. PGC-1α can be acetylated by Sirtuin-1 to regulate cellular bioenergetics and inflammasome during stress. |
| (PGC-1α) | ||
| Mtco3 | cytochrome c oxidase subunit III (Cox 3) | Maintains structure and stability of Cox 3 which transfers terminal electrons from cytochrome c on inner mitochondrial membrane to reduce O2 to H2O during ATP formation. |
| Tfam | mitochondrial transcription factor A | Plays an essential role in maintenance of mtDNA and mitigates Ca2+ overloading and ROS production. Maintains the mtDNA copy for transcription and ATP production. TFAM is downstream of PGC-1α activation. |
| Nr3c1 | glucocorticoid receptor (GR) | GR receptor responds to stress hormones (cortisol, corticosterone) synthesized in adrenal gland. Catecholamines increase GR expression. GR is associated with regulation of up to 2000 genes for metabolism, growth, cognition, immunity and inflammation. |
| Inflammation Processes | ||
| Nlrp3 | NOD-, LRR- and pyrin domain-containing protein 3 | NLRP3 inflammasome upregulation mediates caspase-1, IL-1β & IL-18 activation in response to injury/infection (DAMPs/PAMPs). NF-κB upregulates its expression. |
| Il1b | interleukin 1-beta | Driver of the inflammatory and immune response via inflammasome activation and other pathways. |
| (IL-1β) | ||
| Sdc1 | syndecan-1 | Cell surface proteoglycan that interacts with extracellular matrix. Syndecans play a role in inflammation, mainly by regulating leukocyte infiltration, cytokine function and progression. Receptor expression and shedding of syndecan-1 can change during inflammation, injury or infection. |
AR = adrenergic receptor; HR = heart rate; Ach = acetylcholine; GABA = gamma-aminobutyric acid; AMP = adenosine monophosphate; ATP = adenosine triphosphate; ADP = adenosine diphosphate; Pi = inorganic phosphate; mtDNA = mitochondrial DNA; Ca2+ = calcium; ROS = reactive oxygen species; DAMPs = damage-associated molecular patterns; PAMPs = pattern-associated molecular pattern molecules; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
2.9. Statistical analysis
A priori power analysis was conducted using G-power3 program to determine sample size with effect size for outcome measure cardiac Ampk expression from previous study [20] (Cohen's d = 2.28; α = 0.05; N2/N1 = 1; n = 5; Power (1-β) = 0.88). SPSS Statistical Package 27 was used for all analysis. Data normality was assessed graphically and numerically with Shapiro-Wilks test. Normally distributed data was analysed using one-way analysis of variance (ANOVA) with Tukey or Dunnett's post-hoc test, while non-parametric data was analysed using Kruskal-Wallis test. 5-Parameter-logistic curve fitting was applied to inflammatory, vascular injury and stress assays data using MILLIPLEX Analyst 5.1 software. Gene expression data are expressed as median and interquartile range and graphically presented as box-and-whisker plots with maximum and minimum data points (whiskers) for relative expression. All other data is expressed as mean(standard deviation). Statistical significance was defined as p < 0.05.
3. Results
3.1. Survival and haemodynamics
All animals and were included for analysis. MAP and HR were comparable at baseline (111(11)mmHg and 363(23)bpm) and at 6-hr (118(12)mmHg and 380(25)bpm). Similarly, no significant differences were observed between baseline and 3-days (117(10)mmHg and 342(47)bpm).
3.2. Hematology and blood lactate in the three-day group
Total leukocyte numbers remained unchanged over 3-days (Table 2). However, lymphocytes decreased by ∼70% at both timepoints, and neutrophils increased significantly. Platelet numbers decreased by 34% at 6-hr, and 50% by 3-days, while haemoglobin and haematocrit decreased 10% at 6-hr and ∼30% at 3-days (Table 2). Blood lactate was significantly 1.6-fold higher at 3-days.
Table 2.
Haematology, lactate and blood chemistry after surgical trauma in the 3-day animal group.
| Parameter | Baseline | 6-hr | 3-days |
|---|---|---|---|
| White Blood Cells (x109/L) | 8.96(2.09) | 9.18(2.43) | 7.45(3.71) |
| Lymphocytes (x109/L) | 6.13(1.57) | 1.92(0.47)¶ | 2.23(1.35)¶ |
| Monocytes (x109/L) | 0.48(0.22) | 0.71(0.30) | 0.38(0.21) |
| Neutrophils (x109/L) | 2.35(1.39) | 6.55(1.73)# | 4.84(2.29) |
| Lymphocytes (%) | 69(10) | 21(2)¶ | 31(9)¶ |
| Monocytes (%) | 6(3) | 7(2) | 5(11) |
| Neutrophils (%) | 25(12) | 72(3)¶ | 64(10)¶ |
| Red Blood Cells (x1012/L) | 8.12(0.46) | 7.55(0.53) | 5.34(1.12)* |
| Haemoglobin (g/dL) | 15.2(0.6) | 13.9(1.2) | 9.6(1.7)* |
| Haematocrit (%) | 44.9(2.8) | 40.6(3.0) | 30.5(6.3)* |
| Platelets (x109/L) | 322(58) | 211(81) | 164(57)* |
| Lactate (mM) | 1.2(0.2) | 1.0(0.3) | 1.9(0.4)* |
| Potassium (mM) | 4.67(0.42) | 4.27(0.33) | 4.17(0.25) |
| Calcium (mM) | 1.35(0.02) | 1.20(0.13)† | 1.25(0.05) |
Data represent mean(standard deviation). n = 6 for all measurements. *p < 0.05 compared to Baseline and 6-hr; ¶p < 0.001 compared to Baseline; #p < 0.01 compared to Baseline; †p < 0.05 compared to Baseline.
3.3. Plasma stress, inflammatory and adhesion injury markers
Plasma corticosterone, IL-1β, IL-10, MIP-1α, sICAM-1, sE-selectin and vWF all increased significantly over 3-days (Table 3). Circulating IL-1α, IL-2, IL-6, MCP-1 and IFN-γ were also elevated but did not reach statistical significance. Adiponectin increased at 6-hrs (2.5-fold; p < 0.05); RANTES was high at baseline then decreased; and plasma troponin remained below detectable limits (Table 3).
Table 3.
Stress, inflammatory, and vascular injury markers in plasma before and after surgical trauma in the 3-day animal group.
| Marker | Baseline | 6-hr | 3-days |
|---|---|---|---|
| Corticosterone (μg/ml) | 72(23) | 887(1440)† | 1753(1624)¶ |
| ACTH (ng/ml) | 38(37) | 2(5)# | 5(12)† |
| IL-1α (pg/ml) | ND | ND | 58(142) |
| IL-1β (pg/ml) | ND | ND | 202(328)* |
| IL-2 (pg/ml) | ND | ND | 396(970) |
| IL-6 (ng/ml) | ND | ND | 13(31) |
| IL-10 (pg/ml) | ND | 483(284) | 1149(976)# |
| MIP-1α (pg/ml) | ND | 28(49) | 144(188)† |
| MCP-1 (pg/ml) | ND | 444(870) | 1048(1129) |
| IFN-γ (pg/ml) | ND | ND | 183(448) |
| RANTES (pg/ml) | 768(87) | 313(93)¶ | 514(364) |
| sICAM-1 (ng/ml) | 5(1) | 34(9)† | 53(27)¶ |
| sE-Selectin (ng/ml) | 44(14) | 160(30)¶ | 174(38)¶ |
| vWF (ng/ml) | 815(429) | 1573(325)# | 1453(154)# |
| Adiponectin (ng/ml) | 761(246) | 1872(576)‡ | 749(290) |
| Troponin I | ND | – | ND |
Data represent mean(standard deviation). n = 6 for all measurements. ACTH = Adrenocorticotropic hormone; IL=Interleukin; MIP = Macrophage Inflammatory Protein; MCP = Monocyte Chemoattractant Protein; IFN=Interferon; RANTES = Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; sICAM-1 = Soluble Intercellular Adhesion Molecule-1; vWF = von Willebrand Factor; ND=Not Detected. IL-4, IL-12p70, IL-13, and TNF-α were not detected in any samples. Troponin I was not measured at 6-hr. *p < 0.025 compared to Baseline and 6-hr; ¶p < 0.001 compared to Baseline; #p < 0.01 compared to Baseline; †p < 0.05 compared to Baseline; ‡p < 0.001 compared to Baseline and 3-days.
3.4. Tissue inflammatory protein mediators in heart, liver and gut
At 3-days postoperatively, cardiac IL-1α, IL-4, MIP-1α, and MCP-1 increased significantly, while IL-1β, IL-4, and IL-10 decreased, with no change in IL-2, IL-13, TNF-α, IL-12p70 or RANTES (Table 4). In liver, interleukins -1α, -β, and −4, MIP-1α, sICAM-1, sE-selectin, and vWF significantly increased, whereas IL-2, IL-12p70, IL-13, IFN-γ and RANTES fell. In gut, IL-1α and IL-β were 20-fold higher, IL-10 and MIP-1α were 3-fold higher, and MCP-1 was 5-fold higher at 3-days compared to baseline (all p < 0.05; Table 4).
Table 4.
Cytokine and chemokine levels in heart, liver and gut 3-days after surgical trauma.
| Marker (pg/ml) | HEART |
LIVER |
GUT |
|||
|---|---|---|---|---|---|---|
| Baseline | 3-days | Baseline | 3-days | Baseline | 3-days | |
| IL-1α | 11(7) | 27(9)# | 164(39) | 450(229)† | 50(12) | 91(34)† |
| IL-1β | 135(18) | 88(28)# | 222(12) | 1310(676)# | 23(10) | 451(191)* |
| IL-2 | 14(2) | 15(5) | 28(3) | 18(3)# | 4(9) | 4(3) |
| IL-4 | 425(158) | 197(83)† | 100(14) | 174(73)# | 15(12) | 22(4) |
| IL-6 | 265(67) | 1493(3034) | 525(51) | 947(801) | 18(14) | 283(456) |
| IL-10 | 147(24) | 91(39)† | 169(10) | 215(61) | 5(4) | 31(11)* |
| IL-12p70 | 19(3) | 19(7) | 14(1) | 5(1)* | 2(5) | 1(2) |
| IL-13 | 10(4) | 11(7) | 13(3) | 6(1)* | 2(3) | 2(3) |
| MIP-1α | 2(1) | 13(14)# | 13(1) | 66(38)# | 8(3) | 25(7)* |
| MCP-1 | 86(12) | 428(605)† | 104(12) | 117(33) | 88(20) | 437(278)† |
| TNF-α | 8(5) | 6(2) | ND | 2(3) | ND | ND |
| IFN-γ | ND | ND | 329(272) | ND† | ND | ND |
| RANTES | 35(17) | 44(19) | 475(96) | 147(109)* | 1379(2369) | 731(478) |
Data represent mean(standard deviation). n = 6 for all measurements. ND=Not Detected; IL=Interleukin; MIP = Macrophage Inflammatory Protein; MCP = Monocyte Chemoattractant Protein; TNF = Tumour Necrosis Factor; IFN=Interferon; RANTES = Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted. *p < 0.05 compared to Baseline; #p < 0.01 compared to Baseline; †p < 0.001 compared to Baseline.
3.5. Coagulation
EXTEM clot times (CT) and kinetics did not change over 3-days (Table 5). EXTEM amplitude (A10) and maximum clot firmness (MCF) decreased significantly ∼20% at 6-hr, with no change in maximum lysis (ML). In contrast, A10 and MCF returned to baseline at 3-days but ML decreased significantly by 97%. No hyperfibrinolysis was detected (Table 5). INTEM CT increased up to 10-fold, and α-angle fell by ∼50% at both timepoints. Clot formation time (CFT) increased 26-fold at 6-hr and 10-fold at 3-days. Clot firmness (A10, MCF) significantly decreased by 75–88% at 6-hr, and 45–50% at 3-days, with no hyperfibrinolysis. FIBTEM CT increased 2.6-fold with an accompanying fall in α-angle and CFT at 6-hr, but not 3-days. A10 and MCF increased significantly at 3-days, with no evidence of hyperfibrinolysis (Table 5). FIBTEM A10/EXTEM A10 ratio, which provides a measure of the fibrinogen contribution to clot amplitude [25,27], was 24% at baseline, 38% after 6-hr, and 64% after 3-days. The ratio of platelet count/plasma fibrinogen also decreased 63% after 6-hr and 82% at 3-days relative to baseline. Consistent with FIBTEM clot amplitude, plasma fibrinogen levels increased significantly 1.7-fold at 6-hr and 2.9-fold at 3-days. There were no significant changes in platelet aggregation (Table 5).
Table 5.
Coagulation profile and platelet function in 6-hr and 3-days surgical trauma groups.
| Test | Parameter | Baseline | 6-hr | 3-days |
|---|---|---|---|---|
| EXTEM | CT (sec) | 42(3) | 46(19) | 45(19) |
| CFT (sec) | 32(3) | 42(22) | 23(4) | |
| α (°) | 84(1) | 83(4) | 85(1) | |
| A10 (mm) | 74(2) | 58(22)* | 73(12) | |
| MCF (mm) | 77(1) | 63(23)* | 78(11) | |
| LI30 (%) | 100(0) | 100(0) | 100(0) | |
| ML (%) | 6.5(1.9) | 5.3(8.8) | 0.2(0.4)# | |
| INTEM | CT (sec) | 100(28) | 1332(1205) | 1007(1316) |
| CFT (sec) | 34(8) | 872 (n = 1) | 328(155)¶ (n = 3) | |
| α (°) | 84(2) | 47(40)† | 43(14)† | |
| A10 (mm) | 73(2) | 9(8)‡ | 36(14)¶ | |
| MCF (mm) | 77(1) | 19(1)¶ | 42(27)† | |
| LI30 (%) | 100(0) | 100(0) | 100(0) | |
| ML (%) | 5(1) | 25(50) | 20(39) | |
| FIBTEM | CT (sec) | 37(3) | 98(139) | 31(17) |
| CFT (sec) | 1545(1990) | 937(800) | 24(13) | |
| α (°) | 81(1) | 78(6)* | 86(1) | |
| A10 (mm) | 18(2) | 22(15) | 47(4)^ | |
| MCF (mm) | 18(2) | 25(16) | 51(5)^ | |
| LI30 (%) | 99(2) | 100(0) | 100(0) | |
| ML (%) | 2(3) | 1(3) | 0(0) | |
| Plasma Coagulation | PT (sec) | 17.9(1.6) | 12.7(2.4)¶ | 14.9(1.1) |
| aPTT (sec) | 85(33) | 64(77) | 88(67) | |
| Fibrinogen (g/dL) | 2.35(0.33) | 4.10(1.01)# | 6.77(0.61)^ | |
| ADP-Platelet Function (%) | Aggregation | 91(26) | 55(52) | 82(59) |
| Disaggregation | 5(6) | 5(12) | 0.5(1.2) |
Data represent mean(standard deviation). n = 6 for all measurements unless otherwise indicated. Blood sampling for coagulation testing was performed at time of sacrifice. CT=Clot Time; CFT=Clot Formation Time; α = Alpha Angle; A10 = Clot Amplitude 10min after clot initiation; MCF = Maximum Clot Firmness; LI30 = Lysis Index at 30min; ML = Maximum Lysis; PT=Prothrombin Time; aPTT = Activated Partial Thromboplastin Time. *p < 0.05 compared to 3-days; ¶p < 0.001 compared to Baseline; #p < 0.01 compared to Baseline; †p < 0.05 compared to Baseline; ‡p < 0.01 compared to Baseline and 3-days; ^p < 0.05 compared to Baseline and 6-hr.
3.6. Differential gene expression at 6-hr and 3-days
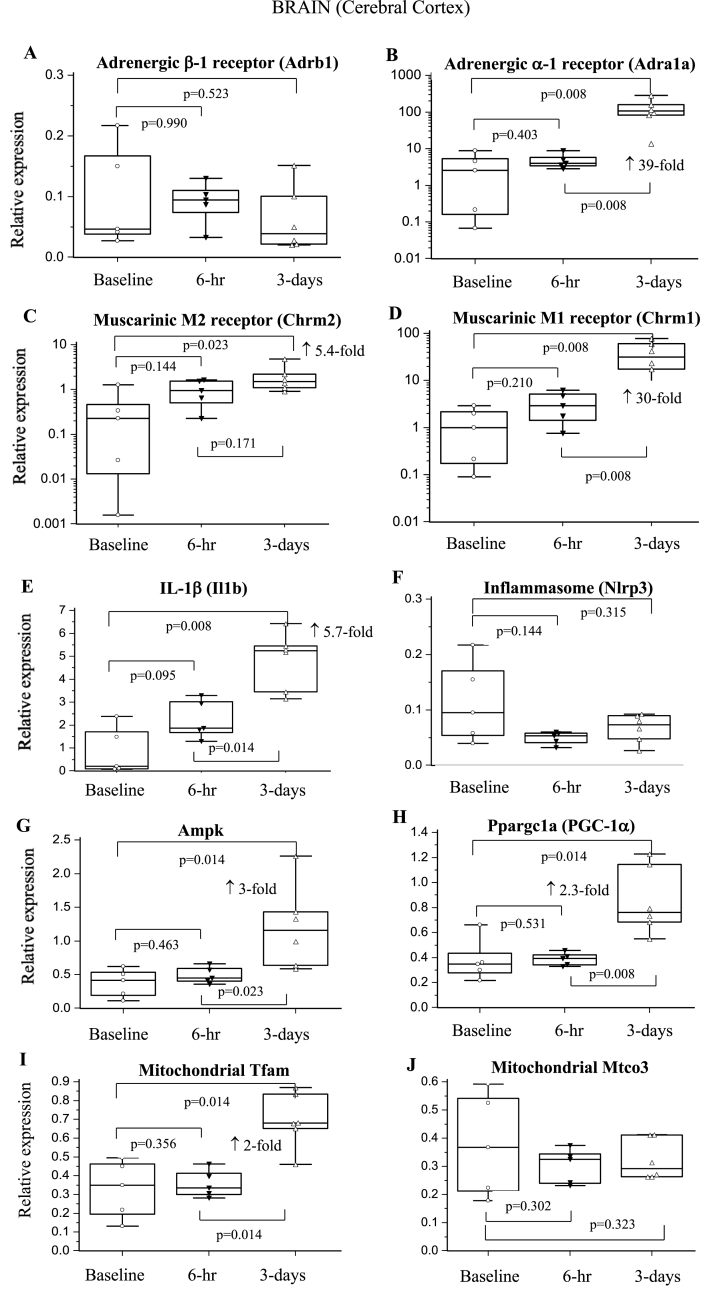
3.6.1. Brain cortex
No change occurred in β-1-adrenergic receptor (AR) expression over 3-days, whereas α-1-AR expression increased significantly 39-fold (Fig. 2AB). M1 and M2 muscarinic receptor expression increased significantly at 3-days (30-fold and 5.4-fold, respectively) (Fig. 2CD). Metabolic and mitochondrial genes showed delayed increases after surgery. Ampk, PGC-1α, and Tfam increased 2–3 times at 3-days (p = 0.014 vs baseline), with no change in Mtco3 (Fig. 2G–J). Nlrp3 did not change over 3-days, whereas Il1b increased 2.6- and 5.7-fold at 6-hr and 3-days, respectively (Fig. 2EF).
Fig. 2.
Relative expression of genes of the sympathetic and parasympathetic nervous system, inflammation and metabolism in brain cortex at 6-hrs and 3-days after a laparotomy. A: Adrb1; B: Adra1a; C: Chrm2; D: Chrm1; E: Il1b; F: Nlrp3; G: Ampk; H: Ppargc1a; I: Tfam; J: Mtco3. Data are expressed as median and interquartile range and graphically presented as box-and-whisker plots with maximum and minimum data points (whiskers) for relative expression. n = 6 for all measurements. The functions of the different genes are found in Table 1.
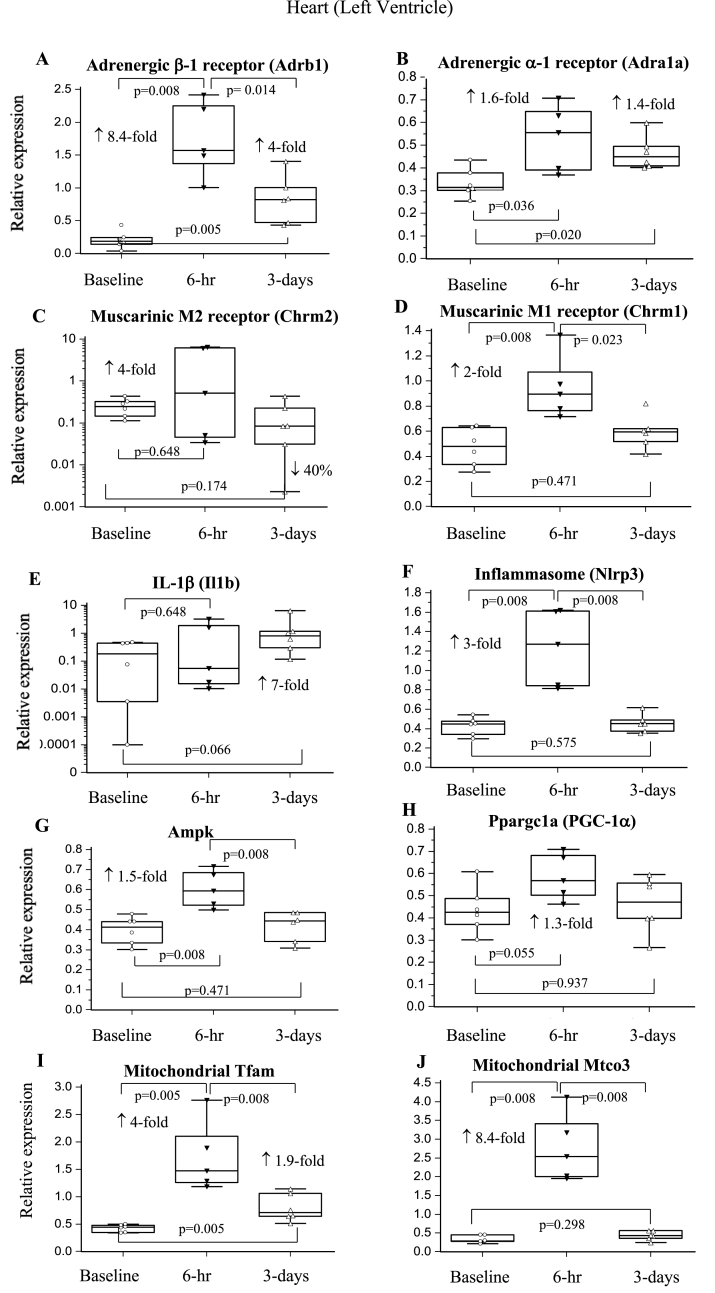
3.6.2. Heart
β-1-AR expression increased significantly 8.4-fold 6-hr postoperatively, and remained 4-fold higher than baseline at 3-days (Fig. 3A). α-1-AR increased 1.4- to 1.6-fold over the same time period (Fig. 3AB). M1 receptor expression increased 2-fold at 6-hr (p = 0.008) before returning to baseline (Fig. 3D). Similarly, metabolic and mitochondrial genes significantly increased at 6-hr then returned to baseline at 3-days (Fig. 3G–J). A comparable pattern was seen for glucocorticoid receptor (GR; Nr3c1) and syndecan-1 which increased 5-fold (p = 0.008) and 3.3-fold (p = 0.008) at 6-hr, respectively, and returned to baseline at 3-days (not graphically shown). Nlrp3 inflammasome expression increased significantly 6-hr after surgery before decreasing, while Il1b remained 7-fold higher than baseline at 3-days (p = 0.066; Fig. 3EF).
Fig. 3.
Relative expression of genes of the sympathetic and parasympathetic nervous system, inflammation and metabolism in heart left ventricle at 6-hrs and 3-days after a laparotomy. A: Adrb1; B: Adra1a; C: Chrm2; D: Chrm1; E: Il1b; F: Nlrp3; G: Ampk; H: Ppargc1a; I: Tfam; J: Mtco3. Data are expressed as median and interquartile range and graphically presented as box-and-whisker plots with maximum and minimum data points (whiskers) for relative expression. n = 6 for all measurements. The functions of the different genes are found in Table 1.
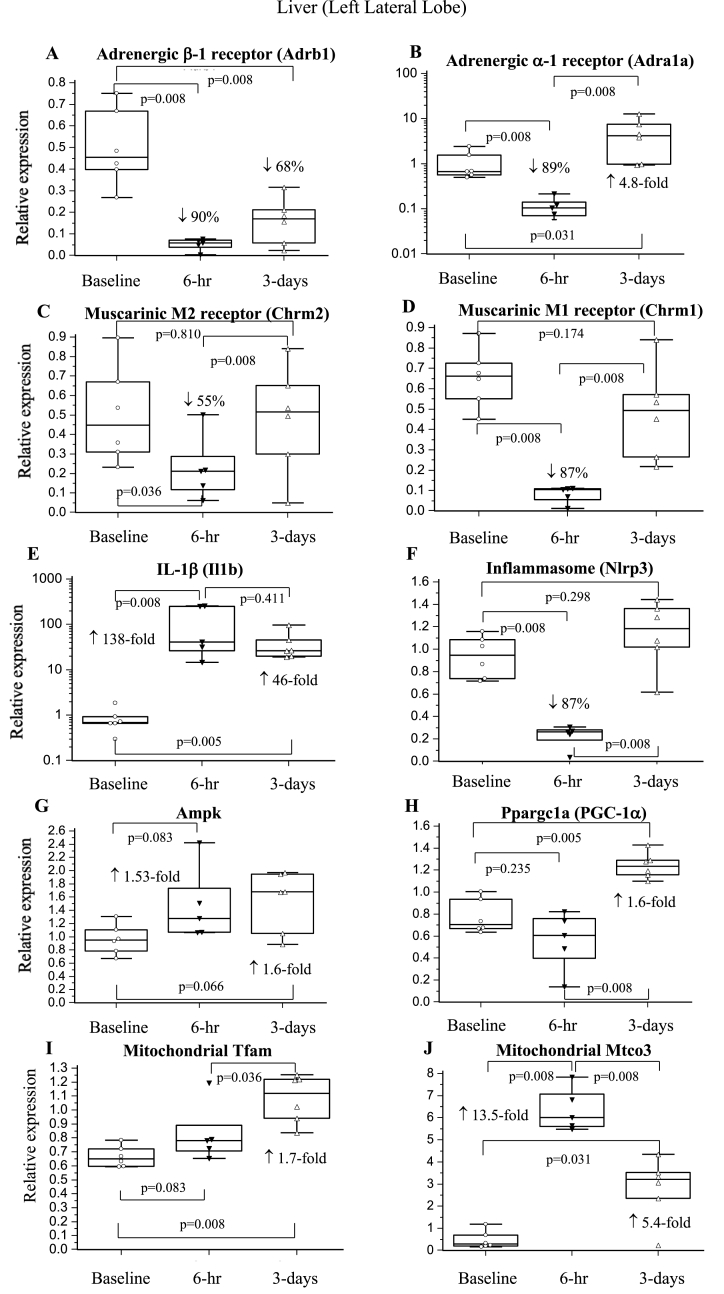
3.6.3. Liver
Expression of both β-1-AR and α-1-AR fell significantly by ∼90% at 6-hr (Fig. 4AB). β-1-AR remained low at 3-days whereas α-1-AR increased above both baseline and 6-hr levels. M2 and M1 receptor expression decreased by 55% and 87% at 6-hr and returned to baseline by 3-days (Fig. 4CD). GR expression increased 2.5-fold at 6-hr (p = 0.008) and remained elevated at 3-days (p = 0.005). Mtco3 increased early at 6-hr (p = 0.008) while 1.6- to 1.7-fold increases in Ampk, PGC-1α, and Tfam occurred later at 3-days (Fig. 4G–J). Syndecan-1 fell 71% at 6-hr (p = 0.008) and returned to baseline by 3-days (p = 0.379). Similarly, Nlrp3 inflammasome expression decreased by 87% at 6-hr before increasing to baseline at 3-days (Fig. 4F). In contrast, Il1b increased 138-fold 6-hr after surgery and remained significantly elevated at 3-days (Fig. 4E).
Fig. 4.
Relative expression of genes of the sympathetic and parasympathetic nervous system, inflammation and metabolism in liver at 6-hrs and 3-days after a laparotomy. A: Adrb1; B: Adra1a; C: Chrm2; D: Chrm1; E: Il1b; F: Nlrp3; G: Ampk; H: Ppargc1a; I: Tfam; J: Mtco3. Data are expressed as median and interquartile range and graphically presented as box-and-whisker plots with maximum and minimum data points (whiskers) for relative expression. n = 6 for all measurements. The functions of the different genes are found in Table 1.
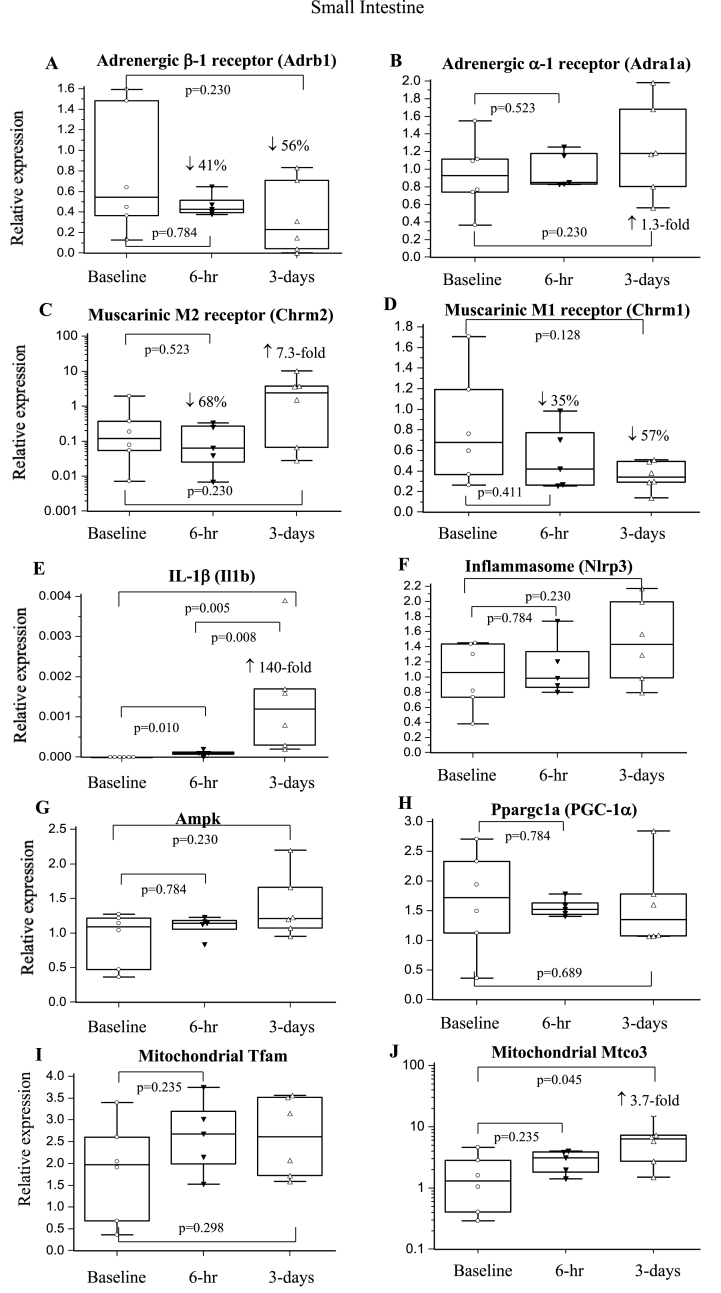
3.6.4. Small Intestine
β-1-AR expression decreased by 41% and 56% at 6-hr and 3-days, respectively, with no changes in α-1-AR expression (Fig. 5AB). M1 expression fell 35–57%, and M2 also fell 68% at 6-hr before increasing 7.4-fold at 3-days (p > 0.05). Mtco3 increased significantly 3.7-fold at 3-days, whereas Ampk, PGC-1α, Tfam, and Syndecan-1 remained unchanged (Fig. 5G–J). Il1b increased at 6-hr (p = 0.010) with a further significant 140-fold increase at 3-days, despite no change in Nlrp3 (Fig. 5EF).
Fig. 5.
Relative expression of genes of the sympathetic and parasympathetic nervous system, inflammation and metabolism in the small intestine at 6-hrs and 3-days after a laparotomy. A: Adrb1; B: Adra1a; C: Chrm2; D: Chrm1; E: Il1b; F: Nlrp3; G: Ampk; H: Ppargc1a; I: Tfam; J: Mtco3. Data are expressed as median and interquartile range and graphically presented as box-and-whisker plots with maximum and minimum data points (whiskers) for relative expression. n = 6 for all measurements. The functions of the different genes are found in Table 1.
4. Discussion
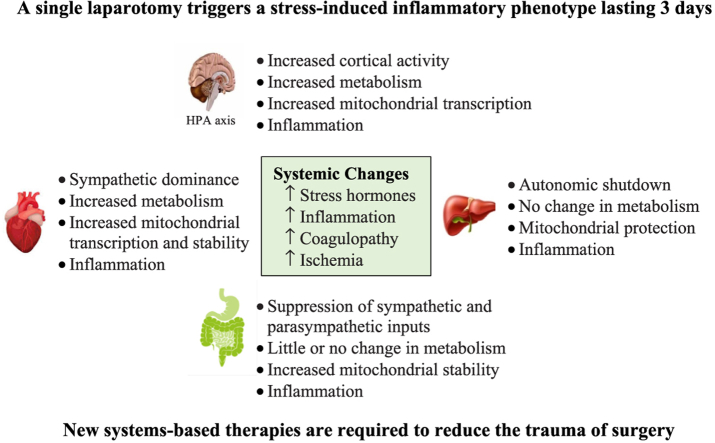
The surgical stress response consists of neuroendocrine, inflammatory, metabolic and immune components [9,12]. Research into tissue-specific gene expression and function of the major organs underpinning the stress response following major surgery is scant. We report that a single laparotomy led to a trauma-induced whole-body inflammatory phenotype that included: 1) an increase in brain cortical excitability, 2) sympathetic dominance of the heart with increased metabolism, 3) an apparent autonomic shutdown of liver, 4) suppression of gut sympathetic and parasympathetic receptor expression with little or no change in metabolism, and 5) a ‘systemic’ switch to a proinflammatory state in all organs. The surgical stress-related inflammatory phenotype will now be discussed.
4.1. Surgical stress response, systemic inflammation, immune activation and coagulopathy
Surgical stress was confirmed by a significant 24-fold increase in stresshormone corticosterone over 3-days (Table 3). Corticosterone in rats, like cortisol in humans, is produced by the adrenal cortex after activation by ACTH via the hypothalamic-pituitary-adrenal (HPA) axis [[10], [11], [12],28,29]. Corticosterone increases the turnover of glucose, free fatty acids and amino acids to support the early ‘stressed’ state, and promotes the early release of neutrophils from bone marrow into the circulation (Table 2) [12,30]. Interestingly, ACTH release was not sustained (Table 3), suggesting that mechanisms controlling corticosterone levels may be different or sensitised differently in the early postoperative period [13,28]. Glucose homeostasis may have been further supported by circulating adiponectin, which significantly increased 6-hr after surgery (Table 2). Adiponectin enhances insulin sensitivity, increases glucose utilisation, decreases liver gluconeogenesis, increases fatty acid oxidation, and protects against ischaemia [31]. Blood lactate, however, also increased indicating that aerobic glucose metabolism was insufficient and required a ‘boost’ from anaerobic pathways to replenish ATP (Table 2). Unfortunately, we did not measure tissue lactate levels.
Surgical stress was accompanied by systemic inflammation, immune activation and acute coagulopathy, with no change in platelet aggregation (Table 2, Table 3, Table 4, Table 5). Systemic inflammation was indicated by significant increases in cytokines and chemokines, and endothelial injury markers sICAM-1, sE-selectin and vWF (Table 3). Circulating neutrophils were also significantly increased 6-hr postoperatively with partial resolution by 3-days (Table 2). The partial resolution may have been related to increased IL-10 (Table 3), which reduces neutrophil recruitment and deactivates monocytes/macrophages [21]. Surgery was also associated with a complex coagulopathy. Clot viscoelastic analysis indicated decreased clot firmness and reduced clot lysis with no hyperfibrinolysis (Table 5). Clot growth also comprised abnormal ratios of fibrinogen to platelets compared to baseline, which appeared to track increases in plasma fibrinogen and falls in total platelets (Table 2). We conclude that the prothrombotic tendency of blood in the presence of inflammation appears to be a secondary consequence of stress-related liver fibrinogen production and decreases in circulating platelets. An acute hypercoagulable status is well-documented in patients after elective laparoscopic and open abdominal surgery with no excessive bleeding [32].
4.2. Molecular physiology and differential gene expression in the major organs
4.2.1. Increased brain excitability in response to stress
An interesting result was the 30-fold significant increase in cortical M1 muscarinic receptor expression 3-days after a laparotomy (Fig. 2D). The M1 receptor subtype is highly expressed in the cortical regions, including the hippocampus, and primarily associated with excitatory synapses [33]. Their activation is involved in regulating neuronal excitability, synaptic plasticity, learning and memory (cognition), and long-term memory consolidation [34]. α-1-AR, which increased 39-fold, is also involved in memory consolidation that follows initial learning and retrieval after stressful events [35,36]. The ∼5-fold higher expression of M2 receptor, which modulates Ach release via glutamatergic synapses and GABAergic interneurons [37], further supports excitability enhancement (Fig. 2C). Since stress by itself increases extracellular glutamate levels and cortical excitability [38], we hypothesise that the acute upregulation of M1 and α-1-AR expression may be associated with mediating a stress-induced vulnerability of the brain to the stressors of surgery.
Increased cortical excitability was further supported by upregulation of metabolism indicated by increases in Ampk, PGC-1α and Tfam expression (Fig. 2G–I). Unfortunately, we did not measure brain cytokines, however, the significant 6-fold expression of cortical Il1b mRNA at 3-days reflects a pro-inflammatory state (Table 3). Systemic and cortical inflammation is known to promote cortical hyperexcitability, alter synaptic network transmission, and can cause neuronal damage [39]. The effect of isoflurane anaesthesia on cortical changes cannot be overlooked, as there is growing evidence linking general anaesthesia to cognitive dysfunction [40].
4.2.2. The heart switches to a sympathetically-driven inflammatory phenotype
Another notable result was the initial 8.4-fold increase in cardiac β-1-AR expression, and persistent elevation at 3-days (Fig. 3A). β-1 receptors are the dominant subtype in heart and their activation increases HR and contractility. Significant smaller increases in α-1-AR expression (Fig. 3B) may also have contributed to increased contractility and/or increased vascular tone. Sympathetic-drive was partially compensated early after surgery by increased expression of parasympathetic M2 and M1 receptors at 6-hr (Fig. 3CD). The net effect was a stable hemodynamic profile, with maintenance of MAP and only marginal increases in HR. Further analysis found that the ratio of expression of the major β-1/M2 subtypes in heart was 2:1 at 6-hr, and 4:1 at 3-days, which indicates a sympathetic dominance of pump function over the 3-days. This was associated with increased myocardial metabolism, inferred from: 1) significant increases in expression of Ampk and PGC-1α, which have been shown to maintain and protect the heart during ischaemic stress [41], and 2) increased Tfam and Mtco3 expression, which increases mitochondrial transcription and cytochrome oxidase subunit stability for efficient ATP production [42,43]. Increases in glucocorticoid receptor expression further supports increased corticosterone uptake and activation of metabolic signalling pathways in heart, which may assist to decrease myocardial damage through reduced apoptosis, and increase coronary flow after ischaemia/reperfusion [44,45].
Increased cardiac inflammation was confirmed by the significant increase in Nlrp3 inflammasome at 6-hr before decreasing at 3-days (Fig. 3EF) despite Il1b remaining 7-fold higher at 3-days. This suggests IL-1β production at 3-days must be via inflammasome-independent pathways compared to 6-hrs. In addition, there were increased tissue cytokines and chemokines at 3-days (IL-1α, IL-4, IL-6, MIP-1α, MCP-1) (Table 4). This proinflammatory state was accompanied by an increase in syndecan-1 gene expression at 6-hr, but not 3-days. Syndecan-1 expression is normally low in cardiac tissue, but increases after injury, such as a myocardial ischaemia/infarction, and is further exacerbated by inflammation [46]. Despite upregulation of metabolism and inflammation in heart, there was no myocardial cell death with no detection of troponin (Table 3).
4.2.3. Liver autonomic shutdown with inflammation
Six hours after laparotomy, the liver underwent a dramatic 90% decrease of α-1-AR and β-1-AR expression, and 55–87% decrease of M1 and M2 receptor expression (Fig. 4A–D). The suppression of sympathetic and parasympathetic receptors implies a surgery-induced autonomic shutdown analogous to a hibernation state. Metabolism also remained quiescent as there were no significant changes in Ampk, Tfam or PGC-1α (Fig. 4G–J). Following decreased neural receptor inputs, liver syndecan-1 and Nlrp3 inflammasome expression fell by ∼70%. In contrast, Mtco3 increased 14-fold at 6-hr, which may be a protective strategy to maintain the stability of mitochondrial electron transport for ATP turnover during surgical stress. In addition, the significant increase in GC receptor expression implies upregulation of corticosterone-induced metabolism such as maintenance of hepatic gluconeogenesis and/or glycogen availability, as Fujiwara and colleagues have reported in conscious dogs infused with cortisol [47]. Hepatic shutdown, however, did not protect against inflammation. This was demonstrated by the 138-and 46-fold increases in Il1b gene expression at 6-hr and 3-days (Fig. 4E), and the significant increases in cytokines and chemokines in liver (Table 4). We further showed that this early upregulation of Il1b mRNA did not involve the Nlrp3 inflammasome for activation, since Nlrp3 expression decreased by 87% (Fig. 4F). As with heart, this new finding suggests alternative pathways for IL-1β activation in liver [48].
4.2.4. Small intestine and stress-induced inflammation
Our study showed β-1-AR and M1 muscarinic receptor expression in small intestine decreased by 50–60% over 3-days (Fig. 5AD). M2, the major muscarinic receptor subtype in gut [49], also fell by 68% at 6-hr, before increasing 7.4-fold at 3-days (Fig. 5C). However, these changes were not significant because of large baseline variations in gut. In general, activation of sympathetic β-1 adrenergic receptors decreases intestinal motility, while parasympathetic muscarinic receptors increase motility [50,51]. Without direct scintigraphic imaging, it is difficult to interpret the net neurotransmitter effect on gut motility. However, assuming similar sensitivities, we predict a net decrease in motility at 6-hr (↓β-1, ↓M1/↓M2), and a return to baseline or net increase at 3-days (↑↑M1/↓M2↓β-1).
As with the other organs, the small intestine was highly proinflammatory at 3-days. Il1b gene expression increased 140-fold, again with no change in Nlrp3 expression (Fig. 5EF), indicating an alternative pathway of activation. Il1b upregulation was supported by IL-1β protein concentration, and significant increases in chemokines MCP-1 and MIP-1α (Table 4). The proinflammatory phenotype was associated with little or no changes in metabolism (Fig. 5G–I), however, the upregulation of gut Mtco3 over 3-days (Fig. 5J) may have been a protection strategy against possible ischaemic injury, as in liver. The links between dramatic falls in neurotransmitter receptor expression, inflammation and metabolism in peripheral organs requires further study.
4.3. Clinical implications
In humans undergoing major surgery, the stressresponse is activated early and a number of secondary complications can ensue, such as post-operative cognitive impairment, delirium, ischaemia, ileus and increased susceptibility to infection [2,[9], [10], [11], [12], [13]]. The clinical manifestations of one or more these complications are largely determined by the patient's previous health history, medications, age, disease severity, extent of trauma (if applicable), and the type and duration of surgery [9]. Recent studies have reported that 20% of high-risk patients undergoing non-cardiac surgery developed one or more cardiac complications within a year [8], and in other major surgeries, up to 20% of patients will develop organ dysfunction, 20–30% will develop short-term or persistent cognitive decline, and ∼5% will develop a post-operative infection [9,12]. Our study showing early systemic and brain, cardiac, liver and gut inflammation suggests that it may underlie many of these post-operative, stress-related complications. The clinical significance can be summed-up with an iceberg analogy, with the tip being systemic inflammation, and underneath the surface being secondary injury processes responding to the potentially “harmful stimuli” from the trauma of surgery itself. Our study also has relevance to the ongoing debate in military medicine regarding the risk of performing exploratory laparotomies in field medical centres for suspected haemorrhage and/or peritonitis before definitive care [24,52,53]. These findings suggest that care should be exercised in this decision-making process given the further complications that can arise from a laparotomy.
4.4. Limitations
A potential limitation of the present study is that, unlike the heterogeneity of human patients undergoing major surgery, our animals were healthy adult males of similar age and physiology. Future studies could include aged rats and also extend the monitoring period from 3- to 30-days with cognitive testing, and examine molecular changes in different parts of the brain (e.g. nucleus tractus solitarius, hippocampus, hypothalamus). Another limitation of the present study is that we did not measure the presence of circulating DAMPs, including microparticles, over the 3-days. Lastly, we did not investigate limited versus higher volumes of fluid therapy, which itself can exacerbate inflammation, coagulopathy, organ dysfunction and clinical outcome [24].
5. Conclusions
We conclude that a laparotomy with surgical catheterisation led to a surgical-induced inflammatory phenotype involving neuroendocrine stress, systemic inflammation, immune activation, metabolic changes and acute coagulopathy. A standout result was the pervasive nature of inflammation in brain, heart, liver and gut, presumably sustained by injury signals from the surgical site and the CNS.
Data availability statement
The datasets supporting the conclusions of this manuscript can be made available by emailing the corresponding author.
Provenance and peer review
Not commissioned, externally peer reviewed.
Funding
This work was supported by James Cook University College of Medicine and Dentistry Research Support Grant (2020); and USSOCOM under Award No. W81XWH-USSOCOM-BAA-15-1. The opinions, interpretations, and conclusions are those of the authors, and are not necessarily endorsed by the US Department of Defense.
Ethical approval
The study was approved by the Institutional Animal Ethics Committee (IACUC:A2296/A2386) and US Animal Care and Review Use Office (ACURO), and complies with the Guide for Care and Use of Laboratory Animals (2011). Animals were assessed twice daily using the IACUC- and ACURO-approved monitoring sheet and humane endpoints of Morton [17], Toth, and Nemzek as per previous studies [20]. The study was conducted and is reported according to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (Supplementary Content 3).
Author contribution
All authors contributed to the funding acquisition, design, conceptualization, implementation, literature analysis, methodology, data analysis, writing, reading, review and final approval of the submitted version of the MS. HL, JM, LD and EB all contributed to the experimental work. GD was responsible for the oversight and leadership of the team, and accepts full responsibility for the work.
Guarantor
Geoffrey Phillip Dobson was responsible for the oversight and leadership of the team, and accepts full responsibility for the work. He accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
We thank James Cook University, College of Medicine and Dentistry, and US Department of Defense for their continued support of our research work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102970.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weiser T.G., Haynes A.B., Molina G., Lipsitz S.R., Esquivel M.M., Uribe-Leitz T., Fu R., Azad T., Chao T.E., Berry W.R., Gawande A.A. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385 doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2.Devereaux P.J., Chan M.T., Alonso-Coello P. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. J. Am. Med. Assoc. 2012;307(21):2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 3.Pearse R.M., Moreno R.P., Bauer P., Pelosi P., Metnitz P., Spies C., Vallet B., Vincent J.L., Hoeft A., Rhodes A. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–1065. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semel M.E., Lipsitz S.R., Funk L.M., Bader A.M., Weiser T.G., Gawande A.A. Rates and patterns of death after surgery in the United States, 1996 and 2006. Surgery. 2012;151(2):171–182. doi: 10.1016/j.surg.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Du W., Glasgow N., Smith P., Clements A., Sedrakyan A. Major inpatient surgeries and in-hospital mortality in New South Wales public hospitals in Australia: a state-wide retrospective cohort study. Int. J. Surg. 2018;50:126–132. doi: 10.1016/j.ijsu.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Anderson O., Davis R., Hanna G.B., Vincent C.A. Surgical adverse events: a systematic review. Am. J. Surg. 2013;206(2):253–262. doi: 10.1016/j.amjsurg.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Xu J., Murphy S.L., Kochanek K.D., Arias E. Mortality in the United States, 2018. NCHS Data Brief. 2020;355:1–8. [PubMed] [Google Scholar]
- 8.Sazgary L., Puelacher C., Lurati Buse G., Glarner N., Lampart A., Bolliger D., Steiner L., Gurke L., Wolff T., Mujagic E., Schaeren S., Lardinois D., Espinola J., Kindler C., Hammerer-Lercher A., Strebel I., Wildi K., Hidvegi R., Gueckel J., Hollenstein C., Breidthardt T., Rentsch K., Buser A., Gualandro D.M., Mueller C., Investigators B.-P. Eur Heart J Acute Cardiovasc Care; 2020. Incidence of Major Adverse Cardiac Events Following Non-cardiac Surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson G.P. Trauma of major surgery: a global problem that is not going away. Int. J. Surg. 2020;81:47–54. doi: 10.1016/j.ijsu.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desborough J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000;85(1):109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 11.Dunser M.W., Hasibeder W.R. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J. Intensive Care Med. 2009;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 12.Cusack B., Buggy D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020;20(9):321–328. doi: 10.1016/j.bjae.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finnerty C.C., Mabvuure N.T., Ali A., Kozar R.A., Herndon D.N. The surgically induced stress response. JPEN - J. Parenter. Enter. Nutr. 2013;37(5 Suppl) doi: 10.1177/0148607113496117. 21S-9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letson H.L., Morris J.L., Biros E., Dobson G.P. Conventional and specific-pathogen free rats respond differently to anesthesia and surgical trauma. Sci. Rep. 2019;9(1):9399. doi: 10.1038/s41598-019-45871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson G.P., Morris J., Biros E., Letson H.L. Specific pathogen-free animals for civilian and military trauma: a cautionary note in the translation of new drug therapies. Shock. 2020;54(2):232–236. doi: 10.1097/SHK.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 16.Dobson G.P. Small animal model species are not created equal. Crit. Care Med. 2012;40(2):711. doi: 10.1097/CCM.0b013e31823c8965. author reply 711-2. [DOI] [PubMed] [Google Scholar]
- 17.Morton D.B. A systematic approach for establishing humane endpoints. ILAR J. 2000;41(2):80–86. doi: 10.1093/ilar.41.2.80. [DOI] [PubMed] [Google Scholar]
- 18.Toth L.A. Defining the moribund condition as an experimental endpoint for animal research. ILAR J. 2000;41(2):72–79. doi: 10.1093/ilar.41.2.72. [DOI] [PubMed] [Google Scholar]
- 19.Nemzek J.A., Xiao H.Y., Minard A.E., Bolgos G.L., Remick D.G. Humane endpoints in shock research. Shock. 2004;21(1):17–25. doi: 10.1097/01.shk.0000101667.49265.fd. [DOI] [PubMed] [Google Scholar]
- 20.Letson H.L., Morris J.L., Biros E., Dobson G.P. Adenosine, lidocaine, and Mg2+ fluid therapy leads to 72-hour survival after hemorrhagic shock: a model for studying differential gene expression and extending biological time. J. Trauma Acute Care Surg. 2019;87(3):606–613. doi: 10.1097/TA.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 21.Davenport L., Letson H.L., Dobson G.P. Immune-inflammatory activation after a single laparotomy in a rat model: effect of adenosine, lidocaine and Mg2+ infusion to dampen the stress response. Innate Immun. 2017;23(5):482–494. doi: 10.1177/1753425917718921. [DOI] [PubMed] [Google Scholar]
- 22.Letson H.L., Dobson G.P. Buprenorphine analgesia: new adverse effects emerge. Shock. 2020;54(2) doi: 10.1097/SHK.0000000000001492. (dobson) [DOI] [PubMed] [Google Scholar]
- 23.Dobson G.P., Letson H.L. Far forward gaps in hemorrhagic shock and prolonged field care: an update of ALM fluid therapy for field use. J. Spec. Oper. Med. 2020;20(3):78–84. doi: 10.55460/06VT-9IH4. [DOI] [PubMed] [Google Scholar]
- 24.Banz V.M., Jakob S.M., Inderbitzin D. Review article: improving outcome after major surgery: pathophysiological considerations. Anesth. Analg. 2011;112(5):1147–1155. doi: 10.1213/ANE.0b013e3181ed114e. [DOI] [PubMed] [Google Scholar]
- 25.Letson H.L., Dobson G.P. Correction of acute traumatic coagulopathy with small-volume 7.5% NaCl adenosine, lidocaine and Mg2+ (alm) occurs within 5 min: a ROTEM analysis. J. Trauma Acute Care Surg. 2015;78(4):773–783. doi: 10.1097/TA.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 26.Messora M.R., Nagata M.J.H., Furlaneto F.A.C., Dornelles R.C.M., Bomfim S.R.M., Deliberador T.M., Garcia V.H., Bosco A.F. A standardized research protocol for platelet-rich plasma (PRP) preparation in rats. RSBO. 2011;8(3):299–304. [Google Scholar]
- 27.Dobson G.P., Morris J.L., Davenport L.M., Letson H.L. Traumatic-induced coagulopathy as a systems failure: a new window into hemostasis. Semin. Thromb. Hemost. 2020;46(2):199–214. doi: 10.1055/s-0039-1701018. [DOI] [PubMed] [Google Scholar]
- 28.Miller T., Gibbison B., Russell G.M. Hypothalamic–pituitary–adrenal function during health, major surgery, and critical illness. BJA Educat. 2017;17(1):16–21. [Google Scholar]
- 29.Kwon Y.S., Jang J.S., Hwang S.M., Tark H., Kim J.H., Lee J.J. Effects of surgery start time on postoperative cortisol, inflammatory cytokines, and postoperative hospital day in hip surgery: randomized controlled trial. Medicine (Baltim.) 2019;98(24) doi: 10.1097/MD.0000000000015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronchetti S., Ricci E., Migliorati G., Gentili M., Riccardi C. How glucocorticoids affect the neutrophil life. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19124090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukumaran S., Dubois D.C., Jusko W.J., Almon R.R. Glucocorticoid effects on adiponectin expression. Vitam. Horm. 2012;90:163–186. doi: 10.1016/B978-0-12-398313-8.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulrych J., Kvasnicka T., Fryba V., Komarc M., Malikova I., Burget F., Brzezkova R., Kvasnicka J., Jr., Krska Z., Kvasnicka J. 28 day post-operative persisted hypercoagulability after surgery for benign diseases: a prospective cohort study. BMC Surg. 2016;16:16. doi: 10.1186/s12893-016-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarr E. Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci. Ther. 2012;18(5):369–379. doi: 10.1111/j.1755-5949.2011.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S., Kumar A., Tripathi T., Kumar A. Muscarinic and nicotinic acetylcholine receptor agonists: current scenario in Alzheimer's disease therapy. J. Pharm. Pharmacol. 2018;70(8):985–993. doi: 10.1111/jphp.12919. [DOI] [PubMed] [Google Scholar]
- 35.Bernardi R.E., Lattal K.M. A role for α₁-adrenergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behav. Neurosci. 2010;124(2):204–210. doi: 10.1037/a0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez D.M. alpha1-Adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Front. Pharmacol. 2020;11:581098. doi: 10.3389/fphar.2020.581098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas C.L., Baghdoyan H., Lydic R. Prefrontal cortex acetylcholine release, EEG slow waves, and spindles are modulated by M2 autoreceptors in C57BL/6J mouse. J. Neurophysiol. 2002;87(6):2817–2822. doi: 10.1152/jn.2002.87.6.2817. [DOI] [PubMed] [Google Scholar]
- 38.Houtepen L.C., Schur R.R., Wijnen J.P., Boer V.O., Boks M.P., Kahn R.S., Joels M., Klomp D.W., Vinkers C.H. Acute stress effects on GABA and glutamate levels in the prefrontal cortex: a 7T (1)H magnetic resonance spectroscopy study. Neuroimage Clin. 2017;14:195–200. doi: 10.1016/j.nicl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odoj K., Brawek B., Asavapanumas N., Mojtahedi N., Heneka M.T., Garaschuk O. Brain Behav Immun; 2021. In Vivo Mechanisms of Cortical Network Dysfunction Induced by Systemic Inflammation. [DOI] [PubMed] [Google Scholar]
- 40.Belrose J.C., Noppens R.R. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol. 2019;19(1):241. doi: 10.1186/s12871-019-0903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyck J.R.B., Lopaschuk G.D. AMPK alterations in cardiac physiology and pathology: enemy or ally? J. Physiol. 2006;574(Pt 1):95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canto C., Auwerx J. PGC-1alpha, Sirt1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunkel G.H., Chaturvedi P., Tyagi S.C. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail. Rev. 2016;21(5):499–517. doi: 10.1007/s10741-016-9561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oakley R.H., Cidlowski J.A. Glucocorticoid signaling in the heart: a cardiomyocyte perspective. J. Steroid Biochem. Mol. Biol. 2015;153:27–34. doi: 10.1016/j.jsbmb.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wester M., Heller A., Gruber M., Maier L.S., Schach C., Wagner S. Glucocorticoid stimulation increases cardiac contractility by SGK1-dependent SOCE-activation in rat cardiac myocytes. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0222341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhoutte D., Schellings M.W., Gotte M., Swinnen M., Herias V., Wild M.K., Vestweber D., Chorianopoulos E., Cortes V., Rigotti A., Stepp M.A., Van de Werf F., Carmeliet P., Pinto Y.M., Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115(4):475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara T., Cherrington A.D., Neal D.N., McGuinness O.P. Role of cortisol in the metabolic response to stress hormone infusion in the conscious dog. Metabolism. 1996;45(5):571–578. doi: 10.1016/s0026-0495(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 48.Netea M.G., van de Veerdonk F.L., van der Meer J.W., Dinarello C.A., Joosten L.A. Inflammasome-independent regulation of IL-1-family cytokines. Annu. Rev. Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 49.Iino S., Nojyo Y. Muscarinic M(2) acetylcholine receptor distribution in the Guinea-pig gastrointestinal tract. Neuroscience. 2006;138(2):549–559. doi: 10.1016/j.neuroscience.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Nasser Y., Ho W., Sharkey K.A. Distribution of adrenergic receptors in the enteric nervous system of the Guinea pig, mouse, and rat. J. Comp. Neurol. 2006;495(5):529–553. doi: 10.1002/cne.20898. [DOI] [PubMed] [Google Scholar]
- 51.Boeckxstaens G.E., de Jonge W.J. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58(9):1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 52.Stone H.H., Strom P.R., Mullins R.J. Management of the major coagulopathy with onset during laparotomy. Ann. Surg. 1983;197:532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts D.J., Stelfox H.T., Moore L.J., Cotton B.A., Holcomb J.B., Harvin J.A. Accuracy of published indications for predicting use of damage control during laparotomy for trauma. J. Surg. Res. 2020;248:45–55. doi: 10.1016/j.jss.2019.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this manuscript can be made available by emailing the corresponding author.