Summary
Generation of patient-derived neurons provides an unprecedented approach in modeling neurological diseases. Here, we describe the direct conversion of adult fibroblasts into motor neurons via lentiviral delivery of transcription factors. Compared with iPSC-based approach, directly converted neurons from donors retain features associated with age, making them ideal systems for modeling age-related neurological diseases. Low yield is the major challenge of this protocol. High quality lentiviruses and optimized cell culture conditions are critical to improve the final yield.
For complete details on the use and execution of this protocol, please refer to Ding et al. (2020), Ding et al. (2021), and Liu et al. (2016)
Subject areas: Cell Biology, Cell culture, Developmental biology, Neuroscience, Tissue Engineering
Graphical abstract
Highlights
-
•
Detailed protocol to generate MNs from human adult fibroblasts
-
•
Protocol assesses the identity and efficiency of MN generation in vitro
-
•
Descriptions of troubleshooting common problems during the reprogramming process
Generation of patient-derived neurons provides an unprecedented approach in modeling neurological diseases. Here, we describe the direct conversion of adult fibroblasts into motor neurons via lentiviral delivery of transcription factors. Compared with iPSC-based approach, directly converted neurons from donors retain features associated with age, making them ideal systems for modeling age-related neurological diseases. Low yield is the major challenge of this protocol. High quality lentiviruses and optimized cell culture conditions are critical to improve the final yield.
Before you begin
Timing: 24–48 days
For more information about the protocols, please refer to (Ding et al., 2020, 2021; Liu et al., 2016) for direct reprogramming procedure.
-
1.
Use the exact reagents mentioned in the key resources table.
-
2.
Prepare all stock solutions listed below and make the necessary working aliquots.
-
3.
Order the antibodies found in the key resources table for quality control.
Note: All types of cell culture media are good for up to 2 weeks at 4°C. It is recommended to prepare the medium when needed at the specific step of the protocol.
CRITICAL: All procedures are performed in a BSL-2 certified laboratory using a Laminar Airflow Hood with standard aseptic techniques. Cultures are grown and maintained in a humidified incubator at 37 °C with 5% CO2.
Key resources table
| REAGENT and RESOURCES | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Cell lines | ||
| Human embryonic kidney (HEK) 293T cells | American Type Culture Collection | ATCC Cat# CRL-3216, RRID:CVCL_0063 |
| Healthy skin fibroblasts | Coriell Institute for Medical Research | Coriell Cat# GM04506, RRID:CVCL_7413 |
| Healthy skin fibroblasts | Coriell Institute for Medical Research | Coriell Cat# GM03652, RRID:CVCL_7397 |
| Healthy skin fibroblasts | Coriell Institute for Medical Research | Coriell Cat# GM08400, RRID:CVCL_7483 |
| Mouse Primary Astrocytes | N/A | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Fetal Bovine Serum (FBS) | Corning | Cat# 35-010-CV |
| Penicillin/Streptomycin (Pen/Strep) | Thermo Fisher Scientific | Cat# 15140122 |
| Dulbecco's Modified Eagle Medium (DMEM) | Thermo Fisher Scientific | Cat# 11995073 |
| DMEM/nutrient mixture F-12 (DMEM-F12) with HEPES; without L-glutamine | Cytiva | Cat# SH30126.01 |
| Neurobasal medium | Thermo Fisher Scientific | Cat# 21103049 |
| Gelatin | Bio-Rad | Cat# 118860A |
| Matrigel | Corning | Cat# 354230 |
| B-27 Supplement (50 ×) | Invitrogen | Cat# 17504044 |
| N-2 Supplement (100×) | Invitrogen | Cat# 17502001 |
| Forskolin (FSK) | Sigma Aldrich | Cat# F6886 |
| Dorsomorphin (DM) | EMD Millipore | Cat# 171260 |
| Heat Stable Recombinant Human Basic Fibroblast Growth Factor (bFGF) | Gibco | Cat#PHG0367 |
| Brain Derived Neurotrophic Factor (BDNF) | PeproTech | Cat# 450-02 |
| Glial-Derived Neurotrophic Factor (GDNF) | PeproTech | Cat# 450-10 |
| Neurotrophin-3 (NT3) | PeproTech | Cat# 450-03 |
| Bovine serum albumin (BSA) | Thermo Fisher Scientific | Cat# bp9706 |
| Hoechst 33342 (HST) | Thermo Fisher Scientific | Cat# H3570 |
| Triton X-100 (TX-100) | Thermo Fisher Scientific | Cat# 85111 |
| Paraformaldehyde (PFA) | Sigma Aldrich | Cat# 158127 |
| Trypsin-EDTA (0.25%) | Thermo Fisher Scientific | Cat# 25200056 |
| Polyethyleneimine (PEI), 25 kDa linear | Polysciences | Cat# 23966-2 |
| Polybrene (Hexadimethrine bromide) | Sigma Aldrich | Cat# H9268-10g |
| Ara-C (Arabinosylcytosine) | Sigma Aldrich | Cat# C1768 |
| Antifade Mounting Medium | Vector Laboratories | Cat#H-1400-10 |
| Na2HPO4 | Sigma Aldrich | Cat# S-0876 |
| NaCl | Thermo Fisher Scientific | Cat# S271-10 |
| KCl | Thermo Fisher Scientific | Cat# P-217 |
| K2HPO4.3H2O | Sigma Aldrich | Cat# P-5504 |
| PVA mountain medium | N/A | N/A |
| DMSO, Dimethyl Sulfoxide | Thermo Fisher Scientific | Cat# D1391 |
| Antibodies | ||
| Rabbit anti-tubulin Beta 3 Class III (TUBB3), monoclonal (1:2000) | BioLegend | BioLegend Cat# 801201, RRID:AB_2313773 |
| Chicken anti-microtubule-associated protein 2 (MAP2) (1:10,000), polyclonal | BioLegend | Cat# 822501, RRID:AB_2564858 |
| Goat anti-CHAT, monoclonal (1:200) | Millipore | Millipore Cat# AB144P, RRID:AB_2079751 |
| Rabbit anti-SYN1, monoclonal (1:200) | Cell Signaling Technology | Cat# 5297 |
| Mouse anti-HB9, monoclonal (1:500) | DSHB | Cat# 81.5C10-c |
| Rabbit anti-Islet1 (ISL1), monoclonal (1:250) | Abcam | Cat# ab109517; RRID: AB_10866454 |
| Donkey anti-Mouse IgG (H + L), Alexa Fluor 488 (1:500) | Jackson ImmunoResearch | Cat# 715-545- 150, RRID: AB_2340846 |
| Sheep anti-Mouse IgG (H + L), Alexa Fluor 594 (1:500) | Jackson ImmunoResearch | Cat# 515-515-062, RRID:AB_2340331 |
| Donkey Anti-Rabbit IgG (H+L), Alexa Fluor 488 (1:500) | Jackson ImmunoResearch | Cat# 711-545-152, RRID:AB_2313584 |
| Donkey Anti-Rabbit IgG (H+L), Alexa Fluor 594 (1:500) | Jackson ImmunoResearch | Cat# 711-585- 152, RRID:AB_2340621 |
| Recombinant DNA | ||
| pRSV-Rev | Addgene | RRID:Addgene_12253 |
| pMDLg/pRRE | Addgene | RRID:Addgene_12251 |
| pMD2.G | Addgene | RRID: Addgene_12259 |
| pCSC-NGN2-IRES-GFP-T2A-Sox11 | Addgene | RRID:Addgene_90214 |
| pCSC-ISL1-T2A-LHX3 | Addgene | RRID:Addgene_90215 |
| Others | ||
| 15 mL polystyrene Conical tube | Thermo Fisher Scientific | Cat# 14-959-53A |
| 50 mL polystyrene Conical tube | Thermo Fisher Scientific | Cat# 14-432-22 |
| 24-well cell culture plates | Thermo Fisher Scientific | Cat#142485 |
| 10 cm Cell Culture Dishes | Thermo Fisher Scientific | Cat# 130182 |
| 0.45 μm Sterile Filter Unit (PVDF membrane) | EMD Millipore | Cat# SLHVM33RS |
| 0.22-μm vacuum filter | Millipore | Cat# SLGP033RB |
Materials and equipment
All equipment and materials should be sterile or autoclaved. Some liquids that cannot be autoclaved need to be filtered with 0.22 μm filters. All materials to be prepared freshly and stored at 4°C for a short-term storage no longer than two weeks. Scaling up and down according to the amount of medium needed.
| HEK293 cell medium | Final concentration | Amount |
|---|---|---|
| Dulbecco’s Modified Eagle Medium (DMEM, 1×) | N/A | 44.5 mL |
| Fetal bovine serum (FBS) | 10% | 5 mL |
| Penicillin/Streptomycin (100×) | 1% | 0.5 mL |
| Total | N/A | 50 mL |
CRITICAL: Store HEK293 cell medium at 4°C up to 2 weeks.
| Fibroblast medium | Final concentration | Amount |
|---|---|---|
| DMEM | N/A | 42 mL |
| Fetal bovine serum (FBS) | 15% | 7.5 mL |
| Penicillin/Streptomycin (100×) | 1% | 0.5 mL |
| Total | N/A | 50 mL |
CRITICAL: Store fibroblast medium at 4°C up to 2 weeks.
| Astrocyte medium | Final concentration | Amount |
|---|---|---|
| DMEM | N/A | 42 mL |
| Fetal bovine serum (FBS) | 15% | 7.5 mL |
| Penicillin/Streptomycin (100×) | 1% | 0.5 mL |
| Total | N/A | 50 mL |
CRITICAL: Store astrocyte medium at 4°C up to 2 weeks.
| Neural induction medium | Final concentration | Amount |
|---|---|---|
| DMEM:F12 (1:1) | N/A | 24 mL |
| Neurobasal media | N/A | 24 mL |
| Penicillin/Streptomycin (100×) | 1% | 0.5 mL |
| N2 (100×) | 1× | 0.5 mL |
| B27 (50×) | 1× | 1 mL |
| FSK (20 mM) | 10 mM | 25 μL |
| DM (10 mM) | 1 mM | 5 μL |
| bFGF (200 μg/mL) | 10 ng/mL | 2.5 μL |
| Total | N/A | 50 mL |
CRITICAL: Prepare all stock aliquots of FSK, DM and bFGF at indicated concentration and keep them in −80°C freezer. Store the complete medium at 4°C up to 2 weeks.
| Neural maturation medium | Final concentration | Amount |
|---|---|---|
| DMEM:F12 (1:1) | N/A | 24 mL |
| Neurobasal media | N/A | 24 mL |
| Penicillin/Streptomycin (100×) | 1% | 0.5 mL |
| N2 (100×) | 1× | 0.5 mL |
| B27 (50×) | 1× | 1 mL |
| FSK (20 mM) | 5 mM | 12.5 μL |
| BDNF (20 μg/mL) | 10 ng/mL | 25 μL |
| GDNF (20 μg/mL) | 10 ng/mL | 25 μL |
| NT3 (20 μg/mL) | 10 ng/mL | 25 μL |
| Total | N/A | 50 mL |
CRITICAL: Prepare all stock aliquots of FSK, BDNF, GNDF, and NT3 at indicated concentration and keep them in −80°C freezer. Store the complete medium at 4°C up to 2 weeks.
| PEI solution stock | Final concentration | Amount |
|---|---|---|
| PEI (transfection grade, 25 kDa, Linear) | 1 μg/μL | 50 mg |
| MilliQ water | N/A | 50 mL |
| Total | N/A | ∼50 mL |
CRITICAL: Dissolve PEI in sterile distilled water. Incubate the tube at 55°C in a water bath and vortex until PEI is completely dissolved. Adjust pH to 7.0 if necessary. Cool PEI solution to room temperature (∼25°C) and sterilize the solution using a 0.22 μm vacuum filter. Aliquot and store at −80°C for up to 1 year.
| Gelatin stock | Final concentration | Amount |
|---|---|---|
| Gelatin | 1 mg/mL | 0.5 g |
| MilliQ water | N/A | 500 mL |
| Total | N/A | 500 mL |
CRITICAL: Autoclave stock and keep it at room temperature or at 4°C for up to 6 months..
| 10× PBS stock | Final concentration | Amount |
|---|---|---|
| NaCl | 1.37 M | 80 g |
| KCl | 27 mM | 2.0 g |
| Na2HPO4 | 100 mM | 14.4 g |
| K2HPO4.3H2O | 18 mM | 2.4 g |
| MilliQ water | N/A | ∼950 mL |
| Total | N/A | 1000 mL |
CRITICAL: Adjust pH to 7.4 with HCl. The autoclaved 10× PBS stock can be stored at room temperature for 1 year. Before use, dilute 10× PBS stock using MilliQ water to make 1× PBS working solution.
Step-by-step method details
Lentivirus production and titration
Timing: 14 days(5–7 days for step 1; 4 days for step 2; 4 days for step 3)
Lentiviruses can infect both proliferating and non-proliferating cells. The genetically modified lentiviral vectors lack dispensable genes from the HIV-1 genome and separate cis-acting sequences from trans-acting factors required for viral particle production, infection, and integration. A third-generation packaging system provides maximal biosafety but requires the transfection of four separate plasmids, including three helper plasmids and one lentiviral vector expressing genes of interest (Dull et al., 1998). To ensure the high reprogramming efficiency, it is critical to prepare high-quality supercoiled plasmids and make lentiviruses with excellent titers. For detailed information about lentivirus preparation, titration and applications, please see our previous studies (Ding et al., 2013, 2016, 2018; Ding and Kilpatrick, 2013).
-
1.Expanding HEK293T cells.
-
a.Culture HEK293T cells in HEK culture medium one week before starting the preparation of viruses. Pass them at about 80% confluency twice.
-
b.For each 10 cm plate, seed about 8 × 10 6 HEK cells in 10 mL medium and gently mix well to let cells distribute evenly on the surface. The next day, the cell density should be about 80% confluency for transfection (Figure 1A).
-
a.
-
2.
Transfection and lentivirus collection
Before transfection, prepare high quality supercoiled plasmids (Ding and Kilpatrick, 2013). Lentiviral plasmid DNA will be delivered by the Polyethyleneimine (PEI)-mediated transfection method.-
a.We use the third-generation lentiviral packing system which consists of three helper plasmids, including pRSV-Rev (3rd generation lentiviral packaging plasmid contains Rev), pMDLg/pRRE (3rd generation lentiviral packaging plasmid contains Gag and Pol), and pMD2.G (VSV-G envelope expressing plasmid). Two lentiviral vectors expressing reprogramming factors are plasmids pCSC-SP-PW-CMV-Ngn2-IRES-GFP-T2A-mSOX11 (Plasmid#1) and pCSC-ISL1-T2A-LHX3 (Plasmid#2).
-
b.For each 10 cm plate, add 4 μg of plasmid#1 or plasmid#2; 2 μg of pMDLg/pRRE plasmid; 1 μg of pMD2.G plasmid and 0.5 μg of pRSV-Rev plasmid to 1 mL DMEM (-FBS) media and mix well.
-
c.Add 22.5 μL transfection reagent PEI (1 μg/μL) to plasmids mixture and immediately mix well by gently pipetting up and down 5 times or vortexing for 15 s. Incubate at room temperature for 15–20 min.Note: The ratio of PEI to the mixture of total DNA is 1:3. For example, the total amount of DNA is 7.5 μg for a 10 cm plate. Add 22.5 μL of PEI (equivalent to 22.5 μg) into the plasmid mixture in 1 mL DMEM.
-
d.Add 1 mL of transfection dropwise to each 10 cm plate of HEK293 cells and gently mix to distribute transfection regent evenly.
-
e.After 8 h transfection or overnight, replace with 10 mL HEK culture medium for each 10 cm plate.
-
f.Collect lentiviral supernatant completely (around 10 mL) at 48 h post-transfection and add 10 mL fresh culture medium. Store this first collection of lentiviral supernatant at 4°C.
-
g.Another 24 h later (72 h post-transfection), collect lentiviral supernatant again and store at 4°C. Discard the plates in a biohazard bag and trash it after autoclave.Note: Transfection efficiency can be examined under a fluorescence microscope if the lentiviral vector contains GFP reporter (e.g. plasmid #1). At lentivirus collection, most HEK cells (>90%) should be GFP (+) (Figure 1B).
-
h.Pool two collections of the same lentivirus together and add 6 μg/mL polybrene. Filter the viral supernatants with 0.45 μm vacuum filters. Store viral supernatants at 4°C until ready to use (within two weeks) or freeze at −80°C for longer term storage.
 CRITICAL: The ratio of PEI to plasmids should be 4:1 to 3:1. Lesser or higher ratios decrease the efficiency of virus production. When you add transfection mixture to HEK293 cells, just gently move the plate on the base up-down and left-right for 10 times. Do not move circular to prevent pooling transfection mixture in the center.
CRITICAL: The ratio of PEI to plasmids should be 4:1 to 3:1. Lesser or higher ratios decrease the efficiency of virus production. When you add transfection mixture to HEK293 cells, just gently move the plate on the base up-down and left-right for 10 times. Do not move circular to prevent pooling transfection mixture in the center.
-
a.
-
3.Titration to verify the quality of lentivirus.
-
a.The virus can be titrated based on GFP reporter (plasmid#1) (Figures 1C and 1D) or immunocytochemistry (ICC) of lentivirus expressed factors (plasmid#2) (Figures 1E and 1F) as previously reported (Ding and Kilpatrick, 2013).
-
b.Seed HEK cells in a 24 well plate at a density of 7–10 × 104 cells/well/0.5 mL medium. Cells will reach ∼70 % confluency the next day at the time of titration.
-
c.Make a serial dilution (e.g., 1:10, 1:100 etc.) of viral supernatant with HEK culture medium.
-
d.Add 50 μL of undiluted and diluted lentivirus to desired wells.Note: Before addition of lentivirus, do not need to remove original culture medium from 24-well plate. Set one well as a negative control without transduction.
-
e.Change the medium after 8 h or overnight (∼16 h) exposure to lentivirus.
-
f.Check the percentage of positive cells after 48–72 h.Note: For lentivirus expressing GFP reporter, examine GFP (+) cells directly under a fluorescence microscope in living cells. For lentivirus that does not express fluorescent reporters, cells need to be fixed and perform ICC to examine lentivirus-expressed factors.
-
g.Routinely, transduced positive cells should be more than 90% in undiluted wells and more than 30% in 10-fold diluted wells (Figures 1C–1F).
 CRITICAL: A good titer of lentiviral supernatants should be no less than 1 × 106 IFU/mL. (IFU, Infectious Unit).
CRITICAL: A good titer of lentiviral supernatants should be no less than 1 × 106 IFU/mL. (IFU, Infectious Unit).
-
a.
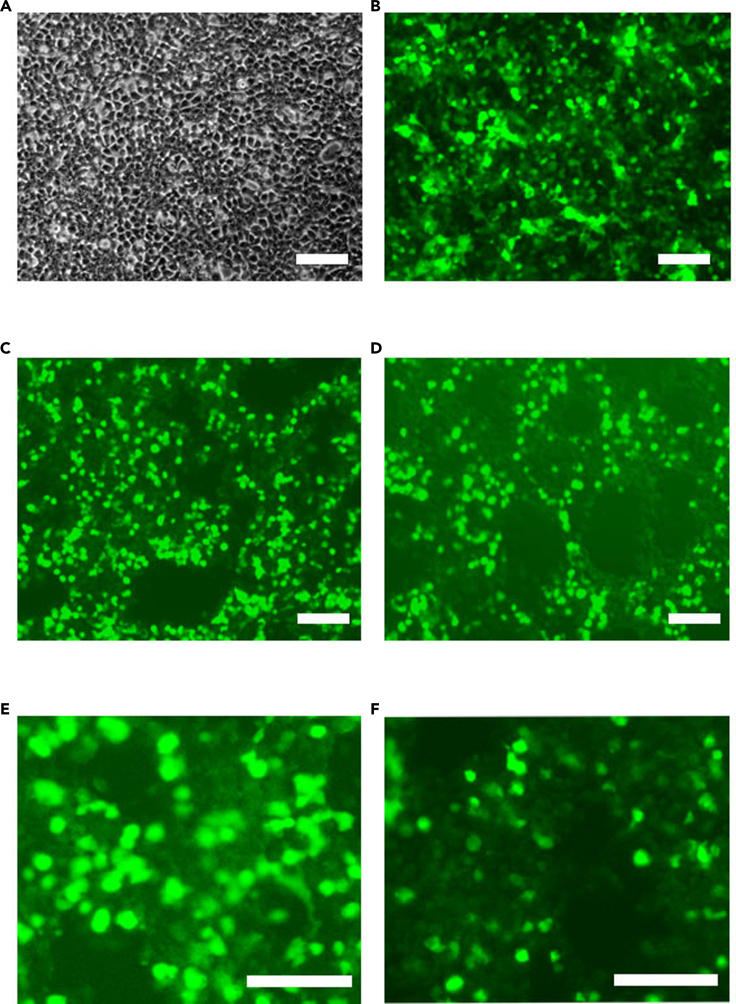
Figure 1.
Lentivirus preparation and titration
(A) HEK cells at transfection with ∼80% confluency.
(B) At virus collection (72 h post-transfection) almost all HEK cells are transfected positive (plasmid #1 with co-expressed GFP).
(C) Virus titration with undiluted virus (#1) at 72 h post-transduction (> 90% cells are GFP positive).
(D) Virus titration with 1:10 diluted virus (#1) at 72 h post-transduction (∼40% cells are positive).
(E) Virus titration of undiluted virus (#2) at 72 h post-transduction with immunocytochemistry (ICC) of ISL1 (∼90% cells are positive).
(F) Virus titration with 1:10 diluted virus (#2) at 72 h post-transduction with ICC of ISL1 (∼40% cells are positive). From A to F, scale bares, 100 μm.
Human fibroblast culture
Timing: 5–10 days
-
4.Fibroblast culture and expansion. If starting to culture fibroblast from a frozen stock, it is recommended to split cells twice before seeding on Matrigel coated plates for reprogramming.Note: You can freeze fibroblasts in DMEM contains 15% FBS and 10% DMSO using cryotubes at a concentration of 1 × 106/mL/vial.Note: Passage number of fibroblasts is important for conversion. Cells with high passage numbers (>15) show slow growth and the low conversion rate.
-
a.Quickly thaw a frozen stock of human fibroblast in a 37°C water bath.
-
b.When there is just a small bit of ice left in the vial, immediately transfer cells into a 15 mL tube containing 5 mL of fibroblast culture medium and gently mix.
-
c.Centrifuge at 200 g for 3 min to collect cells.
-
d.Remove the supernatant and resuspend cell pellet into 10 mL fibroblast culture medium and seed cells in one 10 cm plate.
-
e.Change media every two or three days until close to full confluency.
-
f.Split fibroblast. Wash the cells with 1× PBS and detach them by trypsinization with 0.05% trypsin (make 1:5 dilution of 0.25% trypsin with 1× PBS) at 37°C for 4 min.
-
g.When cells reach about 80% confluency, they will be ready for setting direct reprogramming.
 CRITICAL: The growth speed varies dramatically between different cell lines. Some aged or patient fibroblasts may grow slowly. During culture of these slow-growth cells, the medium must be changed regularly every two or three days to facilitate the cell growth and maintain antibiotics effective in the culture. The splitting ratio could be from 1: 2 to 1: 5 depending on the growth.
CRITICAL: The growth speed varies dramatically between different cell lines. Some aged or patient fibroblasts may grow slowly. During culture of these slow-growth cells, the medium must be changed regularly every two or three days to facilitate the cell growth and maintain antibiotics effective in the culture. The splitting ratio could be from 1: 2 to 1: 5 depending on the growth.
-
a.
Fibroblast transduction and motor neurons induction
Timing: 15–20 days(4–16 h for step 5; 24 h for step 6; Timing: 14 days for step 7)
-
5.Prepare Matrigel-coated plates.
-
a.Dilute Matrigel at 1:100 with cold DMEM medium.
-
b.Add 5 mL of diluted Matrigel to each 10 cm plate and gently shake to ensure to cover all the bottom surface of the plate.
-
c.Incubate at 37°C more than 3 h or overnight.Note: Matrigel is sensitive to temperature. When working with Matrigel, place the aliquot of Matrigel on ice or ice pack within the biosafety cabinet.
-
a.
-
6.Seed proper amount of fibroblasts onto Matrigel-coated plates.
-
a.Fibroblasts at about 90% confluency will be harvested by trypsinization.
-
b.Seed ∼1 × 106 cells for a Matrigel-coated 10 cm plate.Note: A 10 cm plate with ∼90% confluency usually give rise to ∼3 million fibroblast cells. It is enough for seeding three 10 cm plates. The seeding numbers should be optimized according to cell growth to ensure that the cell density will be around 60%–80% confluency in next day at transduction.
-
a.
-
7.This step describes the induction of directly reprogrammed motor neurons from fibroblasts. The neural induction media is used at this stage.
-
a.The day after seeding fibroblast onto the Matrigel-coated plate, cell density should be around 60%–80% confluency at transduction (Figure 2).
-
b.Mix the desired volume of two lentiviruses (1:1) in the presence of 6 μg/mL polybrene.
-
c.Add proper amount of lentiviral supernatants to the plate to transduce fibroblasts. For a 10 cm plate, add ∼20 mL of total lentivirus expressing 4 reprograming factors.Note: The lentiviral dosage should be optimized based on the viral titer and the growth of the cell line. Do not need to remove original culture medium before addition of lentiviruses to the plate.
-
d.Cells are exposed to lentiviruses overnight (∼16 h) in incubator.
-
e.The next morning, replace the media with fresh fibroblast medium. This is the first day post-viral infection (1 dpi).
-
f.The next day (2 dpi), change the neural induction medium.
-
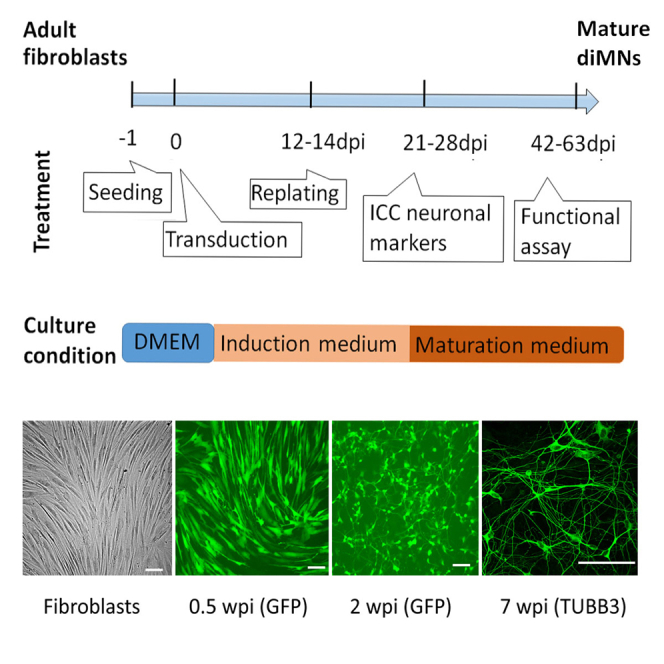
g.Change half volume of neural induction medium every the other day at 4, 6, 8, 10, 12 dpi.Note: For a good transduction, most (>90%) fibroblast cells are GFP (+) and then gradually become neuron-like cells within 2 weeks post-viral infection (wpi) (Figure 3).
-
a.
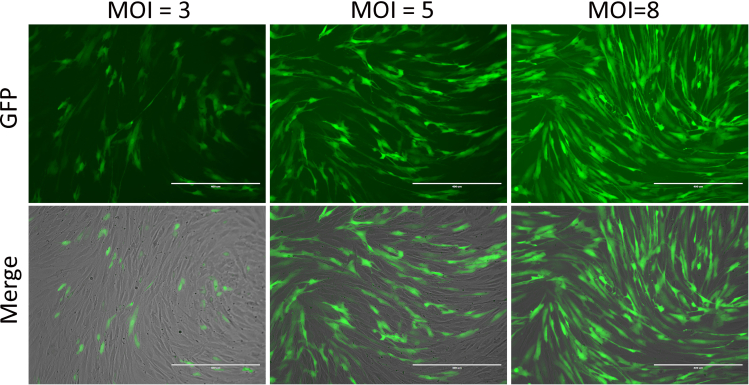
Figure 2.
Transduction of human fibroblasts with proper amount of lentiviruses
Micrographs of fibroblast cells (GM04506) transduced with lentivirus expressing reprogramming factors with indicated MOI (Multiplicity of Infection) at 3 days post-viral infection (dpi). The transduced positive cells can be identified with co-expressed GFP. Scale bars: 400 μm.
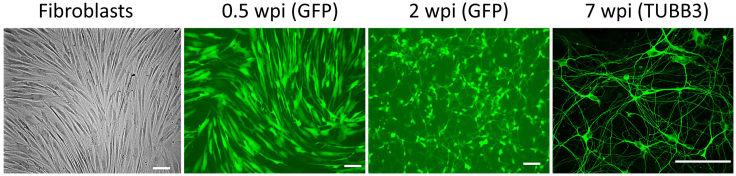
Figure 3.
Representative morphology of fibroblasts and directly induced motor neurons (diMNs) at indicated weeks post-viral infection (wpi)
Transduced cells are identified with co-expressed green fluorescent protein (GFP) at early time points (0.5 and 2 wpi). Transduced fibroblasts gradually become neuron-like morphology within 2 wpi. More complicated branches and very long axons can be detected at late developmental stages with immunostaining of tubulin Beta 3 Class III (TUBB3). Scale bars: 100 μm. Adapted from (Ding et al., 2020) with permission.
CRITICAL: Addition of proper amount of high-quality lentiviruses is very important to ensure good reprogramming efficiency. The proper MOI (Multiplicity of Infection) could be used are 5–8 (Figure 2). As the properties of cells (such as growth speed) will influence the transduction efficiency, different cell lines may need different dosages to obtain optimized transduction efficiency. However, too much viruses could cause more cell death. Transduction efficiency and cell survival should be balanced.
Purify reprogramed neurons by replating
Timing: 5–7 days(3 h for step 9)
This step describes purification of reprogrammed neurons from fibroblasts by replating cells with gelatin coated plates. The attachment of fibroblast cells will occur earlier and more tightly than reprogrammed neurons. Purified neurons will be seeded onto coverslips with a monolayer of astrocytes for long-term culture (2–10 wpi) in neural maturation medium. For primary astrocytes isolation and monolayer astrocyte preparation, please refer (Schildge et al., 2013).
-
8.Culture astrocyte cells. Isolate mouse astrocytes from postnatal day 1 (P1) pups and culture them in DMEM with 15% FBS. Prepare frozen stocks at ∼1 × 106 cells/vial. Start culturing of astrocytes at least 5 days before replating.
-
a.Thaw astrocytes frozen stocks and culture them in astrocyte culture media in 10 cm cell culture plates.Note: Seed cells from one vial of frozen stock (1 × 106 cells) on one 10 cm plate. It will take about 3 days to reach around 90% confluency.
-
b.Coat 24-well plates contain cover slips with Matrigel (1:100) at least for 3 h in 37°C for seeding astrocytes.
-
c.When astrocytes reach ∼90% confluency in the 10 cm plate, trypsinize astrocytes with 0.025% trypsin (make 1:10 dilution of 0.25% trypsin with 1× PBS) for 3 min at 37°C.
-
d.Add astrocyte medium to neutralize trypsin and detach cells.
-
e.Transfer cell suspension to a 15 mL tube and centrifuge at 200 g for 3 min to collect cells.
-
f.Remove supernatant and resuspend the cell pellet into 12 mL astrocyte medium.
-
g.Seed astrocytes onto coverslips in a 24-well plate at 0.5 mL/well.
-
h.Change medium every two days until astrocytes form monolayer on coverslips.Note: One full 10 cm plate of astrocytes is enough for seeding a 24-well plate. If astrocytes reach full confluency on coverslips before neurons are ready for replating, add 10 μM Ara-C in culture medium to inhibit cell amplification.
-
a.
-
9.Infected fibroblasts (GFP +) at 14 dpi will show typical neuron-like morphology (Figure 3). These cells could be directly used for early developmental analysis or replated onto monolayer astrocytes for long-term culture.
-
a.Coat culture plates with 5 mL of 0.1% gelatin for 30 min at 37°C.Note: For one 10 cm plate of reprogrammed cells, need two 10 cm plates for replating to ensure efficient purification.
-
b.Remove culture medium from culture plates with induced neurons and wash once with PBS.
-
c.Add 3 mL of 0.025% trypsin (make 1:10 dilution of 0.25% trypsin with 1× PBS) to a 10 cm plate and incubate for 3 min at 37°C to detach the cells.
-
d.Add 7 mL fibroblast culture medium to quench trypsin activity. Gently pipet up and down to break cell aggregates.
-
e.Transfer cell suspension to gelatin-coated plates (about 5 mL each).Note: Remove gelatin just before use. Avoid air drying coated plates.
-
f.Incubate plates at 37°C and keep checking the attachment of cells under microscope.Note: It will take 15–30 min to see differential adhesion which means most fibroblasts attach at the bottom and the reprogrammed neurons (GFP+) are still floating.
-
g.Collect cell suspensions and transfer to a 15 mL tube. Pool cell suspensions from two plates together.
-
h.Centrifuge at 300 g for 3 min to collect cells.
-
i.Remove the supernatant and gently resuspend the cell pellets into neural maturation medium with desired volume based on the yield and the following experimental plans.Note: The neurons generated from one 10 cm plate are enough for seeding a 24-well plate with the cell density ∼2 × 104 cells/well.
 CRITICAL: The proper length of incubating time is important during replating. Too short, most fibroblasts are still floating, and the purity of neurons is low. If incubation time is too long, more neurons could also attach at the bottom and may lead to poor yield. The best time point is that the majority of fibroblasts attached at the bottom and few neurons attached.
CRITICAL: The proper length of incubating time is important during replating. Too short, most fibroblasts are still floating, and the purity of neurons is low. If incubation time is too long, more neurons could also attach at the bottom and may lead to poor yield. The best time point is that the majority of fibroblasts attached at the bottom and few neurons attached.
-
a.
Neuronal identity verification
Timing: 3 days
-
10.This step describes how to validate the MN identity of reprogrammed neurons using ICC of different markers.
-
a.Take coverslips from 24-well plates at desired time points.Note: As early as 2 wpi, the generic neuronal markers of TUBB3 and early MN marker HB9 could be examined. At 4 wpi or later, MAP2 and mature MN marker CHAT could be examined (Figure 4).
-
b.Fix cultured cells with 4% paraformaldehyde (PFA) in PBS for 15 min at room temperature.
-
c.Wash twice with PBS.
-
d.Incubate fixed cells in blocking buffer (PBS containing 0.2% Triton X-100 and 3% BSA) for 1 h at room temperature for permeabilization and blocking.
-
e.Cells are then incubated with primary antibodies in blocking buffer at 4°C overnight (∼16 h).Note: The antibodies include anti-TUBB3 and anti-MAP2 as neuronal markers, anti-HB9 as an early MN marker, anti-CHAT as a late MN marker, and anti-SYN1 as a presynaptic marker.
-
f.After removal of primary antibodies, wash coverslips with PBS for 10 min × 4.
-
g.Incubated with corresponding fluorophore-conjugated secondary antibodies in dark at room temperature for 2 h.
-
h.Wash coverslips with PBS for 10 min × 3.
-
i.The nuclei were stained with Hoechst 33342 (1:3000) in PBS at room temperature for 15 min.
-
j.Briefly wash coverslips once with PBS.
-
k.Mount coverslips with mounting medium on microslides.
-
l.Air dry the coverslips and visualize cells under a confocal microscope.
 CRITICAL: The examination of different markers need to use samples at different time points. The best time frame for staining nuclear HB9 is 2–3 wpi, and the best time for staining CHAT is 4 wpi or later (Figures 4). For staining presynaptic markers and electrophysiology assay, need reprogrammed MNs reach the full maturation at 6 wpi or later (Figure 5). For the changes of properties at different developmental stages during the reprogramming process, please refer to our previous report (Ding et al., 2020).
CRITICAL: The examination of different markers need to use samples at different time points. The best time frame for staining nuclear HB9 is 2–3 wpi, and the best time for staining CHAT is 4 wpi or later (Figures 4). For staining presynaptic markers and electrophysiology assay, need reprogrammed MNs reach the full maturation at 6 wpi or later (Figure 5). For the changes of properties at different developmental stages during the reprogramming process, please refer to our previous report (Ding et al., 2020).
-
a.
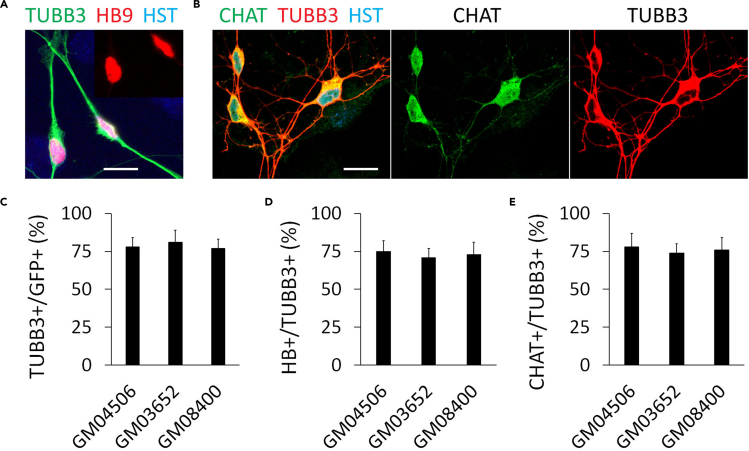
Figure 4.
Neuronal identity verification
(A) Confocal micrograph shows the highly expressed nuclear HB9 (motor neuron and pancreas homeobox 1, the early MN marker) at 2 wpi. The inset shows the nuclear HB9 alone. DNA dye hoechst33342 (HST) stained nuclei. Scale bar: 20 μm.
(B) Confocal micrographs show the robust expression of the mature MN marker CHAT (choline acetyltransferase) at 8 wpi. Scale bar: 20 μm.
(C) Quantification of the reprogramming efficiency was shown as the percentage of GFP (+) cells expressed TUBB3.
(D) Fractions of diMNs among the reprogrammed neurons was shown as the percentage of TUBB3 (+) cells expressed HB9.
(E) Fractions of diMNs among the reprogrammed neurons was shown as the percentage of TUBB3 (+) cells expressed CHAT. From C to E, data are presented as mean ± SEM. More than 300 cells of each line were counted from triplicates.
Adapted from (Ding et al., 2020, 2021) with permission.
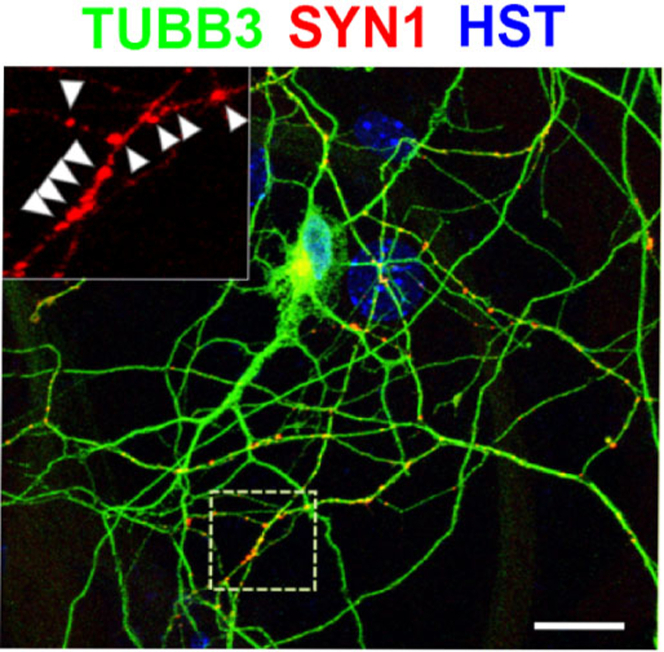
Figure 5.
Micrograph shows the robust expression of the presynaptic marker synapsin 1 (SYN1) at 9 wpi
The inset shows discrete SYN1-positive puncta (indicated by arrow heads). DNA dye hoechst33342 (HST) stained nuclei. Scale bar: 50 μm. Adapted from (Ding et al., 2021) with permission.
Expected outcomes
Fibroblasts are transduced with reprogramming factors can be identified with co-expressed GFP at 3 days post-viral infection (dpi) (Figure 3), and they gradually become neuron-like morphology (Figure 4). These neurons robustly express generic neuronal marker TUBB3 (Figures 3, 4, and 5), early MN marker nuclear HB9 (Figure 4), late MN marker CHAT (Figure 4), and presynaptic marker synapsin 1 (SYN1). About 80% transduced positive cells can be successfully converted into neurons (TUBB3+/GFP+) (Figure 4C). Among these neurons, about 75% cells express MN markers (HB9+/TUBB3+ and CHAT+/TUBB3+) (Figures 4D and 4E). At a late stage, such as 9 wpi, neurons show complicated branches and presynaptic markers, such as synapsin 1 (SYN1) (Figure 5).
Limitations
For aged or diseased fibroblast cell lines, the final yield could be poor due to the slow growth of cells and the low transduction efficiency. Preparation of lentiviruses with high quality and optimization of cell culture conditions are critical to improve the yield.
Troubleshooting
Problem 1
Detachment of cells during reprogramming (steps 5–7).
Potential solution
Coat culture plates with freshly prepared Matrigel (up to 1:50) at least 3 h and seed fibroblast at proper density. The confluency of fibroblast should be around 60%–80% at transduction.
Problem 2
Low quality of viruses (steps 1–3).
Potential solution
When performing the viral preparation, we recommend using low passage (less than 20) of HEK293T cells and checking on the quality of plasmids and transfection reagents. Proper ratio of helper plasmids, lentiviral vectors, and transfection reagent will improve lentivirus titers.
Problem 3
Low transduction efficiency (step 7).
Potential solution
Prepare the high quality of virus (> 1×106 IFU/mL) and add proper amount of virus (MOI 5–8). Add 6 μg/mL of polybrene to the lentiviral supernatant and mix well. Add the lentiviral mixture directly to cultured fibroblasts without removal of culture medium. Let cells exposed to virus for 8 h or overnight.
Problem 4
Low yield of differentiated neurons (steps 8 and 9).
Potential solution
Increase the transduction efficiency and the starting materials. Use 10 cm plates instead of smaller plates. The reprogrammed neurons from multiple plates could be pooled together at replating step to increase the total yield.
Problem 5
Poor survival of reprogrammed neurons (step 10).
Potential solution
Culture proper amount of neurons onto Matrigel coated coverslips with a monolayer of astrocytes. Given substantial neurons will die within 72 h after replating, seed proper amount of neurons in each well (about 2 × 104 cells/well of 24-well plate) is important to improve the neuronal survival. Co-culture with a monolayer of astrocytes is required for long-term survival. We have cultured reprogrammed neurons up to 10 weeks.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Dr. Baojin Ding (Baojin.Ding@Louisiana.edu).
Materials availability
The majority of materials required in this protocol are commercially available.
Acknowledgments
We thank members of the Ding laboratory for help and discussion. This work was supported by NIH grant (NIH/NINDS NS112910 to B.D.) and Department of Defense (DoD) Peer Reviewed Medical Research Program (PRMRP) Discovery Award (W81XWH2010186 to B.D.).
Author contributions
Conceptualization, methodology, funding acquisition, and supervision, B.D.; investigation and writing, B.D., M.S., and M.A.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate/analyze any data sets or code that were submitted to any repositories.
References
- Ding B., Akter M., Zhang C.L. Differential influence of sample sex and neuronal maturation on mRNA and protein transport in induced human neurons. Front. Mol. Neurosci. 2020;13:46. doi: 10.3389/fnmol.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Cave J.W., Dobner P.R., Mullikin-Kilpatrick D., Bartzokis M., Zhu H., Chow C.W., Gronostajski R.M., Kilpatrick D.L. Reciprocal autoregulation by NFI occupancy and ETV1 promotes the developmental expression of dendrite-synapse genes in cerebellar granule neurons. Mol. Biol. Cell. 2016;27:1488–1499. doi: 10.1091/mbc.E15-07-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Dobner P.R., Mullikin-Kilpatrick D., Wang W., Zhu H., Chow C.W., Cave J.W., Gronostajski R.M., Kilpatrick D.L. BDNF activates an NFI-dependent neurodevelopmental timing program by sequestering NFATc4. Mol. Biol. Cell. 2018;29:975–987. doi: 10.1091/mbc.E16-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Kilpatrick D.L. Lentiviral vector production, titration, and transduction of primary neurons. Methods Mol. Biol. 2013;1018:119–131. doi: 10.1007/978-1-62703-444-9_12. [DOI] [PubMed] [Google Scholar]
- Ding B., Tang Y., Ma S., Akter M., Liu M.L., Zang T., Zhang C.L. Disease modeling with human neurons reveals LMNB1 dysregulation underlying DYT1 dystonia. J. Neurosci. 2021;41:2024–2038. doi: 10.1523/JNEUROSCI.2507-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Wang W., Selvakumar T., Xi H.S., Zhu H., Chow C.W., Horton J.D., Gronostajski R.M., Kilpatrick D.L. Temporal regulation of nuclear factor one occupancy by calcineurin/NFAT governs a voltage-sensitive developmental switch in late maturing neurons. J. Neurosci. 2013;33:2860–2872. doi: 10.1523/JNEUROSCI.3533-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.L., Zang T., Zhang C.L. Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Rep. 2016;14:115–128. doi: 10.1016/j.celrep.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildge S., Bohrer C., Beck K., Schachtrup C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. 2013 doi: 10.3791/50079. 23380713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze any data sets or code that were submitted to any repositories.