Abstract
Background
Oncoplastic breast‐conserving surgery (O‐BCS) involves removing the tumour in the breast and using plastic surgery techniques to reconstruct the breast. The adequacy of published evidence on the safety and efficacy of O‐BCS for the treatment of breast cancer compared to other surgical options for breast cancer is still debatable. It is estimated that the local recurrence rate is similar to standard breast‐conserving surgery (S‐BCS) and also mastectomy, but the aesthetic and patient‐reported outcomes may be improved with oncoplastic techniques.
Objectives
Our primary objective was to assess oncological control outcomes following O‐BCS compared with other surgical options for women with breast cancer. Our secondary objective was to assess surgical complications, recall rates, need for further surgery to achieve adequate oncological resection, patient satisfaction through patient‐reported outcomes, and cosmetic outcomes through objective measures or clinician‐reported outcomes.
Search methods
We searched the Cochrane Breast Cancer Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (via OVID), Embase (via OVID), the World Health Organization's International Clinical Trials Registry Platform and ClinicalTrials.gov on 7 August 2020. We did not apply any language restrictions.
Selection criteria
We selected randomised controlled trials (RCTs) and non‐randomised comparative studies (cohort and case‐control studies). Studies evaluated any O‐BCS technique, including volume displacement techniques and partial breast volume replacement techniques compared to any other surgical treatment (partial resection or mastectomy) for the treatment of breast cancer.
Data collection and analysis
Four review authors performed data extraction and resolved disagreements. We used ROBINS‐I to assess the risk of bias by outcome. We performed descriptive data analysis and meta‐analysis and evaluated the quality of the evidence using GRADE criteria. The outcomes included local recurrence, breast cancer‐specific disease‐free survival, re‐excision rates, complications, recall rates, and patient‐reported outcome measures.
Main results
We included 78 non‐randomised cohort studies evaluating 178,813 women. Overall, we assessed the risk of bias per outcome as being at serious risk of bias due to confounding; where studies adjusted for confounding, we deemed these at moderate risk.
Comparison 1: oncoplastic breast‐conserving surgery (O‐BCS) versus standard‐BCS (S‐BCS)
The evidence in the review found that O‐BCS when compared to S‐BCS, may make little or no difference to local recurrence; either when measured as local recurrence‐free survival (hazard ratio (HR) 0.90, 95% confidence interval (CI) 0.61 to 1.34; 4 studies, 7600 participants; very low‐certainty evidence) or local recurrence rate (HR 1.33, 95% CI 0.96 to 1.83; 4 studies, 2433 participants; low‐certainty evidence), but the evidence is very uncertain due to most studies not controlling for confounding clinicopathological factors. O‐BCS compared to S‐BCS may make little to no difference to disease‐free survival (HR 1.06, 95% CI 0.89 to 1.26; 7 studies, 5532 participants; low‐certainty evidence). O‐BCS may reduce the rate of re‐excisions needed for oncological resection (risk ratio (RR) 0.76, 95% CI 0.69 to 0.85; 38 studies, 13,341 participants; very low‐certainty evidence), but the evidence is very uncertain. O‐BCS may increase the number of women who have at least one complication (RR 1.19, 95% CI 1.10 to 1.27; 20 studies, 118,005 participants; very low‐certainty evidence) and increase the recall to biopsy rate (RR 2.39, 95% CI 1.67 to 3.42; 6 studies, 715 participants; low‐certainty evidence). Meta‐analysis was not possible when assessing patient‐reported outcomes or cosmetic evaluation; in general, O‐BCS reported a similar or more favourable result, however, the evidence is very uncertain due to risk of bias in the measurement methods.
Comparison 2: oncoplastic breast‐conserving surgery (O‐BCS) versus mastectomy alone
O‐BCS may increase local recurrence‐free survival compared to mastectomy but the evidence is very uncertain (HR 0.55, 95% CI 0.34 to 0.91; 2 studies, 4713 participants; very low‐certainty evidence). The evidence is very uncertain about the effect of O‐BCS on disease‐free survival as there were only data from one study. O‐BCS may reduce complications compared to mastectomy, but the evidence is very uncertain due to high risk of bias mainly resulting from confounding (RR 0.75, 95% CI 0.67 to 0.83; 4 studies, 4839 participants; very low‐certainty evidence). Data on patient‐reported outcome measures came from single studies; it was not possible to meta‐analyse the data.
Comparison 3: oncoplastic breast‐conserving surgery (O‐BCS) versus mastectomy with reconstruction
O‐BCS may make little or no difference to local recurrence‐free survival (HR 1.37, 95% CI 0.72 to 2.62; 1 study, 3785 participants; very low‐certainty evidence) or disease‐free survival (HR 0.45, 95% CI 0.09 to 2.22; 1 study, 317 participants; very low‐certainty evidence) when compared to mastectomy with reconstruction, but the evidence is very uncertain. O‐BCS may reduce the complication rate compared to mastectomy with reconstruction (RR 0.49, 95% CI 0.45 to 0.54; 5 studies, 4973 participants; very low‐certainty evidence) but the evidence is very uncertain due to high risk of bias from confounding and inconsistency of results. The evidence is very uncertain for patient‐reported outcome measures and cosmetic evaluation.
Authors' conclusions
The evidence is very uncertain regarding oncological outcomes following O‐BCS compared to S‐BCS, though O‐BCS has not been shown to be inferior. O‐BCS may result in less need for a second re‐excision surgery but may result in more complications and a greater recall rate than S‐BCS. It seems that O‐BCS may give better patient satisfaction and surgeon rating for the look of the breast, but the evidence for this is of poor quality, and due to lack of numerical data, it was not possible to pool the results of different studies. It seems O‐BCS results in fewer complications compared with surgeries involving mastectomy.
Based on this review, no certain conclusions can be made to help inform policymakers. The surgical decision for what operation to proceed with should be made jointly between clinician and patient after an appropriate discussion about the risks and benefits of O‐BCS personalised to the patient, taking into account clinicopathological factors. This review highlighted the deficiency of well‐conducted studies to evaluate efficacy, safety and patient‐reported outcomes following O‐BCS.
Plain language summary
Oncoplastic breast‐conserving surgery (O‐BCS) for women with primary breast cancer
Background
Traditional surgery for early breast cancer is standard breast‐conserving surgery (S‐BCS) which aims to keep as much of the breast as possible. For women with large tumours compared to their breast size it can be difficult to conserve the breast whilst ensuring all the tumour is removed and may mean that mastectomy is needed. The most important part of surgical treatment for breast cancer is removing all cancer. In recent years, oncoplastic breast surgery techniques have been used to conserve the breast whilst removing breast cancer by applying the principles of plastic surgery, resulting in better cosmetic results. Oncoplastic breast‐conserving surgery (O‐BCS) may also result in better patient satisfaction and quality of life.
Traditionally, surgeons have either preserved the breast tissue by removing the cancerous lump (S‐BCS) or reconstructing immediately after mastectomy. O‐BCS involves removing cancer and either moving/adjusting the remaining breast tissue around (volume displacement) or bringing in tissue from elsewhere to fill the defect after breast cancer removal (volume replacement). There are many techniques that fall under O‐BCS that we have listed in full in other parts of the review; however, all are similar in their principle.
Review question
We reviewed the evidence about the effects of O‐BCS (that is, removing some of the breast tissue and then reconstructing the remaining breast by either mobilising the breast tissue (mammaplasty or volume displacement) or bringing the tissue from elsewhere (partial breast reconstruction or volume replacement)) compared to other S‐BCS (that is, removing the tumour in the breast without the need for further breast adjustment) or mastectomy (that is, removing all the breast tissue with or without reconstruction). We studied the effect on cancer‐related (local recurrence, disease‐free survival and overall survival), quality of life and cosmetic outcomes in women with breast cancer.
Study characteristics
The evidence is current to August 2020. We included 78 studies involving 178,813 patients with breast cancer. We split the studies into those that compared O‐BCS to S‐BCS, O‐BCS to mastectomy alone and O‐BCS to mastectomy with reconstruction. Some studies contributed to more than one comparison.
Key results
It seemed that O‐BCS resulted in similar rates of local recurrence (that is, whether cancer returned in the same breast) and disease‐free survival (free of any breast cancer after initial treatment) when compared to S‐BCS, and resulted in less need for a second re‐excision surgery (which may be required if the tumour is not fully removed in the first operation). O‐BCS may result in more complications and more biopsies in the years after the surgery compared to S‐BCS. It seems that O‐BCS may give better patient satisfaction and surgeon rating for the look of the breast, but the evidence for this is of poor quality, and due to lack of numerical data, it was not possible to pool the results of different studies.
It was not possible to conclude whether or not cancer outcomes of local recurrence and disease‐free survival for O‐BCS were similar to mastectomy with or without reconstruction as there were not many good‐quality studies. It seems O‐BCS has fewer complications than surgeries involving mastectomy.
In practice, the decision to select O‐BCS should be done through shared decision making with the surgeon, discussing the potential risks and benefits.
Certainty of evidence
The certainty of the evidence in this review was very low. The studies had several methodological flaws. Differences between groups in cancer stage and other cancer treatments that were used may have affected the results. This is likely to have an impact on the findings, and future research is needed to investigate the topic further.
Summary of findings
Background
Description of the condition
Breast cancer is the most commonly diagnosed cancer in women worldwide (Bray 2018). Globally, incidence rates are increasing but mortality rates are decreasing with improved treatments, leaving many more breast cancer survivors (WHO 2010). In the UK an estimated 691,000 women are alive after a diagnosis of breast cancer, and this is predicted to rise to 840,000 women in 2020 (Breast Cancer Care 2020). There are over 3.8 million breast cancer survivors in the USA, including those who have finished treatment or are in the process of receiving treatment (BCRF 2019).
For the majority of women with primary breast cancer, the first treatment is breast surgery with curative intent (Breast Cancer Care 2020). As survival improves following breast cancer treatment, it has become imperative to improve quality of life, and long‐term appearance and aesthetic outcomes after surgery have become increasingly relevant.
Description of the intervention
Surgery for breast cancer has evolved considerably over the years, from the radical mastectomy of Halsted 1894 to the development and acceptance of breast‐conserving therapy as standard of care in recent years. Breast‐conserving surgery (BCS) usually refers to lumpectomy or wide local excision (WLE). BCS followed by radiotherapy has been found to be equivalent in disease‐free and overall survival when compared with mastectomy, and hence has become the standard of care for early‐stage breast cancer (Agarwal 2014; Fisher 2002; Van Maaren 2016; Vila 2015). A WLE may be difficult for patients with a large tumour‐to‐breast‐size ratio, resulting in poor cosmetic outcomes or patients may opt for a simple mastectomy (that is, the removal of the breast tissue up to the chest wall) (Regano 2009). There is large variation across countries in the rates of BCS (Munzone 2014; Sun 2018).
The primary goal of oncological surgery is cancer resection; that is, where the tumour, along with a margin of normal tissue is excised. There is also, however, increasing awareness that aesthetic outcomes of these procedures are extremely important. Patient expectations are increasing as they become aware that they need not be left with deformities after breast cancer surgery. Good aesthetic outcomes have been linked with significant improvements in patient satisfaction and quality of life (Kim 2015; Waljee 2008).
There are many breast reconstruction options for aesthetic improvement. Women being offered a mastectomy have the option of full breast reconstruction, using either implants or their own (autologous) tissue. Breast reconstruction can be done at the same time as the mastectomy (one‐stage) or as a separate operation (two‐stage). For women undergoing BCS for large tumours, the options include either volume displacement or partial volume replacement techniques using either implants or autologous tissue (ACS 2016).
Oncoplastic breast‐conserving surgery (O‐BCS) is the term used for oncological resection (breast tumour excisions) combined with plastic surgery techniques (Almasad 2008; Clough 2003; Rainsbury 2007; Regano 2009). O‐BCS can be broadly divided into the two fundamentally different techniques: 1) volume displacement techniques use breast tissue (glandular or dermoglandular) from the same breast and places (redistributes) it into the surgical site (also known as mammoplasty); and 2) volume replacement techniques use tissue, other than the breast, to compensate for volume loss after breast tumours have been excised.
The uniting principle of these two techniques is to conserve the breast shape/size.
Volume displacement techniques can include various techniques (Holmes 2011), for example:
wise pattern therapeutic mammaplasty;
vertical scar mammaplasty (and its variations);
circumareolar/Benelli’s/round block mammoplasty;
racquet handle/lateral mammaplasty.
Similarly, there are many techniques for autologous partial volume replacement techniques. The following techniques are recognised as partial volume replacement techniques, where the differentiating factor between which flap is used is usually the location of the tumour.
-
Defects in the lower aspects of the breast can be addressed using local flaps such as:
abdominal adipofascial flaps (Kijima 2014; Ogawa 2007);
-
thoracoepigastric flaps (Hamdi 2014; Kijima 2011; Takeda 2005);
superior epigastric artery perforator flap;
medial intercostal artery perforator;
internal mammary artery perforator;
anterior intercostal artery perforator.
-
Defects in the lateral half of the breast can be reconstituted with lateral chest wall perforator flaps such as:
lateral intercostal artery perforator (Hamdi 2006; Hu 2018);
lateral thoracic artery perforator (McCulley 2015);
thoracodorsal artery perforator flap (Munhoz 2011).
-
Defects in any breast quadrant can be addressed using distant flaps. Most often these are pedicled flaps, but free flaps could also be used for partial breast reconstruction such as:
mini‐latissimus dorsi (Raja 1997);
omental flaps (Zaha 2014);
other free flaps for partial breast reconstruction e.g. transverse upper gracilis flaps (McCulley 2011).
Many early‐stage breast cancers can be successfully treated by WLE; however, the lesions with large tumour‐to‐breast‐size ratio remain a challenge for breast surgeons to treat with BCS alone. O‐BCS allows the excision of tumours that cannot be excised by, or would result in poor cosmetic outcomes from S‐BCS. It allows these women to avoid mastectomy.
In this Cochrane Review, we will compare any O‐BCS technique to other surgical techniques used for BCS because any of the aforementioned techniques may be offered to women with breast cancer under varying circumstances. For small cancers, it is likely that WLE with or without partial reconstruction (using either autologous tissue or an implant) will be offered. In contrast, for large cancers, the options could include WLE with or without partial reconstruction; or mastectomy with or without reconstruction.
How the intervention might work
For women with early‐stage breast cancer, studies have shown that there is no detectable difference in overall survival or disease‐free survival in those who have BCS plus radiotherapy and those who have a mastectomy (Poggi 2003; Van Maaren 2016). There has been increased adoption of the practice in many countries to facilitate breast‐conserving therapy and avoid unnecessary mastectomies (Kaufman 2019). The emphasis remains on safe and adequate cancer resection, whilst aiming to achieve better aesthetic outcomes to improve quality of life.
There is evidence indicating that cosmesis, patient satisfaction and quality of life improve with BCS compared to mastectomy (Kim 2015; Waljee 2008). The options for surgical resection for breast cancer are dictated by the size of the tumour. There is an indirect correlation between the percentage of breast volume excised and cosmesis, which can have an impact on the satisfaction levels after BCS (Cochrane 2003). O‐BCS techniques aim to keep the breast shape and size similar despite oncological resection; therefore it would be logical to expect better patient satisfaction.
Why it is important to do this review
Although oncoplastic surgery has rapidly gained acceptance and is widely practised, cohesive evidence is still lacking on both the short‐term and long‐term outcomes, particularly for partial breast reconstruction.
Since the most recent systematic review of oncoplastic breast surgery concluded its search in 2015 (Yiannakopoulou 2016), there have been over 30 articles published regarding partial breast reconstruction. A summary of evidence from this literature will help clinicians understand the indications and clinical, oncological and cosmetic outcomes of such techniques. This Cochrane Review will update our understanding of this rapidly evolving area of clinical practice and address the questions unexplored by previous reviews. In addition, this review will focus on volume displacement and replacement techniques as separate subsets of O‐BCS, and compare these techniques with other alternatives.
Objectives
Our primary objective was to assess oncological control outcomes following O‐BCS compared with other surgical options for women with breast cancer. Our secondary objective was to assess surgical complications, recall rates, need for further surgery to achieve adequate oncological resection, patient satisfaction through patient‐reported outcomes, and cosmetic outcomes through objective measures or clinician‐reported outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all randomised controlled trials (RCTs) assessing oncoplastic breast‐conserving surgery (O‐BCS) but anticipated that there would be no RCTs on the topic. We, therefore, expanded the inclusion criteria to include comparative non‐randomised studies (i.e. cohort studies, case‐control studies and prospectively designed patient registries).
We included studies published in all languages from 1980 onwards as this is the date at which partial breast reconstruction was introduced.
We excluded single‐arm studies, expert opinion and duplicate studies.
Types of participants
We included women with primary breast cancer who underwent any O‐BCS using either volume displacement or partial replacement breast reconstruction for cancer compared with women who underwent any other surgical technique for cancer.
We excluded men and people who have undergone surgery for benign breast conditions.
Types of interventions
Experimental interventions
Any oncoplastic breast‐conserving surgery techniques including:
-
volume displacement techniques
wise pattern therapeutic mammaplasty
vertical scar mammaplasty (and its variations)
circumareolar/Benelli’s/Round block mammoplasty
racquet handle/lateral mammaplasty
-
partial volume replacement techniques
abdominal adipofascial flaps/advancement flaps
-
lateral chest wall perforator flaps
lateral intercostal artery perforator flap
lateral thoracic artery perforator
thoracodorsal artery perforator flap
latissimus dorsi mini‐flap
-
thoracoepigastric flaps
superior epigastric artery perforator flap
medial intercostal artery perforator
internal mammary artery perforator
anterior intercostal artery perforator
omental flaps
free flaps for partial breast reconstruction
We included any other techniques if mentioned in the literature.
Comparator interventions
Any other surgical treatment. The comparators were stratified into partial resection and mastectomy. These include:
standard breast‐conserving surgery (S‐BCS) e.g. wide local excision (WLE), quadrantectomy, segmentectomy, partial mastectomy;
partial volume replacement using non‐autologous tissue;
mastectomy with no reconstruction;
mastectomy with breast reconstruction using an implant alone;
mastectomy with breast reconstruction using autologous tissue including pedicled and free flaps.
The main analyses were:
any O‐BCS versus S‐BCS
any O‐BCS versus mastectomy without reconstruction
any O‐BCS versus mastectomy with reconstruction procedures
Co‐interventions
We recognised that some women with breast cancer may also undergo hormonal therapy, chemotherapy or radiotherapy, or a combination of therapies. We collected data on whether patients received these co‐interventions; we did not, however, conduct a subgroup analysis as no study reported outcomes based on these. This information can be found in Table 4, which describes confounding variables; differences in these co‐interventions informed the risk of bias for each study.
1. Confounding variables.
| Study name | Clinicopathological variables: significantly different | Clinicopathological variables: demonstrated balance | Clinicopathological variables: matched | Clinicopathological variables: statistical adjustment | Co‐interventions: significantly different | Co‐interventions: demonstrated balance | Co‐interventions: matched | Co‐interventions: statistical adjustment |
| Acea‐Nebril 2005 | Size (BCS) | Age (BCS, Mx), size (Mx) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Acea‐Nebril 2017 | Age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality | BMI, histological type, immunohistochemical receptors | ‐ | ‐ | Neoadjuvant CT, axillary management | ‐ | ‐ | ‐ |
| Acosta‐Marin 2014 | Preoperative bra size, tumour size, | Age, BMI | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Amitai 2018 | Age, axillary node status, immunohistochemical receptors, | Smoking status, BMI, histological type, tumour size | ‐ | ‐ | ‐ | Adjuvant RT | ‐ | ‐ |
| Angarita 2020 | Age, BMI, race, smoking status, alcohol consumption, COPD, PCI, HTN, bleeding disorder, steroid use, previous vascular disease, previous cardiac surgery, dialysis,hemiplegia, TIA, CVA, ASA status, histological type | Weight loss, transfusion, diabetes mellitus | ‐ | ‐ | Axillary management, neoadjuvant chemotherapy, anaesthetic technique | ‐ | ‐ | ‐ |
| Atallah 2015 | Age, BMI, menopausal status, tumour size, location, histological type, immunohistochemical receptors | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Bali 2018 | Tumour size | Age, histological type, immunohistochemical receptors, tumour locations | ‐ | ‐ | Neoadjuvant CT, adjuvant CT | Adjuvant RT | ‐ | ‐ |
| Borm 2019 | Age, tumour size, tumour grade, axillary node status, immunohistochemical receptors (ER status), | Immunohistochemical receptors (PR, HER2) | ‐ | ‐ | Adjuvant CT, adjuvant ET | Neo‐adjuvant CT, adjuvant RT | ‐ | ‐ |
| Carter 2016 | Age (BCS, Mx, Mx+R), BMI (BCS), tumour size (BCS, Mx, Mx+R), tumour stage (BCS, Mx, Mx+R), tumour grade (BCS), axillary node status (BCS), immunohistochemical receptors (HER2), multifocality (BCS, Mx, Mx+R). | BMI (Mx, Mx+R), Tumour grade (Mx, Mx+R), axillary node status (Mx, Mx+R), immunohistochemical receptors (ER, PR‐ Mx), lymphovascular invasion | In LR calculation multivariate analysis | ‐ | Neoadjuvant CT (BCS, Mx, Mx+R), adjuvant RT (Mx/MxR), adjuvant CT (BCS, Mx, Mx+R) | Adjuvant RT (BCS) | ‐ | ‐ |
| Cassi 2016 | ‐ | Age, BMI, tumour size | ‐ | ‐ | ‐ | Adjuvant RT | ‐ | ‐ |
| Chakravorty 2012 | Histological type, tumour size, grade, sample weight | Age, axillary node status | ‐ | ‐ | Neoadjuvant CT | Adjuvant RT, adjuvant CT | ‐ | ‐ |
| Chauhan 2016 (1) | Age, tumour size, tumour location | Histological type, grade, axillary node status, immunohistochemical receptors | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chauhan 2016 (2) | Age, tumour size, tumour location | Axillary node status | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Crown 2015 | Tumour size, immunohistochemical receptors | Age, histological type | ‐ | ‐ | ‐ | Adjuvant RT | ‐ | ‐ |
| Crown 2019 | Tumour size, immunohistochemical receptors | Age, smoking, BMI, histological type | ‐ | ‐ | Neoadjuvant CT | Adjuvant CT | ‐ | ‐ |
| DeLorenzi 2016 (1) | Tumour size and multifocality | Menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion | Age (within 5 years), year of surgery (within 2 years), tumour size (T) and multifocality | Adjuvant CT, Adjuvant RT, Adjuvant ET | ‐ | ‐ | ||
| DeLorenzi 2016 (2) | Multifocality | Grade, immunohistochemical receptors | Age (within 5 years), year of surgery (within 2 years), number of positive axillary lymph nodes, tumour subtype, multifocality | Adjuvant RT | Adjuvant CT, Adjuvant ET | ‐ | ‐ | |
| DeLorenzi 2018 | Menopausal, grade | Age, BMI, tumour size, immunohistochemical receptors, multifocality | ‐ | ‐ | ‐ | Adjuvant RT, any adjuvant therapy | ‐ | ‐ |
| Di Micco 2017 | Age, axillary node status | Smoking status, BMI, histological type, tumour size, immunohistochemical receptor, tumour location | Radiation boost, adjuvant CT | Neoadjuvant CT, adjuvant ET, axillary management, adjuvant RT | ||||
| Dolan 2015 | Age, tumour size, axillary node status | Histological type, grade, immunohistochemical receptor | ‐ | ‐ | Adjuvant CT | Adjuvant RT, adjuvant ET, axillary management | ‐ | ‐ |
| Down 2013 | Tumour size | Age, histological type, grade | ‐ | ‐ | ‐ | Adjuvant RT | ‐ | ‐ |
| Eichler 2013 | Tumour size | Age, histological type, grade | ‐ | ‐ | Neoadjuvant CT | Adjuvant CT | ‐ | ‐ |
| Fan 2019 | ‐ | Histological type | Age, BMI, stage | ‐ | ‐ | Neoadjuvant CT, adjuvant RT, adjuvant CT, adjuvant ET | ‐ | |
| Farooqi 2019 | Tumour size, | Age, histological type | ‐ | ‐ | ‐ | Neoadjuvant CT | ‐ | ‐ |
| Gendy 2003 | Histological type, tumour size | Age, grade, axillary node status | ‐ | ‐ | Adjuvant RT | ‐ | ‐ | ‐ |
| Gicalone 2007 (1) | Age | BMI, histological type, tumour size, grade, axillary node status, immunohistochemical receptor | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gicalone 2007 (2) | Age | BMI, tumour size, tumour location | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Gicalone 2015 | ‐ | Age, smoking status, diabetes, BMI, other medical comorbidities, histological type, tumour size | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Gulcelik 2013 | ‐ | Age, tumour size, immunohistochemical receptor | ‐ | ‐ | ‐ | Adjuvant CT, Adjuvant ET, axillary management, adjuvant RT | ||
| Hamdi 2008 | Age, histological type, tumour size, | ‐ | ‐ | ‐ | ‐ | Axillary management | ‐ | ‐ |
| Hart 2015 | Age, BMI | Stage | ‐ | ‐ | Adjuvant RT | ‐ | ‐ | ‐ |
| Hashimoto 2019 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hilli‐Betz 2014 | Tumour size, preoperative bra size | Axillary node status | ‐ | ‐ | ‐ | Axillary management | ‐ | ‐ |
| Hu 2019 | ‐ | Age, tumour size, immunohistochemical receptor | ‐ | ‐ | ‐ | Neoadjuvant CT, axillary management | ‐ | ‐ |
| Jiang 2015 | ‐ | Age, weight, histology type,tumour size, grade, stage, tumour location | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kahn 2013 | ‐ | ‐ | ‐ | ‐ | ‐ | Adjuvant CT (BCS, Mx, Mx+R) | ‐ | ‐ |
| Keleman 2019 | Preoperative bra size, axillary node status | Age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor | ‐ | ‐ | Neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management | Adjuvant RT | ‐ | ‐ |
| Kelsall 2017 | ‐ | Axillary node status | Age, tumour size, date of surgery, breast size | ‐ | Adjuvant RT | Neoadjuvant CT | Adjuvant CT, adjuvant ET | ‐ |
| Kimball 2018 | Age, medical comorbidities, histological type | BMI | ‐ | ‐ | Adjuvant RT, adjuvant CT, axillary management | ‐ | ‐ | |
| Klit 2017 | Age (BCS, Mx), BMI (BCS, Mx), tumour size (BCS, Mx), axillary node status (BCS, Mx) | ‐ | ‐ | ‐ | Axillary management, Adjuvant RT (BCS) | Adjuvant CT (BCS, Mx), Adjuvant RT (Mx) | ‐ | ‐ |
| Lansu 2014 | ‐ | Age, tumour size, tumour location | ‐ | ‐ | Neoadjuvant CT | Adjuvant CT, Adjuvant ET, axillary management, adjuvant RT | ‐ | |
| Lee 2018 | Tumour size (BCS, Mx, Mx+R), stage (BCS, Mx, Mx+R) | Age (BCS, Mx, Mx+R), BMI (BCS, Mx, Mx+R) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Losken 2009 | Age, histological type, stage | BMI | ‐ | ‐ | Adjuvant CT, axillary surgery | Adjuvant RT | ‐ | ‐ |
| Losken 2014 | Age, BMI | Histological type, tumour size, stage, immunohistochemical receptor | ‐ | ‐ | Neoadjuvant CT | ‐ | ‐ | |
| Malhaire 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mansell 2015 | Age (both), histological type (BCS), tumour size (BCS), grade (BCS), axillary node status (BCS), immunohistochemical receptor (ER, PR) | Histological type (Mx), tumour size (Mx), grade (Mx), axillary node status (Mx), immunohistochemical receptor (HER2) | ‐ | ‐ | Adjuvant RT (MxR), adjuvant CT (BCS), adjuvant ET (BCS) | Adjuvant RT (BCS), adjuvant CT (MxR), adjuvant ET (MxR) | ‐ | ‐ |
| Mansell 2017 | Age (both), histological type (BCS), tumour size (BCS), grade (BCS), axillary node status (BCS), immunohistochemical receptor (ER) | Histological type (Mx), tumour size (Mx), grade (Mx), axillary node status (Mx), immunohistochemical receptor (HER2) | ‐ | ‐ | Adjuvant RT (MxR), adjuvant CT (BCS), adjuvant ET (BCS) | Adjuvant RT (BCS), adjuvant CT (MxR), adjuvant ET (MxR) | ‐ | ‐ |
| Matrai 2014 | Tumour size | Age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status | Matched of clinicopathological factors ‐ details not given | ‐ | Adjuvant CT | Axillary surgery, adjuvant RT, adjuvant ET | ‐ | ‐ |
| Mazouni 2013 | Immunohistochemical receptor (ER), tumour location | Histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR) | ‐ | ‐ | ‐ | Axillary surgery, neoadjuvant CT, adjuvant RT | ‐ | ‐ |
| Morrow 2019 | Age (all), histological type (BCS, Mx), tumour size (BCS, Mx), grade (BCS, Mx), axillary node status (Mx, MxIR) | Histological type (MxIR), tumour size (MxIR), grade (MxIR), axillary node status (BCS), immunohistochemical receptors | ‐ | ‐ | Adjuvant CT (BCS), Adjuvant RT (all) | Adjuvant CT (Mx, MxIR), adjuvant ET (Mx, MxIR) | ‐ | ‐ |
| Mukhtar 2018 | Tumour size | "No significant difference in patient or tumour characteristics" | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mustonen 2004 | Tumour size | Age | ‐ | ‐ | Adjuvant, RT | Adjuvant CT | ‐ | ‐ |
| Nakada 2019 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nakagomi 2019 | Age, tumour size, stage | Histological type, axillary node status, immunohistochemical receptor status | ‐ | ‐ | Neoadjuvant CT | ‐ | ‐ | ‐ |
| Niinikoski 2019 (2) | Age, tumour size, grade, axillary node status, immunohistochemical status (ER, TN), multifocality, | Histological type, immunohistochemical receptor status (PR, HER2) | ‐ | ‐ | Adjuvant CT, adjuvant ET | Adjuvant RT, axillary management | ‐ | ‐ |
| Ojala 2017 | Tumour size, tumour location, axillary node status, multifocality, histological type, | Age, grade | ‐ | ‐ | Axillary management | Adjuvant RT | ‐ | ‐ |
| Ozmen 2016 | Age, BMI, multifocality | ‐ | ‐ | ‐ | ‐ | Adjuvant RT | ‐ | ‐ |
| Ozmen 2020 | Age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality | histological type, immunohistochemical receptor status (PR, HER2, TN) | ‐ | ‐ | Adjuvant RT, axillary management | ‐ | ‐ | ‐ |
| Palsodittlir 2018 | Age, smoking status, tumour size | Histological type, axillary node status | ‐ | ‐ | Adjuvant ET | ‐ | ‐ | ‐ |
| Peled 2014 | Age, BMI | Smoking status, diabetes | ‐ | ‐ | ‐ | Neoadjuvant CT | ‐ | ‐ |
| Piper 2016 | Tumour stage | BMI, histological type | Age | ‐ | ‐ | ‐ | ‐ | ‐ |
| PlaFarnos 2018 | Multifocality | ‐ | ‐ | ‐ | Previous breast surgery | ‐ | ‐ | ‐ |
| Potter 2020 | Age (Mx, Mx+R), diabetes (Mx, Mx+R), BMI (Mx, Mx+R), other medical comorbidities ( Mx, Mx+R), histological type (Mx, Mx+R), grade (Mx, Mx+R), axillary node status (Mx, Mx+R), immunohistochemical receptors (BCS, Mx, Mx+R), multifocality (BCS, Mx, Mx+R) | Smoking status (Mx, Mx+R) | ‐ | ‐ | Neoadjuvant CT (Mx, Mx+R), adjuvant RT (Mx, Mx+R), adjuvant CT (Mx, Mx+R), axillary surgery (Mx, Mx+R) | ‐ | ‐ | ‐ |
| Ren 2014 | ‐ | Histological type, tumour location | Age, tumour size, axillary lymph node status, immunohistochemical receptor, (ER, HER2) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Rose 2019 | Menopausal status, axillary node status | ‐ | ‐ | Age, lymphovascular invasion, grade, tumour size, multifocality, immunohistochemical receptor (ER, HER2) | Axillary surgery | Adjuvant RT, adjuvant CT, adjuvant ET | ‐ | ‐ |
| Rose 2020 | ‐ | ‐ | ‐ | Age, follow‐up time, menopausal status, tumour size, bra size, tumour location, bra size, BMI, smoking status, marital status, living arrangement and education | ‐ | ‐ | ‐ | Adjuvant RT, adjuvant CT, adjuvant ET, immunotherapy, axillary surgery |
| Santos 2015 | BMI, histological type, axillary node status, | Age, menopausal status, tumour size, grade, immunohistochemical receptor, tumour location | ‐ | ‐ | ‐ | Axillary surgery | ‐ | ‐ |
| Scheter 2019 | Age, smoking status, tumour size, , | Preoperative bra size, axillary lymph node status | Diabetes, BMI | ‐ | ‐ | Axillary surgery, adjuvant CT, adjuvant ET, adjuvant RT | ‐ | ‐ |
| Sherwell‐Cabello 2006 | Tumour size | Age, other comorbidities, axillary node status, tumour location | ‐ | ‐ | Neoadjuvant CT | ‐ | ‐ | ‐ |
| Srivastava 2018 | ‐ | ‐ | Margin of excision | ‐ | ‐ | ‐ | ‐ | ‐ |
| Tang 2016 | Age, BMI, tumour size, stage | ‐ | ‐ | ‐ | Axillary management | ‐ | ‐ | |
| Tenofsky 2014 | ‐ | Age, BMI, tumour size | ‐ | ‐ | Adjuvant RT | ‐ | ‐ | ‐ |
| Tong 2016 | Age, diabetes, BMI, other comorbidities, preoperative bra size | Smoking status, tumour size, stage | ‐ | ‐ | Neoadjuvant RT, adjuvant RT | Neoadjuvant CT, adjuvant CT | ‐ | ‐ |
| Viega 2010 | ‐ | Age, BMI, tumour location | ‐ | ‐ | ‐ | Adjuvant RT, adjuvant CT | ‐ | ‐ |
| Viega 2011 | ‐ | Age, BMI, tumour size, tumour location | ‐ | ‐ | ‐ | Adjuvant RT, adjuvant CT, axillary management | ‐ | ‐ |
| Vieira 2016 | ‐ | Age, histological type, stage, immunohistochemical receptor | Tumour size, grade | ‐ | ‐ | Neoadjuvant CT, adjuvant RT | ‐ | ‐ |
| Wijgman 2017 | Tumour size, tumour location | Age, menopausal status, smoking, diabetes, BMI, other medical comorbidities, histological type, sample volume resected, sample weight resected | ‐ | ‐ | Adjuvant CT, adjuvant ET | Neoadjuvant CT, adjuvant RT, axillary surgery | ‐ | ‐ |
| Wong 2017 | Tumour size | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zhou 2019 | Tumour size | Age, BMI, preoperative bra size, histological type, tumour location, multifocality, axillary node status | ‐ | ‐ | ‐ | Adjuvant RT, axillary management | ‐ | ‐ |
BCS: breast‐conserving surgery BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy HER2: human epidermal growth factor receptor 2 IR: immediate reconstruction Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy TN: triple negative
Types of outcome measures
Primary outcomes
The primary outcomes focused on oncological control by O‐BCS by assessing the following.
Local recurrence: locoregional recurrence (that is, ipsilateral breast tumour recurrence), defined as cancer detected in the same breast where cancer had been diagnosed. Some studies reported this as local recurrence‐free survival ‐ defined as the time from the date of treatment to the first date of local relapse.
Disease‐free survival: breast cancer‐specific disease‐free survival, defined as the time from the date of completing initial treatment (that is, completing the surgical procedure) to the first date of a local, regional, or distant relapse, diagnosis of a second primary breast cancer, or death due to this.
Overall survival: overall survival, defined as the time from the date of treatment to death from any cause, or number of deaths from any cause.
Follow‐up was described as 1 year, 1 to 5 years, 5 years, and 10 years if reported as dichotomous outcomes; or longest reported follow‐up if hazard ratios were reported.
Secondary outcomes
The secondary outcomes focused on oncological, surgical and cosmetic outcomes by assessing the following.
Re‐excision rates: need for further breast surgery due to inadequate cancer resection (for example, re‐excision for further margin resection or completion mastectomy).
Complications: surgical complications, for example, flap necrosis, infection, wound dehiscence and any other complications reported in the literature.
Recall rates: defined as abnormal surveillance on mammogram resulting in additional imaging or biopsy.
Time to adjuvant therapy: time in days from surgery to initiation of adjuvant chemotherapy and/or radiotherapy.
Patient‐reported outcome measures: such as patient satisfaction, that derive from validated questionnaires (for example, Breast‐Q; Cohen 2016).
Cosmetic evaluation: surgeon‐reported cosmetic outcomes that derive from subjective or objective validated scales (for example, the Harris scale and Breast Analyzing Tool; Harris 1979; Krois 2017).
Search methods for identification of studies
Electronic searches
We searched the following databases on 7 August 2020.
The Cochrane Breast Cancer's Specialised Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined on the Group's website (breastcancer.cochrane.org/sites/breastcancer.cochrane.org/files/public/uploads/specialised_register_details.pdf). We extracted trials with the following key words and considered them for inclusion in the review: abdominal adipofascial flaps, lateral chest wall perforator flaps, lateral intercostal artery perforator flap, latissimus dorsi mini‐flap, omental flaps, thoracoepigastric flaps, superior epigastric artery perforator flap, medical intercostal artery perforator, internal mammary artery perforator, anterior intercostal artery perforator, advancement/random pattern or rotation flaps, free flaps for partial breast reconstruction, breast‐conserving surgery, oncoplastic breast surgery, partial volume replacement breast, partial breast reconstruction and partial mastectomy. We will search for papers including women with breast cancer who are undergoing any kind of oncoplastic breast‐conserving surgery, as it is often the case for breast‐conserving surgeries to be grouped together.
CENTRAL (in the Cochrane Library, August 2020). See Appendix 1.
MEDLINE (via Ovid SP) from 1980 to August 2020. See Appendix 2.
Embase (via Ovid SP) from 1980 to August 2020. See Appendix 3.
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials. See Appendix 4.
ClinicalTrials.gov. See Appendix 5.
Searching other resources
Bibliographic searching
We screened the studies in the reference lists of identified relevant trials or reviews (for example Chen 2018; De La Cruz 2016; Haloua 2013; Losken 2014; Yoon 2016). We obtained a copy of the full‐text article for each reference reporting a potentially eligible study.
Data collection and analysis
Selection of studies
We uploaded our references into Covidence. Two review authors (AN and JH) independently examined each title and abstract to determine whether reports appear to meet the inclusion criteria based on the protocol, and resolved any differences by discussion. For those studies with multiple publications of duplicate data sets, the study with the shorter follow‐up time or fewer participant numbers for outcomes of interest was excluded so as not to duplicate data in the analysis.
We obtained copies of potentially eligible reports and two review authors (AN and JH) examined the full‐text articles independently. We used Cochrane Task Exchange to help with translations for six studies (2 Spanish, 1 French, 1 Hungarian and 2 Chinese (Mandarin)). We did not have any potentially relevant studies that we were unable to translate. The review author team reviewed all potentially eligible reports and decided which studies should be included in the review. We recorded the selection process in a PRISMA flow diagram (Page 2021); we recorded excluded studies in the 'Characteristics of excluded studies table.
Data extraction and management
The review author team designed and agreed upon the uniform criteria for data extraction and create a standardised form in Excel prior to review commencement. Three review authors (AN, JH and SA) independently undertook data extraction, with at least two authors reviewing each study. Any differences were resolved by discussion, and when needed we consulted a fourth review author (PR) to help resolve any disagreements. For those studies with more than one publication, we extracted data from all publications and considered the version with the longest follow‐up as the primary reference for the study and excluded the other from the analysis.
We tabulated the study characteristics for each included study to determine whether we were able to synthesise these data and present them in text or tabular form. We included the following information from the individual studies on standardised data extraction forms.
-
General Information
Author names, countries and year of publication
Study design and level of evidence
Conflicts of interest and funding
-
Demographics
Number of participants
Number of breasts treated
Age of participants
Smoking history
History of diabetes
History of steroid intake or immunosuppression
body mass index (BMI)
-
Breast factors
Preoperative breast/bra size
-
Oncological parameters
Type of cancer (invasive or in situ)
Grade
Stage
Axillary nodal status
Hormone receptor status (oestrogen receptor, progesterone receptor), HER2 status
Size of tumour including any associated additional foci
Location of tumour (which quadrant)
-
Tumour–nipple distance
Solitary, multifocal or multicentric
Presence of lymphovascular invasion
-
Cancer treatment
Adjuvant radiotherapy
Prior neoadjuvant or adjuvant chemotherapy
Previous breast surgery
-
Technical surgical details
Incision used
Reconstruction performed
Flap included a skin paddle used to reconstruct a skin defect
-
Postsurgical details
Median follow‐up duration
Loss to follow‐up expressed as a percentage
-
Primary outcomes as described above
Local recurrence
Survival (for example, disease‐specific (breast cancer) and overall survival)
-
Secondary outcomes, as described above
Patient‐reported outcome measures (for example, patient satisfaction)
Time to adjuvant therapy (days)
Surgical complications
Recall rates
Need for further surgery to address aesthetics/symmetry
Surgeon‐reported cosmetic outcomes
-
Surgical outcomes
-
Early complications, for example:
completion mastectomy rates
flap necrosis
infection
readmission
generic surgical complications
-
Late complications, for example:
correction of symmetry (contralateral augmentation/reduction or nipple reconstruction)
correction of deformity (lipomodelling, scar revision etc.)
any other breast procedures
-
-
Cosmetic outcomes
Clinician‐reported
Patient‐reported outcome measures, such as satisfaction and quality of life
Any symmetrisation surgery
-
For non‐randomised studies
Methods used to control for confounders
Adjusted and unadjusted outcome measures
List of variables included in analyses for adjusted estimates
If reports related to the same study appear in multiple publications, we combined them under the overall study ID.
Assessment of risk of bias in included studies
We planned to use Cochrane's risk of bias tool for RCTs (RoB 1; Higgins 2011) and the ROBINS‐I tool for non‐randomised studies (Sterne 2016). We planned to compare study protocols with final papers where possible and would have noted if key information was missing across all study types. However, there were no RCTs in this review nor any protocols.
Non‐randomised studies
Three review authors (AN, SA and JH) applied the ROBINS‐I tool, as described in Sterne 2016, to assess the risk of bias of effect of assignment in the results of non‐randomised studies that compare health effects of two or more interventions. We resolved disagreements by discussion. We used the ROBINS‐I tool for cohort studies, case‐control studies and prospective patient registries. We completed separate ROBINS‐I tables to generate an overall risk of bias for each outcome: local recurrence, disease‐free survival, overall survival, re‐excision rates, complications, recall rates, time to adjuvant therapy, cosmetic evaluation, and patient‐reported outcome measures. We assessed the risk of bias according to the following domains.
Pre‐intervention bias
-
Due to confounding: for example comorbidities of patients, associated ductal carcinoma in situ, the predominance of small tumour size or small tumour:breast ratio (no established cut‐offs exist for defining size), lack of pathology reporting in published literature, smoking status, age, ethnicity, genetic risk for breast cancer.
For oncological outcomes (local recurrence, disease‐free survival and overall survival we would expect the following confounders to be controlled for: oncological parameters of tumour (type, size, grade, stage, nodal status, hormonal status) and cancer treatment.
For re‐excision rates we would expect the following confounders to be controlled for: oncological parameters of tumour (especially tumour size and location) and cancer treatment.
For complication rates we would expect the following confounders to be controlled for: age, comorbidities, oncological parameters of tumour (especially stage and size) and cancer treatment (especially axillary surgery and adjuvant radiotherapy).
For time to adjuvant therapy we would expect the following confounders to be controlled for: comorbidities and cancer treatment.
For patient‐reported outcome measures we would expect the following confounders to be controlled for: oncological parameters of tumour (especially tumour size and location) and cancer treatment.
For cosmetic evaluation, we would expect the following confounders to be controlled for: oncological parameters of tumour (especially tumour size and location) and cancer treatment.
In the selection of participants into the study
At‐intervention bias
In the classification of the intervention
Post‐intervention bias
-
Due to deviations from the intended intervention
This includes bias due to differences in surgeon technique and experience between control and intervention within studies.
Due to missing data
In the measurement of outcomes: for example, cosmetic assessment being subjective and not using validated anonymised questionnaires
In the selection of the reported results
We scored each of these domains as having low, moderate, serious, or critical risk of bias. Based on these scores, we determined an overall risk of bias for each study per outcome. If we graded any domain as serious, we deemed the overall risk of bias as serious.
We summarised the risk of bias judgements across different studies for each of the domains listed and summarised results in separate risk of bias tables (Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13).
2. Risk of bias for local recurrence.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects may be determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time) | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Amitai 2018 | S‐BCS | Serious | Moderate | Low | Low | Moderate | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, axillary node status, immunohistochemical receptors). Adjuvant RT demonstrated balanced, most co‐interventions missing | Selection may be related to the outcome (those with Mx eventually excluded) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Objective outcome measure | No indication of selected reporting | |||
| Borm 2019 | S‐BCS | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Most clinicopathological variables significantly different: age, tumour size, tumour grade, axillary node status, immunohistochemical receptors (ER status). Important co‐interventions (adjuvant CT, adjuvant ET) not balanced across intervention group and may affect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, multifocality). Adjusted for in LR calculation. Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/Mx+R), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Cassi 2016 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, BMI, tumour size), most missing. Important co‐interventions balanced across intervention group (adjuvant RT) some information missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Chakravorty 2012 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, axillary node status) and some different (histological type, tumour size, grade, sample weight), most missing. Important co‐interventions balanced across intervention group (adjuvant RT, adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Chauhan 2016 (1) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, axillary node status, immunohistochemical receptors) and some different (age, tumour size, tumour location), most missing Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Chauhan 2016 (2) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Axillary node status demonstrated balance and some clinicopathological variables different (age, tumour size, tumour location), most missing. Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| DeLorenzi 2016 (1) | S‐BCS | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion) or matched (age (within 5 years), year of surgery (within 2 years), tumour size. Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant RT, adjuvant ET) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| DeLorenzi 2018 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, BMI, tumour size, immunohistochemical receptors, multifocality), some significantly different (menopausal, grade) Some co‐interventions balanced across intervention group (adjuvant RT, any adjuvant therapy) | Selection may be related to the outcome (Mx eventually excluded) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Down 2013 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, histological type, grade), tumour size different, some missing adjuvant RT balanced across intervention group, some co‐interventions missing | All patients included. Patients were selected for intervention if cosmetic outcome with control would be bad (selection bias but does not affect this outcome) | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Fan 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors matched (age, BMI, stage) or demonstrated balance (histological type), some missing Important co‐interventions demonstrated balance (neoadjuvant CT, adjuvant RT, adjuvant CT, adjuvant ET) | All participants eligible included, control selected for | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods | All patients followed up for 30 days and for re‐excisions specifically | Objective outcome measure | No indication of selected reporting | |||
| Gulcelik 2013 | S‐BCS | Moderate | Low | Low | Moderate | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, tumour size, immunohistochemical receptor), most missing. Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET, axillary management, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Included from the beginning of uptake of intervention | All patients included but some did not have sufficient follow‐up so excluded. Details not given | Objective outcome measure | No indication of selected reporting | |||
| Hashimoto 2019* | S‐BCS | Serious | Low | Low | No information | No information | Low | Moderate | Serious |
| Rate of advanced cases of cancer higher in intervention ‐ | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | ‐ | Objective outcome measure | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor) some different (preoperative bra size, axillary node status) but unlikely to affect outcome Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | All intervention participants eligible included, random patients selected for control | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Two experienced breast surgeons | Patients missed due to loss to follow‐up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | Objective outcome measure | No indication of selected reporting | |||
| Lee 2018 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Centre with large numbers | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Losken 2009 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different: age, histological type, stage. Important co‐interventions demonstrated balance, some significantly different: adjuvant CT, axillary surgery | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Experienced surgeon | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Malhaire 2015 | S‐BCS | No information | Serious | Low | Low | Low | Low | Moderate | Serious |
| ‐ | Selection based on localisation techniques | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. All surgeons had training in O‐BCS | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Mansell 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, histological type, tumour size, grade, axillary node status, immunohistochemical receptor (ER, PR). Important co‐interventions balanced, some significantly different: adjuvant CT, adjuvant ET | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2015 | Objective outcome measure | No indication of selected reporting | |||
| Matrai 2014 | S‐BCS | Serious | Serious | Low | Low | Moderate | Low | Moderate | Serious |
| Tumour size significantly different. Some variables demonstrated balance (age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status). Matching of patients reported but not defined: "the same clinicopathological parameters of 60 traditional breast‐conserving surgeries operated by the same breast surgeon were used". Important co‐interventions including adjuvant RT demonstrated balance. Adjuvant CT significantly different | Unclear why these 60 patients selected (not consecutive, some retrospective and some prospective), controls matched | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Experienced surgeon | Groups followed up for different amounts of time: "The mean follow‐up time was 32.2 months in the BCS group compared to only 8.7 months in the OPS group" | Objective outcome measure | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Important co‐interventions predefined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Niinikoski 2019 (2) | S‐BCS | Serious | Moderate | Low | Low | Moderate | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, tumour size, grade, axillary node status, immunohistochemical status (ER, TN), multifocality Important co‐intervention demonstrated balance (adjuvant RT), some significantly different (adjuvant CT and ET) | All participants eligible included. Excluded on basis on diagnosis by biopsy/incidental. Excluded those without adjuvant therapy nor axillary surgery. Also excluded if follow‐up < 3 years | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. | Some loss to follow‐up for local recurrence free survival: 140/611 in intervention group, 249/1189 in control group | Objective outcome measure | No indication of selected reporting | |||
| Piper 2016 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (BMI, histological type), age matched for and stage significantly different Important co‐interventions missing | Patients without negative margins excluded, minimum 2 years follow‐up (O‐BCS done more recently) | Classification of interventions clear and determined at the start of intervention: "All reduction mammoplasties were performed either via an inferior or superior‐medial pedicle approach, with a Wise pattern or vertical skin pattern incision, based on tumour location" | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Vieira 2016 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance. Matched for demographic and oncological aspects Important co‐interventions demonstrated balance, missing data on axillary management of cases (97.4% for control group) | All O‐BCS participants eligible included, matched standard breast conserving surgery: "cases were matched to decrease a possible bias selection" | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | Mx | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Adjusted for in local recurrence calculation. Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/MxR), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Gendy 2003 | Mx | Moderate | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balanced (Age, grade, axillary node status), some significantly different (histological type, tumour size), some missing. Important co‐interventions different across intervention group, likely to influence outcome. All those that recurred had had RT | All contactable participants | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Lee 2018 | Mx | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Nakagomi 2019 | Mx | Serious | Low | Low | Low | Low | Serious | Moderate | Serious |
| Some variables demonstrated balance (histological type, axillary node status, immunohistochemical receptor status), some significantly different (age, tumour size, stage), many missing. Important co‐interventions missing (RT, axillary management), neoadjuvant CT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention: latissimus dorsi mini flap or mastectomy | All patients received the surgical intervention described in methods. | All patients included followed up | Objective outcome measure but details of follow‐up time not given | No indication of selected reporting | |||
| Ren 2014 | Mx | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (histological type and location) or matched (age, tumour size, axillary lymph node status, immunohistochemical receptor, (ER, HER2)). Co‐intervention information missing for control group | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | Most patients included: "The median follow‐up time was 83 months in s‐BCS and 81 months in mastectomy" | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | Mx + R | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Adjusted for in LR calculation. Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/MxR), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| DeLorenzi 2016 (2) | Mx + R | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (grade, immunohistochemical receptors) or matched (age (within 5 years), year of surgery (within 2 years), number of positive axillary lymph nodes, tumour subtype). Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant ET), adjuvant RT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Lee 2018 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Mansell 2017 | Mx + R | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Some clinicopathological significantly different: age, immunohistochemical receptor (ER, PR). Other important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (HER2). Important co‐interventions demonstrated balance, adjuvant RT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2018 | Objective outcome measure | No indication of selected reporting | |||
| Mustonen 2004 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Age demonstrated balance, tumour size significantly different, most missing. Adjuvant CT balanced, adjuvant radiotherapy significantly different, other co‐interventions missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Ozmen 2020 | Mx + R | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. All interventions done by a single surgeon with more than 30 years of experience in breast surgery. | Most patients included: "Median follow‐up time was 56 (14‐116) months." | Objective outcome measure | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy HER2: human epidermal growth factor receptor 2 Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy
LR: local recurrence
3. Risk of bias for disease‐free survival.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time), co‐interventions significantly different | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Borm 2019 | S‐BCS | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Most clinicopathological variables significantly different: age, tumour size, tumour grade, axillary node status, immunohistochemical receptors (ER status). Important co‐interventions (adjuvant CT, adjuvant ET) not balanced across intervention group and may affect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Objective outcome measure | No indication of selected reporting | |||
| DeLorenzi 2016 (1) | S‐BCS | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion) or matched (age (within 5 years), year of surgery (within 2 years), tumour size. Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant RT, adjuvant ET) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| DeLorenzi 2018 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, BMI, tumour size, immunohistochemical receptors, multifocality), some significantly different (menopausal, grade). Some co‐interventions balanced across intervention group (adjuvant RT, any adjuvant therapy) | Selection may be related to the outcome (mastectomy eventually excluded) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Gulcelik 2013 | S‐BCS | Moderate | Low | Low | Moderate | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, tumour size, immunohistochemical receptor), most missing. Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET, axillary management, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Included from the beginning of uptake of intervention | All patients included but some did not have sufficient follow‐up so excluded. Details not given | Objective outcome measure | No indication of selected reporting | |||
| Mansell 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, histological type, tumour size, grade, axillary node status, immunohistochemical receptor (ER, PR). Important co‐interventions balanced, some significantly different: adjuvant CT, adjuvant ET | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2016 | Objective outcome measure | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Important co‐interventions predefined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Rose 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors statistically adjusted for. Location of surgeries different in intervention and control. Some co‐interventions balanced, some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | Most patients included | Objective outcome measure | No indication of selected reporting | |||
| Vieira 2016 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance. Matched for demographic and oncological aspects. Important co‐interventions demonstrated balance, missing data on axillary management of cases (97.4% for control group) | All OPS participants eligible included, matched BCS 'cases were matched to decrease a possible bias selection' | Classification of interventions clear and determined at the start of intervention: standard surgical treatment was quadrantectomy combined with level III axillary node dissection with was performed in 97.4% of patients | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Nakagomi 2019 | Mx | Serious | Low | Low | Low | Low | Serious | Moderate | Serious |
| Some variables demonstrated balance (histological type, axillary node status, immunohistochemical receptor status), some significantly different (age, tumour size, stage), many missing. Important co‐interventions missing (RT, axillary management), neoadjuvant CT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention: lattisimus dorsi mini flap or mastectomy | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure but details of follow‐up time not given | No indication of selected reporting | |||
| DeLorenzi 2016 (2) | Mx + R | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (grade, immunohistochemical receptors) or matched (age (within 5 years), year of surgery (within 2 years), number of positive axillary lymph nodes, tumour subtype). Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant ET), adjuvant RT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Mansell 2017 | Mx + R | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Some clinicopathological significantly different: age, immunohistochemical receptor (ER, PR). Other important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (HER2). Important co‐interventions demonstrated balance, adjuvant RT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2019 | Objective outcome measure | No indication of selected reporting | |||
| Ozmen 2020 | Mx + R | Serious | Moderate | Low | Moderate | Low | Low | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery. | Most patients included: "Median follow‐up time was 56 (14‐116) months." | Objective outcome measure | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy HER2: human epidermal growth factor receptor 2 PR: progesterone receptor R: reconstruction RT: radiotherapy
4. Risk of bias for overall survival.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time), co‐interventions significantly different | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Borm 2019 | S‐BCS | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Most clinicopathological variables significantly different: age, tumour size, tumour grade, axillary node status, immunohistochemical receptors (ER status). Important co‐interventions (adjuvant CT, adjuvant ET) not balanced across intervention group and may effect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/Mx+R), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| DeLorenzi 2016 (1) | S‐BCS | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion) or matched (age (within 5 years), year of surgery (within 2 years), tumour size. Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant RT, adjuvant ET) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| DeLorenzi 2018 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (Age, BMI, tumour size, immunohistochemical receptors, multifocality), some significantly different (menopausal, grade). Some co‐interventions balanced across intervention group (adjuvant RT, any adjuvant therapy) | Selection may be related to the outcome (mastectomy eventually excluded) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Gulcelik 2013 | S‐BCS | Moderate | Low | Low | Moderate | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, tumour size, immunohistochemical receptor), most missing. Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET, axillary management, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Included from the beginning of uptake of intervention | All patients included but some did not have sufficient follow‐up so excluded. Details not given | Objective outcome measure | No indication of selected reporting | |||
| Lee 2018 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Centre with large numbers | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Mansell 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, histological type, tumour size, grade, axillary node status, immunohistochemical receptor (ER, PR). Important co‐interventions balanced, some significantly different: adjuvant CT, adjuvant ET | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2017 | Objective outcome measure | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance: Histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Important co‐interventions predefined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Niinikoski 2019 (2) | S‐BCS | Serious | Moderate | Low | Low | Moderate | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, tumour size, grade, axillary node status, immunohistochemical status (ER), multifocality. Important co‐intervention demonstrated balance (adjuvant RT), some significantly different (adjuvant CT and ET) | All participants eligible included. Excluded on basis on diagnosis by biopsy/incidental. Excluded those without adjuvant therapy nor axillary surgery. Also excluded if follow‐up < 3 years | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | Some loss to follow‐up for local recurrence free survival: 140/611 in intervention group, 249/1189 in control group | Objective outcome measure | No indication of selected reporting | |||
| Piper 2016 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (BMI, histological type), age matched for and stage significantly different. Important co‐interventions missing | Patients without negative margins excluded, minimum 2 years follow‐up (O‐BCS done more recently) | Classification of interventions clear and determined at the start of intervention: "All reduction mammoplasties were performed either via an inferior or superior‐medial pedicle approach, with a Wise pattern or vertical skin pattern incision, based on tumour location" | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Rose 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors statistically adjusted for. Location of surgeries different in intervention and control. Some co‐interventions balanced (adjuvant RT, adjuvant CT, adjuvant ET), axillary surgery different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | Most patients included | Objective outcome measure | No indication of selected reporting | |||
| Vieira 2016 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance. Matched for demographic and oncological aspects. Important co‐interventions demonstrated balance, missing data on axillary management of cases (97.4% for control group) | All O‐BCS participants eligible included, matched s‐BCS: "cases were matched to decrease a possible bias selection" | Classification of interventions clear and determined at the start of intervention: "Oncoplastic procedures used encompass Clough level I and II techniques", "The ‘standard lumpectomy’ performed in this study, consists of removal of the tumour, with or without simple closure of the glandular tissue, without mobilization of surrounding tissue." | All patient received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | Mx | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/Mx+R), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Lee 2018 | Mx | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Centre with large numbers | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Ren 2014 | Mx | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (histological type and location) or matched (age, tumour size, axillary lymph node status, immunohistochemical receptor, (ER, HER2)). Co‐intervention information missing for control group | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | Most patients included: "The median follow‐up time was 83 months in BCT and 81 months in mastectomy." | Objective outcome measure | No indication of selected reporting | |||
| Carter 2016 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/MxR), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| DeLorenzi 2016 (2) | Mx + R | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (Grade, immunohistochemical receptors) or matched (age (within 5 years), year of surgery (within 2 years), number of positive axillary lymph nodes, tumour subtype) Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant ET), adjuvant RT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| Lee 2018 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, BMI), some significantly different (tumour size and stage). No breakdown between control and study groups for data on cancer treatment | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Centre with large numbers | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Mansell 2017 | Mx + R | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Some clinicopathological significantly different: age, immunohistochemical receptor (ER, PR). Other important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor ( HER2). Important co‐interventions demonstrated balance, adjuvant RT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up until June 2020 | Objective outcome measure | No indication of selected reporting | |||
| Ozmen 2020 | Mx + R | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery. | Most patients included: "Median follow‐up time was 56 (14‐116) months." | Objective outcome measure | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy HER2: human epidermal growth factor receptor 2 Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy
5. Risk of bias for re‐excision rates.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2005 | S‐BCS | Serious | Moderate | Low | Moderate | Low | Low | Moderate | Serious |
| Size significantly different, most clinicopathological variables missing | Selection into the study may have been related to intervention. Selection to which intervention the women had was based on tumour characteristic. This difference at selection may have an effect on the outcome. | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | Deviation from intended intervention (minor changes in operation technique in some patients) but does not impact this outcome | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time) and co‐interventions significantly different but minimal impact on this outcome | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Amitai 2018 | S‐BCS | Serious | Serious | Low | Low | Moderate | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, axillary node status, immunohistochemical receptors). Adjuvant RT demonstrated balanced, most co‐interventions missing | Selection may be related to the outcome (those with Mx eventually excluded) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Atallah 2015* | S‐BCS | Moderate | No information | Low | No information | No information | Low | Moderate | Moderate |
| Some clinicopathological variables demonstrated balance (age, BMI, menopausal status, tumour size, location, histological type, immunohistochemical receptors), some missing | ‐ | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | ‐ | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Bali 2018 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, histological type, immunohistochemical receptors, tumour locations). Tumour size significantly different, most missing. Important co‐interventions (neo‐adjuvant and adjuvant CT) not balanced across intervention group but unlikely to effect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. The margins for determining re‐excisions changed overtime | No indication of selected reporting | |||
| Cassi 2016 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance, most missing. Important co‐interventions balanced across intervention group (adjuvant RT) some information missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Chakravorty 2012 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, axillary node status) and some different (histological type, tumour size, grade, sample weight), most missing. Important co‐interventions balanced across intervention group (adjuvant RT, adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Chauhan 2016 (1) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, axillary node status, immunohistochemical receptors) and some different (age, tumour size, tumour location), most missing. Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Chauhan 2016 (2) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Axillary node status demonstrated balance and some clinicopathological variables different (age, tumour size, tumour location), most missing. Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Crown 2015 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (age, histological type), some significantly different (tumour size, immunohistochemical receptors). Different years of intervention. Adjuvant RT balanced | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given and separated by years | All patients received the surgical intervention described in the methods. Study period chosen to allow for learning period after adoption of O‐BCS | Most patients followed up | Objective outcome measure ‐ due to margin status | No indication of selected reporting | |||
| DeLorenzi 2016 (1) | S‐BCS | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion) or matched (age (within 5 years), year of surgery (within 2 years), tumour size). Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant RT, adjuvant ET) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. Decided on mastectomy after multi disciplinary team discussion | No indication of selected reporting | |||
| Di Micco 2017 | S‐BCS | Moderate | Serious | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (Smoking status, BMI, histological type, tumour size, immunohistochemical receptor, tumour location), some significantly different (Age, axillary node status). Some co‐interventions balanced across intervention group (neoadjuvant CT, adjuvant ET, axillary management, adjuvant RT), some different (radiation boost, adjuvant CT) | Selection may be related to the outcome (Mx eventually) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. Decided on re‐excision after MDT discussion | No indication of selected reporting | |||
| Dolan 2015 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, immunohistochemical receptor) and some different (age, tumour size, axillary node status), some missing. Some co‐interventions balanced across intervention group (adjuvant RT, adjuvant ET, axillary management), adjuvant CT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Down 2013 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, histological type, grade), tumour size different, some missing. Adjuvant RT balanced across intervention group, some co‐interventions missing | All patients included. Patients were selected for intervention if cosmetic outcome with control would be bad (selection bias but does not affect this outcome) | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Fan 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors matched (age, BMI, stage) or demonstrated balance (histological type), some missing. Important co‐interventions demonstrated balance (neoadjuvant CT, adjuvant RT, adjuvant CT, adjuvant ET) | All participants eligible included, control selected for | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Surgeries done by experienced plastic and breast surgeons. | All patients followed up for 30 days and for re‐excisions specifically | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Farooqi 2019* | S‐BCS | Serious | No information | Low | No information | Low | Low | Moderate | Serious |
| Tumour size significantly different. Neoadjuvant CT balanced, most co‐interventions missing | ‐ | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | All patients followed up for 30 days and for re‐excisions specifically | Objective outcome measure (tumour at ink) | No indication of selected reporting | |||
| Gicalone 2007 (1) | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (BMI, histological type, tumour size, grade, axillary node status, immunohistochemical receptor), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All operations done by 2 experienced surgeons | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2007 (2) | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (BMI, tumour size, tumour location), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All operations done by 2 experienced surgeons | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2015 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (age, smoking status, diabetes, BMI, other medical comorbidities, histological type, tumour size), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Both intervention and control done by experienced surgeons. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gulcelik 2013 | S‐BCS | Moderate | Low | Low | Moderate | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (age, tumour size, immunohistochemical receptor), most missing. Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET, axillary management, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Included from the beginning of uptake of intervention | All patients included but some did not have sufficient follow‐up so excluded. Details not given | Objective outcome measure | No indication of selected reporting | |||
| Hamdi 2008 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors different (age, histological type, tumour size), most missing. Axillary management demonstrated balance | Not clear if/why all patients in the time period not selected | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All reconstruction done by plastic surgeons whilst tumourectomy by gynaecologist | All patients included followed up | All positive margins (tumour cells at surgical margin) re‐excised | No indication of selected reporting | |||
| Jiang 2015 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balanced (age, weight, histology type,tumour size, grade, stage, tumour location) | 60 women were picked, study says randomised but not clear how therefore classified as cohort. Risk of selection | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Moderate | Low | Low | Serious | Low | Moderate | Serious |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor) some different (preoperative bra size, axillary node status) but unlikely to affect outcome. Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | All intervention participants eligible included, random patients selected for control | Classification of interventions clear and determined at the start of intervention. The types of intervention were: therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern), dermoglandular rotation (medial, lateral mammoplasty), periareolar (round block, omega) or standard BCS | All patients received the surgical intervention described in the methods. Operations done by experienced breast surgeons. | Patients missed due to loss to follow up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | Objective outcome measure | No indication of selected reporting | |||
| Lansu 2014 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance (age, tumour size, tumour location), some missing. Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET, axillary management, adjuvant RT), some significantly different (neoadjuvant CT) | Patients had to be disease‐free and alive at the time of inclusion | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Losken 2014 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balance. Age and BMI significantly different. Neoadjuvant CT significantly different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Operation done by experienced surgeon. | All patients included followed up (requirement for patients to have at least 2 months follow‐up data from time of surgery) | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Malhaire 2015 | S‐BCS | No information | Serious | Low | Low | Low | Low | Moderate | Serious |
| ‐ | Selection based on localisation techniques | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. All surgeons had training in O‐BCS. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Mansell 2015 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, histological type, tumour size, grade, axillary node status, immunohistochemical receptor (ER, PR). Important co‐interventions significantly different: adjuvant CT, adjuvant ET | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Matrai 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Tumour size significantly different. Some variables demonstrated balance (age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status). Matching of patients reported but not defined: "the same clinicopathological parameters of 60 traditional breast‐conserving surgeries operated by the same breast surgeon were used." Important co‐interventions including adjuvant RT demonstrated balance. Adjuvant CT significantly different | Unclear why these 60 patients selected (not consecutive, some retrospective and some prospective), controls matched | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Operation done by experienced surgeon. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Important co‐interventions predefined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Mukhtar 2018 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factor significantly different: shows that when tumour size is matched for then there is no difference in re‐excisions due to O‐BCS. No information on other clinicopathological factors or co‐interventions | All participants eligible included. Possible bias in assignment: "Surgical procedures were performed according to surgeon recommendation and patient choice." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Niinikoski 2019 (2) | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different: age, tumour size, grade, axillary node status, immunohistochemical status (ER, TN), multifocality. Important co‐intervention demonstrated balance (adjuvant RT), some significantly different (adjuvant CT and ET) | All participants eligible included. Excluded on basis on diagnosis by biopsy/incidental. Excluded those without adjuvant therapy nor axillary surgery. Also excluded if follow‐up < 3 years | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Ojala 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (tumour size, tumour location, axillary node status, multifocality, histological type). Important co‐interventions missing, adjuvant RT demonstrated balance, axillary management significantly different | All participants eligible included: "All patients having breast conserving surgery (BCS) due to primary breast cancer at the Helsinki and Uusimaa Hospital District during 2010 were included in this study" | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Palsodittlir 2018 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (e.g. tumour size), some missing (grade, stage, location of tumour). Adjuvant ET balanced, some co‐interventions missing: radiotherapy, chemotherapy, axillary management | All women included according to selection criteria. Selection criteria excluded level 2 O‐BCS procedures assigning these as minimal: "Level 1 and level 2 oncoplastic procedures (minimal gland mobilization techniques) were not included in the study group." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Piper 2016 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (BMI, histological type), age matched for and stage significantly different. Important co‐interventions missing | Patients without negative margins excluded, minimum 2 years follow‐up (OPS done more recently) | Classification of interventions clear and determined at the start of intervention: "All reduction mammoplasties were performed either via an inferior or superior‐medial pedicle approach, with a Wise pattern or vertical skin pattern incision, based on tumour location" | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Tang 2016 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (tumour size, stage, BMI, age). Some co‐interventions balanced (axillary management), some missing (medical cancer treatment) | All participants eligible included | Classification of interventions clear and determined at the start of intervention: "Standard Breast Conservation Surgery (SBCS) group had surgery conducted according to the National Surgical Adjuvant Breast and Bowel Project (NSABP) standard guidelines." | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Tenofsky 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different, some missing (histological type, grade, stage, axillary node status). Important co‐interventions significantly different (adjuvant RT), some missing (neoadjuvant RT + CT, adjuvant CT + ET, axillary management) | Quote: "Patients were excluded if they received a mastectomy within 6 months of the lumpectomy, and/or if they received 6 months of follow‐up after their procedure." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. Operation done by experienced surgeon. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Vieira 2016 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance. Matched for demographic and oncological aspects. Important co‐interventions demonstrated balance, missing data on axillary management of cases (97.4% for control group) | All OPS participants eligible included, matched s‐BCS: "cases were matched to decrease a possible bias selection" | Classification of interventions clear and determined at the start of intervention: "Oncoplastic procedures used encompass Clough level I and II techniques", "The ‘standard lumpectomy’ performed in this study, consists of removal of the tumour, with or without simple closure of the glandular tissue, without mobilization of surrounding tissue." | All patients received the surgical intervention described in methods. | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Wijgman 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (tumour size), some missing. Important co‐interventions demonstrated balance, some different | All participants eligible included | Classification of interventions clear and determined at the start of intervention: "Oncoplastic procedures used encompass Clough level I and II techniques", "The ‘standard lumpectomy’ performed in this study, consists of removal of the tumour, with or without simple closure of the glandular tissue, without mobilization of surrounding tissue." | All patients received the surgical intervention described in the methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Wong 2017* | S‐BCS | Serious | Low | Low | No information | Low | Low | Moderate | Serious |
| Tumour size significantly different, most clinicopathological variables missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy
6. Risk of bias for complications.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2005 | S‐BCS | Serious | Moderate | Low | Moderate | Low | Low | Moderate | Serious |
| Size significantly different, most clinicopathological variables missing | Selection into the study may have been related to intervention. Selection to which intervention the women had was based on tumour characteristic. This difference at selection may have an effect on the outcome. | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | Deviation from intended intervention (minor changes in operation technique in some patients) but does not impact this outcome | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality), Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time) and co‐interventions significantly different | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Acosta‐Marin 2014 | S‐BCS | Serious | Serious | Low | low | Serious | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, BMI) and some significantly different (preoperative bra size, tumour size), most missing | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data ‐ missed women with complications in short term. If major may have had to have mastectomy and therefore excluded | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Amitai 2018 | S‐BCS | Serious | Serious | Low | Low | Serious | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, axillary node status,immunohistochemical receptors), adjuvant RT demonstrated balanced, most co‐interventions missing | Selection may be related to the outcome (those with mastectomy eventually excluded) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data ‐ missed women with complications in short term. If major may have had to have mastectomy and therefore excluded | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Angarita 2020 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, race, smoking status, alcohol consumption, COPD, PCI, HTN, bleeding disorder, steroid use, previous vascular disease, previous cardiac surgery, dialysis, hemiplegia, TIA, CVA, ASA status, histological type). Adjusted risk analysis for some comorbidities not extractable for our study, Important co‐interventions (axillary management, neoadjuvant chemotherapy, anaesthetic technique) not balanced across intervention group but unlikely to effect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. Difficulties with how complications recorded in the database. If axillary surgery undergone affects the study and this was not balanced across the two. Authors accounted for difficulties/differences in the database | No indication of selected reporting | |||
| Carter 2016 | S‐BCS | Serious | Low | Low | low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality), Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/Mx+R), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Cassi 2016 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance, most missing, Important co‐interventions balanced across intervention group (adjuvant RT), some information missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Chauhan 2016 (1) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, axillary node status, immunohistochemical receptors) and some different (age, tumour size, tumour location), most missing, Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Chauhan 2016 (2) | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Axillary node status demonstrated balance and some clinicopathological variables different (age, tumour size, tumour location), most missing, Important co‐interventions predefined and uniform across studies | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Crown 2019 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (age, smoking, BMI, histological type), some significantly different (tumour size, immunohistochemical receptors). Different years of intervention, adjuvant CT balanced across intervention group, neoadjuvant CT significantly different, some co‐interventions missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given and separated by years | All patients received the surgical intervention described in the methods. Study period chosen to allow for learning period after adoption of O‐BCS | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| DeLorenzi 2016 (1) | S‐BCS | Low | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (menopausal, histological type, grade, axillary node status, immunohistochemical receptors, lymphovascular invasion) or matched (age (within 5 years), year of surgery (within 2 years), tumour size). Important co‐interventions balanced across intervention group (adjuvant CT, adjuvant RT, adjuvant ET) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. Decided on mastectomy after multi‐disciplinary discussion | No indication of selected reporting | |||
| Di Micco 2017 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (smoking status, BMI, histological type, tumour size, immunohistochemical receptor, tumour location), some significantly different (age, axillary node status). Some co‐interventions balanced across intervention group (neoadjuvant CT, adjuvant ET, axillary management, adjuvant RT), some different (radiation boost, adjuvant CT) | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Dolan 2015 | S‐BCS | Serious | Low | Low | Low | Low | Low | Serious | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, immunohistochemical receptor) and some different (age, tumour size, axillary node status), some missing, Some co‐interventions balanced across intervention group (adjuvant RT, adjuvant ET, axillary management), adjuvant CT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | Only reports complications requiring re‐excisions | |||
| Down 2013 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, histological type, grade), tumour size different, some missing. Adjuvant RT balanced across intervention group, some co‐interventions missing | All patients included. Patients were selected for intervention if cosmetic outcome with control would be bad (selection bias but does not affect this outcome) | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2007 (1) | S‐BCS | Moderate | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (BMI, histological type, tumour size, grade, axillary node status, immunohistochemical receptor), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All operations done by 2 experienced surgeons. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2007 (2) | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (BMI, tumour size, tumour location), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All operations done by 2 experienced surgeons. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2015 | S‐BCS | Moderate | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (age, smoking status, diabetes, BMI, other medical comorbidities, histological type, tumour size), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Both intervention and control done by experienced surgeons. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Jiang 2015 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balanced (age, weight, histology type, tumour size, grade, stage, tumour location) | 60 women were picked, study says randomised but not clear how; therefore classified as cohort. Risk of selection | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Moderate | Low | Low | Serious | Low | Moderate | Serious |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor), some different (preoperative bra size, axillary node status) but unlikely to affect outcome. Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | All intervention participants eligible included, random patients selected for control | Classification of interventions clear and determined at the start of intervention. The types of intervention were: Therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern), Dermoglandular rotation (medial, lateral mammoplasty), Periareolar (round block, omega) or standard BSC | All patients received the surgical intervention described in the methods. Operations done by experienced breast surgeons. | Patients missed due to loss to follow‐up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Kimball 2018 | S‐BCS | Serious | Moderate | Low | Moderate | Low | Moderate | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (BMI) and some different (age, medical comorbidities, histological type), some missing ‐ issue with the database, Important co‐interventions significantly different (adjuvant RT, adjuvant CT, axillary management) | Selection based on coding ‐ not standardised for O‐BCS yet | Classification of intervention based on codes ‐ not uniform across sites. Types of intervention: partial mastectomy (‘lumpectomy’) and three breast reconstructive/repair procedures | All patient received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery. Notes that uptake of novel techniques not uniform across centres | All patients included followed up | Coding not uniform for complications | No indication of selected reporting | |||
| Lansu 2014 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors balance (age, tumour size, tumour location), some missing. Important co‐interventions demonstrated balance, some significantly different | Patients had to be disease‐free and alive at the time of inclusion | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Matrai 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Tumour size significantly different. Some variables demonstrated balance (age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status). Matching of patients reported but not defined: "the same clinicopathological parameters of 60 traditional breast‐conserving surgeries operated by the same breast surgeon were used". Important co‐interventions including adjuvant RT demonstrated balance. Adjuvant CT significantly different | Unclear why these 60 patients selected (not consecutive, some retrospective and some prospective), controls matched | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Operation done by experienced surgeon. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Nakada 2019 | S‐BCS | No information | Moderate | Low | Low | Low | Moderate | Moderate | Serious |
| ‐ | Participants were excluded if they were lost to follow‐up before 5 years | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention. Lovy grading criteria | No indication of selected reporting | |||
| Ojala 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (tumour size, tumour location, axillary node status, multifocality, histological type). Important co‐interventions missing, adjuvant RT demonstrated balance, axillary management significantly different | All participants eligible included: "All patients having breast conserving surgery (BCS) due to primary breast cancer at the Helsinki and Uusimaa Hospital District during 2010 were included in this study" | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Ozmen 2016* | S‐BCS | Serious | Moderate | Low | No information | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, BMI, multifocality), adjuvant RT balanced, most co‐interventions missing | Selection into the study may have been related to intervention as BCS data were collected before introduction of O‐BCS technique before 2010. O‐BCS patients after 2010 only | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Palsodittlir 2018 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (e.g. tumour size), some missing (grade, stage, location of tumour), adjuvant ET balanced, some co‐interventions missing: radiotherapy, chemotherapy, axillary management | All women included according to selection criteria. Selection criteria excluded level 2 O‐BCS procedures assigning these as minimal: "Level 1 and level 2 oncoplastic procedures (minimal gland mobilisation techniques) were not included in the study group." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| PlaFarnos 2018* | S‐BCS | Serious | Moderate | Low | No information | No information | Low | Moderate | Serious |
| Multifocality significantly different, most clinicopathological variables missing | Selection into the study may have been related to intervention ‐ it is not clear how the 60 patients in the O‐BCS group and 120 in the control were selected for in that time period | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | ‐ | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Scheter 2019 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors statistically adjusted for or demonstrated balance. Some significantly different: age, smoking status, tumour size. Some missing: axillary node status, grade, stage. Important co‐interventions demonstrated balance (medical cancer treatment and axillary management) | Patients were excluded if they proceeded to have a mastectomy after the intervention: "Patients who had subsequently proceeded to total mastectomy were excluded from the study." | Classification of interventions clear and determined at the start of intervention. Technique clearly described in methods: 'Patients with centrally located tumours who required NAC re‐ section and had medium‐ or large‐sized ptotic breasts were offered immediate OBR using a breast reduction pattern technique. Patients in the control group underwent primary closure of the NAC area in a horizontal or oblique scar and no oncoplastic reconstruction.' | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Sherwell‐Cabello 2006 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (age, medical comorbidities), some significantly different (tumour size, stage, axillary node status), some missing. Neoadjuvant chemotherapy is significantly different between groups. No information on other important co‐interventions (radiotherapy, adjuvant treatment, axillary management) | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected: "All patients diagnosed with breast cancer treated under conventional conservative surgery or oncoplastic patterns at the Institute of Breast Diseases, FUCAM AC, with a complete clinical history and had answered a questionnaire of aesthetic satisfactory in person or by phone were included. Those who did not continue their follow‐up at the institution were eliminated from the study." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Tang 2016 | S‐BCS | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors demonstrated balance (tumour size, stage, BMI, age). Some co‐interventions balanced (axillary management), some missing (medical cancer treatment) | All participants eligible included | Classification of interventions clear and determined at the start of intervention: 'Standard Breast Conservation Surgery(SBCS) group had surgery conducted according to the National Surgical Adjuvant Breast and Bowel Project(NSABP) standard guidelines. ' | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Tenofsky 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different, some missing (histological type, grade, stage, axillary node status). Important co‐interventions significantly different (adjuvant RT), some missing (neoadjuvant RT + CT, adjuvant CT + ET, axillary management) | Participants were excluded if they went on to require mastectomy 6 months after procedure, or if lost to follow‐up within 6 months: "Patients were excluded if they received a mastectomy within 6 months of the lumpectomy, and/or if they received 6 months of follow‐up after their procedure." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. Operation done by experienced surgeon. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Wijgman 2017 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (tumour size), some missing. Important co‐interventions demonstrated balance, some different | All participants eligible included | Classification of interventions clear and determined at the start of intervention: 'Oncoplastic procedures used encompass Clough level I and II techniques', 'The ‘standard lumpectomy’ performed in this study, consists of removal of the tumour, with or without simple closure of the glandular tissue, without mobilization of surrounding tissue. ' | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Zhou 2019 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (tumour size), some missing. Some co‐interventions balanced (adjuvant RT, axillary management), some missing (all other cancer treatment) | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected. Patients also excluded if failure to complete follow‐up | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Acea‐Nebril 2005 | Mx | Serious | Moderate | Low | Moderate | Low | Low | Moderate | Serious |
| Size significantly different, most clinicopathological variables missing | Selection into the study may have been related to intervention. Selection to which intervention the women had was based on tumour characteristic. This difference at selection may have an effect on the outcome. | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | Deviation from intended intervention (minor changes in operation technique in some patients) but does not impact this outcome | All patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Carter 2016 | Mx | Serious | Low | Low | low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR), multifocality). Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/Mx+R), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Gendy 2003 | Mx | Moderate | Moderate | Low | low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balanced (age, grade, axillary node status), some significantly different (histological type, tumour size), some missing. Important co‐interventions different across intervention group, unlikely to influence outcome | All contactable participants | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All surgeries done by an experienced surgeon/under their supervision. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Potter 2020 | Mx | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (age, diabetes, BMI, other medical comorbidities, histological type,grade, axillary node status, immunohistochemical receptors, multifocality). Tumour size missing. Clinicopathological factors e.g. size shown to effect the aesthetic outcome. Important co‐interventions significantly different (neoadjuvant CT, adjuvant RT, adjuvant CT, axillary surgery) | Selection from participants in other studies (iBRA‐2 and TeaM studies ) | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. As per protocols for other studies. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Carter 2016 | Mx + R | Serious | Low | Low | low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different (age, BMI, tumour size, stage, axillary node status, immunohistochemical receptors (ER, PR, TN), multifocality). Important co‐interventions not balanced across intervention group and may affect the outcome (neoadjuvant CT (all), adjuvant RT (Mx/MxR), adjuvant CT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Mustonen 2004 | Mx + R | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Age demonstrated balance, tumour size significantly different, most missing. Adjuvant CT balanced, adjuvant radiotherapy significantly different, other co‐interventions missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Reperfusion measured different with transverse rectus abdominus muscle and latissimus dorsi flaps | No indication of selected reporting | |||
| Ozmen 2020 | Mx + R | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | Most patients included: "Median follow‐up time was 56 (14‐116) months." | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Peled 2014 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (diabetes, smoking status), some significantly different (e.g. age, BMI), some important clinicopathological variables missing (tumour size, grade, stage, location of tumour). Neoadjuvant chemotherapy and adjuvant radiotherapy balanced, other important co‐interventions missing, including axillary management | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Potter 2020 | Mx + R | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (age, diabetes, BMI, other medical comorbidities, histological type,grade, axillary node status, immunohistochemical receptors, multifocality). Tumour size missing. Clinicopathological factors e.g. size shown to effect the aesthetic outcome. Important co‐interventions significantly different (neoadjuvant CT, adjuvant RT, adjuvant CT, axillary surgery) | Selection from participants in other studies (iBRA‐2 and TeaM studies ) | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Tong 2016 | Mx + R | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (age, diabetes, BMI, other comorbidities, preoperative bra size), some missing. Important co‐interventions significantly different (neoadjuvant RT, adjuvant RT), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. | All patients included followed up, but median follow‐up was significantly different between groups. "Median follow‐up was 4 months longer for the oncoplastic breast reconstruction group than for the immediate breast reconstruction group (18.7 months versus. 14.0 months, respectively; P < 0.001)" | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy S‐BCS: standard breast‐conserving surgery
COPD: chronic obstructive pulmonary disease
PCI: primary coronary intervention
HTN: hypertension
TIA: transient ischaemic attack
CVA: cerebral vascular accident
ASA: American Society of anesthesiology
BIRADS: Breast Imaging‐Reporting and Data System
7. Risk of bias for recall rates.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Amitai 2018 | S‐BCS | Serious | Moderate | Low | Low | Moderate | Moderate | Moderate | Serious |
| Most clinicopathological variables significantly different (age, axillary node status, immunohistochemical receptors), adjuvant RT demonstrated balanced, most co‐interventions missing | Selection may be related to the outcome (those with mastectomy eventually excluded) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Dolan 2015 | S‐BCS | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (histological type, grade, immunohistochemical receptor) and some different (age, tumour size, axillary node status), some missing. Some co‐interventions balanced across intervention group (adjuvant RT, adjuvant ET, axillary management), adjuvant CT different | All participants eligible included | Classification of interventions clear and determined at the start of intervention, details of operations described | All patients received the surgical intervention described in the methods | Most patients followed up | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Fan 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Important clinicopathological factors matched (age, BMI, stage) or demonstrated balance (histological type), some missing. Important co‐interventions demonstrated balance (neoadjuvant CT, adjuvant RT, adjuvant CT, adjuvant ET) | All participants eligible included, control selected for | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. Surgeries done by experienced plastic and breast surgeons. | All patients followed up for 30 days and for re‐excisions specifically | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Hu 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Important clinicopathological factors balanced (age, tumour size, immunohistochemical receptor). Important co‐interventions demonstrated balance (neoadjuvant CT, axillary management), most missing | All intervention included, control matched for on certain domains | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. All operations done by an experienced surgeon | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention. BIRADs tool used to limit bias | No indication of selected reporting | |||
| Losken 2009 | S‐BCS | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Some variables demonstrated balance, some significantly different: age, histological type, stage. Important co‐interventions demonstrated balance, some significantly different: adjuvant CT, axillary surgery | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. All operations done by an experienced surgeon | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Piper 2016 | S‐BCS | Serious | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Some variables demonstrated balance (BMI, histological type), age matched for and stage significantly different. Important co‐interventions missing | Patients without negative margins excluded, minimum 2 years follow‐up (O‐BCS done more recently) | Classification of interventions clear and determined at the start of intervention: "All reduction mammoplasties were performed either via an inferior or superior‐medial pedicle approach, with a Wise pattern or vertical skin pattern incision, based on tumour location" | All patients received the surgical intervention described in methods | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting | |||
| Tenofsky 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Some variables demonstrated balance, some significantly different, some missing (histological type, grade, stage, axillary node status). Important co‐interventions significantly different (adjuvant RT), some missing (neoadjuvant RT + CT, adjuvant CT + ET, axillary management) | Participants were excluded if they went on to require mastectomy 6 months after procedure, or if lost to follow‐up within 6 months: "Patients were excluded if they received a mastectomy within 6 months of the lumpectomy, and/or if they received, 6 months of follow‐up after their procedure." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. Operation done by experienced surgeon. | All patients included followed up | Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting |
RT: radiotherapy
ET: endocrine therapy
CT: chemotherapy
BMI: body mass index
BIRADs: Breast Imaging‐Reporting and Data System
8. Risk of bias for time to adjuvant therapy.
| Study | Control | Confouding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2017 | S‐BCS | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time) but does not impact this outcome | All patients followed up | Objective outcome measure (from date of surgery to date of treatment) | No indication of selected reporting | |||
| Borm 2019 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Most clinicopathological variables significantly different: age, tumour size, tumour grade, axillary node status, immunohistochemical receptors (ER status). Important co‐interventions (adjuvant CT, adjuvant ET) not balanced across intervention group and may effect the outcome | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | All patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Cassi 2016 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some clinicopathological variables demonstrated balance, most missing. Important co‐interventions balanced across intervention group (adjuvant RT,) some information missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Di Micco 2017 | S‐BCS | Moderate | Serious | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (smoking status, BMI, histological type, tumour size, immunohistochemical receptor, tumour location), some significantly different (age, axillary node status). Some co‐interventions balanced across intervention group (neoadjuvant CT, adjuvant ET, axillary management, adjuvant RT), some different (radiation boost, adjuvant CT) | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Objective outcome measure | No indication of selected reporting | |||
| Kahn 2013 | S‐BCS | Serious | Low | Moderate | No information | Low | Serious | Moderate | Serious |
| Clinicopathological factors missing. Adjuvant CT balanced | All participants eligible included, consecutive patients to reduce selective bias | Some plane mobilisation without skin reduction counted as WLE. This is standard practice so minor risk of bias due to this | ‐ | All patients included followed up | Date calculated from when MDT decided to give CT, this is not an objective date and could be different across the two groups | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Moderate | Low | Low | Moderate | Low | Moderate | Moderate |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor) some different (preoperative bra size, axillary node status) but unlikely to affect outcome. Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | All intervention participants eligible included, random patients selected for control | Classification of interventions clear and determined at the start of intervention. The types of intervention were: therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern), Dermoglandular rotation (medial, lateral mammoplasty), Periareolar (round block, omega) or standard BSC | All patients received the surgical intervention described in the methods | Patients missed due to loss to follow‐up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | Objective outcome measure | No indication of selected reporting | |||
| Kimball 2018 | S‐BCS | Serious | Moderate | Moderate | Low | Low | Moderate | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (BMI) and some different (age, medical comorbidities, histological type), some missing ‐ issue with the database. Important co‐interventions significantly different (adjuvant RT, adjuvant CT, axillary management) | Selection based on coding ‐ not standardised for OPS yet | Classification of intervention based on codes ‐ not uniform across sites. Types of intervention: partial mastectomy (‘lumpectomy’) and three breast reconstructive/repair procedures | All patient received the surgical intervention described in methods | All patients included followed up | From coding in insurance companies | No indication of selected reporting | |||
| Klit 2017 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some differences in clinicopathological characteristics (age, BMI, tumour size, axillary node status), unlikely to have major impact on outcome. Important co‐interventions significantly different (axillary management), some balanced (adjuvant CT) | Excluded patients with unclear resection margins, needs for further surgery (could influence outcome) | Classification of interventions clear and determined at the start of intervention. Surgical treatment consisted of mastectomy, lumpectomy or O‐BCS in combination with either sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND) | All patients received the surgical intervention described. The surgical and adjuvant treatments were standardized according to DBCG guidelines | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Matrai 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Tumour size significantly different. Some variables demonstrated balance (age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status). Matching of patients reported but not defined: "the same clinicopathological parameters of 60 traditional breast‐conserving surgeries operated by the same breast surgeon were used". Important co‐interventions including adjuvant RT demonstrated balance. Adjuvant CT significantly different | Unclear why these 60 patients selected (not consecutive, some retrospective and some prospective), controls matched | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Low | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Important co‐interventions predefined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Unclear date from which time until adjuvant therapy calculated | No indication of selected reporting | |||
| Morrow 2019 | S‐BCS | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (histological type (MxIR), tumour size (MxIR), grade (MxIR), axillary node status, immunohistochemical receptors), some significantly different (age (all), histological type (BCS, Mx), tumour size (BCS, Mx), grade (BCS, Mx), axillary node status (Mx, MxIR)). Important co‐interventions significantly different (adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Palsodittlir 2018 | S‐BCS | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (e.g. tumour size), some missing (grade, stage, location of tumour). Adjuvant ET balanced, some co‐interventions missing: radiotherapy, chemotherapy, axillary management | All women included according to selection criteria. Selection criteria excluded level 2 O‐BCS procedures assigning these as minimal: "Level 1 and level 2 oncoplastic procedures (minimal gland mobilization techniques) were not included in the study group." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Rose 2019 | S‐BCS | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Important clinicopathological factors statistically adjusted for. Location of surgeries different in intervention and control. Accounted for by measuring time to adjuvant therapy in all locations. Some co‐interventions balanced (adjuvant RT, adjuvant CT, adjuvant ET), axillary surgery different | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | Most patients included | Objective outcome measure: time from day of surgery to first day of therapy | No indication of selected reporting | |||
| Tenofsky 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance, some significantly different, some missing (histological type, grade, stage, axillary node status). Important co‐interventions significantly different (adjuvant RT), some missing (neoadjuvant RT + CT, adjuvant CT + ET, axillary management) | Participants were excluded if they went on to require mastectomy 6 months after procedure, or if lost to follow‐up within 6 months: "Patients were excluded if they received a mastectomy within 6 months of the lumpectomy, and/or if they received 6 months of follow‐up after their procedure." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Kahn 2013 | Mx | Serious | Low | Moderate | No information | Low | Serious | Moderate | Serious |
| Clinicopathological factors missing. Adjuvant CT balanced | All participants eligible included, consecutive patients to reduce selective bias | Some plane mobilisation without skin reduction counted as WLE. This is standard practice so minor risk of bias due to this | ‐ | All patients included followed up | Date calculated from when the multi‐disciplinary team decided to give CT, this is not an objective date and could be different across the two groups | No indication of selected reporting | |||
| Klit 2017 | Mx | Serious | Serious | Low | Low | Low | Low | Moderate | Serious |
| Some differences in clinicopathological characteristics (age, BMI, tumour size, axillary node status), unlikely to have major impact on outcome. Important co‐interventions significantly different (axillary management), some balanced (adjuvant CT) | Excluded patients with unclear resection margins, needs for further surgery (could influence outcome) | Classification of interventions clear and determined at the start of intervention. Surgical treatment consisted of mastectomy, lumpectomy or OBS in combination with either SLNB or ALND | All patients received the surgical intervention described. The surgical and adjuvant treatments were standardised according to DBCG guidelines | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Morrow 2019 | Mx | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (histological type (MxIR), tumour size (MxIR), grade (MxIR), axillary node status (BCS), immunohistochemical receptors), some significantly different (age (all), histological type (BCS, Mx), tumour size (BCS, Mx), grade (BCS, Mx), axillary node status (Mx, MxIR)). Important co‐interventions significantly different (adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Potter 2020 | Mx | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (age, diabetes, BMI, other medical co‐morbidities, histological type,grade, axillary node status, immunohistochemical receptors, multifocality). Tumour size missing. Important co‐interventions significantly different (neoadjuvant CT, adjuvant RT, adjuvant CT, axillary surgery) | Selection from participants in other studies (iBRA‐2 and TeaM studies ) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Kahn 2013 | Mx + R | Serious | Low | Moderate | No information | Low | Serious | Moderate | Serious |
| Clinicopathological factors missing. Adjuvant CT balanced | All participants eligible included, consecutive patients to reduce selective bias | Some plane mobilisation without skin reduction counted as WLE. This is standard practice so minor risk of bias due to this | ‐ | All patients included followed up | Date calculated from when MDT decided to give CT, this is not an objective date and could be different across the two groups | No indication of selected reporting | |||
| Morrow 2019 | Mx + R | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Some variables demonstrated balance (histological type (MxIR), tumour size (MxIR), grade (MxIR), axillary node status (BCS), immunohistochemical receptors), some significantly different (Age (all), histological type (BCS, Mx), tumour size (BCS, Mx), grade (BCS, Mx), axillary node status (Mx, MxIR)). Important co‐interventions significantly different (adjuvant RT) | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Potter 2020 | Mx + R | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Important clinicopathological factors significantly different (age, diabetes, BMI, other medical co‐morbidities, histological type,grade, axillary node status, immunohistochemical receptors, multifocality). Tumour size missing. Important co‐interventions significantly different (neoadjuvant CT, adjuvant RT, adjuvant CT, axillary surgery) | Selection from participants in other studies (iBRA‐2 and TeaM studies ) | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | All patients included followed up | Objective outcome measure | No indication of selected reporting | |||
| Tong 2016 | Mx + R | Serious | Low | Low | Low | Moderate | Moderate | Moderate | Serious |
| Some variables demonstrated balance, some significantly different (age, Diabetes, BMI, other comorbidities, preoperative bra size), some missing. Important co‐interventions significantly different (neoadjuvant RT, adjuvant RT), some missing | All participants eligible included | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods | All patients included followed up, but median follow‐up was significantly different between groups. "Median follow‐up was 4 months longer for the oncoplastic breast reconstruction group than for the immediate breast reconstruction group (18.7 months versus 14.0 months, respectively; P < 0.001)" | Objective outcome measure. Unclear why delay is defined as over 6 weeks "Complications that delayed the initiation of adjuvant chemotherapy or radiation therapy for greater than 6 weeks postoperatively were recorded." Outcome measure likely only minimally influenced by knowledge of intervention | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy Mx: mastectomy MxIR: mastectomy and immediate reconstruction PR: progesterone receptor R: reconstruction RT: radiotherapy
DBCG: Danish Breast Cancer Co‐operative Group SLNB ‐ Sentinel lymph node biopy ALND ‐ Axillary lymph node dissection iBRA‐2: Immediate breast reconstruction and therapy audit TeaM: Tamoxifen Exemestane Adjuvant Multinational Study
9. Risk of bias for cosmetic evaluation.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acosta‐Marin 2014 | S‐BCS | Serious | Serious | Low | Low | Serious | Serious | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, BMI) and some significantly different (preoperative bra size, tumour size), most missing | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data ‐ missed women with complications in short term. If major may have had to have mastectomy and therefore excluded | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2007 (2) | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors demonstrated balance (BMI, tumour size, tumour location), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All operations done by 2 experienced surgeons. | All patients included followed up | 2 person panel of experts, bias likely to influence outcome | No indication of selected reporting | |||
| Hilli‐Betz 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (axillary node status) and some different (tumour size, pre‐operative bra size), some missing. Axillary management demonstrated balance, most co‐interventions missing | Women were invited to return, not all of them did | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | Objective software/panel assessment | No indication of selected reporting | |||
| Jiang 2015 | S‐BCS | Moderate | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors balanced (age, weight, histology type,tumour size, grade, stage, tumour location) | 60 women were picked, study says randomised but not clear how therefore classified as cohort. Risk of selection | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | 3 person panel of experts, bias likely to influence outcome | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Serious | Low | Low | Low | Critical | Serious | Critical |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor) some different (preoperative bra size, axillary node status) but unlikely to affect outcome. Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | Not all patients responded | Classification of interventions clear and determined at the start of intervention. The types of intervention were: Therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern), Dermoglandular rotation (medial, lateral mammoplasty), Periareolar (round block, omega) or standard BSC | All patients received the surgical intervention described in the methods. Operations done by experienced breast surgeons. | Patients missed due to loss to follow up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | 3 person panel of experts, bias likely to influence outcome | Details not given | |||
| Lansu 2014 | S‐BCS | Moderate | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Important clinicopathological factors balance (age, tumour size, tumour location), some missing. Important co‐interventions demonstrated balance, some significantly different | Patients had to be disease free and alive at the time of inclusion | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described | Most patients responded and followed up | Objective BCCT.core score | No indication of selected reporting | |||
| Santos 2015 | S‐BCS | Serious | Serious | Low | Low | Low | Moderate | Moderate | Serious |
| Some variables matched and demonstrated balance, stage significantly different: BMI, histological type, axillary node status. Intervention and control from different locations. Axillary management balanced, important co‐interventions missing: medical cancer treatment | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected | Classification of interventions clear and determined at the start of intervention: 'first group underwent level 2 O‐BCS techniques (bilateral surgeries with mammaplasty techniques', 'second group underwent lumpectomy with incisions over the tumour, without removing skin (except in cases where the tumours where close to skin)' | All patients received the surgical intervention described in methods | All patients included followed up | Objective BCCT.core score. Cosmesis also evaluated by two independent plastic surgeons and two breast surgeons using Garbay's criteria | No indication of selected reporting | |||
| Scheter 2019 | S‐BCS | Serious | Serious | Low | Low | Low | Serious | Serious | Serious |
| Important clinicopathological factors statistically adjusted for or demonstrated balance. Some significantly different: age, smoking status, tumour size. Some missing: axillary node status, grade, stage. Important co‐interventions demonstrated balance (medical cancer treatment and axillary management) | Patients were excluded if they proceeded to have a mastectomy after the intervention: "Patients who had subsequently proceeded to total mastectomy were excluded from the study." | Classification of interventions clear and determined at the start of intervention. Technique clearly described in methods: 'Patients with centrally located tumours who required NAC re‐ section and had medium‐ or large‐sized ptotic breasts were offered immediate OBR using a breast reduction pattern technique. Patients in the control group underwent primary closure of the NAC area in a horizontal or oblique scar and no oncoplastic reconstruction.' | All patients received the surgical intervention described in methods | All patients included followed up | 13 person panel of experts, bias likely to influence outcome | Selective reporting of certain domains | |||
| Viega 2011 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Serious | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (age, BMI, tumour size, tumour location) and "matched for demographic and oncologic aspects". Important co‐interventions demonstrated balance (adjuvant RT, adjuvant CT, axillary management), says some demographic and oncological aspects matched for | Unclear why these 45 patients were selected | Classification of interventions clear and determined at the start of intervention: "All patients underwent quadrantectomy and in most of them sentinel lymph node biopsy was performed. Breast reconstruction procedures included local flaps or breast reduction techniques. Neither distant flaps nor prosthesis were used." | All patients received the surgical intervention described in methods | Some patients were lost to follow‐up at 12 months: PParticipation rates at the follow‐up assessments of oncoplastic group were 100% at the 6th month and 88.9% at the 12th month follow‐up." | 4 person panel of experts, bias likely to influence outcome but tried to limit by standardisation and blinding of methods: "The aesthetic results of control group and oncoplastic group at 6 and 12 months postoperatively were evaluated through photographs of pre and postoperative, by a panel of four independent raters, according to the criteria shown on Table 4, modified from Garbay et al" | No indication of selected reporting | |||
| Gendy 2003 | Mx | Moderate | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors balanced (age, grade, axillary node status), some significantly different (histological type, tumour size), some missing. Important co‐interventions different across intervention group | All contactable participants | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All surgeries done by an experienced surgeon/under their supervision | All patients included followed up | 5 person panel of experts, bias likely to influence outcome | No indication of selected reporting | |||
| Ozmen 2020 | Mx +R | Serious | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patient received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery. | Most patients included: "Median follow‐up time was 56 (14‐116) months." | Cosmetic evaluation reporting tool validated but vulnerable to bias from subjective knowledge of intervention. Carried out by a single surgeon: "The cosmetic evaluation was conducted by a plastic surgeon who was not part of the surgical team." | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ER: oestrogen receptor ET: endocrine therapy Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy
NAC: neoadjuvant chemotherapy
10. Risk of bias for patient‐reported outcome measures.
| Study | Control | Confounding | Selection | Classification of intervention | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of reported results | Overall |
| Acea‐Nebril 2017 | S‐BCS | Serious | Serious | Low | Moderate | Moderate | Serious | Critical | Critical |
| Some clinicopathological variables significantly different (age, menopausal status, tumour size, tumour stage, axillary lymph node status, location of tumour, multifocality). Some co‐interventions balanced (neoadjuvant CT and axillary management), some missing | Selection into the study may have been related to intervention and follow‐up time may miss initial time as questionnaire at 12 and 24 months. Only reported intervention results | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | Deviation from intended co‐intervention (adjuvant therapy time) | Analysis unlikely to have removed risk of bias from missing data ‐ not all women returned the form. Reasons due to recurrence, death, completion mastectomy (all things that would have likely affected PROMs) | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | Selective questionnaire results reported only of intervention | |||
| Acosta‐Marin 2014 | S‐BCS | Serious | Serious | Low | Low | Moderate | Serious | Moderate | Serious |
| Some clinicopathological variables demonstrated balance (age, BMI) and some significantly different (preoperative bra size, tumour size), most missing | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention. Operative details given clearly | All patients received the surgical intervention described in the methods | Analysis unlikely to have removed risk of bias from missing data ‐ missed women with complications in short term. If major may have had to have mastectomy and therefore excluded | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Di Micco 2017 | S‐BCS | Serious | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors demonstrated balance (smoking status, BMI, histological type, tumour size, immunohistochemical receptor, tumour location), some significantly different (age, axillary node status). Some co‐interventions balanced across intervention group (neoadjuvant CT, adjuvant ET, axillary management, adjuvant RT), some different (radiation boost, adjuvant CT) | Selection may be related to the outcome (mastectomy eventually) | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients followed up | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Eichler 2013 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Critical | Moderate | Critical |
| Some clinicopathological variables demonstrated balance (age, histological type, grade), tumour size different, some missing. Adjuvant CT balanced across intervention group, neoadjuvant CT significantly different | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Not all patients responded to questionnaire | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Gicalone 2007 (2) | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors demonstrated balance (BMI, tumour size, tumour location), some missing | Women chose their operation after being told the potential risks and benefits. Bias in assignment | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods | All patients included followed up. All surgeries done by an experienced surgeon/under their supervision | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Hilli‐Betz 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Some clinicopathological variables demonstrated balance (axillary node status) and some different (tumour size, preoperative bra size), some missing. Axillary management demonstrated balance, most co‐interventions missing | Women were invited to return, not all of them did | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Jiang 2015 | S‐BCS | Moderate | Moderate | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors balanced (age, weight, histology type,tumour size, grade, stage, tumour location) | 60 women were picked, study says randomised but not clear how therefore classified as cohort. Risk of selection | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Keleman 2019 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Serious | Serious | Serious |
| Some variables demonstrated balance (age, smoking status, diabetes, BMI, type of cancer, tumour size, grade, stage, immunohistochemical receptor) some different (preoperative bra size, axillary node status) but unlikely to affect outcome. Important co‐intervention of adjuvant RT demonstrated balance, some significantly different (neoadjuvant CT, adjuvant CT, adjuvant ET, axillary management) but less of an impact on outcome | Not all patients responded | Classification of interventions clear and determined at the start of intervention. The types of intervention were: therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern), Dermoglandular rotation (medial, lateral mammoplasty), Periareolar (round block, omega) or standard BCS | All patients received the surgical intervention described in the methods. Operations done by experienced breast surgeons | Patients missed due to loss to follow‐up and did not respond to outcome, equal numbers in both groups so impact may be similar across groups | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | Selective details not given | |||
| Lansu 2014 | S‐BCS | Moderate | Moderate | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors balance (age, tumour size, tumour location), some missing. Important co‐interventions demonstrated balance, some significantly different | Patients had to be disease‐free and alive at the time of inclusion | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described | All patients included followed up | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Matrai 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Tumour size significantly different. Some variables demonstrated balance (age, histological type, grade, tumour location, bra size, immunohistochemical receptor, axillary lymph node status). Matching of patients reported but not defined: "the same clinicopathological parameters of 60 traditional breast‐conserving surgeries operated by the same breast surgeon were used". Important co‐interventions including adjuvant RT demonstrated balance. Adjuvant CT significantly different | Unclear why these 60 patients selected (not consecutive, some retrospective and some prospective), controls matched | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods. Operation done by experienced surgeon | All patients included followed up | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Mazouni 2013 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Critical | Moderate | Critical |
| Important clinicopathological factors balance: histological type, tumour size, grade, axillary node status, immunohistochemical receptor (PR). Some clinicopathological factors statistically different: tumour location. Some factors missing: age, BMI, preoperative bra size. Important co‐interventions pre‐defined and uniform across studies (axillary surgery, neoadjuvant CT, adjuvant RT) | Most participants eligible included, Patients who subsequently underwent mastectomy excluded from survey | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | Most patients responded | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Ojala 2017 | S‐BCS | Serious | Moderate | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors significantly different (tumour size, tumour location, axillary node status, multifocality, histological type). Important co‐interventions missing, adjuvant RT demonstrated balance, axillary management significantly different | All participants eligible included: "All patients having breast conserving surgery (BCS) due to primary breast cancer at the Helsinki and Uusimaa Hospital District during 2010 were included in this study, most had PROMs" | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | Most patients responded (279/293 conventional, 86/86 O‐BCS) | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Palsodittlir 2018 | S‐BCS | Serious | Moderate | Low | Low | Moderate | Critical | Serious | Critical |
| Some variables demonstrated balance, some significantly different (e.g. tumour size), some missing (grade, stage, location of tumour). Adjuvant ET balanced, some co‐interventions missing: radiotherapy, chemotherapy, axillary management | All women included according to selection criteria. Selection criteria excluded level 2 O‐BCS procedures assigning these as minimal: "Level 1 and level 2 oncoplastic procedures (minimal gland mobilisation techniques) were not included in the study group." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | Some patients did not respond to questionnaire, and so were not included: "Question lists were sent to 448 women in total. Of those, 75 were in the O‐BCS group and 373 in the S‐BCS group. Response rate was 68% in the OBCS group but 43% in the SBCS group." | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | Selective reporting of detailed questionnaire | |||
| PlaFarnos 2018* | S‐BCS | Serious | Moderate | Low | No information | No information | Serious | Serious | Serious |
| Multifocality significantly different, most clinicopathological variables missing | Selection into the study may have been related to intervention and may miss initial follow‐up period Selection into the study may have been related to intervention ‐ it is not clear how the 60 patients in the O‐BCS group and 120 in the control were selected for in that time period |
Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | ‐ | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | Details of Breast‐Q domains not reported | |||
| Rose 2020 | S‐BCS | Moderate | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors statistically adjusted for: age, tumour size, tumour location, bra size, BMI. Location of surgeries different in intervention and control. Co‐intervention statistically adjusted for: axillary management, some medical cancer treatment | Patients selected based on those that responded to questionnaire sent out via post or email: "the response rates for the BCS and OBS cohorts were 48.4% (631/1304) and 48.0% (96/200), respectively" | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | All patients included | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Santos 2015 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Some variables matched and demonstrated balance, stage significantly different: BMI, histological type, axillary node status. Intervention and control from different locations. Axillary management balanced, important co‐interventions missing: medical cancer treatment | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected | Classification of interventions clear and determined at the start of intervention: "first group underwent level 2 OP techniques (bilateral surgeries with mammaplasty techniques', 'second group underwent lumpectomy with incisions over the tumour, without removing skin (except in cases where the tumours where close to skin)" | All patients received the surgical intervention described in methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Scheter 2019 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors statistically adjusted for or demonstrated balance. Some significantly different: age, smoking status, tumour size. Some missing: axillary node status, grade, stage. Important co‐interventions demonstrated balance (medical cancer treatment and axillary management) | Patients were excluded if they proceeded to have a mastectomy after the intervention: "Patients who had subsequently proceeded to total mastectomy were excluded from the study." | Classification of interventions clear and determined at the start of intervention. Technique clearly described in methods: "Patients with centrally located tumours who required NAC re‐ section and had medium‐ or large‐sized ptotic breasts were offered immediate OBR using a breast reduction pattern technique. Patients in the control group underwent primary closure of the NAC area in a horizontal or oblique scar and no oncoplastic reconstruction." | All patients received the surgical intervention described in methods | Almost all patients included in follow‐up: The questionnaire response rate was high: 11 of the 12 patients in each group (92%) | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Sherwell‐Cabello 2006 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Some variables demonstrated balance (age, medical comorbidities), some significantly different (tumour size, stage, axillary node status), some missing. Neoadjuvant chemotherapy is significantly different between groups. No information on other important co‐interventions (radiotherapy, adjuvant treatment, axillary management) | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected: "All patients diagnosed with breast cancer treated under conventional conservative surgery or oncoplastic patterns at the Institute of Breast Diseases, FUCAM AC, with a complete clinical history and had answered a questionnaire of aesthetic satisfactory in person or by phone were included. Those who did not continue their follow‐up at the institution were eliminated from the study." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Srivastava 2018* | S‐BCS | No information | No information | Low | No information | No information | Serious | Serious | Serious |
| ‐ | ‐ | Classification of interventions clear and determined at the start of intervention. Some aspects maybe determined retrospectively | ‐ | ‐ | Validated reporting tool but vulnerable bias from subjective knowledge of intervention | Selective questionnaire results reported | |||
| Tang 2016 | S‐BCS | Moderate | Moderate | Low | Low | Low | Critical | Moderate | Critical |
| Important clinicopathological factors demonstrated balance (tumour size, stage, BMI, age). Some co‐interventions balanced (axillary management), some missing (medical cancer treatment) | All participants eligible included | Classification of interventions clear and determined at the start of intervention: "Standard Breast Conservation Surgery (SBCS) group had surgery conducted according to the National Surgical Adjuvant Breast and Bowel Project (NSABP) standard guidelines." | All patients received the surgical intervention described in methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Tenofsky 2014 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Some variables demonstrated balance, some significantly different, some missing (histological type, grade, stage, axillary node status). Important co‐interventions significantly different (adjuvant RT), some missing (neoadjuvant RT + CT, adjuvant CT + ET, axillary management) | Participants were excluded if they went on to require mastectomy 6 months after procedure, or if lost to follow‐up within 6 months: "Patients were excluded if they received a mastectomy within 6 months of the lumpectomy, and/or if they received 6 months of follow‐up after their procedure." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. Operations done by experienced surgeon. | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Viega 2011 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Critical | Moderate | Critical |
| Important clinicopathological factors demonstrated balance (age, BMI, tumour location) and "matched for demographic and oncologic aspects." Important co‐interventions demonstrated balance (adjuvant RT, adjuvant CT), "some demographic and oncological aspects matched for" | Not clear how patients were enrolled. For case group, allocation to type of procedure was based on patient choice | Classification of interventions clear and determined at the start of intervention: "All patients underwent quadrantectomy, and in most of them, sentinel lymph node biopsy was performed. Breast reconstruction procedures were performed by the same plastic surgery team with the use of adjacent tissues, local flaps, or breast reduction techniques. Neither distant flaps nor prostheses were used." | All patient received the surgical intervention described in methods | Some patients were lost in follow‐up: 5 in case group. "Participation rates at the follow‐up assessments were 95.5 per cent at the 6‐month follow‐up and 88.9 per cent at the 12‐month follow‐up." | PROMs reporting tool validated but not for breast cancer and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Viega 2010 | S‐BCS | Moderate | Serious | Low | Low | Moderate | Critical | Moderate | Critical |
| Important clinicopathological factors demonstrated balance (age, BMI, tumour location) and 'matched for demographic and oncologic aspects '. Important co‐interventions demonstrated balance (adjuvant RT, adjuvant CT), 'some demographic and oncological aspects matched for | Not clear how patients were enrolled. For case group, allocation to type of procedure was based on patient choice | Classification of interventions clear and determined at the start of intervention: "All patients underwent quadrantectomy, and in most of them, sentinel lymph node biopsy was performed. Breast reconstruction procedures were performed by the same plastic surgery team with the use of adjacent tissues, local flaps, or breast reduction techniques. Neither distant flaps nor prostheses were used." | All patients received the surgical intervention described in methods. All surgeries by same team of surgeons | Some patients were lost in follow‐up: 5 in case group. "Participation rates at the follow‐up assessments were 95.5 per cent at the 6‐month follow‐up and 88.9 per cent at the 12‐month follow‐up." | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Zhou 2019 | S‐BCS | Serious | Serious | Low | Low | Low | Critical | Moderate | Critical |
| Some variables demonstrated balance, some significantly different (tumour size), some missing. Some co‐interventions balanced (adjuvant RT, axillary management), some missing (all other cancer treatment) | Patients selected based on those that responded to questionnaire, not clear if/why all patients in the time period not selected. Patients also excluded if failure to complete follow‐up | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in the methods | All patients included followed up | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Gendy 2003 | Mx | Moderate | Serious | Low | Low | Moderate | Serious | Serious | Serious |
| Important clinicopathological factors balanced (age, grade, axillary node status), some significantly different (histological type, tumour size), some missing. Important co‐interventions different across intervention group | All contactable participants | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods. All surgeries done by an experienced surgeon/under their supervision. | Not all patients responded to questionnaire | Various validated scales but subject to bias | All PROMs not mentioned for all patients | |||
| Hart 2015 | Mx + R | Serious | Serious | Low | Low | Moderate | Critical | Moderate | Critical |
| Some clinicopathological variables significantly different (age, BMI), stage balanced, some missing. Adjuvant RT significantly different, most co‐interventions missing | Only some patients responded | Classification of interventions clear and determined at the start of intervention, operative details given clearly | All patients received the surgical intervention described in the methods | Not all patients responded to questionnaire | PROMs reporting tool not validated and vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Kelsall 2017 | Mx + R | Serious | Moderate | Low | Low | Low | Serious | Moderate | Serious |
| Important variables matched (age, tumour size, date of surgery, breast size) or demonstrated balance (axillary node status). Important co‐interventions demonstrated balance (adjuvant CT, adjuvant ET and neoadjuvant CT) and some significantly different (adjuvant RT) | Selection based on patient reported outcome measures data | Classification of interventions clear and determined at the start of intervention. Surgery was either O‐BCS (requiring therapeutic mammaplasty or volume replacement with a local chest wall perforator flap); or mastectomy with immediate reconstruction | All patient received the surgical intervention described in methods | All patients included followed up | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting | |||
| Ozmen 2020 | Mx + R | Serious | Serious | Low | Low | Low | Serious | Moderate | Serious |
| Important clinicopathological factors balance, some different (age, menopausal status, BMI, tumour size, grade, axillary node status, immunohistochemical receptor status (ER), multifocality), some missing. Important co‐interventions significantly different (adjuvant RT and axillary management), some missing (neoadjuvant RT + CT, adjuvant CT + ET) | Women chose their operation after being told the potential risks and benefits. Bias in assignment: "Both two procedures were explained to patients, and their choices were recorded." | Classification of interventions clear and determined at the start of intervention | All patients received the surgical intervention described in methods. All operations done by a single surgeon with more than 30 years of experience in breast surgery. | Most patients responded | PROMs reporting tool validated but vulnerable to bias from subjective knowledge of intervention | No indication of selected reporting |
BMI: body mass index CT: chemotherapy ET: endocrine therapy Mx: mastectomy PR: progesterone receptor R: reconstruction RT: radiotherapy
PROM: Patient‐reported outcome measure
When considering treatment effects, we took into account the risk of bias for studies that contribute to each outcome.
Confounding and adjustment
We identified the confounding factors that the researchers had considered, recorded whether they had been measured and what researchers had done to control for bias. That is, any design features used for this purpose (for example, matching or restriction to particular subgroups) and the methods of analysis (for example, stratification, regression modelling with propensity scores or covariates). We have displayed as a table a list of confounders mentioned by the studies (Table 4), and detail how the studies dealt with them; for example, restricted participant selection, demonstrated a balance between groups for the confounder, matched on the confounder or adjusted for the confounder in statistical analyses to quantify the effect size.
Measures of treatment effect
We reported time‐to‐event outcomes (that is, local recurrence, overall survival) as hazard ratios (HRs) with 95% confidence intervals (CIs). We estimated HRs using the methods of Parmar 1998 if possible. We used this method to extract HRs for local recurrence from three studies (Niinikoski 2019 (2); Piper 2016; Ren 2014), for disease‐free survival from four studies (DeLorenzi 2018; Mazouni 2013; Ozmen 2020; Vieira 2016), and for overall survival from six studies (DeLorenzi 2018; Gulcelik 2013; Mazouni 2013; Ozmen 2020; Ren 2014; Vieira 2016). We were unable to estimate HRs from three studies as there were not enough data to calculate the HR (Acea‐Nebril 2017; Chakravorty 2012; Lee 2018).
For local recurrence, the data were reported as either local recurrence rate or local recurrence‐free survival. We extracted both, but were not able to combine these two outcomes. If it was not possible to estimate HRs from all studies, we treated the number of events (that is, recurrences, deaths) from treatment date to 1 year, from treatment date to between 1 year and < 5 years, from treatment date to 5 years, and from treatment date to 10 years of follow‐up as dichotomous outcomes.
We reported continuous outcomes (that is, patient‐reported outcome measures, quality of life) as mean differences (MDs) with 95% CIs.
We reported dichotomous outcomes (that is, re‐excision rates, local or distant recurrence (if not a time‐to‐event outcome), any complications of surgery) as risk ratios (RRs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the study as this systematic review used aggregated data and not individual data. We planned to exclude cross‐over and cluster‐RCTs but there were none.
Dealing with missing data
When studies reported one primary outcome but other primary outcome data were missing, we contacted the authors to request further information. If data were missing to the extent that we could not include the study in a meta‐analysis and our attempts to retrieve data have been exhausted, we would present the results in the review and discuss in the context of the findings. We planned to discuss the impact of missing data and imputation methods in the Discussion section of the review, and if necessary conduct a sensitivity analysis.
Assessment of heterogeneity
If we could combine results in a meta‐analysis, we assessed heterogeneity using the I² statistic (Higgins 2003), and interpreted this according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity*
50% to 90%: may represent substantial heterogeneity*
75% to 100%: considerable heterogeneity*
* In cases of moderate or high heterogeneity, we explored potential sources of heterogeneity by performing sensitivity analyses.
Assessment of reporting biases
We searched for protocols of included studies using PubMed and other trial registries, when possible. If more than 10 trials were included in a meta‐analysis, we assessed publication bias and other reporting biases by visual inspection of funnel plots for primary outcomes (Higgins 2021).
Data synthesis
If it was appropriate to perform a meta‐analysis (wherein the population, intervention, comparison and outcomes are deemed similar enough to pool), we synthesised data using RevMan Web (RevMan5). We used a fixed‐effect model for data synthesis and explored the impact of model choice through sensitivity analysis. We pooled HRs using the generic inverse variance method.
When meta‐analysis was not possible, we considered other methods of analysis following guidance from the Cochrane Handbook on synthesising and presenting data using other methods (McKenzie 2021). When results provided a direction of effect we used the vote counting method. This method provides no information on the magnitude of effects nor does it account for differences in the relative sizes of the studies.
If the data were too diverse to permit combining of effect sizes in a meaningful or valid manner, we presented the results of individual studies in table and graphical formats and used a narrative approach to summarise the data. We provided a narrative synthesis of the findings from the included studies, structured around the type of intervention, target population characteristics, type of outcome and intervention content. We followed the Cochrane guidelines for a narrative summary (Ryan 2013).
If sufficient evidence of high certainty were available for local recurrence rates, we planned to compare the results to a typical non‐inferiority standard of "less than 5% ipsilateral breast tumour recurrence at 5 years follow‐up", which is set for any breast conservation therapy by the Association of Breast Surgery (UK) at the British Association of Surgical Oncology (BASO) 'Surgical guidelines for the management of breast cancer' (Association of Breast Surgery 2012).
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis comparing and discussing the two main techniques of O‐BCS — volume displacement and partial volume replacement — with any other options in BCS (if there were a minimum of 5 studies). This meant we conducted the following further analyses.
Volume displacement techniques versus S‐BCS
Volume displacement versus mastectomy alone
Volume displacement versus mastectomy plus reconstruction
Volume replacement techniques versus S‐BCS
Volume replacement versus mastectomy alone
Volume replacement versus mastectomy plus reconstruction
We planned that if data were available, we would present one particular technique of O‐BCS versus any other available option for breast cancer surgery. In addition, we planned to present data from studies that compare the various types of O‐BCS with each other, specifically relating to those listed in the experimental interventions section, but there were no data for this. Further subgroup analysis may be possible in future reviews.
Sensitivity analysis
We conducted the following sensitivity analyses.
Quality assessment of included studies (removing studies that are at high risk of bias for RCTs or critical risk of bias for non‐randomised studies from the meta‐analysis, whilst noting all studies in a narrative synthesis)
Fixed‐effect model versus random‐effects model
We commented if sensitivity analysis changed any of the meta‐analysis in the main analysis that had moderate or high heterogeneity.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence of the main outcomes. We used the overall ROBINS‐I judgement to feed into the GRADE assessment. We calculated the estimate of absolute risk for outcomes displayed as HRs using an average of unadjusted baseline control event rates from the included studies. Two review authors (AN and SH) used GRADEpro GDT software to develop the summary of findings tables using the following main outcomes.
Local recurrence at 5 years: shown as local recurrence‐free survival and local recurrence rate
Breast cancer‐specific disease‐free survival at 5 years
Re‐excisions: need for further breast surgery due to inadequate cancer resection
Complications
Recall rates: number of biopsies needed in follow‐up period
Patient‐reported outcome measures, such as patient satisfaction
Results
Description of studies
Results of the search
We identified 7910 references through our electronic and manual searches. After removing duplicate records, we retrieved 7902 references. After screening the full text, we identified the 78 observational studies to include in the review. Searching of the reference lists of eligible publications did not reveal additional publications for inclusion. Summarised in Figure 1.
1.

Study flow diagram
We excluded three publications (Angarita 2019; Kelemen 2016; Niinikoski 2019 (1)) because they were published as conference abstracts and then later published as journal articles (Angarita 2020; Keleman 2019; Niinikoski 2019 (2)). One publication Cil 2016 was an earlier dataset of Angarita 2020, which was a larger more recent dataset. Data were extracted from these publications but they were excluded from analyses to avoid duplication of results.
Included studies
Design
All 78 included studies were non‐randomised cohort studies. Four studies were described as case controls, but according to the Cochrane Handbook (Higgins 2021), they were cohort studies due to selecting participants based on intervention rather than outcome (Atallah 2015; Ozmen 2016; PlaFarnos 2018; Vieira 2016). Sixty studies were retrospective (77%) and 18 (23%) prospective studies.
Setting
The majority of the studies were based in the USA and UK. For a full breakdown of the countries see Table 14. Sixty‐three studies were single‐centre (81%), ten (13%) were multi‐centre and five (6%) were large international/national database reviews.
11. Countries of studies.
| Countries | Number |
| Belgium | 1 |
| Brazil | 4 |
| China | 4 |
| Denmark | 3 |
| Europe | 2 |
| Finland | 3 |
| France | 6 |
| Germany | 3 |
| Hungary | 2 |
| Iceland | 1 |
| India | 2 |
| Israel | 2 |
| Italy | 2 |
| Japan | 3 |
| Korea | 2 |
| Mexico | 1 |
| Netherlands | 2 |
| Pakistan | 1 |
| Spain | 3 |
| Turkey | 3 |
| UK | 13 |
| USA | 14 |
| Venezuela | 1 |
Most articles were published in English. Six papers were translated into English from Mandarin (Jiang 2015; Tang 2016); Hungarian (Matrai 2014); Spanish (Acea‐Nebril 2005; Sherwell‐Cabello 2006), and French (Gicalone 2015).
Population
We included 78 observational studies with 178,813 participants in the review. All participants were patients with primary breast cancer. The details of inclusion and exclusion can be found in the individual study details. Some papers only included subsets of patients with primary breast cancer, such as those with certain histological types of cancer (e.g. DeLorenzi 2018) or size (e.g. Di Micco 2017) or location (e.g. Gulcelik 2013) or co‐intervention (e.g.Chauhan 2016 (1); Chauhan 2016 (2)), but we did not differentiate and included all studies of patients with breast cancer that had a surgical intervention as part of their treatment. The age range was 23 to 86 years in the intervention group and 23 to 90 years in the comparison group. The relationship of clinicopathological factors of participants within studies varied, which is displayed in detail in Table 4. Future reviews may consider evaluating these differences as subgroups.
Intervention
We identified two distinct types of intervention: volume replacement and volume displacement O‐BCS. Some studies did not differentiate these methods and combined the techniques as O‐BCS.
Twenty‐one studies combined volume displacement techniques, and we assumed one study (Farooqi 2019), where the details were unclear, to be in this category (27%). Two studies (3%) analysed both volume displacement and replacement techniques and analysed them separately (Bali 2018; Lee 2018 3%).
We classified 44 studies (56%) as volume displacement O‐BCS only. Borm 2019 involved 288 participants that underwent volume displacement surgery and one participant underwent volume replacement. Therefore, we classified this study in the volume displacement category. The breakdown of techniques is displayed in the Characteristics of included studies tables.
We classified 11 (14%) studies as volume replacement O‐BCS only. Seven of these studies evaluated the latissimus dorsi mini‐flap (Fan 2019; Hashimoto 2019; Mustonen 2004; Ozmen 2016; Ozmen 2020; Ren 2014; Zhou 2019). The breakdown of techniques in all studies is displayed in the Characteristics of included studies tables.
The co‐interventions varied among studies and were determined by local guidance and cancer multidisciplinary team decisions. The relationship within the studies is shown in Table 4.
Comparison
We identified three distinct types of control: BCS, mastectomy alone and mastectomy with reconstruction. The breakdown of techniques in all studies is displayed in the Characteristics of included studies tables; some had multiple groups of comparison. The combinations of intervention and comparisons can be seen in Table 15.
12. Matrix of interventions and controls.
| Intervention | |||
| Control | Volume displacement | Volume replacement | Both |
| BCS | 39 | 6 | 13 |
| Mx | 0 | 3 | 0 |
| Mx+R | 3 | 2 | 1 |
| Mx+‐R | 0 | 0 | 1 |
| BCS/Mx | 2 | 0 | 0 |
| BCS/Mx+‐R | 0 | 0 | 2 |
| BCS/Mx/Mx+R | 2 | 0 | 2 |
| Mx/Mx+R | 1 | 0 | 0 |
BCS: breast‐conserving surgery Mx: mastectomy R: reconstruction
O‐BCS versus S‐BCS: 16 studies compared any O‐BCS (volume displacement and replacement together) to a form of BCS (Angarita 2020; Chauhan 2016 (1); Chauhan 2016 (2); DeLorenzi 2016 (1); DeLorenzi 2018; Dolan 2015; Down 2013; Farooqi 2019; Hamdi 2008; Mukhtar 2018; Palsodittlir 2018; Rose 2019; Rose 2020; Tang 2016; Viega 2010; Viega 2011). These studies contributed to the main analysis of O‐BCS versus S‐BCS. One study compared O‐BCS to BCS and analysed volume displacement and replacement techniques separately (Bali 2018), and so contributed to both the main analysis of O‐BCS versus S‐BCS and both subgroup analyses. Thirty‐six studies compared volume displacement O‐BCS only compared to S‐BCS (Acea‐Nebril 2017; Acosta‐Marin 2014; Amitai 2018; Atallah 2015; Borm 2019; Cassi 2016; Chakravorty 2012; Crown 2015; Crown 2019; Di Micco 2017; Eichler 2013; Gicalone 2007 (1); Gicalone 2007 (2); Gicalone 2015; Gulcelik 2013; Hilli‐Betz 2014; Jiang 2015; Keleman 2019; Kimball 2018; Lansu 2014; Losken 2009; Losken 2014; Malhaire 2015; Matrai 2014; Mazouni 2013; Niinikoski 2019 (2); Ojala 2017; Palsodittlir 2018; Piper 2016; Santos 2015; Scheter 2019; Sherwell‐Cabello 2006; Tenofsky 2014; Vieira 2016; Wijgman 2017; Wong 2017), and contributed to the main analysis of O‐BCS versus S‐BCS and the subgroup analysis of volume displacement O‐BCS versus S‐BCS. Six studies compared volume replacement O‐BCS to S‐BCS (Fan 2019; Hashimoto 2019; Hu 2019; Nakada 2019; Ozmen 2016; Zhou 2019), and so contributed to the main analysis of O‐BCS versus S‐BCS and the subgroup analysis of volume replacement O‐BCS versus S‐BCS.
O‐BCS versusmastectomy (Mx): three studies compared volume replacement O‐BCS to mastectomy without reconstruction (Gendy 2003; Nakagomi 2019; Ren 2014), and contributed to the main analysis of O‐BCS versus mastectomy without reconstruction and the subgroup analysis of volume replacement O‐BCS versus mastectomy.
O‐BCS versusmastectomy + reconstruction (Mx + R): one study compared any O‐BCS (volume displacement and replacement together) to mastectomy with reconstruction (Kelsall 2017), and contributed to the main analysis of O‐BCS versus mastectomy with reconstruction. Three studies compared volume displacement only to mastectomy with reconstruction (Hart 2015; Peled 2014; Tong 2016), and contributed to the main analysis of O‐BCS versus mastectomy with reconstruction and the subgroup analysis of volume displacement O‐BCS versus mastectomy plus reconstruction. Two studies compared volume replacement only to mastectomy with reconstruction (Mustonen 2004; Ozmen 2020), and contributed to the main analysis of O‐BCS versus mastectomy with reconstruction and the subgroup analysis of volume replacement O‐BCS versus mastectomy plus reconstruction.
O‐BCS versusmastectomy with or without reconstruction (Mx +/‐ R): one study compared any O‐BCS (volume displacement and replacement together) to mastectomy with or without reconstruction (DeLorenzi 2016 (2)). We have included these studies in the main analyses of O‐BCS versus mastectomy and O‐BCS versus mastectomy with reconstruction, but given they combine mastectomy with and without reconstruction as a control group they are separated when pooling.
O‐BCS versusBCS/Mx: one study compared any O‐BCS (volume displacement and replacement together) to S‐BCS and mastectomy without reconstruction (Klit 2017). One study compared volume displacement O‐BCS to S‐BCS and mastectomy without reconstruction (Acea‐Nebril 2005), and contributed to both the main analyses of O‐BCS versus S‐BCS and O‐BCS versus mastectomy as well as the subgroup analyses of volume displacement O‐BCS versus S‐BCS and versus mastectomy.
O‐BCS versusBCS/Mx +/‐ R: two studies compared any O‐BCS (volume displacement and replacement together) to BCS and mastectomy with or without reconstruction combined (Mansell 2015; Mansell 2017). We have included these studies in the main analyses of O‐BCS versus mastectomy and O‐BCS versus mastectomy with reconstruction, but given they combine mastectomy with and without reconstruction as a control group they are separated when pooling.
O‐BCS versusMx/Mx + R: one study compared volume displacement O‐BCS with mastectomy with and without reconstruction (Potter 2020), and so contributed to O‐BCS versus mastectomy and O‐BCS versus mastectomy with reconstruction.
O‐BCS versusBCS/Mx/Mx + R: two studies compared any O‐BCS (volume displacement and replacement together) to S‐BCS, mastectomy alone and mastectomy with reconstruction (Carter 2016; Kahn 2013), so contributed to all three main analyses. One study compared any O‐BCS to S‐BCS, mastectomy alone and mastectomy with reconstruction and analysed volume displacement and replacement techniques separately (Lee 2018), so contributed to all three main analyses and both subgroup analyses. One study compared volume displacement O‐BCS to S‐BCS, mastectomy alone and mastectomy with reconstruction (Morrow 2019), and contributed to all three main analyses and the subgroup analyses of volume displacement.
Primary outcomes
Local recurrence was evaluated in 30 studies (38%), disease‐free survival in 13 studies (16%), and overall survival in 17 studies (22%). We wrote to all authors of studies that reported one of the primary outcomes but not others. We received four responses, but nobody was able to provide further data.
Secondary outcomes
Re‐excision rates were evaluated in 42 studies (53%), complications in 41 studies (52%), and recall rates were evaluated in 7 studies (9%). Time to adjuvant therapy was evaluated in 16 studies (20%), patient‐reported outcomes in 28 studies (35%), and aesthetic outcomes in 11 studies (14%).
Ongoing studies
Of the nine ongoing studies, one is a RCT based in the UK (ACTRN12612000638831), planning to compare O‐BCS with S‐BCS. It was last updated in 2016 and may no longer be ongoing; we contacted the authors for further information.
There are three trials registered in Egypt by the same author and institution which planned to compare O‐BCS with S‐BCS (NCT02901223; NCT02923635; NCT03012152). They planned to measure the outcome of margins in all specimens and patient‐reported outcome measures. All three trials were last updated in 2017 with no published results. It appears there are similarities between the studies. We contacted authors for further information.
The remaining six studies are observational studies.
One is in China (NCT04030845) comparing O‐BCS with any other breast reconstruction reporting local recurrence, overall survival, complications and patient‐reported outcomes using a visual analogue scale.
Two are in the Netherlands (Catsman 2018; NTR6901), both comparing O‐BCS with S‐BCS. Catsman 2018 will evaluate re‐excisions and patient‐reported outcome measures with patient questionnaires (Breast‐Q; Cohen 2016), EORTC‐QLQ (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; Aaronson 1993) and aesthetic outcome with photographs of the breast given to a panel and analysed with BCCT.core software (BCCT.core). NTR6901 will analyse patient satisfaction and postoperative complications.
One study based in Austria (NCT01396993), will compare O‐BCS with S‐BCS and assess patient‐reported local recurrence, disease‐free survival, overall survival, patient‐reported outcome measures (using a breast image scale, BREAST‐Q; Cohen 2016), complications and aesthetic outcome (using the breast symmetry index; Fitzal 2007).
One study based in Denmark (NCT02159274), will compare O‐BCS with S‐BCS and assess patient‐reported outcomes focusing on shoulder function and lymphoedema. They will also compare aesthetic outcomes (using the breast retraction assessment; Pezner 1985).
Studies awaiting classification
One study describes itself to be a RCT (Srivastava 2018), however as insufficient information on methods was available, we decided to categorise this as awaiting classification. The study has not been published as a full study to our knowledge.
Excluded studies
We excluded 45 studies during the full‐text review, amongst which 20 had study designs that did not meet eligibility criteria, seven had interventions that were not eligible, and five had comparators that were not eligible. For further information see Figure 1.
Risk of bias in included studies
We assessed risk of bias for all studies using ROBINS‐I (Sterne 2016). We have displayed each risk of bias assessment divided into each outcome studied as per the tool. The summary and details can be found per outcome in the corresponding figures and tables.
2.
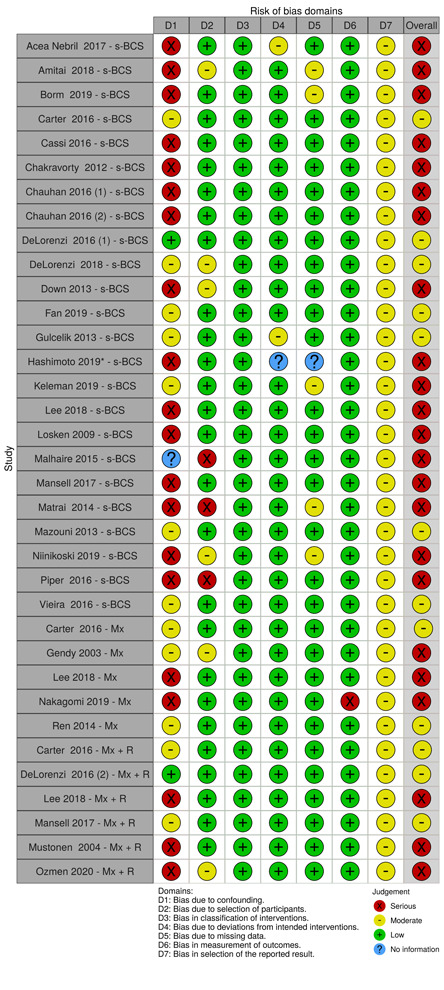
ROBINS‐1 risk of bias for local recurrence
3.
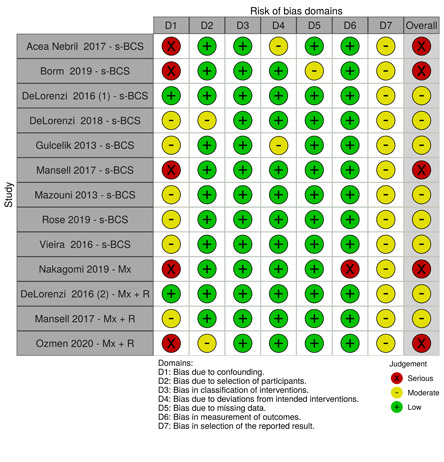
ROBINS‐1 risk of bias for disease‐free survival
4.
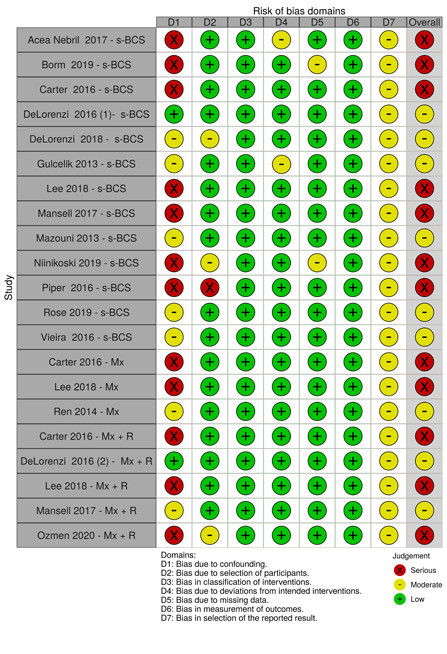
ROBINS‐1 risk of bias for overall survival
5.
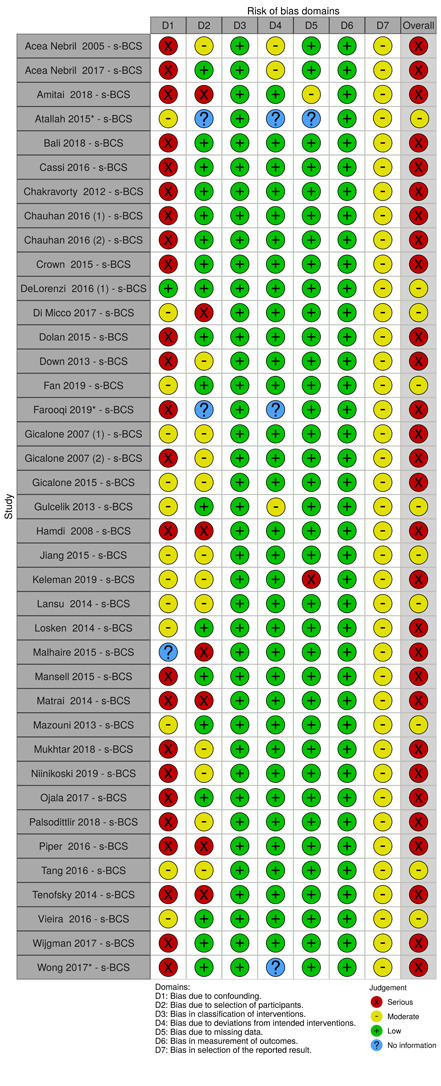
ROBINS‐1 risk of bias for re‐excisions
6.
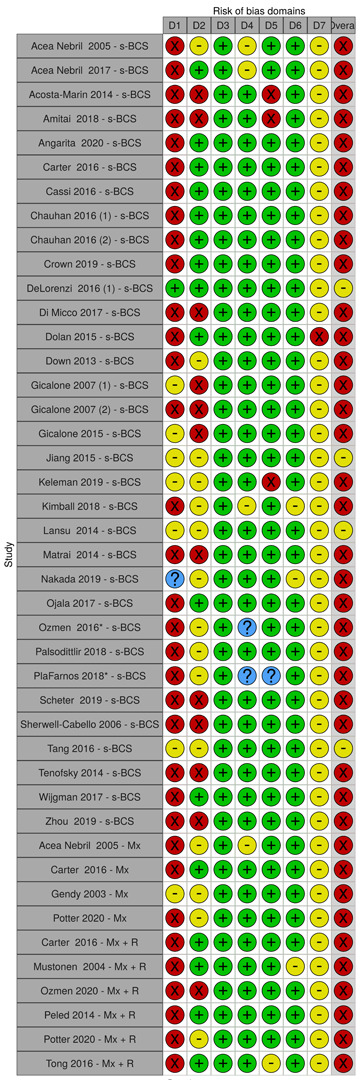
ROBINS‐1 risk of bias for complications
7.
ROBINS‐1 risk of bias for recall rates
8.
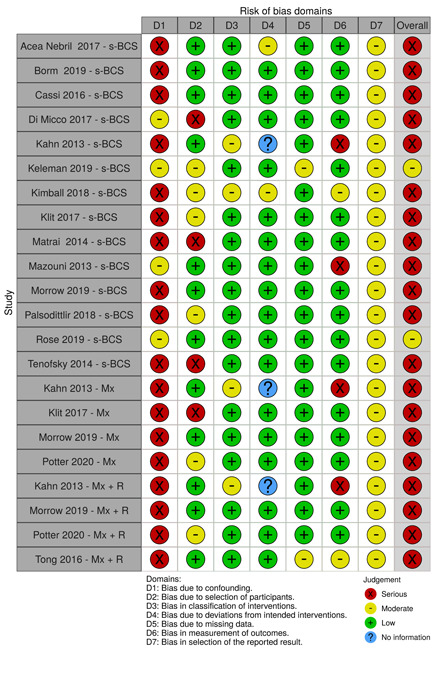
ROBINS‐1 risk of bias for time to adjuvant therapy
9.
ROBINS‐1 risk of bias for patient reported outcome measures
10.
ROBINS‐1 risk of bias for cosmetic evaluation
Overall
Overall we rated the risk of bias for local recurrence (Figure 2, Table 5), disease‐free survival (Figure 3, Table 6), overall survival (Figure 4, Table 7), re‐excision rates (Figure 5, Table 8), complications (Figure 6, Table 9), recall rates (Figure 7, Table 10), and time to adjuvant therapy (Figure 8, Table 11) as serious in most studies. The major implication for risk of bias was confounding bias with details of confounding in Table 4.
For patient‐reported outcome measures (Figure 9, Table 13) and cosmetic evaluation (Figure 10, Table 12) overall, we rated the risk of bias for recall rates as serious/critical in most studies. For those with critical risk of bias, the major implication for risk of bias was measurement of outcome bias due to the use of unvalidated tools. If validated but still subjective tools were used then we deemed risk of bias serious due to knowledge of the intervention impacting the outcome.
Bias due to confounding
We judged the risk of bias due to confounding to be serious in most studies for most outcomes. This is due to differences in clinicopathological factors and co‐interventions, e.g. radiotherapy, chemotherapy and endocrine therapy; details are displayed in Table 4.
For local recurrence (Figure 2, Table 5), disease‐free survival (Figure 3, Table 6), and overall survival (Figure 4, Table 7), if comparisons differed in clinicopathological factors, such as tumour stage, size and grade (e.g. Lee 2018; Mansell 2017; Piper 2016), or co‐interventions (e.g. Carter 2016; Keleman 2019; Mansell 2017), we deemed them at serious risk of bias. We deemed some studies at low risk of bias (e.g. DeLorenzi 2016 (1); DeLorenzi 2016 (2)), as important clinicopathological factors were matched for and co‐interventions were balanced across the groups. We deemed studies moderate (e.g. Fan 2019; Mazouni 2013; Vieira 2016) if they demonstrated balance in some clinicopathological and co‐interventions across the studies. It should be noted that Mazouni 2013 includes only patients undergoing surgery following primary systemic treatments; given that this was balanced between the O‐BCS and control group, we deemed this at moderate risk of bias. Differences in adjuvant radiotherapy are more significant (higher risk of bias) for the comparison O‐BCS versus S‐BCS, as it is usually standard practice to give radiotherapy with BCS, whereas radiotherapy can be avoided with mastectomies.
For re‐excisions (Figure 5, Table 8), if clinicopathological factors, especially tumour size and tumour location were different across the groups, then we deemed studies at serious risk of bias (e.g. Chakravorty 2012; Hamdi 2008; Wijgman 2017).
For complications (Figure 6, Table 9) if clinicopathological factors especially tumour stage and patient comorbidities/factors (e.g. Crown 2019; Gicalone 2007 (1); Ozmen 2016) and co‐interventions, especially axillary surgery and adjuvant radiotherapy (Di Micco 2017; Kimball 2018; Tang 2016) were imbalanced, we deemed studies at serious risk of bias.
For patient‐reported outcome measures (Figure 9, Table 13) and cosmetic evaluation (Figure 10, Table 12), we judged the risk of bias due to confounding to be serious in most studies, especially if differences in size and location (e.g. Lee 2018; Mansell 2017; Piper 2016) or co‐interventions, especially radiotherapy (e.g. Carter 2016; Keleman 2019; Mansell 2017).
Bias due to selection of participants
We judged the selection bias to be low in most outcomes as all/most eligible participants in a period of time were included. We deemed some studies (Amitai 2018; DeLorenzi 2018; Niinikoski 2019 (2); Ren 2014 etc.) at moderate risk of bias as some participants were not included or controlled for in a way that could have affected the selection, e.g. excluding patients that needed mastectomy eventually and women choosing after being counselled on potential outcomes. If studies excluded patients based on needing mastectomy, eventually we deemed the risk of bias moderate for oncological outcomes, recall rates and time to adjuvant therapy (as these outcomes would be slightly affected by the exclusion of such patients) but serious for re‐excision rates, complications, patient‐reported outcome measures and cosmetic evaluation, given patients who had a mastectomy have had a re‐excision, may have had it due to a complication, will have their overall satisfaction and cosmesis affected by the intervention initially chosen. We deemed some studies at serious risk of bias (e.g. Malhaire 2015; Matrai 2014; Piper 2016) for reasons such as: patients were selected to certain arms as selection was based on localisation techniques or it was unclear why these patients were selected, or patients without negative margins were excluded. For patient‐reported outcome measures (Figure 9, Table 13) and cosmetic evaluation (Figure 10, Table 12), we judged the selection bias to be serious in most cases as there is a natural bias in those patients that respond to questionnaires.
Bias due to classification of interventions
We judged risk of bias to be low in all studies as classification of interventions was clear and determined at the start of the intervention.
Bias due to deviation from intended intervention
We judged risk of bias to be low/moderate in all studies as there was no evidence of deviation from the intended operation as these studies were cohort studies and were selected based on their intervention. Acea‐Nebril 2017 mentioned a deviation from the intended co‐intervention (time to adjuvant therapy) in the intervention group.
We evaluated surgeon experience and whether the study had taken into account learning curves after the introduction of a new technique in the study. Most studies did not comment on this. The study period for Crown 2015 and Crown 2019 began after allowing time for the surgeons to adapt to the new O‐BCS technique accounting for confounding created by learning curves, therefore we judged them to be at low risk of bias. Some studies, such as Gicalone 2007 (1), Keleman 2019 and Tenofsky 2014 ensured all surgeries were done by or under the supervision of experienced surgeons in the operations studied. We deemed two studies at moderate risk of bias due to the study period starting from the beginning of uptake of O‐BCS and for including centres with varying levels of experience in O‐BCS (Gulcelik 2013; Kimball 2018).
Bias due to missing data
We judged risk of bias due to missing data to be low because in most studies all patients enrolled were followed up. Some studies reported some loss to follow‐up, but with similar numbers in both groups, so the impact may be similar across groups (Amitai 2018; Borm 2019; Gendy 2003; Gulcelik 2013; Keleman 2019).
Bias in measurement of outcomes
We judged risk of bias to be low in all cases for local recurrence (Figure 2, Table 5), disease‐free survival (Figure 3, Table 6), overall survival (Figure 4, Table 7), re‐excision rates (Figure 5, Table 8), and complications (Figure 6, Table 9) as all are an objective outcome measure. For disease‐free survival, length of follow‐up time details were not clear for Nakagomi 2019 and so we deemed this to be at serious risk of bias. For complications, some studies reported difficulties in recording complications in large databases (e.g. Angarita 2020), so we judged these to be at moderate risk of bias. For recall rates we judged risk of bias to be moderate in all cases as recall rates are usually based on radiological imaging, which can be subject to bias. Four studies used the BI‐RADS (Breast Imaging‐Reporting and Data System) scale to reduce this risk of bias (Amitai 2018; Dolan 2015; Fan 2019; Hu 2019).
For time to adjuvant therapy, we judged risk of bias to be low in most cases as time to adjuvant therapy is an objective outcome measure in days. However, we deemed Tong 2016 at critical risk of bias as they reported a general 'delay in time to adjuvant therapy', which was poorly defined.
We judged risk of bias to be serious when patient‐reported outcome measures were measured used a validated reporting tool (e.g. BREAST‐Q; Cohen 2016) (Acea‐Nebril 2017; Di Micco 2017; PlaFarnos 2018) or EORTC (Aaronson 1993) (Keleman 2019; Lansu 2014 etc.) but this is still very vulnerable to bias from subjective knowledge of the intervention. We deemed studies at critical risk of bias that used non‐validated tools (e.g. Eichler 2013; Jiang 2015; Palsodittlir 2018).
For cosmetic evaluation, we judged risk of bias to be moderate when aesthetic outcome was judged by the objective BCCT.core software (Hilli‐Betz 2014; Lansu 2014; Santos 2015). We judged those with a large panel who were unaware of the surgery with validated scoring tools at serious risk of bias (it is very difficult to actually blind surgeons) (e.g. Scheter 2019). We deemed those with small unblinded panels with self‐designed tools judging cosmetic outcome to have a critical risk of bias (e.g. Viega 2011).
Bias in the selection of the reported results
For local recurrence (Figure 2, Table 5), disease‐free survival (Figure 3, Table 6), overall survival (Figure 4, Table 7), re‐excision rates (Figure 5, Table 8), complications (Figure 6, Table 9), recall rates (Figure 7, Table 10), and time to adjuvant therapy (Figure 8, Table 11), we judged the selection of reported results as moderate in all cases as there was no indication of selected reporting and no indication that an outcome would have been logically collected (given what is reported in the study) but then not reported. There was no difference between the methods sections and results reported in any of the papers, but no study had a prior protocol. For patient‐reported outcome measures (Figure 9, Table 13), and cosmetic evaluation (Figure 10, Table 12), we judged selection of reported results as moderate in most cases as there was no indication of selected reporting, but no study had a prior protocol. There were a few that did not report all outcomes that we deemed serious (e.g. Keleman 2019; Palsodittlir 2018; Gendy 2003).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Any O‐BCS compared to S‐BCS for women with primary breast cancer.
| Any O‐BCS compared to S‐BCS for women with primary breast cancer | ||||||
| Patient or population: women with primary breast cancer Setting: mixed multicentre/single‐centre studies with initial inpatient procedure and outpatient follow‐up Intervention: any O‐BCS Comparison: S‐BCS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with S‐BCS | Risk with any O‐BCS | |||||
| Local recurrence‐free survival (up to 5 years) |
Study population | HR 0.90 (0.61 to 1.34) | 7600 (4 observational studies) | ⊕⊝⊝⊝ Very lowa,b | We calculated estimates of risk with BCS using an average of non‐adjusted baseline control rates from included studies. | |
| 55 per 1000 | 50 per 1000 (34 to 73) | |||||
| Local recurrence rates (up to 5 years) |
Study population | HR 1.33 (0.96 to 1.83) | 2443 (4 observational studies) | ⊕⊕⊝⊝ Lowb,c | We calculated estimates of risk with BCS using an average of non‐adjusted baseline control rates from included studies. | |
| 57 per 1000 | 75 per 1000 (55 to 102) | |||||
| Disease‐free survival (up to 5 years) | Study population | HR 1.06 (0.89 to 1.26) | 5532 (7 observational studies) | ⊕⊕⊝⊝ Lowb,c | We calculated estimates of risk with BCS using an average of non‐adjusted baseline control rates from included studies. | |
| 98 per 1000 | 104 per 1000 (88 to 122) | |||||
| Re‐excision rate: total re‐excisions | Study population | RR 0.76 (0.69 to 0.85) | 13,341 (38 observational studies) | ⊕⊝⊝⊝ Very lowa,d,e | We also assessed the risk of completion mastectomy (RR 1.00, 95% CI 0.85 to 1.15); O‐BCS may have no effect on the completion mastectomy rate but the evidence is very uncertain. | |
| 134 per 1000 | 101 per 1000 (92 to 114) | |||||
| Complications | Study population | RR 1.19 (1.10 to 1.27) | 118,005 (20 observational studies) | ⊕⊝⊝⊝ Very lowa,b,f | O‐BCS may increase or have no effect on the rate of complications but the evidence is very uncertain. | |
| 34 per 1000 | 41 per 1000 (38 to 44) | |||||
| Recall rate | Study population | RR 2.39 (1.67 to 3.42) | 715 (6 observational studies) | ⊕⊕⊝⊝ Lowa | O‐BCS may increase recall rate slightly. | |
| 100 per 1000 | 240 per 1000 (167 to 343) | |||||
| Patient‐reported outcome measures | There is no significant difference in quality of life patient‐reported outcome measures using BREAST‐Q. However, there may be better patient‐reported cosmetic satisfaction with O‐BCS | ‐ | 5665 (24 observational studies) |
⊕⊝⊝⊝ Very lowa,g | The evidence is very uncertain about the effect of any O‐BCS on patient‐reported outcome measures. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; O‐BCS: oncoplastic breast‐conserving surgery; RR: risk ratio; S‐BCS: standard breast‐conserving surgery. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels due to study limitation: serious risk of bias due to confounding. bDowngraded by one level due to imprecision: wide confidence levels crossing line of no effect. cDowngraded by one level due to study limitation: moderate risk of bias due to confounding. dDowngraded by one level due to heterogeneity: I2 = 43%, P < 0.0001. eDowngraded by one level due to publication bias detected. fDowngraded by one level due to heterogeneity: I2 = 60%, P = 0.0003. gDowngraded by two levels due to study limitations: serious/critical risk due to measurement of outcome.
Summary of findings 2. Any O‐BCS compared to mastectomy for women with primary breast cancer.
| Any O‐BCS compared to mastectomy for women with primary breast cancer | ||||||
| Patient or population: women with primary breast cancer Setting: mixed multicentre/single‐centre studies with initial inpatient procedure and outpatient follow‐up Intervention: any O‐BCS Comparison: mastectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Mx | Risk with any O‐BCS | |||||
| Local recurrence‐free survival (up to 5 years) | Study population | HR 0.55 (0.34 to 0.91) | 4713 (2 observational studies) | ⊕⊝⊝⊝ Very lowa,b | Estimates of risk with BCS were calculated using an average of non‐adjusted baseline control rates from included studies. | |
| 161 per 1000 | 92 per 1000 (58 to 148) | |||||
| Cumulative local recurrence rate | (0 studies) | ‐ | No studies evaluated local recurrence as cumulative rate | |||
| Disease‐free survival | Study population | RR 0.58 (0.41 to 0.82) | 1193 (1 observational study) | ⊕⊝⊝⊝ Very lowc,d | Dichotomous data used as no studies reported time‐to‐event data | |
| 139 per 1000 | 81 per 1000 (57 to 114) | |||||
| Re‐excision rates | (0 studies) | Re‐excisions are not often needed for mastectomy, therefore this outcome is not relevant for this comparison. | ||||
| Complications | Study population | RR 0.75 (0.67 to 0.83) | 4839 (4 observational studies) | ⊕⊝⊝⊝ Very lowc,e | O‐BCS may reduce complications compared to mastectomy but the evidence is very uncertain. | |
| 312 per 1000 | 234 per 1000 (209 to 259) | |||||
| Recall rates | (0 studies) | Recall biopsies are not often needed for mastectomy, therefore this outcome is not relevant for this comparison. | ||||
| Patient‐reported outcome measures | There are insufficient data to make any conclusions | ‐ | (1 observational study) | ‐ | There are insufficient data to make any conclusions | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCS: breast‐conserving surgery; CI: confidence interval; HR: hazard ratio;Mx: mastectomy; O‐BCS: oncoplastic breast‐conserving surgery; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level due to study limitation: moderate risk of bias due to confounding. bDowngraded by two levels due to heterogeneity: I2 = 81%, P = 0.02. cDowngraded by two levels due to study limitation: serious risk of bias due to confounding. dDowngraded by one level due to imprecision: optimal size not met. eDowngraded by two levels due to heterogeneity: I2 = 61%, P < 0.0001.
Summary of findings 3. Any O‐BCS compared to mastectomy plus reconstruction for women with primary breast cancer.
| Any O‐BCS compared to mastectomy plus reconstruction for women with primary breast cancer | ||||||
| Patient or population: women with primary breast cancer Setting: mixed multicentre/single‐centre studies with initial inpatient procedure and outpatient follow‐up Intervention: any O‐BCS Comparison: mastectomy plus reconstruction (Mx+R) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Mx+R | Risk with any O‐BCS | |||||
| Local recurrence‐free survival | Study population | HR 1.37 (0.72 to 2.62) | 3785 (1 observational study) | ⊕⊝⊝⊝ Very lowa,b | Any O‐BCS may result in little to no difference in local recurrence‐free survival compared to Mx+R. Estimates of risk with BCS were calculated using an average of non‐adjusted baseline control rates from included studies. Also calculated HR for LRR (local recurrence rates) for studies with a comparison of Mx+/‐R where the vast majority were reconstructed; HR 1.59 (0.71 to 3.55) | |
| 43 per 1000 | 58 per 1000 (31 to 108) | |||||
| Cumulative local recurrence rate | 0 studies | Re‐excisions are not often needed for mastectomy, therefore this outcome is not relevant for this comparison and therefore not studied. | ||||
| Disease‐free survival | Study population | HR 0.45 (0.09 to 2.22) | 317 (1 observational study) | ⊕⊝⊝⊝ Very lowa,c | Estimates of risk with BCS were calculated using an average of non‐adjusted baseline control rates from included studies. Also calculated HR for DFS for studies with a comparison of Mx+/‐R where the vast majority were reconstructed; HR 1.03 (0.75 to 1.42) | |
| 189 per 1000 | 90 per 1000 (19 to 371) | |||||
| Re‐excision rates | 0 studies | ‐ | Re‐excisions are not often needed for mastectomy, therefore this outcome is not relevant for this comparison. | |||
| Complications | Study population | RR 0.49 (0.45 to 0.54) | 4973 (5 observational studies) | ⊕⊝⊝⊝ Very lowa,d | ||
| 492 per 1000 | 241 per 1000 (221 to 266) | |||||
| Recall rates | 0 studies | ‐ | Recall biopsy is not often needed for mastectomy, therefore this outcome is not relevant for this comparison. | |||
| Patient‐reported outcome measures | The evidence is too methodologically diverse and of high risk of bias due to measurement of outcomes to combine | ‐ | (3 observational studies) | ‐ | There is insufficient evidence to make a conclusion | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCS: breast‐conserving surgery; CI: confidence interval; DFS: disease‐free survival; HR: hazard ratio;Mx: mastectomy; Mx+R: mastectomy with reconstruction; Mx+/‐R: mastectomy with or without reconstruction; O‐BCS: oncoplastic breast‐conserving surgery; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels due to study limitation: serious risk of bias due to confounding. bDowngraded by one level due to imprecision: optimal size not met. cDowngraded by one level due to imprecision: 95% CI overlaps no effect. dDowngraded by two levels due to heterogeneity: I2 = 85%, P < 0.001.
The 78 studies with 92 comparisons, enrolled 178,813 women. The matrix of different comparisons can be found in Table 15. The certainty of evidence ratings for the main outcomes are presented in Table 1, Table 2 and Table 3.
Comparison 1: O‐BCS versus S‐BCS
Primary outcomes
Local recurrence
Twenty‐four studies involving 16,126 participants evaluated local recurrence for oncoplastic breast‐conserving surgery (O‐BCS) versus standard breast‐conserving surgery (S‐BCS). One study evaluated local recurrence (Atallah 2015) but we did not include it in the analysis due to a lack of follow‐up time.
For seven of these studies, including 10,043 participants, we were able to extract hazard ratios (HRs). Four of the studies reported local recurrence‐free survival and the HR was 0.90 (95% confidence interval (CI) 0.61 to 1.34; I2 = 0%, P = 0.77; 4 studies, 7600 participants; very‐low certainty evidence; Analysis 1.1). We downgraded the certainty of evidence two levels due to high risk of bias due to confounding in most of the studies and one level due to imprecision, as the 95% CI overlaps the line of no effect. Four studies reported local recurrence rates and the HR was 1.33 (95% CI 0.96 to 1.83; I2 = 0%, P = 0.68; 4 studies, 2443 participants; low‐certainty evidence; Analysis 1.1). We downgraded the certainty of evidence by one level due to confounding and one level due to imprecision as the 95% CI overlaps the line of no effect.
1.1. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 1: Local recurrence‐free survival (time to recurrence)
To see the impact of the studies where data were not extractable as HRs, we extracted the data as dichotomous event rates and analysed with time points of 1 year (risk ratio (RR) 0.73, 95% CI 0.25 to 2.10; I2 = 0%, P = 0.84; 3 studies, 637 participants; ); 1 to 5 years (RR 0.83, 95% CI 0.66 to 1.04; ; I2 = 27%, P = 0.16; 15 studies, 9014 participants); and 5‐year follow‐up (RR 1.07, 95% CI 0.82 to 1.39; I2 = 26%, P = 0.2; 10 studies, 6672 participants) in Analysis 1.2. We created a funnel plot for these studies, which suggests publication bias (Figure 11).
1.2. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 2: Local recurrence
11.

Funnel plot of comparison: 1 Any O‐BCS versus breast‐conserving surgery, outcome: 1.3 Local recurrence: O‐BCS versus S‐BCS.
Disease‐free survival
Eight studies involving 6411 participants evaluated disease‐free survival for O‐BCS versus S‐BCS. One study (Lee 2018) evaluated disease‐free survival (DFS) but no data were extractable.
For seven of these studies, we were able to extract HRs for DFS and the HR was 1.06 (95% CI 0.89 to 1.26; I2 = 18%; P = 0.29; 7 studies, 5532 participants; low‐certainty evidence; Analysis 1.3). We downgraded the level of evidence by one level due to imprecision as the 95% CI overlaps the line of no effect and one level due to confounding.
1.3. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 3: Disease‐free survival (HR)
To see if extracting the data as dichotomous event rates changed the analysis, we analysed at time points of 1 to 5 years (RR 0.99, 95% CI 0.74 to 1.34; ; I2 = 0%, P = 0.49; 3 studies, 946 participants), 5 years (RR 1.19, 95% CI 0.99 to 1.44; I2 = 41%, P = 0.13; 6 studies, 5054 participants) and 10 years (RR 1.21, 95% CI 1.04 to 1.40; I2 = 0%, P = 0.33; 2 studies, 2163 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 4: Disease‐free survival (RR)
Overall survival
Thirteen studies involving 13,887 participants evaluated overall survival for O‐BCS versus S‐BCS. One study evaluated overall survival (OS) (Chakravorty 2012), but no data were extractable.
For eight of these studies, we were able to extract HRs for OS and the HR was 1.02 (95% CI 0.82 to 1.28;I2 = 0%; P = 0.95; 8 studies, 10,078 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 5: Overall survival (HR)
To see if extracting the data as dichotomous event rates changed the analysis, we analysed with time points of 1 to 5 years (RR 0.82, 95% CI 0.61 to 1.10; I2 = 0%, P = 0.42; 3 studies, 4970 participants) and 5 years (RR 0.82, 95% CI 0.67 to 1.00; I2 = 1%, P = 0.43; 12 studies, 8730 participants; Analysis 1.6). We created a funnel plot for these studies, which suggests publication bias (Figure 12).
1.6. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 6: Overall survival (RR)
12.

Funnel plot of comparison: 1 Any O‐BCSversus breast‐conserving surgery, outcome: 1.7 Overall survival: O‐BCS versus S‐BCS.
Secondary outcomes
Re‐excision rates
Thirty‐eight studies evaluated participants that need further surgery due to inadequate cancer resection. Fifteen studies reported the total number of women that underwent any further surgery (Amitai 2018; Atallah 2015; Cassi 2016; Di Micco 2017; Fan 2019; Farooqi 2019; Hamdi 2008; Jiang 2015; Lansu 2014; Matrai 2014; Ojala 2017; Tang 2016; Tenofsky 2014; Vieira 2016; Wong 2017). Eighteen studies evaluated women that eventually had further partial re‐excisions or total mastectomy separately (Chakravorty 2012; Chauhan 2016 (1); Chauhan 2016 (2); Dolan 2015; Down 2013; Gicalone 2007 (1); Gicalone 2007 (2); Gicalone 2015; Gulcelik 2013; Keleman 2019; Malhaire 2015; Mansell 2015; Mazouni 2013; Mukhtar 2018; Niinikoski 2019 (2); Palsodittlir 2018; Piper 2016; Wijgman 2017). In four studies (Acea‐Nebril 2017; Bali 2018; Crown 2015; Losken 2014) they reported women who initially underwent partial re‐excision and some went on to have a mastectomy, the total number of women that underwent any surgery was extracted so as not to duplicate participants in the results. DeLorenzi 2016 (1) reported women who underwent mastectomy only.
Four studies also evaluated re‐excision rates but we did not include them in the analysis; we excluded Acea‐Nebril 2005, Crown 2019 and Mansell 2017 as they were the publications of subsets of participants (those with sufficient follow‐up) of studies already included in the analysis (Acea‐Nebril 2017; Crown 2015; Mansell 2015). We excluded Kahn 2013 as they reported re‐excisions for the intervention alone.
The RR for O‐BCS for needing any further surgery due to inadequate cancer resection compared to S‐BCS was 0.76 (95% CI 0.69 to 0.85; I2 = 43%, P = 0.003; 38 studies, 13,341 participants; very‐low certainty; Analysis 1.7 ). We downgraded the certainty of evidence by one level each for risk of bias due to confounding, inconsistency of the results due to heterogeneity and publication bias. The RR for O‐BCS for needing completion mastectomy compared to O‐BCS was 1.00 (95% CI 0.85 to 1.18; I2 = 50%; P = 0.003; 24 studies, 10,863 participants). We created a funnel plot for these studies, which suggests publication bias (Figure 13).
1.7. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 7: Re‐excision rates
13.

Funnel plot of comparison: 1 Any O‐BCS versus breast‐conserving surgery, outcome: 1.8 Re‐excision rates: O‐BCS versus S‐BCS.
Complications
Thirty‐four studies evaluated complications in O‐BCS versus S‐BCS. Crown 2015 reported complications for the intervention but we excluded it from the analysis as it was the same cohort as Crown 2019.
Amitai 2018 and Nakada 2019 reported fat necrosis rates only. Dolan 2015, Ojala 2017 and Zhou 2019 reported women who required reoperation for complications only. Hilli‐Betz 2014 reported postoperative pain only. Six studies (Down 2013; Gicalone 2007 (1); Gicalone 2007 (2); Kimball 2018; Tang 2016; Tenofsky 2014) reported a breakdown of certain complications but not the total rate of complications. DeLorenzi 2016 (1) reported complications for the intervention only.
Twenty‐six studies reported a breakdown of the complications ‐ we presented these in Table 16 and Table 17. Twenty of these studies reported the rate of complications; we included these in the meta‐analysis. The RR was 1.19 (95% CI 1.10 to 1.27; I2 = 60%, P = 0.0003; 20 studies, 118,005 participants; very‐low certainty evidence; Analysis 1.8). We created a funnel plot for these studies, which suggests publication bias (Figure 14). We downgraded the certainty of evidence by one level each due to risk of bias due to confounding, inconsistency due to heterogeneity of the results, imprecision and publication bias.
13. Complications: O‐BCS of those compared to S‐BCS.
| Study | Wound infection | Flap necrosis | Dehisence | Fat necrosis | Seroma | Skin | Haematoma | Bleeding | Needed surgery |
| Acea‐Nebril 2017‐VD | 1 (0.5%) | 4 (2.3%) | ‐ | ‐ | 3 (1.7%) | ‐ | 4 (2.3%) | 3 (1.7%) | (1.7%) ‐ bleeding only |
| Acea‐Nebril 2005‐VD | 2 (4%) | 3 (6%) | ‐ | ‐ | 3 (6%) | ‐ | 4 (8%) | ‐ | ‐ |
| Acosta‐Marin 2014‐VD | 1 (1.9%) | ‐ | ‐ | 1 (1.9%) | ‐ | 1 (1.9%) | ‐ | ‐ | ‐ |
| Amitai 2018‐VD | ‐ | ‐ | ‐ | 15 (22%) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Angarita 2020‐VD | 209 (2.3%) | ‐ | 67 (0.7%) | ‐ | ‐ | ‐ | ‐ | 22 (0.3%) | ‐ |
| Carter 2016‐VD | 45 (4.8%) | ‐ | ‐ | ‐ | 126 (13.4%) | ‐ | 18 (1.9%) | ‐ | ‐ |
| Chauhan 2016 (1)‐VD and VR | 4 (7%) | 2 (3.5%) | ‐ | ‐ | 1 (1.8%) | ‐ | 1 (1.8%) | ‐ | ‐ |
| Chauhan 2016 (2)‐VD and VR | 1 (3.67%) | 1 (3.7%) | ‐ | ‐ | 0 (0%) | ‐ | 1 (3.7%) | ‐ | ‐ |
| Jiang 2015‐VD | ‐ | ‐ | 1 (3.3%) | ‐ | 3 (10%) | ‐ | ‐ | ‐ | ‐ |
| Tang 2016‐VD and VR | 0 (0%) | 0 (0%) | 2 (3%) | 0 (0%) | 10 (15%) | 10 (15%) | 3 (4.48%) | 0 (0%) | ‐ |
| Crown 2019‐VD | 15 (3.3%) | ‐ | 14 (3.1%) | ‐ | 8 (1.8%) | 2 (0.4%) | ‐ | ‐ | ‐ |
| DeLorenzi 2016 (1)‐Both | 13 (2.8%) | 6 (1.3%) | 16 (3.5%) | 12 (2.6%) | ‐ | ‐ | 11 (2.4%) | ‐ | ‐ |
| Dolan 2015‐VD and VR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 5 (7%) |
| Down 2013‐VD and VR | 2 (5.4%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | breakdown only |
| Gicalone 2007 (1)‐VD | ‐ | 21 (67%) | 5 (16%) | ‐ | ‐ | ‐ | 2 (6.45%) | ‐ | ‐ |
| Gicalone 2007 (2)‐VD | ‐ | ‐ | 2 (5.13%) | ‐ | ‐ | 1 (2.6%) | 1 (2.6%) | ‐ | ‐ |
| Gicalone 2015‐VD | 4 (9.52%) | ‐ | ‐ | ‐ | ‐ | 2 (4.8%) | 1 (2.4%) | ‐ | ‐ |
| Keleman 2019‐VD | 8 (2.3%) | 3 (0.9%) | ‐ | 2 (0.57%) | 5 (1.4%) | ‐ | 2 (0.6%) | ‐ | ‐ |
| Kimball 2018‐VD | 17.7 (2.5%) | 33.3 (4.7%) | ‐ | ‐ | ‐ | ‐ | 6.4% (includes seroma) | ‐ | < 1% |
| Mazouni 2013 ‐ VD | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 4 (2%) |
| Nakada 2019‐VR | ‐ | 68 (16%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Palsodittlir 2018‐VD and VR | ‐ | 0 (0%) | ‐ | ‐ | ‐ | ‐ | 0 (0%) | ‐ | ‐ |
| Scheter 2019‐VD | ‐ | ‐ | 2 (16.7%) | 1 (8.3%) | ‐ | ‐ | ‐ | ‐ | 0 |
| Tenofsky 2014‐VD | 5 (8.6%) | ‐ | 4 (6.9%) | 15 (25.9%) | 10 (17.2%) | 21 (36.2%) | 10 (17.2%) | ‐ | ‐ |
| Wijgman 2017‐VD | 11 (4%) | ‐ | ‐ | ‐ | 17 (6.2%) | ‐ | 31 (11.4%) | ‐ | 6 (1.9%) |
| Zhou 2019‐VR | ‐ | ‐ | ‐ | ‐ | 3 (9.3%) | ‐ | 0 (0%) | ‐ | 3 (9.3%) |
O‐BCS: oncoplastic breast‐conserving surgery S‐BCS: standard breast‐conserving surgery VD: volume displacement VR: volume replacement
14. Complications: S‐BCS.
| Study | Wound infection | Flap/skin necrosis | Dehisence | Fat necrosis | Seroma | Skin | Haematoma | Bleeding | Needed surgery |
| Acea‐Nebril 2017‐ BCS | 13 (2%) | 1 (0.1%) | ‐ | ‐ | 21 (3.3%) | ‐ | 22 (3.3%) | ‐ | |
| Acea‐Nebril 2005‐ BCS | 2 (3.5%) | 0 | ‐ | ‐ | 8 (13.9%) | ‐ | 5 (8.7%) | ‐ | ‐ |
| Acosta‐Marin 2014‐ BCS | 0 | ‐ | ‐ | 0 | ‐ | 0 | ‐ | ‐ | ‐ |
| Amitai 2018‐ BCS | ‐ | ‐ | ‐ | 3 (1%) | ‐ | ‐ | ‐ | 22 | ‐ |
| Angarita 2020‐ BCS | 1842 (1.8%) | ‐ | 126 (0.1%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Carter 2016‐ BCS | 32 (1.4%) | ‐ | ‐ | ‐ | 406 (18%) | ‐ | 57 (2.5%) | ‐ | ‐ |
| Chauhan 2016 (1)‐ BCS | 2 (3.5%) | ‐ | ‐ | ‐ | 1 (1.8%) | ‐ | 1 (1.75%) | ‐ | ‐ |
| Chauhan 2016 (2) ‐ BCS | 2 (4.3%) | 1 (2.2%) | ‐ | ‐ | 2 (4.3%) | ‐ | ‐ | ‐ | ‐ |
| Jiang 2015‐ BCS | ‐ | ‐ | 4 (13.3%) | ‐ | 2 (6.7%) | ‐ | ‐ | 0 (0%) | ‐ |
| Tang 2016‐ BCS | 3 (2.6%) | 0 (0%) | 16 (13.7%) | 2 (1.71%) | 57 (48.7%) | 21 (17.9%) | 17 (14.5%) | 0 (0%) | ‐ |
| Crown 2019‐ BCS | 21 (8.4%) P = 0.01 | ‐ | 13 (4.7%) | ‐ | 12 (4.4%) | 2 (0.7%) | ‐ | ‐ | ‐ |
| DeLorenzi 2016 (1)‐ BCS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3 (2.6%) |
| Dolan 2015‐ BCS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Down 2013‐ BCS | 3 (2.5%) | ‐ | ‐ | 1 (0.8%) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gicalone 2007 (1)‐ BCS | ‐ | ‐ | 1 (2.3%) | ‐ | ‐ | ‐ | 3 (6.7%) | ‐ | ‐ |
| Gicalone 2007 (2)‐ BCS | ‐ | ‐ | 3 (3.4%) | ‐ | ‐ | 0 (0%) | 1 (1.14%) | ‐ | ‐ |
| Gicalone 2015‐ BCS | 5 (8.8%) | ‐ | ‐ | ‐ | ‐ | 0 (0%) | 2 (3.5%) | ‐ | ‐ |
| Keleman 2019‐ BCS | 7 (2%) | 2 (0.6%) | ‐ | 1 (0.3%) | 9 (2.6%) | ‐ | 4 (1.1%) | ‐ | <1% |
| Kimball 2018‐ BCS | 298 (1.7%) | 702 (4%) | ‐ | ‐ | ‐ | ‐ | 1000 (5.7%) | ‐ | ‐ |
| Nakada 2019‐ BCS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2 (1%) |
| Palsodittlir 2018‐ BCS | ‐ | 26 (4.6%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1 (0.2%) |
| Scheter 2019‐ BCS | ‐ | 5 (0.8%) | ‐ | ‐ | ‐ | ‐ | 11 (1.67%) | ‐ | 16 (2.4$%) |
| Tenofsky 2014‐ BCS | ‐ | ‐ | 1 (8.3%) | 0 (0%) | ‐ | ‐ | ‐ | ‐ | 0 (0%) |
| Wijgman 2017‐ BCS | 8 (9.5%) | 0 | 4 (4.8%) | 8 (9.5)%) | 15 (17.2%) | 21 (25%) | 8 (9.5%) | ‐ | ‐ |
| Zhou 2019‐ BCS | 12 (2.6%) | ‐ | ‐ | ‐ | 23 (5%) | ‐ | 59 (12.8%) | ‐ | 1 (0.2%) |
BCS: breast‐conserving surgery
1.8. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 8: Complications
14.

Funnel plot of comparison: 1 Any O‐BCS versus breast‐conserving surgery, outcome: 1.9 Complications: O‐BCS versus S‐BCS.
Recall rates
Seven studies evaluated recall rates (Amitai 2018; Dolan 2015; Fan 2019; Hu 2019; Losken 2009; Piper 2016; Tenofsky 2014). All studies evaluated the requirement for biopsies and we were able to extract dichotomous data from all but Tenofsky 2014, which reported a mean number of biopsies per woman. The risk ratio was 2.39 (95% CI 1.67 to 3.42 ; I² = 0% P = 0.53; 6 studies, 715 participants; low‐certainty evidence; Analysis 1.9). We downgraded the certainty of evidence by two levels to low due to serious risk of bias. Details on recall imaging in studies were too methodologically diverse to combine and are summarised in Table 18.
1.9. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 9: Recall rates
15. Recall rates: O‐BCS versus S‐BCS.
| Intervention details | Intervention results | S‐BCS results | |
| Amitai 2018 | VD | 7/12 due to lump needed more imaging | 7/14 due to a lump needed more imaging |
| Dolan 2015 | Both VD and VR | Imaging per patient: 2.19 ultrasound: 20/71 VD: 16/61 VR: 4/10 | Imaging per patient: 2.146, ultrasound 17/119 |
| Fan 2019 | VR | 3.2% | 1% |
| Hu 2019 | VR | 1/18 1.4% | 0 |
| Losken 2009 | VD | Further US, MRI imaging: 41.0% | Further US, MRI imaging: 47.0%, 6.0% |
MRI: magnestic resonance imaging O‐BCS: oncoplastic breast‐conserving surgery S‐BCS: standard breast‐conserving surgery US: ultrasound VD: volume displacement VR: volume replacement
Time to adjuvant therapy
Fourteen studies evaluated time to adjuvant therapy. Twelve studies defined this as from initial surgery to first adjuvant therapy appointment. Of these, three studies reported time to any adjuvant therapy (Keleman 2019; Matrai 2014; Palsodittlir 2018), six reported time to chemotherapy and radiotherapy separately (Acea‐Nebril 2017; Borm 2019; Di Micco 2017; Kimball 2018; Morrow 2019; Rose 2019), one reported time to chemotherapy only (Klit 2017), and two reported time to radiotherapy alone (Cassi 2016; Tenofsky 2014). Mazouni 2013 was found to have an unclear definition of when the timing began and Kahn 2013 defined it as from multidisciplinary team meeting, which is an unreliable time point. Therefore, we excluded these studies from the analysis.
Of these, seven studies provided extractable mean and standard deviation (SD) data (Acea‐Nebril 2017; Borm 2019; Cassi 2016; Klit 2017; Matrai 2014; Rose 2019; Tenofsky 2014) and contributed to Analysis 1.10. For time to any adjuvant therapy, the mean difference (MD) was 2.60 days (95% CI ‐5.48 to 10.68; 1 study, 120 participants). For time to adjuvant chemotherapy, the MD was ‐1.13 days (95% CI ‐2.55 to 0.29; I2 = 56%, P = 0.08; 4 studies, 4566 participants). For time to adjuvant radiotherapy, the MD was 9.67 days (95% CI 7.21 to 12.14; I2 = 54%, P = 0.07; 5 studies, 3720 participants).
1.10. Analysis.

Comparison 1: Any O‐BCS versus S‐BCS, Outcome 10: Time to therapy
The studies that reported data as the median number of days to adjuvant therapy are shown in Table 19.
16. Time to adjuvant therapy: O‐BCS versus S‐BCS unextractable values.
| Intervention details | Time to any adjuvant therapy: intervention | Time to any adjuvant therapy: control | P value | Time to adjuvant chemotherapy: intervention | Time to adjuvant chemotherapy: control | P value | Time to adjuvant radiotherapy: intervention | Time to adjuvant radiotherapy: control | P value |
| Di Micco 2017‐ VD | ‐ | ‐ | ‐ | Median (range) 39 (21 to 78) | 40 (11 to 81) | 0.551 | Median (range) 57 (36 to 153) | 53 (25 to 126) | 0.025 |
| Keleman 2019‐ VD | Median (range) 29.4 (28 to 84) | 28.7 (28 to 84) | 0.31 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kimball 2018‐ VD | ‐ | ‐ | ‐ | Median (IQR) 37 (23.5 to 51.5) | 36 (26 to 49) | 0.0004 | Median (IQR) 41 (28 to 56) | 34 (22 to 48) | 0.0002 |
| Morrow 2019‐ VD | ‐ | ‐ | ‐ | Less than 31 days: 14.9% | Less than 31 days: 22.1% | 0.171 | Median (range) 51 (35 to 125) | 50 (10 to 447) | 0.088 |
| Palsodittlir 2018‐ VD and VR | Median (range) 47.5 (22 to 111) | 50 (15 to 202) | 0.05 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
IQR: interquartile range O‐BCS: oncoplastic breast‐conserving surgery S‐BCS: standard breast‐conserving surgery VD: volume displacement VR: volume replacement
Patient‐reported outcome measures
Twenty‐three studies, evaluating 5665 participants reported outcomes for O‐BCS versus S‐BCS.
Five studies (Acea‐Nebril 2017; Di Micco 2017; PlaFarnos 2018; Rose 2020; Scheter 2019) used the validated Breast‐Q questionnaire (Cohen 2016). Of these Acea‐Nebril 2017 and PlaFarnos 2018 gave details about Breast‐Q for the intervention only. The comparative studies were synthesised using the vote‐counting method per BREAST‐Q(Cohen 2016) domain (Figure 15). The outcomes were measured/given in various ways and so we extracted the direction of effect for each Breast‐Q component, taking into account whether the study authors found the result significant or not. We downgraded these results to very low due to the very high risk of bias due to confounding and measurement of outcome.
15.
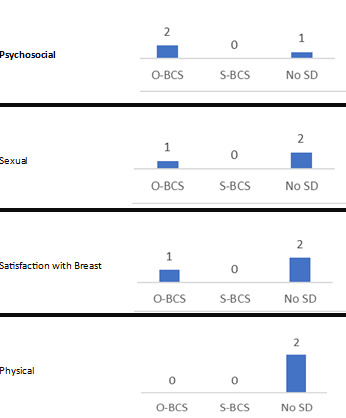
Harvest plot for vote counting: O‐BCS versus S‐BCS ‐ PROMs (Breast‐Q). Each column represents the number of studies that significantly favoured either O‐BCS, S‐BCS or found no significant difference for each Breast‐Q component.
Three studies used some form of the European Organisation for Research and Treatment of Cancer (EORTC) (Aaronson 1993) Breast questionnaires (Keleman 2019; Lansu 2014; Matrai 2014). Keleman 2019 and Matrai 2014 only reported some scales and we, therefore, deemed these at critical risk of bias.
Two studies used other validated patient‐reported outcome measures scales: Ojala 2017 used the Breast Cancer Treatment Outcome Scale (Stanton 2001), and Viega 2010 used the short‐form 36 (Garratt 1993) and Rosenberg EPM self‐esteem score (Rosenberg 1989). These studies are summarised in Table 20.
17. Patient‐reported outcome measures: O‐BCS versus S‐BCS.
| Study: intervention details | Outcome measure | Intervention: quality of life | Intervention: cosmetic | Intervention: other | Control: quality of life | Control: cosmetic | Control: other | P value | Conclusion |
| Keleman 2019 ‐ VD | EORTC | Median (range) emotional functioning score: 91.6 (50‐100), social functioning score: 83.4 (33‐100) | Median (range): body image score: 91.6 (50‐100) | ‐ | Emotional functioning score: 83.4 (50‐100), Social functioning score: 75.0 (50‐100) | Body image score was 75.0 (33‐100) | ‐ | < 0.01/< 0.01/< 0.01 | OPS significantly better in emotional/social/body image |
| Lansu 2014 ‐ VD | EORTC QLQ C30 and BR23 and Young Boost Trial | C30 function scale: 75.9 (22.57) C30 symptom scale: 17.31 (10.2) C30 QOL: 63.45(35.77) BR23 fuction scale: 70.19(16.30) BR23 symptom scale: 20.51 (12.35) | YBT 26.94 (15.03) | ‐ | C30 function scale: 92.34 (5.89) C30 symptom scale: 14.51 (11.18) C30 QOL: 87.96(7.30) BR23 function scale: 84.17(7.3) BR23 symptom scale: 11.9 (8.32) | YBT 31.35 (23.79) | ‐ | 0.28/0.57/0.05/0.06/0.07/0.93 | BCS significantly better in C30 QOL but otherwise no SD |
| Matrai 2014 ‐ VD | Q 47‐53 of Hungarian EORTC and self‐designed cosmetic | The quality of life questions, "Did you feel any arm or shoulder pain?" (P = 0.0399), "Did you have difficulty raising or moving your arm to the side?" (P = 0.0060) and “Did you feel any pain in the affected chest area?” (P = 0.0304) showed a significant advantage in the OPS group. | 8.73 (1.023) 61.7% had 9/10 or 10/10 | ‐ | ‐ | 7.35 (1.5) ‐ 23.3% had 9/10 or 10/10 | ‐ | < 0.001 | Significantly better PR cosmetic score in OPS than control. Significantly less shoulder disability and chest pain in OPS group. |
| Acosta‐Marin 2014 ‐ VD | Self‐designed | ‐ | Mean: 4.4 ‐ 88.4% (4/5 (good) or 5/5 (excellent)) | ‐ | ‐ | Mean: 4.2 83.4% (4/5 (good) or 5/5 (excellent)) | ‐ | 0.644 | No SD |
| Jiang 2015 ‐ VD | Self‐designed | ‐ | 28 (93.3%) satisfied | ‐ | ‐ | 25 (83.3%) satisfied | ‐ | ‐ | More satisfied in OPS |
| Tang 2016 ‐ VD and VR | Self‐designed | ‐ | 62/67 satisfied | ‐ | ‐ | 92/117 satisfied | ‐ | 0.025 | Significantly more satisfied in OPS |
| Eichler 2013 ‐ VD | Self‐designed | Overall satisfaction (4‐5/5): 86% | Satisfied with overall appearance: 83% | ‐ | Overall satisfaction (4‐5/1‐5): 87% | Satisfied with overall appearance: 87% | No significant difference in overall, shape, appearance, size, quality of life, sensitivity in nipple, swelling, self‐confidence. Significant better satisfaction with appearance/amount of scar tissue in BCS compared to OPS | Overall satisfaction: 0.48 Cosmetic evaluation: 0.91 Scar satisfaction: 0.013 |
No SD in most domains. Scar satisfaction better in BCS |
| Gicalone 2007 (2) ‐ VD | Self‐designed | ‐ | 32/39 (4‐5/5) | ‐ | 63/88 (4‐5/5) | ‐ | 0.23 | No SD of cosmetic satisfaction between patient groups | |
| Hilli‐Betz 2014 ‐ VD | Self‐designed | ‐ | 92.8% ‐ very satisfied with the cosmetic appearance of their breasts. No difference in physical attractiveness | More PROMS in paper | ‐ | 83.5% ‐ very satisfied with the cosmetic. No difference in physical attractiveness | More PROMS in paper | 0.189/0.435 | No SD in PROMs in paper |
| Mazouni 2013 ‐ VD | Self‐designed | ‐ | Moderately satisfied: 12.5%; satisfied: 37.5%; very satisfied: 50% | ‐ | ‐ | Moderately satisfied: 14.5%, satisfied: 47.9%, very satisfied: 37.6% | ‐ | 0.52 | No SD in cosmetic difference |
| Palsodittlir 2018 ‐ VD and VR | Self‐designed | ‐ | 97% happy with aesthetic outcome of surgery | ‐ | ‐ | 89% happy | ‐ | ‐ | Greater proportion of patients in OPS happy with the aesthetic outcome |
| Santos 2015 ‐ VD | Self‐designed | ‐ | 35 excellent (61.4%) | ‐ | ‐ | 45 excellent (69.2%) | ‐ | 0.242 | No SD in patient‐reported cosmetic score |
| Sherwell‐Cabello 2006 ‐ VD | Self‐designed | Overall QOL 4.77 | 4.81 | ‐ | 4.81 | 4.72 | ‐ | 0.256 | No SD in any parameters. High levels of aesthetic acceptance and mild psychological and social impact on patients. |
| Tenofsky 2014 ‐ VD | Self‐designed | ‐ | 8 complained of unfavourable cosmetic outcomes (13.8%) | ‐ | ‐ | 6 (7.1%) complained of unfavourable cosmetic outcome | ‐ | 0.191 | No SD in patient‐reported complaints of cosmetic outcome |
| Viega 2011 ‐ VD | Self‐designed | ‐ | 10 (9‐10)/10 | ‐ | ‐ | 10 (5‐10)/10 | ‐ | < 0.001 | OPS is better than standard BCS |
| Zhou 2019 ‐ VR | Self‐designed and DASH | ‐ | 28 (87.5) extremely satisfied | DASH 10.57 | ‐ | 22 (78.6) extremely satisfied | DASH 7.86 | NS/0.06 | No SD in overall outcome or DASH questionnaires scores |
O‐BCS: oncoplastic breast‐conserving surgery S‐BCS: standard breast‐conserving surgery VD: volume displacement VR: volume replacement
PROM: Patient‐reported outcome measures
DASH: The Disabilities of the Arm, Shoulder and Hand Questionnaire
EORTC: The European Organisation for Research and Treatment of Cancer
YBT: Young Boost Trial
Thirteen studies (Acosta‐Marin 2014; Eichler 2013; Gicalone 2007 (2); Hilli‐Betz 2014; Jiang 2015; Mazouni 2013; Palsodittlir 2018; Santos 2015; Sherwell‐Cabello 2006; Tang 2016; Tenofsky 2014; Viega 2011; Zhou 2019) used self‐designed unvalidated questionnaires to assess patient‐reported outcome measures. The results of these studies are also summarised in Table 20. We deemed these studies to have too high risk of bias and methodological diversity to synthesise in any form. We downgraded these results to very low due to very high risk of bias and inconsistent results.
Cosmetic evaluation
Nine studies evaluating 1461 participants reported a cosmetic evaluation for O‐BCS versus S‐BCS (Acosta‐Marin 2014; Gicalone 2007 (2); Hilli‐Betz 2014; Jiang 2015; Keleman 2019; Lansu 2014; Santos 2015; Scheter 2019; Viega 2011).
Three studies used the computer programme BCCT.core to objectively assess aesthetic outcomes (Hilli‐Betz 2014; Lansu 2014; Santos 2015). We synthesised these studies using the vote‐counting method (Figure 16).
16.
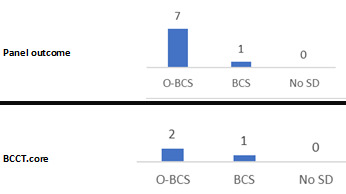
Harvest plot for vote counting: O‐BCS versus S‐BCS ‐ cosmetic evaluation. Each column represents the number of studies that significantly favoured either O‐BCS, S‐BCS or found no significant difference for BCCT.core scores or panel assessment.
Eight studies used an expert panel and self‐designed assessment of aesthetic outcome (Acosta‐Marin 2014; Gicalone 2007 (1); Hilli‐Betz 2014; Keleman 2019; Lansu 2014; Santos 2015; Scheter 2019; Viega 2011) and results are provided in Table 21. These studies have a lot of methodological diversity but we deemed it appropriate to use the vote‐counting method to synthesise results in Figure 16.
18. Cosmetic‐reported outcomes: O‐BCS versus S‐BCS ‐ subjective panel assesment.
| Study | Intervention details | Assesment details | Intervention results | Control results | P‐value | Conclusion |
| Acosta‐Marin 2014 | VD | Self‐designed, 4 person panel (1 plastic surgeon, 1 breast surgeon, 2 surgical oncologists) | Mean 4.5/5 | 4.1/5 | < 0.005 | O‐BCS better than control (S‐BCS) |
| Jiang 2015 | VD | Self‐designed ‐ grade 1‐3 (1 best, 1 and 2 satisfactory) by 1 doctor, 1 nurse, 1 non‐professional | 93.3% satisfactory | 83.3% satisfactory | ‐ | O‐BCS better than control (S‐BCS) |
| Gicalone 2007 (2) | VD | Self‐designed, panel (1 surgeon, 1 oncologist) | 33; 4‐5/5 | 11; 4‐5/5 | 0.006 | O‐BCS (Donught mastopexy) significantly better cosmetic results than standard lumpectomy (S‐BCS) |
| Hilli‐Betz 2014 | VD | Self‐designed, 1 surgeon evaluation | Excellent in 8.7%, good in 63.8%, moderate in 24.6%, and poor in 0.0% | Excellent in 32.2%, good in 60.9%, moderate in 5.6%, and poor in 0.6% | 0.191 | O‐BCS (Dermoglandular rotation) significantly worse than standard segmentectomy |
| Keleman 2019 | VD | Self‐designed ‐ 3 surgeons panel | Median (range) 4.4 (3‐5)/5 | Median (range) 3.2 (1‐5)/5 | 0.001 | O‐BCS significantly better than control |
| Santos 2015 | VD | 2 plastic surgeon panel | Excellent in 50.9%, good in 40.4%, moderate in 7%, and poor in 1.8% | Excellent in 18.5%, good in 61.5%, moderate in 18.5%, and poor in 1.5% | < 0.001 | O‐BCS significantly better than control |
| Scheter 2019 | VD | Self‐designed, 13 plastic surgeon panel (1‐10) 10 excellent | Shape: 7.9; Symmetry: 7.9 Volume: 8.1 | Shape: 5.5 Symmetry: 5.4 Volume: 6.2 | 0.002/0.016/0.012 | O‐BCS significantly better than control |
| Viega 2011 | VD and VR | Self‐designed ‐ 2 breast surgeons, 2 plastic surgeons (male and female of each): FBS/MBS/FPS/MPS | 10/9/9/9 | 9/8/6/6 | < 0.001/0.005/< 0.001/< 0.001 | O‐BCS significantly better than control |
O‐BCS: oncoplastic breast‐conserving surgery S‐BCS: standard breast‐conserving surgery VD: volume displacement VR: volume replacement
FBS: female breast surgeon
MBS: male breast surgeon
FPS: female plastic surgeon
MPS: male plastic surgeon
Comparison 2: O‐BCS versus mastectomy without reconstruction
Primary outcomes
Local recurrence
Five studies involving 6682 participants evaluated disease‐free survival for O‐BCS versus mastectomy alone. It was possible to extract HRs for two studies, both of which reported local recurrence‐free survival (Ren 2014; Carter 2016) (HR 0.55; 95% CI 0.34 to 0.91; I2 = 81%, P = 0.02; 2 studies, 4713 participants; very‐low uncertainty evidence Analysis 2.1). We downgraded the evidence by one level for risk of bias due to confounding and two levels due to inconsistency.
2.1. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 1: Local recurrence (HR)
To see the impact of the studies where data were not extractable as HRs, we extracted the data as dichotomous event rates and analysed with time points of 1 to 5 years (RR 0.32, 95% CI 0.24 to 0.41; I2 = 64%, P = 0.1; 2 studies, 4025 participants), 5 years (RR 0.84, 95% CI 0.41 to 1.75; I2 = 33%, P = 0.22; 2 studies, 942 participants) and 10 years follow‐up (RR 6.52, 95% CI 1.42 to 30.06; 1 study, 1193 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 2: Local recurrence (RR)
Disease‐free survival
One study involving 1193 participants evaluated disease‐free survival for O‐BCS versus mastectomy alone (Nakagomi 2019). It reported significantly better disease‐free survival in the intervention group (Analysis 2.3). However, this study was at serious risk of bias due to confounding from clinicopathological factors and uneven distribution of co‐interventions. One study evaluated disease‐free survival (Lee 2018), but no data were extractable from it. Therefore, no studies reported HR and so were unable to contribute to this analysis; there were insufficient data to make any conclusions.
2.3. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 3: Disease‐free survival
Overall survival
Three studies involving 5382 participants evaluated overall survival for O‐BCS versus mastectomy alone (Carter 2016; Lee 2018; Ren 2014). It was possible to extract HRs for two studies (Carter 2016; Ren 2014). The HR for OS was 0.39 (95% CI 0.30 to 0.51; I2 = 71%, P = 0.06; 2 studies, 4713 participants).
To see the impact of the studies where data were not extractable as HRs, we extracted the data as dichotomous event rates and analysed with time points of 1 to 5 years (RR 0.30, 95% CI 0.22 to 0.40; 1 study, 3924 participants) and 5‐year follow‐up (RR 1.71, 95% CI 0.79 to 3.69; I2 = 88%, P = 0.004; 2 studies, 932 participants) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 5: Overall survival (RR)
Secondary outcomes
Re‐excision rates
Re‐excisions for oncological margin control are not often performed when a mastectomy is undertaken, therefore this outcome is not relevant for this comparison.
Complications
Four studies evaluated complications in O‐BCS versus mastectomy without reconstruction (Acea‐Nebril 2005; Carter 2016; Gendy 2003; Potter 2020). The RR of developing a complication compared to mastectomy was 0.75 (95% CI 0.67 to 0.83; I2 = 61%, P = 0.05; 4 studies, 4839 participants; very‐low certainty evidence). We downgraded the certainty of evidence two levels due to risk of bias (confounding) and two levels due to inconsistency of the results.
Acea‐Nebril 2005 and Carter 2016 mentioned a breakdown of complications. This is found in Table 22 and Table 23.
19. Complications: O‐BCS of those compared to mastectomies.
| Study | Intervention type | Wound infection | Flap/skin necrosis | Dehisence | Fat necrosis | Seroma | Skin | Haematoma | Needed surgery |
| Acea‐Nebril 2005 | VD | 2 (4%) | 3 (6%) | ‐ | ‐ | 3 (6%) | ‐ | 4 (8%) | ‐ |
| Carter 2016 | VD and VR | 45 (4.8%) | ‐ | ‐ | ‐ | 126 (13.4%) | ‐ | 18 (1.9%) | ‐ |
| Mustonen 2004 | VR | 1 (8.3%) | 1 (8.3%) | ‐ | ‐ | 3 (25%) | ‐ | ‐ | ‐ |
| Ozmen 2020 | VR | ‐ | ‐ | ‐ | ‐ | ‐ | 3 (5.6%) | ‐ | 5 (2%) |
| Peled 2014 | VD | 6 (16.2%) | ‐ | 4 (10.8%) | ‐ | ‐ | ‐ | ‐ | 1 (2.7%) |
| Potter 2020 | VD | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 8 (2.13%) |
| Tong 2016 | VD | 11 (8.4%) | 0 (0%) | 8 (6.1%) | 5 (3.8%) | ‐ | 18 (13.7%) | 4 (3.1%) | 3 (2.3%) |
O‐BCS: oncoplastic breast‐conserving surgery VD: volume displacement VR: volume replacement
20. Complications: mastectomies.
| Study | Control Details | Wound infection | Flap/skin necrosis | Dehiscence | Fat necrosis | Seroma | Skin | Haematoma | Needed surgery |
| Acea‐Nebril 2005 | Mx | 3 (5.6%) | 0 (0%) | ‐ | ‐ | 14 (26.4%) | ‐ | 2 (3.6%) | ‐ |
| Carter 2016 | Mx | 133 (5.8%) | ‐ | ‐ | ‐ | 305 (13.2%) | ‐ | 66 (2.9%) | ‐ |
| Carter 2016 | Mx+R | 212 (11.6%) | ‐ | ‐ | ‐ | 228 (12.5%) | ‐ | 87 (4.8%) | ‐ |
| Mustonen 2004 | Mx+R | 1 (8.3%) | 1 (8.3%) | ‐ | ‐ | 3 (5.6%) | ‐ | ‐ | 1 (8.3%) |
| Ozmen 2020 | Mx+R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 5 (6.7%) |
| Peled 2014 | Mx+R | 23 (35.9%) | ‐ | 19 (29.7%) | ‐ | ‐ | ‐ | ‐ | 24 (37.5%) |
| Potter 2020 | Mx+R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 96 (9.5%) |
| Tong 2016 | Mx+R | 31 (11.2%) | 41 (14.8%) | 14 (5.1%) | 11 (4%) | 32 (11.6%) includes haematoma | 14 (5.1%) | ‐ | 27.1% |
Mx: mastectomy R: reconstruction
Recall rates
Recall biopsy after mastectomy is often not needed, therefore this outcome is not relevant for this comparison.
Time to adjuvant therapy
Four studies including 5093 participants evaluated time to adjuvant therapy for O‐BCS versus mastectomy alone. Three studies ( (Kahn 2013; Morrow 2019; Potter 2020) defined this as from initial surgery to first adjuvant therapy appointment. Klit 2017 reported time to chemotherapy. Morrow 2019 and Potter 2020 reported time to chemotherapy and radiotherapy separately. Kahn 2013 defined it as from multidisciplinary team meeting, which is an unreliable time point. Potter 2020 defined this as from the final surgery and reported time to chemotherapy and radiotherapy separately. Therefore, we excluded this study from the analysis.
Klit 2017 provided extractable mean and SD data and contributed to Analysis 2.7. This showed no difference between the groups in time to adjuvant therapy, and no conclusions can be made from the results due to the lack of studies reporting outcome data. The studies that reported data as medians and provided P values are shown in Appendix Table 24.
2.7. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 7: Time to therapy
21. Time to adjuvant therapy: O‐BCS versus mastectomy alone.
| Study | Intervention details | Time to adjuvant chemotherapy ‐ intervention | Time to adjuvant chemotherapy ‐ control | P value | Time to adjuvant radiotherapy ‐ intervention | Time to adjuvant radiotherapy ‐ control | P value |
| Morrow 2019 | VD | Less than 31 days: 14.9% | Mx: 16.2% MX+R: 10.8% |
0.787/0.386 | Median (range) 51 (35‐125) | Mx alone: 55 (26‐428) Mx+R: 56 (33‐122) |
0.626/0.747 |
Mx: mastectomy R: reconstruction VD: volume displacement
Patient‐reported outcome measures
One study compared aesthetic outcomes between O‐BCS (49 participants) and mastectomy without reconstruction (58 participants; Gendy 2003). The authors used the Hopwood Body Image score (Hopwood 2001), hospital anxiety and depression scale (Zigmond 1983) and Rosenberg self‐esteem scale (Jordan 2020) to assess patient outcomes. They found objectively and subjectively significantly better sensation in the intervention group. Body image based on the Hopwood Body Image score (Hopwood 2001) was significantly better in the intervention group. There was no significant difference in anxiety/depression. We deemed the study to have a serious risk of bias due to confounding, selection bias and measurement and reporting of the outcome. No conclusions can be made due to the lack of studies reporting this outcome for this comparison.
Cosmetic evaluation
One study involving 107 participants, reported this outcome (Gendy 2003). The authors used a self‐designed questionnaire given to a panel of five surgeons to mark the breasts' aesthetic outcome out of five. They found O‐BCS to be better (median (range) 3.8/5 (1.2 to 5)) than mastectomy alone (2.9 (1 to 4.4)). We deemed the study to have a critical risk of bias due to the measurement of the outcome. No conclusions can be made due to the lack of studies reporting this outcome for this comparison.
Comparison 3: O‐BCS versus mastectomy with reconstruction (Mx+R)
Primary outcomes
Local recurrence
Six studies involving 6337 participants evaluated disease‐free survival for O‐BCS versus mastectomy with reconstruction (Carter 2016; DeLorenzi 2016 (2); Lee 2018; Mansell 2017; Mustonen 2004; Ozmen 2020). It was possible to extract HR for three studies of which one reported local recurrence‐free survival compared to the control group Mx+R alone (Carter 2016), and two reported local recurrence compared to the control group Mx with or without reconstruction (DeLorenzi 2016 (2); Mansell 2017; Analysis 3.1). The HR for local recurrence‐free survival was 1.37 (95% CI 0.72 to 2.62; 1 study, 3785 participants; very low‐certainty evidence) and for local recurrence rate was 1.03 (95% CI 0.75 to 1.42; 2 studies, 1001 participants). We downgraded the evidence by two levels due to high risk of bias due to confounding and one level due to imprecision as the optimal size was not met.
3.1. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 1: Local recurrence‐free survival
To see the impact of the studies where data were not extractable as HRs, we extracted the data as dichotomous event rates and analysed with time points of 1 to 5 years (RR 1.19, 95% CI 0.87 to 1.64; I2 = 0%, P = 0.43; 2 studies, 3449 participants), 5 years with the comparator Mx+R alone (RR 0.53, 95% CI 0.19 to 1.44; I2 = 0%, P = 0.87; 2 studies, 830 participants) and 5 years with the comparator Mx+/‐R (RR 1.54, 95% CI 0.74 to 3.21; I2 = 14%, P = 0.28; 2 studies, 1001 participants) in Analysis 3.2.
3.2. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 2: Local recurrence
Disease‐free survival
Three studies involving 1318 participants evaluated disease‐free survival for O‐BCS versus mastectomy with reconstruction (DeLorenzi 2016 (2); Mansell 2017; Ozmen 2020). Lee 2018 evaluated disease‐free survival, but we were not able to extract data. It was possible to extract HRs for all other studies (Analysis 3.3): O‐BCS versus Mx+R alone (HR 0.45, 95% CI 0.09 to 2.22; 1 study, 317 participants; very‐low certainty evidence); O‐BCS versus Mx+/‐R (HR 1.03, 95% CI 0.75 to 1.42; 2 studies, 1001 participants). We downgraded the evidence to very low certainty due to the study design, high risk of bias and inconsistency.
3.3. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 3: Disease‐free survival (HR): Mx+R
To see the impact of the studies if we extracted the data as dichotomous event rates, we analysed that available data at time points of 5 years follow‐up with the comparator Mx+R alone (RR 0.74, 95% CI 0.27 to 2.04; 1 study, 317 participants) and 5 years with the comparator Mx+/‐R (RR 0.88, 95% CI 0.66 to 1.18; I2 = 4%, P = 0.31; 2 studies, 1001 participants) in Analysis 3.4.
3.4. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 4: Disease‐free survival (RR): Mx+R
Overall survival
Five studies involving 5616 participants evaluated overall survival for O‐BCS versus mastectomy with reconstruction. It was possible to extract HRs for four studies (Carter 2016; DeLorenzi 2016 (2); Mansell 2017; Ozmen 2020): O‐BSC versus Mx+R alone (HR 1.74, 95% CI 1.23 to 2.47; I² = 0%, P = 0.5; 2 studies, 4102 participants; Analysis 3.5) and O‐BCS versus Mx+/‐R (HR 0.65, 95% CI 0.40 to 1.07; I² = 85%, P = 0.01; 2 studies, 1001 participants; Analysis 3.5).
3.5. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 5: Overall survival (HR): Mx+R
To see the impact of the studies where data were not extractable as HRs, we extracted the data as dichotomous event rates and analysed with time points of 1 to 5 years (RR 1.39, 95% CI 0.97 to 1.98; 1 study, 3387 participants), 5‐year follow‐up with the comparator Mx+R alone (RR 0.52, 95% CI 0.33 to 0.84; I2 = 0%, P = 0.49; 2 studies, 1001 participants) and 5‐year follow‐up with the comparator Mx+/‐R (RR 0.52, 95% CI 0.33 to 0.84; I2 = 87%, P = 0.006; 2 studies, 1001 participants) in Analysis 3.6.
3.6. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 6: Overall survival (RR): Mx+R
Secondary outcomes
Re‐excision rates
Re‐excisions for oncological margin control are not often performed when a mastectomy is undertaken, therefore this outcome is not relevant for this comparison.
Complications
Six studies evaluated complications in O‐BCS versus mastectomy with reconstruction (Carter 2016; Mustonen 2004; Ozmen 2020; Peled 2014; Potter 2020; Tong 2016). The combined RR was 0.49 (95% CI 0.45 to 0.54; I2 = 87%, P < 0.0001; 5 studies, 4973 participants; very‐low certainty evidence) with critical heterogeneity. We downgraded the certainty of evidence to very low due to high risk of bias due to confounding and heterogeneity of the results. All studies mentioned a breakdown of complications and are recorded in Table 22 and Table 23.
Recall rates
Recall after mastectomy is often not needed, therefore this outcome is not relevant for this comparison.
Time to adjuvant therapy
Four studies including 2766 participants evaluated time to adjuvant therapy for O‐BCS versus mastectomy plus reconstruction (Kahn 2013; Morrow 2019; Potter 2020; Tong 2016).
Only Morrow 2019 defined this as from initial surgery to first adjuvant therapy appointment and data are reported in Table 24. Potter 2020 defined this as from the final surgery and reported time to chemotherapy and radiotherapy separately. Kahn 2013 defined it as from multidisciplinary team meeting, which is an unreliable time point. Tong 2016 reported how many patients had complications that resulted in a delay to receiving adjuvant therapy. Therefore, we excluded these three studies from the analysis.
Patient‐reported outcome measures
Three studies evaluated patient‐reported outcomes in O‐BCS compared to mastectomy and reconstruction (Hart 2015; Kelsall 2017; Ozmen 2020), and results are presented in Table 25. Studies were all of serious risk of bias due to measurement of outcome. They are too methodologically diverse to synthesise.
22. Patient‐reported outcome measures: O‐BCS versus Mx+R.
| Study | Intervention details | Assesment details | Intervention results | Control results | P value | Conclusion |
| Hart 2015 | VD | Self‐designed | ‐ | ‐ | 0.03/0.02/0.09/0.03 | Greater gains in satisfaction with body image, more often attributed to their reconstruction than control. Increased ability to wear revealing clothing. More often thought they were perceived as womanly by partner |
| Kelsall 2017 | VD and VR | Hopwood Body Image score/return to activities | Case‐matched: large/small breasts mean body image score: 3.3/5.69 | Body image score: 5.37/5.34 | 0.011/0.715 | OPS better body image score in large breasts |
| Ozmen 2020 | VR | EORTC | Physical function: 88.6 (26.6‐100) emotional function: 83.3 (0‐100) body image: 75 (0‐100) | Physical function: 93.3 (33.3‐100) emotional function: 83.3 (33‐100) body image: 58.3 (0‐100) | < 0.001, 0.71, 0.012, 0.298 | Significantly better physical function, less nausea and vomiting, less sleep disturbance, fewer breast symptoms in M + I. Better body image in MLDF. No SD in emotional or physical function |
Mx: mastectomy O‐BCS: oncoplastic breast‐conserving surgery R: reconstruction VD: volume displacement VR: volume replacement
EORTC: The European Organisation for Research and Treatment of Cancer Questionnaire
Cosmetic evaluation
One study compared aesthetic outcome between O‐BCS (242 participants) and mastectomy with reconstruction (75 participants) (Ozmen 2020). Authors used the Japanese Breast Cancer Society Cosmetic Evaluation Scale (Kijima 2011) assessed by a panel. They found O‐BCS had a significantly better cosmetic outcome. We deemed the study to have serious risk of bias due to selection bias and measurement of the outcome. No conclusions can be made due to the lack of studies reporting this outcome for this comparison.
Subgroup analysis
For each outcome, we evaluated how many evaluated the subgroups of volume displacement and volume replacement techniques to see if this changed the conclusions. Most of the studies used volume displacement techniques only or did not evaluate the techniques separately.
Comparison 1: O‐BCS versus S‐BCS
Local recurrence
Of the 24 studies evaluating local recurrence, 15 studies (62.5%) evaluated local recurrence for the volume displacement subgroup (Acea‐Nebril 2017; Amitai 2018; Borm 2019; Cassi 2016; Chakravorty 2012; Gulcelik 2013; Keleman 2019; Lee 2018; Losken 2009; Malhaire 2015; Matrai 2014; Mazouni 2013; Niinikoski 2019 (2); Piper 2016; Vieira 2016), and three studies (12.5%) evaluated local recurrence for the volume replacement subgroup (Fan 2019; Hashimoto 2019; Lee 2018).
Out of the seven studies we were able to extract HRs from, three studies were volume displacement (Borm 2019; Niinikoski 2019 (2); Piper 2016), and none were volume replacement. Therefore, insufficient evidence was available to conduct a subgroup analysis.
It was possible to see the impact of volume displacement O‐BCS on local recurrence when data were extracted as dichotomous event rates and analysed with time points of 1 to 5 years (RR 0.84, 95% CI 0.51 to 1.39; 8 studies, 2578 participants) and 5‐year follow‐up (RR 0.90, 95% CI 0.63 to 1.27; 8 studies, 4729 participants) in Analysis 4.1.
4.1. Analysis.

Comparison 4: Volume displacement versus S‐BCS, Outcome 1: Local recurrence
Disease‐free survival
Of the nine studies that evaluated disease‐free survival, five studies (56%) evaluated volume displacement techniques (Acea‐Nebril 2017; Borm 2019; Gulcelik 2013; Mazouni 2013; Vieira 2016), whilst none evaluated volume replacement techniques alone. Of these, we were able to extract HRs from four studies (Borm 2019; Gulcelik 2013; Mazouni 2013; Vieira 2016), therefore, insufficient evidence was available to conduct a subgroup analysis.
Overall survival
Of the 13 studies that evaluated overall survival, eight studies (62%) evaluated volume displacement techniques (Acea‐Nebril 2017; Borm 2019; Gulcelik 2013; Lee 2018; Mazouni 2013; Niinikoski 2019 (2); Piper 2016; Vieira 2016). One study (8%) evaluated volume replacement techniques (Lee 2018). For three volume displacement studies, we were able to extract HRs (Borm 2019; Mazouni 2013; Vieira 2016), therefore, insufficient evidence was available to conduct a subgroup analysis. We analysed those studies that were extracted as dichotomous data with sufficient data for the 5‐year time point (RR (non‐event) 0.76, 95% CI 0.59 to 0.98; 7 studies, 4373 participants) in Analysis 4.2. There were insufficient data to comment on volume replacement techniques.
4.2. Analysis.

Comparison 4: Volume displacement versus S‐BCS, Outcome 2: Overall survival
Re‐excision rates
Of the 38 studies that evaluated participants that need further surgery due to inadequate cancer resection, 27 studies (69%) evaluated volume displacement techniques (Acea‐Nebril 2017; Amitai 2018; Atallah 2015; Bali 2018; Cassi 2016; Chakravorty 2012; Crown 2015; Di Micco 2017; Gicalone 2007 (1); Gicalone 2007 (2); Gicalone 2015; Gulcelik 2013; Hamdi 2008; Jiang 2015; Keleman 2019; Lansu 2014; Losken 2014; Malhaire 2015; Mansell 2015; Matrai 2014; Mazouni 2013; Niinikoski 2019 (2); Ojala 2017; Piper 2016; Tenofsky 2014; Vieira 2016; Wijgman 2017; Wong 2017) and two studies (5%) evaluated volume replacement techniques (Bali 2018; Fan 2019). For total re‐excisions in these studies of volume displacement techniques, the RR was 0.77 (95% CI 0.69 to 0.87; 27 studies, 9076 participants) and for total mastectomy, the RR was 1.05 (95% CI 0.86 to 1.28; 16 studies, 7078 participants; Analysis 4.3). There were insufficient data to comment on volume replacement techniques.
4.3. Analysis.

Comparison 4: Volume displacement versus S‐BCS, Outcome 3: Re‐excision rates
Complications
Of the 33 studies that evaluated complications, 21 studies (64%) evaluated volume displacement techniques (Acea‐Nebril 2005; Acea‐Nebril 2017; Acosta‐Marin 2014; Amitai 2018; Cassi 2016; Crown 2019; Di Micco 2017; Gicalone 2007 (1); Gicalone 2007 (2); Gicalone 2015; Jiang 2015; Keleman 2019; Kimball 2018; Lansu 2014; Matrai 2014; Ojala 2017; PlaFarnos 2018; Scheter 2019; Sherwell‐Cabello 2006; Tang 2016; Tenofsky 2014; Wijgman 2017) and three studies (9%) evaluated volume replacement techniques (Nakada 2019; Ozmen 2020; Zhou 2019).
Of the 21 studies that reported the rate of complications included in the meta‐analysis, 14 studies evaluated volume displacement techniques ( Acea‐Nebril 2017; Acosta‐Marin 2014; Cassi 2016; Crown 2019; Di Micco 2017; Gicalone 2015; Jiang 2015; Keleman 2019; Lansu 2014; Matrai 2014; PlaFarnos 2018; Scheter 2019; Sherwell‐Cabello 2006; Wijgman 2017) and one study evaluated volume replacement techniques (Ozmen 2016). For volume displacement techniques, the RR was 1.03 (95% CI 0.9 to 1.18; 14 studies, 4083 participants; Analysis 4.4). There were insufficient data to comment on volume replacement techniques.
4.4. Analysis.

Comparison 4: Volume displacement versus S‐BCS, Outcome 4: Complications
Recall rates
Of the six studies that evaluated recall rates, three studies (50%) evaluated volume displacement techniques (Amitai 2018; Losken 2009; Piper 2016) and two studies (33%) evaluated volume replacement techniques (Fan 2019; Hu 2019). There were insufficient data to comment on both volume displacement and replacement techniques.
Time to adjuvant therapy
Of the seven studies that provided extractable mean and SD data, four of them evaluated volume displacement techniques (Acea‐Nebril 2017; Cassi 2016; Matrai 2014; Tenofsky 2014), and none reported volume replacement techniques. There were insufficient data to comment on both volume displacement and replacement techniques.
Patient‐reported outcome measures
Of the 24 studies that evaluated patient‐reported outcomes, 18 studies (75%) evaluated volume displacement techniques and one study (4%) evaluated volume replacement techniques. Due to the high risk of bias and methodological diversity, it was not possible to conduct a subgroup analysis. The results of each study along with their intervention method are presented in Analysis 1.11 and Table 20.
1.11. Analysis.
Comparison 1: Any O‐BCS versus S‐BCS, Outcome 11: Patient‐reported outcomes (BREAST‐Q)
| Patient‐reported outcomes (BREAST‐Q) | |||||
| Study | Intervention details (type ‐ n) | Intervention BREAST‐Q (n/100) | Control | Statistics | Conclusion |
| Acea‐Nebril 2017 | VD ‐ 60 | > 80 in all domains | ‐ | ‐ | No comparison |
| Di Micco 2017 | VD ‐170 | Median: psychological = 83; satisfaction with breast = 82 evolution = 73, sexual = 70 | ‐ | ‐ | No comparison |
| PlaFarnos 2018 | VD ‐ 70 | Median (IQR): satisfaction with breast: 80 (0‐100); psychosocial well‐being: 76 (0‐100); sexual well being: 46 (26‐100); physical wellbeing: 81 (37‐100) | Median (IQR): satisfaction with breast: 68 (IQR 29‐100); psychosocial well‐being: 82 (0‐100); sexual well‐being: 57 (0‐100); physical well‐being: 75 (17‐100) P = 0.32/0.71/0.08/0.422 |
P value: 0.32/0.705/0.079/0.422 | No significant difference (SD) in any domain |
| Rose 2020 | Both ‐ 96 | No. of patients above median score psychosocial: 2.15 (1.25‐3.69); physical: 0.83 (0.5‐1.39); satisfaction with breast: 0.95 (0.57‐1.59); sexual well‐being 1.42 (0.78‐2.58) |
No. of patients above median score: psychosocial: 2.15 (1.25‐3.69); physical: 0.83 (0.5‐1.39); satisfaction with breast 0.95 (0.57‐1.59); sexual well‐being; 1.42 (0.78‐2.58) |
Odds ratio psychosocial: 2.15 (1.25‐3.69); physical: 0.83 (0.5‐1.39); satisfaction with breast 0.95 (0.57‐1.59); sexual well‐being: 1.42 (0.78‐2.58) | Better psychosocial well‐being in O‐BCS. No SD in any other domain |
| Scheter 2019 | VD ‐ 12 | Mean score per domains: satisfaction with breast: 75.18; psychosocial well‐being: 76.09; sexual well‐being: 78 | Satisfaction with breast: 39.64; psychosocial well‐being: 43.18; sexual well‐being: 41 | 0.001/0.025/0.021 | O‐BCS better in satisfaction of breast, psychosocial wellbeing and sexual well‐being |
Cosmetic evaluation
Of the nine studies evaluating cosmetic evaluation, eight studies evaluated volume displacement techniques only (Acosta‐Marin 2014; Gicalone 2007 (2); Hilli‐Betz 2014; Jiang 2015; Keleman 2019; Lansu 2014; Santos 2015; Scheter 2019). Due to the high risk of bias and methodological diversity it was not possible to conduct a subgroup analysis.
Comparison 2: O‐BCS versus mastectomy without reconstruction
Local recurrence
Of the five studies that evaluated local recurrence for O‐BCS versus mastectomy alone, four studies (80%) evaluated volume replacement only (Gendy 2003; Lee 2018; Nakagomi 2019; Ren 2014) and one study evaluated volume displacement (Lee 2018). There were insufficient data to comment on both volume displacement and replacement techniques.
Disease‐free survival
No studies evaluated volume displacement or replacement alone for disease‐free survival.
Overall survival
Of the three studies that evaluated overall survival for O‐BCS versus mastectomy alone, two studies (66%) evaluated volume replacement (Lee 2018; Ren 2014) and one study evaluated volume displacement (Lee 2018). There were insufficient data to comment on both volume displacement and replacement techniques.
Complications
Of the four studies that evaluated complications in O‐BCS versus mastectomy alone, two studies (50%) evaluated volume displacement techniques (Acea‐Nebril 2005; Potter 2020) and one study (25%) evaluated volume replacement techniques (Gendy 2003). There were insufficient data to comment on both volume displacement and replacement techniques.
Time to adjuvant therapy
No studies evaluated any subgroup alone and provided extractable mean and SD data. Morrow 2019 and Potter 2020 both extracted volume displacement only, details of which can be shown in Table 24. There were insufficient data to comment on both volume displacement and replacement techniques.
Patient‐reported outcome measures
The one study that compared aesthetic outcome between O‐BCS and mastectomy alone analysed volume replacement techniques (Gendy 2003). There were insufficient data for analysis.
Cosmetic evaluation
The one study that compared aesthetic outcome between O‐BCS and mastectomy alone analysed volume replacement techniques (Gendy 2003). There were insufficient data for analysis.
Comparison 3: O‐BCS versus mastectomy with reconstruction
Local recurrence
Of the six studies that evaluated local recurrence for O‐BCS versus mastectomy with reconstruction, three studies (50%) evaluated volume replacement techniques (Lee 2018; Mustonen 2004; Ozmen 2020). There were insufficient data to comment on both volume displacement and replacement techniques.
Disease‐free survival
Of the three studies that evaluated disease‐free survival for O‐BCS versus mastectomy with reconstruction, one study evaluated volume replacement techniques alone (Ozmen 2020); there were no studies for volume displacement techniques. There were insufficient data for analysis.
Overall survival
Of the four studies that provided HR data for overall survival, two studies evaluated volume replacement techniques (Lee 2018; Ozmen 2020). There were insufficient data for analysis.
Complications
Of the five studies that evaluated total complications in O‐BCS versus mastectomy with reconstruction, three studies evaluated volume displacement techniques (Peled 2014; Potter 2020; Tong 2016) and one evaluated volume replacement techniques (Ozmen 2020). There were insufficient data to comment on both volume displacement and replacement techniques.
Time to adjuvant therapy
We included one study in this analysis evaluating volume displacement techniques (Morrow 2019; Table 24). There were insufficient data to conduct a subgroup analysis of this outcome.
Patient‐reported outcome measures
Three studies evaluated patient‐reported outcomes (Hart 2015; Kelsall 2017; Ozmen 2020), and results are summarised in Table 25. Hart 2015 evaluated volume displacement techniques only and Ozmen 2020 evaluated volume replacement techniques only. There were insufficient data to comment on both volume displacement and replacement techniques.
Cosmetic evaluation
The one study comparing aesthetic outcome with mastectomy with reconstruction (Ozmen 2020), evaluated volume replacement techniques only. No conclusions can be made due to the lack of studies reporting this outcome for this comparison.
Sensitivity analysis
It was not possible to conduct a sensitivity analysis of studies at low risk of bias as all studies were viewed with at least a moderate/serious risk of bias.
We used the fixed‐effect model and conducted sensitivity analyses for all the comparisons using the random‐effects model. Most analyses were robust and did not change the conclusions drawn from the findings except in the following cases.
-
Comparison 1: O‐BCS versus S‐BCS
-
Overall survival (5 years)
fixed‐effect: 0.79 (0.65 to 0.96)
random‐effects: 0.82 (0.67 to 1.00)
-
Complication rate
fixed‐effect: 1.19 (1.10 to 1.27)
random‐effects: 1.12 (0.94 to1.33)
-
-
Comparison 2: O‐BCS versus mastectomy alone
-
Local recurrence HR
fixed‐effect: 0.55 (0.34 to 0.91)
random‐effects: 0.87 (0.18 to 4.11)
-
-
Comparison 3: O‐BCS versus mastectomy plus reconstruction
-
Overall survival HR
fixed‐effect 0.39 (0.30 to 0.51)
random‐effects 0.58 (0.18 to 1.85)
-
-
Subgroup analysis
-
Overall survival
fixed‐effect 0.76 (0.59 to 0.98)
random‐effects 0.77 (0.54 to 1.09)
-
Discussion
Summary of main results
In general, the results were inconclusive as many studies included in the analyses did not account for confounding or were downgraded due to inconsistency or imprecision.
O‐BCS versus S‐BCS
When comparing O‐BCS to S‐BCS, there may be little or no difference in local recurrence‐free survival, local recurrence rate or disease‐free survival based on very‐low certainty of evidence. There may be little to no effect on overall survival. O‐BCS may reduce the rate of re‐excision based on very low‐certainty evidence due to the risk of bias from confounding and inconsistent results. This result, however, is plausible as O‐BCS allows larger resections. O‐BCS may increase the number of women who have at least one complication and this is based on very low‐certainty evidence. This result may be due to the novelty of the technique or that it is a more intensive surgical procedure. The evidence from the review suggests that O‐BCS may increase the recall to biopsy rate and this may be due to changes in follow‐up imaging due to the surgery and mobilisation of the breast. The review suggests that days to adjuvant therapy may be increased, only for time to adjuvant radiotherapy, by the use of O‐BCS compared to S‐BCS. This may be explained by delays due to complications. The delay to adjuvant radiotherapy is of the order of 7.21 to 12.1 days, which may be clinically significant.
The results were inconclusive as to whether there was a difference in patient‐reported outcomes between O‐BCS and S‐BCS. Little or no difference was found in the overall quality of life measured by the BREAST‐Q. However, cosmesis, psychosocial well‐being and satisfaction with the breast reported by patients were at times significantly better after O‐BCS. The review was inconclusive about the difference in cosmetic evaluation between O‐BCS and S‐BCS. Two out of three studies reported better BCCT.core scores after O‐BCS, whilst one favoured S‐BCS. Panel assessments favoured the aesthetic outcome of O‐BCS, however, these studies had a critical risk of bias with measurement of outcome methods.
O‐BCS versus mastectomy alone
Evidence from two studies suggests O‐BCS may increase local recurrence‐free survival, but the evidence is very uncertain. No conclusion could be made about disease‐free survival as there were data from only one eligible study. O‐BCS may reduce complications compared to mastectomy, but the evidence is very uncertain due to the high risk of bias mainly due to confounding. There were insufficient data to draw conclusions on time to adjuvant therapy, patient‐reported outcome measures and cosmetic evaluation, as each subgroup was reported in one study only.
O‐BCS versus mastectomy with reconstruction
The results of the review found that O‐BCS may result in little or no difference in recurrence or disease‐free survival when compared to mastectomy with reconstruction. The evidence is very uncertain due to the high risk of bias, inconsistency and imprecision among studies. O‐BCS may reduce the complication rate compared to mastectomy plus reconstruction, but the evidence is very uncertain due to the high risk of bias due to confounding and inconsistency of the results. There were insufficient data to make any conclusions on time to adjuvant therapy, patient‐reported outcome measures and cosmetic evaluation as each subgroup was reported in one study only.
Overall completeness and applicability of evidence
In this systematic review, the evidence was incomplete due to a lack of good‐quality studies in this area that used appropriate methods to adjust for confounding. Additional research is likely to have an important impact on the estimated effect. Decisions regarding choice of surgical method should be made jointly by the surgeon and patient after extensive information on the risks and benefits is provided. Careful consideration of patients for whom to offer O‐BCS is needed.
Strengths of the review
We compared O‐BCS to all other surgical alternatives for breast cancer, which has not been done before.
Our search strategy was comprehensive where the electronic search included publications of relevant studies irrespective of language. We also conducted a manual search of reference lists of relevant studies and screened trial registries.
We categorised interventions, comparators and outcomes as per clinical relevance.
When it was not possible to count the outcome in the main analyses, we presented the results as appendices (for full transparency).
We analysed subgroups and conducted sensitivity analyses to ensure rigorous data analysis that informed our conclusions.
At least two or three review authors checked all data extraction and input to minimise errors.
Our results were assessed carefully with application of the ROBINS‐I tool and GRADE criteria for each of the relevant outcomes.
Main limitations
The main limitations of this systematic review are due to the limited strength of the evidence due to methodological deficiencies of the existing studies.
The evidence in this review came from observational studies (mostly retrospective and of low‐methodological quality), subject to important biases which increased the uncertainty of the results and limited the quality of existing evidence.
It was not possible to calculate the HR for the assessment of survival data for all studies because many studies did not report time‐to‐event analyses in sufficient detail.
We assessed the surgical technique performed as a subgroup analysis, but not enough evidence exists on volume replacement techniques.
The surgical techniques are not standardised in terminology nor methodology.
This was a systematic review that used aggregated data (in which the subject of analysis was the study) and not a meta‐analysis of individual data (in which the subject of analysis is the person or the participant).
For patient‐reported outcome measures and cosmetic evaluation, we used a narrative synthesis or vote counting synthesis. This provides no magnitude of effect nor does it account for the difference in relative study design.
Quality of the evidence
The overall certainty of the evidence was low due to most studies not accounting for confounding variables. There was inconsistency in the body of evidence and in comparisons 2 (O‐BCS versus mastectomy) and 3 (O‐BCS versus mastectomy with reconstruction), there was a lack of evidence resulting in imprecision. For patient‐reported and cosmetic evaluation outcomes, studies did not always use validated or standardised tools, making the risk of bias due to measurement of these outcomes an issue.
Potential biases in the review process
There were several potential biases in the review process. We tried to limit bias in several ways ‐ two or three review authors assessed the eligibility for inclusion and independently assessed the risks of bias. Although the review authors’ views varied, we decided to accept the final conclusions after extensive discussion and reaching a consensus. We ensured an expert in oncoplastic surgery was involved at each of these steps.
We accept that carrying out reviews requires a number of subjective judgements, and it is possible that a different review team may have reached different decisions regarding the assessments of eligibility and risks of bias. We acknowledge that the comparisons and outcomes we have focused on are quite broad. Future reviews may be split into multiple reviews to allow narrower analysis. Feedback from readers will serve to improve the next review update.
Agreements and disagreements with other studies or reviews
We found a meta‐analysis by Chen 2018 comparing S‐BCS versus O‐BCS. They found that O‐BCS significantly reduced the number of re‐excisions. They found that the local and distal recurrence rates were similar in both groups. Both disease‐free survival (HR 1.19, 95% CI 0.96 to 1.49; P = 0.112) and overall survival (HR 1.14, 95% CI 0.76 to 1.69; P = 0.527) did not differ significantly between the two groups. These results are similar to our results. They noted clinicopathological differences between the two groups that could have confounded the results and suggested the need for randomising or matching patients in future studies.
De La Cruz 2016 conducted a comprehensive review but did not focus on comparative studies and only evaluated studies for T1‐T2 cancers. They reported high rates of overall survival and disease‐free survival with low local recurrence, distant recurrence, positive margin rate, re‐excision rate, conversion to mastectomy rate and complication rates, thereby confirming the oncologic safety of this procedure in patients with T1–T2 invasive breast cancer. The oncoplastic techniques evaluated were mainly volume displacement (> 50%) but very few details on surgical technique were available.
Losken 2014 conducted a meta‐analysis comparing O‐BCS to S‐BCS (called breast‐conserving therapy (BCT) in the paper). They combined data from case series with more than 10 patients. They found that re‐excision was more common in the S‐BCS alone group (14.6% versus 4%, P < 0.0001), however, completion mastectomy was more common in the oncoplastic group (6.5% versus 3.79%, P < 0.0001). The average follow‐up was longer in the S‐BCS alone group (64 versus 37 months). Local recurrence was 4% in the oncoplastic group and 7% in the S‐BCS alone group. Satisfaction with the aesthetic outcome was significantly higher in the oncoplastic group (89.5% versus 82.9%, P < 0.001). The conclusions are similar to what our review found, however combining case series from different studies is liable to very high risk of bias. Methodological conclusions drawn from this technique are uncertain.
Yiannakopoulou 2016 evaluated 40 studies of which 15 were on volume replacement. The majority of studies were observational studies. The length of follow‐up was relatively short; long‐term oncological outcome of oncoplastic surgery for breast cancer is not adequately investigated. They recommended further research efforts should focus on level 1 evidence on oncological outcome of oncoplastic surgery
Yoon 2016 conducted a comprehensive literature review but again did not focus on comparative studies and looked at radiotherapy with O‐BCS. Haloua 2013 conducted a literature review but only included poorly designed and underpowered studies.
We acknowledge a recent publication by Rocco 2021, whereby a group of international breast specialists concluded there was low level evidence for outcomes after O‐BCS, a lack of randomised data and absence of standardised tools for patient‐reported outcome measures.
Authors' conclusions
Implications for practice.
The evidence is very uncertain regarding oncological outcomes following O‐BCS compared to S‐BCS, though O‐BCS has not been shown to be inferior. O‐BCS may result in less need for a second re‐excision surgery but may result in more complications and greater recall rate than S‐BCS. It seems that O‐BCS may give better patient satisfaction and surgeon rating for the look of the breast, but the evidence for this is of poor quality, and due to lack of numerical data, it was not possible to pool the results of different studies. It seems O‐BCS results in fewer complications compared with surgeries involving mastectomy.
No firm conclusions can be made to inform policymakers, health professionals or patients based on this review. The surgical decision should be made jointly between clinician and patient after appropriate discussion about the risks and benefits of O‐BCS personalised to the patient, taking into account clinicopathological factors.
Implications for research.
This review highlighted the deficiency of well‐conducted studies to evaluate efficacy, safety and patient‐reported outcomes following O‐BCS.
Well‐designed cohort studies are still needed and randomised controlled trial (RCT) data should be sought. RCTs may not be feasible due to importance of patient choice in surgeries, especially when the motivation for choosing O‐BCS may be patient satisfaction and cosmetic outcomes.
For planning and development of these studies, we suggest the following.
Describe and adjust for all potential confounders (baseline patient characteristics, such as age and comorbidities and tumour characteristics).
Define surgical techniques clearly and ensure surgeries are conducted by experienced surgeons and centres.
Volume replacement and volume displacement techniques should be assessed separately ‐ individual techniques should be noted.
Use standardised criteria, defining endpoints and follow‐up for objective outcomes.
Use validated tools to assess patient‐reported outcomes.
Use objective tools or blinding large panels to assess aesthetic outcomes.
Minimum 5‐year follow‐up is needed to allow conclusions on oncological safety to be made.
Studies should adjust appropriately for follow‐up time in the analysis of outcomes using survival analysis methods.
A standardised categorisation of oncoplastic surgeries is needed to encompass the long list of techniques, often with overlapping but different terminology.
Researching outcomes relevant to health economics, such as quality‐adjusted life years.
History
Protocol first published: Issue 7, 2020
Acknowledgements
The authors would like to acknowledge the support and help from the Cochrane Breast Cancer Group, especially Sam Egger (statistical editor), Cancer Council NSW; Nicola Rocco (external clinical reviewer), Scientific Director at Group for Reconstructive and Therapeutic Advancements (G.Re.TA); Linda Vincent (consumer reviewer), Breast Cancer Patient Advocate, University of California, Breast Science Advocacy Core; and Sandy Finestone (consumer reviewer), PsyD, Association of Cancer Patient Educators.
We would like to thank Ava Tan‐Koay for the development of the search strategies.
We would like to thank Jason Ellar for assistance with design of the data extraction spreadsheet.
We would like to thank the following for their translation services (language translated):
Rujan Shretha ‐ Mandarin Chinese
Vivien Daum ‐ Hungarian
Diana Maria Cespedes Arcani ‐ Spanish
Rafael Guízar ‐ Spanish
Frédérique Thonon ‐ French
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumour* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Mastectomy, Segmental] explode all trees #9 (Oncoplastic breast‐conserving surgery):ti,ab,kw #10 (Oncoplastic breast conserving surgery):ti,ab,kw #11 (Oncoplastic breast conservation):ti,ab,kw #12 Oncoplastic near (breast conserving or breast conservation):ti,ab,kw #13 oncoplastic surger*:ti,ab,kw #14 volume displacement near procedur*:ti,ab,kw #15 volume displacement near tech*:ti,ab,kw #16 MeSH descriptor: [Mammaplasty] explode all trees #17 mammaplast* or mammoplast*:ti,ab,kw #18 therapeutic near (mammaplast* or mammoplast*):ti,ab,kw #19 Wise pattern near (mammaplast* or mammoplast*):ti,ab,kw #20 Vertical scar near (mammaplast* or mammoplast*):ti,ab,kw #21 Circumareolar near (mammaplast* or mammoplast*):ti,ab,kw #22 Benelli near (mammaplast* or mammoplast*):ti,ab,kw #23 Round block near (mammaplast* or mammoplast*):ti,ab,kw #24 Raquet handle near (mammaplast* or mammoplast*):ti,ab,kw #25 lateral near (mammaplast* or mammoplast*):ti,ab,kw #26 volume replacement near procedur*:ti,ab,kw #27 volume replacement near tech*:ti,ab,kw #28 (Abdominal Adipo‐fascial Flap):ti,ab,kw #29 (Abdominal Adipofascial Flap):ti,ab,kw #30 abdominal flap*:ti,ab,kw #31 Adipo‐fascial Flap*:ti,ab,kw #32 Adipofascial Flap*:ti,ab,kw #33 Thoraco‐epigastric Flap*:ti,ab,kw #34 Thoracoepigastric Flap*:ti,ab,kw #35 Superior epigastric artery perforator flap*:ti,ab,kw #36 Medial Intercostal Artery Perforator flap*:ti,ab,kw #37 Internal Mammary Artery Perforator flap*:ti,ab,kw #38 Anterior Intercostal Artery Perforator flap*:ti,ab,kw #39 MeSH descriptor: [Perforator Flap] explode all trees #40 Lateral Intercostal Artery Perforator flap*:ti,ab,kw #41 Lateral Thoracic Artery Perforator flap*:ti,ab,kw #42 Thoracodorsal Artery Perforator Flap*:ti,ab,kw #43 Mini Latissimus Dorsi:ti,ab,kw #44 Omental flap*:ti,ab,kw #45 transverse upper gracilis flap*:ti,ab,kw #46 MeSH descriptor: [Free Tissue Flaps] explode all trees #47 "Advancement Flap*":ti,ab,kw #48 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 #49 #7 AND #48 in Trials
Appendix 2. MEDLINE
| # | Searches |
| 1 | exp Breast Neoplasms/ |
| 2 | (breast adj6 cancer$).tw. |
| 3 | (breast adj6 neoplasm$).tw. |
| 4 | (breast adj6 carcinoma$).tw. |
| 5 | (breast adj6 tumo?r$).tw. |
| 6 | or/1‐5 |
| 7 | exp Surgical Oncology/ |
| 8 | exp Breast Neoplasms/su [Surgery] |
| 9 | or/7‐8 |
| 10 | 6 and 9 |
| 11 | exp Mastectomy, Segmental/mt [Methods] |
| 12 | Oncoplastic breast‐conserving surgery.tw. |
| 13 | Oncoplastic breast conserving surgery.tw. |
| 14 | (oncoplastic adj5 breast‐conserving adj5 surgery).tw. |
| 15 | Oncoplastic breast conservation surgery.tw. |
| 16 | (oncoplastic adj5 breast adj5 (conserving or conservation*) adj5 surgery).tw. |
| 17 | Oncoplastic breast conservation.mp. |
| 18 | (oncoplastic adj5 breast adj5 (conserving or conservation*)).tw. |
| 19 | oncoplastic surger*.tw. |
| 20 | (volume displacement and (procedur* or tech*)).tw. |
| 21 | exp Mammaplasty/ |
| 22 | therapeutic mamm#plast*.mp. |
| 23 | Wise pattern therapeutic mamm#plast*.tw. |
| 24 | Vertical scar mamm#plast*.tw. |
| 25 | Circumareolar mamm#plast*.tw. |
| 26 | Benelli* mamm#plast*.tw. |
| 27 | Round block mamm#plast*.tw. |
| 28 | Raquet handle mamm#plast*.tw. |
| 29 | lateral mamm#plast*.tw. |
| 30 | (volume replacement and (procedur* or tech*)).tw. |
| 31 | Abdominal Adipo‐fascial Flap*.tw. |
| 32 | Abdominal Flap*.tw. |
| 33 | Adipo‐fascial Flap*.tw. |
| 34 | Thoraco‐epigastric Flap*.tw. |
| 35 | Superior epigastric artery perforator flap*.tw. |
| 36 | Medial Intercostal Artery Perforator flap*.tw. |
| 37 | Internal Mammary Artery Perforator flap*.tw. |
| 38 | Anterior Intercostal Artery Perforator flap*.tw. |
| 39 | exp Perforator Flap/ |
| 40 | Lateral Intercostal Artery Perforator flap*.tw. |
| 41 | Lateral Thoracic Artery Perforator flap*.tw. |
| 42 | Thoracodorsal Artery Perforator Flap*.tw. |
| 43 | (mini adj5 Latissimus Dorsi).tw. |
| 44 | Omental flap*.tw. |
| 45 | transverse upper gracilis flap*.tw. |
| 46 | exp Free Tissue Flaps/ |
| 47 | Advancement Flap*.tw. |
| 48 | or/11‐46 |
| 49 | 10 and 48 |
| 50 | randomized controlled trial.pt. |
| 51 | controlled clinical trial.pt. |
| 52 | randomized.ab. |
| 53 | placebo.ab. |
| 54 | Clinical Trials as Topic/ |
| 55 | randomly.ab. |
| 56 | trial.ti. |
| 57 | (crossover or cross‐over).tw. |
| 58 | Pragmatic Clinical Trials as Topic/ |
| 59 | pragmatic clinical trial.pt. |
| 60 | or/50‐59 |
| 61 | Case‐Control Studies/ |
| 62 | Control Groups/ |
| 63 | Matched‐Pair Analysis/ |
| 64 | Retrospective Studies/ |
| 65 | ((case* adj5 control*) or (case adj3 comparison*) or control group*).ti,ab. |
| 66 | or/61‐65 |
| 67 | Cohort Studies/ |
| 68 | Longitudinal Studies/ |
| 69 | Follow‐Up Studies/ |
| 70 | Prospective Studies/ |
| 71 | Retrospective Studies/ |
| 72 | cohort.ti,ab. |
| 73 | longitudinal.ti,ab. |
| 74 | prospective.ti,ab. |
| 75 | retrospective.ti,ab. |
| 76 | or/67‐75 |
| 77 | 49 and 60 |
| 78 | 49 and 66 |
| 79 | 49 and 76 |
| 80 | 77 or 78 or 79 |
| 81 | animals/ not humans/ |
| 82 | 80 not 81 |
| 83 | remove duplicates from 82 |
Appendix 3. Embase
| # | Searches |
| 1 | exp breast/ |
| 2 | exp breast disease/ |
| 3 | (1 or 2) and exp neoplasm/ |
| 4 | exp breast tumor/ |
| 5 | exp breast cancer/ |
| 6 | exp breast carcinoma/ |
| 7 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 8 | or/3‐7 |
| 9 | exp breast cancer/su [Surgery] |
| 10 | exp cancer surgery/ |
| 11 | 9 or 10 |
| 12 | 8 and 11 |
| 13 | exp partial mastectomy/ |
| 14 | oncoplastic breast surgery/ |
| 15 | Oncoplastic breast‐conserving surgery.tw. |
| 16 | Oncoplastic breast conserving surgery.tw. |
| 17 | (oncoplastic adj5 breast‐conserving adj5 surgery).tw. |
| 18 | oncoplastic breast conservation surgery/ |
| 19 | Oncoplastic breast conservation surgery.tw. |
| 20 | (oncoplastic adj5 breast adj5 (conserving or conservation*) adj5 surgery).tw. |
| 21 | Oncoplastic breast conservation.tw. |
| 22 | (oncoplastic adj5 breast adj5 (conserving or conservation*)).tw. |
| 23 | (oncoplastic adj5 (procudur* or tech* or surger*)).tw. |
| 24 | (volume displacement and (procedur* or tech*)).tw. |
| 25 | exp breast reconstruction/ and partial.tw. |
| 26 | therapeutic mamm#plast*.tw. |
| 27 | Wise pattern therapeutic mamm#plast*.tw. |
| 28 | Vertical scar mamm#plast*.tw. |
| 29 | Circumareolar mamm#plast*.tw. |
| 30 | Benelli* mamm#plast*.tw. |
| 31 | Round block mamm#plast*.tw. |
| 32 | Raquet handle mamm#plast*.tw. |
| 33 | lateral mamm#plast*.tw. |
| 34 | (volume replacement and (procedur* or tech*)).tw. |
| 35 | Abdominal Adipo‐fascial Flap*.tw. |
| 36 | Abdominal Flap*.tw. |
| 37 | exp adipofascial flap/ |
| 38 | ((Adipo‐fascial or adipofascial) and Flap*).tw. |
| 39 | ((Thoraco‐epigastric or Thoracoepigastric) and Flap*).tw. |
| 40 | Superior epigastric artery perforator flap*.tw. |
| 41 | Medial Intercostal Artery Perforator flap*.tw. |
| 42 | Internal Mammary Artery Perforator flap*.tw. |
| 43 | Anterior Intercostal Artery Perforator flap*.tw. |
| 44 | exp perforator flap/ |
| 45 | Lateral Intercostal Artery Perforator flap*.tw. |
| 46 | Lateral Thoracic Artery Perforator flap*.tw. |
| 47 | exp thoracodorsal artery perforator flap/ |
| 48 | Thoracodorsal Artery Perforator Flap*.tw. |
| 49 | Mini Latissimus Dorsi.tw. |
| 50 | Omental flap*.tw. |
| 51 | transverse upper gracilis flap*.tw. |
| 52 | exp free tissue graft/ |
| 53 | Advancement Flap*.tw. |
| 54 | or/13‐53 |
| 55 | 12 and 54 |
| 56 | Randomized controlled trial/ |
| 57 | Controlled clinical study/ |
| 58 | Random$.ti,ab. |
| 59 | randomization/ |
| 60 | intermethod comparison/ |
| 61 | placebo.ti,ab. |
| 62 | (compare or compared or comparison).ti. |
| 63 | (open adj label).ti,ab. |
| 64 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 65 | double blind procedure/ |
| 66 | parallel group$1.ti,ab. |
| 67 | (crossover or cross over).ti,ab. |
| 68 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 69 | (assigned or allocated).ti,ab. |
| 70 | (controlled adj7 (study or design or trial)).ti,ab. |
| 71 | (volunteer or volunteers).ti,ab. |
| 72 | trial.ti. |
| 73 | or/56‐72 |
| 74 | exp case control study/ |
| 75 | case control study.ti,ab. |
| 76 | ((case control or case base or case matched or retrospective) adj1 (analys* or design* or evaulation* or research or stud* or survey* or trial*)).ti,ab. |
| 77 | or/74‐76 |
| 78 | exp retrospective study/ |
| 79 | exp prospective study/ |
| 80 | ((cohort or concurrent or incidence or longitudinal or followup or 'follow up' or prospective or retrospective) adj1 (analys* or design* or evaluation* or research or stud* or survey* or trial*)).ti,ab. |
| 81 | or/78‐80 |
| 82 | 55 and 73 |
| 83 | 55 and 77 |
| 84 | 55 and 81 |
| 85 | 82 or 83 or 84 |
| 86 | limit 85 to (human and (conference abstracts or embase)) |
| 87 | remove duplicates from 86 |
Appendix 4. WHO ICTRP
Basic search:
1. Oncoplastic breast‐conserving surger*
2. Breast cancer AND volume displacement
3. Breast cancer AND volume replacement
4. Breast cancer AND flap
Advanced search:
1. Condition: breast cancer Intervention: oncoplastic breast surgery OR oncoplastic technique OR oncoplastic procedure Recruitment Status: ALL
2. Condition: breast cancer Intervention: volume displacement OR wise pattern mammaplasty OR therapeutic mammaplasty OR vertical scar mammaplasty OR Circumareolar mammoplasty OR benelli mammoplasty OR round block mammoplasty OR raquet handle mammoplasty OR lateral mammoplasty Recruitment Status: ALL
3. Condition: breast cancer Intervention: volume replacement OR Abdominal adipo‐fascial flap OR advancement flap OR Lateral intercostal artery perforator flap OR Lateral thoracic artery perforator OR Thoracodorsal artery perforator flap Recruitment Status: ALL
4. Condition: breast cancer Intervention: Latissimus dorsi mini flap OR Thoraco‐epigastric Flap OR Superior epigastric artery perforator flap OR Medial intercostal artery perforator OR Internal mammary artery perforator OR Anterior inter‐costal artery perforator OR omental flap OR transverse upper gracilis flap Recruitment Status: ALL
Appendix 5. ClinicalTrials.gov
Basic search:
1. Condition or disease: Breast cancer Other terms: Oncoplastic breast‐conserving surgery
2. Condition or disease: Breast cancer Other terms: volume displacement technique
3. Condition or disease: Breast cancer Other terms: volume replacement technique
4. Condition or disease: Breast cancer Other terms: flap (consider adding ‘reconstruction’)
Advanced search:
1. Condition or disease: Breast cancer Intervention: Oncoplastic breast‐conserving surgery Study type: all studies
2. Condition or disease: Breast cancer Intervention: volume displacement technique Study type: all studies
3. Condition or disease: Breast cancer Intervention: therapeutic mammoplasty OR wise pattern mammoplasty OR vertical scar mammoplasty OR Circumareolar mammoplasty OR benelli mammoplasty OR round block mammoplasty OR raquet handle mammoplasty OR lateral mammoplasty Study type: all studies
4. Condition or disease: Breast cancer Intervention: volume replacement technique Study type: all studies
5. Condition or disease: Breast cancer Intervention: Abdominal Adipo‐fascial Flap OR advancement flap OR Lateral intercostal artery perforator flap OR Lateral thoracic artery perforator OR Thoracodorsal artery perforator flap Study type: all studies
6. Condition or disease: Breast cancer Intervention: Latissimus dorsi mini flap OR Thoraco‐epigastric Flap OR Superior epigastric artery perforator flap OR Medial intercostal artery perforator OR Internal mammary artery perforator OR Anterior inter‐costal artery perforator OR omental flap OR transverse upper gracilis flap Study type: all studies
Data and analyses
Comparison 1. Any O‐BCS versus S‐BCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Local recurrence‐free survival (time to recurrence) | 8 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Local recurrence‐free survival | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 0.90 [0.61, 1.34] | |
| 1.1.2 Local Recurrence Rates | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.33 [0.96, 1.83] | |
| 1.2 Local recurrence | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 1 year | 3 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 1.2.2 1 to 5 years | 15 | 9014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.04] |
| 1.2.3 5 years | 10 | 6672 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
| 1.3 Disease‐free survival (HR) | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.06 [0.89, 1.26] | |
| 1.4 Disease‐free survival (RR) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 1 to 5 years | 3 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.74, 1.34] |
| 1.4.2 5 years | 6 | 5054 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.99, 1.44] |
| 1.4.3 10 years | 2 | 2163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.5 Overall survival (HR) | 8 | Hazard Ratio (IV, Fixed, 95% CI) | 1.02 [0.82, 1.28] | |
| 1.6 Overall survival (RR) | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 1 to 5 years | 3 | 4970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.09] |
| 1.6.2 5 years | 12 | 8730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.96] |
| 1.7 Re‐excision rates | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Total re‐excisions | 38 | 13341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.85] |
| 1.7.2 Mastectomies | 23 | 10756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 1.8 Complications | 20 | 118005 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.10, 1.27] |
| 1.9 Recall rates | 6 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.67, 3.42] |
| 1.10 Time to therapy | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Any adjuvant therapy | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐5.48, 10.68] |
| 1.10.2 Chemotherapy | 4 | 4566 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐2.55, 0.29] |
| 1.10.3 Radiotherapy | 5 | 3720 | Mean Difference (IV, Fixed, 95% CI) | 9.67 [7.21, 12.14] |
| 1.11 Patient‐reported outcomes (BREAST‐Q) | 5 | Other data | No numeric data | |
| 1.12 Aesthetic outcome BCCT.core | 3 | Other data | No numeric data |
1.12. Analysis.
Comparison 1: Any O‐BCS versus S‐BCS, Outcome 12: Aesthetic outcome BCCT.core
| Aesthetic outcome BCCT.core | |||||
| Study | Intervention type | BCCT.core‐ Intervention | BCCT.core‐ Control | P value | Conclusion |
| Hilli‐Betz 2014 | VD | Excellent: 4.3%, good: 75.4%, moderate: 18.8% | BCCT.core Excellent: 10.6%, good: 77.0%, moderate in 5.6%, poor in 0.6% | < 0.001 | OPS significantly worse in expert panel cosmetic than standard segnentectomy |
| Lansu 2014 | VD | Mean (SD) 2.45 (0.52) | Mean (SD) 2.11 (0.6) | 0.02 | OPS significantly better than control |
| Santos 2015 | VD | BCCT.core: Excellent: 22.8%, good: 54.4%, moderate: 21.1%, bad:1.8%, poor in 1.8% | BCCT.core Excellent: 6.2%, good: 73.8%, moderate: 15.4%, poor: 4.6% | 0.004 | OPS significantly better than control |
Comparison 2. Any O‐BCS versus mastectomy (Mx).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Local recurrence (HR) | 2 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Local recurrence‐free survival | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.55 [0.34, 0.91] | |
| 2.2 Local recurrence (RR) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 1 to 5 years | 2 | 4025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.24, 0.41] |
| 2.2.2 5 years | 2 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.41, 1.75] |
| 2.2.3 10 years | 1 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.52 [1.42, 30.06] |
| 2.3 Disease‐free survival | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 10 years | 1 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.41, 0.82] |
| 2.4 Overall survival (HR) | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.39 [0.30, 0.51] | |
| 2.5 Overall survival (RR) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 1 to 5 years | 1 | 3924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.22, 0.40] |
| 2.5.2 5 years | 2 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.79, 3.69] |
| 2.6 Complications | 4 | 4839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.67, 0.83] |
| 2.7 Time to therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Chemotherapy | 1 | 974 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.23, 2.03] |
2.4. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 4: Overall survival (HR)
2.6. Analysis.

Comparison 2: Any O‐BCS versus mastectomy (Mx), Outcome 6: Complications
Comparison 3. Any O‐BCS versus mastectomy plus reconstruction (Mx+R).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Local recurrence‐free survival | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Local recurrence rate: Mx+/‐R | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.59 [0.71, 3.55] | |
| 3.1.2 Local recurrence‐free survival: Mx+R only | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 1.37 [0.72, 2.62] | |
| 3.2 Local recurrence | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 1 to 5 years | 2 | 3449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.64] |
| 3.2.2 5 years: Mx+R only | 2 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.44] |
| 3.2.3 5 years: Mx+/‐R | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.74, 3.21] |
| 3.3 Disease‐free survival (HR): Mx+R | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Mx+/‐R | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.03 [0.75, 1.42] | |
| 3.3.2 Mx+R only | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.45 [0.09, 2.22] | |
| 3.4 Disease‐free survival (RR): Mx+R | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 5 years: Mx+R only | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.27, 2.04] |
| 3.4.2 5 years: Mx+/‐R | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 3.5 Overall survival (HR): Mx+R | 4 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Mx+R only | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.74 [1.23, 2.47] | |
| 3.5.2 Mx+/‐R | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.40, 1.07] | |
| 3.6 Overall survival (RR): Mx+R | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.6.1 1 to 5 years | 1 | 3387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.97, 1.98] |
| 3.6.2 5 years: Mx only | 2 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.24, 2.28] |
| 3.6.3 5 years: Mx+/‐R | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.84] |
| 3.7 Complications: Mx+R only | 5 | 4973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.45, 0.54] |
3.7. Analysis.

Comparison 3: Any O‐BCS versus mastectomy plus reconstruction (Mx+R), Outcome 7: Complications: Mx+R only
Comparison 4. Volume displacement versus S‐BCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Local recurrence | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 1 to 5 years | 8 | 2578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.51, 1.39] |
| 4.1.2 5 years | 8 | 4729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.27] |
| 4.2 Overall survival | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 5 years | 7 | 4373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.98] |
| 4.3 Re‐excision rates | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Total re‐excisions | 27 | 9076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 4.3.2 Mastectomies | 16 | 7097 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.28] |
| 4.4 Complications | 14 | 4083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acea‐Nebril 2005.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort March 2003 to Dec 2004 Complejo Hospitalario Universitario Juan Canalejo. La Coruña. España 160 participants |
|
| Participants |
Inclusion: women with invasive/in situ breast cancer with tumours less than 3 cm in diameter (T1‐2) OR treated with neoadjuvant chemotherapy and reduced to a size less than 3 cm, axillary clinical stages N0‐N1a‐b Exclusion: women with breast cancer with T3‐4 tumours, impossibility of postoperative radiotherapy (previous radiotherapy, scleroderma, collagen diseases, pregnant women etc.), small breast size, impossibility of disease‐free margins or lack of compression technique by the patient or demand for a commitment to result. |
|
| Interventions |
Intervention: volume displacement ‐ vertical/lower pedicle/single limb vertical/horizontal/rotational/lateral mammoplasty, (n = 50) Control: 1) standard BCS, (n = 57); 2) mastectomy, (n = 53) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared | |
Acea‐Nebril 2017.
| Study characteristics | ||
| Methods | Retrospective single centre cohort Jan 2000 to June 2016 Complejo Hospitalario Universitario a Coruña, Spain 801 participants |
|
| Participants |
Inclusion: women with invasive breast carcinoma/ductal carcinoma in situ (DCIS) undergoing breast conserving surgery Exclusion: patients who underwent mastectomy as the primary intervention, patients that did not give their consent to participate in the study |
|
| Interventions |
Intervention: volume displacement ‐ reduction mammoplasty, (n = 170) Control: BCS ‐ wide local excision, (n = 631) |
|
| Outcomes |
Primary outcomes (median 84 +/‐ 55.6 months):
Secondary outcomes:
Other outcomes:
|
|
| Notes | Some overlap with Acea‐Nebril 2005 in patient group but different controls No funding/disclosures declared |
|
Acosta‐Marin 2014.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort Jan 2011 to Oct 2012 Breast Surgery Department, Centro Clinico de Estereotaxia—CECLINES, Caracas, Venezuela 107 participants |
|
| Participants |
Inclusion: women with early breast cancer undergoing either standard BCS or level II OPS and with 12‐month follow‐up Exclusion:
|
|
| Interventions |
Intervention: volume displacement ‐ round block (40.3%), inverted‐T (26.8%), vertical scar (15.3%), raquet (7.6%), horizontal (5.7%), lower inner‐quadrant mammoplasty (3.8%), (n = 52) Control: standard BCS, (n = 55) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | No disclosures/funding declared | |
Amitai 2018.
| Study characteristics | ||
| Methods | Retrospective single=centre cohort 2009 to 2014 Tel Aviv University, Israel 335 participants |
|
| Participants |
Inclusion: women with breast cancer undergoing either immediate OPS and those undergoing lumpectomy in the same week (the first 4 lumpectomies after an OPS that week) Exclusion: simple local tissue rearrangement. Women undergoing mastectomy eventually for positive lumpectomy margins |
|
| Interventions |
Intervention: volume displacement ‐ breast reduction (64%), mastopexy (30%) augmentation (6%), (n = 67) Control: BCS: lumpectomy, (n = 268) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding disclosed Authors were contacted requesting full dataset for primary outcomes |
|
Angarita 2020.
| Study characteristics | ||
| Methods | Retrospective database review cohort 2005 to 2016 American College of Surgeons National Surgical Quality Improvement Program, USA 109,487 participants |
|
| Participants |
Inclusion: adult women with an International Classification of Diseases Ninth Revision (ICD‐9) code of in situ (ICD‐9 code 233.0) or invasive breast cancer (ICD‐9 code 174.0–9) who underwent a traditional lumpectomy or OPS (soft tissue transfer, mastopexy, or mammoplasty) Exclusion: male patients, metastatic tumours, and concurrent surgery (non‐breast and bilateral procedures) |
|
| Interventions |
Intervention: both VD and VR ‐ adjacent tissue transfer < 10 cm (4.7%), 10 cm2 to 30 cm2 (16.2%), 30 cm2 to60 cm2 (34.9%), mastopexy (23.7%), reduction (20.5%), (n = 9126) Control: BCS: lumpectomy, (n = 100,361) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared | |
Atallah 2015.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort study Hotel‐Diey de France, Beirut, France 2005‐2013 280 participants |
|
| Participants | Inclusion: women with early breast cancer who underwent breast conserving surgery | |
| Interventions |
Intervention: volume displacement ‐ OPS stage 1 and 2, (n = 193) Control: wide local excision, (n = 87) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Conference abstract No disclosures/funding declared |
|
Bali 2018.
| Study characteristics | ||
| Methods | Retrospective single‐centre and surgeon cohort Apr 2014 to Sep 2016 University of Cambridge, England, UK 201 participants |
|
| Participants |
Inclusion: women with breast cancer operated on by a single oncoplastic breast surgeon Exclusion: patients undergoing mastectomy |
|
| Interventions |
Intervention: volume displacement and volume replacement (analysed separately) ‐ mammoplasty (19), chest wall perforator flaps (16), (n = 35) Control: BCS: wide local excision, (n = 166) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared | |
Borm 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2000 to December 2005 Klinikum rechts der Isar, Munich, Germany 965 participants |
|
| Participants |
Inclusion: women with breast cancer undergoing BCS with no distant metastases at the time of diagnosis Exclusion: patients with other malignancies in addition to breast cancer |
|
| Interventions |
Intervention: volume displacement ‐ rotation flap (265), reduction mammoplasty (23). 1 patient received a volume replacement flap
(thoracoepigastric flap), (n = 288) Control: Standard BCS, (n = 677) |
|
| Outcomes |
Primary outcomes (median 67 months (IQR 6 = 51‐84)):
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared | |
Carter 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2007 to December 2014 University of Texas MD Anderson Cancer Center, Houston, Texas, USA 10,407 participants |
|
| Participants |
Inclusion: women who underwent operations for in situ or invasive breast cancer (Tis–T4) Exclusion: male patients, surgeries performed for benign lesions or prophylaxis, lymph node only procedures, patients who did not consent to data collection |
|
| Interventions |
Intervention: both VD and VR ‐ adjacent tissue transfer/rearrangement < 10 cm2/10 to 30 cm2/30 to 60 cm2/other techniques, (n = 1177) Control: 1) standard BCS, (n = 3359) 2) mastectomy, (n = 3263) 3) mastectomy +reconstruction. (n = 2608) |
|
| Outcomes |
Primary outcomes (median 40.8 months (range: 0 ‐ 109.2):
Secondary outcomes:
Other outcomes:
|
|
| Notes | Funded by the Cancer Center Support Grant No disclosures declared Authors were contacted for further outcomes ‐ none available but authors confirmed 'recurrence free survival refers to local recurrence' |
|
Cassi 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2012 to December 2014 University of Rome, Tor Vergata, Rome, Italy 215 participants |
|
| Participants | Inclusion: adult women with breast cancer undergoing breast conserving surgery | |
| Interventions |
Intervention: volume displacement ‐ therapeutic mammoplasty and adjacent tissue transfer following lumpectomy, (n = 61) Control: BCS: lumpectomy, (n = 154) |
|
| Outcomes |
Primary outcomes (median (I)44.8/(C)43.3 months):
Secondary outcomes:
|
|
| Notes | No disclosures/funding declared Authors declared they are employees of the University Hospital Authors were contacted requesting full dataset for primary outcomes |
|
Chakravorty 2012.
| Study characteristics | ||
| Methods | Retrospective single‐centre and surgeon cohort June 2003 to February 2010 Royal Marsden Hospital, London, UK |
|
| Participants | Inclusion: women with breast cancer undergoing either OPS (consecutive patients of mainly one consultant) or standard BCS by the same surgeon | |
| Interventions |
Intervention: volume displacement ‐ wise‐pattern, comma & lateral (77), Grisotti (51) and Benelli (round block) (22) procedures, (n = 150) Control: standard BCS, (n = 440) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Chauhan 2016 (1).
| Study characteristics | ||
| Methods | Prospective single centre January 2012 to December 2014 Command Hospital, Lucknow (tertiary care teaching hospital), India 100 participants |
|
| Participants |
Inclusion: women with locally advanced breast cancer (including stage III A, stage III B and stage IIB) and receiving doxorubicin based neoadjuvant chemotherapy, adjuvant chemotherapy and adjuvant radiotherapy Exclusion: patients with extensive peau d orange, extensive skin involvement( infiltration or ulceration), chest wall involvement or metastatic disease |
|
| Interventions |
Intervention: volume displacement and replacement (analysed together) ‐ VD: periareolar, superior and inferior pedicle techniques, quadrantectomy with glandular remodeling, and dermoglandular flaps, VR: (mini LD myofascial or myocutaneous flap) (n = 57) Control: BCS: lumpectomy or quadrantectomy, (n = 43) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared Differs from Chauhan (2) in participant selection Authors were contacted requesting full dataset for primary outcomes |
|
Chauhan 2016 (2).
| Study characteristics | ||
| Methods | Prospective single‐centre cohort January 2012 to December 2014 Tertiary teaching hospital, India 79 participants |
|
| Participants |
Inclusion: women with early breast cancer (T1/T2, N0/N1) undergoing breast conserving surgery Exclusion:
|
|
| Interventions |
Intervention: volume displacement and replacement (analysed together) ‐ lateral mammaplasty (9), medial mammaplasty (4), radial excision (5), grissotis flap (2) superior ped (5) inferior pedicl (4) donut (3) Mini LD (1), (n = 33) Control: BCS: margin or a formal quadrantectomy, (n = 46) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Differs from Chauhan (1) in participant selection Authors were contacted requesting full dataset for primary outcomes |
|
Crown 2015.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2009 to December 2010 for control January 2013 to September 2014 for intervention Virgina Mason Medical Center, Seattle, USA |
|
| Participants |
Inclusion: women with invasive or non‐invasive breast carcinoma undergoing breast conserving surgery Exclusion: patients who underwent breast surgery between January 2013 and September 2014 performed by surgeons who did not perform OPS |
|
| Interventions |
Intervention: volume displacement ‐ radial ellipse with adjacent tissue transfer (31%), racquet mammoplasty (22%), mastopexy (21%), reduction mammoplasty (15%), neoareolar reduction (3%), and other techniques (8%) Control: BCS |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures Study supported by Benaroya Research Institute at VMC Same participant group as Crown 2019 but greater n as did not need patient follow‐up data to be included in the study |
|
Crown 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2009 to December 2010 for control January 2013 to July 2015 for intervention Virgina Mason Medical Center, Seattle, USA 561 participants |
|
| Participants |
Inclusion: women with breast cancer undergoing breast conserving surgery with adequate follow up and information on complications Exclusion: patients treated with OPS between January 2011 and December 2012 were excluded from the study to allow for the learning period needed during the adoption of new surgical techniques. |
|
| Interventions |
Intervention: volume displacement ‐ mammoplasty (18%), mastopexy (23%), racquet mammoplasty (26%), (n = 288) Control: standard BCS, (n = 273) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared Same as Crown 2015 but have had chart review for all patients, therefore, can extract complications from this study |
|
DeLorenzi 2016 (1).
| Study characteristics | ||
| Methods | Retrospective matched multicentre database review cohort 2000 to 2008 European Institute of Oncology (IEO) Breast Cancer Institutional Database 1362 participants |
|
| Participants |
Inclusion: patients with invasive breast cancer undergoing breast conserving surgery and radiotherapy Exclusion: patients presenting with secondary tumours or local relapses, bilateral tumours, patients that received neoadjuvant chemotherapy |
|
| Interventions |
Intervention: volume displacement and volume replacement (analysed together) ‐ (n = 454) VR: glandular flaps (33.8%), fasciocutaneous flap (3.3%), myocutaneous muscular flap (1.1%), implants (5.9%) VD: mastopexy (28.5%) round‐block approach (14.5%), superior pedicled reduction mammoplasty (2.4%), inferior pedicled reduction mammoplasty (7.7%), other procedures (2.8%) Control: standard BCS, (n = 908) |
|
| Outcomes |
Primaryoutcomes (median 84 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Different control to DeLorenzi 2016 (2) |
|
DeLorenzi 2016 (2).
| Study characteristics | ||
| Methods | Retrospective matched multicentre database review cohort 2000 ‐ 2008 European Institute of Oncology (IEO) Breast Cancer Institutional Database 579 participants |
|
| Participants |
Inclusion: women with breast cancer with tumours larger than 2 cm (T2) undergoing OPS or mastectomy and reconstruction Exclusion:
|
|
| Interventions |
Intervention: volume displacement and replacement (analysed together): (n = 193) VR: glandular flaps (59.6 %), a fasciocutaneous flap (1.5 %) myocutaneous or muscular flap in 2 patients (1 %), implants (4.1 %) VD: mastopexy (18.1%), a round‐block approach (1.5 %), a superior pedicled reduction mammoplasty (2.1 %), an inferior pedicled reduction mammoplasty (7.7 %), other procedures were performed in the remaining 4 patients (4.1 %) Control: nipple areola‐sparing mastectomies (41.7 %), skin‐sparing mastectomies, (58.3 %) 91% immediate postmastectomy reconstruction (definitive silicone implants (273 patients), temporary expanders (74 patients), and muscular flaps (4 cases)), (n = 386) |
|
| Outcomes |
Primaryoutcomes (median 88.8 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Different control to DeLorenzi 2016 (1) |
|
DeLorenzi 2018.
| Study characteristics | ||
| Methods | Retrospective multicentre database review cohort European Institute of Oncology (IEO) Breast Cancer Institutional Database 2000 to 2008 419 participants |
|
| Participants |
Inclusion: patients with DCIS breast cancer who underwent breast conserving surgery (monolateral, bilateral procedures) followed by adjuvant radiation Exclusion: patients presenting with secondary tumours or local relapses, patients requiring re‐excision or completion mastectomy for positive margins |
|
| Interventions |
Intervention: both VD and VR: no breakdown given, (n = 44) Control: standard BCS (n = 375) |
|
| Outcomes |
Primaryoutcomes (median follow‐up (I) 92.4months (C) 110.4 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Different participants to DeLorenzi 2016 (1) |
|
Di Micco 2017.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort June 2009 to November 2014 Royal Marsden Hospital, London, UK 157 participants |
|
| Participants |
Inclusion: large‐breasted women with early breast cancer (tumours < 3 cm) undergoing bilateral reduction mammoplasty or unilateral BCS Exclusion: patients who did not undergo radiotherapy, patients who had bilateral or multicentric cancer, patients who went on to have a mastectomy for involved margins, developed distant disease or were lost to follow‐up were excluded from the evaluation of patient satisfaction |
|
| Interventions |
Intervention: volume displacement ‐ bilateral reduction mammoplasty, (n = 70) Control: standard BCS, (n = 87) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Dolan 2015.
| Study characteristics | ||
| Methods | Retrospective multicentre (2) cohort May 2009 to December 2011 Victoria Infirmary, Glasgow and Western Infirmary Glasgow, UK 187 participants |
|
| Participants |
Inclusion: women with breast cancer undergoing breast conserving surgery Exclusion: patients requiring completion mastectomy for incomplete margins after breast conservation. 1 patient from the OBCS group who had a Grisotti flap for squamous cell carcinoma on her nipple requiring no follow‐up imaging was also excluded. The data for 2 further patients who died within the 2‐year follow‐up period (1 with breast cancer‐related death) were omitted from the WLE group. |
|
| Interventions |
Intervention: volume displacement and replacement (analysed together) (n = 71) VD: benelli (12), wise pattern (44), melon slice (1), le‐jour (1), tennis‐racquet: (3) VR: TEF (6) , T‐DAP (1), matrix rotation (3) Contol: BCS: wide local excision, (n = 116) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Down 2013.
| Study characteristics | ||
| Methods | Retrospective single surgeon cohort July 2006 to April 2010 Norfolk and Norwich University Hospital, Norfolk, United Kingdom 158 participants |
|
| Participants |
Inclusion: patients with early invasive breast cancer/DCIS requiring breast conserving surgery Exclusion: patients requiring mastectomy |
|
| Interventions |
Intervention: volume displacement and replacement, (n = 37) ‐ therapeutic mammoplasties (18), subaxillary fat pad
rotation mammoplasties (14), thoracoepigastric flaps (4), central flap (1) Control: BCS: wide local excision, (n = 121) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Eichler 2013.
| Study characteristics | ||
| Methods | Retrospective single‐centre study 2007 University of Cologne, Germany 143 participants |
|
| Participants | Inclusion: women with breast cancer undergoing breast conserving surgery | |
| Interventions |
Intervention: volume displacement ‐ mastopexy (n = 72) Control: BCS: lumpectomy (n = 71) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Fan 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre matched cohort May 2013 to December 2016 Yonsei University College of Medicine, Seoul, Korea |
|
| Participants | Inclusion: patients with breast cancer undergoing mini latissimus dorsi flap and a matched control group of breast conserving surgery | |
| Interventions |
Intervention: volume replacement ‐ Mini‐LD flap, (n = 29) Control: BCS: partial mastectomy, (n = 29) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Farooqi 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort Aga Khan University Hospital, Karachi, Pakistan August 2016 to 2018 257 participants |
|
| Participants | Inclusion: women with early breast cancer (stages 1‐3 and DCIS) who underwent breast conserving surgery | |
| Interventions |
Intervention: unclear whether volume displacement or replacement, (n = 146) Control: standard breast conserving surgery, (n = 111) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Conference abstract No disclosures/funding declared |
|
Gendy 2003.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort Intervention: 1991 to 1999 Control: 1994 to 1999 Breast Unit, Royal Hampshire County Hospital, Winchester, UK 106 participants |
|
| Participants |
Inclusion: all contactable disease‐free patients who underwent latissimus dorsi mini‐flap reconstruction (1991 to 1999) and standard segmental mastectomy (1994 to 1999) Exclusion: patients who did not consent and complete questionnaire |
|
| Interventions |
Intervention: volume replacement ‐ latissimus dorsi miniflap, (n = 49 out of 89 contacted) Control: skin sparing mastectomy, (n = 57) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes Author RR |
|
Gicalone 2007 (1).
| Study characteristics | ||
| Methods | Prospective single‐centre cohort January 2004 to May 2005 University Hospital of Montpellier, France 74 participants |
|
| Participants |
Inclusion criteria: women with breast cancer with tumours > 15 mm undergoing surgical treatment Exclusion criteria:
|
|
| Interventions |
Intervention: volume displacement ‐ inverted‐T procedure (5), round‐block technique (26), (n = 31) Control: BCS: quadrantectomy, (n = 43) |
|
| Outcomes |
Primary outcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared Different to Giacalone 2007 (2) and Gicalone 2015 as has different intervention |
|
Gicalone 2007 (2).
| Study characteristics | ||
| Methods | Prospective single centre January 2004 to May 2005 University Hospital of Montpellier, France 127 participants |
|
| Participants |
Inclusion: women with breast cancer with tumours >2cm Exclusion:
|
|
| Interventions |
Intervention: VD‐Donut Mastoplexy (n = 39) Control: BCS: standard lumpectomy without concomitant mammoplasty (n = 88) |
|
| Outcomes |
Primaryoutcomes:
Seondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Different to Gicalone 2007 (1) and Gicalone 2015 as has different intervention |
|
Gicalone 2015.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort September 2003 to September 2004 University Hospital of Montpellier, France 99 participants |
|
| Participants |
Inclusion: women with breast cancer whose breast size and/or ptosis, made it possible to consider either conventional surgical treatment or oncoplastic surgery as a first‐line treatment Exclusion:
|
|
| Interventions |
Intervention:
Control: BCS ‐ WLE |
|
| Outcomes |
Primaryoutcomes:
Seondaryoutcomes:
Other outcomes:
|
|
| Notes | No disclosures/funding declared Different to Giacalone 2007 (1) and (2) as has different intervention Translated from French |
|
Gulcelik 2013.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort Ankara Oncology Training and Education Hospital, Ankara, Turkey 2003 to 2010 268 participants |
|
| Participants |
Inclusion: patients with breast cancer and macromastia undergoing breast cancer surgery. Patients with upper inner and upper outer‐quadrant lesions were included in the study. Exclusion: patients who did not attain their follow‐up and were excluded (n = 18) |
|
| Interventions |
Intervention: volume displacement ‐ bilateral reduction mammoplasty (n = 106) Control: quadrantectomy (n = 162) |
|
| Outcomes |
Primaryoutcomes (median (I) 33 months (C) 37 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Hamdi 2008.
| Study characteristics | ||
| Methods | Retrosepective single‐centre cohort 2002 to 2003 Gent University Hospital, Belgium 152 participants |
|
| Participants | Inclusion: patients who received lumpectomies with or without reconstruction | |
| Interventions |
Intervention: volume displacement and replacement, (n = 26)
Control: BCS: quadrantectomy (12), tumourectomy (114), (n = 126) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Hart 2015.
| Study characteristics | ||
| Methods | Prospective single‐centre and surgeon cohort 2009 to 2011 Division of Plastic and Reconstructive Surgery, Emory University, Atlanta, USA 70 participants |
|
| Participants | Inclusion: women with breast cancer treated with mastectomy and immediate BR (control) or lumpectomy with reduction mammoplasty (intervention) | |
| Interventions |
Intervention: oncoplastic reduction mammoplasty, (n = 10) Control: mastectomy + reconstruction: implant‐based reconstruction (40.0%), latissimus dorsi flap (38.3%), and pedicled or free transverse rectus abdominis myocutaneous flaps (21.7%), (n = 60) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Hashimoto 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort April 2012 to November 2017 Osaka International Cancer Institute ‐ Department of Breats and Endocrine Surgery, Osaka, Japan 1333 participants |
|
| Participants | Inclusion: women with breast cancer undergoing standard breast conserving surgery with or without latissimus dorsi flap reconstruction | |
| Interventions |
Intervention: volume replacement ‐ Mini‐latissimus dorsi flap (MLDF), (n = 183) Control: standard breast conserving surgery, (n = 1150) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Conference abstract No disclosures/funding Authors were contacted requesting full dataset for primary outcomes |
|
Hilli‐Betz 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort 2003 to 2011 Hannover Medical School, 30625 Hannover, Germany 230 participants |
|
| Participants | Inclusion: women with breast cancer with tumours in the upper inner, upper outer, and lower inner quadrants undergoing breast conserving surgery | |
| Interventions |
Intervention: volume displacement ‐ dermoglandular rotation flap, (n = 69) Control: BCS: standard lumpectomy, (n = 161) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Hu 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre single surgeon cohort January 2013 to December 2014 Department of Breast Surgery, Oxford University Hospitals NHS Trust, Oxford, UK 36 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery by a single surgeon in a tertiary referral centre who received CWPF or WLE Exclusion:
|
|
| Interventions |
Intervention: Volume replacement ‐ chest wall perforator flap, (n = 18) Control: BCS: wide local excision |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | Authors PG and JH No disclosures/funding declared |
|
Jiang 2015.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort Tangshan People's Hospital, China February 2011 to November 2013 60 participants |
|
| Participants |
Inclusion: Women with breast cancer with:
Exclusion:
|
|
| Interventions |
Intervention: OPS surgery Control: BCS: lumpectomy |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Translated from Chinese |
|
Kahn 2013.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort August 2008 to December 2011 Victoria and Western Infirmary Glasgow, UK 169 participants |
|
| Participants |
Inclusion:
Exclusion: Patients with more than one of the following risk factors for wound healing problems were not offered OBCS:
|
|
| Interventions |
Intervention: Volume displacement and replacement (analysed together), (n = 31) VD: wise pattern (16), benelli (6), lateral excision (3), matrix rotation (3) VR: TEPF (2), V‐Y advancement flap (1) Control: (1) BCS: wide local excision, (n = 66) (2) Mastectomy, (n = 56) (3) Mastectomy + reconstruction, (n = 16) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Keleman 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2010 to January 2017 National Institute of Oncology, Budapest, Hungary 700 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery Exclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ therapeutic mammaplasty (superior, central, inferior pedicle Wise‐pattern) (143), dermoglandular rotation (medial, lateral mammoplasty) (159), periareolar (round block, omega) (48), (n = 350) Control: BCS: Wide local excision/quadrantectomy, (n = 350) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Kelsall 2017.
| Study characteristics | ||
| Methods | Retrospective matched single‐centre cohort 1999 to2014 Nottingham Breast Institute, Nottingham City Hospital, Nottingham, United Kingdom 567 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement and replacement (analysed together), (n = 286) ‐ bilateral therapeutic mammaplasty and a chest wall perforator flaps (LICAP [lateral intercostal artery perforator], LTAP [lateral
thoracic artery perforator] (204) or TDAP [thoracodorsal artery
perforator] (82)) Control: Mastectomy and reconstruction, (n = 281) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Kimball 2018.
| Study characteristics | ||
| Methods | Retrospective multi‐centre database review cohort January 2010 to March 2017 Optum ClinformaticsTM DataMart, Eden Prairie, MN, USA) 18,251 participants |
|
| Participants |
Inclusion: Women with breast cancer undergoing breast‐conserving surgery Exclusion: Patients were excluded if they underwent:
|
|
| Interventions |
Intervention: Volume displacement ‐ lumpectomy & mammoplasty &/or mastopexy, (n = 709) Control: BCS: wide local excision, (n = 17,542) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | Declarations: 2 of the authors were employees of Medtronic and one was a paid consultant, but for services unrelated to this present research Funding: Medtronic provided funds for professional medical writing but had no influence on study design and manuscript preparation |
|
Klit 2017.
| Study characteristics | ||
| Methods | Retrospective multi‐centre database review cohort 2009 to 2013 Danish Breast Cancer Group (DBCG) registery 1798 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Both VD and VR, (n = 445) Control: (1) WLE, (n = 824) (2) Mastectomy, (n = 529) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures declared Funding: The Pink Tribute Foundation |
|
Lansu 2014.
| Study characteristics | ||
| Methods | Retrospective multi‐centre cohort Regional hospitals referring to Institue Verbeeten, Netherlands July 2004 to May 2012 46 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ all patients had conventional RT fractionation scheme and simultaneous boost with OPS breast remodelling and careful closure by mobilising tissue, (n = 19) Control: BCS: wide local excision, all patients had conventional RT fractionation scheme and simultaneous boost, (n = 27) Other interventions not extracted: The following goups were investigated 1) The hypofractionated group (HF): hypofractionated RT fractionation scheme, sequential boost, and conventional BCS (lumpectomy). 2) The oncoplastic surgery hypofractionated group (OSHF): hypofractionated RT fractionation scheme, simultaneous boost and OPS |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Lee 2018.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2008 to December 2013 Kyungpook National University, Daegu, Korea |
|
| Participants | Inclusion: Women with breast cancer undergoing breast cancer surgery by a breast surgeon only or collaborative team of a breast and plastic surgeons | |
| Interventions |
Intervention: Volume displacement and replacement alone, (n = 260) VD: Volume displacement (11.2%), batwing mastopexy (0.3%), glandular reshaping (0.7%), round block technique (1.2%), purse‐string suture technique (1.2%), tennis racket technique (3.3%), local flap (0.6%), rotating flap (2.5%), reduction mammoplasty (1.3%) VR: Volume replacement (24.3%), Intercostal artery perforator flap (1.6%), lateral thoracodorsal perforator flap (1.1%), thoracodorsal artery perforator flap (0.8%), latissimus dorsi myocutaneous flap (10.6%), latissimus dorsi myocutaneous flap with silicone implant (1.5%), transverse rectus abdominis myocutaneous flap (3.7%) Control: (1) BCS, (n = 582) (2) Mastecomy, (n=409) (3) Mastectomy and reconstruction, (n = 253) |
|
| Outcomes |
Primaryoutcomes (median 72.4 (16.76) months):
Otheroutcomes:
|
|
| Notes | No disclosures Funding: A national research foundation of Korea grant, funded by the Korean government and a grant from the national R&D programme for cancer control |
|
Losken 2009.
| Study characteristics | ||
| Methods | Retrospective single‐centre and single surgeon cohort Before 2004 Emory University Hospital, Atlanta, GA, USA 34 patients |
|
| Participants |
Inclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ breast conservation with reduction, (n = 17) Control: standard BCS, (n = 17) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | Different years and outcomes No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Losken 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre and single surgeon cohort 2009 to 2013 Emory University Hospital, Atlanta, GA, USA 222 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery with sufficient follow‐up (> 2 months after confirmed final margin status) Exclusion: If surgical pathology or clinical follow‐up information was unavailable at the time of the review. |
|
| Interventions |
Intervention: Volume displacement ‐ tumour resection with oncoplastic reduction, (n = 83) Control: BCS: wide local excision, (n = 139) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Malhaire 2015.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort May 2005 to September 2011 Institut Curie, 26 rue d’Ulm, France 113 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement, (n = 73), lateral mammaplasty (37), inverted‐T (superior pedicle) (15), omega (5), J‐plasty (4), inverted‐T (inferior pedicle) (4), peri‐areolar (3), infra‐mammary fold (1), medial mammaplasty (4) Control: BCS: wide local excision, (n = 40) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | Authors were contacted requesting full dataset for primary outcomes No disclosures/funding declared |
|
Mansell 2015.
| Study characteristics | ||
| Methods | Retrospective multi‐centre cohort 2009 to 2012 Glasgow Breast Units (Victoria & Western Infirmary), UK 1000 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement and replacement (analysed together) (n = 20) VD: (n = 103: Wise pattern reduction (81), Benelli‐type “round‐block” breast reduction (16), Racquet‐type excision (6), Lejour (1), Grisotti (1), "Melon slice” reduction (1) VR (n=17): Thoracoepigastric flap (10), Breast matrix rotation (5), thoracodorsal artery perforator (TDAP) flap (1) Control: 1)WLE (n = 600) 2) Mastectomy with and without reconstruction (n = 281) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Duplicate with Mansell 2017, this study used for "re‐excisions" |
|
Mansell 2017.
| Study characteristics | ||
| Methods | Retrospective multi‐centre cohort June 2009 to August 2012 Glasgow Breast Units (Victoria & Western Infirmary), UK 1010 patients |
|
| Participants |
Inclusion: Women with breast cancer undergoing breast‐conserving surgery or mastectomy and reconstruction Exclusion: Patients with previous DCIS or breast cancer were excluded |
|
| Interventions |
Intervention: Volume displacement and volume replacement (analysed together), (n = 104) VD: (n = 90); Wise pattern reduction (78), Benelli‐type “roundblock” (6), "Racquettype” excision (3), Lejour (1), Grisotti (1) and “melon slice” reduction (1) VR: (n=14); Thoracoepigastric flap (9), breast matrix rotation (4) and thoracodorsal artery perforator (TDAP) flap (1) Control: (1) WLE (2) Mastectomy with and without reconstruction |
|
| Outcomes |
Primaryoutcomes (median: (I) 56.8 months (C) 57.2 months/54.4 months
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | Different outcomes to Mansell 2015 No disclosures/funding declared |
|
Matrai 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre matched cohort January 2010 to September 2013 Department of Breast and Soft Tissue Surgery of the National Institute of Oncology, Hungary |
|
| Participants |
Inclusion:
Exclusion: Distant metastases |
|
| Interventions |
Intervention: Volume displacement, (n = 60); Inverse T (Wise pattern) (17) Regnault B (15) Round Block (dual plane) (8) Circum‐vertical (5) Lateral matrix rotation (5) Batwing "bat wing" (3) Grisotti (2) Holmström's lobe (2) Medial matrix rotation (2) Control: WLE/quandrantectomy, (n = 60) |
|
| Outcomes |
Primaryoutcomes: (median follow‐up (I) 8.7 (3.05) (C) 32.2 (9.22))
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | Tranlated from Hungarian No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Mazouni 2013.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2002 to November 2010 Institute Gustave Roussy, Villejuif, France 259 participants |
|
| Participants |
Inclusion: Women with invasive breast cancer undergoing BCS after primary CT Exclusion: Patients with metastatic disease |
|
| Interventions |
Intervention: Volume displacement, (n = 45): periareolar mammoplasty (the
round block technique) (13), recentering of the nipple‐areola
complex (3), ablation of the nipple‐areola complex (5),
external radial mammaplasty (2), inferior pedicle mammaplasty (8), vertical mammaplasty (1), superior pedicle
mammaplasty (11), cutaneous resection with a rotation flap
(2) Control: BCS: WLE, (n = 214) |
|
| Outcomes |
Primaryoutcomes (median follow‐up: 46 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Morrow 2019.
| Study characteristics | ||
| Methods | Retrospective multi‐centre database review cohort January 2014 to December 2015 National Managed Clinical Networks/Cancer Networks of the 3 Scottish regions covering the whole of Scotland (WOSCAN: West of Scotland Cancer Network, SCAN: East of Scotland Cancer Network and NOSCAN: North of Scotland Cancer Network), UK |
|
| Participants |
Inclusion: Patients with breast cancer undergoing surgical treatment Exclusion: Patients who had non‐operative treatment only were excluded |
|
| Interventions |
Intervention: Volume disaplacement ‐ theraputic mammoplasty, (n=217) Control: (1) BCS, (n=5241) (2) Mastectomy, (n=1907) (3) Mastectomy and reconstruction, (n=710) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Mukhtar 2018.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort 1992 to 2017 University of California, San Francisco, USA 326 participants |
|
| Participants | Inclusion: Women with breast cancer undergoing breast conserving surgery | |
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Mustonen 2004.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 1998 to June 2001 Kuopio University Hospital, Finland 66 participants |
|
| Participants | Inclusion: Patients with primary (invasive/in situ) breast cancer undergoing immediate breast reconstruction following mastectomy or breast‐conserving surgery | |
| Interventions |
Intervention: Volume replacement: Latissimus‐dorsi mini flap, (n = 12) Control: Mastectomy plus reconstruction, (n = 54) |
|
| Outcomes |
Primaryoutcomes: (medican follow‐up: (I) > 24 months (C) 45.6 months)
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Nakada 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2000 to December 2012 University of Yamanashi, Yamanashi, Japan 1043 participants |
|
| Participants | Inclusion: Patients with breast cancer undergoing breast‐conserving surgery and were followed for more than 5 years after surgery | |
| Interventions |
Intervention: Volume replacement, (n = 417): Pedicled fat flaps: lateral epidermal fat flap (276), Inframammary adipofascial flap (25), rotation of surrounding tissue (116) Control: BCS; Quadrantectomy, (n=626) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Nakagomi 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2000 to December 2017 University of Yamanashi, Yamanashi, Japan 1193 participants |
|
| Participants | Inclusion: Patients with breast cancer undergoing surgery with either lateral thoracoaxillar dermal‐fat flap (ltdf) or mastectomy or BCS | |
| Interventions |
Intervention: Volume replacement: lateral thoracoaxillar dermal fat flap, (n = 487) Control: Mastectomy, (n = 706) Other study groups: BCS without lateral thoracoaxillar dermal fat flap (includes some OPS rechniques) |
|
| Outcomes |
Primaryoutcomes (120 months):
Secondary outcomes:
Otheroutcomes:
|
|
| Notes | Same patient database as Nakada (different outcomes) No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Niinikoski 2019 (2).
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort Breast Surgery Unit, Helsinki University Hospital, Helsinki, Finland January 2010 to December 2012 1800 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement, (n = 611): Racket (184) Round block (171) Upper rotation (67) Lower rotation (50) Superior pedicle (37) inferior pedicle (10) Mastopexy (26) S‐plasty (21) J‐plasty (20) Batwing (17) Wise‐amputation (8) Control: Standard BCS, (n = 1189) |
|
| Outcomes |
Primaryoutcomes (median follow‐up 75 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Ojala 2017.
| Study characteristics | ||
| Methods | Retrospective multi‐centre cohort Helsinki and Uusimaa Hospital District, Finland 2010 379 participants |
|
| Participants |
Inclusion: Patients with invasive breast cancer undergoing breast‐conserving surgery Exclusion: Bilateral disease or previous breast cancer |
|
| Interventions |
Intervention: Volume displacement, (n = 86): Racket mammoplasty (22%) Reduction mammoplasty techniques (22%) Round block (19%) Rotation plasty techniques (19%) Extensive dual plane undermining (14%) Other oncoplastic techniques (5%) Control: Standard BCS, (n = 293) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures declared Funding: Kurt and Doris Palander Foundation Grant |
|
Ozmen 2016.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort Turkey 2005 to 2015 309 participants |
|
| Participants |
Inclusion:
|
|
| Interventions |
Intervention: Volume replacement‐ BCS+MLDF (after 2010) Control: Standard BCS (before 2010) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Poster No funding/disclosures |
|
Ozmen 2020.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort Department of Surgery, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey January 2010 to January 2018 317 participants |
|
| Participants |
Inclusion:
Exclusion: Contraindications for intervention:
|
|
| Interventions |
Intervention: Volume replacement ‐ partial mastectomy plus mini‐latissimus dorsi flap, (n = 242) Control: Mastectomy plus reconstruction ( with implant), (n = 75) |
|
| Outcomes |
Primaryoutcomes (median follow‐up 54 months):
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Palsodittlir 2018.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2008 to Dec 2014 University of Iceland, Reykjavík, Iceland 750 participants |
|
| Participants |
Inclusion: Women with breast cancer undergoing breast‐conserving surgery Exclusion:
|
|
| Interventions |
Intervention: Volume displacement and replacement (analysed together), (n = 85); Volume displacement (89.4%) ‐ glandular rotational flaps or the use of secondary or extended dermoglandular flaps within the breast and may often involve the use of breast reduction techniques Volume replacement (10.6%) ‐ chest wall perforator flaps (lateral intercostal artery perforator (LICAP); intercostal perforator (ICAP) or pedicled flaps (thoracodorsal artery perforator (T‐DAP) or latissimus dorsi ( LD‐miniflap) Control: standard BCS, (n = 665) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No disclosures declared Funding: Visindasjoour Landspitalans (Landspitali Uni Hosp reserch fund) |
|
Peled 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort 2001 to 2010 Department of Surgery, University of California, San Francisco, USA 101 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing partial or complete mastectomy with immediate reconstruction and neo‐adjuvant CT and adjuvant RT |
|
| Interventions |
Intervention: Volume displacement: wise pattern incision for all, (n = 37) Control: Mastectomy plus reconstruction, (n = 64) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Piper 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre matched cohort 2001 to 2009 University of California, San Francisco, USA 98 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery Exclusion: Patients without negative margins at the time of initial surgery |
|
| Interventions |
Intervention: Volume displacement ‐ simultaneous partial mastectomy and bilateral reduction mammoplasty, (n = 49) Control: standard BCS: WLE, (n = 49) |
|
| Outcomes |
Primaryoutcomes (median follow‐up 60 months):
Secondaryoutcomes
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
PlaFarnos 2018.
| Study characteristics | ||
| Methods | Prospective single‐centre cohort June 2014 to June 2016 Hospital de llobregat, Barcelona, Spain 180 participants |
|
| Participants | Inclusion: Women undergoing breast‐conserving surgery for breast cancer | |
| Interventions |
Intervention: Volume displacement ‐ oncological reduction pattern, (n = 60) Control: Standard BCS, (n = 120) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | Conference abstract No funding/disclosures declared Abstract refers to study as case‐control trial ‐ according to Cochrane Handbook classified as Cohort |
|
Potter 2020.
| Study characteristics | ||
| Methods | Prospective multi‐centre cohort July to December 2016 (iBRA), September 2016 to June 2017 (TeaM) Centres invovled in iBRA‐2 and TeaM trials, UK 2916 participants |
|
| Participants |
Inclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ theraputic mammoplasty, (n = 376) Control: 1) Mastectomy, (n = 1532) 2) Mastectomy plus reconstruction, (n = 1008) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | S.P. is a National Institute for Health Research (NIHR) Clinician Scientist (CS‐2016‐16‐019). T.R. has received support from the NIHR through a Doctoral Research Fellowship (DRF‐2014‐07‐079) and Academic Clinical Lectureship The TeaM study was funded by an Association of Breast Surgery research grant. This work was undertaken with the support of the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. |
|
Ren 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre matched cohort 2003 to 2013 Department of Surgery, Jiangsu Cancer Hospital, China 273 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast surgery with either MLDF or mastectomy with resection‐free margins (﹥1mm) Exclusion: Patients with multifocal diseases |
|
| Interventions |
Intervention: Volume replacement ‐ mini‐LD flaps, (n = 91) Control: Mastectomy, (n = 182) |
|
| Outcomes |
Primaryoutcomes (median follow‐up: (I) 83 months (C) 81 months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared Authors were contacted requesting full dataset for primary outcomes |
|
Rose 2019.
| Study characteristics | ||
| Methods | Retrospective multi‐centre matched cohort 2008 to 2013 Hospitals in the southern region of Denmark and northern region of Denmark 1596 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery in the Region of Northern Denmark and Southern Denmark, (n = 197) Exclusion: Patients who had bilateral cancers at the time of surgery (24) |
|
| Interventions |
Intervention: Volume displacement and replacement (analysed together) Control: BCS: WLE |
|
| Outcomes |
Primaryoutcomes (median follow‐up (I) 49.2 months (C) 67.2 months):
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Rose 2020.
| Study characteristics | ||
| Methods | Retrospective multi‐centre cohort January 2008 to December 2013 Danish Breast Cancer Group (DBCG) registry 727 participants |
|
| Participants |
Inclusion: Patients who received BCS for primary breast cancer Exclusion:
|
|
| Interventions |
Intervention: Volume displacement and volume replacement (analysed together) ‐ mammoplasty, perforator flaps and muscle sparing LD, (n = 96) Control: BCS: WLE, (n = 631) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures declared Funding: The Malmö University Hospital Cancer Research Fund, The Einar and Inga Nilsson Foundation, Skåne University Hospital Funds and Donations and The Hospital of Southwest Jutland. Same patient database as Rose 2019 (different outcomes) |
|
Santos 2015.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort 2007 to 2012 Hospital Nossa Senhora das Grac¸as (HNSG) Breast Unit, Curitiba, Brazil 122 participants |
|
| Participants |
Inclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ mammoplasty, (n = 57) Inferior pedicle techniques (38), superior pedicle (17), central quadrantectomy (1) and round block (1) Control: BCS: WLE, (n = 65) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosure/funding declared | |
Scheter 2019.
| Study characteristics | ||
| Methods | Retrospecting single‐centre matched cohort January 2011 to December 2016 Tel‐Aviv Sourasky Medical Center, Tel Aviv, Israel 24 participants |
|
| Participants |
Inclusion: Patient with breast cancer with central tumours undergoing breast‐conserving surgery Exclusion: Patients who had subsequently proceeded to total mastectomy |
|
| Interventions |
Intervention: Volume displacement ‐ mammoplasty, (n = 12) Control: BCS: WLE, (n = 12) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Other outcomes:
|
|
| Notes | Declaration: One author is a speaker for Johnson Medical, no financial or personal declarations No funding declared |
|
Sherwell‐Cabello 2006.
| Study characteristics | ||
| Methods | Prospective single‐centre January 2010 to July 2013 Instituto de Enfermedades de la Mama, FUCAM A.C, Coyoacán D.F, México 170 participants |
|
| Participants |
Inclusion: Women with breast cancer with a complete clinical history and had answered a questionnaire of aesthetic satisfaction in person or by phone were included Exclusion: Those who did not continue their follow‐up at the institution were eliminated from the study |
|
| Interventions |
Intervention: VD, (n = 75) ‐ OPS level 1 (15), lateral (21), internal rotation (1), circular (13), grisotti (8), vertical (13), double (3) Control: Standard BCS, (n = 95) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Tang 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort Affiliated Cancer Hospital of Guangxi Medical University, China January 2011 to December 2013 184 participants |
|
| Participants |
Inclusion: Women with breast cancer undergoing breast cancer surgery Exclusion: Women who underwent mastectomy |
|
| Interventions |
Intervention: Volume displacement and replacment (analysed together), (n = 67); Including round block, omega‐plasty, teniis racket mammoplasty, inverted T‐mammoplasty, inferior pedicle mammoplasty, local pedicled skin flap, partial LD flap Control: BCS ‐ WLE, (n = 117) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Tenofsky 2014.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort December 2006 to April 2011 University of Kansas School of Medicine ‐ Wichita, USA 142 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ mammoplasty & adjacent tissue transfer, (n = 58) Control: BCS ‐ WLE, (n = 84) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Tong 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort January 2005 to April 2013 The University of Texas M. D. Anderson Cancer Center, Texas, USA 408 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume displacement ‐ mammoplasty, (n = 131) Control: Mastectomy plus reconstruction, (n = 277) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Viega 2010.
| Study characteristics | ||
| Methods | Prospective single‐centre matched cohort Hospital das Clinicas Samuel Libanio ‐ Universidade do Vale do Sapucai, Brazil August 2005 to August 2008 87 participants |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery by the mastology team Exclusion:
|
|
| Interventions |
Intervention: Both VD and VR ‐ 11 reduction & 34 local flaps, (n = 45) Control: BCS: Quandrantectomy, (n = 42) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | Some crossover in study group as Veiga 2011 but different outcomes No disclosures/funding declared |
|
Viega 2011.
| Study characteristics | ||
| Methods | Prospective single‐centre matched cohort study Hospital das Clinicas Samuel Libanio ‐ Universidade do Vale do Sapucai, Brazil December 2005 to March 2009 90 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Both VD and VR ‐ 11 reduction & 34 local flaps, (n = 45) Control: BCS: Quandrantectomy, (n = 45) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | Some crossover in study group as Veiga 2010 but different outcomes No disclosures/funding declared |
|
Vieira 2016.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort October 2005 to December 2011 Barretos Cancer Hospital, Brazil 78 participants |
|
| Participants |
Inclusion: Patients with locally advanced breast cancer undergoing neoadjuvant CT and breast‐conserving surgery Exclusion:
|
|
| Interventions |
Intervention: Volume displacement, (n = 26); central quadrectomy (8), dermoglandular rotation flap (7), periareolar quad (5), inferior pedicle (4), superior pedicle (2) Control: BCS: Quandrantectomy, (n = 52) |
|
| Outcomes |
Primaryoutcomes (median follow‐up: (I) 60.01 (18.19) months (C) 64.88 (24.53) months):
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | ||
Wijgman 2017.
| Study characteristics | ||
| Methods | Retrospective multi‐centre database review cohort Netherlands Cancer Registry, The Netherlands January 2010 to December 2014 842 breasts |
|
| Participants |
Inclusion: Patients with breast cancer undergoing breast‐conserving surgery Exclusion:
|
|
| Interventions |
Intervention: Volume displacement, mammoplasty, (n = 314) Control: BCS: WLE, (n = 528) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
Otheroutcomes:
|
|
| Notes | No disclosures/funding declared | |
Wong 2017.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort University of California San Francisco, USA 1992 to April 2017 167 participants |
|
| Participants | Inclusion: Women with invasive lobular carcinoma | |
| Interventions |
Intervention: Volume displacement ‐ oncoplastic reduction mammoplasty, (n = 30) Contol: BCS ‐ lumpectomy, (n = 137) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Notes | No funding/disclosures declared Conference abstract |
|
Zhou 2019.
| Study characteristics | ||
| Methods | Retrospective single‐centre cohort Sun Yat‐sen University Cancer Center, Guangzhou, China October 2015 to March 2017 60 participants |
|
| Participants |
Inclusion:
Exclusion:
|
|
| Interventions |
Intervention: Volume replacement ‐ all mini latissimus dorsi flap (MLDF) (n = 32) Control: BCS: WLE (n = 28) |
|
| Outcomes |
Primaryoutcomes:
Secondaryoutcomes:
|
|
| Notes | No disclosures/funding declared | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adimulam 2014 | Ineligible study design |
| Angarita 2019 | Conference abstract ‐ published in full journal form Angarita 2020 (same participants) |
| Ayoub 2019 | Ineligible outcomes |
| Bogusevicius 2014 | Ineligible study design |
| Chapa 2019 | Ineligible study design |
| Cil 2016 | Dataset with additions published as Angarita 2020 (duplicate participants) |
| Emiroglu 2016 | Ineligible study design |
| Flanagan 2019 | Ineligible intervention |
| Freitas 2019 | Ineligible intervention |
| Fung 2001 | Ineligible intervention |
| Geluk 2020 | Ineligible study design |
| Hamilton 2019 | Ineligible intervention |
| Han 2010 | Ineligible intervention |
| Hashem 2017 | Ineligible comparator |
| IRCT20111207008316N4 | No longer registered |
| Jonczyk 2019 | Ineligible study design |
| Kabir 2015 | No outcomes of interest |
| Kabir 2015a | Ineligible outcomes |
| Kaur 2005 | No outcomes of interest |
| Kawanaka 2019 | Ineligible study design |
| Kelemen 2016 | Duplicate dataset |
| Khan 2018 | Ineligible comparator |
| Lima 2012 | No outcomes of interest |
| Mondani 2019 | Ineligible study design |
| Moustafa 2016 | Ineligible comparator |
| Nano 2005 | Ineligible study design |
| NCT00870415 | Ineligible intervention |
| NCT02376413 | Withdrawn (unavailable to recruit participants) |
| NCT03273348 | Ineligible study design |
| NCT03900299 | Ineligible study design |
| NCT04349527 | Ineligible comparator |
| Niinikoski 2019 (1) | Conference abstract ‐ published in full journal from Niinikoski 2019 (2) (same participants) |
| Nisiri 2018 | Too few participants n = 16 in O‐BCS intervention group |
| Pearce 2020 | Ineligible study design |
| Pukancsik 2017 | Ineligible study design |
| Pukancsik 2019 | Ineligible study design |
| Rietjens 2007 | Ineligible study design |
| Romics 2017 | Ineligible study design |
| Sun 2014 | Ineligible intervention |
| Tang 2013 | Ineligible study design |
| van Paridon 2017 | Ineligible study design |
| Youssef 2017 | Ineligible study design |
| Youssef 2018 | Ineligible comparator |
| Zucca 2012 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Srivastava 2018.
| Methods | Prospective single‐centre cohort All India Institute of Medical Sciences, Surgical Disciplines, New Delhi, India April 2015 to October 2016 64 participants |
| Participants | Inclusion: women with early breast cancer (T1‐T2) undergoing breast conserving surgery |
| Interventions |
Intervention: volume displacement ‐ O‐BCS (oncoplastic breast conserving surgery), (n = 32) Control: standard BCS (breast conserving surgery), (n = 32) |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | No disclosures/funding declared Conference abstract Abstract refers to study as randomised controlled trial ‐ according to Cochrane Handbook classified as Cohort (Higgins 2021) |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000638831.
| Study name | Effect of breast oncoplastic reshaping on the long term cosmetic outcome after breast conservation surgery: a prospective randomised trial |
| Methods | Prospective randomised controlled trial (RCT) Norfolk and Norwich University Hospital, Norwich, England, UK 316 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: breast reshaping ‐ the breast tissue is mobilised superficially and deep from the skin and the pectoral muscles in order to close the defect Control: wide local excision |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | April 2012 |
| Contact information | Maged Hussien MD, FRCS (Gen. Surg) maged.hussien@nnuh.nhs.uk |
| Notes | Recruitment status: no update since 2012 |
Catsman 2018.
| Study name | The COSMAM TRIAL a prospective cohort study of quality of life and cosmetic outcome in patients undergoing breast conserving surgery |
| Methods | Single‐centre prospective cohort The Amphia Hospital, Breda, Netherlands |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention arm 1: level 1 oncoplastic surgery
Intervention arm 2: level 2 oncoplastic surgery Control: standard lumpectomy with/without minor volume replacement Aim at least 75 patients per group |
| Outcomes |
|
| Starting date | July 2015, protocol published 2018 |
| Contact information | cjlmcatsman@gmail.com |
| Notes | Funding: Amphia Hospital Breda, the Netherlands |
NCT01396993.
| Study name | Prospective non‐randomized evaluation of oncoplastic surgery (iTOP) |
| Methods | Prospective cohort study Medical University of Vienna, Vienna, Austria 150 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: Immediate techniques for Oncoplastic surgery (iTOP) ‐ patients undergoing immediate techniques for oncoplastic surgery (level I only parenchymal rotation and breast undermining as well as level II using complex reduction plastics for nipple‐areola‐complex movings) and patients with mastectomy and immediate reconstruction. Breast conserving surgery and immediate defect filling using local flaps (level I) or reduction plastics (level II) as well as mastectomy and immediate reconstruction using free flaps Control: patients undergoing conservative breast surgery. Breast‐conserving therapy without defect correction |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | July 2011 (estimated completion August 2016) |
| Contact information | Florian Fitzal, Professor of Surgery, Medical University of Vienna |
| Notes | Recruitment status: unknown ‐ no update since 2015 |
NCT02159274.
| Study name | Shoulder disability and late symptoms following oncoplastic breast surgery |
| Methods | Observational Cohort Study University of Aarhus, Aarhus, Denmark 408 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: Breast conserving surgery (BCS) with oncoplastic techniques Control: BCS without oncoplastic techniques |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | March 2014 to October 2018 |
| Contact information | Katrine R Hauerslev, MD |
| Notes | Recruitment status: completed ‐ not published |
NCT02901223.
| Study name | The impact of oncoplastic breast surgery on the oncological safety and patient satisfaction |
| Methods | Prospective Cohort Ain Shams University, Egypt, Cairo 70 participants |
| Participants | Inclusion:
Exclusion:
|
| Interventions |
Intervention: oncoplastic group
35 female patients with non‐metastatic breast cancer who had oncoplastic techniques for tumour resection by well‐trained oncoplastic breast surgeons Control: quadrantectomy group 35 female patients with non‐metastatic breast cancer who had standard conservative breast surgery with no use of any plastic techniques by general breast surgeons |
| Outcomes |
Primary outcome measures
|
| Starting date | September 2012 to September 2013 |
| Contact information | Yasser Mohamed abdel‐samii El Ghamrini ‐ Cairo, Egypt |
| Notes | Recruitment: Completed ‐ not published Study published on clinicaltrials.gov October 2016 Similar to NCT02923635 and NCT03012152 |
NCT02923635.
| Study name | A prospective comparative study between oncoplastic breast surgery and standard wide local excision |
| Methods | Prospective Cohort Ain Shams University, Egypt, Cairo 70 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: oncoplastic group ‐ (35 patients) have curative oncoplastic surgery in which plastic techniques integrated with oncological procedures Control: standard wide ‐ (35 patients) have standard curative conservative breast surgery without integration of plastic techniques |
| Outcomes |
Primary outcome measures
Secondary outcome Measures
|
| Starting date | August 2013 to June 2016 (uploaded to clinicaltrials.gov October 2016) |
| Contact information | Yasser Mohamed Abdel‐samii, Ain Shams University |
| Notes | Recruitment status: completed ‐ not published Similar to NCT02901223 and NCT03012152 |
NCT03012152.
| Study name | A comparative study between oncoplastic breast surgery and standard conservative surgery: margin status and patient satisfaction |
| Methods | Prospective cohort Ain Shams University, Egypt, Cairo 70 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: O‐BCS oncoplastic breast conserving surgery(35 patients) have curative oncoplastic surgery in which plastic techniques integrated with oncological procedures Control: standard breast conserving surgery‐ (35 patients) have standard curative conservative breast surgery without integration of plastic techniques |
| Outcomes |
Primary outcome measures
|
| Starting date | May 2013 to September 2016 uploaded onto clinicaltrials.gov January 2017 |
| Contact information | Yasser Mohamed Abdel‐samii, Ain Shams University |
| Notes | Recruitment status: completed ‐ not published SImilar to NCT02901223 and NCT02923635 |
NCT04030845.
| Study name | Patient reported outcome ‐ reconstruction and oncoplastic cohort (PRO‐ROC) |
| Methods | Prospective cohort study 10000 patients |
| Participants |
Inclusion
Exclusion
|
| Interventions |
Intervention: oncoplastic breast‐conserving surgery. The oncoplastic breast‐conserving surgery were mainly those surgeries using volume displacement or volume replacement techniques. Control: breast reconstruction ‐ mainly included autologous tissue flaps (latissimus dorsi myocutaneous flaps, pedicled transverse rectus abdominis myocutaneous flaps, free transverse rectus abdominis musculocutaneous flaps, deep inferior epigastric artery perforator flaps, etc.), implant based breast reconstruction, autologous flaps combined with implant reconstruction, fat graft, etc. |
| Outcomes |
Primary outcome measures
Secondary outcome measures
Other outcome measures
|
| Starting date | July 2019 (estimated finish date December 2024) |
| Contact information | |
| Notes | Recruitment status: recruiting |
NTR6901.
| Study name | Patient satisfaction after oncoplastic breast surgery in the context of breast conserving therapy |
| Methods | Observational Cohort Zuyderland Medical Centre, Netherlands 110 participants |
| Participants |
Inclusion
Exclusion
|
| Interventions | |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2018 to May 2019 |
| Contact information | Nadine Hillbergm, n.hillberg@zuyderland.nl, 0031648531220 |
| Notes | Funding: Zuyderland‐Maastro Grant |
Differences between protocol and review
Criteria for considering studies for this review
Authors planned to exclude studies with fewer than 20 women with O‐BCS. The original reasoning had been to eliminate bias created by learning curves of the surgeons performing the procedure. It was then agreed, prior to full‐text review, that to avoid creating study selection bias by this restrictive criterion we agreed to remove this restriction.
Authors included studies in all languages and did not limit to English only.
In the control, authors expanded the wide local excision (WLE) group to include any breast conservation surgery. Some studies used terminology such as "lumpectomy", "quadrantectomy", "segmentectomy" or "partial mastectomy" that in practice are almost identical operations to a WLE, but the term "breast‐conserving surgery" better encompasses all of these operations.
Outcomes
Local recurrence was reported as 'local recurrence rate' or 'local recurrence‐free survival' so both were extracted but not pooled as authors felt they were two different outcomes.
For primary outcomes follow‐up, we included the addition of '1 to 5 years' and '10 years' to display all studies and be clear on follow‐up periods.
We replaced the secondary outcome 'need for further surgery to address aesthetics or symmetry (for example, symmetrisation or fat transfer)' with 'time to adjuvant therapy; time in days from surgery to initiation of adjuvant chemotherapy and/or radiotherapy.' This was done prior to data extraction as it was felt this outcome was more important to assess whether oncoplastic surgery results in a hastening or delay of treatment compared to other surgeries. The need for further aesthetic surgeries or symmetrisation was deemed a less important outcome and repetition of information captured by the patient‐reported cosmetic evaluation and independent cosmetic evaluation. This change in protocol was approved by the editorial group.
Shortened titles of outcomes added for ease of writing in the review. Definitions have not been altered in any way between protocol and review.
Selection of studies
Studies with multiple publications of duplicate data sets: we excluded the study with the shorter follow‐up time or fewer participant numbers for outcomes of interest so as not to duplicate data in the analysis.
Dealing with missing data
We had previously not specified what data sets we would seek from authors and deemed it sensible that, given we included 78 studies with varying outcomes we would take a selective approach. When studies reported one primary outcome but other primary outcome data were missing, we contacted the authors to request further information.
Risk of bias
In our protocol, we planned to use the ROBINS‐I tool. We planned to included bias 'due to centre‐specific experience and post‐operative follow‐up' in the analysis. Risk of bias due to the follow‐up period is covered in the 'selection of participants domain'. Centre experience would have been appropriate to analyse in the subgroup analysis, but not enough studies reported information on this for it to be conducted.
Author contributions
Another author (SA) was added to the review to help with data extraction, risk of bias and uploading of data and references to RevMan.
Author JH (not SH) analysed the risk of bias with AN. Author SH (not JH) constructed the summary of findings tables with AN.
Sensitivity analysis
We did not do any sensitivity analysis with "missing data that require assumptions and/or imputations (removing studies where assumptions have been made)" as we had no studies with missing data or assumptions.
Contributions of authors
Draft the protocol: AN, JH, SH, PGR, RR
Study selection: AN, JH
Extract data from studies: AN, JH, SA
Enter data into RevMan: AN, SA
Carry out the analysis: AN, SH
Interpret the analysis: AN, JH, SH, PGR, RR
Draft the final review: AN, JH, PGR, SA, SH, RR
Disagreement resolution: PGR, RR
Update the review: AN, PGR
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Akriti Nanda: none known. Jesse Hu: none known. Sarah Hodgkinson: none known. Sanah Ali: none known Richard Rainsbury: none known. Pankaj Roy: none known.
New
References
References to studies included in this review
Acea‐Nebril 2005 {published data only}
- Acea-Nebril B, López S, Cereijo C, Bazarra A, Pais P, Uriarte I, et al. Impacto asistencial de las técnicas oncoplásticas conservadoras en un programa quirúrgico para enfermas con cáncer de mama. Cirugía Española 2005;78(3):175-82. [DOI] [PubMed] [Google Scholar]
- Ayoub F, Latifi L, Trapszo P, Seetharam S. Comparison of re-excision rates between standard wide local excision and therapeutic mammoplasty in a district general hospital. European Journal of Surgical Oncology 2019;45(2):e104. [Google Scholar]
Acea‐Nebril 2017 {published data only}
- Acea-Nebril B, Cereijo-Garea C, Garcia-Novoa A, Varela-Lamas C, Builes-Ramirez S, Bouzon-Alejandro A, et al. The role of oncoplastic breast reduction in the conservative management of breast cancer: Complications, survival, and quality of life. Journal of Surgical Oncology 2017;115(6):679-86. [DOI] [PubMed] [Google Scholar]
Acosta‐Marin 2014 {published data only}
- Acosta-Marin V, Acosta-Freites V, Contreras A, Ravelo R, Fuenmayor G, Marin C, et al. Oncoplastic breast surgery: initial experience at the Centro Clinico de Estereotaxia-CECLINES, Caracas, Venezuela. Ecancermedicalscience 2014;8:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amitai 2018 {published data only}
- Amitai Y, Golan O, Barnea Y, Klausner J, Menes TS. Follow-up of patients undergoing oncoplastic surgery - more palpable masses and benign biopsies. Breast Disease 2018;37(3):115-21. [DOI] [PubMed] [Google Scholar]
Angarita 2020 {published data only}
- Angarita FA, Acuna SA, Cordeiro E, McCready DR, Cil TD. Does oncoplastic surgery increase immediate (30‐day) postoperative complications? An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Breast Cancer Research and Treatment 2020;182(2):429-38. [DOI] [PubMed] [Google Scholar]
- Angarita FA, Acuna SA, Cordeiro E, McCready DR, Cil TD. Does oncoplastic surgery increase immediate (30-day) postoperative complications? An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Breast Cancer Research and Treatment 2020;182(2):429-38. [DOI] [PubMed] [Google Scholar]
Atallah 2015 {published data only}
- Atallah D, Moubarak M, Safi J, El Kassis N. Safety of oncoplastic surgery in early breast cancer: a case–control study. The Breast 2015;24(Suppl 1):S147. [Google Scholar]
Bali 2018 {published data only}
- Bali R, Kankam HK, Borkar N, Provenzano E, Agrawal A. Wide local excision versus oncoplastic breast surgery: differences in surgical outcome for an assumed margin (0, 1, or 2 mm) distance. Clinical Breast Cancer 2018;18(5):e1053-7. [DOI] [PubMed] [Google Scholar]
Borm 2019 {published data only}
- Borm KJ, Schonknecht C, Nestler A, Oechsner M, Waschulzik B, Combs SE, et al. Outcomes of immediate oncoplastic surgery and adjuvant radiotherapy in breast cancer patients. BMC Cancer 2019;19(1):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2016 {published data only}
- Carter SA, Lyons GR, Kuerer HM, Bassett RL Jr, Oates S, Thompson A, et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Annals of Surgical Oncology 2016;23(10):3190-8. [DOI] [PubMed] [Google Scholar]
Cassi 2016 {published data only}
- Calì Cassi L, Vanni G, Petrella G, Orsaria P, Pistolese C, Lo Russo G, et al. Comparative study of oncoplastic versus non-oncoplastic breast conserving surgery in a group of 211 breast cancer patients. European Review for Medical and Pharmacological Sciences 2016;20(14):2950-4. [PubMed] [Google Scholar]
Chakravorty 2012 {published data only}
- Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della Rovere G, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. European Journal of Surgical Oncology 2012;38(5):395-8. [DOI] [PubMed] [Google Scholar]
Chauhan 2016 (1) {published data only}
- Chauhan A, Sharma MM. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Medical Journal, Armed Forces India 2016;72(1):12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chauhan 2016 (2) {published data only}
- Chauhan A, Sharma MM, Kumar K. Evaluation of surgical outcomes of oncoplasty breast surgery in locally advanced breast cancer and comparison with conventional breast conservation surgery. Indian Journal of Surgical Oncology 2016;7(4):413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crown 2015 {published data only}
- Crown A, Wechter DG, Grumley JW. Oncoplastic breast-conserving surgery reduces mastectomy and postoperative re-excision rates. Annals of Surgical Oncology 2015;22(10):3363-8. [DOI] [PubMed] [Google Scholar]
Crown 2019 {published data only}
- Crown A, Scovel LG, Rocha FG, Scott EJ, Wechter DG, Grumley JW. Oncoplastic breast conserving surgery is associated with a lower rate of surgical site complications compared to standard breast conserving surgery. American Journal of Surgery 2019;217(1):138-41. [DOI] [PubMed] [Google Scholar]
DeLorenzi 2016 (1) {published data only}
- De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. European Journal of Surgical Oncology 2016;42(1):71-7. [DOI] [PubMed] [Google Scholar]
DeLorenzi 2016 (2) {published data only}
- De Lorenzi F, Loschi P, Bagnardi V, Rotmensz N, Hubner G, Mazzarol G, et al. Oncoplastic breast-conserving surgery for tumors larger than 2 centimeters: is it oncologically safe? A matched-cohort analysis. Annals of Surgical Oncology 2016;23(6):1852-9. [DOI] [PubMed] [Google Scholar]
DeLorenzi 2018 {published data only}
- De Lorenzi F, Di Bella J, Maisonneuve P, Rotmensz N, Corso G, Orecchia R, et al. Oncoplastic breast surgery for the management of ductal carcinoma in situ (DCIS): is it oncologically safe? A retrospective cohort analysis. European Journal of Surgical Oncology 2018;44(7):957-62. [DOI] [PubMed] [Google Scholar]
Di Micco 2017 {published data only}
- Di Micco R, O'Connell RL, Barry PA, Roche N, MacNeill FA, Rusby JE. Standard wide local excision or bilateral reduction mammoplasty in large-breasted women with small tumours: Surgical and patient-reported outcomes. European Journal of Surgical Oncology 2017;43(4):636-41. [DOI] [PubMed] [Google Scholar]
Dolan 2015 {published data only}
- Dolan R, Patel M, Weiler-Mithoff E, Mansell J, Stallard S, Doughty JC, et al. Imaging results following oncoplastic and standard breast conserving surgery. Breast Care (Basel) 2015;10(5):325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Down 2013 {published data only}
- Down SK, Jha PK, Burger A, Hussien MI. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast Journal 2013;19(1):56-63. [DOI] [PubMed] [Google Scholar]
Eichler 2013 {published data only}
- Eichler C, Kolsch M, Sauerwald A, Bach A, Gluz O, Warm M. Lumpectomy versus mastopexy – a post-surgery patient survey. Anticancer Research 2013;33(2):731-6. [PubMed] [Google Scholar]
Fan 2019 {published data only}
- Fan KL, Yang S, Park S, Park TH, Song SY, Lee N, et al. Postoperative cancer surveillance following oncoplastic surgery with latissimus dorsi flap: a matched case-control study. Annals of Surgical Oncology 2019;26(13):4681-91. [DOI] [PubMed] [Google Scholar]
Farooqi 2019 {published data only}
- Farooqi N, Vohra L, Jiwani U. Outcomes of oncoplastic breast surgery compared to breast-conserving surgery in breast cancer patients (abstract no. 580724). In: The 20th Annaul Meeting - American Society of Breast Surgeons (April 30 - May 5), Dallas, TX. 2019.
Gendy 2003 {published data only}
- Gendy RK, Able JA, Rainsbury RM. Impact of skin-sparing mastectomy with immediate reconstruction and breast-sparing reconstruction with miniflaps on the outcomes of oncoplastic breast surgery. British Journal of Surgery 2003;90(4):433-9. [DOI] [PubMed] [Google Scholar]
Gicalone 2007 (1) {published data only}
- Giacalone PL, Dubon O, Roger P, El Gareh N, Rihaoui S, Daures JP. Doughnut mastopexy lumpectomy versus standard lumpectomy in breast cancer surgery: a prospective study. European Journal of Surgical Oncology 2007;33(3):301-6. [DOI] [PubMed] [Google Scholar]
Gicalone 2007 (2) {published data only}
- Giacalone PL, Roger P, Dubon O, El Gareh N, Rihaoui S, Taourel P, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Annals of Surgical Oncology 2007;14(2):605-14. [DOI] [PubMed] [Google Scholar]
Gicalone 2015 {published data only}
- Giacalone PL, Roger P, Dubon O, El Gareh N, Daurés JP, Laffargue F. Lumpectomy versus oncoplastic surgery for breast-conserving therapy of cancer. A prospective study about 99 patients. Annales de Chirurgie 2016;131(4):256-61. [DOI] [PubMed] [Google Scholar]
Gulcelik 2013 {published data only}
- Gulcelik MA, Dogan L, Yuksel M, Camlibel M, Ozaslan C, Reis E. Comparison of outcomes of standard and oncoplastic breast-conserving surgery. Journal of Breast Cancer 2013;16(2):193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamdi 2008 {published data only}
- Hamdi M, Sinove Y, DePypere H, Van Den Broucke R, Vakaet L, Cocquyt V, et al. The role of oncoplastic surgery in breast cancer. Acta Chirurgica Belgica 2008;108(6):666-72. [DOI] [PubMed] [Google Scholar]
Hart 2015 {published data only}
- Hart AM, Pinell-White X, Egro FM, Losken A. The psychosexual impact of partial and total breast reconstruction: a prospective one-year longitudinal study. Annals of Plastic Surgery 2015;75(3):281-6. [DOI] [PubMed] [Google Scholar]
Hashimoto 2019 {published data only}
- Hashimoto Y, Ishitobi M, Okuno J, Tashima H, Kurita T, Tokui R, et al. Comparison of oncological outcomes in breast-conserving surgery with immediate latissimus dorsi flap reconstruction versus breast-conserving surgery alone. The Breast 2019;44(Suppl 1):S108. [Google Scholar]
Hilli‐Betz 2014 {published data only}
- Hille-Betz U, Vaske B, Henseler H, Soergel P, Kundu S, Makowski L, et al. Dermoglandular rotation flaps for breast-conserving therapy: aesthetic results, patient satisfaction, and morbidity in comparison to standard segmentectomy. International Journal of Breast Cancer 2014;2014:152451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2019 {published data only}
- Hu J, Cuffolo G, Parulekar V, Chan V, Tenovici A, Roy PG. The results of surveillance imaging after breast conservation surgery and partial breast reconstruction with chest wall perforator flaps; a qualitative analysis compared with standard breast-conserving surgery for breast cancer. Clinical Breast Cancer 2019;19(3):e422-7. [DOI] [PubMed] [Google Scholar]
Jiang 2015 {published data only}
- Jiang R. Clinical study of oncoplastic breast- conserving surgery for the treatment of 30 cases. Chinese Journal of Clinical Oncology 2015;42:112. [Google Scholar]
Kahn 2013 {published data only}
- Khan J, Barrett S, Forte C, Stallard S, Weiler-Mithoff E, Doughty JC, et al. Oncoplastic breast conservation does not lead to a delay in the commencement of adjuvant chemotherapy in breast cancer patients. European Journal of Surgical Oncology 2013;39(8):887-91. [DOI] [PubMed] [Google Scholar]
Keleman 2019 {published data only}
- Kelemen P, Pukancsik D, Ujhelyi M, Kovacs E, Udvarhelyi N, Kenessey I, et al. 72. Comparing oncoplastic breast surgery with conventional breast conserving therapies. Oncological, cosmetic and quality of life outcomes of 350 cases. In: European Journal of Surgical Oncology. Vol. 42. 2016:PS95.
- Kelemen P, Pukancsik D, Ujhelyi M, Savolt A, Kovacs E, Ivady G, et al. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: A single-centre retrospective study. European Journal of Surgical Oncology 2019;45(2):118-24. [DOI] [PubMed] [Google Scholar]
Kelsall 2017 {published data only}
- Kelsall JE, McCulley SJ, Brock L, Akerlund MT, Macmillan RD. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: Case-matched patient reported outcomes. Journal of Plastic, Reconstructive & Anaesthetic Surgery 2017;70(10):1377-85. [DOI] [PubMed] [Google Scholar]
Kimball 2018 {published data only}
- Kimball CC, Nichols CI, Vose JG, Peled AW. Trends in lumpectomy and oncoplastic breast-conserving surgery in the US, 2011-2016. Annals of Surgical Oncology 2018;25(13):3867-73. [DOI] [PubMed] [Google Scholar]
Klit 2017 {published data only}
- Klit A, Tvedskov TF, Kroman N, Elberg JJ, Ejlertsen B, Henriksen TF. Oncoplastic breast surgery does not delay the onset of adjuvant chemotherapy: a population-based study. Acta Oncologica 2017;56(5):719-23. [DOI] [PubMed] [Google Scholar]
Lansu 2014 {published data only}
- Lansu JT, Essers M, Voogd AC, Luiten EJ, Buijs C, Groenendaal N, et al. The influence of simultaneous integrated boost, hypofractionation and oncoplastic surgery on cosmetic outcome and PROMs after breast conserving therapy. European Journal of Surgical Oncology 2015;41(10):1411-6. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee J, Jung JH, Kim WW, Chae YS, Lee SJ, Park HY. Comparison of 5-year oncological outcomes of breast cancer based on surgery type. ANZ Journal of Surgery 2018;88(5):E395-9. [DOI] [PubMed] [Google Scholar]
Losken 2009 {published data only}
- Losken A, Schaefer TG, Newell M, Styblo TM. The impact of partial breast reconstruction using reduction techniques on postoperative cancer surveillance. Plastic and Reconstructive Surgery 2009;124(1):9-17. [DOI] [PubMed] [Google Scholar]
Losken 2014 {published data only}
- Losken A, Pinell-White X, Hart AM, Freitas AM, Carlson GW, Styblo TM. The oncoplastic reduction approach to breast conservation therapy: benefits for margin control. Aesthetic Surgical Journal 2014;34(8):1185-91. [DOI] [PubMed] [Google Scholar]
Malhaire 2015 {published data only}
- Malhaire C, Hequet D, Falcou MC, Feron JG, Tardivon A, Leduey A, et al. Outcome of oncoplastic breast-conserving surgery following bracketing wire localization for large breast cancer. Breast 2015;24(4):370-5. [DOI] [PubMed] [Google Scholar]
Mansell 2015 {published data only}
- Mansell J, Weiler-Mithoff E, Martin J, Khan A, Stallard S, Doughty JC, et al. How to compare the oncological safety of oncoplastic breast conservation surgery - to wide local excision or mastectomy? The Breast 2015;24(4):P497-501. [DOI] [PubMed] [Google Scholar]
Mansell 2017 {published data only}
- Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Mallon E, Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast 2017;32:179-85. [DOI] [PubMed] [Google Scholar]
Matrai 2014 {published data only}
- Mátrai Z, Gulyás G, Kovács E, Sándor Z, Polgár C, Bartal A, et al. Oncoplastic versus conventional breast conserving surgery. A comparison of clinicopathological findings, cosmetic results and quality of life of 60 cases [Onkoplasztikus versus hagyományos emlőmegtartó sebészet. 60 eset összehasonlító klinikopatológiai, kozmetikai és életminőségi vizsgálata]. Hungarian Oncology 2014;58(2):116–127. [PubMed] [Google Scholar]
Mazouni 2013 {published data only}
- Mazouni C, Naveau A, Kane A, Dunant A, Garbay JR, Leymarie N, et al. The role of oncoplastic breast surgery in the management of breast cancer treated with primary chemotherapy. Breast 2013;22(6):1189-93. [DOI] [PubMed] [Google Scholar]
Morrow 2019 {published data only}
- Morrow ES, Stallard S, Doughty J, Malyon A, Barber M, Dixon JM, et al. Oncoplastic breast conservation occupies a niche between standard breast conservation and mastectomy - A population-based prospective audit in Scotland. European Journal of Surgical Oncology 2019;45(10):1806-11. [DOI] [PubMed] [Google Scholar]
Mukhtar 2018 {published data only}
- Mukhtar RA, Wong J, Piper M, Zhu Z, Fahrner-Scott K, Mamounas M, et al. Breast conservation and negative margins in invasive lobular carcinoma: the impact of oncoplastic surgery and shave margins in 358 patients. Annals of Surgical Oncology 2018;25(11):3165-70. [DOI] [PubMed] [Google Scholar]
Mustonen 2004 {published data only}
- Mustonen P, Lepisto J, Papp A, Berg M, Pietilainen T, Kataja V, et al. The surgical and oncological safety of immediate breast reconstruction. European Journal of Surgical Oncology 2004;30(8):817-23. [DOI] [PubMed] [Google Scholar]
Nakada 2019 {published data only}
- Nakada H, Inoue M, Furuya K, Watanabe H, Ikegame K, Nakayama Y, et al. Fat necrosis after breast-conserving oncoplastic surgery. Breast Cancer 2019;26(1):125-30. [DOI] [PubMed] [Google Scholar]
Nakagomi 2019 {published data only}
- Nakagomi H, Inoue M, Nakada H, Ohmori M, Nakayama Y, Furuya K, et al. Lateral thoracoaxillar dermal-fat flap for breast conserving surgery: the changes of the indication and long-term results. Breast Cancer 2019;26(5):595-601. [DOI] [PubMed] [Google Scholar]
Niinikoski 2019 (2) {published data only}
- Niinikoski L, Leidenius MH, Vaara P, Voynov A, Heikkila P, Mattson J, et al. Resection margins and local recurrences in breast cancer: Comparison between conventional and oncoplastic breast conserving surgery. European Journal of Surgical Oncology 2019;45(6):976-82. [DOI] [PubMed] [Google Scholar]
Ojala 2017 {published data only}
- Ojala K, Meretoja TJ, Leidenius MH. Aesthetic and functional outcome after breast conserving surgery - comparison between conventional and oncoplastic resection. European Journal of Surgical Oncology 2017;43(4):658-64. [DOI] [PubMed] [Google Scholar]
Ozmen 2016 {published data only}
- Ozmen V, Sarsenov D, Ozmen T, Ilgun S, Alco G, Ordu C, et al. Mini latissimus dorsi flap increases breast conserving surgery rate in early breast cancer patients. Cancer Research 2016;76(4 Suppl):Abstract P2-12-17. [Google Scholar]
Ozmen 2020 {published data only}
- Ozmen V, Ilgun S, Celet Ozden B, Ozturk A, Aktepe F, Agacayak F, et al. Comparison of breast cancer patients who underwent partial mastectomy (PM) with mini latissimus dorsi flap (MLDF) and subcutaneous mastectomy with implant (M + I) regarding quality of life (QOL), cosmetic outcome and survival rates. World Journal of Surgical Oncology 2020;18(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palsodittlir 2018 {published data only}
- Palsdottir EP, Lund SHL, Asgeirsson KSA. Oncoplastic breast-conserving surgery in Iceland: a population-based study. Scandinavian Journal of Surgery 2018;107(3):224-9. [DOI] [PubMed] [Google Scholar]
Peled 2014 {published data only}
- Peled AW, Sbitany H, Foster RD, Esserman LJ. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast Journal 2014;20(3):302-7. [DOI] [PubMed] [Google Scholar]
Piper 2016 {published data only}
- Piper M, Peled AW, Sbitany H, Foster RD, Esserman LJ, Price ER. Comparison of mammographic findings following oncoplastic mammoplasty and lumpectomy without reconstruction. Annals of Surgical Oncology 2016;23(1):65-71. [DOI] [PubMed] [Google Scholar]
PlaFarnos 2018 {published data only}
- Pla Farnos MJ, Fernandez-Montoli ME, Garay Garcia L, Garcia Tejedor MA, Campos Delgado M, Verdaguer Menendez-Arango P, et al. Clinical and pathological variables associated to an oncoplastic procedure in breast cancer surgery. European Journal of Cancer 2018;92(Suppl 3):S68. [Google Scholar]
Potter 2020 {published data only}
- Potter S, Trickey A, Rattay T, O'Connell RL, Dave R, Baker E, et al. Therapeutic mammaplasty is a safe and effective alternative to mastectomy with or without immediate breast reconstruction. British Journal of Surgery 2020;107(7):832-44. [DOI] [PubMed] [Google Scholar]
Ren 2014 {published data only}
- Ren ZJ, Li XJ, Xu XY, Xia L, Tang JH. Oncoplastic breast conserving surgery with nipple-areolar preservation for centrally located breast cancer: a retrospective cohort study. Asian Pacific Journal of Cancer Prevention 2014;15(12):4847-9. [DOI] [PubMed] [Google Scholar]
Rose 2019 {published data only}
- Rose M, Svensson H, Handler J, Hoyer U, Ringberg A, Manjer J. Oncoplastic breast surgery compared to conventional breast-conserving surgery with regard to oncologic outcome. Clinical Breast Cancer 2019;19(6):423-432 e5. [DOI] [PubMed] [Google Scholar]
Rose 2020 {published data only}
- Rose M, Svensson H, Handler J, Hoyer U, Ringberg A, Manjer J. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Research and Treatment 2020;180(1):247-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2015 {published data only}
- Santos G, Urban C, Edelweiss MI, Zucca-Matthes G, Oliveira VM, Arana GH, et al. Long-term comparison of aesthetical outcomes after oncoplastic surgery and lumpectomy in breast cancer patients. Annals of Surgical Oncology 2015;22(8):2500-8. [DOI] [PubMed] [Google Scholar]
Scheter 2019 {published data only}
- Shechter S, Friedman O, Inbal A, Arad E, Menes T, Barsuk D, et al. Oncoplastic partial breast reconstruction improves patient satisfaction and aesthetic outcome for central breast tumours. ANZ Journal of Surgery 2019;89(5):536-40. [DOI] [PubMed] [Google Scholar]
Sherwell‐Cabello 2006 {published data only}
- Sherwell-Cabello S, Maffuz-Aziz A, Villegas-Carlos F, Dominguez-Reyes C, Labastida-Almendaro S, Rodriguez-Cuevas S. Feasibility and cosmetic outcome of oncoplastic surgery in breast cancer treatment. Cirugia y Cirujanos 2015;83(3):199-205. [DOI] [PubMed] [Google Scholar]
Tang 2016 {published data only}
- Tang W, Liu J, Yang H, Jiang Y, Wei W. Clinical comparative study of oncoplastic and standard breast-conserving surgery in the treatment of early breast cancer. Chinese Journal of Clinical Oncology 2016;43:235-9. [Google Scholar]
Tenofsky 2014 {published data only}
- Tenofsky PL, Dowell P, Topaloversuski T, Helmer SD. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Americal Journal of Surgery 2014;207(3):398-402; discussion 402. [DOI] [PubMed] [Google Scholar]
Tong 2016 {published data only}
- Tong WM, Baumann DP, Villa MT, Mittendorf EA, Liu J, Robb GL, et al. Obese women experience fewer complications after oncoplastic breast repair following partial mastectomy than after immediate total breast reconstruction. Plastic and Reconstructive Surgery 2016;137(3):777-91. [DOI] [PubMed] [Google Scholar]
Viega 2010 {published data only}
- Veiga DF, Veiga-Filho J, Ribeiro LM, Archangelo I Jr, Balbino PF, Caetano LV, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plastic and Reconstructive Surgery 2010;125(3):811-7. [DOI] [PubMed] [Google Scholar]
Viega 2011 {published data only}
- Veiga DF, Veiga-Filho J, Ribeiro LM, Archangelo-Junior I, Mendes DA, Andrade VO, et al. Evaluations of aesthetic outcomes of oncoplastic surgery by surgeons of different gender and specialty: a prospective controlled study. Breast 2011;20(5):407-12. [DOI] [PubMed] [Google Scholar]
Vieira 2016 {published data only}
- Vieira RA, Carrara GF, Scapulatempo Neto C, Morini MA, Brentani MM, Folgueira MA. The role of oncoplastic breast conserving treatment for locally advanced breast tumors. A matching case-control study. Annals Of Medicine & Surgery (London) 2016;10:61-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijgman 2017 {published data only}
- Wijgman DJ, Ten Wolde B, Groesen NR, Keemers-Gels ME, den Wildenberg FJ, Strobbe LJ. Short-term safety of oncoplastic breast conserving surgery for larger tumors. European Journal of Surgical Oncology 2017;43(4):665-71. [DOI] [PubMed] [Google Scholar]
Wong 2017 {published data only}
- Wong JM, Piper ML, Ewing C, Alvarado M, Esserman LJ, Sbitany H, et al. The use of oncoplastic surgical techniques to increase successful breast conservation in invasive lobular carcinoma of the breast. Cancer Research 2018;78(Suppl 4):Abstract P2-12-16. [Google Scholar]
Zhou 2019 {published data only}
- Zhou L, Wang Y, Cai R, Huang J, Li X, Xie Z, et al. Pedicled descending branch latissimus dorsi mini-flap in repairing partial mastectomy defect: Shoulder functional and esthetic outcomes. Journal of Surgical Oncology 2019;120(3):518-26. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adimulam 2014 {published data only}
- Adimulam G, Challa VR, Dhar A, Chumber S, Seenu V, Srivastava A. Assessment of cosmetic outcome of oncoplastic breast conservation surgery in women with early breast cancer: a prospective cohort study. Indian Journal of Cancer 2014;51(1):58-62. [DOI] [PubMed] [Google Scholar]
Angarita 2019 {published data only}
- Angarita F, Elmi M, Cordeiro E, McCready D, Cil T. Oncoplastic surgery is not associated with increased complications when compared to standard breast-conserving surgery: An analysis of the NSQIP Database. Annals of Surgical Oncology 2019;26(2 Suppl):119-20. [Google Scholar]
Ayoub 2019 {published data only}
- Ayoub F, Latifi L, Trapszo P, Seetharam S. Comparison of re-excision rates between standard wide local excision and therapeutic mammoplasty in a district general hospital. European Journal of Surgical Oncology 2019;45(2):E104. [Google Scholar]
Bogusevicius 2014 {published data only}
- Bogusevicius A, Cepuliene D, Sepetauskiene E. The integrated evaluation of the results of oncoplastic surgery for locally advanced breast cancer. Breast Journal 2014;20(1):53-60. [DOI] [PubMed] [Google Scholar]
Chapa 2019 {published data only}
- Chapa L, Chadha M, Jacobs J, Zaretsky E, Boolbol S. Breast-conserving surgery with partial breast reconstruction or reduction mammoplasty followed by whole breast radiation therapy (WBRT). Annals of Surgical Oncology 2019;26(2 Suppl):124. [Google Scholar]
Cil 2016 {published data only}
- Cil TD, Cordeiro E. Complications of oncoplastic breast surgery involving soft tissue transfer versus breast-conserving surgery: an analysis of the NSQIP Database. Annals of Surgical Oncology 2016;23(10):3266-71. [DOI] [PubMed] [Google Scholar]
Emiroglu 2016 {published data only}
- Emiroglu M, Sert I, Karaali C, Aksoy SO, Ugurlu L, Aydin C. The effectiveness of simultaneous oncoplastic breast surgery in patients with locally advanced breast cancer. Breast Cancer 2016;23(3):463-70. [DOI] [PubMed] [Google Scholar]
Flanagan 2019 {published data only}
- Flanagan MR, Zabor EC, Romanoff A, Fuzesi S, Stempel M, Mehrara BJ, et al. A comparison of patient-reported outcomes after breast-conserving surgery and mastectomy with implant breast reconstruction. Annals of Surgical Oncology 2019;26(10):3133-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitas 2019 {published data only}
- Freitas NM, Watanabe PD, Simionatto TF, Yagi NA, Campedelli AF, Martins E, et al. Oncoplastic surgery and the influence of the surgical clip on breast, heart and lung volumes of patients submitted to boost during the planning of breast cancer radiotherapy after lumpectomy. Cancer Research 2020;80(4 Suppl):Abstract P4-12-31. [Google Scholar]
Fung 2001 {published data only}
- Fung KW, Lau Y, Fielding R, Or A, Yip AW. The impact of mastectomy, breast-conserving treatment and immediate breast reconstruction on the quality of life of Chinese women. ANZ Journal of Surgery 2001;71(4):202-6. [DOI] [PubMed] [Google Scholar]
Geluk 2020 {published data only}
- Geluk C, Duijnhoven F, Hoornweg M. Direct or delayed oncoplastic reconstruction after wide local excision for breast cancer in breast conserving therapy: a single centre cohort study of 252 cases. European Journal of Cancer 2020;46(2):e46-7. [Google Scholar]
Hamilton 2019 {published data only}
- Hamilton SN, Nichol A, Wai E, Gondara L, Velasquez Garcia HA, Speers C, et al. Local relapse after breast-conserving therapy versus mastectomy for extensive pure ductal carcinoma in situ >=4 cm. International Journal of Radiation Oncology, Biology and Physics 2019;103(2):381-8. [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han J, Grothuesmann D, Neises M, Hille U, Hillemanns P. Quality of life and satisfaction after breast cancer operation. Archives of Gynecology and Obstetrics 2010;282(1):75-82. [DOI] [PubMed] [Google Scholar]
Hashem 2017 {published data only}
- Hashem T, Farahat A. Batwing versus Wise pattern mammoplasty for upper pole breast tumours: a detailed comparison of cosmetic outcome. World Journal of Surgical Oncology 2017;15(1):Article no. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20111207008316N4 {published data only}
- Mehdi Asadi. Evaluation of therapeutic results and patients' satisfaction of oncoplastic techniques in surgery of breast cancer. Iranian Registry Clinical Trials 2018.
Jonczyk 2019 {published data only}
- Jonczyk M, Jean J, Graham R, Chatterjee A. New era of modern breast cancer surgery: An 11-year analysis of surgical trends with adoption of breast reconstruction: NSQIP database 2005-2016 analysis. Annals of Surgical Oncology 2019;26(Suppl 1):S69-70 (Abstract no. P8).
Kabir 2015 {published data only}
- Kabir SA, Mansell J, Romics L. P086. High incomplete excision rate is strongly associated with lobular subtype, node positivity and tumour size, but independent of hormonal and HER-2 status. In: European Journal of Surgical Oncology. PS51 edition. Vol. 41. 2015:6.
Kabir 2015a {published data only}
- Kabir SA, Weiler-Mithoff E, Mansell J, Stallard S, Doughty J, Romics L. P085. Indication for breast conservation for lobular cancer may be extended when oncoplastic techniques used. European Journal of Surgical Oncology 2015;41(6):S50-1. [Google Scholar]
Kaur 2005 {published data only}
- Kaur N, Petit JY, Rietjens M, Maffini F, Luini A, Gatti G, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Annals of Surgical Oncology 2005;12(7):539-45. [DOI] [PubMed] [Google Scholar]
Kawanaka 2019 {published data only}
- Kawanaka T, Kubo A, Tonoiso C, Takahashi A, Furutani S, Ikushima H, et al. Use of local boost radiation therapy after breast-conserving surgery with volume replacement oncoplastic method. International Journal of Radiation Oncology 2019;105(1 Suppl):E43. [Google Scholar]
Kelemen 2016 {published data only}
- Kelemen P, Pukancsik D, Ujhelyi M, Kovacs E, Udvarhelyi N, Kenessey I, et al. 72. Comparing oncoplastic breast surgery with conventional breast conserving therapies. Oncological, cosmetic and quality of life outcomes of 350 cases. European Journal of Surgical Oncology 2016;42(9):PS95. [Google Scholar]
Khan 2018 {published data only}
- Khan S, Epstein M, Savalia N, Silverstein M. Extreme oncoplasty: Breast conservation for patients who traditionally require mastectomy. Breast Journal 2018;25(2 Suppl 1):131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lima 2012 {published data only}
- Lima FR, Veiga Filho J, Ribeiro LM, Morais TB, Rocha LR, Juliano Y, et al. Oncoplastic approach in the conservative treatment of breast cancer: analysis of costs. Acta Cirúrgica Brasileira 2012;27(5):311-4. [DOI] [PubMed] [Google Scholar]
Mondani 2019 {published data only}
- Mondani J, Ansari A, Gaber A, Ali Z, Kaushik M. Oncoplastic and reconstructive surgery is a valid option for the treatment of elderly breast cancer patients. British Journal of Surgery - ASIT Poster Presentations 2019;106(Suppl 6):21-169 (Abstract no. 0691). [Google Scholar]
Moustafa 2016 {published data only}
- Moustafa A. Volume displacement techniques for filling partial mastectomy defects. European Journal of Surgical Oncology 2016;42(9):PS121. [Google Scholar]
Nano 2005 {published data only}
- Nano MT, Gill PG, Kollias J, Bochner MA, Carter N, Winefield HR. Qualitative assessment of breast reconstruction in a specialist breast unit. ANZ Journal of Surgery 2005;75(6):445-53. [DOI] [PubMed] [Google Scholar]
NCT00870415 {published data only}
- NCT00870415. Breast-conserving surgery techniques in treating women with breast cancer. clinicaltrials.gov/ct2/show/NCT00870415 (first received 27 March 2009).
NCT02376413 {published data only}
- NCT02376413. Oncoplastic breast-conserving surgery in non-metastatic breast cancer patients. clinicaltrials.gov/ct2/show/NCT02376413 (first received 3 March 2015).
NCT03273348 {published data only}
- NCT03273348. Role of oncoplastic breast surgery in breast cancer treatment. clinicaltrials.gov/ct2/show/NCT03273348 (first received 6 September 2017).
NCT03900299 {published data only}
- NCT03900299. Evaluating new surgical technique in management of female patients with operable multifocal breast cancer. clinicaltrials.gov/ct2/show/NCT03900299 (first received 3 April 2019).
NCT04349527 {published data only}
- NCT04349527. Comparison of the cosmetic results, quality of life and patient satisfaction achieved with round-block and retroglandular oncoplastic breast conserving surgeries. clinicaltrials.gov/ct2/show/NCT04349527 (first received 16 April 2020).
Niinikoski 2019 (1) {published data only}
- Niinikoski L, Leidenius M, Vaara P, Voynov A, Heikkila P, Meretoja T. Reoperations due to inadequate surgical margins and risk of local recurrence in breast cancer: comparison between conventional and oncoplastic breast conserving surgery. European Journal of Surgical Oncology 2019;45(2):e22-e23. [DOI] [PubMed] [Google Scholar]
Nisiri 2018 {published data only}
- Nisiri A, Pour RO, Zadeh HM, Ramim T. Comparison of surgical margin after breast cancer surgery between oncoplastic technique and conventional breast-conserving surgery. International Journal of Cancer Management 2018;11(4):e9696. [Google Scholar]
Pearce 2020 {published data only}
- Pearce BC, Fiddes RN, Paramanathan N, Chand N, Laws SA, Rainsbury RM. Extreme oncoplastic conservation is a safe new alternative to mastectomy. European Journal of Surgical Oncology 2020;46(1):71-6. [DOI] [PubMed] [Google Scholar]
Pukancsik 2017 {published data only}
- Pukancsik D, Kelemen P, Ujhelyi M, Kovacs E, Udvarhelyi N, Meszaros N, et al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: An aesthetic and functional prospective cohort study. European Journal of Surgical Oncology 2017;43(2):303-10. [DOI] [PubMed] [Google Scholar]
Pukancsik 2019 {published data only}
- Pukancsik D, Kelemen P, Ujhelyi M, Kovacs E, Meszaros N, Kasler M, et al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: an aesthetic and functional prospective cohort study. European Journal of Surgical Oncology 2019;45(2):e109. [DOI] [PubMed] [Google Scholar]
Rietjens 2007 {published data only}
- Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, Garusi C, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast 2007;16(4):387-95. [DOI] [PubMed] [Google Scholar]
Romics 2017 {published data only}
- Romics L, Macaskill J, Fernandez T, Morrow E, Simpson L, Pitsinis V, et al. Oncoplastic breast conservations - the Scottish audit: surgical techniques, oncological outcomes, complication rates and variations in practice across the country based on the analysis of 589 patients. Cancer Research 2018;78(4 Suppl):Abstract P4-13-01. [Google Scholar]
Sun 2014 {published data only}
- Sun Y, Kim SW, Heo CY, Kim D, Hwang Y, Yom CK, et al. Comparison of quality of life based on surgical technique in patients with breast cancer. Japanese Journal of Clinical Oncology 2014;44(1):22-7. [DOI] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang JH, Yao YF, Qin JW, Xu XM, Li L. Clinical observation of the immediate breast reconstruction following breast-conserving surgery for centrally located breast cancer. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 2013;35(7):518-20. [PubMed] [Google Scholar]
van Paridon 2017 {published data only}
- Paridon MW, Kamali P, Paul MA, Wu W, Ibrahim AM, Kansal KJ, et al. Oncoplastic breast surgery: achieving oncological and aesthetic outcomes. Journal of Surgical Oncology 2017;116(2):195-202. [DOI] [PubMed] [Google Scholar]
Youssef 2017 {published data only}
- Youssef M, Namour A, Youssef O, Ahmed M. Oncoplastic breast surgery is oncologically safe in locally advanced breast cancer after neoadjuvant chemotherapy, an Egyptian experience. Indian Journal of Surgical Oncology 2017;72(Suppl 1):S30-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2018 {published data only}
- Youssef MM, Namour A, Youssef OZ, Morsi A. Oncologic and cosmetic outcomes of oncoplastic breast surgery in locally advanced breast cancer after neoadjuvant chemotherapy, experience from a developing country. Indian Journal of Surgical Oncology 2018;9(3):300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zucca 2012 {published data only}
- Zucca Matthes AG, Uemura G, Kerr L, Matthes AC, Michelli RA, Folgueira MA, et al. Feasibility of oncoplastic techniques in the surgical management of locally advanced breast cancer. International Journal of Surgery 2012;10(9):500-5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Srivastava 2018 {published data only}
- Srivastava A. BR 23 Score and aesthetic outcome in ladies with early breast cancer undergoing oncoplasty versus conventional breast conservation surgery. European Journal of Cancer 2018;92(Suppl 3):S83-4. [Google Scholar]
References to ongoing studies
ACTRN12612000638831 {published data only}
- ACTRN12612000638831. Effect of breast oncoplastic reshaping on the long term cosmetic outcome after breast conservation surgery: a prospective randomised trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362438 (first received 26 April 2012).
Catsman 2018 {published data only}
- Catsman CJ, Beek MA, Voogd AC, Mulder PG, Luiten EJ. The COSMAM TRIAL a prospective cohort study of quality of life and cosmetic outcome in patients undergoing breast conserving surgery. BMC Cancer 2018;18(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01396993 {published data only}
- NCT01396993. Prospective non-randomized evaluation of oncoloplastic surgery (iTOP). clinicaltrials.gov/ct2/show/NCT01396993 (first received 19 July 2011).
NCT02159274 {published data only}
- NCT02159274. Shoulder disability and late symptoms following oncoplastic breast surgery. clinicaltrials.gov/ct2/show/NCT02159274 (first received 9 June 2014).
NCT02901223 {published data only}
- NCT02901223. The impact of oncoplastic breast surgery on the oncological safety and patient satisfaction. clinicaltrials.gov/ct2/show/NCT02901223 (first received 15 September 2016).
NCT02923635 {published data only}
- NCT02923635. A prospective comparative study between oncoplastic breast surgery and standard wide local excision. clinicaltrials.gov/ct2/show/NCT02923635 (first received 4 October 2016).
NCT03012152 {published data only}
- NCT03012152. A comparative study between oncoplastic breast surgery and standard conservative surgery: margin status and patient satisfaction. clinicaltrials.gov/ct2/show/NCT03012152 (first received 6 January 2017).
NCT04030845 {published data only}
- NCT04030845. Patient report outcome-reconstruction and oncoplastic cohort (PRO-ROC). clinicaltrials.gov/ct2/show/NCT04030845 (first received 24 July 2019).
NTR6901 {published data only}
- NTR6901. Patient satisfaction after oncoplastic breast surgery. www.trialregister.nl/trial/6667 (first received 13 December 2017).
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365-76. [DOI] [PubMed] [Google Scholar]
ACS 2016
- American Cancer Society. Surgery for Breast Cancer. www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer.html (accessed 15 March 2020).
Agarwal 2014
- Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy versus mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surgery 2014;149(3):267-74. [DOI] [PubMed] [Google Scholar]
Almasad 2008
- Almasad J. Breast reconstruction in conserving breast cancer surgery. Saudi Medical Journal 2008;29(11):1548-53. [PubMed] [Google Scholar]
Association of Breast Surgery 2012
- Rainsbury D, Willett A, editor(s), Association of Breast Surgery (ABS) and British Association of Plastic Reconstructive and Aesthetic Surgeons (BAPRAS). Oncoplastic Breast Reconstruction: Guidelines for Best Practice. Available at www.bapras.org.uk/docs/default-source/commissioning-and-policy/final-oncoplastic-guidelines-healthcare-professionals.pdf?sfvrsn=0.
BCCT.core [Computer program]
- BCCT.core software. INESC TEC. INESC TEC, 2007.
BCRF 2019
- Breast Cancer Research Foundation. Breast Cancer Statistics 2019. www.bcrf.org/breast-cancer-statistics-and-resources (accessed 15 March 2020).
BI‐RADS
- American College of Radiology (ACR, D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al). ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System.. Reston, VA, American College of Radiology, 2013. [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians 2018;68(6):393-424. [DOI] [PubMed] [Google Scholar]
Breast Cancer Care 2020
- Breast Cancer Now: The Research & Care Charity. Facts and Statistics. breastcancernow.org/about-us/media/facts-statistics (accessed 15 March 2020).
Chen 2018
- Chen JY, Huang YJ, Zhang LL, Yang CQ, Wang K. Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: a meta-analysis. Journal of Breast Cancer 2018;21(3):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clough 2003
- Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Annals of Surgery 2003;237(1):26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2003
- Cochrane RA, Valasiadou P, Wilson AR, Al-Ghazal SK, Macmillan RD. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. British Journal of Surgery 2003;90(12):1505-9. [DOI] [PubMed] [Google Scholar]
Cohen 2016
- Cohen WA, Mundy LR, Ballard TN, Klassen A, Cano SJ, Browne J, et al. The BREAST-Q in surgical research: a review of the literature 2009-2015. Journal of Plastic, Reconstructive & Aesthetic Surgery 2016;69(2):149-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 20 June 2020. Available at covidence.org.
De La Cruz 2016
- De La Cruz L, Blankenship SA, Chatterjee A, Geha R, Nocera N, Czerniecki BJ, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Annals of Surgical Oncology 2016;23(10):3247-58. [DOI] [PubMed] [Google Scholar]
Excel [Computer program]
- Microsoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. Retrieved from https://office.microsoft.com/excel.
Fisher 2002
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine 2002;347(16):1233-41. [DOI] [PubMed] [Google Scholar]
Fitzal 2007
- Fitzal F, Krois W, Trischler H, Wutzel L, Riedl O, Kühbelböck U, et al. The use of a breast symmetry index for objective evaluation of breast cosmesis. Breast 2007;16(4):429-35. [DOI] [PubMed] [Google Scholar]
Garratt 1993
- Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF-36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ 1993;306(6890):1440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Haloua 2013
- Haloua MH, Krekel NM, Winters HA, Rietveld DH, Meijer S, Bloemers FW, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Annals of Surgery 2013;257(4):609-20. [DOI] [PubMed] [Google Scholar]
Halsted 1894
- Halsted WS. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889 to January 1894. Annals of Surgery 1894;20(5):497-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamdi 2006
- Hamdi M, Van Landuyt K, Frene B, Roche N, Blondeel P, Monstrey S. The versatility of the inter-costal artery perforator (ICAP) flaps. Journal of Plastic, Reconstructive & Aesthetic Surgery 2006;59(6):644-52. [DOI] [PubMed] [Google Scholar]
Hamdi 2014
- Hamdi M, Craggs B, Stoel AM, Hendrickx B, Zeltzer A. Superior epigastric artery perforator flap: anatomy, clinical applications, and review of literature. Journal of Reconstructive Microsurgery 2014;30(7):475-82. [DOI] [PubMed] [Google Scholar]
Harris 1979
- Harris J, Levene MR, Svensson GB, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. International Journal of Radiation Oncology, Biology, Physics 1979;5(2):257-61. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343(5928):9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Holmes 2011
- Holmes DR, Schooler W, Smith R. Oncoplastic approaches to breast conservation. International Journal of Breast Cancer 2011;2011:ID: 303879, 16 pages. [DOI: 10.4061/2011/303879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopwood 2001
- Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. European Journal of Cancer 2001;37(2):189-97. [DOI] [PubMed] [Google Scholar]
Hu 2018
- Hu J, Tenovici A, Parulekar V, Bhattacharyya M, Roy PG. The impact of partial breast reconstruction with lateral chest wall perforator flaps on post-operative cancer surveillance. Available at abs.amegroups.com/article/view/4317/4913. [DOI: 10.21037/abs.2018.04.01] [DOI]
Jordan 2020
- Jordan CH. Zeigler-Hill V, Shackelford TK (eds). Encyclopedia of Personality and Individual Differences. Springer, 2020. [Google Scholar]
Kaufman 2019
- Kaufman CS. Increasing role of oncoplastic surgery for breast cancer. Current Oncology Reports 2019;21(12):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kijima 2011
- Kijima Y, Yoshinaka H, Hirata M, Mizoguchi T, Ishigami S, Arima H, et al. Immediate reconstruction using a modified thoracodorsal adipofascial cutaneous flap after partial mastectomy. Breast 2011;20(5):464-7. [DOI] [PubMed] [Google Scholar]
Kijima 2014
- Kijima Y, Yoshinaka H, Hirata M, Arima H, Nakajo A, Shinden Y, et al. Oncoplastic surgery combining partial mastectomy and immediate volume replacement using a thoracodorsal adipofascial cutaneous flap with a crescent-shaped dermis. Surgery Today 2014;44(11):2098-105. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim MK, Kim T, Moon HG, Jin US, Kim K, Kim J, et al. Effect of cosmetic outcome on quality of life after breast cancer surgery. European Journal of Surgical Oncology 2015;41(3):426-32. [DOI] [PubMed] [Google Scholar]
Krois 2017
- Krois W, Romar AK, Wild T, Dubsky P, Exner R, Panhofer P, et al. Objective breast symmetry analysis with the breast analyzing tool (BAT): improved tool for clinical trials. Breast Cancer Research and Treatment 2017;164(2):421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Losken 2014
- Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Annals of Plastic Surgery 2014;72(2):145-9. [DOI] [PubMed] [Google Scholar]
McCulley 2011
- McCulley SJ, Macmillan RD, Rasheed T. Transverse Upper Gracilis (TUG) flap for volume replacement in breast conserving surgery for medial breast tumours in small to medium sized breasts. Journal of Plastic, Reconstructive & Aesthetic Surgery 2011;64(8):1056-60. [DOI] [PubMed] [Google Scholar]
McCulley 2015
- McCulley SJ, Schaverien MV, Tan VK, Macmillan RD. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery 2015;68(5):686-91. [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Munhoz 2011
- Munhoz AM, Montag E, Arruda E, Brasil JA, Aldrighi JM, Gemperli R, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast 2011;20(3):233-40. [DOI] [PubMed] [Google Scholar]
Munzone 2014
- Munzone E. Highlights from the Ninth European Breast Cancer Conference, Glasgow, 19-21 March 2014. Ecancermedicalscience 2014;8:426. [DOI: 10.3332/ecancer.2014.426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogawa 2007
- Ogawa T, Hanamura N, Yamashita M, Ri Y, Kuriyama N, Isaji S. Usefulness of breast-volume replacement using an inframammary adipofascial flap after breast-conservation therapy. American Journal of Surgery 2007;193(4):514-8. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372(71):9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pezner 1985
- Pezner RD, Patterson MP, Hill LR, Vora N, Desai KR, Archambeau JO, Lipsett JA. Breast retraction assessment: an objective evaluation of cosmetic results of patients treated conservatively for breast cancer. International Journal of Radiation Oncology, Biology and Physics 1985;11(3):575-8. [DOI] [PubMed] [Google Scholar]
Poggi 2003
- Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98(4):697-702. [DOI] [PubMed] [Google Scholar]
Rainsbury 2007
- Rainsbury R. Surgery insight: oncoplastic breast-conserving reconstruction - indications, benefits, choices and outcomes. Nature Clinical Practice. Oncology 2007;4(11):657-64. [DOI] [PubMed] [Google Scholar]
Raja 1997
- Raja AM, Straker FV, Rainsbury R. Extending the role of breast conserving surgery by immediate volume replacement. British Journal of Surgery 1997;84(1):101-5. [PubMed] [Google Scholar]
Regano 2009
- Regano S, Hernanz F, Ortega E, Redondo-Figuero C, Manuel G. Oncoplastic techniques extend breast-conserving surgery to patients with neoadjuvant chemotherapy response unfit for conventional techniques. World Journal of Surgery 2009;33(10):2082-6. [DOI] [PubMed] [Google Scholar]
RevMan5 [Computer program]
- The Cochrane Collaboration Review Manager (RevMan) Version 5.4. The Cochrane Collaboration, 2020. Available at: revman.cochrane.org.
Rocco 2021
- Rocco N, Catanuto G, Cinquini M, Audretsch W, Benson J, Criscitiello C, et al. Should oncoplastic breast conserving surgery be used for the treatment of early stage breast cancer? Using the GRADE approach for development of clinical recommendations. Breast Surgical Oncology 2021;57:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 1989
- Rosenberg M. Society and the Adolescent Self-Image. Revised edition. Middletown, CT: Wesleyan University Press, 1989. [Google Scholar]
Ryan 2013
- Ryan R. Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Review Group: data synthesis and analysis (2013). cccrg.cochrane.org (accessed 2 June 2020).
Stanton 2001
- Stanton AL, Krishnan L, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer 2001;91(12):2273-81. [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I). BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2018
- Sun L, Legood R, dos-Santos-Silva I, Gaiha SM, Sadique Z. Global treatment costs of breast cancer by stage: a systematic review. PLOS ONE 2018;13(11):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeda 2005
- Takeda M, Ishida T, Ohnuki K, Suzuki A, Kiyohara H, Moriya T, et al. Breast conserving surgery with primary volume replacement using a lateral tissue flap. Breast Cancer 2005;12(1):16-20. [DOI] [PubMed] [Google Scholar]
Van Maaren 2016
- Maaren MC, Munck L, Bock GH, Jobsen JJ, Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncology 2016;17(8):1158-70. [DOI] [PubMed] [Google Scholar]
Vila 2015
- Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤ 40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 2015;24(3):175-81. [DOI] [PubMed] [Google Scholar]
Waljee 2008
- Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. Journal of Clinical Oncology 2008;26(20):3331-7. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Breast Cancer: Prevention and Control. World Health Organization 2010.
Yiannakopoulou 2016
- Yiannakopoulou EC, Mathelin C. Oncoplastic breast conserving surgery and oncological outcome: systematic review. European Journal of Surgical Oncology 2016;42(5):625-30. [DOI] [PubMed] [Google Scholar]
Yoon 2016
- Yoon JJ, Green WR, Kim S, Kearney T, Haffty BG, Eladoumikdachi F, et al. Oncoplastic breast surgery in the setting of breast-conserving therapy: a systematic review. Advances in Radiation Oncology 2016;1(4):205-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaha 2014
- Hisamitsu Z. Partial breast reconstruction for the medial quadrants using the omental flap. Annals of Surgical Oncology 2014;21(10):3358. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nanda 2020
- Oncoplastic breast‐conserving surgery for women with primary breast cancer. The Cochrane Database of Systematic Reviews 2020;6:e. [DOI] [PMC free article] [PubMed] [Google Scholar]





