Summary
The current human exposome primarily emphasizes the total environmental exposure during an entire life. The characteristics of “lifelong” and “all environmental factors” make it very challenging to bring the exposomic study into real-life applications. Herein, we mainly discuss the typical application scenarios of exposomics and how to conduct an exposomic study to establish relationships between the exposome and human health. To increase the feasibility and efficiency, we propose that (1) an exposomic study can start with health events during critical-window periods; (2) both data- and hypothesis-driven exposomics should be combined to prioritize the risk of environmental factors; and (3) reliable statistical analysis of high-dimensional data of external and internal exposure factors are urgently needed. With standardization of the exposomic study, it will be critical to build a “wonderland” for human health.
Keywords: exposome, exposomics, health risk assessment, human health, critical window
There are increasing evidences that environmental factors make enormous contributions to human health. Exposome was originally defined as something that encompassed the life-course environmental exposure from the prenatal period onward,1 or the cumulative measure of environmental influences and associated biological responses throughout the lifespan, of which exposures can be from the environment, diet, behavior, and endogenous processes.2 Briefly, exposome, developed as a partner of the genome to reveal the causes of diseases, aims to characterize all lifelong environmental exposure factors and predict their impacts on human health endpoints such as various diseases, of which the study is called exposomics. The exposome can include all physical, chemical, microbiological factors, and even social activities. Compared with conventional environmental health risk assessment, the exposome aims to provide a 'perfect' solution by characterizing all environmental factors and minimizing the confounding effects.3 However, due to its ambitious aim of including the total environmental exposure during an entire life, it is very challenging to bring exposomics into real-life applications. Here, we discuss the following three questions and propose possible ways to improve the feasibility of exposomic studies.
What type of studies can exposomics be applied to?
Exposomics can be applied to any studies with health outcomes that are known to be probably affected by multiple environmental factors. Exposomic studies could facilitate causal inference, especially for those endpoints with complex or unknown causes, such as the development of children, aging, and diseases of metabolic, cardio-pulmonary, and reproductive systems. To conduct any exposomic studies, an epidemiological study should be designed first. At the same time, a systematic meta-analysis of previous studies that examine one or several environmental factors can provide information for further exposomic studies. In addition, more analysis is needed to understand the critical development window of a disease of concern or adverse health outcome. The critical window can be the important development stages of one disease or other health outcomes. For example, the Human Early-Life Exposome (HELIX) study combined six existing longitudinal population-based birth cohort studies in Europe, aiming to implement novel exposure assessment and associate it with child health outcomes (https://humanexposomeproject.com/). In contrast with the lifelong concept, we consider that exposomics can be applied to studies of causal events occurring during relatively short periods or its critical window, although the human exposome is very diverse, dynamic, and spatiotemporally driven.4,5 With proper manipulation of the cohort to minimize the dynamic changes in environmental exposure, it should be useful to conduct such research with limited time points. The lessons we learn from a short time window can also help us design more appropriate lifelong exposomic studies. Furthermore, due to the high cost and duration of exposomic studies and the high likelihood of failure, one exposomic study should consider multiple health outcomes if possible. In sum, we consider that exposomics has great potential to associate one or multiple health endpoints with complex or unknown causes during the critical development window of one disease or other health outcomes.
What factors should be considered and how should they be monitored in an exposomic study?
Previous exposure studies such as ongoing exposome projects (https://humanexposomeproject.com/) have investigated both the external (e.g., persistent organic pollutants, endocrine disrupters, metals, ambient air pollutants, bacteria, and noise) and internal (e.g., epigenome, transcriptome, proteome, metabolome, gut microflora, inflammation, and oxidative stress) exposome. Among them, chemical exposure is the biggest challenge. The latest EPA ToxCast database contains over 800,000 compounds (https://www.epa.gov/chemical-research/comptox-chemicals-dashboard). Together with the bioactivation of those chemicals, it is almost impossible to characterize all the exposure chemicals. Therefore, an exposomic study needs to consider a few rules of thumb. First, the scope of exposome should be well defined during the early experimental design, which requires tailored selection depending on the health outcome. Although the current exposome mainly emphasizes the global nontargeted analysis using an advanced analytical platform such as high-resolution mass spectrometry, we believe both targeted and untargeted environmental factors should be equally important. A substantial portion of the targeted environmental factors might not be able to be captured by the global nontargeted analysis due to the limitation of each method. It is necessary to collect all previous epidemiological or toxicological information on the environmental factors related to the health outcome. Such targeted lists together with the available untargeted characterization from advanced analysis can increase the chance of success in an exposomic study. Second, it is important to prioritize the exposure factors by ranking their potential risk. Both exposure and toxicity or adverse outcomes can be considered for the risk ranking. Although the experimental or epidemiological information might not be available for most environmental factors, such prioritization is possible using advanced quantitative structure-activity relationship models, toxicology datasets, and exposure models.6 Third, more efforts are needed to characterize and quantify exposure biomarkers of environmental chemicals, diet, behavior, and endogenous physiological processes. Due to the xenobiotic transformation in human, exposure biomarkers of most environmental factors have not been well characterized. More time-effective exposure biomarker identification platforms such as the integration of biotransformation prediction, in vitro bioactivation, and human monitoring can be adopted in the future to fill the gap. Surrogate biomarkers for groups of compounds with similar exposure trends can be considered to reduce the target numbers. Regarding the analytical methods, besides the non-target identification, the development of targeted or suspect screening methods should also be emphasized. More technologically feasible and cost-effective methods are still needed to meet the requirements of large samples in exposomic studies to enable routine analysis. Fourth, besides the external exposure, the internal change should also be characterized with cutting-edge high-throughput omics technologies (e.g., proteomics and metabolomics) by using different bio-specimens (e.g., urine and blood). Last, the sampling frequency and spatial resolution are other important factors to be considered in the exposomic study. The stability of the exposed environmental factors determines how often they should be monitored. For example, the monitoring for persistent organic compounds can be less frequent than for the labile chemicals. Some factors, such as meteorological parameters and atmospheric pollutions, can be potentially characterized using regional monitoring,7 while most chemical exposure needs more personalized monitoring. Geographic information system, personalized sampling, and wireless sensing techniques for common exposure factors can also be involved to increase the spatiotemporal resolution.8
How does one establish relationships between the exposome and human health?
A relevant epidemiological study design, especially one with strong ability of causal inference, is also important for establishing relationships between the exposome and health endpoints. For thousands of features detected in the exposomic analyses,3 advanced statistical tools should be the primary driver for associating the exposome with human health. Multiple dimension data, including external exposome, internal exposome, and health outcomes, should be integrated and associated. In addition, the interactions of exposome factors such as chemical-chemical, chemical-microbe, and chemical-behavior factors should be taken into consideration.3 The correlation in the high-dimension data is still the key driver in associating all exposome factors. These analytical methods can go beyond traditional epidemiological methods and borrow experiences from genome-wide association studies (GWAS) associating huge genetic variants with a specific trait. For example, an analytical framework for multivariate mediation analysis was introduced to identify mediation pathways of 61 mediators in the relationship between 38 environmental toxicants (phthalates, phenols, polycyclic aromatic hydrocarbons, and metals) and gestational age at delivery.9 More method development and standardization are needed. Additionally, for exposomic studies, macro-scale, multi-factor, multi-indicator, and long-term considerations are usually adopted to coordinate the enormous challenges of human health, which is easy to criticize, because the causal contributions of some screened variables cannot be validated efficiently, especially distinguishing the confounding effects. Therefore, theoretical and experimental validation to confirm causal correlations is also necessary. For example, computational toxicity tools and in vitro or in vivo screening can be used to estimate potential risk at the exposome level by studying the effect of low-level environmental mixtures.10
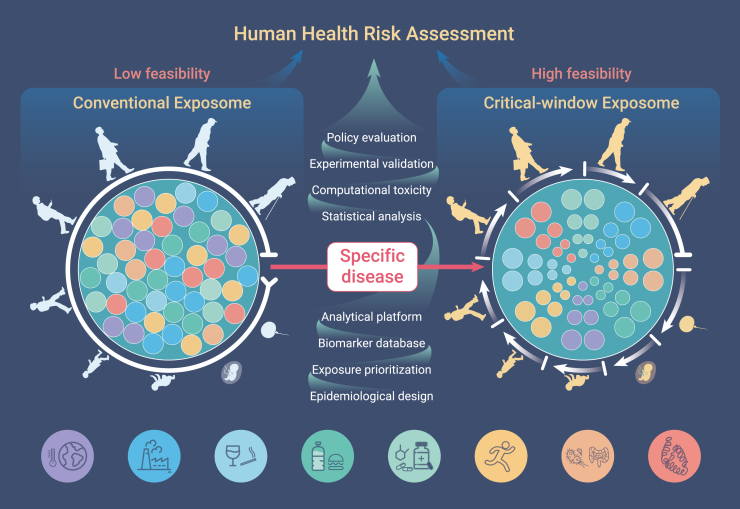
In conclusion, exposomics has great application potential for studies with one or multiple health endpoints with complex or unknown causes and can help find a critical exposure window for policy intervention. Although exposomic studies are still in their infancy with many unknowns, they are highly expected to start booming in the historical context of human health. However, it is still impossible to accomplish a lifelong exposome of the total environment at present, and the era of data-driven study is still far away, which seems to be overly emphasized in the current definitions.1,2 To increase its operability and reduce public confusion, we prefer to propose that (1) the current exposomic studies should focus more on the causal relations occurring during the critical time window or any suitable health outcome that can be investigated with a feasible/acceptable time window; (2) the multi-factor hypothesis-driven exposomic studies should be the mainstream to address human health issues; and (3) more standardizations of exposomic study and high-dimensional data analysis or visualization are urgently needed, which can only be completed with active collaboration between epidemiologists, biologists, clinicians, statisticians, chemists, economists, and sociologists. Supported by advanced monitoring technology of exposomics to overcome various barriers, human health assessment can evolve from the conventional health risk assessment to its next generation (Figure 1). In this way, we believe that the exposome is one of the best ways to build a “wonderland” for human health.
Figure 1.
Critical-window exposome with prioritized exposure factors increases the feasibility of the conventional exposome in human health risk assessment.
The critical window can be defined by the development of one specific disease. The risk importance of exposome factors from both exogenous and endogenous processes for the specific disease should be meta-analyzed and prioritized. To achieve this goal, appropriate epidemiological design, followed by exposure prioritization, biomarker databases, analytical platforms, statistical analysis, computational toxicity, experimental validation, and even policy evaluation, needs to be further standardized.
Acknowledgments
We would like to express our gratitude to organizers of the Exposome Forum of China Reproductive Health Forum (Dr. Zhiwen Li, Dr. Hongtian Li, Dr. Le Zhang, Dr. Tao Xue, and Mrs. Zhaoxia Xiong), Mrs. Xueling Meng for optimizing Figure 1, Special Committee of Environment and Reproductive Health of Chinese Environmental Mutagen Society, the working group of environmental exposure and human health of the China Cohort Consortium (http://chinacohort.bjmu.edu.cn/).
Declaration of interests
The authors declare no competing interests.
Published Online: October 6, 2021
Contributor Information
Jicheng Gong, Email: jicheng.gong@pku.edu.cn.
Bin Wang, Email: binwangpku@foxmail.com.
References
- 1.Wild C.P. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers. Prev. 2009;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 2.Miller G.W., Jones D.P. The nature of nurture: refining the definition of the exposome. Toxicol. Sci. 2014;137:1–2. doi: 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen R., Schymanski E.L., Barabasi A.L., et al. The exposome and health: where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escher B., Stapleton H., Schymanski E. Tracking complex mixtures of chemicals in our changing environment. Science. 2020;367:388–392. doi: 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang C., Wang X., Li X.Y., et al. Dynamic human environmental exposome revealed by longitudinal personal monitoring. Cell. 2018;175:277–291. doi: 10.1016/j.cell.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao F., Li L., Chen Y., et al. Risk-based chemical ranking and generating a prioritized human exposome database. Environ. Health Perspect. 2021;129:47014. doi: 10.1289/EHP7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di N., Li S., Xiang H., et al. Associations of residential greenness with depression and anxiety in rural Chinese adults. Innovation. 2020;1:100054. doi: 10.1016/j.xinn.2020.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia P., Lakerveld J., Wu J., et al. Top 10 research priorities in spatial lifecourse epidemiology. Environ. Health Perspect. 2019;127:74501. doi: 10.1289/EHP4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aung M., Song Y., Ferguson K., et al. Application of an analytical framework for multivariate mediation analysis of environmental data. Nat. Commun. 2020;11:5624. doi: 10.1038/s41467-020-19335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M., Jia S., Dong T., et al. Metabolomic and transcriptomic analysis of MCF-7 cells exposed to 23 chemicals at human-relevant levels: estimation of individual chemical contribution to effects. Environ. Health Perspect. 2020;128:127008. doi: 10.1289/EHP6641. [DOI] [PMC free article] [PubMed] [Google Scholar]