Abstract
Background
Post-stroke cognitive impairment can occur after damage to various brain regions, and cognitive deficits depend on infarct location. The Mini-Mental State Examination (MMSE) is still widely used to assess post-stroke cognition, but it has been criticized for capturing only certain cognitive deficits. Along these lines, it might be hypothesized that cognitive deficits as measured with the MMSE primarily involve certain infarct locations.
Aims
This comprehensive lesion-symptom mapping study aimed to determine which acute infarct locations are associated with post-stroke cognitive impairment on the MMSE.
Methods
We examined associations between impairment on the MMSE (<5th percentile; normative data) and infarct location in 1198 patients (age 67 ± 12 years, 43% female) with acute ischemic stroke using voxel-based lesion-symptom mapping. As a frame of reference, infarct patterns associated with impairments in individual cognitive domains were determined, based on a more detailed neuropsychological assessment.
Results
Impairment on the MMSE was present in 420 patients (35%). Large voxel clusters in the left middle cerebral artery territory and thalamus were significantly (p < 0.01) associated with cognitive impairment on the MMSE, with highest odds ratios (>15) in the thalamus and superior temporal gyrus. In comparison, domain-specific impairments were related to various infarct patterns across both hemispheres including the left medial temporal lobe (verbal memory) and right parietal lobe (visuospatial functioning).
Conclusions
Our findings indicate that post-stroke cognitive impairment on the MMSE primarily relates to infarct locations in the left middle cerebral artery territory. The MMSE is apparently less sensitive to cognitive deficits that specifically relate to other locations.
Keywords: Mini-Mental State Examination, MRI, lesion-symptom mapping, cognitive impairment, ischemic stroke, cerebral infarction
Introduction
Post-stroke cognitive impairment is a common and debilitating consequence of ischemic stroke. 1 Cognitive screening tools play an essential role in the diagnostic process in stroke care and research.2,3 Compared to other screening tools such as the Montreal Cognitive Assessment (MoCA), 4 the Mini-Mental State Examination (MMSE) 5 has limited sensitivity to cognitive deficits occurring in the context of cerebrovascular disease, particularly in those without overt dementia.6,7 This predominantly relates to the cognitive domains assessed by the MMSE. It is considered sensitive to impairment of language and orientation. 3 However, a known limitation is that it is less sensitive to impairment of executive functioning and attention, 7 memory, 3 abstract reasoning, and visuospatial abilities. 7 This is relevant because stroke can cause impairment in any of these cognitive domains. 8 Nevertheless, the MMSE is still applied in contemporary stroke research as a measure of post-stroke cognitive impairment (e.g. Kwon et al. 9 and Liu et al. 10 ), and evidence from older studies and large clinical trials remains based on the MMSE. 11
Infarct location is an important determinant of the heterogeneity of post-stroke cognitive impairment, 12 reflecting the functional specialization of the brain. 13 It is unclear whether the MMSE is sensitive to cognitive consequences of infarcts across the brain or cognitive deficits as measured with the MMSE primarily involve certain infarct locations. Research on this topic is scarce and inconclusive. Previous studies suggested that the MMSE might be less sensitive to right hemispheric lesions,14,15 while others reported no interhemispheric differences.16,17 These studies were limited to relatively small study samples (N = 126–253) and only compared hemispheric lateralization of infarcts rather than specific infarct locations. If the MMSE primarily captures cognitive impairment due to specific infarct locations, this may help explain why the MMSE is a relatively insensitive tool for post-stroke cognitive impairment.
The aim of this study was to investigate whether impairment on the MMSE is associated with specific infarct locations. For this purpose, we performed a large-scale lesion-symptom mapping study on 1198 patients with acute ischemic stroke.
Methods
Study population
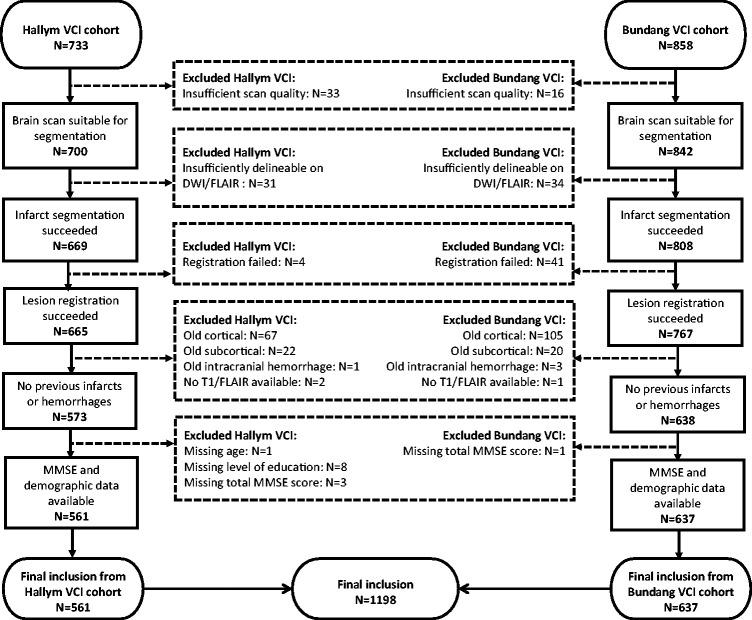
Patients were selected from the Hallym Vascular Cognitive Impairment (VCI) and Bundang VCI cohorts, consisting of patients admitted to Hallym University Sacred Heart Hospital or Seoul National University Bundang Hospital (Republic of Korea) with acute ischemic stroke between January 2007 and December 2018.18,19 A flowchart of patient selection is provided in Figure 1. A total of 1198 patients were selected based on the following inclusion criteria: (1) brain magnetic resonance imaging (MRI) showing the acute symptomatic infarct(s) on diffusion-weighted imaging (DWI) and/or fluid-attenuated inversion recovery (FLAIR), (2) successful infarct segmentation and registration (‘Generation of infarct maps’ section), (3) no previous cortical infarcts, large subcortical infarcts or hemorrhages on MRI (‘Generation of infarct maps’ section), and (4) data on key demographics and MMSE.
Figure 1.
Flowchart of patient selection.
Generation of infarct maps
Brain MRI, including DWI and FLAIR sequences, was performed with a 3 T MRI scanner in the first week after stroke onset. Details regarding the MRI protocol are provided in the Supplementary material. Infarct segmentation and subsequent registration to the MNI-152 brain template were performed in accordance with a previously published protocol. 20 First, acute infarcts were manually segmented on DWI (97.5%) or FLAIR (2.5%) sequences using in-house developed software built in MeVisLab (MeVis Medical Solutions AG, Bremen, Germany). 21 Next, all scans and the corresponding lesion maps were transformed to the T1 1-mm MNI-152 brain template 22 with the RegLSM tool (www.metavcimap.org/features/software-tools; most recent version described in Weaver et al. 23 ). Quality checks of all registration results were performed by one rater (N.A.W.). Manual adaptations were made in case of minor registration errors (45%, N = 537; see Supplementary material).
Presence of chronic vascular lesions, specifically old cortical infarcts, large subcortical infarcts (>15 mm), large hemorrhages (>10 mm), and lacunes was assessed by an experienced rater (N.A.W.) and classified according to the STRIVE criteria. 24 Acute infarcts were categorized into stroke subtypes for summary purposes (definitions provided in Supplementary material).
Neuropsychological assessment
All selected patients underwent neuropsychological assessment within a year after stroke onset. Cognitive performance was evaluated in the hospital setting using the Korean version of the MMSE 25 and the Korean version of the 60-min VCI Harmonization Standards-Neuropsychology Protocol (K-VCIHS-NP). 19 Both were administered on the same date by trained clinical neuropsychologists who were blinded to patients’ clinical and neuroradiological profiles. At the discretion of the attending physician, patients were excluded if they suffered from disabilities that would interfere with neuropsychological testing including neurological deficits such as severe aphasia or motor weakness or impairment of hearing or vision.
Impairment on the MMSE was defined as scoring <5th percentile based on age- and education-corrected Korean normative data. 25 Examples of impaired MMSE scores for different ages and education levels are shown in Supplementary Table 1. As a frame of reference, we also investigated which infarct patterns were associated with impairment in individual cognitive domains based on a more detailed neuropsychological assessment with age- and education-corrected normative data. Neuropsychological tests were assigned to five cognitive domains: attention and executive functions, language, processing speed, verbal memory, and visuospatial abilities. The cognitive profile of the study cohort and categorization of neuropsychological scores are shown in Supplementary Table 2. Assignment to cognitive domains was based on seminal work, 26 with some minor modifications to the K-VCIHS-NP. Cognitive domain-specific impairment was defined as scoring <5th percentile in >50% of available neuropsychological tests per cognitive domain. The number of available tests was determined on a per-subject basis.
Statistical analyses
Voxel-based lesion-symptom mapping (VLSM) was performed to relate acute infarct location to impairment on the MMSE, using the Fisher’s exact test in Python (SciPy 1.4.1). Dichotomized scores for presence of impairment on the MMSE and cognitive domain-specific impairment were used as outcome measures. Voxels damaged in <5 patients were excluded. Odds ratios and significance levels were calculated for each included voxel. False discovery rate correction (FDR; threshold p < 0.01) was applied to correct for multiple comparisons.
We performed a sensitivity analysis in which we repeated the VLSM analyses after excluding patients with evidence of pre-stroke cognitive impairment using a validated Korean version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE; excluded if ≥ 3.6).27,28 This was done to evaluate whether the observed associations were related to the acute stroke event or might be attributed to pre-existing conditions.
Ethics statement
Data were prospectively collected in accordance with study protocols approved by the Institutional Review Boards of the Hallym University Sacred Heart Hospital and Seoul National University Bundang Hospital. Use of the acquired data for the current study was also approved by these Institutional Review Boards.
Data availability statement
Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Clinical characteristics of the 1198 included patients are listed in Table 1. The mean age was 67 years (SD = 12), 513 patients were female (43%), and median years of education was 9 (IQR = 6). Regarding infarct locations, 963 patients (80%) had supratentorial infarcts, of whom 399 (33% of total) had small subcortical infarcts, and 294 patients (25%) (also) had infratentorial infarcts. MMSE assessment generally took place after three months (median (IQR): 99 (69) days). Impairment on the MMSE was present in 420 patients (35%). Impairment on the MMSE occurred most commonly in patients with infarcts in multiple regions, i.e. in bilateral hemispheres or infra- and supratentorial (N = 70/149; 47% (95%CI: 39–55)) and left-hemispheric infarcts (N = 186/473; 39% (35–44)), yet also frequently occurred in patients with right-hemispheric (N = 116/369; 31% (27–36)) and infratentorial infarcts (N = 48/207; 23% (18–30)). Four patients (0.3%) had recurrent stroke in the time interval between the index stroke and neuropsychological assessment; only the index stroke was considered in the analyses.
Table 1.
Demographic and clinical characteristics of the study population (N = 1198)
| Demographics and clinical characteristics | |
|---|---|
| Age (years), mean (SD) | 67.3 (11.7) |
| Female, N (%) | 513 (42.8) |
| Years of education, median (IQR) | 9 (6) |
| Hand preference a | |
| Right, N (%) | 1150 (97.5) |
| Left, N (%) | 10 (0.8) |
| Ambidextrous, N (%) | 19 (1.6) |
| Stroke severity a | |
| NIHSS at admission, median (IQR) | 3 (4) |
| Vascular risk factors, N (%) | |
| Hypertension a | 813 (67.9) |
| Hyperlipidemiaa | 383 (32.2) |
| Current smoker a | 291 (24.5) |
| Past smoker a | 177 (15.0) |
| Diabetes Mellitus a | 373 (31.2) |
| Atrial fibrillation b | 151 (13.4) |
| Cognitive assessment | |
| Time interval between stroke onset and cognitive assessment (days) a , median (IQR) | 99 (69) |
| IQCODE c , median (IQR) | 3.2 (0.5) |
| IQCODEc < 3.6, indicative of no pre-stroke cognitive impairment, N (%) | 704 (78.0) |
| Brain MRI | |
| Time interval between stroke onset and MRI (days) a , median (IQR) | 4 (4) |
| Total infarct volume in ml d , median (IQR) | 2.6 (12.5) |
Data missing in < 30 cases.
Data missing in 70 cases.
Data missing in 296 cases.
Normalized volumes after registration to the MNI-152 template.
Valid percent is indicated in cases with missing data.
IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly; IQR: interquartile range; MRI: magnetic resonance imaging; SD: standard deviation.
Infarct distribution was symmetrical, and subcortical regions were more commonly damaged than cortical regions (Figure 2(a)). Because of the large sample size, brain coverage was very high: 71% of the MNI-152 template (1,300,080/1,827,240 voxels) was included. Only some parts of the midbrain, temporal lobes, and anterior cerebral artery territory were excluded due to infrequent involvement (i.e. < 5 patients).
Figure 2.
Lesion prevalence map and voxel-based lesion-symptom mapping results for cognitive impairment on the Mini-Mental State Examination (MMSE): (a) lesion prevalence map of infarcts in the total sample (N = 1198), only including voxels damaged in ≥5 subjects. The color indicates how many subjects had an infarct in a particular voxel; (b) voxel-wise odds ratios (OR) for MMSE impairment are shown, calculated using the Fisher’s exact test. Threshold for statistical significance was p < 0.01 after false discovery rate correction. Included voxels (N ≥ 5) with no significant association are shown in cyan. For orange-red colored voxels, presence of infarcts was significantly associated with an increased OR for MMSE impairment; (c) examples of interpretation of analysis results from panel B. L: left; R: right.
VLSM revealed that large voxel clusters in frontal, parietal, and temporal lobes covering the left middle cerebral artery territory (based on visual inspection of the MNI-152 template) and the thalamus were significantly associated with impairment on the MMSE, with highest odds ratios (>15) for voxel clusters in the thalamus and superior temporal gyrus (Figure 2(b)). In comparison, domain-specific impairments were related to various infarct patterns across both hemispheres. For example, infarcts in the left medial temporal lobe were related to verbal memory impairment, and infarcts in the right parietal lobe were related to impairment of visuospatial functioning (Figure 3).
Figure 3.
Voxel-based lesion-symptom mapping results for individual cognitive domains based on neuropsychological assessment. Voxel-wise odds ratios (OR) for cognitive impairment on individual cognitive domains are shown, calculated using the Fisher’s exact test. Threshold for statistical significance was p < 0.01 after false discovery rate correction. Included voxels (N ≥ 5) with no significant association are shown in cyan. For orange-red colored voxels, presence of infarcts was significantly associated with an increased OR for impairment. L: left; R: right.
The sensitivity analysis on patients without evidence of pre-stroke cognitive impairment (IQCODE < 3.6; N = 704) showed a similar pattern of odds ratios for MMSE impairment as the main results, with highest odds ratios (>15) in left frontotemporal regions and the thalamus. However, statistical power was attenuated due to several factors, most importantly, the smaller sample size, decreased brain coverage, and lower prevalence of impairment on the MMSE in the selected sample. Details are provided in Supplementary Figure 1.
Discussion
In this large-scale lesion-symptom mapping study, we found that impairment on the MMSE is primarily related to infarcts located in the left middle cerebral artery territory and thalamus. Meanwhile, domain-specific impairments were related to various infarct patterns across both hemispheres including the left medial temporal lobe (verbal memory) and right parietal lobe (visuospatial functioning).
Previous studies reported that impairment on the MMSE was more frequently observed in patients with left hemispheric lesions compared to those with right hemispheric lesions both in the context of stroke 14 and other neurological diseases. 15 We indeed found a strong left-hemispheric lateralization of infarct locations significantly related to impairment on the MMSE. Although a substantial number of patients with MMSE impairment had right hemispheric infarcts, we found no voxel-based associations, suggesting a more diffuse effect of right hemispheric infarcts on MMSE performance. Furthermore, we extend previous findings by pinpointing specific infarct patterns associated with impairment on the MMSE. Specifically, our reference analyses of individual cognitive domains revealed much more widespread lesion correlates, which suggest that the MMSE insufficiently captures cognitive impairment caused by damage to brain regions beyond the left middle cerebral artery territory and thalamus. To illustrate, we showed that infarcts in the left medial temporal and occipital lobes were related to verbal memory impairment, while these regions were not related to impairment on the MMSE. The observed infarct patterns also strengthen the face validity of our approach (e.g. involvement of Broca and Wernicke areas for language, right parieto-occipital areas for visuospatial abilities, language areas, and left medial temporal lobe for verbal memory). Of note, a previous lesion-symptom mapping study (N = 410) demonstrated that performance deficits on the MoCA did relate to a more elaborate pattern of infarct locations, also involving the right hemisphere. 29
Our sensitivity analysis showed that very similar infarct patterns (i.e. locations and effect sizes) were found after excluding subjects with potential pre-stroke cognitive impairment. This suggests that the observed associations are linked to the acute stroke event rather than pre-existing cognitive problems. Lack of statistical significance in the sensitivity analyses can be explained by several factors that were outlined in the Results section and further explored in Supplementary Figure 1.
There are numerous statistical approaches available for lesion-symptom mapping. 30 Because our primary aim was to identify infarct locations that are related to impairment on the MMSE, we chose a straightforward univariate VLSM approach that offers a high sensitivity to detect potentially relevant voxels, provides easily interpretable effect sizes (i.e. odds ratios), and is well suited for dealing with binary outcomes. 31 Multivariate techniques can potentially establish more fine-grained neuroanatomical correlates of a cognitive function, but the interpretation of effect sizes is less straightforward, and application for binary outcomes is less common. 30 Based on these considerations, univariate VLSM was deemed the most suitable approach for this study.
Strengths of this study include the large sample size, extensive brain coverage, the detailed spatial resolution of the voxel-wise approach, and the domain-specific analyses used as a frame of reference. Some potential limitations must also be noted. First, the time interval between stroke onset and cognitive assessment was relatively wide, ranging from the acute phase to one-year post-stroke. The observed associations might therefore have been influenced by factors such as delirium in the acute stage, recurring stroke events, co-occurring pathologies, or cognitive recovery during this time window. However, cognitive assessment generally took place after three months, which should minimize the impact of these early or chronic contributing factors. Third, although we excluded patients with chronic infarcts and hemorrhages to reduce the effect of prior vascular lesions on the lesion-deficit associations, we did not evaluate the potential influence of other imaging markers, for example, for Alzheimer’s disease or cerebral small vessel disease. Finally, the generalizability of our findings to other sociodemographic populations is undetermined.
Our study indicates that post-stroke impairment on the MMSE primarily relates to infarct locations in the left middle cerebral artery territory. The MMSE is apparently less sensitive to cognitive deficits that relate to other specific infarct locations. Hence, our findings provide yet another argument to consider other tests, such as the MoCA, rather than the MMSE to screen for post-stroke cognitive impairment.
Supplemental Material
Supplemental material, sj-pdf-1-wso-10.1177_1747493020984552 for Post-stroke cognitive impairment on the Mini-Mental State Examination primarily relates to left middle cerebral artery infarcts by Nick A Weaver, Angelina K Kancheva, Jae-Sung Lim, J Matthijs Biesbroek, Irene MC Huenges Wajer, Yeonwook Kang, Beom J Kim, Hugo J Kuijf, Byung-Chul Lee, Keon-Joo Lee, Kyung-Ho Yu, Geert Jan Biessels and Hee-Joon Bae in International Journal of Stroke
Acknowledgements
The authors thank Gözdem Arikan for her help with the infarct segmentations and Jaeseol Park and Eunbin Ko for their help in organizing the neuropsychological data.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: G.J.B.’s work was supported by Vici Grant 918.16.616 from ZonMw, The Netherlands, Organisation for Health Research. J.M.B.’s work was supported by a Young Talent Fellowship from the UMC Utrecht Brain Center, The Netherlands.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Nick A Weaver https://orcid.org/0000-0001-8425-8436
Matthijs Biesbroek https://orcid.org/0000-0001-7017-2148
References
- 1.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 2.Kosgallana A, Cordato D, Chan DKY, et al. Use of cognitive screening tools to detect cognitive impairment after an ischaemic stroke: a systematic review. SN Compr Clin Med 2019; 1: 255–262. [Google Scholar]
- 3.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–2241. [DOI] [PubMed] [Google Scholar]
- 4.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 6.Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014; 45: 3008–3018. [DOI] [PubMed] [Google Scholar]
- 7.Pendlebury ST, Cuthbertson FC, Welch SJV, et al. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke 2010; 41: 1290–1293. [DOI] [PubMed] [Google Scholar]
- 8.Lo JW, Crawford JD, Desmond DW, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019; 93: e2257–e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon HS, Lee D, Lee MH, et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J Neurol 2020; 267: 688–93. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Chen H, Zhao K, He W, Lin S, He J. High levels of plasma fibrinogen are related to post-stroke cognitive impairment. Brain Behav 2019; 9: e01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkinjuntti T, Román G, Gauthier S, et al. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004; 35: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 12.Casolla B, Caparros F, Cordonnier C, et al. Biological and imaging predictors of cognitive impairment after stroke: a systematic review. J Neurol 2019; 266: 2593–604. [DOI] [PubMed] [Google Scholar]
- 13.Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci 2017; 131: 715–728. [DOI] [PubMed] [Google Scholar]
- 14.Kupke T, Revis ES, Gantner AB. Hemispheric bias of the Mini-Mental State Examination in elderly males. Clin Neuropsychol 1993; 7: 210–214. [DOI] [PubMed] [Google Scholar]
- 15.Dick JPR, Guiloff RJ, Stewart A. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry 1984; 47: 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelros P. Characteristics of Mini-Mental State Examination 1 year after stroke. Acta Neurol Scand 2005; 112: 88–92. [DOI] [PubMed] [Google Scholar]
- 17.Bour A, Rasquin S, Boreas A, et al. How predictive is the MMSE for cognitive performance after stroke? J Neurol 2010; 257: 630–637. [DOI] [PMC free article] [PubMed]
- 18.Lim JS, Kim N, Jang MU, et al. Cortical hubs and subcortical cholinergic pathways as neural substrates of poststroke dementia. Stroke 2014; 45: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 19.Yu KH, Cho SJ, Oh MS, et al. Cognitive impairment evaluated with vascular cognitive impairment harmonization standards in a multicenter prospective stroke cohort in Korea. Stroke 2013; 44: 786–788. [DOI] [PubMed] [Google Scholar]
- 20.Biesbroek JM, Kuijf HJ, Weaver NA, Zhao L and Duering M; Meta VCI Map Consortium, Biessels GJ. Brain infarct segmentation and registration on MRI or CT for lesion-symptom mapping. J Vis Exp 2019; 25; 151. [DOI] [PubMed]
- 21.Ritter F, Boskamp T, Homeyer A, et al. Medical image analysis. IEEE Pulse 2011; 2: 60–70. [DOI] [PubMed] [Google Scholar]
- 22.Fonov V, Evans AC, Botteron K, et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011; 54: 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver NA, Zhao L, Biesbroek JM, et al. The Meta VCI Map consortium for meta-analyses on strategic lesion locations for vascular cognitive impairment using lesion-symptom mapping: design and multicenter pilot study. Alzheimers Dement (Amst) 2019; 11: 310–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y. A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly. Kor J Psychol 2006; 25: 1–12. [Google Scholar]
- 26.Lezak MD, Howieson DB, Loring DW, Hannay JH, Fischer JS. Neuropsychological assessment, New York: Oxford University Press, 2004. [Google Scholar]
- 27.Lee DW, Lee JY, Ryu SG, et al. Validity of the Korean Version of Informant Questionnaire on cognitive decline in the elderly (IQCODE). J Korean Geriatr Soc 2005; 9: 196–202.
- 28.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24: 145–153. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Biesbroek JM, Shi L, et al. Strategic infarct location for post-stroke cognitive impairment: a multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab 2018; 38: 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnath HO, Sperber C, Rorden C. Mapping human brain lesions and their functional consequences. Neuroimage 2018; 165: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007; 19: 1081–1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-wso-10.1177_1747493020984552 for Post-stroke cognitive impairment on the Mini-Mental State Examination primarily relates to left middle cerebral artery infarcts by Nick A Weaver, Angelina K Kancheva, Jae-Sung Lim, J Matthijs Biesbroek, Irene MC Huenges Wajer, Yeonwook Kang, Beom J Kim, Hugo J Kuijf, Byung-Chul Lee, Keon-Joo Lee, Kyung-Ho Yu, Geert Jan Biessels and Hee-Joon Bae in International Journal of Stroke
Data Availability Statement
Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.