Abstract
Acute cerebral infarct (ACI) is a severe subtype of ischemic stroke. microRNAs (miRNAs) are implicated in the pathogenesis of ACI. This study investigated the expression pattern and clinical implication of miR-124 in ACI patients. Serum samples were collected from 108 healthy people and 108 ACI patients at 24 h, 48 h, and 72 h. Serum miR-124 expression was tested using qRT-PCR. The levels of interleukin (IL)-6, IL-8, and C-reactive protein (CRP) were detected using ELISA kits. The correlations between miR-124 expression and infarct classification, infarct size, risk factors, and inflammatory factors were analyzed. The diagnostic efficacy of miR-124 in ACI was analyzed by the ROC curve. ACI patients were assigned to the miR-124 high/low expression group and the incidence of poor prognosis was compared between the two groups. miR-124 expression was poorly expressed in the serum of ACI patients. The area under the ROC curve of miR-124 in the diagnosis of ACI was 0.9527, the specificity was 91.67%, and the sensitivity was 93.52%. miR-124 expression in ACI patients was not affected by infarct classification, infarct size, low-density lipoprotein level, and homocysteine level. miR-124 expression was negatively correlated with IL-6, IL-8, and CRP in ACI patients. Low expression of miR-124 was positively correlated with the poor prognosis of ACI. miR-124 was poorly expressed in the serum of ACI patients and served as a biomarker for the diagnosis and prognosis. This study shall confer a promising novel target for the diagnosis and treatment of ACI.
Keywords: acute cerebral infarct, diagnosis, miR-124, IL-6, IL-8, C-reactive protein, ROC curve, prognosis
Introduction
Acute cerebral infarction (ACI) refers to the disruption of blood supply to brain tissues caused by intracranial and extracranial artery stenosis or occlusion, resulting in ischemic necrosis or cerebromalacia because of focal tissue ischemia and hypoxia. 1 Without rapid clinical decision-making, ACI may result in severe outcomes, such as disability or even death; consequently, early diagnosis of ACI is crucial for the improvement of curative effects and the reduction of potential sequelae and mortality. 2 Currently, ACI diagnosis mainly relies on neuroimaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), nevertheless, the availability of this expensive device is restricted and image interpretation may be inconsistent in the early stage of stroke, which limits the efficiency of decision-making in ACI. 3 The lack of rapid and effective clinical decision-making in ACI could result in serious consequences. The blood sample is the most readily available material in monitoring brain changes in acute ischemic stroke and rehabilitation of ischemic stroke. Finding novel blood biomarkers for early diagnosis of ACI has critical clinical implications and application value to identify the etiology, guide individualized treatments, and predict disease progression and prognosis. 4 This study is expected to explore effective diagnostic factors and therapeutic targets as the aid of complementary methods to the current diagnostic strategy, and thus provide a new idea for therapeutic targets of ACI via survival and prognostic analysis.
microRNAs (miRNAs) are a group of endogenous small non-coding RNAs (about 22 nt) that can repress the biological functions of target genes by binding to the 3′’UTR of mRNAs. 5 miRNAs are stably expressed in the serum and the detection method of circulating miRNAs is sensitive and accurate, which means that miRNAs have the potential to act as the effective biomarkers for the diagnosis of various diseases. 6 Accumulating studies have unveiled the aberrant expression of miRNAs in the brain tissues and peripheral blood of ACI patients, implying that miRNAs are implicated in the pathogenesis, diagnosis, and prognosis of ACI. 2,7,8 miR-124 is identified as one of the most abundant miRNAs in the human brain that modulates variant biological functions of the central nervous system (CNS), and dysregulated miR-124 is tightly associated with stroke. 9 The elevated miR-124 expression has the property to reduce the infarct size and facilitate the recovery of neurological functions post-ischemic stroke. 10 Notably, miR-124 participates in the pathophysiological process of ischemic brain injury, and plasma miR-124 is identified as a potential biomarker for the time-sensitive diagnosis of ACI. 3 However, whether serum miR-124 expression in ACI is correlated with cerebral infarction classification, risk factors, and inflammatory factors remains unidentified yet. Moreover, whether miR-124 can assist in the diagnosis and prognosis of ACI needs to be further determined. Thereupon, this study measured miR-124 expression in the serum of ACI patients and explored its clinical implication and possible regulatory roles, which shall confer a certain reference value for the diagnosis and prognosis of ACI.
Materials and Methods
Ethics Statement
The study was approved by the Clinical Ethical Committee of the East District of Weifang Traditional Chinese Medicine Hospital. All subjects had signed the informed consent.
Subjects and Grouping
Totally 108 ACI patients (≤ 24 h between the onset of stroke and hospitalization) admitted to the East District of Weifang Traditional Chinese Medicine Hospital from January 2015 to December 2015 were recruited in this study. The diagnosis of ACI was strictly based on the diagnostic criteria in Chinese Guidelines of Diagnosis and Treatment of Acute Ischemic Stroke and confirmed by cranial CT or MRI. Inclusive criteria were as follows: (1) first onset of disease; and (2) the onset time was less than 24 hours. Patients with malignancies, severe liver and kidney dysfunction, intracranial hemorrhage, and acute infarction for over 24 hours were excluded. All patients received standardized treatments (anticoagulant: heparin injection or oral anticoagulants, antiplatelet drugs: aspirin, clopidogrel, sodium ozagrel) immediately. The healthy control subjects, 108 unrelated individuals, were also selected from the physical examination center of the East District of Weifang Traditional Chinese Medicine Hospital. For the control group, patients with coronary heart disease, transient ischemic attack, hemorrhagic stroke, Parkinson’s disease, dementia, tumor, or acute infection were excluded. According to the modified TOAST classification, 11 the causes of ACI were divided into atherothrombosis (AT), small artery disease (SAD), and cardioembolism (CE). According to the T2-weighted image of brain MRI, the maximum infarct size was determined as follows: large infarct (L, over 1 lobe of the brain, > 5.0 cm in diameter), medium infarct (M, less than one lobe, 3.1-5.0 cm), small infarct (S, 1.6-3.0 cm), and lacunar infarct (LI, < 1.5 cm). Blood samples were collected at 24, 48, and 72 h after the onset of ACI. For the control group, fasting blood was collected on the morning of the second day post-admission. The blood was centrifuged at 1000 g for 10 min and stored at −80°C. The biochemical indexes including body mass index (BMI), blood pressure, atrial fibrillation, prothrombin time (PT), activated partial thromboplastin time (APTT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), lipoprotein (a) (Lp(a)), homocysteine (Hcy) were examined in all subjects.
Glasgow Score
Glasgow outcome scale was adopted to evaluate the short-term prognosis of ACI patients on the 30th day. Briefly, the evaluation was based on the 5 ranks of GOS: 5, mild disturbance of consciousness; 4, moderate disturbance of consciousness; 3, severe disturbance of consciousness; 2, persistent vegetative state; 1, death.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the serum using the RNAzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and reverse transcribed into cDNA using the cDNA reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc., MA, USA). SYBR® Premix Ex TaqTM kit (Takara Bio, Inc., Otsu, Japan) was used for quantitative PCR amplification. The relative expression of the gene was calculated based on the 2-ΔΔCt method, 12 with U6 as the internal control. Each sample was repeated 3 times independently. Table 1 shows the PCR primers.
Table 1.
Primer Sequences for qRT-PCR.
| Name of primer | Sequences |
|---|---|
| miR-148a-3p-F | GCGGCGGTAAGGCACGCGGTG |
| miR-148a-3p-R | ATCCAGTGCAGGGTCCGAGG |
| U6-F | CTCGCTTCGGCAGCACATATACTA |
| U6-R | ACGAATTTGCGTGTCATCCTTGCG |
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA kits [human LDL (ab270212), HDL (ab125961), Lp(a) (ab212165), Hcy (ab228559), IL-6 (ab178013), IL-8 (ab214030), and C-reactive protein (CRP) (ab260058)] were all from Abcam (Cambridge, MA, USA). Each well of enzyme-coated plates was added with 10 µL serum samples and 40 µL sample diluent, followed by a warm bath at 37°C for 30 min. The plates were washed 5 times and then added with 50 µL reagents, followed by a warm bath at 37°C for 30 min. Following washing, the plates were added with 50 µL chromogenic agent A and 50 µL chromogenic agent B in the dark at 37°C for 15 min. The reaction was terminated by adding 50 µL termination solution to each well, at which time the color changed from blue to yellow. The absorbance at a wavelength of 450 nm was determined with the Multiskan™ FC microplate reader (Thermo Fisher Scientific). The related factors were calculated according to the standard curve.
Statistical Analysis
Statistical analysis was conducted using SPSS21.0 (IBM Corp. Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). The counting data are expressed as the number of cases and percentage, and the measurement data are expressed as mean ± standard deviation. The chi-square test was used for the analysis of counting data between two groups. The t-test was applied for the analysis of measurement data between two groups. The 1-way analysis of variance (ANOVA) was applied to analyze the measurement data among multi-groups, followed by Tukey’s multiple comparisons test. The diagnostic value of miR-124 in ACI was analyzed using the receiver operating characteristic (ROC) curve. Pearson correlation analysis was used to observe the correlation between the measurement data. Kaplan-Meier curve was used to show the prognosis, and the log-rank test was used to determine the difference in the survival rate between groups. The P < 0.05 was indicative of statistical significance.
Results
Clinical Characteristics of Patients
Table 2 shows the clinical data of 108 patients in the ACI group. For TOAST classification, there were 94 cases (87.1%) of atherosclerosis (AT), 9 cases (8.3%) of small artery disease (SAD), and 5 cases (4.6%) of cardioembolism (CE). For infarct size, there were 25 cases (23.2%) of large infarct (L), 23 cases (21.3%) of medium infarct (M), 51 cases (47.2%) of small infarct (S), and 9 cases (8.3%) of lacunar infarct (LI). There was no significant difference in age and gender between the two groups (P > 0.05). In clinical indicators, no significant difference was observed in HDL and LP(a) between the two groups (P > 0.05), but there existed significant differences in BMI, LDL, and Hcy (P < 0.05).
Table 2.
Clinical Data of ACI Patients and Healthy Controls.
| ACI (n = 108) | Control (n = 108) | X 2/t | P | ||
|---|---|---|---|---|---|
| Age | 66.32 ± 11.51 | 64.46 ± 12.77 | 1.12 | 0.26 | |
| Male/female | 56/52 | 62/46 | 0.67 | 0.41 | |
| Etiological sub-types | AT | 94 (87.1%) | – | – | – |
| SAD | 9 (8.3%) | – | – | – | |
| CE | 5 (4.6%) | – | – | – | |
| Sub-types of infarct size | L | 25 (23.2%) | – | – | – |
| M | 23 (21.3%) | – | – | – | |
| S | 51 (47.2%) | – | – | – | |
| LI | 9 (8.3%) | – | – | – | |
| Smoking | 57 (52.8%) | 49 (45.4%) | 1.19 | 0.28 | |
| Drinking | 34 (31.5%) | 38 (35.2%) | 0.33 | 0.56 | |
| Hypertension | 39 (36.1%) | 26 (%) | 3.72 | 0.05 | |
| Atrial fibrillation | 14 (13.0%) | 8 (7.4%) | 1.82 | 0.18 | |
| BMI | 22.73 ± 2.74 | 19.36 ± 2.27 | 9.84 | < 0.001 | |
| PT (s) | 11.78 ± 1.34 | 12.09 ± 1.39 | 1.67 | 0.10 | |
| APTT (s) | 27.89 ± 2.95 | 28.63 ± 3.53 | 1.67 | 0.10 | |
| TC/ (mmoL/L) | 4.83 ± 0.37 | 3.61 ± 0.43 | 22.35 | < 0.001 | |
| TG/ (mmoL/L) | 1.82 ± 0.24 | 0.88 ± 0.33 | 23.94 | < 0.001 | |
| LDL (mmoL/L) | 3.59 ± 0.90 | 2.32 ± 0.73 | 11.39 | < 0.001 | |
| HDL (mmoL/L) | 1.16 ± 0.43 | 1.21 ± 0.24 | 1.06 | 0.29 | |
| Lp(a) (mg/mL) | 152.34 ± 36.47 | 142.29 ± 39.52 | 1.94 | 0.05 | |
| Hcy (mmoL/L) | 14.43 ± 5.43 | 11.14 ± 3.78 | 5.17 | < 0.001 | |
Serum miR-124 Showed High Diagnostic Efficiency for ACI
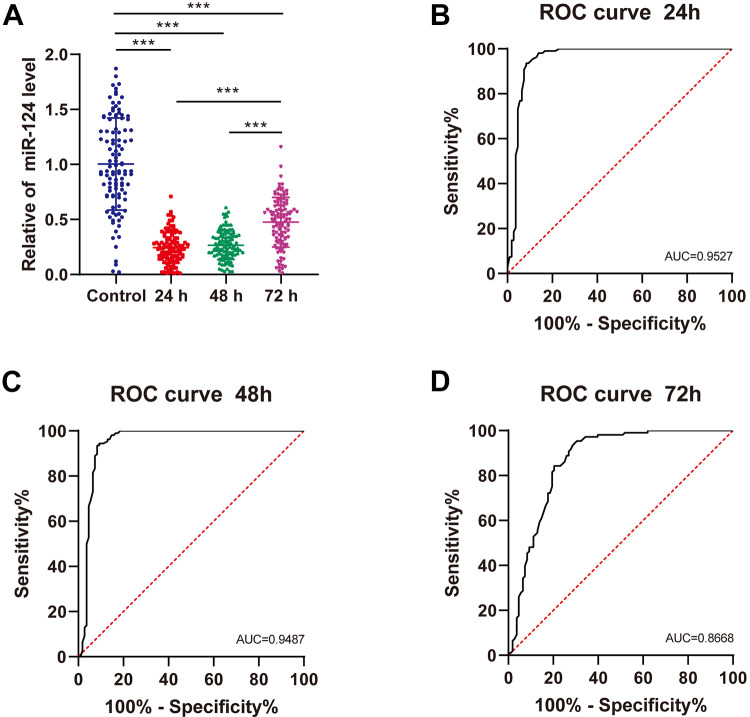
miR-124 expression in the serum of healthy controls and ACI patients at 24, 48, and 72 h was detected using qRT-PCR (Figure 1A). Compared with healthy controls, the ACI patients showed evidently reduced miR-124 expression at 24, 48, and 72 h (P < 0.001); and the serum miR-124 expression of ACI patients was notably increased at 72 h. The area under the ROC curve (AUC) of miR-124 expression in the serum of ACI patients at 24 h was 0.9527, the specificity was 91.67%, and the sensitivity was 93.52% (Figure 1B). The AUC of serum miR-124 expression in ACI patients at 48 h was 0.9487, the specificity was 91.67%, and the sensitivity was 93.52% (Figure 1C). The AUC of serum miR-124 expression in ACI patients at 72 h was 0.8668, the specificity was 71.30%, and the sensitivity was 93.52% (Figure 1D). Taken together, miR-124 was downregulated in the serum of ACI patients and had high clinical diagnostic efficiency.
Figure 1.
miR-124 was downregulated in the serum of ACI patients and had high clinical diagnostic efficiency. A, The miR-124 expression in the serum of healthy controls and ACI patients at 24, 48, and 72 h was detected using qRT-PCR. B-D, The ROC curve of Serum miR-124 expression at 24, 48, and 72 h in the diagnosis of ACI. Data in panel A were analyzed using 1-way ANOVA, followed by Tukey’s multiple comparisons test. *** P < 0.001.
Effect of Cerebral Infarction Classification and Risk Factors on miR-124 Expression
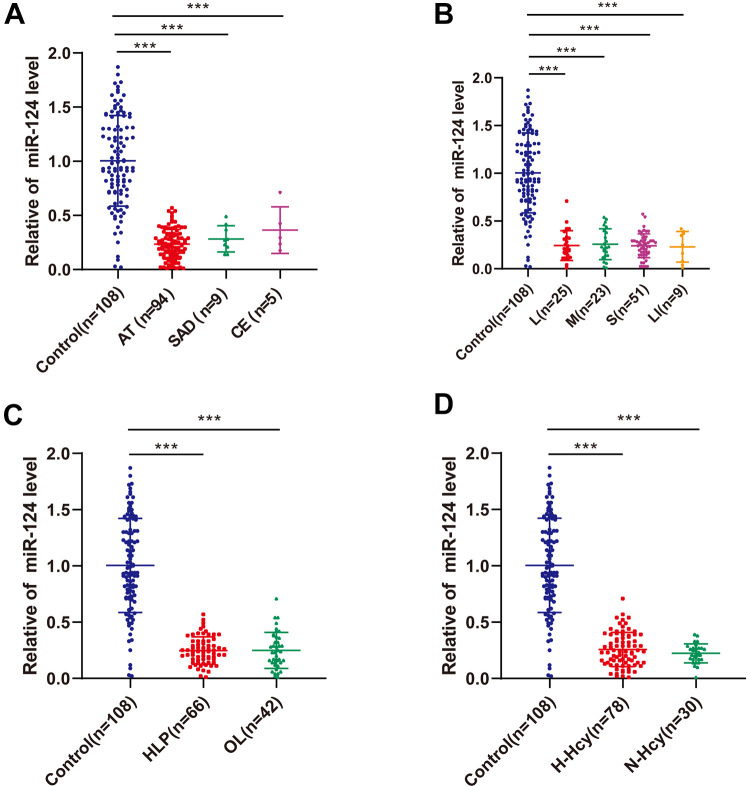
Serum miR-124 expression in ACI patients with variant etiologies was compared, and no significant difference was observed among groups (Figure 2A). Also, miR-124 expression showed no significant difference in patients with different cerebral infarct sizes (Figure 2B), but miR-124 expression in these groups was higher than that in the control group (P < 0.001). According to LDL and Hcy levels, ACI patients were assigned into the hyperlipidemia group (HLP, LDL ≥ 3.64 mmoL/L) and ortholiposis group (OL), as well as hyperhomocysteinemia group (H-Hcy, Hcy ≥ 10 μmoL/L), and normal homocysteine group (N-Hcy). qRT-PCR exhibited no significant difference in miR-124 expression between groups (Figure 2C-D). It was indicated that the serum miR-124 expression of ACI patients was not affected by the etiology, infarct size, and LDL and Hcy levels (P > 0.05).
Figure 2.
Effect of cerebral infarction classification and risk factors on miR-124 expression. A, The expression of serum miR-124 in healthy controls and ACI patients with different ACI classification was detected using qRT-PCR. B, The expression of serum miR-124 in healthy controls and ACI patients with different cerebral infarct sizes was detected using qRT-PCR. C, The expression of serum miR-124 in healthy controls and ACI patients in the hyperlipidemia group (HLP) and ortholiposis group (OL) was detected using qRT-PCR. D, The expression of serum miR-124 in healthy controls and ACI patients in the hyperhomocysteinemia group (H-Hcy) and normal homocysteine group (N-Hcy) was detected using qRT-PCR. Data were counting data and analyzed using 1-way ANOVA, followed by Tukey’s multiple comparisons test. *** P < 0.001.
Correlation Between Serum miR-124 and Inflammatory Factors in ACI Patients
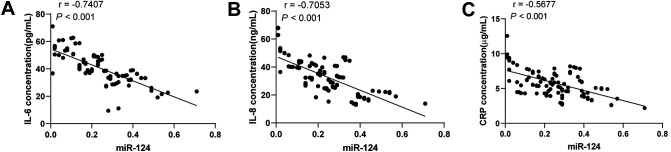
Next, we detected the serum levels of inflammatory factors IL-6, IL-8, and CRP using ELISA kits, and performed Pearson correlation analysis between miR-124 expression and cerebral infarction-related inflammatory factors (Figure 3A-C). The results showed that miR-124 was negatively correlated with IL-6, IL-8, and CRP (P < 0.001).
Figure 3.
miR-124 in ACI patients was negatively correlated with IL-6, IL-8, and CRP. The levels of IL-6, IL-8, and CRP were detected using ELISA kits. A, miR-124 was negatively correlated with IL-6. B, miR-124 was negatively correlated with IL-8. C, miR-124 was negatively correlated with CRP. Pearson correlation analysis was used for data analysis.
Serum miR-124 Expression Was Correlated With the Survival Prognosis and GOS Score of ACI Patients
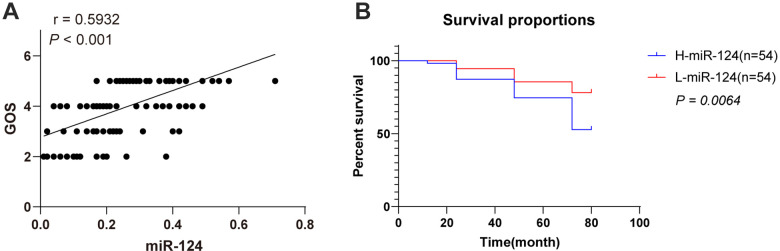
The correlation of serum miR-124 expression with short-term prognosis was analyzed by Pearson’s correlation coefficient based on the serum expression of miR-124 and GOS scores in ACI patients, and the result showed that miR-124 expression was positively correlated with GOS scores (P < 0.001, Figure 4A). According to the median value of serum miR-124 expression in ACI patients (0.24, 0.01-0.72), all participants were allocated to the H-miR-124 group (miR-124 high expression group) and L-miR-124 group (miR-124 low expression group). The patients with death outcomes within 5 years in each group were recorded, and the survival prognosis was analyzed (Figure 4B). The patients with low miR-124 expression exhibited an unfavorable survival prognosis (P < 0.05), indicating that miR-124 was correlated with the survival prognosis of ACI.
Figure 4.
Serum miR-124 expression was correlated with the survival prognosis of ACI patients. A, Pearson’s correlation coefficient based on the serum expression of miR-124 and GOS scores in ACI Patients. B, Kaplan-Meier curve showed the survival rate of ACI patients with different serum miR-124 expressions. A log-rank test was used to compare the differences between groups.
Discussion
ACI seriously impairs the physiology and psychology of patients and also poses heavy burdens to families and society. 13 Identification of ACI-specific serum markers is crucial for early diagnosis of ACI, which is conducive for physicians to make clinical decisions rapidly. 6 Emerging studies have unveiled the differential expression of miRNAs in the serum of ACI patients. 8,14 This study demonstrated the downregulation of miR-124 in the serum of ACI patients, with favorable diagnostic and prognostic value.
Dysregulated miRNA expression is implicated in the pathogenesis of ACI. For example, Chang et al have indicated that the upregulated miR-151a-3p expression in the serum can predict the severity of neurological impairment of ACI clinically. 15 Aberrant miR-124 expression leads to pathological conditions of CNS, and it is commonly identified as a diagnostic and prognostic biomarker of CNS disorders including stroke. 16 This study collected the serum of healthy people and ACI patients for the detection of miR-124 expression. Compared with the healthy controls, the ACI patients showed reduced miR-124 expression at 24, 48, and 72 h, and enhanced miR-124 expression at 72 h. The AUC of miR-124 in the diagnosis of ACI was 0.9527, the specificity was 91.67%, and the sensitivity was 93.52%. Consistently, Sun et al have demonstrated the downregulation of miR-124 in ACI patients and suggested that dynamic monitoring of miR-124 has the functionality to prevent and treating ischemic stroke. 3 In another study by Badacz et al, patients with low echo (vulnerable plaques) were characterized by low expression of miR-124-4p (P = 0.038) compared with internal carotid artery echo plaques, and risks of cerebral ischemia were higher for patients with vulnerable plaques. 17 In brief, miR-124 expression was declined in ACI patient serum and exhibited high clinical diagnostic efficiency.
Although a study by Gacon et al suggested that low expression of miR-124-3p was observed in ACI patients, but not in patients with acute ischemia in coronary artery territory; and indicated that miR-208b-3p, miR-34a-5p, miR-122-5p, and miR-499-5p were independently associated with the risk factors of future cerebral ischemia, but they failed to show that miR-124-5p was independently associated with risk factors of future cerebral ischemia. 18 We compared the serum miR-124 expression of ACI patients with different etiologies and different infarct sizes and found that miR-124 expression had no significant difference. Some scholars hold that the primary contributor of ACI is the lipid deposition in the cerebral arterial wall, mainly LDL. 19 The elevated Hcy level is implicated in the onset of ACI and can be exploited as a diagnostic marker of ACI. 20 Our results exhibited that LDL and Hcy levels did not affect the miR-124 expression. Taken together, serum miR-124 expression of ACI patients was not affected by the etiology, infarct size, and LDL and Hcy levels. To a certain extent, this study excluded the confounding factors of miR-124, such as the ACI classification, infarct size, LDL, Hcy, etc. which indicated that miR-124 can serve as a serum marker of ACI.
ACI triggers a series of complicated events at the gene, molecular, and cellular levels, and inflammation plays a vital in these events. 21 Circulating cytokines, especially the IL family, are recognized as critical mediators of inflammation. 22 CRP is an acute-phase protein, which is synthesized by hepatocytes when the body is under inflammation stimulation. 7 IL-6, IL-8, and CRP are common inflammatory factors in ACI. 22 Hence, the contents of IL-6, IL-8, and CRP in ACI patients were detected. Pearson correlation analysis was performed between miR-124 and cerebral infarction-related inflammatory factors. It was observed that miR-124 was negatively correlated with IL-6, IL-8, and CRP, which further proved the feasibility of miR-124 as an indicator of ACI. miR-124 is reported to mediate anti-inflammatory pathways in ischemic stroke. 23 miR-124 expression is negatively correlated with infarct size and plasma CRP level, and upregulation of miR-124 represses inflammation in ACI. 24 miR-124, tumor necrosis factor-α, and IL-1β in the serum have the ability to function as early auxiliary diagnostic indicators for vulnerable carotid plaque in ACI patients, and the combination of these 3 shows the optimal diagnostic efficacy. 25 Moreover, plasma miR-124 acts as a molecular marker for predicting the mortality of acute stroke. 26 Accordingly, we assigned ACI patients to the miR-124 high expression group and miR-124 low expression group according to the median value of miR-124 expression. The patients with death outcomes within 5 years in each group were recorded. The results exhibited that low miR-124 expression indicated an unfavorable prognosis, implying the correlation of miR-124 with the survival prognosis of ACI. Enhanced plasma miR-124 expression is correlated with the prognosis of acute ischemic stroke patients receiving thrombolysis. 27 Altogether, the low expression of miR-124 in ACI patients is also a prognostic factor.
To sum up, miR-124 was downregulated in the serum of ACI patients and accepted as a biomarker for the diagnosis and prognosis of ACI. Based on existing studies, this study speculated that miR-124 played a role in ACI by regulating inflammatory factors. However, it simply revealed that miR-124 had a certain correlation with inflammatory factors, and did not make in-depth exploration on the specific molecular mechanism of miR-124 in ACI. In future studies, we shall investigate the underlying mechanism of miR-124 in inflammatory factors, as well as the potential mechanism of miR-124 in ACI. Additionally, the treatment of hemorrhagic stroke is completely different from that of ischemic stroke, so we will collect samples from patients with different sub-types of the hemorrhagic stroke to clarify the clinical significance of miR-124 in the following studies.
Footnotes
Authors’ Note: XJZ contributed to the study concepts, study design, and definition of intellectual content; XJZ, LZQ contributed to the literature research; LZQ contributed to the manuscript preparation and XJZ contributed to the manuscript editing and review; XJZ, LZQ contributed to the experimental studies and data acquisition; XJZ, LZQ contributed to the data analysis and statistical analysis. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Sun Y, He W, Geng L. Neuroprotective mechanism of HIF-1alpha overexpression in the early stage of acute cerebral infarction in rats. Exp Ther Med. 2016;12(1):391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu X, Zhang X, Li D, Zhu Z. Plasma level of miR-99b may serve as potential diagnostic and short-term prognostic markers in patients with acute cerebral infarction. J Clin Lab Anal. 2020;34(3):23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun M, Hou X, Ren G, Zhang Y, Cheng H. Dynamic changes in miR-124 levels in patients with acute cerebral infarction. Int J Neurosci. 2019;129(7):649–653. [DOI] [PubMed] [Google Scholar]
- 4. Dewdney B, Trollope A, Moxon J, Thomas Manapurathe D, Biros E, Golledge J. Circulating microRNAs as biomarkers for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2018;27(3):522–530. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. Metazoan microRNAs. Cell. 2018;173(1):20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Zhang Y, Xu F. Function and mechanism of microRNA-210 in acute cerebral infarction. Exp Ther Med. 2018;15(2):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teng L, Meng R. Long non-coding RNA MALAT1 promotes acute cerebral infarction through miRNAs-mediated hs-CRP regulation. J Mol Neurosci. 2019;69(3):494–504. [DOI] [PubMed] [Google Scholar]
- 8. Ye Z, Hu J, Xu H, et al. Serum exosomal microRNA-27-3p aggravates cerebral injury and inflammation in patients with acute cerebral infarction by targeting PPARγ. Inflammation. 2021;44(3):1035–1048. [DOI] [PubMed] [Google Scholar]
- 9. Sun Y, Luo ZM, Guo XM, Su DF, Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 2015;9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang WY, Jiang C, Ye HB, et al. miR-124 upregulates astrocytic glutamate transporter-1 via the Akt and mTOR signaling pathway post ischemic stroke. Brain Res Bull. 2019;149:231–239. [DOI] [PubMed] [Google Scholar]
- 11. Han SW, Kim SH, Lee JY, et al. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism. Eur Neurol. 2007;57(2):96–102. [DOI] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 13. Li Z, Xin Z. Expression and significance of S-100β, CysC and NF-κB in patients with acute cerebral infarction. Exp Ther Med. 2021;21(2):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Lin M, Ren H, Yu Z, Guo T, Gu B. Expression and clinical significance of serum miR-497 in patients with acute cerebral infarction. Clin Lab. 2019;65(4). [DOI] [PubMed] [Google Scholar]
- 15. Chang H, Lu Z. The expression of serummiR-151a-3p in patients with acute cerebral infarction and its correlation with pro-inflammatory factors. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016;28(3):272–276. [PubMed] [Google Scholar]
- 16. Sun Y, Gui H, Li Q, et al. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther. 2013;19(10):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badacz R, Przewlocki T, Gacon J, et al. Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Postepy Kardiol Interwencyjnej. 2018;14(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gacon J, Badacz R, Stepien E, et al. Diagnostic and prognostic micro-RNAs in ischaemic stroke due to carotid artery stenosis and in acute coronary syndrome: a four-year prospective study. Kardiol Pol. 2018;76(2):362–369. [DOI] [PubMed] [Google Scholar]
- 19. Meng X, Wen R, Li X. Values of serum LDL and PCT levels in evaluating the condition and prognosis of acute cerebral infarction. Exp Ther Med. 2018;16(4):3065–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu W, Guan Y, Xu K, et al. Plasma homocysteine levels predict the risk of acute cerebral infarction in patients with carotid artery lesions. Mol Neurobiol. 2016;53(4):2510–2517. [DOI] [PubMed] [Google Scholar]
- 21. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des. 2012;18(28):4289–4310. [DOI] [PubMed] [Google Scholar]
- 22. Xu P, Xin J, Song L, et al. Serum miR-133 as a potential biomarker in acute cerebral infarction patients. Clin Lab. 2020;66(10). [DOI] [PubMed] [Google Scholar]
- 23. Martinez B, Peplow PV. Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke. Neural Regen Res. 2017;12(6):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Zhang J, Han R, Liu H, Sun D, Liu X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J Clin Neurosci. 2015;22(2):291–295. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Xu L. Combined value of serum miR-124, TNF-alpha and IL-1beta for vulnerable carotid plaque in acute cerebral infarction. J Coll Physicians Surg Pak. 2020;30(4):385–388. [DOI] [PubMed] [Google Scholar]
- 26. Rainer TH, Leung LY, Chan CPY, et al. Plasma miR-124-3p and miR-16 concentrations as prognostic markers in acute stroke. Clin Biochem. 2016;49(9):663–668. [DOI] [PubMed] [Google Scholar]
- 27. He XW, Shi YH, Liu YS, et al. Increased plasma levels of miR-124-3p, miR-125b-5p and miR-192-5p are associated with outcomes in acute ischaemic stroke patients receiving thrombolysis. Atherosclerosis. 2019;289:36–43. [DOI] [PubMed] [Google Scholar]