Abstract
Purpose: Procalcitonin (PCT) may be an effective biomarker in the management of lower respiratory tract infections (LRTI) when combined with antimicrobial stewardship support. We assessed the impact of a PCT protocol with clinical pharmacy support for LRTI using a clinical decision support system (CDSS) for monitoring. Methods: This was a single-center retrospective cohort study conducted at a large, nonteaching hospital in Nashville, TN. All patients who met eligibility requirements and were initiated on the PCT protocol for a suspected LRTI between February and March 2018 were included and matched to historical control patients from 2016 to 2017 on a 1:1 basis based on antibiotics, indication, and time of year. Results: During this 2-month period, a total of 126 patients met eligibility requirements for inclusion in the PCT group and were matched to historical control patients. Patients in the PCT group received decreased median antibiotic days of therapy (DOT) compared to controls (11 vs 14, P = .004). There was no change in median length of stay (LOS) between groups. The acceptance rate for patient-specific antibiotic de-escalation recommendations from the clinical pharmacist was 62.5%. Conclusion: PCT protocols that utilize clinical pharmacist interpretation and a CDSS may be an effective intervention of the antimicrobial stewardship program (ASP) for decreasing antibiotic DOT for LRTI.
Keywords: procalcitonin, PCT, lower respiratory tract infections, LRTI, clinical decision support system, CDSS
Introduction
An estimated 30% to 50% of antibiotics given to hospitalized patients may be unnecessary. 1 One of the most common indications for prescribing antibiotics is the suspicion of bacterial lower respiratory tract infections (LRTI).2,3 A multitude of diagnostic barriers exist in this setting. LRTI often present with ambiguous signs and symptoms that can be attributed to other etiologies such as acute exacerbation of chronic obstructive pulmonary disease (COPD) or congestive heart failure.4,5 Adding to this uncertainty, diagnostic imaging may often be non-specific when trying to distinguish pneumonia from other etiologies.3,6,7 Another diagnostic barrier is the inability to identify an infectious pathogen. Standard diagnostic tests have demonstrated a low yield of identification of bacterial pathogens. Jain et al, in a large population-based, active surveillance study of adult patients hospitalized for community-acquired pneumonia (CAP) utilizing extensive diagnostic methods, demonstrated that the majority of patients did not have a pathogen identified. When a pathogen was identified, it was more commonly viral than bacterial. 8 Finally, while 5-day courses of antibiotics are generally recommended for CAP and 7-day courses for hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) in patients that achieve stability, actual durations of therapy often exceed these guidelines.9 -11 One contributing factor may be nebulous criteria for clinical resolution as determined by the provider.
The Centers for Disease Control and Prevention as part of its Core Elements recommend antimicrobial stewardship programs (ASP) to develop interventions to improve antibiotic utilization including durations of therapy. 12 For the aforementioned reasons, LRTI is an area with singular potential for decreasing unnecessary antimicrobial consumption. Biomarkers may be useful tools for clinicians in the treatment of LRTI both in the discrimination of bacterial from other etiologies as well as in the determination of the response to treatment and appropriate duration of therapy.10,11,13,14
Procalcitonin (PCT), a precursor of calcitonin, is a biomarker that is upregulated by cytokines and therefore rises in response to proinflammatory, bacterial infection.15,16 The magnitude of the rise in PCT has been shown to correspond to the severity of the infection. In the absence of a precipitating factor, serum PCT levels are undetectable. 4 PCT has a short half-life of 25 to 30 hours; so rapidly declining PCT levels correspond with the resolution of inflammation.15,16 PCT is blocked by interferon gamma and does not increase in the presence of viral infection or non-infectious inflammation. 4 These properties suggest that trending PCT levels may be a particularly useful complement to standard clinical criteria for guidance in initiation, adjustment, and discontinuation of antimicrobials for LRTI. Potential drawbacks to utilizing PCT include the potential for false positive and false negative tests. Severe trauma, circulatory shock, burns, inhalation injuries, and pancreatitis have been shown to generate positive PCT levels in the absence of bacterial infection. 15 Additionally, localized infections, such as empyema, as well as atypical bacterial infections may produce PCT levels that are below the diagnostic threshold.15,17
In 2016, the Infectious Disease Society of America (IDSA) antimicrobial stewardship guidelines acknowledged that PCT algorithms had shown reductions in antibiotic use in the intensive care unit (ICU) in Europe. They went on to recommend that “If implemented, each ASP must develop processes and guidelines to assist clinicians in interpreting and responding appropriately to results, and must determine if this intervention is the best use of its time and resources.”11,18 While the potential utility of PCT for antimicrobial stewardship may be apparent, the best processes for utilization is an area that has not been fully elucidated in the literature. The Procalcitonin Antibiotic Consensus Trial (ProACT) was a large, patient-level, 1:1 randomized study conducted in 14 hospitals with high adherence to quality measures in the United States and assessed the impact of PCT in patients with LRTI for whom the physician was uncertain of starting antibiotic therapy. Physicians were provided with PCT levels in real-time along with protocol antibiotic guidelines. There was no significant difference in the primary endpoint of mean antibiotic-days for patients in the PCT group (4.2 vs 4.3; P = .87). 19 In contrast, a single-center, controlled, pre-post study of PCT-guided therapy for LRTI at a large, academic tertiary care hospital in Baltimore, Maryland using daily infectious disease (ID) pharmacist and ID physician review and non-binding advice provided via email or secure text demonstrated a reduction in median antibiotic duration from 7 days to 6 (P = .045) for pneumonia and from 4 days to 3 (P = .01) for acute COPD exacerbation. 20 The purpose of this investigation is to determine the impact of a PCT protocol for LRTIs with accompanying clinical pharmacy recommendations utilizing a clinical decision support system (CDSS).
Materials and Methods
This was a single-center, retrospective cohort study conducted at a large, nonteaching hospital in Nashville, Tennessee that includes a level II trauma center and certified stroke center. This study received institutional review board (IRB) approval with an opt-out consent form given to all patients who were still hospitalized at the time of data collection.
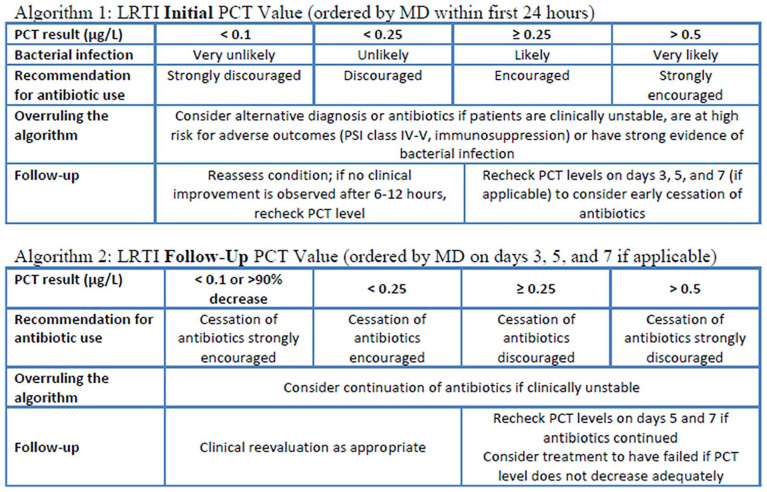
A PCT protocol for LRTI was developed by the ASP and approved in July 2017. It consists of two algorithms, one for the initial PCT level and one for follow-up PCT levels (Appendix 1). 3 PCT labs are performed in-house with turnaround times of approximately 1 hour. The protocol is ordered at the prescriber’s discretion but is recommended to be initiated within 24 hours of suspicion of LRTI. Likewise, antibiotic regimens are ordered at the prescriber’s discretion but are recommended not to be delayed for return of PCT values to avoid hindering timely antibiotic administration as well as the potential for PCT levels to not be elevated in the setting of early bacterial infection. 18 Importantly, recent guidelines for the management of CAP and HAP/VAP have recommended that PCT not be used in determining whether to withhold antibiotics in the setting of clinically suspected pneumonia.10,11 Per protocol, initial PCT levels >0.5 µg/L indicate that a bacterial infection is very likely and continuation of antibiotics is strongly encouraged. Initial PCT levels between 0.25 µg/L and 0.5 µg/L indicate that a bacterial infection is likely and continuation of antibiotics is encouraged. Initial PCT levels between 0.1 µg/L and 0.25 µg/L indicate that a bacterial infection is unlikely and continuation of antibiotics is discouraged. If initial PCT levels are <0.1 µg/L, a bacterial LRTI is very unlikely and continuation of antibiotics is strongly discouraged. Pre-specified criteria for overruling the algorithm are explicitly stated and include: if the patient is clinically unstable, if the patient is at a high risk for adverse outcomes, or if there is alternative strong evidence for a bacterial infection. Recommendations for follow-up PCT levels are also explicitly stated. For patients in which continuation of antibiotics was discouraged or strongly discouraged, follow-up PCT levels are recommended if the clinical condition has not improved after 6 to 12 hours. For patients in which continuation of antibiotics was encouraged or strongly encouraged, follow-up PCT levels are recommended on days 3, 5, and 7, if applicable. The interpretations of follow-up PCT levels are the same as initial levels with regard to continuation or discontinuation of antibiotics with the added caveat that a decrease of >90%, regardless of the particular level, warrants a recommendation that cessation of antibiotics is strongly encouraged. If there is not an appropriate decrease in PCT levels by day 7, then treatment failure should be considered. Furthermore, additional patient-specific recommendations that clinically overrule the written protocol can be provided by the clinical pharmacist and include a concern for early bacterial infection or suspicion of localized infection in a patient with a PCT below the diagnostic threshold as well as comorbidities that may elevate the PCT in the absence of bacterial infection in a patient with values that are typically indicative of bacterial infection. In addition, the clinical pharmacist may recommend aggressive diagnostic workup such as multiplex polymerase chain reaction (PCR) for common respiratory viral pathogens.
Using a CDSS, clinical pharmacists trained on the protocol were alerted to PCT levels in real-time. The written protocol with the patient-specific PCT level, protocol interpretation and possible patient-specific clinical pharmacist recommendations was then provided to the prescribing physician. The protocol was designed for all PCT levels to result in this communication to physicians regardless of whether a change in therapy was recommended. While it always contains standard protocol recommendations, the clinical pharmacist trained on the protocol can make more specific regimen recommendations or deviate from the protocol recommendations based on clinical criteria in an effort to be consistent with recommendations that PCT measurements be combined with ASP support. 18 The protocol form contains “Agree” and “Disagree” boxes as well as a designated space for physicians to state their rationale for disagreement with the recommendations. The prescribing physician was requested to sign and date the protocol and return it to the submitting pharmacist. This form was used for internal communication, was never added to the patient’s chart, and was not part of the permanent medical record. All recommendations and subsequent interventions were documented in the CDSS associated with the patient’s chart. Clinical pharmacists trained on the protocol were available 7 days per week from 7 am to 7 pm.
Case patients consisted of patients that received antibiotics for a suspected LRTI and were initiated on the PCT protocol. All patients age 18 years or older who were initiated on the protocol for a suspected LRTI with a resulting PCT level were assessed for eligibility. The CDSS was used to identify patients with a resulting PCT level between February and March 2018. Patients with only one resulting PCT were unable to be trended; however, they were included in the final analysis. Patients were excluded if they opted out using the IRB approved opt-out consent form or if they had an initial PCT level prior to the defined study period.
Cases were matched to historical controls based on antibiotics, indication, and time of year on a 1:1 basis. The CDSS and chart reviews were used to match each case patient to a control patient age 18 years or older who was initiated with the same antibiotic regimen for the same LRTI indication between February and March 2016 or February and March 2017. Time of year was included in matching criteria in an effort to preserve consistency in regards to circulating respiratory viruses. Positive bacterial and viral culture results were not included as matching criteria as the PCT level may influence the etiological workup of the potential infection including the recommendation by the clinical pharmacist for a respiratory multiplex PCR panel. Age was not included as a matching criterion in an effort to maintain an adequate control sample although this variable is recognized as a potential confounder. When multiple patients met criteria for matching, a random number generator was utilized to choose the control patient using Microsoft Excel (2016).
Data collected included the following: pertinent patient demographics, antibiotic DOT including intended outpatient regimens upon discharge, pertinent labs, vital signs, microbiology data, and imaging, as well as pharmacist clinical recommendations, and provider responses. Microbiology data included respiratory pathogen PCR panel and pertinent positive respiratory and blood cultures. All systemic antibiotics were included in DOT including in an aggregate fashion for combination regimens. Nominal data for patient demographics, antibiotics, and microbiology results were analyzed using the Chi-square test. The Shapiro–Wilk test for normality demonstrated a non-normal distribution for antibiotic DOT (P < .001) and LOS (P < .001). As a result, median antibiotic DOT and LOS were analyzed using the Mann–Whitney test.
The primary endpoint was median antibiotic DOT. Secondary endpoints include prescriber acceptance rates of pharmacist recommendations as determined by the internal communication tool and hospital LOS.
Results
One hundred and thirty-two patients receiving antibiotics for LRTI with PCT levels were identified using the CDSS. Three patients opted out of the study and three patients were excluded due to initial PCT levels being ordered prior to the study period. A total of 126 case patients were included in the final analysis and matched to historical controls. Patients were similar in regards to initial antibiotic regimens, indications, gender, chest radiography, and ICU admission (Table 1). There were significantly fewer case patients aged 65 years or older (63 vs 83, P = .011). Significantly more case patients had a pertinent positive microbiology result (36 vs 30, P = .010) and these were predominantly viral. Control patients had more positive bacterial isolates. Significantly fewer case patients had a leukocytosis (60 vs 87, P = .001). Antibiotic regimens were similar between groups (Table 2).
Table 1.
Patient Demographics.
| PCT group n (%) |
Control group n (%) |
P-value | |
|---|---|---|---|
| Characteristic | |||
| Age > 65 years | 63 (50) | 83 (65.9) | .011 |
| ICU admission | 45 (35.7) | 44 (34.9) | .539 |
| Sex, male | 60 (47.6) | 55 (43.7) | .527 |
| Leukocytosis (WBC > 11k) | 60 (47.6) | 87 (69) | .001 |
| Microbiology | |||
| Positive result | 36 (28.6) | 30 (23.8) | .010 |
| Viral only | 21 (16.7) | 7 (5.6) | |
| Bacterial only | 12 (9.5) | 18 (14.3) | |
| Viral and bacterial | 2 (1.6) | 1 (0.8) | |
| Fungal | 0 (0) | 4 (3.2) | |
| Fungal and viral | 1 (0.8) | 0 (0) | |
| Indication | |||
| CAP | 43 (34.1) | 44 (34.9) | |
| HAP | 12 (9.5) | 11 (8.7) | |
| VAP | 0 (0) | 0 (0) | |
| HCAP | 46 (36.5) | 47 (37.3) | |
| Aspiration pneumonia | 16 (12.7) | 15 (11.9) | |
| COPD | 8 (6.3) | 9 (7.1) | |
Table 2.
Initial Antibiotic Regimens.
| PCT group n |
Control group n |
|
|---|---|---|
| Antibiotic regimen | ||
| Monotherapy | 30 | 31 |
| Combination therapy | 96 | 95 |
| Antibiotic class | ||
| Penicillin (antipseudomonal) | 17 | 15 |
| Cephalosporin (3rd generation) | 31 | 31 |
| Cephalosporin (antipseudomonal) | 50 | 51 |
| Carbapenem (antipseudomonal) | 6 | 6 |
| Monobactam | 2 | 2 |
| Fluoroquinolone (respiratory) | 11 | 11 |
| Macrolide (advanced generation) | 37 | 38 |
| Tetracycline | 3 | 3 |
| Glycopeptide | 63 | 63 |
| Oxazolidinone | 2 | 1 |
| Lincosamide | 4 | 4 |
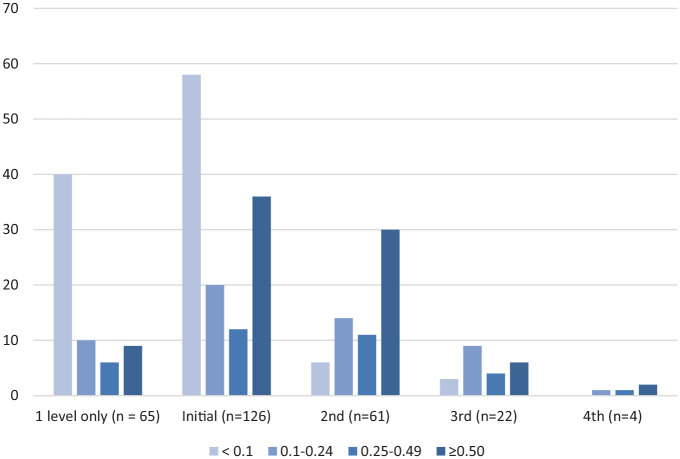
Initial PCT levels are described in Table 3. Forty-two patients (33.3%) had initial PCT levels collected on days 0 to 1 of antibiotics as recommended in the protocol. Seventy-eight (61.9%) patients had initial PCT levels below the set diagnostic threshold of 0.25 µg/L for bacterial LRTI. A patient-specific recommendation beyond the standard protocol recommendation to discontinue or de-escalate an antibiotic regimen based on this initial PCT was made in 43 (55.1%) of these patients and was accepted in 30 (38.5%) of these patients. A recommendation that deviated from the protocol based on clinical criteria warranting continued antibiotics was made in 23 (29.5%) of these patients. All PCT levels are described in Figure 1. Sixty-one (48.4%) patients had at least one follow-up PCT level and 22 (17.5%) patients had at least two follow-up PCT levels. Of these, 21 (25.3%) patients had specific recommendations for de-escalation extending beyond standard recommendations with an acceptance rate of 47.6%. Five (6.0%) of these patients had recommendations that deviated from the protocol based on clinical criteria.
Table 3.
Initial PCT.
| n (%) | |
|---|---|
| Day of antibiotics first PCT ordered | |
| 0-1 | 42 (33.3) |
| 2-3 | 71 (56.3) |
| ≥4 | 13 (10.3) |
| Initial PCT (µg/L) | |
| <0.10 | 58 (46.0) |
| 0.10-0.24 | 20 (12.3) |
| 0.25-0.50 | 12 (9.5) |
| >0.50 | 36 (28.6) |
Figure 1.
Description of PCT levels (µg/L).
The primary endpoint of median antibiotic DOT was decreased in the PCT group compared with matched controls (11 vs 14, P = .004). There was no change in median LOS between groups (Table 4). Acceptance rates for specific antibiotic de-escalation recommendations was 62.5%.
Table 4.
Results.
| PCT group | Control group | P-value | |
|---|---|---|---|
| Median antibiotic DOT | 11 | 14 | .004 |
| Median LOS | 5 | 5 | >.99 |
| Antibiotic recommendations based on PCT | |||
| De-escalation recommendations | 64 (50.8%) | ||
| De-escalation recommendations accepted | 40 (62.5%) | ||
Discussion
The utility of an effective and efficient tool for determining the need for initiation and continuation of antibiotics in patients that present with the often-vague symptoms of LRTI is apparent. PCT has demonstrated potential for filling this role but with the stipulation that clinical judgment should not be abandoned. 18 Therefore PCT is likely unsuitable for strictly enforced protocols. Since the clinical pharmacist is often a core member of the ASP, we initiated a PCT protocol in which the clinical pharmacist provides clinical recommendations in addition to the standard interpretation of the level. Utilization of a CDSS provided real-time alerts of PCT levels and thus allowed for timely antibiotic recommendations. This intervention resulted in a significant decrease in antibiotic DOT but did not affect overall LOS. It is noteworthy that despite the consistency of the time of year between case and control groups, there was an increased number of confirmed viral etiologies in the PCT group. It is likely that this is a contributing factor for the decreased DOT but also likely that the PCT led to more aggressive workup for viral etiologies in this group.
Limitations to this protocol include the fact that it is subject to the availability of both clinical pharmacists and providers. The aim of the intervention is to have a clinical pharmacist trained to interpret the result of the test available during the usual hours of the prescriber but this is subject to variability depending on practice models and individual prescriber preference. While prescribers were always able to access PCT values once they returned, the ASP clinical interpretation and recommendation could often be delayed resulting in a decrease in DOT saved. Automatic protocols or CDSS models that immediately alert the prescriber could potentially overcome these shortcomings. The CDSS has its own limitations. PCT levels resulted as a nonspecific value. The determination of whether a PCT was to be included in the LRTI protocol was at the discretion of the clinical pharmacist. A separate PCT protocol for sepsis is active at the hospital and this was utilized according to the clinical pharmacist’s judgment even if the primary source of sepsis was LRTI. A major limitation of this study was that it was a small study conducted at a single center. Differences in prescriber and ASP models as well as availability of resources such as a CDSS will affect the overall impact of this type of intervention. In addition, measures of severity of illness such as APACHE II scores and comorbidities were not taken into account. More case patients had viral diagnoses. The impact of positive viral respiratory samples on antibiotic prescribing is apparent although the influence of PCT levels on this etiologic workup was not recorded. Age was not included as a matching criterion and there were significantly more elderly patients in the control group, which would be expected to have an impact on the results. Finally, the decrease in median antibiotic DOT was modest. The determination of whether this protocol is a wise use of resources must be made based on the individual ASP perception of needs and opportunities.
Conclusion
A multidisciplinary approach to PCT monitoring and interpretation may be an effective method to decrease antibiotic DOT for LRTI. Resources such as a CDSS may be useful for implementing protocols efficiently. Further studies are needed to fully address the limitations acknowledged in this study including the applicability to other institutions.
Acknowledgments
Sarah Fraker, MS, CHDA—Statistician
Appendix
Appendix 1. PCT Protocol for LRTI
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Quentin Minson  https://orcid.org/0000-0002-4498-3120
https://orcid.org/0000-0002-4498-3120
References
- 1. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuetz P, Briel M, Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA. 2013;309(7):717-718. [DOI] [PubMed] [Google Scholar]
- 3. Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15(15):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang D, Angus D, Chang C, et al. Design and rationale of the Procalcitonin Antibiotic Consensus Trial (ProACT), a multicenter randomized trial of procalcitonin antibiotic guidance in lower respiratory tract infection. BMC Emerg Med. 2017;17(25):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9(107):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Self WH, Courtney DM, McNaughton CD, Wunderink RG, Kline JA. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med. 2013;31:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Upchurch C, Grijalva C, Wunderink R, et al. Community-acquired pneumonia visualized on CT scans but not chest radiographs. Chest. 2018;153(3):601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi S, Hatfield K, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2017;66(9):1333-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalil A, Metersky M, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Core elements of hospital antibiotic stewardship programs, antibiotic use, CDC. Centers for Disease Control and Prevention. https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html. Published 2018. Accessed June 1, 2018. [Google Scholar]
- 13. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95-107. [DOI] [PubMed] [Google Scholar]
- 14. Çolak A, Yılmaz C, Toprak B, Aktoğu S. Procalcitonin and CRP as biomarkers in discrimination of community-acquired pneumonia and exacerbation of COPD. J Med Biochem. 2017;36(2):122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foushee J, Hope N, Grace E. Applying biomarkers to clinical practice: a guide for utilizing procalcitonin assays. J Antimicrob Chemother. 2012;67(11):2560-2569. [DOI] [PubMed] [Google Scholar]
- 16. Walsh T, DiSilvio B, Hammer C, et al. Impact of procalcitonin guidance with an educational program on management of adults hospitalized with pneumonia. Am J Med. 2018;131(2):201.e1-201.e8. [DOI] [PubMed] [Google Scholar]
- 17. Self W, Balk R, Grijalva C, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barlam T, Cosgrove S, Abbo L, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Townsend J, Adams V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States Medical Center: results of a clinical trial. Open Forum Infect Dis. 2018;5(12):ofy327. [DOI] [PMC free article] [PubMed] [Google Scholar]