Abstract
Purpose
The purpose was to determine whether a simplified procalcitonin (PCT) algorithm guided by pharmacist recommendations reduces antibiotic duration of therapy in critically ill patients with suspected sepsis.
Methods
This was a single-centered pre-post study conducted at a 1368-bed community teaching hospital in the United States. A prospective cohort with pharmacist intervention utilizing a simplified PCT algorithm was compared with a retrospective historical cohort with standard therapy. Adult patients admitted to the intensive care unit (ICU) with suspected sepsis who received intravenous antibiotics were included. A pharmacist recommended continuation or discontinuation of antibiotics based on the PCT level per our algorithm and full clinical assessment of the patient. Primary outcome was total duration of antibiotic therapy. Secondary outcomes were ICU and hospital length of stay (LOS), reinitiation of antibiotic therapy within 72 hours of discontinuation, and 28-day in-hospital mortality.
Results
From September 2017 to May 2018, 360 patients were screened for eligibility. Of these, 26 patients were included in the PCT group and 26 patients in the standard therapy group. Baseline characteristics were similar between groups. A significant difference in duration of antibiotic therapy was detected with a median of 9 days in the PCT group versus 12 days in the standard therapy group (P = .02). There were no significant differences in secondary endpoints of ICU and hospital LOS, reinitiation of antibiotics at 72 hours, or 28-day mortality.
Conclusion
Use of a simplified PCT algorithm with pharmacist-guided recommendations significantly reduced the duration of antibiotic therapy in critically ill patients with sepsis.
Keywords: procalcitonin; pharmacist; critically ill; sepsis, antibiotics
Background
Sepsis and septic shock are life-threatening conditions that are prevalent in our health care system, affecting millions of people every year. 1 Early identification and appropriate management are key to improving morbidity and mortality. 1 Antibiotic therapy is the mainstay of sepsis management; guidelines highlight not only the importance of initial selection but also appropriate duration of therapy and de-escalation strategies with the assistance of culture data, clinical signs and symptoms, and biomarkers of infection severity and resolution. 1
Biomarkers such as procalcitonin (PCT) are promising tools to help guide decision-making and monitoring of patient response to antibiotic therapy. 2 Procalcitonin is a precursor to calcitonin and has been described as a “hormokine” (displaying properties of both hormone and cytokine) that is upregulated during state of infection, particularly sepsis, by microbial toxins and pro-inflammatory mediators. 2 Procalcitonin is markedly elevated within 2 to 4 hours in severe forms of infection and begins to decline when inflammatory substances subside during recovery from infection.2,3 Unlike other biomarkers, such as C-reactive protein, PCT levels can correlate with the extent and severity of infection, and its course may predict mortality risk in critically ill patients.3,4
Extended durations of antibiotic therapy can lead to the development of resistant bacterial strains, increased costs, and prolongation of length of stay (LOS). 5 There is accumulating evidence that use of a PCT algorithm to guide antibiotic therapy can lead to reduction in duration of therapy, cost, and even mortality. 5 Current literature suggests these methods appear to be safe without increasing the risk for recurrent infections or treatment failures. 5 Procalcitonin algorithms have been studied in multiple settings, including outpatient, emergency department, and intensive care units (ICUs). These studies focused mostly on the disease states of chronic obstructive pulmonary disease, pneumonia, and sepsis.6,7
Majority of the studies in critically ill patients utilizing PCT algorithm were conducted in Europe.8 –11 There is a lack of prospective randomized controlled trials in the United States. The current published studies reveal low adherence rates to PCT which may be due to the complexity of the studied algorithms and need for repeated PCT levels.8 –11 One observational study evaluating PCT practice patterns and outcomes in the United States suggested a lack of improved outcomes or reduced antibiotic use in the real-world setting. 12 However, the majority of patients did not have repeat PCT levels or guidance of a simple algorithm. 12 Recently, our institution incorporated a PCT algorithm with pharmacists' interventions in patients with community-acquired pneumonia, and observed reduction in antibiotic duration of therapy. 13 This current study evaluated the impact of pharmacist recommendations utilizing a more simplified PCT algorithm on critically ill patients and expanded indications to sepsis or septic shock.
Methods
Study Design and Patients
This study was conducted at a 1368-bed tertiary care community teaching hospital with more than 150 ICU beds. The study compared a prospective cohort admitted to the ICU from March 1, 2018, to May 31, 2018, with a retrospective cohort from September 1, 2017, to February 2, 2018. Patients were included if they were aged 18 years or older, admitted to an ICU, and were initiated on antibiotic therapy for suspected sepsis, severe sepsis, or septic shock using the 2012 Surviving Sepsis definition. 14 Exclusion criteria were as follows: surgery within the previous 7 days, trauma, burn, pancreatitis, autoimmune disorders, immunocompromised patients (defined as active cancer, tuberculosis, human immunodeficiency virus, or cystic fibrosis), end-stage renal disease (defined as kidney failure with glomerular filtration rate <15 mL/min or need for renal replacement therapy), 15 infections requiring long-term antibiotics (tuberculosis, osteomyelitis, and endocarditis), cardiogenic shock, or receiving OKT3 antibodies, monoclonal antibodies, polyclonal antibodies, interleukin, or immune globulin.
Procedures
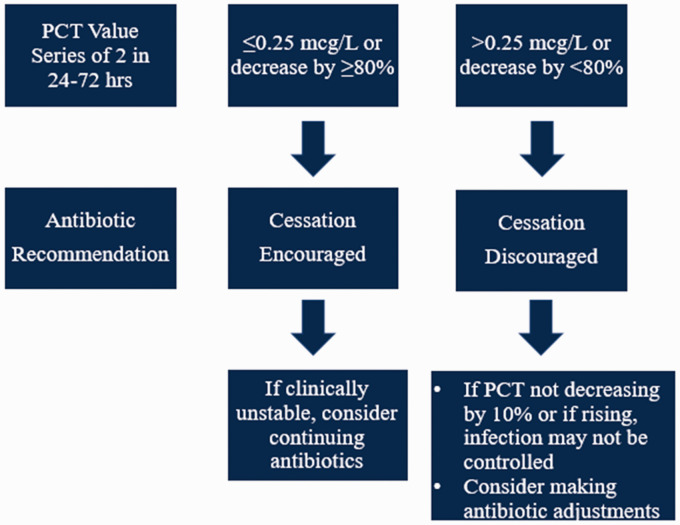
Patients were screened from a list generated daily and identified as being admitted to an ICU with active antibiotic orders for suspected sepsis. If eligibility criteria were met, the critical care clinical pharmacist recommended to the provider to check a series of 2 PCT levels and begin utilizing the PCT study algorithm (Figure 1). The approach of only 2 PCT levels was chosen to increase awareness and utilization of serial PCT levels, which was theorized to simplify the utilization of PCT. Upon review of the repeat PCT value, clinical pharmacists made a recommendation to the ICU team regarding de-escalation or continuation of antibiotic therapy per the PCT algorithm in addition to full clinical assessment of the patient based on multidisciplinary approach. The patient was followed throughout their ICU stay and when transferred to the medical floors. A retrospective chart review of a comparison group of patients was screened for the same inclusion and exclusion criteria that did not utilize the PCT algorithm and compared with the prospective cohort for all primary and secondary endpoints.
Figure 1.
PCT Algorithm.
Note. PCT = procalcitonin.
Data Collection
Baseline demographics, comorbidities, admitting diagnosis, and dates of hospital and ICU admission and discharge were collected. Other clinical criteria that were evaluated included maximum temperature over 24 hours, white blood cell count, rates of mechanical ventilation, Sequential Organ Failure Assessment score, concomitant steroids, and positive cultures.
Outcomes and Statistical Analysis
The primary outcome was duration of antibiotic therapy. Secondary outcomes included ICU LOS, hospital LOS, reinitiation of antibiotic therapy within 72 hours, and 28-day in-hospital mortality. All data were collected via documentation in the electronic medical record (Cerner Millennium®). Shapiro-Wilk test was utilized to test for normality of the data. Mann-Whitney U test or t test was used to analyze continuous variables. Chi-square or Fisher's exact test was used to analyze categorical variables. A sample size of 23 patients in each group was calculated to provide 90% power and α of 0.05 to detect a 2-day difference in duration of antibiotic therapy.
Results
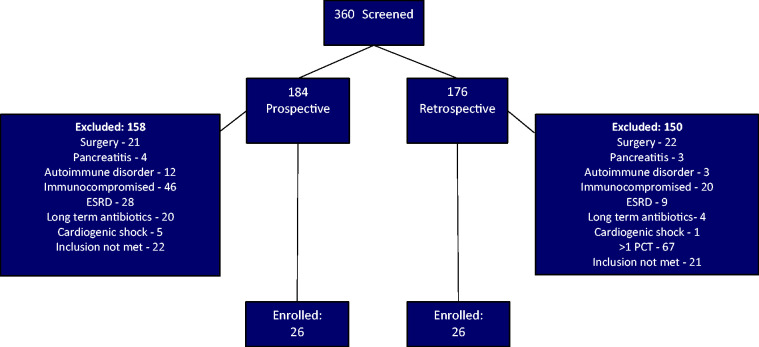
A total of 52 patients were enrolled in the study; 26 patients in the prospective cohort and 26 patients in the retrospective cohort (Figure 2). All baseline characteristics were similar between the groups (Table 1). The most common source of sepsis was pneumonia (69% in the PCT group and 65% in the standard therapy group). More patients in the standard therapy group had a positive blood culture, which was not statistically significant. These positive blood cultures were all coagulase-negative Staphylococcus which were deemed as contaminants by the clinical team. The PCT group had 1 coagulase-negative Staphylococcus and 1 methicillin-resistant Staphylococcus aureus.
Figure 2.
Patient Inclusion.
Note. PCT = procalcitonin; ESRD = end-stage renal disease.
Table 1.
Baseline Characteristics.
| Procalcitonin group (n = 26) | Standard therapy group (n = 26) | P value | |
|---|---|---|---|
| Age, mean ± SD | 72.6 ± 9.4 | 73.2 ± 13.7 | .92 |
| Male gender, n (%) | 16 (61.5) | 12 (46.2) | .21 |
| Comorbidities, n (%) | |||
| CAD | 11 (42) | 12 (46) | .46 |
| CHF | 13 (50) | 13 (50 | 1 |
| CVA | 4 (15) | 5 (19) | .71 |
| CKD | 3 (11.5) | 8 (31) | .09 |
| COPD | 12 (46) | 10 (38) | .57 |
| DM | 14 (54) | 9 (35) | .16 |
| Cirrhosis | 0 (0) | 2 (8) | .15 |
| Mechanical ventilation, n (%) | 11 (42) | 9 (35) | .78 |
| SOFA score, median [IQR] | 6 [3.3-9.8] | 7 [4.0-9.5] | .39 |
| Source of sepsis, n (%) | |||
| Pulmonary | 18 (69) | 17 (65) | .78 |
| Intra-abdominal | 1 (4) | 4 (15) | .55 |
| Urinary tract | 4 (15) | 4 (15) | .85 |
| Skin and soft tissue | 1 (4) | 2 (8) | .55 |
| Unknown | 2 (8) | 1 (4) | .55 |
| Tmax, median [IQR] | 99 [98–99.8] | 99 [98–100] | .96 |
| WBC, median [IQR] | 14.6 [11.4–18.5] | 13.4 [9.6–18.0] | .2 |
| Positive sputum culture, n (%) | 9 (35) | 8 (31) | .49 |
| MRSA | 2 | 2 | |
| Stenotrophomonas maltophilia | 2 | 1 | |
| Candida albicans | 2 | 0 | |
| Pseudomonas aeruginosa | 1 | 1 | |
| Klebsiella pneumoniae | 1 | 3 | |
| Serratia marcescens | 0 | 1 | |
| Corynebacterium | 1 | 0 | |
| Blood | 2 (8) | 6 (23) | .09 |
| MRSA | 1 | 0 | |
| Coagulase-negative Staphylococcus | 1 | 6 | |
| Urine | 8 (31) | 7 (27) | .76 |
| Escherichia coli (ESBL+) | 0 | 1 | |
| Escherichia coli | 0 | 2 | |
| Klebsiella pneumoniae | 1 | 0 | |
| Proteus mirabilis | 1 | 1 | |
| Stenotrophomonas maltophilia | 1 | 0 | |
| Pseudomonas aeruginosa | 1 | 3 | |
| Enterobacter cloacae | 1 | 0 | |
| Providencia stuartii | 1 | 0 | |
| Candida tropicalis | 1 | 0 | |
| Candida glabrata | 1 | 0 | |
| Other | 1 (4) | 2 (8) | .48 |
| MRSA (foot wound) | 1 | 0 | |
| Bacteroides ovatus (abdominal abscess) | 0 | 1 | |
| Escherichia coli (abdominal fluid) | 0 | 1 | |
| Concomitant corticosteroids, n (%) | 17 (65) | 13 (50) | .53 |
Note. CAD = coronary artery disease; CHF = congestive heart failure; CVA = cerebrovascular accident; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment; Tmax = maximum temperature over 24 hours; WBC = white blood cells; ESBL = extended-spectrum beta-lactamase; MRSA = methicillin-resistant Staphylococcus aureus.
The primary endpoint of duration of antibiotic therapy was significantly lower in the PCT group, with a median of 9 days, [IQR 7-13], versus 12 days, [IQR 9-18], in the standard therapy group (P = .02). See Table 2 for all results. Secondary endpoints were not statistically significant: ICU LOS (4 days in each group), hospital LOS (11 days in each group), reinitiation of antibiotics within 72 hours (zero patients in each group), and 28-day in-hospital mortality (3 patients in the PCT group vs 2 patients in the standard therapy group, P = .49).
Table 2.
Results.
| Procalcitonin group (n = 2 6) | Standard therapy group (n = 23) | P value | |
|---|---|---|---|
| Duration of therapy, median [IQR] | 9 [7–13] | 12 [9–18] | .02 |
| Intensive care unit length of stay (d), median [IQR] | 4 [3–7] | 4 [3–6] | .46 |
| Hospital length of stay (d), median [IQR] | 11 [7–16] | 11 [7–18] | .41 |
| Reinitiation of antibiotics at 72 h, n (%) | 0 (0) | 0 (0) | 1.0 |
| 28-day mortality, n (%) | 3 (12) | 2 (7) | .49 |
| Adherence to algorithm, n (%) | 21 (81) | n/a | n/a |
Note. IQR = interquartile range.
Discussion
This single-center study evaluating a simplified PCT algorithm demonstrated a decrease in antibiotic duration of therapy in critically ill patients with sepsis. This was achieved with no increase in negative outcomes, as there were no significant differences in ICU and hospital LOS, reinitiation of antibiotics within 72 hours of discontinuation, or in-hospital mortality. However, this study was not powered to detect these secondary endpoints.
Several large randomized controlled trials have evaluated the use of a PCT algorithm for antibiotic therapy guidance in critically ill patients. In the 2010 Procalcitonin to Reduce Patients' Exposure to Antibiotics in Intensive Care Units (PRORATA) trial, the use of a PCT-guided algorithm versus standard care showed a significant reduction in antibiotic days and noninferiority for 28- and 60-day mortality. 16 The 2016 Stop Antibiotics on guidance of Procalcitonin Study (SAPS) trial is the largest and most significant trial to date, which not only saw a significantly lower antibiotic usage in the PCT algorithm group but also demonstrated a reduction in mortality at 28 days (20% vs 25%, P = .0122) and 1 year (36% vs 43%, P = 0.0188). 17
Procalcitonin is a promising biomarker in critically ill patients to guide antibiotic therapy. However, adherence to a PCT algorithm in real-world practice can prove to be a challenge. The PRORATA trial had 47% adherence, and in the SAPS trial, 44% of patients who met stopping criteria had antibiotics stopped within 24 hrs.16,17 Both of these studies were conducted in Europe. In a 2017 large retrospective cohort study of critically ill patients with sepsis evaluating PCT practice patterns and outcomes in the United States, investigators observed that approximately 5% of patients with sepsis in 2012 had a PCT level measured. 12 Furthermore, repeat PCT levels were checked in only 1 in 3 of those patients. This suggests that PCT use may be idiosyncratic and clinician dependent, and coordination between providers may yield an increased benefit in utilization of this biomarker. 12
The PCT algorithm utilized in our study was a simplified version of a previously studied algorithm that we hypothesized would provide better compliance by the providers. 16 Since adherence to PCT algorithms has been less than ideal in previous studies, with an adherence rate of <50% even in large prospective trials, a simplified version was chosen to facilitate decision-making between disciplines. Our previous study demonstrated adherence rates of 97%. In the current study, pharmacist recommendations were accepted in 21 of 26 attempts in the prospective cohort, demonstrating adherence to the algorithm 81% of the time. Our institution has clinical pharmacists incorporated in a multidisciplinary fashion which facilitated and expedited recommendations to continue or discontinue antibiotics to the team.
The median durations of antibiotic therapy in this study were long in both the PCT group and standard therapy group at 9 days and 12 days, respectively. This is recognized as a challenge at our institution, and implementation of a PCT algorithm is one possible tool to foster a culture more centered on antimicrobial stewardship. Because we previously experienced success with a PCT algorithm in our institution in reducing antibiotic therapy for critically ill patients with pneumonia, simplification of the algorithm and expanding its use to sepsis helped with awareness and adherence among our critical care providers. 13
This study has several limitations. This was a single-center study with a small sample size. The design utilized a prospective cohort versus a retrospective cohort rather than randomizing and comparing 2 prospective comparator groups. There is possibility of selection bias because patients were being screened for criteria by investigators from a generated list. In addition, only 2 serial PCT levels were required for the algorithm rather than using multiple serial levels as previously studied in larger trials.
Conclusion
Use of a simplified PCT algorithm with pharmacist-guided recommendations significantly reduced the duration of antibiotic therapy in a cohort of critically ill patients with sepsis. These results coincide with those of larger randomized controlled trials using PCT algorithms that showed decrease in antibiotic therapy. The implementation of the simplified algorithm into ICUs with clinical pharmacists making recommendations also demonstrated higher adherence (81%) compared with previous studies. Large prospective multicenter studies are needed to prove the validity of the simplified algorithm before it is widely adopted.
Acknowledgments
The authors would like to thank Matthew Ferraro, PharmD.
Authors' Note: Results of this research were presented at the Florida Residency Conference in Tampa, FL, on May 18, 2018, and in the 48th Society of Critical Care Medicine Congress on February 19, 2019, in San Diego, CA. The research abstract was published in January 2019 Critical Care Medicine journal supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Julie Willmon https://orcid.org/0000-0003-1015-8939
Bibidh Subedi https://orcid.org/0000-0002-4376-7696
References
- 1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017; 45(3):486–552. [DOI] [PubMed] [Google Scholar]
- 2. Riedel S. Procalcitonin and role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis. 2012; 73:221–227. [DOI] [PubMed] [Google Scholar]
- 3. Lee H. Procalcitonin as a biomarker for infectious disease. Korean J Intern Med. 2013; 28:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schuetz P, Birkhahn R, Sherwin R, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the Multicenter Procalcitonin Monitoring Sepsis (MOSES) study. Crit Care Med. 2017; 45:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017(10):CD007498. doi:10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014; 34:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009; 302(10):1059–1066. [DOI] [PubMed] [Google Scholar]
- 8. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008; 177:498–505. [DOI] [PubMed] [Google Scholar]
- 9. Hochreiter M, Kohler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009; 13:R83. doi:10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009; 394:221–226. [DOI] [PubMed] [Google Scholar]
- 11. Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis: a randomized controlled trial. Am J Respir Crit Care Med. 2014; 190(10):1102–1110. doi:10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 12. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017; 64(11):1509–1515. doi:10.1093/cid/cix179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subedi B, Louzon P, Zappas K, Onyia W, DeBoer K. Impact of pharmacist-led procalcitonin-guided antibiotic therapy in critically ill patients with pneumonia. Hosp Pharm. 2020;55(3):204–210. [DOI] [PMC free article] [PubMed]
- 14. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3:1–150. [Google Scholar]
- 16. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicenter randomised controlled trial. Lancet. 2010; 375(9713):463–474. [DOI] [PubMed] [Google Scholar]
- 17. De Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016; 16(7): 819–2714. doi:10.1016/S1473-3099(16)00053. [DOI] [PubMed] [Google Scholar]