Abstract
Objective
Use of ampicillin in outpatient parenteral antimicrobial therapy (OPAT) has historically been complicated by frequent dosing and limited stability. The purpose of this study was to evaluate stability of ampicillin using high-pressure liquid chromatography (HPLC) in an OPAT dosing model using continuous infusion at room temperature over 24 hours immediately following preparation compared with batches stored under refrigeration for 24 hours, 72 hours, and 7 days.
Methods
An HPLC method was developed and validated as stability indicating using guidance in USP general Chapter <1225>. Four ampicillin batches were prepared for each experimental condition (immediate use and refrigerated storage for 24 hours, 72 hours, and 7 days). A pump was used to recirculate the solutions through medical-grade tubing for 24 hours. Triplicate 1-mL aliquots were removed from each batch at time 0, 4, 8, 12, and 24 hours and analyzed for ampicillin concentration.
Results
Each batch was assayed for initial concentration (20.34-21.50 mg/mL), and percent recovery compared with that concentration thereafter. For the duration of infusion, the average recoveries were 96.4%, 95.8%, 94.6%, and 90.3% for immediate use, 24-hour storage, 72-hour storage, and 7-day storage, respectively. The recovery remained above 90% for all batches and time points, except for 7-day storage, which fell below 90% after 4 hours of circulation.
Conclusion
Ampicillin can be prepared and stored in a refrigerator for up to 72 hours prior to continuously infusing at room temperature over 24 hours with less than a 10% loss of potency over the dosing period. This model supports twice weekly OPAT delivery of ampicillin.
Keywords: drug stability, infectious diseases, intravenous therapy
Introduction
Ampicillin, in combination with either gentamicin or ceftriaxone, is the treatment of choice for enterococcal endocarditis, when susceptibilities and tolerance allow. 1 According to the 2015 Infectious Diseases Society of America (IDSA) guidelines on infective endocarditis in adults, treatment durations are 4 to 6 weeks, often completed by shifting treatment to the outpatient setting.1,2 First released in 2004, the IDSA outpatient parenteral antimicrobial therapy (OPAT) guidelines highlighted ampicillin administration as a challenge to outpatient administration due to frequent administration of 2 g every 4 hours and short stability. 3 Early ampicillin stability studies were performed with imprecise testing methodologies, including microbiologic assays, iodometric analyses, and calorimetric assays. 4
More recent studies have utilized high-pressure liquid chromatography (HPLC), often labeled as the gold standard for stability testing, and have found different results, demonstrating longer stabilities.5–7 Several studies have concluded that ampicillin solutions are stable for at least 24 hours at room temperature, supporting administration via continuous infusion.5–7 Specifically, Maher and colleagues evaluated a buffered ampicillin solution of 12 mg/mL using HPLC and found a stability of at least 48 hours at room temperature and 72 hours under refrigeration. 5 Juste and colleagues evaluated an ampicillin infusion solution of 24 mg/mL in normal saline (NS) at room temperature using HPLC and concluded this to be physically compatible. 6 Zhang and colleagues concluded that ampicillin at a concentration of 10 mg/mL was stable for 3 days under refrigeration using HPLC. 7 Additional reports have indicated that ampicillin is stable for at least 3 days under refrigeration (4°C).8,9 The IDSA OPAT guidelines were updated in 2018 to reflect the 3-day stability but do not acknowledge any information regarding continuous infusion after refrigerated storage. 9
To our knowledge, extended storage at refrigerated temperature followed by continuous infusion at room temperature has not been studied. For improved workflow for OPAT, preparation of ampicillin infusion solutions should ideally be made several days in advance, allowing for once or twice weekly delivery to the patient. The purpose of this study was to determine the stability of 12-g ampicillin in 500 mL of NS at room temperature after extended refrigerated conditions for 24 hours, 72 hours, and 7 days, compared with a control solution prepared and immediately infused at room temperature.
Methods
Equipment and Chromatographic Conditions
A Shimadzu HPLC system, equipped with autosampler, column oven, in-line degasser, and ultraviolet (UV) detection set at 240 nm, was used for all chromatographic measurements (Shimadzu Scientific, Kyoto, Japan). Ampicillin calibration solutions (12–24 mg/mL) were prepared in NS on each day of the stability investigation and during method validation (US Pharmacopeia Reference Standard, Rockville, MD, USA). The chromatographic separation utilized a gradient with 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B) at a flow rate of 0.150 mL/min (Honeywell Burdick & Jackson, Morris Township, NJ, USA). The gradient program started at 2% B and ramped to 99% B over 7 minutes, with a 4-minute reequilibration at 2% B. The analytical column was a UCT C18 column (2.1 × 100 mm; 1.8 micron), maintained at 50°C (UCT, Inc., Bristol, PA, USA). All injected samples were filtered using a 0.22-micron syringe filter prior to injection. All sample injection volumes were 1 μL, and the autosampler was purged with 2-propanol between injections (Fisher Scientific, Waltham, MA, USA).
Validation of Stability-Indicating HPLC Method
The methods used in this experiment were developed through accessing other relevant stability-indicating studies available in the literature.10–12 The final HPLC-UV method was validated for system suitability, linearity, accuracy, precision, and specificity. To evaluate system suitability, theoretical plates, tailing factor, and peak resolution were calculated using the ampicillin peak area and LCMS solutions software (v 3.0; Kyoto, Japan). These parameters were evaluated for 6 different concentrations (12, 14.4, 16.8, 19.6, 21.5, and 24 mg/mL) using 5 independent sets of samples (ampicillin diluted in NS). In addition, these samples were used for the determination of intraday precision (as indicated by percent relative standard deviation) and intraday accuracy, as indicated by percent error. On each day of the stability investigation, linearity in the calibration range was assessed using the coefficient of variation, R 2 , with a target of R2 > 0.99. The concentration range of 12 to 24 mg/mL was chosen because our initial sample was expected to be no higher than 24 mg/mL, and subsequent samples were expected to be lower than initial concentration, but the extend of degradation was unknown. Furthermore, this range was also chosen to capture concentrations of ampicillin resulting from forced degradation experiments. The 4 days of calibration data were also used to assess interday precision and accuracy, as data from each concentration in the calibration curve were pooled at the end of the study. Finally, forced degradation experiments were conducted to evaluate specificity, utilizing strong base, strong acid, oxidation, and heat for 48 hours. Ampicillin stock solutions of 24 mg/mL were prepared in clear 20-mL scintillation vials in 0.1 M NaOH, 0.1 M HCl, and 3% H2O2 and left at room temperature with ambient lighting (Fisher Scientific; Acros Organics, Morris, NJ, USA), and one prepared in NS and placed in a 60°C water bath. Aliquots were removed at 2, 24, and 48 hours; diluted 1:1 using mobile phase A; and analyzed using the aforementioned HPLC-UV method, comparing with a fresh ampicillin reference solution with a target concentration of 12 mg/mL.
Stability Investigation
For each experimental condition (immediate use, 24-hour storage, 72-hour storage, and 7-day storage), 4 batches of ampicillin were prepared. These batches represented 4 different lots from 2 manufacturers, and care was taken to ensure that each lot was represented on each experimental day (Lots 1D9002, 1D9001U, Mylan Pharmaceuticals, Canonsburg, PA, USA; Lots JN7738, JN7735, Sandoz, Holzkirchen, Germany). We chose to include 4 different lots from 2 different manufactures in this study because it increases its external validity, making the results more applicable to wider range of product. Each batch was prepared by adding 10-mL sterile water for injection (SWFI) into 2-g vials of ampicillin, and then, 3 vials of the reconstituted product representing a total of 6 g of ampicillin were injected into 250-mL polyvinyl chloride bag of NS (Baxter Healthcare Corporation, Deerfield, IL, USA). After transfer to the laboratory, 1-mL aliquots were removed from each ampicillin batch to establish baseline concentrations of the drug, and those batches slated for storage were placed in a laboratory refrigerator (3.63 ± 0.50°C). The experimental setup involved connecting each ampicillin batch to a multichannel pump (MCP 3000 Digital Multichannel Pump, Model #13-310-662; Fisher Scientific) using medical-grade tubing (Tygon ND100-65 Med/Surgical Tubing, 1/16″ ID, 1/8″ OD, 1/32″ wall; Component Supply Company, Sparta, TN, USA). The pump was set to continuously recirculate the ampicillin preparations through 4 independent channels, at the lowest pump flow rate setting. Furthermore, a fifth channel was used to recirculate a 250-mL bag of NS to serve as a “blank” for that experiment. Triplicate aliquots of 1 mL were removed from each batch and the blank periodically during the recirculation, specifically at 4, 8, 12, and 24 hours and analyzed for ampicillin concentrations using HPLC-UV. These aliquots were filtered using 0.22-micron syringe filters and directly analyzed without further dilution. The laboratory room (20.49 ± 0.59°C) and refrigerator (3.62 ± 0.50°C) temperatures were monitored throughout the study by recording the refrigerator temperature daily and the room temperature at the initiation of and during sampling for each recirculation experiment. Ampicillin concentration in each study sample was calculated using a freshly prepared calibration curve (12- to 24-mg/mL ampicillin in NS) for each study day using Microsoft Excel.
Results and Discussion
High-Pressure Liquid Chromatography-Ultraviolet Validation as Stability Indicating
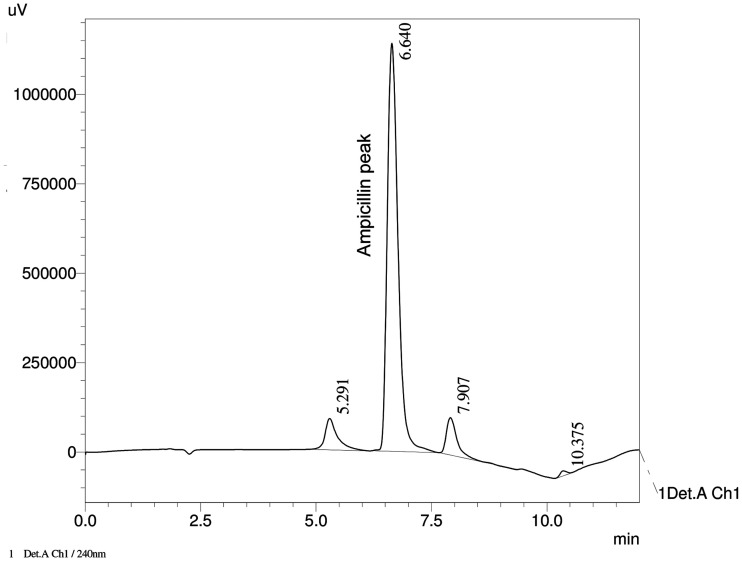
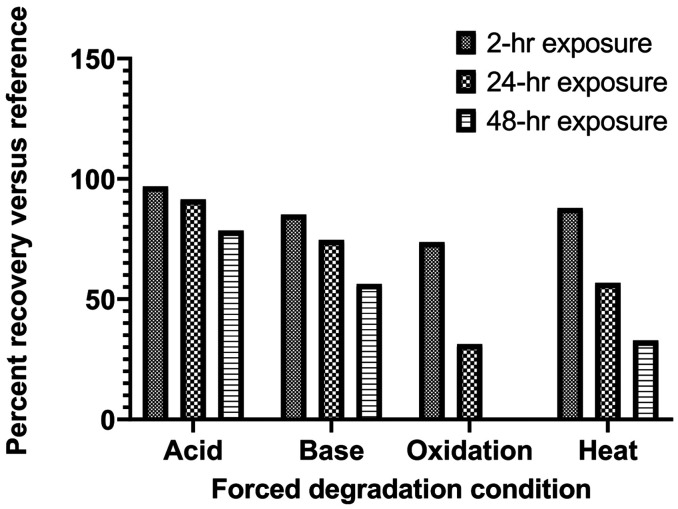
An example chromatogram (Figure 1) shows the ampicillin peak to be well resolved from other matrix peaks. The method's system suitability evaluation yielded an average of 3672 theoretical plates, with average resolution of 4.706 and average tailing factor of 1.299. The R2 in the calibration range of 12 to 24 mg/mL was consistently above 0.99 for the duration of the stability investigation, suggesting an excellent linear relationship between peak area and ampicillin absorbance at 240 nm. Furthermore, average interday and intraday precision and percent error were <2% for all concentrations in the calibration range, providing confidence in the accuracy and reproducibility of the analytical measurements in the study. These data are presented in Table 1. Under forced degradation, ampicillin showed a>10% loss of chemical potency after 2-hour exposure to 0.1 M NaOH, 3% H2O2, and heat. It was most stable under acidic conditions, where the concentration of the treated sample versus the reference fell below 90% recovery after 48 hours. The results of the forced degradation experiments are summarized in Figure 2. Of note, no ampicillin was detected in the 48-hour sample treated with H2O2.
Figure 1.
Example chromatogram of ampicillin stability study sample at 240 nm (actual sample pictured is from 24-hour infusion following 72-hour refrigerated storage).
Table 1.
Interday and Intraday Precision and Accuracy Data for Validated HPLC-UV Assay for Quantification of Ampicillin in Sterile Water for Injection.
| Theoretical concentration (mg/mL) | Average calculated concentration (mg/mL)± standard deviation | % relative standard deviation | % error |
|---|---|---|---|
| Interday precision and accuracy (n = 4 data points per concentration) | |||
| 24 | 23.94 ± 0.065 | 0.270 | 0.249 |
| 21.6 | 21.54 ± 0.258 | 1.199 | 0.258 |
| 19.2 | 19.35 ± 0.315 | 1.627 | 0.795 |
| 16.8 | 16.86 ± 0.127 | 0.755 | 0.328 |
| 14.4 | 14.35 ± 0.246 | 1.711 | 0.320 |
| 12 | 11.95 ± 0.188 | 1.571 | 0.384 |
| Intraday precision and accuracy (n = 5 data points per concentration) | |||
| 24 | 23.76 ± 0.145 | 0.561 | 0.998 |
| 21.6 | 21.57 ± 0.130 | 0.551 | 0.488 |
| 19.2 | 19.50 ± 0.221 | 1.022 | 1.552 |
| 16.8 | 17.034 ± 0.095 | 0.497 | 1.395 |
| 14.4 | 14.34 ± 0.108 | 0.656 | 0.680 |
| 12 | 11.79 ± 0.063 | 0.455 | 1.715 |
Note. HPLC = high-pressure liquid chromatography; UV = ultraviolet.
Figure 2.
Forced degradation of ampicillin following exposure to 0.1 M HCI (acid), 0.1 M NaOH (base), 3% H2O2 (oxidation), and a 60°C water bath (heat).
Stability Investigation
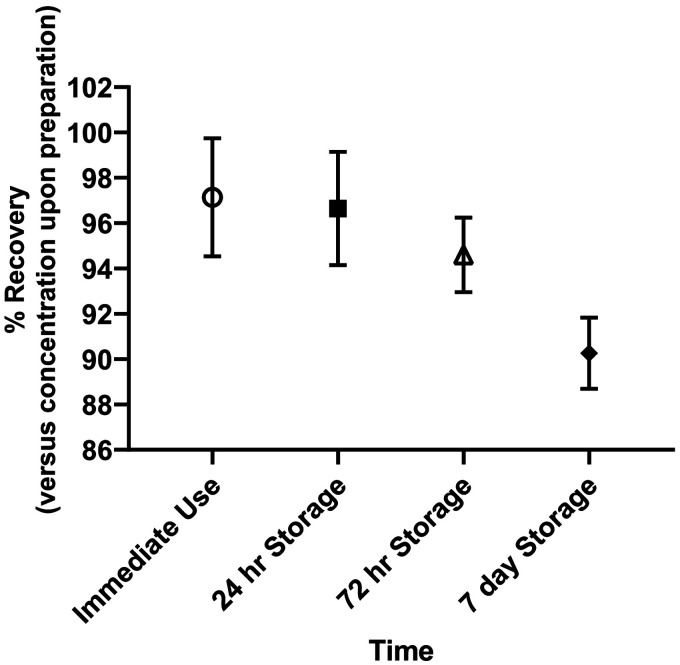
The sample concentrations with average recovery versus initial concentration are included in Table 2. Average initial concentrations ranged from 20.34- to 21.17-mg/mL ampicillin. The average initial concentrations of ampicillin were lower than 24 mg/mL because the 250-mL NS bags used for dilution contain “overfill,” making their volume slightly higher than the 250 mL expected. In addition, the use of 10-mL SWFI to reconstitute the drug introduces additional dilution above the benchmark 250 mL. For the immediate use experiment, the average recovery exceeded 94% after 24 hours with the lowest individual data point at 91.28% recovery. Storing the preparation for 24 hours prior to infusion did not have a negative effect on ampicillin recovery, as this also exceeded 94% at the end of the 24-hour infusion. The preparations also tolerated 72-hour storage prior to pump recirculation, with the average recovery>92% after 24 hours. Those preparations stored for 7 days did not fare as well as the short-term storage samples, with some data points falling <90% recovery by the 8-hour sampling mark. After 24 hours, those preparations stored for 7 days had decreased in potency by 10.7% to 12.6%. Figure 3 shows the average recovery for the 24-hour infusion with 95% confidence intervals. This depiction supports the tabulated data in showing that the 72-hour storage is the longest, among the test conditions, where the ampicillin preparation can be refrigerated and still survive the 24-hour infusion process. Throughout the course of each recirculation experiment, the infusion solutions were monitored for changes in physical stability. The samples remained clear, colorless, and free of visible particulate formation. Finally, these data indicate no evidence of drug adsorption to the medical-grade tubing used in these experiments. This study is limited to being in vitro in design; in vivo efficacy was not studied. In addition, the continuous infusion phase of the study was performed at a controlled room temperature. It is possible that ambient temperatures during infusion could exceed room temperature. Results of this study cannot be extrapolated beyond the parameters investigated. Efforts should be made to ensure the infusion bag does not exceed room temperature, such as insulating the storage container, avoidance of direct sunlight, and avoidance of close contact to the skin without an insulator. Finally, our laboratory is not set up to test for microbial stability and is the responsibility of the compounding center to be compliant with USP 797 guidelines. Our study is within the boundaries of medium risk (not more than 30 hours at room temperature and not more than 9 days under refrigeration). 13
Table 2.
Ampicillin Concentrations (mg/mL) ± Standard Deviation (n = 12 for Each Time Point; Average Percent Recovery Shown in Parenthesis) in Drug Preparations Stored Under Refrigerated Conditions Followed by 24-Hour Continuous Infusion at Room Temperature.
| Immediate use | 24-h storage | 72-h storage | 7-d storage | |
|---|---|---|---|---|
| Initial concentration | 21.50 ± 0.512 | 20.34 ± 0.672 | 21.17 ± 0.718 | 20.98 ± 0.644 |
| After 4-h infusion | 20.91 ± 0.840 (97.22) | 19.69 ± 0.718 (96.81) | 20.23 ± 0.690 (95.55) | 19.11 ± 0.469 (91.11) |
| After 8-h infusion | 20.94 ± 1.03 (97.33) | 19.53 ± 0.639 (96.03) | 20.04 ± 0.704 (94.78) | 18.98 ± 0.489 (90.47)a |
| After 12-h infusion | 20.88 ± 1.07 (97.06) | 19.45 ± 0.597 (95.62) | 19.91 ± 0.697 (94.06) | 18.88 ± 0.490 (90.00)a |
| After 24-h infusion | 20.23 ± 0.768 (94.09) | 19.27 ± 0.705 (94.77) | 19.61 ± 0.614 (92.63) | 18.51 ± 0.445 (88.24)a |
aAt least 1 data point fell below 90% recovery.
Figure 3.
Ampicillin recovery 95% confidence intervals for duration of 24-hour pump recirculation following preparation (immediate use) and refrigerated storage (24 hours, 72 hours, and 7 days).
Conclusions
The developed and validated HPLC-UV method for ampicillin quantification in NS is linear (12-24 mg/mL), precise, accurate, and stability indicating. The method was successfully applied to the investigation of stored ampicillin solutions in NS, prior to pump recirculation. Ampicillin can be prepared and stored in a refrigerator for up to 72 hours prior to continuously infusing at room temperature over 24 hours with less than a 10% loss of potency over the dosing period. Drug loss during 7-day refrigerated storage followed by continuous infusion exceeded a 10% loss and is not recommended. This model supports twice weekly OPAT delivery of ampicillin.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stacy D Brown https://orcid.org/0000-0001-5566-1728
References
- 1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi:10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2. Cervera C, del Rio A, Garcia L, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infec Microbiol Clin. 2011;29:587–592. doi:10.1016/j.eimc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 3. Tice AD, Rehm SJ, Dalvisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38:1651–1672. doi:10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 4. Lewis PO, Jones A, Amodei RJ, Youssef D. Continuous infusion ampicillin for the outpatient management of enterococcal endocarditis: a case report and literature review. J Pharm Prac. 2020;33:392–394. doi:10.1177/0897190018804964. [DOI] [PubMed]
- 5. Maher M, Jensen KJ, Lee D, Nix DE. Stability of ampicillin in normal saline and buffered normal saline. Int J Pharm Compd. 2016;20:338–342. [PubMed] [Google Scholar]
- 6. Juste JL, Roca M, Soy D, et al. Ampicillin solution stability for outpatient antibiotic therapy (OPAT) in patients with enterococcal endocarditis. Eur Hosp Pharm. 2001;7:145–148. [Google Scholar]
- 7. Zhang Y, Trissel LA. Stability of ampicillin sodium, nafcillin sodium, and oxacillin sodium in autodose infusion system bags. Int J Pharm Compd. 2002;6:226–229. [PubMed] [Google Scholar]
- 8. Allen LV., Jr Stiles ML, Prince SJ, Smeeding J. Stability of 14 drugs in the latex reservoir of an elastomeric infusion device. Am J Health-syst Pharm. 1996;53:2740–2743. doi:10.1093/ajhp/53.22.2740. [DOI] [PubMed] [Google Scholar]
- 9. Norris AH, Shrestha NK, Allison GM, et al. 2018 infectious diseases society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68:e1–e35. doi:10.1093/cid/ciy745 [DOI] [PubMed] [Google Scholar]
- 10. Abdelrahman MM, Naguib IA, Elsayed MA, Zaazaa HA. Chromatographic methods for quantitative determination of ampicillin, dicloxacillin and their impurity 6-aminopenicillianic acid. J Chromatogr Sci. 2018;56:209–215. doi:10.1093/chromsci/bmx101. [DOI] [PubMed] [Google Scholar]
- 11. Kumar V, Bhutani H, Singh S. ICH guidance in practice: validated stability-indicating HPLC method for simultaneous determination of ampicillin and cloxacillin in combination drug products. J Pharm Biomed Anal. 2007;43:769–773. doi:10.1016/j.jpba.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 12. Ishida M, Kobayashi K, Awata N, Sakamoto F. Simple high-performance liquid chromatography determination of ampicillin in human serum using solid-phase extraction disk cartridges. J Chromatogr B Biomed Sci Appl. 1999;727:245–248. doi:10.1016/s0378-4347(98)00588-x. [DOI] [PubMed] [Google Scholar]
- 13.Pharmaceutical Compounding—Sterile Preparations. (General chapter 797). United States Pharmacopeia, 42th rev., and The National Formulary, 37nd ed. Rockville, MD: United States Pharmacopeia; 2008. [Google Scholar]