Summary
We investigated the decline of activities of daily living with symptomatic progression in patients with mucopolysaccharidosis type II (MPS II) and investigated the associated factors. Clinical data were retrospectively collected from the medical records of 28 patients with MPS II who visited our hospital between October 2007 and August 2019. Activities of daily living were assessed over time using a 5-point scale (from stage 1, indicating independent, to stage 5, indicating total assistance + medical care); the relationships of the interval years from stage 2 (mild symptoms) to stage 4 (total assistance) with therapeutic intervention, anti-drug antibodies (ADA), urinary glycosaminoglycans (uGAG), and genotypes were analyzed. Eight are attenuated types, and 20 are severe types. Further, 20 underwent enzyme replacement therapy (ERT) alone, 5 underwent hematopoietic stem cell transplantation (HSCT) alone, and 3 underwent both therapy. The mean interval years (standard deviation) from stage 2 to 4 was 3.5 (0.7) and 7.3 (3.3) in patients who started undergoing ERT (n = 6) and HSCT (n = 3) at stage 2, respectively, whereas it was 3.1 (1.5) in patients who received no treatment until they reached stage 4 (n = 8). The study findings revealed the process of changes in the activities of daily living over a long duration in patients with MPS II undergoing different treatments. In severe type, the activity deteriorated regardless of the stage at which ERT was initiated. The activity declined slower in patients who received HSCT at an early stage.
Keywords: Activities of daily living, Enzyme replacement therapy, Hematopoietic stem cell transplantation, Hunter syndrome, Long-term follow-up, Mucopolysaccharidosis
1. Introduction
Mucopolysaccharidosis type II (MPS II) is an extremely rare X-linked genetic condition that occurs in 0.88 per 100,000 births and is the most common type of MPS in Japan [1]. Organs in the entire body are damaged by the accumulation of mucopolysaccharides in lysosomes and various symptoms emerge, including central nervous disorders, characteristic facial appearance, joint contracture, hepatomegaly, short stature, and cardiac valvulopathy. In severe type, symptoms progress at an early stage, with walking difficulty observed from the age of 10 years onward, leading to significant activity decline requiring total assistance, bed ridden, and respiratory assistance. In patients who remain untreated, cardiovascular and respiratory symptoms result in death by the age of 20 years [2]. Patients with attenuated type show only a small number of symptoms, such as joint contracture, and follow varied disease courses, resulting in some cases not being diagnosed until adulthood.
Since 2006, enzyme replacement therapy with idursulfase (ERT) has been used to treat this disorder in Japan in addition to hematopoietic stem cell transplantation (HSCT). ERT has facilitated the treatment of patients for whom HSCT is not an option owing to the absence of a donor and safety concerns. In approximately 14 years since the initiation of ERT for treating this disorder, treatment outcomes have been described in several reports. A study analyzing 146 patients with MPS who underwent HSCT reported that HSCT improved symptoms related to activities of daily living (ADL), such as range of joint motion [3]. The Hunter Outcome Survey (HOS) that investigated the long-term effects of ERT treatment in 29 countries worldwide reported improvements in the urinary glycosaminoglycan (uGAG) level, 6-min walking test (6MWT) results, left ventricular mass index, forced expiratory volume in 1 s, and hepatosplenomegaly [2]. A study using the same registry compared the median survival duration and showed a difference of approximately 10 years between the ERT-treated (33.0 years) and untreated (21.2 years) groups; furthermore, a Cox model adjusted for the condition at the time of treatment revealed a 54% lower mortality risk in the ERT-treated group compared with the untreated group [4]. According to a report on a post-marketing surveillance of the safety and efficacy of idursulfase in Japan, the 7-year survival rate was 82.7% (91.2% in attenuated type and 76.7% in severe type), and clinical manifestations of hepatosplenomegaly, skin disease, joint disorder, and respiratory disease were improved, with decreased uGAG levels and improved 6MWT results [5].
Although these studies have demonstrated that treatments resulted in improvements in various test results and survival benefits, changes in ADL over time have not yet been described. According to a report based on a questionnaire survey regarding ADL, patients with severe type reported ADL deterioration with age and required total assistance around the age of 20 years, whereas patients with attenuated type showed no major impact on their ADL in their 20s [6]. However, it should be noted that this was a cross-sectional study and gradual changes of each individual were not investigated. Considering that the symptom progression in MPS patients varies from individual to individual, long-term follow-up of individual patients is crucial to understand changes in quality of life, including social participation, caregiver assistance, and requirement of medical devices.
In the present study, we attempted to demonstrate the manner in which activity restriction progresses with age in individual patients with MPS II—an aspect that has been unelucidated previously. The severity staging data based on a comprehensive evaluation of neurodevelopment and assistance dependency for each fiscal year were collected from medical records of patients with MPS II at a single center specializing in inherited metabolic disorders. We retrospectively determined the rate of disease progression in patients with MPS II undergoing various therapeutic interventions and analyzed its relationship with factors, i.e., anti-drug antibodies (ADA), uGAG, and genotypes.
2. Materials and methods
2.1. Patient population
In total, 28 patients with MPS II who visited the Osaka City University Hospital between January 2007 and August 2019 were included in the present study. Medical records were collected from all patients from the initial visit to the last follow-up visit. For those who participated in clinical trials, information after participation in the trials was not collected.
2.2. Stage classification
In terms of information regarding restrictions on daily life owing to symptoms, we captured the staging results, which were annually evaluated to receive financial aid from national health insurance for the treatment of lysosomal storage diseases. This stage classification was originally defined by research group on lysosomal storage diseases under the research program on rare and intractable diseases sponsored by the Ministry of Health, Labor, and Welfare in Japan. Table 1 shows the criteria of the stages. Unlike the measures—such as 6MWT, respiratory/cardiopulmonary function, and range of joint motion—that are frequently used in terms of therapeutic effects, this is a comprehensive staging system based on the extent to which the daily life of a patient is affected by symptom progression. Although the stage is typically recorded annually, in cases where it was not explicitly stated in the patient records, we deduced the stage based on the descriptions in the medical records. In the present study, DQ was measured using The Kyoto Scale of Psychological Development (KSPD), which was validated to correlate with Bayley III [7].
Table 1.
Staging criteria.
| Infancy/early childhood | Young age/adulthood | |
|---|---|---|
| Stage 1 | Patients have physical abnormalities⁎ [1] but can engage in activities nearly equivalent to those by children of the same age in months (or years). | Patients have symptoms⁎ [2] but can work (or attend a school). |
| Stage 2 | Patients have mild developmental delay compared with children of the same age in months (or years) due to physical abnormalities⁎ [1] or motor (intellectual) disabilities. | Patients can independently perform tasks in daily life but are unable to work (or attend school). |
| Stage 3 | Patients have moderate developmental delay due to physical abnormalities⁎ [1] or motor (intellectual) disabilities (DQ = 35–50). | Patients require partial assistance in daily life (moderate disabilities). |
| Stage 4 | Patients have severe developmental delay due to physical abnormalities or motor (intellectual) disabilities (DQ < 35). | Patients require total assistance in daily life (severe disabilities). |
| Stage 5 | Patients are bedridden and require advanced medical care for respiratory/cardiovascular/hepatic/renal insufficiency. | Patients are bedridden and require advanced medical care, such as suctioning. |
1. Physical abnormalities: e.g., bottle feeding disorder, hypersensitivity, convulsion, visual impairment, characteristic facial appearance, joint contracture, skeletal maldevelopment, hepatosplenomegaly, heart failure symptoms, and kidney failure symptoms.
2. Symptoms: e.g., dementia/psychiatric symptom, spastic paralysis, joint contracture, cerebellar ataxia, involuntary movements, visual impairment, muscle weakness, deafness, convulsion, pain episode, heart failure symptoms, and kidney failure symptoms.
2.3. Data analysis
The age-related data (age during the observation period, age at diagnosis, age at treatment initiation, age at respective stages, and age at death) and ADA titers are presented as median (interquartile range, IQR). The treatment duration, interval years of stage transition, and uGAG level, are presented as mean (SD) values. We performed t-tests to examine differences in the interval years of stage transition, with/without therapeutic intervention, and uGAG level according to the phenotype. A p-value of <0.05 was considered to indicate statistical significance. Kaplan–Meier survival analyses were used to compare survival differences among 3 different treatment type groups.
3. Results
3.1. Patient profile
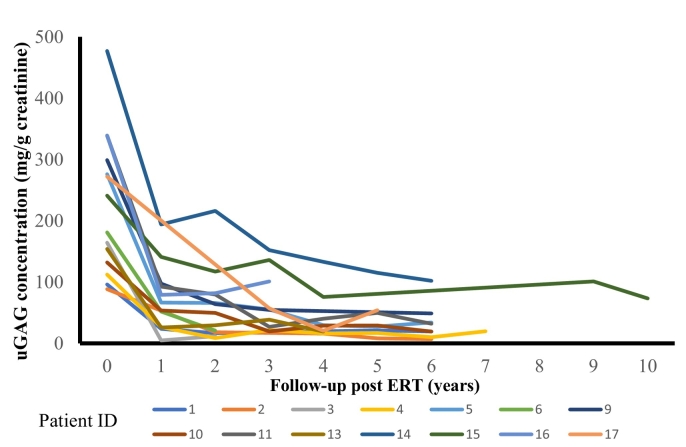
The age at observation, phenotype, genotype, age at diagnosis, age at treatment initiation, interval years from stage 2 to 4, maximum ADA level and uGAG level for all 28 patients are listed in the Supplementary table 1 and summarized in Table 2. Fig. 1 shows changes in uGAG level after enzyme replacement therapy initiation.
Table 2.
Phenotype, age, age at diagnosis, age at death, genotype, treatment, ADA, and uGAG data of all patients.
| n | Total | n | Attenuated type | n | Severe type | |
|---|---|---|---|---|---|---|
| Age [median (IQR)] | 28 | 21.0 (13.5–25.0) | 8 | 34.0 (23.0–41.0) | 20 | 16.5 (11.0–21.0) |
| Age at diagnosis [median (IQR)] | 28 | 4.0 (2.0–6.0) | 8 | 7.0 (3.5–11.0) | 20 | 3.0 (2.0–5.0) |
| Age at death [median (IQR)] | 5 | 21.0 (15.5–29.0) | 2 | 29.0 (25.0–33.0) | 3 | 17.0 (14.0–21.0) |
| n | n | n | ||||
| Genotype | ||||||
| Missense mutation | 13 | 3 | 10 | |||
| Recombination | 5 | 0 | 5 | |||
| Frameshift | 5 | 3 | 2 | |||
| Nonsense mutation | 3 | 1 | 2 | |||
| Large deletion | 1 | 0 | 1 | |||
| Unknown | 1 | 1 | 0 | |||
| Treatment | ||||||
| ERT alone | 20 | 6 | 14 | |||
| Age at initiation [median (IQR)] | 7.0 (3.0–12.0) | 25.5 (12.0–30.0) | 4.0 (2.0–7.0) | |||
| Treatment duration [mean (SD)] | 9.5 (4.5) | 10.5 (4.1) | 9.1 (4.5) | |||
| HSCT alone | 5 | 1 | 4 | |||
| Age when treatment was performed [median (IQR)] | 5.0 (4.5–6.5) | 5.0 | 4.5 (3.0–6.0) | |||
| Follow-up time after HSCT [mean (SD)] | 18.6 (2.7) | 20.0 | 18.3 (2.9) | |||
| ERT + HSCT | 3 | 1 | 2 | |||
| Antibody | ||||||
| Negative | 7 | 5 | 2 | |||
| Positive | 14 | 1 | 13 | |||
| Antibody titer [median (IQR)] | 3300 (20–163,840) | 10 | 6400 (35–245,290) | |||
| uGAG level |
14 |
Mean (SD) (mg/g creatinine) |
4 |
Mean (SD) (mg/g creatinine) |
10 |
Mean (SD) (mg/g creatinine) |
| Baseline | 226.5 (109.3) | 115.1 (29.5) | 271 (97.2) | |||
| Lowest (post ERT) | 36.6 (29.1) | 9.6 (4.3) | 38.1 (47.5) | |||
ADA = anti-drug antibody; ERT = enzyme replacement therapy; HSCT = hematopoietic stem cell transplantation, uGAG = urinary glycosaminoglycan; IQR = interquartile range.
Fig. 1.
Changes in uGAG level after enzyme replacement therapy initiation.
Urinary glycosaminoglycans: creatinine (uGAG:Cr) ratios in 14 patients are shown. Values were annually recorded after the initiation of idursulfase administration.
3.2. Stage transition
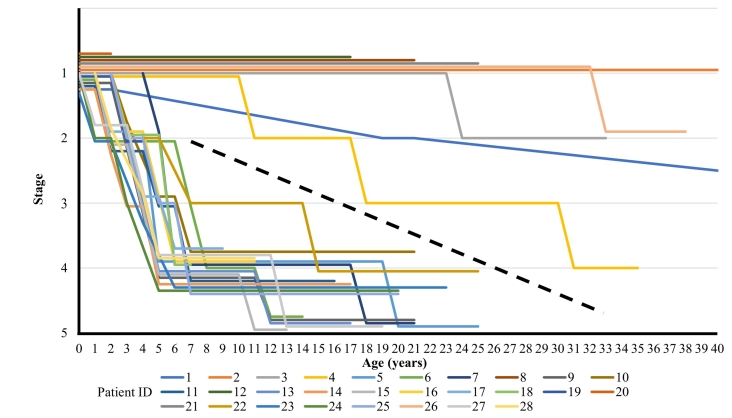
Fig. 2 shows a graph of stage transition at different ages (up to 40 years) in 28 patients. The dotted line is to help distinguish patients with attenuated and severe types. The lines denoting stage transition were flat or had a negative slope, indicating that there were no patients whose condition improved over time, regardless of whether each type of treatment was implemented. Attenuated type patient showed 10 years interval to reach next stage, meanwhile, severe type patients demonstrated rapid progression from stage 2 to 4 within few years. To elucidate the factors associated with the rapid progression, attenuated type patients were excluded from subsequent analysis.
Fig. 2.
Stage transitions in all patients (up to 40 years of age).
The age and stage transitions up to 40 years of age in all patients are shown. The stage data (stages 1–5) for individual patients are shown in a staggered layout to avoid overlapping.
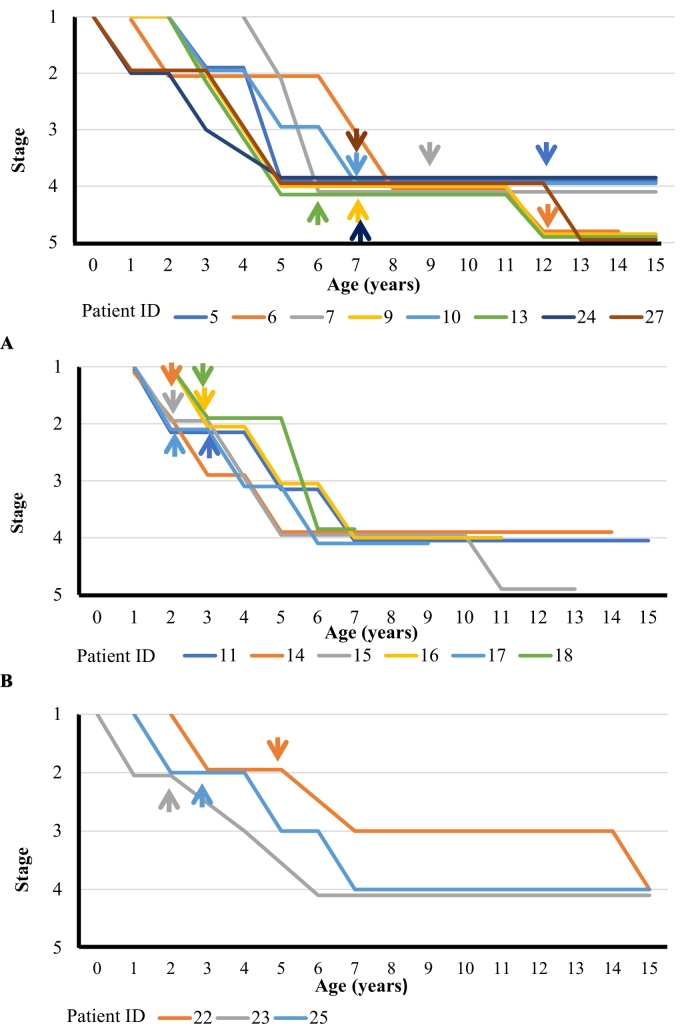
Stage transitions in patients with severe type who received different treatments are shown in Fig. 3. Panels A, B, and C show the data from patients for whom the treatment was initiated at or after stage 4 (the late treatment group), those for whom ERT alone was initiated at stage 2 (the early ERT group), and those who underwent HSCT alone at stage 2 (the early HSCT group), respectively. The age at reaching each stage and the interval years of stage transition in patients receiving different treatments are listed in Table 3.
Fig. 3.
Age and stage transitions grouped by treatment.
The age and stage transition up to 15 years of age in patients undergoing different treatments are shown. The stage data (stages 1–5) for individual patients are shown in a staggered layout to avoid overlapping. Panel A: the late treatment group consisting of patients who were left untreated until stage 4 (n = 8); Panel B: the early ERT group consisting of patients who started receiving ERT at stage 2 (n = 6); and Panel C: the early HSCT group consisting of patients who underwent HSCT at stage 2 (n = 3). ERT, enzyme replacement therapy; HSCT, hematopoietic stem cell transplantation.
Table 3.
List of ages reaching different stages, period before transition between different stages, ADA titers, genotypes, and uGAG levels by treatment.
| Untreated until stage 4 (Late-treated) |
ERT initiated at stage 2 (Early ERT) |
HSCT at stage 2 (Early HSCT) |
||||
|---|---|---|---|---|---|---|
| n | Median age (IQR) | n | Median age (IQR) | n | Median age (IQR) | |
| Treatment age | 8 | 7.0 (7.0–10.5) | 6 | 2.5 (2.0–3.0) | 3 | 4.0 (2.0–5.0) |
| Age at stage 2 | 8 | 3.0 (1.5–3.0) | 6 | 2.0 (2.0–3.0) | 3 | 2.0 (1.0–3.0) |
| Age at stage 4 | 8 | 5.0 (5.0–6.5) | 6 | 6.0 (5.0–6.0) | 3 | 7.0 (6.0–15.0) |
| Age at stage 5 | 6 | 12.5 (12.0–18.0) | 1 | 11.0 | 0 | – |
| Age at death | 3 | 17.0 (14.0–21.0) | 0 | – | 0 | – |
| n | Mean year (SD) | n | Mean year (SD) | n | Mean year (SD) | |
| Stage 2–4 interval | 8 | 3.1 (1.5) | 6 | 3.5 (0.7) | 3 | 7.3 (3.3) |
| Missense mutation | 2 | 3.0 (1.0) | 3 | 4.0 (0.8) | 2 | 8.5 (3.5) |
| Other type mutation | 6 | 3.2 (1.7) | 3 | 3.0 (0.0) | 1 | 5.0 |
| Stage 4–5 interval | 6 | 9.0 (3.8) | 1 | 6.0 | 0 | – |
| Stage 5 to death | 3 | 4.0 (0.8) | 0 | – | 0 | – |
| Pt | Pt | |||||
| ID | ADA titer | ID | ADA titer | |||
| Missense mutation | 13 | 2 | 17 | 0 | ||
| 10 | 50 | 11 | 2 | |||
| 18 | 328,000 | |||||
| Other type mutations | 27 | 20 | 16 | 163,840 | ||
| 7 | 160 | 15 | 655,360 | |||
| 6 | 200 | 14 | 20,971,520 | |||
| 5 | 40,960 | |||||
| 9 | 163,840 | |||||
| Mean (SD) | Mean (SD) | |||||
| n | (mg/g creatinine) | n | (mg/g creatinine) | n | ||
| uGAG baseline level | 5 | 208 (66.8) | 5 | 334 (81.2) | 0 | – |
| uGAG lowest level | 5 | 27.1 (11.3) | 5 | 67.8 (24.0) | 0 | – |
ADA = anti-drug antibody; ERT = enzyme replacement therapy; HSCT = hematopoietic stem cell transplantation, uGAG = urinary glycosaminoglycan.
Fig. 3A shows stage transitions in the late treatment group (n = 8). Patients in this group reached stage 2 at the age of 3.0 (1.5–3.0) years and stage 4 at the age of 5.0 (5.0–6.5) years. The interval years from stage 2 to 4 without treatment was 3.1 (1.5). With treatment initiation at an age of 7.0 (7.0–10.5) years, 6 patients reached stage 5 at the age of 12.5 (12.0–18.0) years, which was 9.0 (3.8) years after reaching stage 4. Further, 3 patients died at the age of 17.0 (14.0–21.0) years, which was 4.0 (0.8) years after reaching stage 5. In the late treatment group, 2 patients showed the missense mutation genotype, and 5 patients showed other mutation types (recombination, frameshift, nonsense mutation, large deletion). The interval years from stage 2 to 4 was 3.0 (1.0) for patients with the missense mutation genotype and 3.2 (1.7) for those with other mutation types. (Table 3).
Fig. 3B shows stage progression in the early ERT group (n = 6). Patients in this group reached stage 2 at the age of 2.0 (2.0–3.0) years, started undergoing ERT at the age of 2.5 (2.0–3.0) years, and reached stage 4 at the age of 6.0 (5.0–6.0) years. The interval years from stage 2 to 4 was 3.5 (0.7). The p-value from the t-test for comparison of the interval years from stage 2 to 4 between the early ERT and late treatment groups was 0.5. In this cohort, only one person reached stage 5 at 11 years of age—6.0 years after reaching stage 4 (Pt ID 15). Among the ERT-treated patients, 3 had the missense mutation genotype and 3 had other mutation types. The interval years from stage 2 to 4 was 4.0 (0.8) for patients with the missense mutation genotype and 3.0 (0.0) for patients with other mutation types.
In the late treatment group, ADA titers were 2 and 50 for patients with missense mutations (n = 2) and 20, 160, 200, 40,960, and 163,840 for patients with other mutation types (n = 5). In the early ERT group, ADA titers were 0, 2, and 328,000 for patients with missense mutations (n = 3) and 163,840, 655,360, and 20,971,520 for patients with other mutation types (n = 3). ADA titers in the missense mutation group tended to be lower than those in the other mutation group.
In the late treatment group (n = 5), the uGAG baseline level, and lowest level, were 208 (66.8) mg/g creatinine, and 27.1 (11.3) mg/g creatinine, respectively. In the early ERT group (n = 5), the corresponding values were 334 (81.2) mg/g creatinine, 67.8 (24.0) mg/g creatinine, and 0.21 (0.07), respectively. No comparable data were available for the early HSCT group. (Table 3).
Fig. 3C shows stage transitions in the early HSCT group (n = 3). Patients in this group reached stage 2 at an age of 2.0 (1.0–3.0), underwent HSCT at an age of 4.0 (2.0–5.0), and reached stage 4 at an age of 7.0 (6.0–15.0). The interval years from stage 2 to 4 was 7.3 (3.3). The p-value from the t-test for comparison of the interval years from stage 2 to 4 transition between the late treatment and early HSCT groups was 0.02. No patients reached stage 5. Two patients exhibited the missense mutation genotype and 1 had other mutation type (frameshift). The interval years from stage 2 to 4 was 8.5 (3.5) for patients with the missense mutation and 5.0 in the patient with other mutation type. (Table 3).
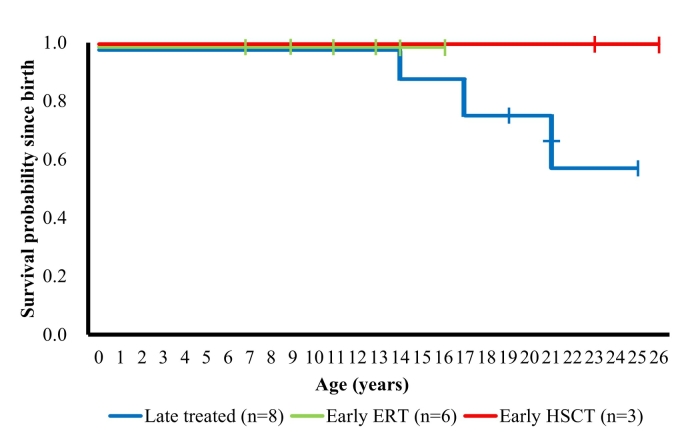
Survival curves for patients with severe type in different treatment groups are shown in Fig. 4. Survival rates in the early ERT and early HSCT groups were maintained at 1.0, while deaths occurred at the ages of 14, 17, and 21 years in the late treatment group.
Fig. 4.
Survival curves by treatment.
Kaplan–Meier survival curves for the late treatment group (n = 8), early ERT group (n = 6), and early HSCT group (n = 3) of patients are shown. Survival was defined as the period from birth to death; data collection was censored at the final recording point for patients who were alive at the time of this analysis. ERT, enzyme replacement therapy; HSCT, hematopoietic stem cell transplantation.
3.3. Treatment-related adverse events
A patient with severe type (Pt ID 28) started receiving ERT immediately after the diagnosis as stage 3 at 4 years of age and underwent cord blood transplantation at 5 years of age from unrelated donor. Due to engraftment failure, he underwent unrelated bone marrow transplantation at 6 years of age. He further required donor leukocyte infusion therapy to correct mixed chimerism. Some ERT-treated patients developed mild allergic reactions at the beginning of treatment and received antiallergic agents as premedication. None of these patients discontinued/terminated ERT due to the adverse events. There were no treatment-related deaths.
3.4. Causes of death
Of the 28 patients, 5 died, 2 were attenuated and 3 were severe types. Four patients died related to MPS II symptoms. Pt ID 21, a patient with attenuated type, underwent HSCT at 5 years of age, remained at stage 1 until adulthood, and continued to work; however, the patient died at 25 years of age due to a traffic accident. Pt ID 3, a patient with attenuated type, started receiving ERT at 28 years of age. he died at 33 years of age due to heart failure with poor treatment adherence. An older brother of this patient (Pt ID 2), with the same genotype, started receiving ERT at 30 years of age and remained at stage 1 as of 43 years of age without any signs of heart failure.
Pt ID 6, a patient with severe type, exhibited a rapid stage progression from stage 2 at 6 years of age to stage 4 at 8 years of age. ERT was initiated at 12 years of age during stage 5, and the patient died of asphyxiation at 14 years of age. In the younger brother of this patient (Pt ID 9), with severe type, ERT was initiated during stage 4 at 7 years of age, and 10 years have passed since the patient reached stage 5 at 12 years of age. Pt ID 13, a patient with severe type, started ERT during stage 4 at 6 years of age. The condition worsened to stage 5 when the patient developed hypoxic–ischemic encephalopathy due to asphyxiation at 12 years of age, and the patient died of septicemia at 17 years of age. Pt ID 7, a patient with severe type, reached stage 2 at 5 years of age and started receiving ERT at 9 years of age; however, aspiration pneumonitis worsened due to a swallowing disfunction. Subsequently, the patient reached stage 5 at 18 years of age and died of respiratory failure at 21 years of age.
4. Discussion
The present study analyzed changes in restrictions to daily life over time in patients with MPS II. Patients were followed-up since early childhood, including some who were followed-up over several decades. The comparison between the early ERT and late treatment groups of patients with severe type demonstrated the possible survival benefit of early ERT initiation, although it did not contribute to the maintenance of independent life. Patients who underwent HSCT early remained capable of independent life for an extended duration and did not require advanced medical care. The genotype strongly correlated with ADA and uGAG level and was not an independent predictor of therapeutic prognosis. Our observation of long-term courses of individual patients provided a deeper understanding of the clinical course and outcomes of MPS II.
Early diagnosis and early treatment initiation are critical for MPS, and the importance of newborn screening has been advocated [8]. Moreover, a cross-sectional study investigating ADL in patients with MPS has shown a higher ADL score in those who started receiving ERT or underwent HSCT early than in those who received these treatments late [6]. In our cohort study, we compared stage progression between the early ERT and late treatment groups of patients with severe type, with ages at the treatment initiation being 2.5 (2.0–3.0) and 7.0 (7.0–10.5) years, respectively, and found no difference in interval years from stage 2 to 4 (Table 3). Lampe et al. investigated clinical course of 22 patients with severe type, who were treated with ERT for ≥2 years. They reported that the somatic signs/symptoms were improved but the cognitive function deteriorated in 17 patients (77%) [9]. Given the fact that the idursulfase does not cross the blood–brain barrier (BBB), the progression from stage 2 to 4 was mainly due to the consequence of cognitive disfunction. Therefore brain target therapies are crucial to improve ADL in patient with severe type MPS II.
In the late treatment group, 6 of 8 patients progressed to stage 5, and 3 of them died. Of 9 patients with severe type in whom treatment was initiated at stage 2, only 1 progressed to stage 5 with no mortality. A study on the survival rate in HOS reported that the median survival duration in the ERT-treated group was extended by 10 years compared with that in the untreated group. Kaplan–Meier plots showed that the survival rate in the ERT-treated patients gradually decreased from their 10s to 40s, whereas the survival rate in untreated patients showed a sharp reduction from pre-teen to teenage years [4]. Although the patients were not classified according to the timing of intervention or phenotype in HOS, our survival curves (Fig. 4) showed that deaths in the late-treated patients started occurring from pre-teen to teenage years, similar to that observed in untreated patients in HOS. These findings suggest that the ERT intervention after stage 4 has no effect on extending survival.
A study investigating the prognosis of patients with MPS II reported that respiratory failure was the most common cause of death. Indeed, causes of death of the all 3 patients in the late treatment group were respiratory failure or asphyxia in our cohort. Of the 5 survivors in the late treatment group, 3 reached stage 5 due to requirement of respiratory assistance. In contrast, 8 of 9 patients for whom treatment was initiated at stage 2 were still independent from respiratory assistance. These findings consistent with the possible survival benefit of early ERT initiation. The survival rate decreased only in the late treatment group (Fig. 4). Since the ages of the patients in early ERT group were younger than those in late treatment group patients, further observation will define the benefit of early ERT in the future.
Among patients with severe type, the ADA titer in the missense mutation group tended to be lower than that in the other mutation group. A study analyzing 36 patients with attenuated MPS II who underwent ERT suggested that ADA are more likely to be elicited in the mutations which has major structural changes. And also, the study showed that the ADA had no effects on vital capacity and 6MWT results, so the ADA detection may be a genotype marker [10]. Another previous study discussing relationships of ADA with clinical outcomes and safety showed no association between ADA and outcomes [11]. Consistent with these findings, in our study, the ADA titer in the other mutations group tended to be higher than that in the missense mutation group (Table 3). Although the transition from stage 2 to 4 does not seem to be associated with the ADA titers, the transition mainly relies on cognitive decline, on which the enzyme does not have any effects.
A systematic review of treatment of MPS II concluded that impact of IgG and neutralizing antibodies on clinical outcomes could not be determined due to conflicting evidence [12].
Owing to efficiency in diagnosing MPS, uGAG measurements are used in clinical practice [13]. Our data revealed that the baseline level and lowest level after treatment initiation in patients with attenuated type were significantly lower than those in patients with severe type (Table 2), suggesting the potential usefulness of the uGAG measurement for differentiation of patients with attenuated and severe types (Table 3). A study measuring uGAG levels in 11 patients with MPS II showed a rapidly decreasing trend at week 8 after ERT initiation. Significant changes from baseline continued until week 52, after which no significant differences were observed [14]. In our study, uGAG levels decreased in a similar manner to their study in both patients with attenuated and severe types upon ERT. Furthermore, we did not observe association between interval years of stage transition and uGAG levels (data not shown). Therefore, uGAG level may not be suitable to predict the ADL deterioration.
A study investigating genotypes and changes in cognitive function in 13 patients with MPS II who underwent ERT showed that cognitive deterioration in the missense mutation group was slightly slower than that in the other mutation group [15]. In our cohort, we did not observe obvious difference in interval years from stage 2 to 4 between missense mutation patients and other mutation patients (Table 3). Among early ERT and late treated groups, there is no significant difference interval years from stage 2 to 4 between missense mutation patients and other mutation patients.
In Japan, HSCT has been used widely for treatment of MPS II since before the development of ERT. In a retrospective study comparing ADL, intelligence quotient, and brain images in 26 patients with MPS II undergoing HSCT with those in natural course, early HSCT was effective on brain lesions and cardiovascular symptoms and helped maintain ADL [16]. A recent report showed that HSCT resulted in greater improvements in range of joint motion and ADL compared with ERT [3], [17]. Furthermore, Tanjuakio et al. reported that ADL in patients with MPS II who underwent HSCT before 5 years of age remained higher than that in those who underwent HSCT at or after 5 years of age [6]. Although these studies suggest the potential of HSCT to improve ADL, its effectiveness on neurological symptoms remains unclear. In 3 patients who received early HSCT in our cohort, the stage progressions were slow but they all suffered from intellectual disabilities. Nevertheless, none of them reached stage 5 or died, and early HSCT appeared to have favorable effects on maintaining ADL and extending survival. According to a study investigating patients with lysosomal storage disease who underwent HSCT in Japan, the overall survival and event-free survival rates in 55 patients with MPS II were 79.2% and 60.9%, respectively [ 18]. Patients included in that study underwent HSCT between 1985 and 2010, and the event-free survival rate in all 216 participants improved over time from 65.6% before 1999 to 73.2% after 2000. However, in addition to the difficulty involved in securing HSCT donors, caution should be exercised before performing the procedure owing to concerns regarding adverse events. In Japan, guidelines for MPS type II published in 2019, clear indications of HSCT are not described. Therefore, the decisions will be made by incorporating risk-benefit assessments of treatments as well as patients' preferences.
The limitation of this study is that interrater reliability was not validated in this staging system. The stage classification for adults is contradictory to child's classification and cannot distinguish whether the patient needs assistance due to cognitive decline or physical disability. Therefore, patients with attenuated type were not thoroughly assessed in this scale. In contrast, this classification is relevant for patients with severe type, who start manifesting cognitive decline and eventually require advanced medical care.
5. Conclusion
The study findings demonstrated the process of long-term activity changes in patients with MPS II undergoing various treatments. For patients with severe type, early initiation of ERT was beneficial for survival, but not for ADL maintenance. The activity declined slower in patients who received HSCT at an early stage.
Acknowledgements
The authors thank Dr. Akemi Tanaka (deceased), who had been engaged in MPS research since before the discovery of treatment methods, for the contribution to this study.
This work was supported by Health and Labor Sciences Research Grant (20FC0201). T.H.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2021.100816.
Appendix A. Supplementary data
Supplementary table shows the profile of all patients and changes in DQ by age 20.
References
- 1.Khan S.A., Peracha H., Ballhausen D. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab. 2017;121(3):227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muenzer J., Giugliani R., Scarpa M., Tylki-Szymańska A., Jego V., Beck M. Clinical outcomes in idursulfase-treated patients with mucopolysaccharidosis type II: 3-year data from the hunter outcome survey (HOS) Orphanet J. Rare Dis. 2017;12(1):161. doi: 10.1186/s13023-017-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubaski F., Yabe H., Suzuki Y. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol. Blood Marrow Transplant. 2017;23(10):1795–1803. doi: 10.1016/j.bbmt.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton B.K., Jego V., Mikl J., Jones S.A. Survival in idursulfase-treated and untreated patients with mucopolysaccharidosis type II: data from the hunter outcome survey (HOS) J. Inherit. Metab. Dis. 2017;40(6):867–874. doi: 10.1007/s10545-017-0075-x. [DOI] [PubMed] [Google Scholar]
- 5.Ueda K., Hokugo J. Safety and efficacy of idursulfase in the treatment of mucopolysaccharidosis II (Hunter syndrome): a post-marketing study in Japan. Expert Opin. Drug Saf. 2020;19(7):891–901. doi: 10.1080/14740338.2020.1751120. [DOI] [PubMed] [Google Scholar]
- 6.Tanjuakio J., Suzuki Y., Patel P. Activities of daily living in patients with hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol. Genet. Metab. 2015;114(2):161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yumi Kono, Naohiro Yonemoto, Satoshi Kusuda et al. Developmental assessment of VLBW infants at 18 months of age: A comparison study between KSPD and Bayley III doi:10.1016/j.braindev.2015.10.010. [DOI] [PubMed]
- 8.Tomatsu S., Fujii T., Fukushi M. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110(1–2):42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampe C., Bosserhoff A.K., Burton B.K. Long-term experience with enzyme replacement therapy (ERT) in MPS II patients with a severe phenotype: an international case series. J. Inherit. Metab. Dis. 2014;37(5):823–829. doi: 10.1007/s10545-014-9686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbier A.J., Bielefeld B., Whiteman D.A.H., Natarajan M., Pano A., Amato D.A. The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase. Mol. Genet. Metab. 2013;110(3):303–310. doi: 10.1016/j.ymgme.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Concolino D., Deodato F., Parini R. Enzyme replacement therapy: efficacy and limitations. Ital. J. Pediatr. 2018;44(Suppl 2):120. doi: 10.1186/s13052-018-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley L.A., Haddow H.R.M., Palomaki G.E. Treatment of mucopolysaccharidosis type II (Hunter syndrome): results from a systematic evidence review. Genet Med. 2017;19(11):1187–1201. doi: 10.1038/gim.2017.30. [DOI] [PubMed] [Google Scholar]
- 13.Khan S.A., Mason R.W., Giugliani R. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018;125(1–2):44–52. doi: 10.1016/j.ymgme.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glamuzina E., Fettes E., Bainbridge K. Treatment of mucopolysaccharidosis type II (Hunter syndrome) with idursulfase: the relevance of clinical trial end points. J. Inherit. Metab. Dis. 2011;34(3):749–754. doi: 10.1007/s10545-011-9280-1. [DOI] [PubMed] [Google Scholar]
- 15.Seo J.H., Okuyama T., Shapiro E., Fukuhara Y., Kosuga M. Natural history of cognitive development in neuronopathic mucopolysaccharidosis type II (Hunter syndrome): contribution of genotype to cognitive developmental course. Mol. Genet. Metab. Rep. 2020;24 doi: 10.1016/j.ymgmr.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A., Okuyama T., Suzuki Y. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107(3):513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y., Taylor M., Orii K., Fukao T., Orii T., Tomatsu S. Assessment of activity of daily life in mucopolysaccharidosis type ii patients with hematopoietic stem cell transplantation. Diagnostics (Basel) 2020;10(1):46. doi: 10.3390/diagnostics10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S., Yabe H., Takakura H. Hematopoietic stem cell transplantation for inborn errors of metabolism: a report from the research committee on transplantation for inborn errors of metabolism of the japanese Ministry of Health, Labour and Welfare and the working Group of the Japan Society for hematopoietic cell transplantation. Pediatr. Transplant. 2016;20(2):203–214. doi: 10.1111/petr.12672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table shows the profile of all patients and changes in DQ by age 20.