Abstract
Background: Diazepam is one of the most widely prescribed tranquilizers for the therapy of alcohol withdrawal syndrome (AWS), which includes the symptoms of anxiety, fear, and emotional tension. However, diazepam therapy often turns out to be ineffective, and some patients experience dose-dependent adverse drug reactions, reducing the efficacy of therapy. Aim: The purpose of our study was to investigate the effects of CYP2C19*17 genetic polymorphisms on the steady-state concentration of diazepam in patients with AWS. Materials and Methods: The study was conducted on 50 Russian male patients suffering from the AWS. For the therapy of psychomotor agitation, anxiety, fear, and emotional tension, patients received diazepam in injections at a dosage of 30.0 mg/day for 5 days. Genotyping was performed by real-time polymerase chain reaction. The efficacy and safety assessment was performed using psychometric scales and scales for assessing the severity of adverse drug reactions. Therapeutic drug monitoring (TDM) was performed using the high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS) method. Results: Based on the results of the study, we revealed the differences in the efficacy of therapy in patients with different CYP2C19 −806C>T genotypes: (*1/*1) −12.0 [−15.0; −8.0], (*1/*17+*17/*17) −7.0 [−14.0; −5.0], P < .001, as well as the results of TDM: (CC) 250.70 [213.34; 308.53] ng/mL (*1/*17+*17/*17) 89.12 [53.26; 178.07] ng/mL, P < .001. Conclusion: Thus, our study enrolling 50 patients with AWS, showed the effects of CYP2C19*17 genetic polymorphisms on the efficacy and safety rates of diazepam. Furthermore, we revealed the statistically significant difference in the levels of plasma steady-state concentrations of diazepam in patients carrying different genotypes.
Keywords: pharmacogenetics, benzodiazepines, diazepam, biotransformation, personalized medicine, CYP2C19, alcohol withdrawal syndrome
Introduction
Today benzodiazepines (BZDs) are among the most widely prescribed medicinal products in the world. 1 They have the largest and the best evidence base in the treatment of AWS, and are considered the gold standard. 2 Currently the problem of personalized approach to the prescription of BZDs is poorly developed in the scientific community. The wide use of these medications creates a misleading impression that it does not require the personalized approach. Therefore, despite the high frequency of administration of BZDs, their dose selection is currently empirically based. According to the available scientific data, in a subset of patients, AWS worsens despite escalating doses of BZDs. 3 Such patients represent a severe alcohol withdrawal state and a serious challenge to practitioners due to the acuity and refractoriness of the disorder.4,5 The incidence rates of this state are unknown, but patients suffering from the resistant AWS were found to have a higher rate of intubation, longer ICU stays, and a greater risk of nosocomial infections in comparison with the patients with the AWS who response to BZDs.5,6 Meanwhile, the use of the exceedingly high doses of BZDs in this cohort of patients may result in the occurrence of adverse drug reactions (ADRs). 7 Common side effects among all BZDs include drowsiness, lethargy, and fatigue. At higher dosages, impaired motor coordination, dizziness, vertigo, slurred speech, blurry vision, mood swings, and euphoria can occur, as well as hostile or erratic behavior in some instances.7,8
Today it is well known that clinical responses to BZDs vary widely between individuals. 1 The studies of the pharmacogenetics of BZDs usually focus on the genes of cytochrome P450 (CYP) enzymes, which are among the factors that contribute to the pharmacokinetic (PK) variability of drugs. Diazepam is mainly metabolized via CYP2C19 and CYP3A4 to its major active metabolite, desmethyldiazepam. Recent research revealed the effect of CYP2C19 and CYP3A4 genetic polymorphisms on the pharmacokinetics of BZDs.9 -12 Studies found that the impact of polymorphisms in CYP3A4 on different BZDs metabolism have been mixed, while polymorphisms of CYP2C19 appear to have an impact on the metabolism of several BDZs.
The differences in the activity of these enzymes are genetically determined. Currently there is a lack of data on the pharmacogenetics of BZDs in patients with AWS. 13 The activity of CYP2C19 isozyme in carriers of the minor *17 allele (T) by the polymorphic marker −806C>T (rs12248560) of gene CYP2C19 is increased. Thus, the biotransformation of each substrate of CYP2C19, including drugs, is increased, as well as the elimination of these substrates. 14
The results of previous studies which were conducted by our research group and focused on investigation of the CYP2D6 15 and CYP3A5 16 genetic polymorphisms confirm the importance, relevance and possibility to investigate the personalized approach to the prescription of BZDs (and specifically diazepam) in such cohort of patients. The objective of our study was to investigate the effect of CYP2C19*17 genetic polymorphisms on the steady-state concentration of diazepam as well as its impact on the efficacy and safety rates of therapy in patients with AWS.
Materials and Methods
Clinical Characteristics of Patients
The study included 50 male patients (average age—42.6 ± 10.3 years). Inclusion criteria were the diagnosis of “Mental and behavioral disorders due to use of alcohol. Withdrawal state, uncomplicated” (F10.30, according to ICD-10); written informed consent obtained from the patient; an initial phase of AWS (abstinence from alcohol for at least 8 hours, but no longer than 48 hours prior to the inclusion in study); presence of anxiety, fear or emotional tension in the clinical presentation of the patient; Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) score more than 10; and 5-days diazepam therapy. Exclusion criteria were presence of any other mental disorders or severe somatic disorders (except chronical alcoholic hepatitis and toxic encephalopathy); presence of any other psychotropic medications in treatment regimen except diazepam; creatinine clearance values <50 mL/min, creatinine concentration in plasma ≥1.5 mg/dL (133 mmol/L); body weight <60 kg or >100 kg; age of 75 years or more, and presence of any contraindications for diazepam use.
For the therapy of anxiety, fear, and emotional tension in the clinical presentation of AWS, patients received diazepam in intramuscular injections at a dose of 10.0 mg three times a day at equal intervals of 8 hours (30.0 mg per day).
Local Ethical Committee
The research was approved by the local ethical committee of the Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare (The protocol No. 08 from February 18, 2016).
Therapy Efficacy and Safety Evaluation
To evaluate the diazepam efficacy, several international well-validated psychometric scales were used: Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) 17 and Visual Analogue Scale (VAS). The safety profile was evaluated using The UKU Side-Effect Rating Scale (UKU). 18 Patients were examined on days 1 and 5 of diazepam therapy.
Genotyping
Venous blood samples collected in vacuum tubes VACUETTE® (Greiner Bio-One, Austria) on the fifth day of diazepam therapy were used for genotyping. The real-time polymerase chain reaction was performed using DNA amplifiers “Dtlite” of DNA Technology (Moscow, Russia), CFX96 Touch Real Time System with CFX Manager software of Bio-Rad Laboratories Inc. (Hercules, CA, USA) and sets “SNP-screen” of “Syntol” (Moscow, Russia). It was used to determine the single nucleotide polymorphisms (SNP’s) 681G>A of the gene CYP2C19*2 (rs4244285) and −806C>T of the gene CYP2C19*17 (rs12248560). In every “SNP-screen” set, two allele-specific hybridizations were used, which allowed determining two alleles of studied polymorphism separately on two fluorescence channels.
Therapeutic Drug Monitoring
For the therapeutic drug monitoring, venous blood samples were collected on the day 5 of diazepam therapy (by morning before the drugs injection). The plasma calibration standards (St) and quality control samples (QC) were made from a stock solution prepared by consistent dissolving of substantial amounts in methanol with subsequent dilution to the relevant concentrations. Calibration curve was created using 5, 10, 20, 50, 100, 200, 500, 1000, 2000 ng/mL calibration standards along with 5 ng/mL (LLOQ), 15 ng/mL (Low QC), 1000 ng/mL (Medium QC), and 1500 ng/mL (High QC) quality control samples (QC). Diazepam (250 ng/mL in acetonitrile) was used as the internal standard.
Sample preparation
Samples were prepared using a protein precipitation method. A 1.5 mL tube was filled with 0.200 mL of analyzed plasma sample and 600 µL of acetonitrile containing the internal standard. The mixture was shaken on Vortex for 10 minutes, and then samples were centrifuged at 14 500 g for 10 minutes at 4°С. Then the supernatant was transferred to an autosampler vial. Samples were analyzed using the HPLC system Agilent 1260 (Agilent Technologies, CA, USA) and tandem mass selective detector Agilent 6460 (Agilent Technologies, CA, USA) with Jet Stream Electrospray Ionization Source.
Conditions of chromatographic analysis
Stationary phase: column Agilent Poroshell 120 EC-C18 (2.7 µm, 3.0 mm, 50 mm) with the precolumn InfinityLab Poroshell 120 EC-C18 (2.7 µm, 3.0 mm, 5.0 mm; Agilent Technologies, CA, USA). The column temperature was 50°С. The mobile phase consisted of the A eluent (10 mM ammonium formate in 0.1% formic acid) and B eluent (methanol in 0.1% formic acid). The flow rate was 0.4 mL/min. The gradient elution process was performed. The analysis time was 9.0 minutes for every sample. The volume of the inserted sample was 0.002 mL. Retention time under the given conditions was 4.75 min for diazepam and 4.84 min for the internal standard.
Conditions of mass-spectrometry determination
We used positive mode electrospray ionization for mass-selective detection. Detector registered following MRM-transitions: from 349.0 m/z [MН+] to 206.1 m/z (collision cell energy 40 V) and from 349.0 m/z [MН+] to 184.0 m/z (collision cell energy 32 V) for diazepam; from 285.1 m/z [MН+] to 193.1 m/z (collision cell energy 32 V) and from 285.1 m/z [MН+] to 154.1 m/z (collision cell energy 24 V) for the internal standard. The voltage on fragmentor for diazepam and internal standard was 156 V and 166 V, respectively. The voltage on capillary was 3.5 kV, the temperature of desiccant gas was 350°С, nitrogen flow was 6000 mL/min. Nebulizers pressure was 45 psi, sheath gas temperature was 375°С, sheath gas flow was 11 L/min.
Method validation
The methodology used in the study met FDA Guidance for Industry: Bioanalytical method validation.19,20 Calibration dependence was linear for diapason at 0.5 to 200 ng/mL. Correlation coefficients were normal (at least 0.99). We evaluated the intra- and inter-cycles precision and accuracy rates. Precision and accuracy rates were normal (no more than 20% at LLOQ, no more than 15% for other points). The matrix effect had no influence.
Statistical Analysis
Statistical analysis of the results was performed with non-parametric methods using the Statsoft Statistica v. 10.0 (Dell Statistica, Tulsa, OK, USA). The normality of samples distribution was evaluated using W-Shapiro–Wilk test and taken into account when choosing a method. The differences were considered statistically significant at Р < .05 (power in excess of 80%). To compare two independent groups Mann–Whitney U-test was used with Benjamini–Hochberg multiple testing correction. To determine the correlation between quantitative characteristics Spearman rank correlation coefficient (rS) was calculated. Calculation of sample size using “pwr” package. A power analysis showed that 45 patients are sufficient to minimize type 2 errors (<0.2). Research data are presented as the median and interquartile range (Me [Q1; Q3]) or, in case of a normal distribution, as the arithmetic mean and standard deviation (Mean ± SD).
Results
The CYP2C19 genotyping by polymorphic marker −806C>T (rs12248560) performed in 50 patients have revealed the following: the number of patients with *1/*1 (CC) genotype accounted for 30 (60%), *1/*17 (CT) - 16 (32%), *17/*17 (TT) - 4 (8%).
The distribution of genotypes corresponded to Hardy–Weinberg equilibrium for the European population (χ2 = 0.75, P-value = .38).
The results of data analysis performed for psychometric scales (CIWA-Ar, VAS) and side-effect rating scale (UKU) in patients who received diazepam are presented in Table 1.
Table 1.
Changes in the Psychometric Scales and Side-Effect Rating Scale Scores in Patients With Different Genotypes by Polymorphic Marker –806C>T (rs12248560) From Day 1 to Day 5 of the Inpatient Treatment.
| Scale | CC | CT+TT | P |
|---|---|---|---|
| CIWA-Ar | −12.0 [−15.0; −8.0] | −7.0 [−14.0; −5.0] | <.001 |
| VAS | −8.5 [7.0; 10.0] | −6.0 [5.0; 9.0] | <.001 |
| UKU | 8.0 [6.0; 12.0] | 6.0 [6.0; 12.0] | <.001 |
Note. P—obtained in Benjamini–Hochberg multiple testing correction (based on the results of Mann–Whitney U-test). CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol scale; VAS = Visual Analogue Scale; UKU = The UKU Side-Effect Rating Scale.
Changes in CIWA-Ar scale scores across patients with different genotypes by polymorphic marker −806C>T (rs12248560) are shown in Figure 1. The severity of AWS (as evaluated by the difference in CIWA-Ar scores before the therapy and after it) differ between the different genotypes groups: (CC) −12.0 [−15.0; −8.0], (CT+TT) −7.0 [−14.0; −5.0], P < .001. Changes in the UKU scores across patients are shown in Figure 2. The severity of ADRs (as evaluated by the difference in UKU scores before the therapy and after it) was different between the patients with different genotypes: (CC) 8.0 [6.0; 12.0], (CT+TT) 6.0 [6.0; 12.0], P < .001.
Figure 1.
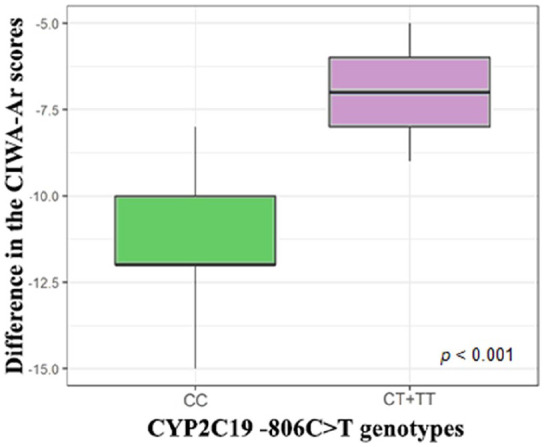
Changes in the CIWA-Ar scores across patients with different genotypes by polymorphic marker −806C>T (rs12248560).
Note. CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol scale used to evaluate the efficacy of therapy; UKU = The UKU Side-Effect Rating Scale used to evaluate the safety of therapy.
Figure 2.
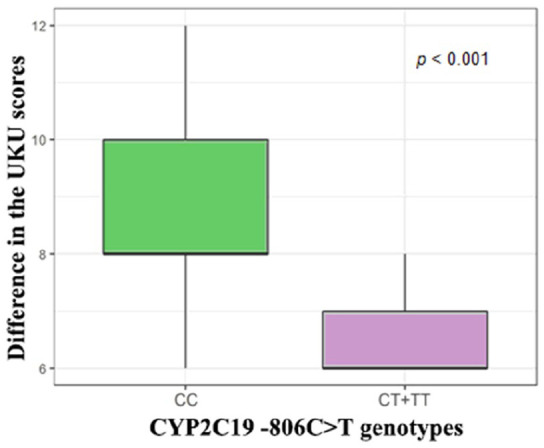
Changes in the UKU scores across patients with different genotypes by polymorphic marker −806C>T (rs12248560).
Note. CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol scale used to evaluate the efficacy of therapy; UKU = The UKU Side-Effect Rating Scale used to evaluate the safety of therapy.
We revealed a statistically significant difference in plasma steady-state concentration of diazepam across patients with different genotypes: (CC) 250.70 [213.34; 308.53], (CT+TT) 89.12 [53.26; 178.07], P < .001 (Figure 3).
Figure 3.
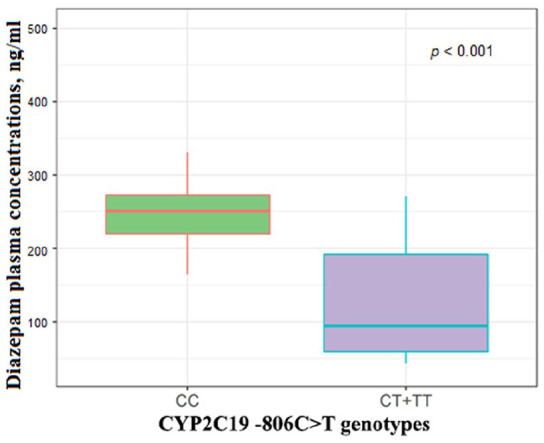
Differences in plasma steady-state concentrations of diazepam in patients with different genotypes.
Discussion
The results revealed the difference between the efficacy and safety profiles of diazepam in patients with AWS carrying different genotypes of the CYP2C19 gene by polymorphic marker −806C>T (rs12248560).
Patients carrying the CT and TT genotypes showed a slower increase in the UKU scale scores, which demonstrate a lower risk of ADR occurrence than the CC genotype carriers. This could be due to the enhanced activity of the CYP2C19 isoenzyme in patients carrying the minor allele T by polymorphic marker −806C>T (rs12248560) of the CYP2C19 gene. The increased activity of the CYP2C19 isoenzyme leads to the enhanced biotransformation rates of diazepam, which in turn leads to a decrease in plasma concentration of the drug and to a reduced risk of undesirable side effects.
The efficacy of diazepam therapy (as evaluated by the psychometric scales) was also different depending on AWS patients’ genotypes: the difference in CIWA-Ar scores before the therapy and after it was higher in patients with the CC genotype in comparison with those who carried the CT and TT genotypes. This is presumably related to the enhanced CYP2C19 isoenzyme activity in patients carrying the CT and TT genotypes. This, in turn, leads to the increased biotransformation rates of diazepam, a decrease in concentration rates of the drug in plasma and the less pronounced effect of the medication.
Therapeutic drug monitoring revealed that carriers of the wild-type genotype have a higher level of plasma diazepam concentrations, which is probably due to the increased diazepam biotransformation in the carriers of the minor allele T.
Thus, based on the study results, one would assume that those patients who carry the CT and TT genotypes, have a higher risk of the decreased therapeutic effect of diazepam, which leads to persistence of the anxiety, fear, and emotional tension. To reduce this risk, such cohort of patients requires the prescription of medications, which are not metabolized by CYP2C19, or administration of higher doses of diazepam, which will compensate for the accelerated elimination of the drug and maintain the level of its steady-state concentration within the therapeutic window. This can positively affect the profile of the overall therapy efficacy, which will increase compliance. Furthermore, it is possible that the carriers of the minor nonmutant genotype by polymorphic marker −806C>T (rs12248560) of CYP2C19 gene have a higher risk of ADRs, such as drowsiness, lethargy, fatigue, impaired motor coordination, dizziness, vertigo, slurred speech, and blurry vision.
Overall, pharmacogenomic testing can be beneficial to optimize diazepam treatment outcomes for the patients with AWS by avoiding ADRs and maximizing efficacy.
Conclusion
The study conducted in 50 patients with AWS revealed the correlation between the CYP2C19*17 genetic polymorphisms and the efficacy and safety of diazepam. Furthermore, a statistically significant difference in plasma steady-state concentrations of diazepam was revealed.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Valentin Yurievich Skryabin  https://orcid.org/0000-0002-4942-8556
https://orcid.org/0000-0002-4942-8556
References
- 1. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. [DOI] [PubMed] [Google Scholar]
- 2. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benedict NJ, Wong A, Cassidy E, et al. Predictors of resistant alcohol withdrawal (RAW): a retrospective case-control study. Drug Alcohol Depend. 2018;192:303-308. [DOI] [PubMed] [Google Scholar]
- 4. Hack JB, Hoffman RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38(Suppl 9):S494-S501. [DOI] [PubMed] [Google Scholar]
- 6. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Griffin CE, III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223. [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitz A. Benzodiazepine use, misuse, and abuse: a review. Ment Health Clin. 2016;6(3):120-126. doi: 10.9740/mhc.2016.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skryabin VY, Zastrozhin MS, Torrado MV, et al. How do CYP2C19*2 and CYP2C19*17 genetic polymorphisms affect the efficacy and safety of diazepam in patients with alcohol withdrawal syndrome? Drug Metab Pers Ther. 2020;35(1). doi: 10.1515/dmpt-2019-0026. [DOI] [PubMed] [Google Scholar]
- 10. Fukasawa T, Yasui-Furukori N, Suzuki A, Inoue Y, Tateishi T, Otani K. Pharmacokinetics and pharmacodynamics of etizolam are influenced by polymorphic CYP2C19 activity. Eur J Clin Pharmacol. 2005;61(11):791-795. [DOI] [PubMed] [Google Scholar]
- 11. Zastrozhin MS, Skryabin VY, Miroshkin SS, Bryun EA, Sychev DA. Pharmacogenetics of alcohol addiction: current perspectives. Appl Clin Genet. 2019;12:131-140. doi: 10.2147/TACG.S206745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean L. Diazepam therapy and CYP2C19 genotype In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, eds. Medical Genetics Summaries. Bethesda, MD: National Center for Biotechnology Information (US); 2012-2016. [Google Scholar]
- 13. Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Curr Pharm Des. 2010;16(19): 2141-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande N, Sharanya V, Ravi Kanth VV, et al. Rapid and ultra-rapid metabolizers with CYP2C19*17 polymorphism do not respond to standard therapy with proton pump inhibitors. Meta Gene. 2016;9:159-164. doi: 10.1016/j.mgene.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zastrozhin MS, Savchenko LM, Bryun EA, et al. Effects of CYP2D6 genetic polymorphisms on the efficacy and safety of fluvoxamine in patients with depressive disorder and comorbid alcohol use disorder. Pharmacogenomics Pers Med 2018;11:113-119. doi: 10.2147/PGPM.S160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zastrozhin MS, Grishina E, Ryzhikova KA, et al. The influence of CYP3A5 polymorphisms on haloperidol treatment in patients with alcohol addiction. Pharmgenomics Pers Med. 2018;11:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nuss MA, Elnicki DM, Dunsworth TS, Makela EH. Utilizing CIWA-Ar to assess use of benzodiazepines in patients vulnerable to alcohol withdrawal syndrome. W V Med J. 2004;100(1):21-25. [PubMed] [Google Scholar]
- 18. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1-100. [DOI] [PubMed] [Google Scholar]
- 19. Food and Drug Administration. Draft Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: U.S. Food and Drug Administration; 1999. [Google Scholar]
- 20. Melnik EV, Petukhov AE, Kozin SV, Pomerantseva TY, Ramenskaya GV. Validated HPLC-MS/MS method for quantification of ethylmethylhydroxypyridine succinate in rat brain and its application to a pharmacokinetic study. J Chromatogr B: Analyt Technol Biomed Life Sci. 2018;1096:180-186. doi: 10.1016/j.jchromb.2018.08.029. [DOI] [PubMed] [Google Scholar]


