Abstract
Purpose:
To compare the efficacy, safety, and tolerability of waterfree cyclosporine formulation (CyclASol) at 2 concentrations (0.1% and 0.05% of cyclosporine [CsA]) to vehicle when applied twice daily for 16 weeks in patients with dry eye disease (DED). An open-label Restasis (Allergan, Irvine, CA) arm was included to allow a direct comparison with an approved therapy.
Design:
An exploratory phase II, multicenter, randomized, vehicle-controlled clinical trial, double-masked between CyclASol and vehicle with an open-label comparator.
Participants:
Two hundred and seven eligible patients with a history of dry eye disease were randomized 1:1:1:1 to 1 of 4 treatment arms (CyclASol 0.05%, n = 51; CyclASol 0.1%, n = 51; vehicle, n = 52, and Restasis, n = 53).
Methods:
After a 2-week run-in period with twice-daily dosing of Systane Balance (Alcon, Fort Worth, TX), patients were randomized to the respective treatment arm and dosed twice daily for 16 weeks.
Main Outcome Measures:
The study was set up to explore efficacy on a number of sign and symptom end points including total and subregion corneal fluorescein staining, conjunctival staining, visual analog scale (VAS) for dry eye symptoms VAS severity, and Ocular Surface Disease Index (OSDI) questionnaire.
Results:
CyclASol showed a consistent reduction in corneal and conjunctival staining compared with both vehicle and Restasis over the 16-week treatment period, with an early onset of effect (at day 14). A mixed-effects model–based approach demonstrated that the CyclASol drug effect was statistically significant over vehicle (total corneal staining P < 0.1, central corneal staining P < 0.001, conjunctival staining P < 0.01). This model-based analysis suggests a significant CyclASol effect for OSDI as symptom parameter (P < 0.01). The numbers of ocular adverse events were low in all treatment groups.
Conclusions:
CyclASol showed efficacy, safety, and tolerability at 2 concentrations in moderate-to-severe DED. In a direct head-to-head against open-label Restasis, CyclASol was found to have an earlier onset of action, as early as after 2 weeks of treatment, in relieving the signs of DED, as measured by corneal and conjunctival staining. The central region of the cornea, an important area for visual function in dry eye sufferers, was shown to have the most benefit from treatment. Excellent safety, tolerability, and comfort profile supports this new CsA formulation as having a positive benefit-to-risk ratio.
Dry eye disease (DED) is a chronic disease and, depending on the duration and severity, impacts quality of life (QoL) as negatively as other severe diseases.1 Cyclosporine A (CsA) is an anti-inflammatory agent with immunomodulatory properties and is often used in the treatment of moderate-to-severe DED. A challenge in the topical administration of CsA to the eyes is its high hydrophobicity, which impedes the use of common aqueous ophthalmic vehicles. Therefore, most studies dissolved CsA in olive oil or oil-based emulsions, which are poorly tolerated and result in low ocular availability owing to the short retention time on the ocular surface.2 Although the safety and efficacy of the commercially available emulsions Restasis (0.05% CsA, Allergan, Irvine, CA) and Ikervis (0.1% CsA, Santen, Tampere, Finland) could be demonstrated in several clinical trials, a high percentage of drug-related adverse events (AEs), probably related to the vehicle, were reported.3,4
Waterfree cyclosporine formulation (CyclASol) has been formulated to take advantage of the efficacy of CsA without the use of water, oils, surfactants, or preservatives. Using the novel EyeSol technology based on semifluorinated alkanes, the new formulation is a nonaqueous and clear solution, designed for improved ocular tolerability, local bioavailability, and early onset of efficacy. The clear solution spreads rapidly over the ocular surface owing to its low surface tension and minimizes visual disturbances compared with oily eye drops, ointments, and emulsions because of its refractive index being similar to water. At the same time, the small drop size (approximately 10 μl) minimizes overflow and destabilization of the tear film. An ex vivo model using rabbit corneas supports the higher bioavailability potential whereby after initial application, 0.05% CyclASol passes the corneal barrier in as little as 2.5 hours, while Restasis still had not penetrated after 8.5 hours.5 Work by Agarwal and colleagues6 in a porcine ex vivo model provides further evidence of this by the observation that 0.05% CyclASol had a significantly greater local bioavailability based on penetration into the cornea compared to Restasis and Ikervis (8-fold and 2-fold, respectively). The latter study also showed a dose response: CsA concentrations using CyclASol 0.1% were found to be double that of the 0.05% solution.6 These 2 formulations (0.05% and 0.1%) were chosen for clinical assessment because the clinically effective CsA concentration range was already well established. This was supported by an animal concentration response study with CyclASol, which had shown that 0.2% CsA does not correspond to greater efficacy than 0.1% CsA (Steven P, unpublished data, 2018).
This clinical study was undertaken to confirm the promising preclinical efficacy data5-8 and positive safety and tolerability data from the first-in-human phase I clinical trial. Additionally, gaining an understanding of the possible treatment effects in comparison to vehicle and a marketed CsA product, optimal dose, and estimation of effect sizes for different outcome measures were key objectives. The disease population targeted for this study was moderate-to-severe patients not currently responding to artificial tears in line with American Academy of Ophthalmology Preferred Practice Guidelines for DED.9 To get an understanding of what the optimal patient population for CyclASol is, the inclusion criteria were kept rather broad in this first exploratory patient study. However, patients with significant meibomian gland dysfunction were excluded because they potentially do not require an anti-inflammatory treatment.
Methods
Study Design
A phase II, multicenter, randomized, vehicle-controlled trial, double-masked between CyclASol and vehicle with an open-label comparator, Restasis, was performed to evaluate the safety, efficacy, and tolerability of CyclASol ophthalmic solution at 2 concentrations (0.05% and 0.1%) in patients with moderate-to-severe DED not responding to artificial tears.
The study was performed across 4 investigational sites in the United States (Andover Eye Associates, Andover, MA; MedRACS, LLC, Quincy, MA; Central Maine Eye Care, Lewiston, ME; and Eye Research Foundation, Newport Beach, CA) and enrolled patients through the already existing patient database. This study was reviewed and approved by the Institutional Review Board Alpha IRB (San Clemente, CA) and performed in accordance with the Health Insurance Portability and Accountability Act of 1996, the Declaration of Helsinki, the protocol, the International Conference on Harmonization guideline on Good Clinical Practices, and all other applicable local regulatory requirements and laws. The study was registered at www.clinicaltrials.gov (NCT02617667).
After informed consent was obtained, patients who met all eligibility requirements began the study with a 2-week run-in period using Systane Balance (Alcon, Fort Worth, TX) lubrication eye drops in each eye twice a day. After 2 weeks, patients returned and upon confirmation of eligibility criteria, patients were randomized into 1 of the 4 treatment arms, CyclASol 0.05%, CyclASol 0.1%, vehicle, or Restasis, in a 1:1:1:1 ratio by the Interactive Voice/Web Response System and were stratified based on total corneal fluorescein staining (CFS) score and visual analog scale (VAS) symptom dryness. Investigators, study staff, and patients were all masked to study treatment with the exception of the Restasis arm, which could not be masked owing to its unique single-use packaging and opacity of the emulsion. All other treatment arms used identical bottles, fill volumes, and labels. Treatment consisted of twice-daily dosing in each eye for a duration of 16 weeks. Patients attended a total of 6 study visits: visit 0, day −14, screening; visit 1, day 0, baseline/randomization; visit 2, week 2; visit 3, week 4; visit 4, week 12; and visit 5, week 16, study exit.
Patients
A total of 418 patients over the age of 18, with a patient-reported history of DED in both eyes, were screened from the 4 selected investigational sites. Patients were enrolled into the study if 1 eye (the same eye) met the following main inclusion criteria at screening and time of randomization: total CFS ≥6 (National Eye Institute [NEI] scale), VAS dryness ≥40, total lissamine green conjunctival score of ≥2 (Oxford scale), and Schirmer test I score between ≥2 mm and ≤8 mm.
Patients were excluded from participating if clinically significant slit-lamp findings or abnormal lid anatomy was observed at screening, including trauma, Stevens-Johnson syndrome, active blepharitis, meibomian gland dysfunction, or lid margin inflammation requiring therapeutic treatment; DED secondary to scarring; ocular or periocular malignancy; intraocular surgery or ocular laser surgery within the previous 6 months; active ocular allergies; use of contact lenses within 3 months before screening; ongoing ocular or systemic infection; history of herpetic keratitis; history of no response to previous topical CsA; and/or use of topical CsA within 6 months before screening.
Assessments of Outcome Measures
Eyes were eligible for primary analysis if they met all inclusion criteria and none of the exclusion criteria. In the case that both eyes were eligible for analysis, the “worse eye” was the eye with the highest total CFS score (NEI scale) at baseline. If the total CFS score at baseline was the same in both qualifying eyes, then the right eye was designated as the “worse eye.” Signs and symptoms of dry eye, in addition to safety parameters, were assessed in both eyes at screening, at baseline (day 0, predose), and again during the 4 follow-up visits (week 2, week 4, week 12, and week 16).
The study was set up to explore efficacy on a number of end points, including but not limited to total CFS (NEI scale, sum of 5 subregions, maximum score 15), subregion CFS (NEI scale, maximum score of 3; 0 corresponds to no staining, 3 corresponds to maximal staining), conjunctival staining (lissamine, Oxford scale, sum of temporal and nasal zone, each zone maximum score was 5, where 0 indicates no staining and 5 maximal staining, maximum score is 10), unanesthetized Schirmer I test, tear film break-up time, VAS severity for several parameters (dryness, burning/stinging, sticky feeling, foreign body sensation, itching, blurred vision, sensitivity to light, pain and frequency for dryness; scale 0 to 100%, where 0% corresponds to no symptoms and 100% corresponds to maximum symptoms), Ocular Surface Disease Index (OSDI) questionnaire, tear osmolarity, Human Leukocyte Antigen-Antigen D Related (HLA-DR) determination on ocular surface (methodology as previously described),10,11 and dry eye symptoms as recorded in a diary.
Treatment-emergent AEs (TEAEs) were defined as AEs occurring after the first dose of randomized study treatment was administered. The investigator determined severity and relationship to the drug. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 18.1.
Statistical Methods
The prespecified primary treatment comparisons were between CyclASol (0.05% and 0.1%) and vehicle in the worse eye. All other comparisons (e.g., against Restasis) were secondary. The 2 prespecified primary end points were change from baseline in total CFS (clinical sign, NEI scale) and VAS dryness (patient symptom) in the worse eye after 16 weeks of treatment. The primary efficacy end points (e.g., change from baseline to visit 5 [week 16] in total CFS [NEI scale] and dryness severity VAS) were analyzed separately using an analysis of covariance model (ANCOVA) with terms for baseline value and treatment (including all 4 treatment groups). Treatment group least square mean differences from the model were presented with P values and 2-sided 95% confidence intervals (CIs). Other comparisons between treatment groups were performed using a similar model. Primary efficacy analysis was performed on the full analysis set population for worse eye, which included all randomized patients having received at least 1 dose of study medication and evaluated for all available data. These patients were analyzed as treated. However, all patients were treated as randomized; therefore the full analysis set population is equivalent to an intent-to-treat population.
This exploratory study was expected to enroll 50 patients in each of the 4 treatment arms, for a total of 200 randomized patients. Assuming a 5% dropout rate, 47 patients per treatment group were expected to complete the study. No formal sample size calculation was performed owing to the study’s exploratory nature and the unknown vehicle effect. A sample size of 47 patients per group would have allowed to detect a difference of 0.93 units in change from baseline in CFS between CyclASol and vehicle with a 2-sided 95% CI if the common standard deviation in total CFS were 2.3 units (NEI scale).
Model-based Approach
The repeated measurements of the response variables were modeled in NONMEM (ICON Development Solutions [Ellicott City, MD] version 7.3) using a nonlinear mixed-effects modeling approach. Nonlinear mixed-effects models include both fixed and random effects. Whereas fixed effects are constant across individuals, random effects vary and account for interindividual variability. The goal of this analysis was to identify signs and symptoms that revealed a robust improvement in CyclASol treatment groups relative to vehicle either in the whole population or in subpopulations prone to respond to treatment. Moreover, it was evaluated whether a dose–response relationship could be observed. Response signs such as total, inferior, and central CFS (NEI scale) and conjunctival staining in both regions (Oxford scale) and response symptoms (VAS severity and frequency of dryness, VAS worst baseline symptom, total OSDI score, questions 6–9 of the OSDI, OSDI reading question) were used for the analysis.
Model development started with the simplest model structure and proceeded to more complex models. First, vehicle models of increasing complexity were inferred using data from the vehicle group only. At this stage it was evaluated how to best deal with those response variables for which 2 eyes could qualify for analysis (i.e., it was tested whether the data could be better described by clustering on the level of individual patients or individual eyes). Once a suitable vehicle model was identified, it was evaluated on the combined data set. Subsequently, it was tested whether the inclusion of a drug effect led to an additional improvement of the model fit. The modeling analysis was prespecified in aModeling and Simulation analysis plan.
Results
From the 418 patients screened, 207 were enrolled into the study, with the first patient enrolled in January 2016 and the last patient being evaluated in August 2016. The 207 patients were randomized into their respective treatment groups as follows: 51 to the CyclASol 0.05% group, 51 to the CyclASol 0.1% group, 52 to the vehicle group, and 53 to the Restasis group. A total of 211 patients were screen failures for the following reasons: unmet inclusion criteria (150), met exclusion criteria (51), investigator discretion (3), unknown (3), unacceptable laboratory values (2), withdrawal of consent (1), and other (1). A total of 98% of the patients in both CyclASol groups, 98% in Restasis and 92% in the vehicle-treated group, completed the study (Table 1). The distribution of age, gender, and baseline disease characteristics between all 4 treatment groups were well balanced, with no significant differences emerging (Table 2). The mean age across all treatment groups was 62.4±11.4 years and of the 207 patients, 153 (73.9%) were female.
Table 1.
Study Population Characteristics
| CyclASol 0.05% | CyclASol 0.1% | Vehicle | Restasis | |
|---|---|---|---|---|
| Randomized patients, n | 51 | 51 | 52 | 53 |
| Patients completing the study, n (%) | 50 (98.0) | 50 (98.0) | 48 (92.3) | 52 (98.1) |
| Patients discontinuing the study, n (%) | 1 (2.0) | 1 (2.0) | 4 (7.7) | 1 (1.9) |
| Reason for discontinuation, n (%) | ||||
| Adverse events | 1 (2.0) | 0 | 2 (3.8) | 1 (1.9) |
| Subject choice | 0 | 0 | 2 (3.8) | 0 |
| Other | 0 | 1 (2.0) | 0 | 0 |
Table 2.
Demographics and Baseline Characteristics for the Full Analysis Set
| CyclASol 0.05% (n = 51) | CyclASol 0.1% (n = 51) | Vehicle (n = 52) | Restasis (n = 53) | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 13 (25.5%) | 15 (29.4%) | 13 (25.0%) | 13 (24.5%) |
| Female | 38 (74.5%) | 36 (70.6%) | 39 (75.0%) | 40 (75.5%) |
| Age | ||||
| Mean (years) | 64.3±10.72 | 61.1±12.29 | 61.3±10.45 | 62.8±11.90 |
| <65 years, n (%) | 26 (51.0%) | 32 (62.7%) | 31 (59.6%) | 35 (66.0%) |
| ≥65 years, n (%) | 25 (49.0%) | 19 (37.3%) | 21 (40.4%) | 18 (34.0%) |
| Total CFS (NEI scale) | 8.78±2.138 | 8.75±1.885 | 8.71±2.145 | 8.83±1.889 |
| Central CFS (NEI scale) | 1.49±0.612 | 1.37±0.631 | 1.40±0.721 | 1.36±0.653 |
| Total conjunctival staining (Oxford scale) | 4.12±1.381 | 3.94±1.348 | 4.04±1.220 | 4.70±1.624 |
| Unanesthetized Schirmer test I | 5.10±2.128 | 5.16±2.158 | 4.63±2.327 | 4.42±1.896 |
| MMP9, n (%) | ||||
| Positive | 27 (52.9%) | 18 (35.3%) | 26 (50.0%) | 32 (60.4%) |
| Negative | 24 (47.1%) | 33 (64.7%) | 26 (50.0%) | 21 (39.6%) |
| Severity of dryness VAS | 64.18±15.058 | 64.51±14.251 | 64.63±15.449 | 63.94±15.318 |
| Total OSDI score | 38.02±18.653 | 40.37±19.317 | 37.91±16.477 | 35.38±16.641 |
CFS = corneal fluorescein staining; MMP9 = Matrix Metallopeptidase 9; NEI = National Eye Institute; OSDI = Ocular Surface Disease Index; VAS = visual analog scale.
Protocol deviations were classified before database lock and unmasking. A total of 350 protocol deviations were recorded for the study. The majority were minor protocol deviations relating to improper protocol procedures, timing of re-consent, patient failure to follow instructions, patient noncompliance, use of prohibited concomitant medication, out-of-window study visits, study drug instillation and assignment error, and study drug randomization process. Eight major protocol deviations were recoded; these were failure to follow instructions (2) and use of prohibited concomitant medication (6). No protocol deviations affected proper enrollment of patients, and all patients were treated as randomized.
Efficacy
The change from baseline at visit 5 (week 16) in total CFS (NEI scale) was the primary sign end point. The CyclASol and vehicle groups exhibited a consistent and statistically significant decrease in the total CFS score after the 16-week treatment period (Fig 1A). Improvements in CFS staining in both CyclASol groups began early, indicating an onset effect as early as after 4 weeks. When compared directly with Restasis, both CyclASol groups demonstrated a large and statistically significant reduction in mean total CFS staining at week 4 (CyclASol 0.05% −1.92±2.108; CyclASol 0.1% −1.88±2.046 vs. Restasis −0.85±2.476, with P = 0.0104 and P = 0.0100, respectively); in addition, the reduction in the CyclASol 0.1% group (−2.18±2.378) was significant (Restasis: −1.17±2.603, P = 0.0272) at week 12. Additional analysis using a nonlinear mixed-effects modeling approach showed that CyclASol trends for a statistically significant drug effect when compared with the vehicle (P < 0.1). The best modeling results were obtained when accounting for interindividual variability on the patient level rather than the level of individual eyes (i.e., if both eyes of a patient qualified for analysis, the data from both eyes were regarded as equal measurements of the underlying disease and its progression). Moreover, the baseline total CFS value was identified as a significant linear covariate on the maximal drug effect for patients with a baseline total CFS value larger than the population median value of 9. Consequently, the maximal drug effect in patients with a baseline value of 13 was predicted to be almost twice as large as the maximal drug effect in patients with a median baseline value.
Figure 1.
A, Mean change from baseline for total corneal fluorescein staining over the treatment period for worse eye in the full analysis set (FAS) population. B, Mean change from baseline for central corneal fluorescein staining over the treatment period for worse eye in the FAS population. (1) Statistically significant vs. Restasis (P ≤ 0.05) in analysis of covariance (ANCOVA); (2) statistically significant vs. vehicle (P ≤ 0.05) in ANCOVA. The National Eye Institute (NEI) scale was used to calculate the score. The cornea is divided into 5 regions: central, inferior, superior, nasal and temporal. Each region is graded from 0 to 3, where 0 indicates no staining and 3 maximal staining. The total score is the sum of all 5 regions (maximum score of 15).
The central area of the cornea proved to have the most benefit upon treatment with CyclASol. Both CyclASol groups showed a consistently larger reduction in staining when compared with vehicle. In particular, the change from baseline at week 4 in the worse-eye analysis of the central region showed that CyclASol 0.1% significantly reduced the extent of staining (and thus corneal damage) when compared with vehicle (CyclASol 0.1% −0.31±0.761; vehicle −0.08±0.752, P = 0.0299). At the same visit, both CyclASol 0.05% (−0.34±0.658, P = 0.0112) and CyclASol 0.1% (−0.31±0.761, P = 0.0025) were found to have a statistically significant reduction in central corneal staining when compared with Restasis (0.06±0.802), further demonstrating the early-onset effect of CyclASol (Fig 1B). Nonlinear mixed-effects modeling of the central CFS identified CyclASol as having a highly significant drug effect compared with vehicle (P < 0.001). Again, the best modeling results were obtained when accounting for interindividual variability on the patient level. The influence of the baseline central CFS value on the drug effect was not evaluated.
Improvements were also observed in the total conjunctival staining score (Oxford) using lissamine green staining. Both CyclASol groups showed a consistent reduction in staining throughout the treatment period. CyclASol 0.05% had the most statistically significant change at week 12 (−0.82±1.438, P = 0.0223) in comparison with vehicle (−0.24±1.238) and at week 4 (−0.74±1.103, P = 0.0321) when compared with Restasis (−0.38±1.270) in the worse-eye analysis (Fig 2). The drug effect of CyclASol on conjunctival lissamine green staining became statistically significant over vehicle (P < 0.01) when analyzed by nonlinear mixed-effects modeling.
Figure 2.
Mean change from baseline for total lissamine green conjuctival staining over the treatment period for worse eye in the full analysis set population. (1) Statistically significant vs. Restasis (P ≤ 0.05) in analysis of covariance (ANCOVA); (2) statistically significant vs. vehicle (P ≤ 0.05) in ANCOVA. Staining followed the Oxford grading scale and was the sum score of the nasal and temporal zones. Each zone is graded from 0 to 5, where 0 indicates no staining and 5 maximal staining. Maximum total score is 10.
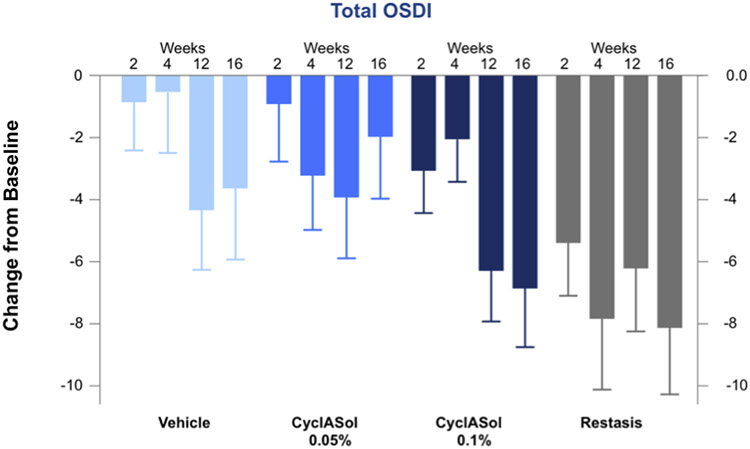
All treatment groups showed an improvement in dryness symptoms, as measured by VAS (the primary symptom end point), with no statistical difference between CyclASol and vehicle groups. Respectively, total OSDI scores improved across all treatment groups (Fig 3), with CyclASol 0.1% showing a more pronounced effect on total OSDI compared with vehicle. In particular, both CyclASol groups demonstrated a more numerically pronounced effect in OSDI subscale questions 6–9 (relating to reading, driving at night, working with a computer or bank [i.e., automated teller] machine, and watching TV) in comparison with vehicle. The improvement in the reading question from the OSDI reached statistical significance over vehicle when both CyclASol groups were pooled. Numerically, patients in the Restasis group seemed to have the largest response in OSDI and these differences reached significance over the 0.05% CyclASol group. However, there were design limitations regarding the symptom comparison with Restasis: the open-label nature of the arm likely introduced bias toward more positive responses in the Restasis arm. Analysis using the nonlinear mixed-effects model indicates a highly significant drug effect for CyclASol in total OSDI (P < 0.001). The baseline total OSDI score was identified as a significant covariate in the CyclASol groups, with patients with a higher baseline score exhibiting a stronger improvement. Moreover, nonlinear mixed-effects models also indicate a significant drug effect in OSDI questions 6–9 (P < 0.01) when compared with vehicle. When restricting the analysis to patients who had a baseline score of 15 or greater for questions 6–9, for both CyclASol groups, a highly significant drug effect (P < 0.001) was found, with the maximal drug effect being 30% larger than the maximal drug effect obtained without the baseline restriction.
Figure 3.
Mean change from baseline for the total Ocular Surface Disease Index (OSDI) score over the treatment period for worse eye in the full analysis set population.
The remaining measures had no significant change during the treatment period. Of note, HLA-DR was found to decrease across all groups, with a more pronounced effect in the active treatment arms (CyclASol and Restasis) as expected, whereas tear production was shown to increase across all treatment groups. No significant drug effect could be identified for inferior corneal staining or the VAS parameters when using the nonlinear mixed-effects model.
Safety
A total of 65 patients (31.4%) reported TEAEs, all of which were of mild-to-moderate intensity: 18 patients (35.3%) in the CyclASol 0.05% group, 12 patients (23.5%) in the CyclASol 0.1% group, 14 patients (26.9%) in the vehicle group, and 21 patients (39.6%) in the Restasis group. All ocular TEAEs that occurred in at least 2 patients are listed in Table 3.
Table 3.
Treatment-Emergent Adverse Events Observed in At Least 2 Patients
| CyclASol 0.05% (n = 51) | CyclASol 0.1% (n = 51) | Vehicle (n = 52) | Restasis (n = 53) | |
|---|---|---|---|---|
| Patients with any TEAE* | 18 (35.3%) | 12 (23.5%) | 14 (26.9%) | 21 (39.6%) |
| Patients with at least 1 ocular TEAE | 7 (13.7%) | 7 (13.7%) | 8 (15.4%) | 11 (20.8%) |
| Ocular TEAEs† | ||||
| Visual acuity related | 2 (3.9%) | 4 (7.8%) | 1 (1.9%) | 4 (7.5%) |
| Conjunctival hemorrhage | 1 (2.0%) | 0 | 0 | 2 (3.8%) |
| Eye irritation | 0 | 0 | 2 (3.8%) | 1 (1.9%) |
| Vision blurred | 0 | 1 (2.0%) | 0 | 2 (3.8%) |
| Eye pain | 1 (2.0%) | 0 | 1 (1.9%) | 0 |
| Instillation site pain | 1 (2.0%) | 1 (2.0%) | 1 (1.9%) | 2 (3.8%) |
| Conjunctivitis | 0 | 1 (2.0%) | 1 (1.9%) | 1 (1.9%) |
TEAE = treatment-emergent adverse event.
TEAE is defined as an adverse event occurring after the first dose of randomized study treatment.
Medical Dictionary for Regulatory Activities Version 18.1.
In total, 3 patients (1.2%) withdrew from the study as a result of a TEAE. The 3 TEAEs were ocular in nature and 2 were considered at least possibly related to treatment (CyclASol 0.05% group, eye pain; vehicle group, chemical eye injury). Three treatment-emergent serious AEs were reported during the study; however none were considered related to study treatment and all had recovered by the end of the study. The serious AEs reported during the study were myocardial infarction (CyclASol 0.1%), acute coronary syndrome (CyclASol 0.1%), and ovarian cyst (Restasis). No deaths were reported.
Across all treatment groups, no clinically significant findings were detected in any laboratory assessment, and no significant changes were observed by slit-lamp biomicroscopy or dilated funduscopy. No appreciable change in mean visual acuity or intraocular pressure from baseline was observed.
No clear difference between the 2 CyclASol concentrations was observed in signs, symptoms, or safety parameters analyzed for the clinical data, and this finding was supported by the modeling data.
Discussion
This exploratory phase II clinical trial investigated the efficacy, safety, and tolerability of the novel aqueous-free CsA formulations CyclASol 0.05% and CyclASol 0.1% in comparison with vehicle (masked) and Restasis (open-label) in patients with moderate-to-severe DED not responding to artificial tears.
The CyclASol groups showed a consistent reduction in total CFS, CFS in most subregions (NEI scale), and conjunctival staining (Oxford scale) compared with vehicle and Restasis. There were no clear differences in these parameters between the 2 CyclASol concentrations. The improvements in corneal and conjunctival staining parameters in the 2 CyclASol groups started early, between 2 and 4 weeks, depending on the parameter measured. These observations were supported by the modeling analysis, showing statistical significance of the findings. This analysis technique is a powerful and flexible regression tool in an exploratory setting, as it takes all available data across visits and patients into the analysis to account for the temporal progression of the response variables.12,13 Moreover, a covariate analysis of total CFS, total OSDI score, and OSDI score of questions 6–9 suggested that patients with larger baseline values benefited more from treatment with CyclASol than CyclASol-treated patients with lower baseline values.
Most symptom end points improved over the course of the study, with statistical significance, compared with baseline values. CyclASol elicited promising improvements in the total OSDI score compared with vehicle for both concentrations, with 0.1% trending toward a greater effect in relieving symptoms. The visual function subscale had the greatest effect. This is consistent with the observation that central corneal staining improved with CyclASol treatment because the central region is considered highly relevant, as studies have shown that central CFS in dry eye leads to visual function deficiencies.14-16 This result is clinically important, as studies have shown that patients with DED frequently report increased difficulties with regard to reading, driving, and using visual displays,17 which has a significant impact on patients’ QoL.18,19
Both the early onset of effect and the improvement of the central area of the cornea can likely be attributed to the novel formulation. The low surface and interface tensions lead to superior spreading behavior, potentially forming a protective layer on the ocular surface, which by itself may positively affect the signs and symptoms of DED, as shown by the statistically significant improvement over baseline in almost all parameters in this study. Moreover, CyclASol dissolves CsA to form a clear solution; thus it is directly available, which is in contrast to emulsions requiring liberation from micelles. Both features, spreadability and solution, lead to improved local availability and faster onset of action compared with Restasis, as previously shown in preclinical studies5-8 and now confirmed by clinical data. Furthermore, the formulation does not contain any surfactants, buffers, or preservatives, which might counteract the beneficial CsA effects in other formulations.
The CFS results, taken together with the OSDI scores, show that CyclASol effectively improves both the signs and symptoms of DED. The magnitude of improvement in ocular surface staining parameters combined with symptom improvement and its early onset is considered clinically meaningful. The central area of the cornea benefited the most after treatment with CyclASol, adding to the clinical significance of the finding. Although there is generally a low correlation between the signs and symptoms of DED,20-23 there is growing evidence that slower reading speeds are highly correlated to higher corneal staining.24,25 Applying reading tests in future clinical trials could in fact capture the functional impact of dry eye before and after treatment. Reading ability is a symptom of dry eye that has also been found to directly correlate with the signs of dry eye, in particular with corneal staining.25 Moreover, difficulty with reading is highly relevant for patients, as it affects QoL and may even affect employment or decrease work productivity.
Safety and tolerability of both concentrations of CyclASol were found to be excellent and 98% of enrolled patients completed the study. No meaningful imbalances among treatment groups in either ocular or nonocular TEAEs were detected. No TEAEs were categorized as severe by the investigator, with most TEAEs classified as mild in severity. No serious ocular-related TEAEs were reported for any treatment group, and the most common ocular TEAE in patients was reduced visual acuity. The formulation of CyclASol is also likely attributed to the low incidence rate of ocular AEs compared with other CsA formulations. During the 2 Ikervis phase III studies, 42.6% and 42.9% of patients on the active treatment arm reported ocular TEAEs and 26.8% and 30.0% in the vehicle arm, respectively, after 6 months of treatment,26,27 which is more than double what was reported during this clinical phase II study for the Restasis arm. The most common side effect in Restasis clinical trials was found to be ocular burning (17%)28; in comparison, this study had no reports of eye irritation (MedDRA preferred term for ocular burning) in either CyclASol group and reports in only 3.8% for the vehicle group and 1.9% for the Restasis group.
One of the limitations to this trial was that the active competitor treatment arm, Restasis, was open label, owing to differences in drop behavior and packaging (CyclASol is a clear solution in multidose bottle, whereas Restasis is a white opaque emulsion in single-unit-dose packaging). Restasis is currently the state-of-the-art treatment for dry eye, and that in itself possibly introduces inherent bias on patient-reported symptom parameters (e.g., VAS, OSDI, and side effects). Secondly, the vehicle for Restasis is a water-based emulsion, which may have different effects on subjective patient-reported symptoms, and the alternative perception at instillation may introduce bias to the patient-reported outcomes in this group. This bias is supported by the low AE rate observed during this trial compared with published Restasis studies.3,29 Despite this, in the head-to-head comparison of CyclASol and the established therapy Restasis, CyclASol demonstrated a more pronounced effect on the clinical objective signs of DED throughout the study by improving the damage to the ocular surface, as measured by corneal and conjunctival staining. Furthermore, CyclASol elicited an earlier onset of action compared with Restasis in as little as after 2 weeks of treatment.
In summary, this phase II clinical study demonstrates efficacy of CyclASol in DED. Corneal and conjunctival staining sign end points were consistently improved relative to vehicle and Restasis over the 16-week treatment period. In addition to an early onset of action, the central region of the cornea, which is an important area for the visual function, seemed to have the most benefit from treatment with CyclASol. Improvements were also observed in OSDI scores, particularly in the visual function subscale, which has been shown to highly correlate to QoL. Both CyclASol concentrations showed excellent safety, tolerability, and comfort profiles.
Abbreviations and Acronyms:
- AE
adverse event
- CFS
corneal fluorescein staining
- CI
confidence interval
- CsA
cyclosporine A
- DED
dry eye disease
- MedDRA
Medical Dictionary for Regulatory Activities
- NEI
National Eye Institute
- OSDI
Ocular Surface Disease Index
- QoL
quality of life
- TEAE
treatment-emergent adverse event
- VAS
visual analog scale
Footnotes
Presented as a poster at the American Academy of Ophthalmology Annual Meeting in New Orleans, Louisiana, November 11–14, 2017
Financial Disclosures:
The authors made the following disclosures: The sponsor of the study participated in the design of the study, data interpretation and preparation, review and approval of the manuscript. D.L.W.: Research grants – Novaliq, Kala, Allergan, Shire, Novartis.
G.L.T.: Research grants – Novaliq, HanAll, ReGenTree, Oculeve, Inc., Topivert Pharma Ltd, Mentholatum Co., Allergan, Axerovison, Ora, Inc.
H.R.M.: Research grants – Novaliq.
J.D.L.: Research grants – Novaliq.
G.W.O.: Employee – ORA Inc., the Clinical Research Organization used for the study.
J.B.C.: Consultant – ORA Inc.
G.J., M.B., and P.S.: Consultants – Novaliq.
S.K.: Employee – Novaliq.
HUMAN SUBJECTS: Human subjects were included in this study. This study was reviewed and approved by the Institutional Review Board Alpha IRB (San Clemente, CA) and performed in accordance with the Health Insurance Portability and Accountability Act of 1996, the Declaration of Helsinki, the protocol, the International Conference on Harmonization guideline on Good Clinical Practices, and all other applicable local regulatory requirements and laws. The study was registered at www.clinicaltrials.gov (NCT02617667). Patients’ informed consent was obtained.
No animals were used in this study.
References
- 1.Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419. [DOI] [PubMed] [Google Scholar]
- 2.Lallemand F, Felt-Baeyens O, Besseghir K, et al. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56:307–318. [DOI] [PubMed] [Google Scholar]
- 3.Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 study group, two randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107:631–639. [DOI] [PubMed] [Google Scholar]
- 4.Leonardi A, Messmer EM, Labetoulle M, et al. Efficacy and safety of 0.1% ciclosporin A cationic emulsion in dry eye disease: a pooled analysis of two double-masked, randomized, vehicle controlled phase III clinical studies. Br J Ophthalmol. 2018;0:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutescu RM, Panfil C, Merkel UM, et al. Semifluorinated alkanes as a liquid drug carrier system for topical ocular drug delivery. E J Pham Biopharm. 2014;88:123–128. [DOI] [PubMed] [Google Scholar]
- 6.Agarwai O, Scherer D, Günther B, Rupenthal ID. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int J Pharm. 2018;538:119–129. [DOI] [PubMed] [Google Scholar]
- 7.Gehlsen U, Braun T, Notara M, et al. A semifluoronated alkane (F4H5) as novel carrier for cyclosporine A: a promising therapeutic and prophylactic option for topical treament of dry eye. Graefes Clin Exp Ophthalmol. 2017;255:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steven P, Braun T, Krösser S, Gehlsen U. Influence of aging on severity and anti-inflammatory treatment of experimental dry eye disease. Klin Monbl Augenheilkd. 2017;234:662–669. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern® Guidelines. Dry Eye Syndrome. San Francisco, CA: American Academy of Ophthalmology; 2013. [Google Scholar]
- 10.Baudouin C, Brignole F, Becquet F, et al. Flow cytometry in impression cytology specimens. Invest Ophthalmol Vis Sci. 1997;38:1458–1464. [PubMed] [Google Scholar]
- 11.Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41(6):1356–1363. [PubMed] [Google Scholar]
- 12.Schoemaker RC, Cohen AF. Estimating impossible curves using NONMEM. Br J Clin Pharmacol. 1996;42:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson EN, Karlsson MO, Wade JR. Nonlinearity detection: advantages of nonlinear mixed-effects modeling. AAPS PharmSci. 2000;2:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ousler GW III, Brazzell K, Durham T, et al. A correlation between central corneal staining and visual function in patients diagnosed with dry eye. Invest Ophthalmol Vis Sci. 2007;48:410. [Google Scholar]
- 15.Kaido M, Matsumoto Y, Shigeno Y, et al. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci. 2011;52:9516–9522. [DOI] [PubMed] [Google Scholar]
- 16.Koh S Mechanisms of visual disturbance in dry eye. Cornea. 2016;35:83–88. [DOI] [PubMed] [Google Scholar]
- 17.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. [DOI] [PubMed] [Google Scholar]
- 19.Grubbs JR Jr, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measurements in dry eye questionnaires. Cornea. 2014;33:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlett JD, Kieth MS, Sudharshan L, Snedecor SJ. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs of among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44:4753–4761. [DOI] [PubMed] [Google Scholar]
- 22.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009;7:199–211. [DOI] [PubMed] [Google Scholar]
- 24.Ridder WH III, Zhang Y, Huang JF. Evaluation of reading speed and contrast sensitivity in dry eye disease. Optom Vis Sci. 2013;90:37–44. [DOI] [PubMed] [Google Scholar]
- 25.Mathews PM, Ramulu PY, Swenor BS, et al. Functional impairment of reading in patients with dry eye. Br J Ophthamol. 2017;101:481–486. [DOI] [PubMed] [Google Scholar]
- 26.Baudouin C, Figueiredo FC, Messmer EM, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of moderate to severe dry eye. Eur J Ophthalmol. 2017;27:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonardi A, Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26:287–296. [DOI] [PubMed] [Google Scholar]
- 28.Restasis™ PI. Highlights of Prescribing Information: Restasis™ 0.05%. Allergan. http://www.allergan.com. Accessed June 27, 2018. [Google Scholar]
- 29.Stonecipher KG, Torkildsen GL, Ousler GW III, et al. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]