Abstract
Background: There are significant costs associated with proper controlled substance disposal, management, and regulatory compliance. Given the high abuse potential of fentanyl, hydromorphone, and morphine it is imperative that (1) product waste is minimized; and (2) waste procedures are followed to ensure safe disposal. Research is needed to better understand the financial and workforce impacts of drug waste on inpatient hospital units. The primary objective of this study was to quantify the waste associated with administering fentanyl, hydromorphone, and morphine via the intravenous push route. Two categories of waste were evaluated: (1) the quantity (mg/µg) of drug disposed; and (2) workforce time associated with the waste disposal process. Methods: A workflow time study design, a sub-set of continuous direct observation time motion studies, was employed to achieve the research objectives. A data collection tool was developed to capture medication type, waste amount, activity time stamps, total time, and number of interruptions at two separate study sites. Descriptive statistics were conducted on all the data measures. The number of assessments, total values, and mean values were reported for each drug (fentanyl, hydromorphone, and morphine) separately as well as grouped data. Results: A total of 669 distinct waste observations meeting inclusion criteria were collected during a study period of 15 days. In total, 207 mg of hydromorphone and 17 962.50 µg of fentanyl were wasted during this study. Nursing staff time associated with the wasting process totaled 50 990 seconds (849.83 minutes or 14.16 hours). A combined waste (loss) of approximately $1605.39 was associated with controlled substance wasting. The cost per dose wasted in this study was found to be $2.40 for all medications. When a yearly extrapolation model was applied to the four study units, the total combined product and workforce waste cost was $35 425. Conclusion: There are financially significant costs associated with wasting both the product and the valuable time of a skilled workforce. Optimizing product size, taking special note to match product availability with common practice use, would reduce the associated financial burden on our health-systems nationwide.
Keywords: intravenous therapy, cost effectiveness, medication process, purchasing
Introduction
Background
Health-system policies and procedures for handling and disposing of controlled substances vary. Proper disposal is an essential best practice, as controlled substances including fentanyl, hydromorphone, and morphine are associated with particularly high abuse and diversion risk. The Drug Enforcement Administration (DEA) estimates that prescription drug diversion in the United States is a $25 billion-a-year industry. 1
Current federal statute dictates the appropriate disposal of controlled substance medications must occur immediately with documentation, and be witnessed by two licensed healthcare professionals. 1 Depending on the patient care unit, the quantity, and variety of controlled substances administered can create an administrative and regulatory burden on healthcare professionals. Policies requiring thorough documentation, checks-and-balances, and possible audits necessitate an institutional investment of time and resources.
In addition to workforce time, there is a significant cost associated with disposing of physical drug product. A recent retrospective study in 2017 assessed the costs of reducing pharmaceutical waste through supply optimization in three emergency department settings, both urban and suburban. The primary objective of this study was to identify the total waste and annual cost related to intravenous hydromorphone and morphine. Two models were used to quantify the pharmaceutical waste: price optimization scenario and the waste optimization scenario. In the price optimization scenario, the most inexpensive vial was selected without accounting for waste. Meanwhile, in the waste optimization scenario, vials were selected to minimize the amount of unused medication after the order was filled. In both scenarios, the researchers found considerable waste. The price optimization study resulted in 56 171 mg of waste ($139 563.10) whereas the waste minimization scenario resulted in 16 612.99 mg of waste ($161 798.80). This study showed the significant costs related with medical waste within the healthcare system. This information was based on models, however, and limited peer-reviewed literature exists that has used direct observation methods to assess both product and workforce time waste associated with administering intravenous push (IVP) controlled substances. 2
Ultimately, there are significant costs associated with proper controlled substance disposal, management, and regulatory compliance. In the context of the current opioid crisis, and given the high abuse potential of fentanyl, hydromorphone, and morphine, it is imperative that (1) waste is minimized; and (2) waste procedures are followed to ensure safe disposal. Research is needed to better understand the financial and workforce impacts of pharmaceutical waste on inpatient hospital units. This information can assist the healthcare community in making the best use of limited resources.
Objectives
The primary aims of this investigator-initiated research study were to quantify the total waste associated with administering fentanyl, hydromorphone, and morphine via the IVP route. Two categories of waste were evaluated: (1) The quantity (mg/µg) of medication disposed defined as “pharmaceutical waste” or “product waste” (PW); and (2) workforce time associated with the waste disposal process defined as “workforce time waste” (WTW). Although a regulatory requirement, for the purposes of this research, WTW is considered a type of waste with the assumption optimized vial and syringe sizes can limit time spent in the waste disposal process, thereby freeing workforce time to conduct other necessary tasks. Study data were captured as units of time, and converted into cost (USD). Additional secondary measures were collected, including number of distractions (interruptions) during the waste disposal process and the average time taken to document the PW.
Methods
Study Design
A workflow time study design, a sub-set of continuous direct observation time motion studies (TMS), was employed to achieve the research aims. TMS were first described in the early 20th century in industrial engineering in reference to a quantitative data collection method. In TMS, an external observer captures detailed data on the duration and motion required to accomplish a task, or set of tasks. Since the development and validation of TMS, this method has been adopted by biomedical researchers who are seeking to assess and improve clinical workflows. Continuous observation TMS studies have been the method preferred by healthcare researchers, who consider direct observation the “gold standard.” 3 In one comprehensive review of TMS published in the literature, continuous observation TMS represented the majority of all articles in the 10-year review. 4 In the workflow time study schema, an observer measures the time tasks take, while recording the occurrence and duration of unpredicted actions in a series of time-stamps.
To accomplish the research aims and number of desired observations, two research sites were enrolled. Various criteria were considered in scope when selecting units for IVP observation. Staff, including nursing staff, were 18 years and older. Only inpatient units, including but not limited to: medical/surgical, critical care, operating/procedural were considered. Out of scope were outpatient areas, as well as those areas focused in pediatrics, psychiatric care, and OB/GYN units. Both sites are academic teaching centers located in a large metropolitan area. Automated Dispensing Cabinet (ADC) reports were generated to identify four “high-use” units. Over the study period, research observers collected data on the measures in Table 1, and time-stamped data points on each unit, including:
Table 1.
Study Data Measures.
| Quantity (mg/µg) of fentanyl, hydromorphone, and morphine wasted (PW) | Cost (USD) in average wholesale pricing (AWP) associated with fentanyl, hydromorphone, and morphine waste |
| Time spent conducting procedural steps to dispose of fentanyl, hydromorphone, and morphine IVP (WTW) | Time-cost (USD) related to PW procedure |
Note. Additional data, including: number (and duration) of interruptions and time between drug removal and PW documentation were collected for safety, compliance, and/or cost considerations.
Time elapsed while interacting with an ADC machine (e.g., medication removal, patient query, medication return, barcode scanning, biometric identification, and password entry).
Time elapsed while traveling from an ADC machine to a patient room.
Time elapsed during the witnessing process, if applicable.
The two processes assessed in this study will follow the nurse who is wasting a medication by one of two methods, non-integrated ADC waste steps (occurs after medication is administered to patient) and integrated ADC waste steps (occurs before medication is administered to patient or while other activities are conducted). In the non-integrated ADC waste process after administering the dose: (1) the nurse travels from patient bedside to the ADC; (2) the nurse logs into the ADC using biometric identification; (3) the nurse calls for a witness; (4) the nurse documents the medication name, strength, and amount to be wasted; (5) the witness verifies information is correct and signs off via biometric identification; and (6) the nurse disposes of the specified remaining amount in the designated waste container. In the other process, integrated ADC waste occurs when, (1) the nurse pulls a controlled substance from the ADC for a patient; (2) the nurse notices the dose prescribed is smaller than what is contained in the dosage form; he or she decides to waste the excess now; (3) the nurse calls for a witness; (4) the nurse documents the medication name, strength, and amount to be wasted; (5) the witness verifies the information is correct and signs off via biometric identification; and (6) the nurse disposes of the specified amount in the designated waste container.
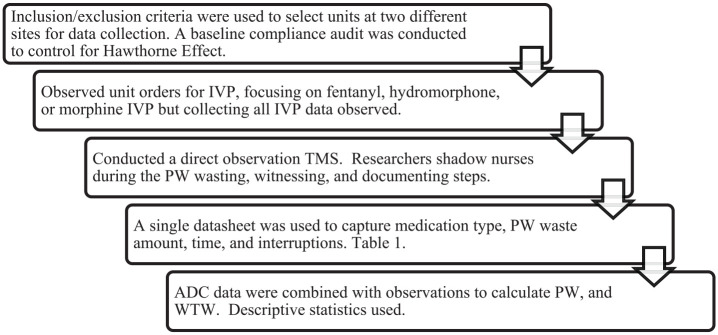
Average times for each activity were calculated for each unit and applied as appropriate (i.e., no travel time included in analysis of an integrated waste). Figure 1 below gives a summary overview of the data collection process. A data collection tool was developed to capture medication type, waste amount, activity time stamps, total time, and number of interruptions. Study data measures can be found in Table 1. The study was approved as exempt by the institutional review board (IRB) for each site as well as the Butler University IRB prior to study initiation.
Figure 1.
Study data collection process.
Statistical Analysis
All the data recorded on waste were converted into cost (USD). The quantity of the PW was converted to cost (USD) using Average Wholesale Price (AWP) of each medication. WTW was converted to cost (USD) using the nurse median salary. Descriptive statistics were conducted on all the data measures. The cost of the total waste, PW per dose, WTW with interruptions, and WTW without interruptions were calculated for each drug (fentanyl, hydromorphone, and morphine) for the study period and extrapolated to 1-year time frame.
Results
A total of 669 distinct waste observations meeting inclusion criteria were collected during the study period across four units (two per hospital). The study period ran from September 13 through September 27, 2019. In total, 207 mg of hydromorphone and 17 962.50 µg* (*calculated to two decimal places based on ADC data) of fentanyl were wasted during this study. This total does not include two (2) doses of morphine that were wasted. Most frequently observed PW amounts for fentanyl and hydromorphone are noted in Table 2. Nursing staff time associated with the wasting process totaled 50 990 seconds, (849.83 minutes or 14.16 hours). Cost analyses were conducted utilizing nursing median salary 5 and Average Wholesale Price (AWP) 6 of each medication. A combined waste (loss) of approximately $1605.39 was associated with controlled substance wasting over 15 days on four (4) nursing units, encompassing 80 beds. This total includes both PW and WTW (nursing) time. Table 3 details total (PW and WTW) waste costs for fentanyl, hydromorphone, and morphine.
Table 2.
Most Frequently Observed Waste Amounts for Fentanyl and Hydromorphone.
| Drug | N | Waste amount | Percentage of total wastes (%) |
|---|---|---|---|
| Fentanyl (50 µg/mL) 2 mL vial | 143 | 50 µg | 49.83 |
| Fentanyl (50 µg/mL) 2 mL vial | 132 | 75 µg | 45.99 |
| Hydromorphone (1 mg/mL) 1 mL vial | 239 | 0.5 mg | 62.89 |
| Hydromorphone (1 mg/mL) 1 mL vial | 68 | 0.8 mg | 17.89 |
Table 3.
Observed Total Cost of Waste.
| Drug | N | Product waste (PW) | Workforce waste (WTW) | Total waste | Total waste per dose |
|---|---|---|---|---|---|
| Fentanyl (50 µg/mL) 2 mL vial | 287 | $226.33 | $217.58 | $443.91 | $1.55 |
| Hydromorphone (1 mg/mL) 1 mL vial | 380 | $886.89 | $270.23 | $1157.12 | $3.05 |
| Morphine (2 mg/mL) 1 mL vial | 2 | $2.66 | $1.70 | $4.36 | $2.18 |
| Total | 669 | $1115.88 | $489.51 | $1605.39 |
To control for Hawthorne Effect (HE), a sample of units at the study sites was audited prior to conducting research observations to determine an estimated baseline compliance rate for adherence to the institutions’ waste policy. The audit was conducted retrospectively, using ADC data and electronic charting data. Doses dispensed from ADCs during a 7- to 14-day timeframe on targeted study units was compared to electronic charting records for the same period and matched to the correct dose. This analysis determined there was no significant change in discrepancy (PW) documentation from baseline. As such, the authors suggest HE had limited impact on the study conclusions.
To capture secondary measures, research observers took notes to characterize the frequency of workflow interruptions. PW reports for the same period were assessed and compared to manually recorded data. Annual reports were pulled to assess and validate PW compliance and further quantify the all PW associated with these controlled substances. An extrapolation model was applied to this yearly data for the same units observed. This yearly extrapolated data is presented in Table 4.
Table 4.
Total Cost of Waste—Year Extrapolation Model.
| Drug | N | Product waste (PW) | Workforce waste (WTW) | Total waste | Total waste per dose |
|---|---|---|---|---|---|
| Fentanyl (50 µg/mL) 2 mL vial | 8327 | $6275.46 | $6256.88 | $12 532.34 | $1.51 |
| Hydromorphone (1 mg/mL) 1 mL vial | 7207 | $17 427.23 | $5037.51 | $22 464.74 | $3.12 |
| Morphine (2 mg/mL) 1 mL vial | 193 | $277.31 | $120.97 | $398.28 | $2.22 |
| Total | 15 727 | $23 980.00 | $11 445.51 | $35 425.51 |
Additional secondary measures were collected, including number of distractions (interruptions) along with associated time and financial cost. A total of 86 observed PWs were interrupted for a variety of reasons, including talking to other nurses or the rounding team, forgetting administration materials (needles, gloves), or assisting another patient. A “cost of interruptions” model was developed by taking the observed time associated with each interruption and applying the average time to each of the observed 669 incidences of PW. A “per medication dose” cost was then calculated for each medication. To compare, the same approach was used to develop a hypothetical per dose cost if no interruptions had occurred throughout the study. In this case, the 583 incidences of PW in which no interruption occurred were used to develop an average. In summary, two extremes are presented; one in which there would always be interruptions, and the other in which no nurse would be interrupted. The resulting data from this “cost of interruptions” model are presented in Table 5. Note, the difference between each scenario can then be assumed to be the estimated cost associated with interruptions during IVP administration of each medication. This same model was then applied to the yearly data extrapolation, and can be found in Table 6.
Table 5.
“Cost of Interruptions” Model.
| Drug | N | Waste per dose with interruptions | Waste per dose without interruptions | Diff (Δ) |
|---|---|---|---|---|
| Fentanyl (50 µg/mL) 2 mL vial | 287 | $2.14 | $1.44 | $0.70 |
| Hydromorphone (1 mg/mL) 1 mL vial | 380 | $3.32 | $2.98 | $0.34 |
| Morphine (2 mg/mL) 1 mL vial | 2 | $2.86 | $1.94 | $0.92 |
Table 6.
“Cost of Interruptions” Model—Year Extrapolation.
| Drug | N | Waste per dose with interruptions | Waste per dose without interruptions | Diff (Δ) |
|---|---|---|---|---|
| Fentanyl (50 µg/mL) 2 mL vial | 8327 | $1.92 | $1.38 | $0.54 |
| Hydromorphone (1 mg/mL) 1 mL vial | 7207 | $3.27 | $3.02 | $0.25 |
| Morphine (2 mg/mL) 1 mL vial | 193 | $2.63 | $2.06 | $0.57 |
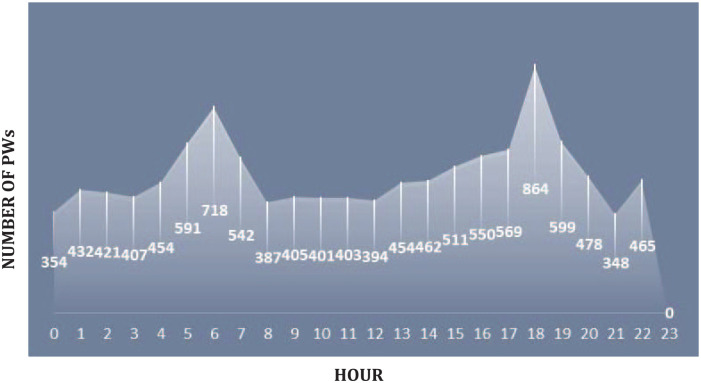
Delays in charting PW were also assessed. The average time between medication removal and disposal was collected using ADC reports. The average time to chart for the 669 study observations was 2 hours, 4 minutes, and 52 seconds from medication pull to PW charting. For 31 instances of PW, it look longer than 8 hours to document PW had occurred on the unit. To capture peak waste times, 24 hours of waste data was compiled (Figure 2). Note, all units are included so documented PW occurrences at each hour total more than those observed in the study. As demonstrated in the figure, there are spikes in waste activity throughout the 24-hour period (shift change occurs at hours 7 and 19).
Figure 2.
24-History of PW documentation.
Note. This is the compiled ADC data for all care units included in the study; it includes PW times for the entire duration of the study and not just those directly observed.
Discussion
Primary Objective
The primary aim of this study was to quantify the total waste (PW + WTW) associated with administering fentanyl, hydromorphone, and morphine via the IVP route. During the duration of this 2-week study, 207 mg of hydromorphone and 17 962.50 µg of fentanyl were wasted. For the study period, approximately 50% of fentanyl doses wasted were 50 µg (dose given was 50 µg, with 100 µg vial available) in volume, while 46% of the time, 75 µg were wasted (25 µg dose given). For hydromorphone, approximately 63% of doses given had 0.5 mg wasted (0.5 mg given, with 1 mg vial available), while 18% wasted 0.8 mg (0.2 mg given). Limited morphine wastes were identified. Further investigation uncovered the use of order sets on the study units that limited morphine administration, or included morphine doses in quantities (2 mg/mL–1 mL vial) that were administered in full, minimizing PW. This fact validates the recommendation that optimizing IVP product size with frequently administered doses can significantly reduce PW.
Using AWP pricing, the cost of PW for the study period was $1115.88. Other studies in the literature have evaluated the PW associated with controlled substance usage. Oh et al conducted an assessment of the cost of reducing PW through supply optimization in emergency departments (EDs). 2 In that study, costs (AWP) of PW were assessed across three EDs (urban, community, and suburban) over 1 year. When calculated per dose, the “Oh” study estimated that $0.75 was wasted for every order of morphine or hydromorphone. Comparatively, when looking at the results from this research, the calculated PW per dose was $1.67. It is important to note the Oh et al study was not observational and only compared dose ordered against vial size and calculated the difference. In addition, this study included fentanyl, but the “Oh” study omitted fentanyl and focused on morphine use. The “Oh” study was simulated over the course of a year (cost savings of $13 422). In comparison, a yearly extrapolation was also done with this research, which yielded $23 980 in PW. This study has validated what previous studies have also concluded; there is significant PW associated with IVP administration of fentanyl, hydromorphone, and morphine. Vial and syringe size optimization, order set review, and a multi-disciplinary procurement and inventory approach are needed to minimize waste associated with these medications. Manufacturers, health-systems, regulators, and other stakeholders must collaborate to ensure PW is minimized.
Although literature exists outlining costs associated with PW, there is little evidence available describing the costs associated with the waste process burden on the workforce (WTW). Controlled substances administration and waste follows specific administrative and regulatory requirements including, but not limited to witnessing and documentation with every controlled substance wasting. These important process steps require financial investment in the workforce. As part of the primary aim of this study, the researchers sought to quantify the WTW (nursing) associated with the waste process. Data was captured as units of time, and converted into cost (USD). During the course of the study period, 50 990 seconds, or 849.83 minutes, were used to appropriately waste fentanyl, hydromorphone, or morphine. Using median salary statistics from the Bureau of Labor Statistics, this time translates into a cost of $489.51 in nursing time. In addition to this financial investment, there is also the opportunity cost associated with using valuable trained nursing staff to waste product, rather than provide direct patient care activities. For more than 14 hours (over approximately 2 weeks of observation), nurses were involved in wasting controlled substances rather than caring for their patients. If extrapolated out to a year using a similar method for the four (4) units as noted above, the workforce cost is $11 445.51.
The total cost (PW + WTW) of waste for fentanyl, hydromorphone, and morphine for the 2-week study period was approximately $1605.39. The cost per dose wasted in this study was found to be $2.40 for all medications. In other words, for every IVP fentanyl, hydromorphone, or morphine medication ordered, the health-system wasted $2.40 in non-value expense. Given the high volume of these medications, and the average PW and WTW of $2.40 a dose, the financial impact can be substantial. To highlight this, the researchers compiled a yearly extrapolation model. ADC data was queried to capture the total number of wastes for fentanyl, hydromorphone, and morphine from October of 2018 to September of 2019. The extrapolation found the total combined PW and WTW cost to be $35 425.51. Annually, the cost increases significantly, and just for the four (4) units, which totaled 80 combined beds. If this model were to be applied to all units at the site for the year, the total impact would certainly be even more costly. With increased hospital size, volume-of-use, and total waste come significantly increased costs to the health-system. Additional secondary measures were also collected as part of this study, including number of distractions (interruptions), and average time to document PW, which has compliance considerations.
Number of Distractions (Interruptions)
Interruptions have been identified as increasing the likelihood of committing safety-critical errors when handling medications. 7 The results from this study suggests there is a real financial cost associated with interrupting the IVP process, in addition to those costs resulting from errors and potential patient harm. For instance, because of the increased nursing time associated with interruptions, the waste cost per dose for fentanyl rose to $2.14 (a $0.59 increase from $1.55 per dose witnessed for all observations), while the same cost for hydromorphone increased to $3.32 (increased by $0.27 from $3.05 per dose witnessed for all observations). To more clearly illustrate the financial burden of interruptions, an additional model was developed to assess cost per dose if no interruptions had occurred throughout the study. In this model, the total cost per dose for fentanyl was $1.44, and hydromorphone was $2.98. Then, taking the difference between the “interruptions” and “no interruptions” models, the calculated cost of nursing interruptions per dose was $0.70 for every dose of fentanyl administered and $0.34 for every dose of hydromorphone administered. The costs associated with morphine were not calculated due to the small observed number of morphine wastes in this study. The results from this research indicate there is a meaningful financial cost associated with workflow interruptions that can be quantified.
Distractions and interruptions are prevalent in healthcare and an inherent risk during process steps, including the wasting of controlled substances. Future studies should be designed to adequately collect, assess, and analyze common distractions and interruptions that occur as part of the IVP administration process. Talking to other nurses or rounding team, forgetting administration materials, and being called away to assist another patient are just a few of the types of interruptions collected as part of this study. Examining this socio-technical phenomenon in detail, while ensuring statistical validity can be applied to the results, would help contribute to a greater understanding of this patient safety concern. Ultimately, the literature supports a comprehensive evaluation and set of mitigation strategies to reduce the likelihood of interruptions, distractions, and medication administration errors. 8 Successful implementation of these strategies would reduce both the financial and safety burden of interruptions and distractions in practice.
Time to Document Waste and Drug Diversion Risk
The availability of more precise clinically relevant vial and pre-filled syringe sizes may help address diversion by limiting the need for PW and related documentation. Timely documentation has been identified in the literature as a top failure mode leading to drug diversion. Current federal statute dictates the appropriate disposal of controlled substance medications must occur immediately with documentation, and be witnessed by two licensed healthcare professionals. 1 Delays in wasting and documentation put organizations at risk for patient safety issues, diversion activities, and regulatory non-compliance. 9 In this observational study, the average time to document PW for the 669 instances was 2 hours, 4 minutes, and 52 seconds. Further evidence of practice variation related to the timeliness of wasting can be seen in Figure 2. Note, there are clear spikes in PW activity throughout the 24-hour period with shift change occurring at hours 7 and 19. These distinct spikes in PW activities the hour prior to shift change seem to indicate that nurses are often collecting controlled substances and batch-wasting. As discussed, ensuring a witness independently and accurately documents the wasting of a controlled substance is an integral part of the proper wasting and disposal process. However, it was noted that in approximately 5% (31 of 669) of our observed instances of PW, the documentation and PW disposal process occurred greater than 8 hours after the medication was pulled. These delays in documentation put the patient, nurse, and organization at possible risk. Carrying “to-be” wasted medication increases the likelihood of medication mix-ups as nurses can mistakenly administer used medication to patients. The Institute for Safe Medication Practices (ISMP) guidelines for ADCs provide some direction for organizations to prevent the mishandling of medications after administration and prior to completion of PW documentation. 10
Additionally, delays in documentation could indicate possible nefarious diversion, and are likely to result in audits. Audit activities require more staff time, and further financial investment on behalf of the organization. These reconciliatory audits are necessary, however, to ensure an organization maintains compliance with legal and regulatory standards. The longer the time between medication pull from the ADC and PW documentation, the higher the risk for medication diversion. 9 And, the economic costs of diversion and abuse is significant. In one report, the estimated cost to public and private medical insurers is $72.5 billion a year. 11 This research supports a further examination of this gap by all health-systems as part of their action plans to minimize drug diversion.
Limitations
As with any research study, research limitations have been identified. Although the observational study was conducted in four different units across two academic-teaching hospitals, every health-system is unique. The technique and research approach outlined can be replicated at any facility, but the specific time, cost, and other data reported in this manuscript is unique to the study sites included in this research. Extrapolation should be conducted with the caveat that variations in care unit layout and practice, including the use of order sets, can influence study results, as they did in this research. Morphine was initially included in the data analysis and collection plan, but practices at the study sites limited morphine dose evaluation.
As with all observation-based studies, not all measures are observed completely, and in this study, ADC data was incorporated to supplement and validate observations. Multiple researchers were used in the data collection phase of the research. With more than one individual collecting data, it is important to ensure proper interrater reliability. No subjective data was collected, and all observers used the same data collection sheet and process. The principle investigator reviewed all data collected to help ensure consistency. Financial projections are based on costs available at the time of the study. Costs, including median salaries and AWP, will fluctuate. AWP was used for comparison to other studies in the literature, with the understanding that contracted procurement costs vary from one health-system to another. Other cost multipliers, including Wholesale Acquisition Cost (WAC) may also be used to calculate total cost of PW for a particular organization. That said, the workforce cost estimates in this study were conservative given the researchers only used one-way transit time to calculate workforce cost. The time associated with only the trip to the ADC was used and incorporated. The trip from the ADC to the nurses’ next task was not included. Finally, in any direct observation study, the HE must be considered, as those being observed may have a tendency to improve performance, and skew results. There is debate as to the significance of the HE in practice. In one published systematic review, the data suggest the size of any effect on being observed depends specifically on the task. 12 Notably, a few of the studies reviewed showed no performance improvement by those individuals being observed. In a previous observational study that compared error rates between various IVP methods, the research team discussed the possibility of improved nursing performance during initial observations. However, they found that after a short, yet undetermined period those being observed tended to revert to baseline practice. Busy shifts, interruptions, and general comfortability after a few hours of being observed were all inferred as possible explanations for this normalization, following an initial performance peak. 13 Over time, compiled results should average close to practice norms, retaining reasonable external validity. Compliance rates at baseline and during the study were electronically evaluated. This analysis determined there was no significant change in discrepancy (waste) documentation from baseline. As such, the authors suggest HE had limited impact on the study conclusions.
Conclusion
As healthcare costs continue to rise, it is essential to limit waste of product and time whenever and wherever possible. For controlled substances, including fentanyl, hydromorphone, and morphine, there are financial costs associated with both PW and WTW. Health-systems are encouraged to coordinate with manufacturers and other stakeholders to ensure product sizes are optimized, aligned with practical use. There are also societal considerations, as controlled substances including fentanyl, hydromorphone, and morphine are associated with particularly high abuse and diversion risk. If PW is addressed, diversion will be more difficult. This study validated previous studies, demonstrating significant PW associated with these controlled substance IVP medications. Further, this research highlighted the significant cost associated with using a skilled nursing workforce to waste controlled substances, noting specific issues with the cost of interruptions and risk associated with prolonged PW documentation.
Additional research should be conducted to investigate the length of time associated with the PW disposal process, focusing on outlier practices. Further evaluation of distractions is important to categorize, and then develop mitigation strategies given the substantial cost associated with interrupting patient care processes. Health-systems are encouraged to use this research approach to evaluate their own processes, collect PW and WTW data, and apply varying cost models to the volume and time figures. Ultimately, this research demonstrates there are significant costs associated with wasting both the pharmaceutical product and the valuable time of a skilled workforce. Optimizing product size, taking special note to match product availability with common practice use, would reduce this financial burden on our health-systems.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) received grant funding from Fresenius Kabi USA, LLC as an investigator initiated research study.
ORCID iD: John Hertig  https://orcid.org/0000-0001-9869-2903
https://orcid.org/0000-0001-9869-2903
References
- 1. Drug Enforcement Administration Diversion Control Division. The 21 United States Code (USC) Controlled Substances Act. 2016 edition. https://www.deadiversion.usdoj.gov/21cfr/21usc/. [Google Scholar]
- 2. Oh A, Rothenburg C, Lord K, et al. 138 assessment of the cost of reducing drug waste through supply optimization. Ann Emerg Med. 2017;70(4): S55-S56. [Google Scholar]
- 3. Barker K, Flynn E, Pepper G, Observation method of detecting medication errors, Am J Health Syst Pharm. 2002;59(23):2314-2316. [DOI] [PubMed] [Google Scholar]
- 4. Lopetegui M, Yen P, Lai A, Jeffries J, Embi P, Payne P. Time motion studies in healthcare: what are we talking about? J Biomed Inform. 2014;49:292-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Bureau of Labor Statistics. Registered nurses. https://www.bls.gov/ooh/healthcare/registered-nurses.htm. Published 2019. Accessed December 2019.
- 6. Lexicomp Online. Lexi-Drugs Online. Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc.; 2019. [Google Scholar]
- 7. Prakash V, Koczmara C, Savage P, et al. Mitigating errors caused by interruptions during medication verification and administration: interventions in a simulated ambulatory chemotherapy setting. BMJ Qual Saf. 2014;23(11):884-892. doi: 10.1136/bmjqs-2013-002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raban MZ, Westbrook JI. Are interventions to reduce interruptions and errors during medication administration effective?: a systematic review. BMJ Qual Saf. 2014;23(5):414-421. doi: 10.1136/bmjqs-2013-002118/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nolan K, Zullo A, Bosco E, Marchese C, Berard-Collins C. Controlled substance diversion in health systems: a failure modes and effects analysis for prevention. Am J Health Syst Pharm. 2019;76(15):1158-1164. doi: 10.1093/ajhp/zxz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Institute for Safe Medication Practices. Guidelines for the safe use of automated dispensing cabinets. https://www.ismp.org/resources/guidelines-safe-use-automated-dispensing-cabinets. Published 2019. Accessed December 2019.
- 11. United States Department of Justice National Drug Intelligence Center. The economic impact of illicit drug use on American society. https://www.justice.gov/archive/ndic/pubs44/44731/44731p.pdf. Published 2011. Accessed December 2019.
- 12. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hertig JB, Degnan DD, Scott CR, Lenz JR, Li X, Anderson CM. A comparison of error rates between intravenous push methods: a prospective, multisite observational study. J Patient Saf. 2018;14(1):60-65. [DOI] [PubMed] [Google Scholar]