Abstract
Mesenchymal stromal/stem cells (MSCs) have a great potential to proliferate, undergo multi‐directional differentiation, and exert immunoregulatory effects. There is already much enthusiasm for their therapeutic potentials for respiratory inflammatory diseases. Although the mechanism of MSCs‐based therapy has been well explored, only a few articles have summarized the key advances in this field. We hereby provide a review over the latest progresses made on the MSCs‐based therapies for four types of inflammatory respiratory diseases, including idiopathic pulmonary fibrosis, acute respiratory distress syndrome, chronic obstructive pulmonary disease, and asthma, and the uncovery of their underlying mechanisms from the perspective of biological characteristics and functions. Furthermore, we have also discussed the advantages and disadvantages of the MSCs‐based therapies and prospects for their optimization.
Keywords: acute respiratory distress syndrome, asthma, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, mesenchymal stem cells
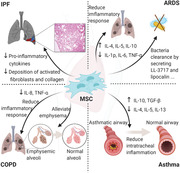
The dominant functions of MSCs in IPF, ARDS, COPD and Asthma.
Abbreviations
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- AT
adipose tissue
- BM
bone marrow
- BM‐MSCs
bone marrow mesenchymal stem cell
- CCR5
CC‐chemokine receptor 5
- CM
computational modeling
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CS
cigarette smoke
- CXCR3
CXC‐chemokine receptor 3
- EPA
eicosapentarnoic acid
- ERK
extracellular signal‐regulated kinase
- EVs
extracellular vesicles
- FasL
Fas ligand
- IA
intraarterial
- IC
intracoronary
- IGF
insulin‐like growth
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- iPSC
induced pluripotent stem cell
- IV
intravenous
- KC
keratinocyte‐derived chemokine
- KGF
keratinocyte growth factor
- MAPK
mitogen‐activated protein kinase
- miRNA
microRNA
- MMP
matrix metalloproteinase
- MSCs
mesenchymal stem cells
- MVs
microvesicles
- NK
natural killer
- OS
overlap syndrome
- OVA
ovalbumin
- SDF‐1
stromal cell‐derived factor‐1
- TNF
tumor necrosis factor
- UC‐MSCs
umbilical cord mesenchymal stem cells
- UCTD
umbilical cord tissue source
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
The respiratory system plays a significant role in gas exchange, immune function, metabolism, and endocrine functions. However, owing to air pollution, smoking, population aging, and other factors, the incidence rate of respiratory diseases, especially respiratory inflammatory diseases, has increased, imposing a great financial burden on both national healthcare systems and citizens.
Respiratory inflammatory diseases include idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and asthma, 1 , 2 , 3 , 4 which are usually characterized by inflammatory cell infiltration, cytokine release, epithelial cell damage, airway remodeling, and pulmonary tissue fibrosis. 5 , 6 , 7 Dysregulation of the inflammatory response in the respiratory system, which is a significant cause of these diseases, is mediated by a complex intercellular interaction with concentration‐dependent regulation of various cytokines (especially inflammatory factors). With increasing cytokine concentrations, especially inflammatory cytokines, a devastating response can negatively affect the respiratory system.
Traditional drug intervention does not exert significant effects on the destructed airway and pulmonary epithelial cells or other pathological damages of the respiratory system caused by the inflammatory response. However, stem cells, especially mesenchymal stem cells (MSCs), have shown great potential in treatments, owing to their capacity to proliferate, undergo multidirectional differentiation, and exert immunoregulatory effects. Several preclinical studies have shown the significant therapeutic effects of systemic or endotracheal MSCs on a number of respiratory inflammation diseases. In some of these diseases, major breakthroughs have been made in the clinical application of stem cell therapy, thereby increasing our understanding of its therapeutic mechanisms and safety evaluation. Not only did Wei Jiang and Erin N Worthington 8 , 9 demonstrated the clinical safety of stem cell therapy, but also showed a statistically significant increase in the cure rate among patients receiving MSCs‐based therapy. As MSCs‐based therapy is being actively explored, the MSC characteristics and functions, which play key roles during the treatment, need to be summarized, and clarified in the context of therapeutic applications of MSCs.
A total of 397 articles, including 83 preclinical studies, and 28 clinical trials that included using MSCs to treatment respiratory immunological disorders, such as IPF, ARDS, COPD, and acute lung injury (ALI), were screened for this review. We summarized the properties and key functions of MSCs to clarify the corresponding treatment mechanism. Finally, we put forward the major challenges to the application of MSCs‐based therapy in respiratory inflammation diseases and further conclude appropriate improvement methods for its expansion.
2. THE CORE MECHANISMS UNDERLYING MSC THERAPY IN RESPIRATORY DISEASES
The properties of MSCs enable them to be used in the treatment of various diseases, including typical inflammatory diseases in the respiratory system. For example, immune compatibility allows MSC transplantation across histocompatibility barriers, which seldom causes immune response. 10 , 11 , 12 Furthermore, like endogenous MSCs, exogenous imported MSCs can migrate to damaged tissues through the SDF‐1‐CXCR4 axis, in which stromal cell‐derived factor‐1 (SDF‐1) produced by the damaged lung tissue can bind to its receptor C‐X‐C motif chemokine receptor 4 (CXCR4) on the MSC to mediate the migration. 4 , 5 Moreover, MSCs can differentiate into type II alveolar epithelial (ATII)‐like cells through the activation of canonical and noncanonical Wnt pathways, thereby promoting the regeneration of damaged lung tissue. 13 , 14 , 15 However, MSC differentiation potential has not been fully studied and, as MSCs can also differentiate into myofibroblasts and exacerbate pulmonary fibrosis under certain experimental conditions, the appropriate culture conditions should be found to allow MSCs to differentiate in the right direction. 16 , 17 , 18 Additionally, the secretome of MSCs plays an important role in tissue regeneration and immunomodulation, which is believed to be the main mechanism by which MSCs can function in lung injury (Figure 1).
FIGURE 1.
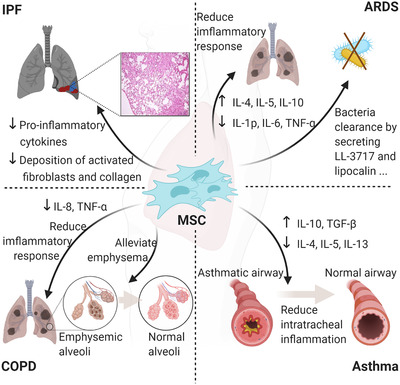
The dominant functions of MSCs in IPF, ARDS, COPD, and asthma
Additionally, MSCs can regulate cell proliferation and control the secretion of various immune cells through cell–cell contact. In this section, we discuss in detail how MSCs perform roles in tissue regeneration and immunomodulation through the MSC secretome and cellular interactions between MSCs and immune cells (Figure 2).
FIGURE 2.
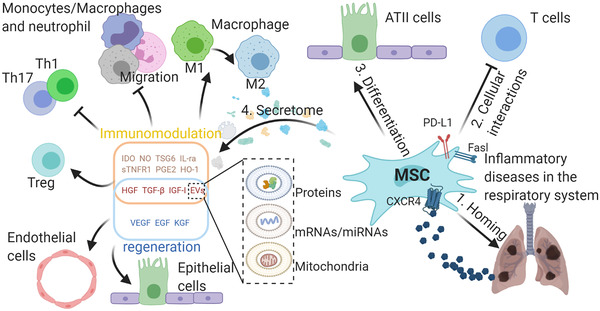
Properties of MSCs in typical inflammatory diseases in respiratory system. Exogenous imported MSCs can migrate to damaged tissues through the SDF‐1‐CXCR4 axis. MSCs can differentiate into ATII‐like cells and inhibit immune cells by cellular interactions and secret a variety of bioactive molecules which have immunomodulation abilities and can promote regeneration of the damaged tissue. The molecules associated with immunomodulation can shift macrophages from M1 to M2 phenotype, inhibit the migration of macrophages, neutrophil, and monocytes, inhibit the differentiation of TH1 and TH17 cells, and promote the formation of Treg cells. The growth factors can protect alveolar epithelial cells and pulmonary vascular endothelial cells from damage. MSCs can transport proteins, mRNAs/miRNAs, and mitochondria to other cells through EVs to exert its functions in immunomodulation and regeneration. The function of EVs is determined by its contents
2.1. MSC secretome
Currently, several studies have found that the paracrine function involving the secretion of soluble molecules and extracellular vesicles (EVs) by MSCs, rather than migration capability, and differentiation potential of MSCs, can promote the regeneration of damaged tissues and confer immunomodulatory capabilities, which may be the main mechanism by which MSCs play a beneficial role.
The soluble molecules produced by MSCs include growth and anti‐inflammatory factors, which mediate most MSC functions in inflammatory diseases of the respiratory system. MSCs can secrete a variety of growth factors to promote the repair of alveolar epithelial cells and pulmonary vascular endothelial cells. Under the stimulation of proinflammatory cytokines, such as tumor necrosis factor‐alpha (TNF‐α) and IL‐1β, the mRNA levels of growth factors produced by MSCs (including HGF, EGF, KGF, and VEGF) increased both in vivo 19 , 20 and in vitro. 21 , 22 , 23 Moreover, the anti‐inflammatory factors produced by MSCs can interact with a variety of immune cell types in lung tissue 8 and, after being stimulated by proinflammatory factors, MSCs produce various anti‐inflammatory factors, such as IDO and TSG6, to relieve inflammation. The exact mechanisms of action of these growth and anti‐inflammatory factors are shown in Table 1.
TABLE 1.
The factors involved in the MSCs‐based therapy for inflammatory diseases in the respiratory system
| Factors | Mechanism analysis | References | |
|---|---|---|---|
| Growth factors | Epidermal growth factor (EGF) |
|
20 , 131 , 208 |
| Keratinocyte growth factor (KGF) |
|
209 , 210 , 211 , 212 | |
| Hepatocyte growth factor (HGF) |
|
8 , 18 , 72 , 74 , 213 , 214 | |
| Vascular endothelial growth factor (VEGF) |
|
74 , 76 , 77 | |
| Anti‐inflammatory factor | Indoleamine 2,3‐dioxygenas (IDO) |
|
39 , 40 , 75 |
| Inducible NO synthase (iNOS) | Produce high concentration of NO and suppress the IL‐2 pathways by inhibiting phosphorylation of signal transducer and activator of transcription 5 (STAT5) thereby resulting in T‐cell proliferation and function inhibition in lung tissue. | 78 , 79 | |
| TNF‐stimulated gene 6 (TSG6) |
|
26 , 36 , 38 , 80 , 81 | |
| Transforming growth factor beta (TGF‐β) |
|
83 , 84 , 85 | |
| Insulin‐like growth factor I (IGF‐I) | Decrease lung inflammation and protect lung tissue by promoting the polarization of macrophages from M1 phenotype to M2 phenotype. | 83 , 164 | |
| Interleukin‐1 receptor antagonist (IL‐1ra) |
|
17 , 42 | |
| Soluble tumor necrosis factor receptor 1 (sTNFR1) | Through the activation of NFκB pathway, MSCs may produce sTNFR1 to decrease a panel of inflammatory cytokines and inflammatory infiltration of macrophages and neutrophils in lung tissue. | 38 | |
| Prostaglandin E2 (PGE2) | Interacts with EP2 and EP4 receptors expressed on the surface of immune cells and exerts its anti‐inflammatory effects by promoting immune cells to express anti‐inflammatory cytokines such as IL‐10 and shift the macrophages into M2 phenotype. | 34 , 35 | |
| Heme oxygenase‐1 (HO‐1) | Relieve lung inflammation by inhibiting the production of proinflammatory cytokines and protect human pulmonary microvascular endothelial cells from oxidative damage. | 86 , 89 |
EVs are naturally occurring, cell‐derived, membrane‐bound spherical structures that are shed or secreted from most cell types under various physiological and pathological conditions. 9 The proteins, RNA (including mRNA and miRNA), and mitochondria contained in the exosomes and microvesicles (MVs) produced by MSCs may explain the beneficial effects of MSCs in typical inflammatory diseases of the respiratory system.
To some degree, the delivered components decide the function of EVs. First, it was indicated that exosomes may function at the protein level, 24 as western blotting detected TSG6 (an anti‐inflammatory factor) in the exosomes secreted by MSCs. The exosomes isolated from TSG6 siRNA‐transfected MSCs lost their ability to reduce lung inflammation and protect tissues from damage in a mouse model of hyperoxia‐induced bronchopulmonary dysplasia. 24 Second, Zhu et al. found that in a mouse model of endotoxin‐induced ALI, inflammation was alleviated, while KGF level was significantly elevated in lung tissue after administration of MSC MVs. After assessing the contents of MVs, there was not enough KGF protein to account for the level found in the lung tissue, indicating that MVs may function at the RNA level, perhaps by transferring KGF mRNA to the injured alveolar epithelium, which subsequently express the protein. 25 Furthermore, microRNAs (miRNAs) are crucial components of EVs. The exosomes secreted by MSCs can prevent hypoxia‐induced pulmonary inflammation, and high expression levels of miRNA‐16 and miRNA‐21 were detected in these exosomes compared with those of the control group. 21 These miRNAs have been shown to mediate the therapeutic effects of exosomes in different models. 22 , 26 Aliotta et al. performed miRNA microarray analysis on exosome‐based miRNAs and some miRNAs associated with anti‐inflammatory and antiproliferative effects, including miRs‐34a,‐122,‐124, and ‐127, were uniquely expressed or upregulated in exosomes secreted by MSCs. 23
MSCs can also transport mitochondria to other cells through EVs. 27 , 28 , 29 MSCs can modulate primary human macrophages through EV‐mediated transfer of functional mitochondria, which enhances macrophage oxidative phosphorylation. In a mouse model of LPS‐induced ALI, modulated macrophages expressed the M2 marker CD206 and reduced TNF‐α production. 29 Mitochondria in EVs produced by MSCs can also be transferred into the alveolar epithelium, increasing ATP production in the epithelium and subsequently stabilizing the epithelium, and reducing lung inflammation. 27
Through mass spectrometry and array analysis, more than 850 unique gene products and more than 150 miRNAs were identified in the cargo of MSC‐derived EVs. 30 Some of them may mediate the protective effects of MSCs, but much remains unknown. Importantly, the content of EVs can change in response to cell activation, such as hypoxia, irradiation, injury, and cellular stress. 30 , 31 Thus, EVs produced under appropriate conditions may have better therapeutic value in cell‐free therapy.
The therapeutic effects of MSCs‐derived exosomes have been evaluated in several completed or ongoing clinical trials. Harrell et al. prepared an inhalation agent from exosome‐derived proteins, including sTNFRI, sTNFRII, IL‐1Ra, and sRAGE. Thirty adult COPD patients received this exosome‐derived protein inhalation agent and the treatment significantly improved their pulmonary status and quality of life. 32 In an ongoing clinical phase I/II trial, 169 ARDS patients have been administered 2.0108, 8.0108, or 16.0108 exosome particles or normal saline per day for 7 consecutive days to test the efficacy of MSC exosomes (NCT04602104). Some other ongoing clinical trials are using exosomes in healthy volunteers to test their safety (NCT04313647). Sengupta et al. tested the efficacy of MSC exosomes in ARDS caused by SARS‐CoV‐2 infection. Reversal of hypoxia, immune reconstitution, and downregulation of cytokine storm was observed in patients who received a single intravenous dose of bone marrow (BM) MSCs‐derived exosomes, with no adverse effects attributable to the treatment. 33 In a randomized double‐blind clinical trial conducted in Russia, COVID‐19 patients received exosomes (0.5×1010‐2.0×1010) or placebo (solution without exosomes). Although this trial was completed in November 2020, the results have not been published yet (NCT04491240).
2.2. Cells regulated by MSCs
Both innate and adaptive immune cells play important roles in the pathogenesis of inflammatory lung diseases. Immune cells, including eosinophils, macrophages, neutrophils, and T lymphocytes, among other immune cells, recruited by chemokines produced by the damaged lung tissue, may contribute to pulmonary inflammation. MSCs can interact with these inflammatory immune cells through their complex paracrine effects and surface molecules to attenuate lung inflammation.
Innate immune cells, including macrophages, neutrophils, dendritic cells, and natural killer cells, can be regulated by MSCs. For example, several factors released by MSCs, such as TSG6 and PGE2, can modulate macrophages, shifting them from a proinflammatory phenotype to an anti‐inflammatory phenotype. 26 , 34 , 35 , 36 , 37 Macrophages can also engulf the EVs produced by MSCs, which contain functional mitochondria that enhance oxidative phosphorylation, thereby enhancing their anti‐inflammatory abilities. 29 MSCs can also inhibit the infiltration of neutrophils in different ways; for example, indoleamine‐2, 3‐dioxygenase (IDO)‐dependent manner has been shown in several preclinical lung disease models. 38 , 39 , 40
By modulating innate immune cells, MSCs can exert indirect regulatory effects on adaptive immune cells. 26 For example, MSCs can switch the mature DCs into a suppressive immature phenotype and promote IL‐10‐positive pDC (plasmacytoid DC) differentiation, which results in the inhibition of effector T‐cells and the formation of Treg cells. 8 , 41 MSCs can secrete a variety of anti‐inflammatory molecules, such as IDO, IL‐1ra, and PD‐L1, to inhibit effector T‐cells directly. 39 , 40 , 42 , 43 , 44 Moreover, proinflammatory cytokine stimulated MSCs can produce ligands for CXC‐chemokine receptor 3 (CXCR3) and CC‐chemokine receptor 5 (CCR5), including CCL5, CXCL9, and CXCL11. These chemokines recruit T cells to the proximity of MSCs and, at the same time, MSCs secrete IDO/iNOS, inhibiting T cells in their vicinity. 26 , 45 By contrast, immunosuppressive factors secreted by MSCs can promote the formation of CD4+CD25+FOXP3+Treg cells (e.g., transforming growth factor‐beta [TGF‐β]) and inhibit the differentiation of Th1 and Th17 cells (e.g., PGE2).
However, MSCs are not always immunosuppressive and do show proinflammatory properties under certain conditions. For example, MSCs can promote the infiltration of monocytes, macrophages, and neutrophils into tumors in a chemokine‐dependent manner. 46 Studies have shown that an inflammatory environment with high levels of interferon gamma (IFNγ) and TNF is crucial for MSCs to exert anti‐inflammatory function. Low levels of IFNγ and TNF can endow MSCs with immunostimulatory potential. 45 This characteristic of MSCs may explain why when MSCs are used to treat pulmonary fibrosis, they are only useful in the early stage of inflammation. However, when fibrosis has already occurred, MSCs cannot relieve inflammation because of the low inflammatory environment. This feature of MSCs emphasizes the necessity of using MSCs for treatment at the right stage. Additionally, for diseases with a low inflammatory environment, 47 pretreatment of MSCs with proinflammatory factors before administration may improve their anti‐inflammatory effects.
3. SYSTEMATIC REVIEW OF MSCS‐BASED THERAPY FOR INFLAMMATION RESPIRATORY DISEASES
In this section, we have reviewed the application of MSCs for the treatment of respiratory inflammatory diseases, including IPF, ARDS, COPD, and asthma, with an emphasis on the significant breakthroughs made in the therapy and advances in our understanding of the mechanisms underlying MSC therapy (Tables 2, 3, 4, 5).
TABLE 2.
The preclinic research of IPF over last decade
| Year | Model | Animal | Cell source | Route | Outcome | References |
|---|---|---|---|---|---|---|
| 2012 | BLM induced | mice | ES | i.v. | Reduced inexpression of ECM and profibrotic genes. | 58 |
| 2014 | BLM induced | mice |
BM‐MSC (STC1 plasmid) |
i.t. | STC1 plasmid transfected to MSCs/STC1 inhalation as promising treatments for IPF | 58 |
| 2015 | BLM induced | mice | BM‐MSC | i.v. | Exhibited fibrosis (MMP‐2) activity, oxidative stress, apoptosis. | 151 |
| 2016 | BLM/orally induced | mice | BM‐MSC | i.v. | Immunomodulation and anti‐fibrotic effect at IPF early stage. | 88 |
| 2018 | BLM induced | mice | BM‐MSC with G‐CSF | i.v. | Promoted BM‐MSCs homing via upregulating CXCR4, increasing antifibrotic effects of BM‐MSCs. | 90 |
| 2018 | BLM induced | mice | BM‐MSC | i.t. | BM‐MSCs from IPF patients are senescent, inducing inflammation and senescence in the neighboring cells | 91 |
TABLE 3.
The preclinic research of ARDS over last decade
| Year | Model | Animal | Cell source | Route | Outcome | References |
|---|---|---|---|---|---|---|
| 2010 | E. coli | mice | hBM‐MSC | i.t. | Improve IL‐37 level and decreased bacteremia and MIP‐2 level | 92 |
| 2010 | BLM induced | rat | BM‐MSC | i.v. | Suppressed inflammation and fibrosis, decreased IL‐6, IL‐10, IL‐1β, JE, TNF‐α, VEGF, TGF‐β | 216 |
| 2010 | CLP induced | mice | BM‐MSC | o.a. | Reduced cytokine levels in sepsis, prevented acute lung injury and organ dysfunction, down‐regulated inflammation (IL‐10, IL‐6) and swiftly up‐regulated phagocytosis and bacterial killing. | 118 |
| 2010 | LPS induced | mice | hBM‐MSC | i.v. or i.t. | ANG‐1 secretion prevented actin stress fiber formation and claudin 18 disorganization through NF‐κB suppression. | 216 |
| 2011 | LPS induced | mice | hBM‐MSC | i.t. | Anti‐inflammatory properties with secreting TSG‐6 | 217 |
| 2011 | E. coli i.t. | mice | hUC‐MSC | i.t. | Attenuated E. coli‐induced ALI primarily by down‐modulating the inflammatory process and enhancing bacterial clearance. | 218 |
| 2011 | LPS induced | mice | hUC‐MSC | i.t. | Ameliorated ALI by diminishing CD4+CD25+ Foxp3+Treg and balancing anti‐ and pro‐inflammatory. | 219 |
| 2012 | CLP induced | mice | BM‐MSC | i.v. | Increased complementary activities in host cell metabolism and inflammatory response. | 220 |
| 2012 | E. coli i.t. | mice | BM‐MSC | i.t. | Enhanced survival and bacterial clearance in Gram‐negative pneumonia | 221 |
| 2012 | LPS induced | rat | hUC‐MSC | i.v. | Increased survival rate and reduced inflammation | 222 |
| 2012 | P. aeruginosa i.p. | mice | hBM‐MSC | i.v. | Increased survival in part by a monocyte‐dependent increase in bacterial phagocytosis | 223 |
| 2012 | hyperoxia | mice | BM‐MSC | i.p. | Produced soluble bioactive factors then regulated pathogenesis of inflammation and fibrosis | 224 |
| 2013 | VIL1 | mice | BM‐MSC | i.t&i.v. | Enhanced recovery via a paracrine mechanism | 119 |
| 2013 | LPS induced | mice | BM‐MSC | i.v. | Improved lung function via inflammatory and remodeling | 225 |
| 2013 | LPS induced | rat | BM‐MSC | i.v. | Improved gas exchanges, alleviated the lung injury, and reduced mortality | 226 |
| 2013 | LPS induced | mice |
hAT‐MSC (IL‐33/IL‐1 RL1) |
i.v. | Reduced protein content, differential neutrophil counts, TNF‐α, IL‐6, and macrophage inflammatory proteins in bronchoalveolar lavage fluid | 227 |
| 2014 | P. aeruginosa | sheep | BM‐MSC | i.t. | Improved oxygenation, decreased pulmonary oedema | 228 |
| 2014 | Hyperoxia | rat | hUC‐MSC | i.t. | VEGF attenuated hypertoxic lung injuries | 229 |
| 2014 | CLP induced | rat |
hBM‐MSC hUC‐MSC |
i.v. | Increased circulating CD3+CD4+CD25+ Treg cells, enhanced suppressive function, and decrease serum levels of IL‐6 and TNF‐α | 230 |
| 2014 | E. coli i.p. | mice | AT‐MSC | Retro‐orbital | Ameliorated immune response with inflammatory cytokines decreasing and IL‐10 increasing, inhibited apoptosis and tissue damage | 231 |
| 2014 | LPS induced | mice | hBM‐MSC | i.v. | Regulated inflammatory response and inhibited lymphocyte proliferation | 232 |
| 2014 | CLP induced | mice |
hUCB‐MSC (pcw poly(I:C)) |
i.v. | Poly(I:C) improved MSCs immunosuppressive abilities | 233 |
| 2015 | CLP induced | mice |
hMens‐MSC (with antibiotics) |
i.t.&i.p. | Enhanced survival by acting on multiples targets | 234 |
| 2015 | E. coli i.t. | rat | hBM‐MSC | i.v. | Decreased injury and reduced bacterial burden potentially via enhanced macrophage phagocytosis and increased alveolar LL‐37 concentrations | 235 |
| 2015 | CLP induced | mice |
hBM‐MSC/murine BM‐MSC |
i.v. | EPC‐EXP+MSC‐HUMAN yielded better lung function and reduced histologic damage | 236 |
| 2015 | VILI | rat | BM‐MSC | i.v. | Diminished injury and restoring lung structure, function | 237 |
| 2015 | E. coli i.t. | mice |
hBM‐MSC/EV‐MSC |
i.t&i.v. | MVs were as effective as the MSCs | 18 |
| 2015 | CLP induced | mice | Demal‐MSC | i.v. | Specific anti‐inflammatory effect on sepsis | 238 |
| 2015 | BLM induced | mice |
BM‐MSC (pc hypoxia) |
i.t. | Hypoxia‐preconditioned MSCs exerted better therapeutic effects and enhanced the survival rate, partially due to the upregulation of hepatocyte growth factor | 239 |
| 2016 | influenza A H5N1 | mice | hBM‐MSC | i.v. | Prevented or reduced injury in vitro and in vivo | 127 |
| 2016 | CLP induced | rat | hWJ‐MSC | i.p. | Klotho protein expression was higher in WJ‐MSCs than in hAD‐MSCs | 112 |
| 2016 | E. coli i.t. | mice |
hBM‐MSC/EV‐MSC |
i.t&i.v. | Innate immune cells enhanced phagocytic activity | 240 |
| 2016 | E. coli i.t. | mice | hUC‐MSC | i.t. | BD2 secreted then ensued acute lung injury through anti‐inflammatory and antibacterial effects | 218 |
| 2016 | LPS induced | rat | BM‐MSC | i.v. | Restored the lung permeability and attenuated lung injury by maintaining VEGF level | 241 |
| 2017 | CLP induced | rat | hUC‐MSC | i.v. | Reduced rodent mortality after induced ARDS‐SS | 242 |
| 2017 | LPS induced | mice | AT‐MSC | Retro‐orbital | Modulated inflammatory response and oxidative damage | 243 |
| 2017 | LPS induced | mice | hMens‐MSC | i.v. | Upregulated PCNA, KGF and down‐regulated caspase‐3, then improved BEAS‐2B cells viability and inhibited LPS‐induced apoptosis | 244 |
| 2017 | LPS induced | mice |
hUC‐MSC (with FTY720) |
i.v. | Showed higher survival and attenuated lung injuries | 245 |
| 2018 | LPS induced | mice | Huc‐MSC (with FTY720) | i.v. | Mitigated LPS‐induced inflammatory lung injury | 246 |
| 2018 | LPS induced | rat | BM‐/AT‐/lung‐derived MSC | i.v. | BM‐MSCs and AT‐MSCs yielded greater effects than lung tissue MSCs | 247 |
| 2018 | LPS induced | rabbit | BM‐MSC | i.t. | Reduced inflammatory cells, pulmonary hemorrhage and edema infiltration | 248 |
| 2019 | HCI/VILI | mice | BM‐MSC | i.t&i.v. | Retained protective effects in small airway epithelial cells | 120 |
| 2018 | LPS induced | mice |
BM‐MSC (oe IL‐10) |
i.t. | IL‐10‐MSCs offered superior protection against LPS‐induced ALI | 249 |
| 2018 | LPS induced | mice |
hAM‐MSC (oe Nrf2) |
i.v. | Nrf2 overexpression reduced lung injury, fibrosis, and inflammation | 250 |
| 2019 | LPS induced | rat | Lung‐derived MSC | i.v. | Upregulated the balance of Tregs and Th17 cells | 251 |
| 2019 | LPS induced | rat |
BM‐MSC (oe HO‐1) |
i.v. | MSCs‐HO‐1 enhanced protective effect | 89 |
| 2019 | HCl/VILI/both | mice |
BM‐MSC (oe HGF/IL‐10) |
i.t. | Effects depending on micro‐environment at the time of administration | 120 |
| 2019 | E. coli i.t. | rabbit |
hUC‐MSC (oe IL‐10) |
i.v. | IL‐10 overexpression in UC‐MSCs enhanced their effects in E. coli pneumosepsis and increased macrophage function | 252 |
| 2019 | LPS induced | mice |
BM‐MSC (kd Lats) |
i.t. | Inhibited Hippo by Lats1 knockdown improved the protective effects against epithelial damage and the therapeutic potential | 253 |
| 2019 | LPS induced | mice |
hBM‐MSC/EVs (pdw serum from injured mice) |
i.v. | EPA‐preconditioned AT‐MSCs yielded further reductions in injury, resulting in greater improvement and higher survival rate | 254 |
| 2019 | CLP induced | mice |
AT‐MSC (pdw EA) |
i.v. | MSCs yielded greater overall improvement in ARDS in comparison to EVs | 255 |
| 2019 | LPS induced | mice |
BM‐MSC (oe p130/E2F4) |
i.t. | Over‐expressing p130 or E2F4 in BM‐MSCs could further improve the injured structure and cells function | 256 |
TABLE 4.
The preclinic research of COPD over last decade
| Year | Model | Animal | Cell source | Route | Outcome | References |
|---|---|---|---|---|---|---|
| 2010 | papain induced | rat | BM‐MSC | i.v | Protected pulmonary emphysema by secretion of reparative growth factors | 77 |
| 2011 | cigarette smoke induced | rat | BMC vs. BM‐MSC | i.v. | BMC better induced AT2 cells and pulmonary vascular endothelial cells proliferation; BM‐MSC and BMC both alleviate emphysema | 200 |
| 2013 | cigarette smoke induced | rat | BM‐MSC | i.t. | Down‐regulated COX2 and E‐2 by p38 and ERK MAP kinase activity inhibition in macrophages | 74 |
| 2014 | elastase or cigarette smoke induced | mice |
BM‐MSC, AT‐MSC, LT‐MSC |
i.v. i.t. |
Decreased keratinocyte‐derived chemokine and TGF‐β levels in all sources, i.v. with better cardiovascular function and phenotype change from M1 to M2. | 10 |
| 2015 | elastase or cigarette smoke induced | mice | hAT‐MSC | i.v. | AT‐MSC decreased mean linear intercept and reduced caspase‐3 activity | 257 |
| 2016 | elastase induced | mice | hBM‐MSC | i.v. | Reduced fibrosis, reduced inflammation | 258 |
| 2016 | cigarette smoke induced | mice |
hBM‐MSC, UCB‐MSC |
i.v. | Reduced inflammation, increased regeneration | 259 |
| 2017 | cigarette smoke induced | mice |
hBM‐MSC/Ipsc |
i.v. |
Reduced inflammation |
260 |
| 2018 | elastase induced | mice | LT‐MSC | i.t. | Activated HGF/c‐Met system, by promoting survival and proliferation of alveolar epithelial cells | 261 |
| 2018 | elastase induced | mice | BM‐MSC | i.t. | Attenuated pulmonary arterial hypertension | 262 |
| 2019 | elastase induced | mice | hWJ | i.v. | Promoted regenerative effect, pathomechanism not investigated | 263 |
| 2019 | overlap syndrome (OS) | rat | BM‐MSC | i.v. | Inhibited emphysema progression by differentiating into endotheliocytes, suppressing the apoptosis of endotheliocytes and oxidative stress | 264 |
TABLE 5.
The preclinic research of Asthma over last decade
| Year | Model | Animal | Cell source | Route | Outcome | References |
|---|---|---|---|---|---|---|
| 2010 | ragweed induced | mice | BM‐MSC | i.v. | BM MSC suppressed Th2‐driven allergic responses by TFG‐ β production | 84 |
| 2012 | ovalbumin induced | mice | h iPSC vs. BM‐MSC | i.v. | iPSC‐MSC owned same therapeutic effect as BM MSC | 265 |
| 2015 | house dust mite induced | mice | BM‐MSC | i.v. | Reduced eosinophilia, Th2 response and activated dendritic cells | 266 |
| 2015 | ovalbumin induced | mice | BM‐MSC | i.v. | Reduced lymphocytes and eosinophils attraction, suppressed lung dendritic cell maturation and Th2 responses | 267 |
| 2016 | ovalbumin induced | mice | BM‐MSC | i.v. | Reduced neutrophils and eosinophils recruitment, goblet cell hyperplasia and lung fibrosis | 268 |
| 2016 | house dust mite induced | mice | BM‐MSC | i.v. | Expressed high levels of COXZ (MSCs), IL‐10 and TGF‐β (M2 macrophages) and low level of IL‐6 | 269 |
| 2017 | house dust mite induced | mice | BM‐MSC | i.t. | Reduced inflammation, remodeling, and improved lung functions | 135 |
| 2017 | ovalbumin induced | mice | hUCB‐MSC | i.v. | Reduced allergic inflammation which mediated by regulatory I cells | 270 |
| 2018 | ovalbumin induced | mice | BM‐MSC vs. AT‐MSC | i.t. | Therapeutic efficiency only after BM‐MSC treated | 136 |
| 2018 | ovalbumin induced | rat | hPD‐MSC | i.v. | Shifted from Notch‐1, ‐2 and jagged‐1 to Notch‐3, ‐4 and delta‐like ligand‐4 signaling | 271 |
| 2018 | ovalbumin induced | mice | mASC‐ | i.t. | Alleviated airway inflammation, improved airway remodeling, and relieved airway hyper‐responsiveness with the restoration of Th1/Th2 cell balance | 16 |
| 2019 | ovalbumin induced | mice | BM‐MSC | i.v. | Simvastatin and BMSCs affected serum IgE, lung IL‐13 and TGF‐β levels more than BMSC. Simvastatin increased BMSCs migration into the lung tissue | 272 |
| 2019 | Th2‐mediated inflammation induced | rats | rBM‐MSC | i.t. | Ameliorated pathological changes presumably by targeting ICAM‐1 and VCAM‐1 | 13 |
3.1. Idiopathic pulmonary fibrosis
IPF is an unexplained and chronic progressive pulmonary fibrosis disease, 48 predominantly occurring in middle‐aged and elderly men with long‐term smoking history. 49 Moreover, its prevalence and mortality has been steadily yearly, 50 and its etiology and clinical manifestations remain unclear. 51 As a significant pathological feature, it causes diffuse alveolar inflammation, characterized by fibroblast proliferation and transformation in the lungs, extracellular matrix expansion, and extensive lung parenchyma refactor. 52 , 53 Pulmonary fibrosis is closely related to immune disorders. Inflammatory stimulation leads to the proliferation of inflammatory cells, releases a large number of cytokines, such as TNF‐α, platelet‐derived growth factor (PDGF), TGF‐β, insulin‐like growth factor 1 (IGF‐1), interleukin (IL)‐4, ET‐1, and connective tissue growth factor, and then stimulates fibroblast pathogenesis and collagen accumulation. 54 As the mechanism is being gradually unraveled, the IPF model construction has constantly improved and the application scope of stem cell therapy in IPF has expanded.
Regarding preclinical research, the bleomycin‐induced fibrosis model is most commonly applied to evaluate the potential therapeutic effect of MSCs on IPF, 55 showing MSC treatment to have a remarkable effect (Table 2). Intravenous (IV) injection of human BM MSCs is more effective than traditional drugs in improving bleomycin‐induced pulmonary fibrosis and the feasibility of combination therapy. 56 Furthermore, administration of BM‐ or umbilical cord (UC)‐MSCs early in the initial inflammatory phase can have a protective effect, reducing the levels of proinflammatory cytokines and deposition of activated fibroblasts and collagen, while improving epithelial repair. Moreover, several well‐designed clinical trials of MSC treatment for IPF patients have been steadily carried out. The most relevant research projects focused on allogeneic BM‐MSCs, 57 and placental‐derived MSCs 58 have also been used in clinical trials. Only minor adverse events have been reported, and there is no evidence of worsening fibrosis. 58 In addition, high‐dose MSCs are safe for IPF treatment and can delay disease progression to a certain extent. 59
3.2. ARDS
ARDS is an acute progressive respiratory failure caused by changes in lung tissue structure owing to various factors. It can be caused by infectious factors, such as bacteria, viral infections, and noninfectious factors, such as inhalation of toxic gases and massive blood transfusions. Severe cases can further develop non‐ARDS. The mortality rate can be as high as 30−40%, and morbidity and mortality increase with age. 60 , 61 ALI can cause alveolar epithelium and vascular endothelial cell damage, as well as increased pulmonary vascular permeability. The main clinical manifestations are low oxygenation, low lung compliance, and high physiological dead space. Its pathogenesis is complicated and the currently recognized mechanism involves various causes leading to increased production of proinflammatory factors in the lungs, followed by massive release and activation of inflammatory factors drive the inflammation in the lungs out of control. 62 Whether the inflammatory response subsides effectively or not is key in the treatment of ARDS patients. Current treatment mainly focuses on the regulation of inflammatory response by stimulating nuclear factor‐kappa B and inhibiting the glucocorticoid receptor‐mediated signal transduction cascade.
Regarding preclinical research, the experimental results obtained by different models have their own advantages and disadvantages, but all have shown the advantages of MSC therapy in ARDS treatment (Table 3). Methods used in these models mainly include treatment with lipopolysaccharide/cefpodoxmine proxetil (CLP)/hypoxia pretreatment and live virus infection, whereas in vivo models have been based on animals, including mice and rats (small animal‐based models), and sheep 63 and pigs, 64 among others(large animal‐based models). Although the underlying mechanism remains unclear, results have shown that MSCs can reduce inflammation significantly and restore lung functions.
To date, 13 clinical trials of MSC therapy in ARDS patients have been registered. Although all of them are in the early phase and are limited by small sample size, they still successfully assessed the safety of MSC administration and treatment efficiency in clinical outcomes, for example, respiratory and systemic parameters, inflammation, and hemodynamics. Several recent clinical studies have confirmed that MSC injection relieves ARDS symptoms in patients with COVID‐19. 65 , 66 However, as most experimental studies, these clinical investigations present substantial heterogeneity concerning inclusion and exclusion criteria, follow‐up length, MSC dose, source, route of administration, and treatment frequency. 67
3.3. Chronic obstructive pulmonary disease
COPD is one of the most common chronic lung diseases in humans. Its high prevalence is one of the leading causes of morbidity and mortality worldwide. COPD is characterized by persistent respiratory symptoms and airflow obstruction, which is caused by chronic bronchitis and destruction of the lung parenchyma (emphysema). Changes in the function of immune cells infiltrating the lungs, oxidative stress, and imbalance in the activity of proteases and their inhibitors are the main causes of major pathological changes in COPD patients. 68
For preclinical research on COPD, a COPD rat/mouse model induced by elastase or cigarette smoke (CS) is most commonly used. These studies have shown that MSCs can alleviate emphysema and inflammation symptoms and have a good therapeutic effect in experimental COPD (Table 4). MSCs from different sources can reduce the mean linear intercept, neutrophil infiltration, and cell apoptosis; increase elastic fiber content, reduce alveolar epithelial and endothelial cell damage, and decrease keratinocyte‐derived chemokine, that is, a mouse analog of IL‐8, and TGF‐β levels in lung tissue. 10 , 69
In terms of alleviating emphysema, the role of MSCs has been mainly reflected in three aspects: reducing the damage of lung tissue, promoting the transformation of macrophages toward the M2 phenotype, and promoting the transfer of mitochondria to airway epithelial cells. In addition, preclinical studies have highlighted that different MSC sources and administration routes can reduce elastase‐induced lung injury to different degrees.
In recent years, clinical trials have shown that the combination of endobronchial valve insertion and intrabronchial stromal cells (i.e., MSCs) is relatively safe for treating critically ill patients, providing support for MSC therapy as a companion therapy. 11 A phase I pilot clinical study showed that systemic MSC infusion may help reduce inflammation in patients with COPD. 12 In a subsequent phase 1 and 2 clinical study, four doses of umbilical cord tissue source‐MSC treatment were shown to significantly alleviate the severity of COPD symptoms. 18
3.4. Asthma
Asthma is a chronic inflammatory disease of the airways caused by endogenous (such as chronic inflammation and oxidative stress) and exogenous factors (such as exposure to CS and air pollution), involving multiple cells and cellular components. 70 The pathological process of asthma mainly involves lung structural cells and effector cells. Effector cells can secrete inflammatory factors that cause inflammation, mediate peripheral tissue destruction, induce high secretion of airway mucus, hypertrophy of smooth muscle, and increase of goblet cell levels. Asthma mainly features airway inflammation, hyper‐responsiveness, and remodeling. 71 Repeated damage and repair of airway and lung tissue accelerate the process of airway remodeling, which will cause continuous damage and inflammation of the airway, aggravating asthma.
In preclinical research on asthma, asthmatic rats/mouse models induced by ovalbumin are generally used. These studies have shown that therapy based on MSCs has a good effect in experimental asthma models (Table 5). In the treatment of asthma, MSCs have been shown to revert pathological changes and intratracheal inflammation. 13 In asthmatic animals, MSCs can effectively reduce pathological changes observed in lungs, such as basement membrane epithelial thickness, subepithelial smooth muscle thickness, and increase goblet cell number, to reduce airway inflammation, hyper‐responsiveness, and remodeling, thereby improving lung function and showing therapeutic potential for use in clinical settings. 14 , 15 , 16 , 17
3.5. Autoimmune lung diseases
Autoimmunity is a misdirected immune response in which the immune system attacks its own body. Systemic autoimmune diseases, such as systemic lupus erythematosus, systemic sclerosis, Crohn's disease, and rheumatoid arthritis, are often complicated by autoimmune lung injury like interstitial lung disease (ILD), causing fibrosis and inflammation in the lung parenchyma. 18 , 72 These autoimmune diseases usually occur in genetically susceptible individuals and thus, external antigens could trigger a misdirected immune response in lungs or other organs. 73 Supporting this, Xiao‐Jun Guan et al. 74 observed that the presence of ILD is related to human leukocyte antigen class II HLA‐DRB1*03‐DQA1*05‐DQB1*02 but not anti‐Jo‐1 auto‐antibodies or myositis subtype for myositis patients. Although immunosuppressive and anti‐inflammatory drugs like corticosteroids and therapy for modulating cytokines, such as 1L‐1β, have been widely used, there is a lack of effective treatment for autoimmune lung diseases.
MSCs‐based therapy appears to be a potential approach to treat lung disorders. Liu et al. 75 defined the immunopathology involved in lung exacerbation during autoimmunity and determined the role of MSCs in reversing the pathological changes associated with these disorders. They found that the addition of MSCs isolated from BM of normal individuals (HBMSCs) increased the number of regulatory T cells and concomitantly reduced the cytotoxic T cell count. Moreover, HBMSCs also decreased proinflammatory chemokine/cytokine secretion, which would help to block IPF progression. Due to the immunomodulatory and regenerative properties of MSCs, their clinical efficacy and safety has been tested in a range of autoimmune disorders. Currently, over 49 ongoing trials are evaluating the efficacy of MSCs in autoimmune diseases. 76 Generally, no unwanted side effects are observed in MSC‐treated patients and 7 of the 11 trials have demonstrated the potent immunomodulatory effects of MSCs. 77
3.6. Lung cancer
Lung cancer is a malignant tumor of lung tissues characterized by uncontrolled cell growth. Globally, 1.8 million people are diagnosed with lung cancer and 1.6 million die of the disease every year. Despite commendable progress in cancer detection and therapy, prognosis of patients with lung cancer remains poor. 78 MSCs‐based therapy has been recognized as a promising approach to treat lung cancer. The antitumor capacity of MSCs has been proven effective in different tumors, including lung cancer. 38 , 79 , 80 , 81 Furthermore, MSCs have the ability to migrate to both primary tumors and metastatic sites, which helps them exert effective tumor‐suppressing effects. 82 , 83
MSCs have been found to have diverse effects on tumor cells. Some studies have reported contrasting effects of MSCs with pro and antitumor properties. For example, Nemeth et al. 84 reported contradictory effects of human MSCs (hMSCs) on tumor growth. They found that hMSCs and hMSC‐conditioned media (CM) reversed the invasion and proliferation of human A549 lung adenocarcinoma and Eca‐109 esophageal cancer cells and induced apoptosis. However, they also observed that hMSCs promoted tumor formation by enhancing vessel formation. Moreover, human UC MSC‐CM can promote epithelial‐mesenchymal transition (EMT), migration, and invasion, but also inhibited proliferation and promoted apoptosis of lung cancer cells at the same time. 85 This study showed that the contrasting effects of MSCs are mediated by exosomes. Therefore, tumor‐promoting effects can be inhibited by blocking the release of exosomes. Besides, knockdown of TGF‐β1 expression in MSCs can reverse the EMT and enhance the antiproliferative and proapoptotic effects. Collectively, these observations suggest MSCs to be a potentially safe therapy for lung cancer. 85
The inherent property of MSCs to migrate to tumor sites makes them suitable to deliver anticancer drugs. 86 Several studies have provided evidence that MSCs can be used to deliver antitumor drugs, such as PTX 87 and DOX, 88 as well as immunomodulatory factors like IL‐12, 89 IL‐24, 90 IFN‐β, 91 IFN‐γ, 92 TRAIL 91 to target cells. Hence, modified MSCs can target tumors and accumulate in neoplastic tissues, thereby inhibiting the tumor growth and lung tumor metastasis. Based on the results of the preclinical studies, an ongoing phase I/II clinical trial is evaluating the antitumor efficacy and safety of TRAIL‐expressing MSCs with the combination of cisplatin and pemetrexed in NSCLC (ClnicalTrial.gov 76 Identifier: NCT03298763).
Notably, administered MSCs also show bi‐directional effects on the treatment of lung cancer. Therefore, there is a need to be cautious about the prospects of MSCs‐based therapy for lung cancer. It is necessary to understand the biology of different types of MSCs and their function in various tumor environments to reduce the adverse effects while carefully harnessing their beneficial effects.
3.7. The H7N9 avian influenza
In the spring of 2013, a novel avian‐origin influenza A (H7N9) virus appearednd spread in China. H7N9 viruses, the product of reassortment of viruses that are of avian origin, can quickly spread between mammalian hosts 93 and confer the risk of human‐to‐human transmission. 94 , 95 The novel H7N9 virus binds to host cell receptors through its surface hemagglutinin to infect cells, but the key molecular basis of virus transmission has not yet been fully elucidated. 96 The novel H7N9 virus can cause severe illness, including acute pneumonia and ARDS, it can also cause the extrapulmonary diseases, including rhabdomyolysis and encephalopathy, through cytokine storms. 97 , 98
In terms of treatment, a universal influenza vaccine is not available, though several influenza types ‐, subtype‐, and strain‐specific vaccines are available and provide protection. 99 Antiviral therapy cannot improve the survival rate of severely patients, such as ARDS patients caused by H7N9. A study has shown 65 that MSC therapy is a safe and effective therapy for patients with severe lung diseases induced by H7N9, and can significantly improve lung injury and fibrosis caused by ARDs. MSCs have the ability to reduce inflammatory effects and defend against cytokine storm. Its good adjuvant treatment effect may be related to the significant reduction of procalcitonin, serum creatinine, and creatine kinase levels in patients. In order to understand the potential of MSCs for treating H7N9‐induced ARDS, further elucidation of the pathogenic mechanism of H7N9 is still needed.
3.8. The COVID‐19
SARS‐CoV‐2 virus is a new coronavirus that has a 5% genetic association with SARS and is a subset of Sarbecovirus. 100 And the name “COVID‐19,″ given by the World Health Organization (WHO), refers to the disease (particularly the pneumonia) associated with the SARS‐CoV‐2 virus. Several studies have shown that Angiotensin‐converting enzyme 2 (ACE2) is the main host cell receptor for SARS‐CoV‐2 entry. 101 , 102 The cellular protease TMRRSS2 is also necessary for the entry of coronavirus into host cells, by which the virus use to prime the S protein. 103 Many human cells, especially alveolar type 2 (AT2) and capillary epithelium, are angiotensin I converting enzyme 2 (ACE‐2) receptor‐positive cells. 103 This is why the respiratory system is the main system through which viruses infect the human body.The inflammatory response caused by SARS‐CoV‐2 infection is the main mechanism for the body to eliminate the virus, while is also the main mechanism for SARS‐CoV‐2 to cause tissue damage and dysfunction to the body and the severity of the response is proportional to the expression of ACE‐2. 104 , 105 The invasion of the virus will cause the secretion of a variety of inflammatory factors, including IL‐2, IL‐6, IL‐7, IL‐17, TNF‐α, and INF‐γ. Cytokine‐induced storm results in pulmonary edema, dysfunction of air‐exchange, ARDS, even acute cardiac injury, and leading to death. 106 , 107
Aiming at the pathogenic mechanism of SARS‐CoV‐2, MSCs possess the potential to treat COVID‐19 as a cell therapy. As mentioned, following the COVID‐19, the patient's immune system will produce an excessive immune response, causing tissue damage. And MSC therapy can revert the complex cytokine storm, which reduces the level of many inflammatory factors including TNF‐α 108 and promotes the endogenous repair of damaged tissues through its stem cell repair properties. 26 , 109 Specifically, MSCs could recover the pulmonary microenvironment, protect alveolar epithelial cells, inhibit the process of pulmonary fibrosis, and cure lung dysfunction and COVID‐19 pneumonia. 110 There have been many case reports and clinical studies confirming that MSCs are safe and effective for the treatment of COVID‐19 patients, especially acute patients, to improve their clinical symptoms and immune function levels. 108 , 110 , 111 Shi Land his colleagues have performed a phase 2 trial that proved the efficacy and safety of human UC‐MSCs for severe COVID‐19 patients’ treatment. 112 However, current research has also highlighted many urgent problems to be solved, which include but are not limited lacking unified source of MSCs, dose, and dosing strategies, such as the number and timing of administrations.
4. DISCUSSION
4.1. Strengths and weaknesses of MSCs‐based therapy
In the last 20 years, several immunomodulatory therapies have been investigated for inflammatory diseases in the respiratory system, although only a few have progressed to clinical application. Several immunomodulation‐based therapies, such as mechanical nebulization, and drug treatment have weak treatment effects combined with side effects, such as diabetes, cataracts, gastrointestinal toxicity, and growth impairment. 113 , 114 , 115 MSCs‐based therapy is considered a promising treatment for acute and chronic respiratory diseases. Thus, the application of MSCs‐based therapy is expected to reduce side effects and improve therapeutic effect. 115
MSCs‐based therapy has obvious advantages. From a therapeutic point of view, the unique advantages of MSCs‐based therapy include the homing, migration, and regenerative properties of MSCs, which allow them to be automatically recruited to the injury site and replace damaged tissue through differentiation and regeneration when systemically administering drugs to patients. 116 , 117 Additionally, MSCs can regulate the inflammation of lung tissues by stimulating the reduction of proinflammatory marker cytokines and increasing anti‐inflammatory ones to restore the balance of cytokine network. 118 , 119
In ALI, lung injury, fibrosis, and inflammatory reactions usually occur jointly. Treatment with modified MSCs can reduce the characteristic lung injury, fibrosis, and inflammation. As these injures are usually interrelated, inhibition of one pathway may affect another, which is also a strength of MSCs. 120 , 121 , 122 MSCs‐based therapy has been proven effective in multiple lung injury models, such as acid injury, E. coli endotoxin‐induced ALI, ventilator‐induced lung injury, hypoxia‐induced lung injury, systemic sepsis, and respiratory virus infection. 120 , 123 , 124 , 125 , 126 , 127 , 128 From a management point of view, owing to its inherent low immunogenicity and limited tumorgenicity risk and ease of management, MSCs can be easily isolated and expanded in culture, 129 , 130 promoting their clinical application.
However, the efficacy of MSCs‐based therapies varies significantly, which may be attributed to significant differences in protein profile of the lung microenvironment during different stages of lung cancer. The lung microenvironment determines the beneficial and harmful effects of MSCs. For example, when the IL‐6 and fibronectin content is high and the antioxidant capacity is low, using MSCs may lead to adverse results. 120 Therefore, it is necessary to check the adaptability and contraindications before administration of MSCs. For chronic diseases, such as asthma, IPF, and COPD, MSCs have shown anti‐inflammatory effects and repair function in animal‐based experiments. 26 , 131 , 132 , 133 Clinical studies have also reported their safety and few adverse effects, as well as improvement of the clinical condition and quality of life in such patients. 132 , 133 , 134 However, the MSC perfusion cannot significantly improve lung function and quality of life in patients with moderate to severe disease. 117 In some diseases, owing to the low level of specific biomolecules and growth factors, MSCs exert a weaker inhibitory effect on the remodeling process, which is more apparent in asthma. 135 , 136 This also implies the importance of screening patients for assessing their responsiveness to MSC treatment before metastasis. 137
However, despite the commendable advances made in the field of MSC therapy, there are several obstacles in the clinical application of MSCs related to the cell preparation, fitness, and functionality of MSCs, as these characteristics are significantly affected by their source, cell culture method, expansion level, dosage of administration, storage, and transportation conditions. 138 , 139 Transplanted MSCs have a low survival rate in the recipient tissue, which may be mainly affected by the culture conditions or may cause cell death owing to the lack of proper interconnection, which restricts their application. 140 Meanwhile, as the mechanism of action of MSCs has not been elucidated and knowledge of immunobiology is not yet perfect, the results of clinical trials often cause controversy. 120 , 137 In addition, the long‐term benefits of MSCs are currently unclear, and results are highly dependent on differences among patients. 141 These uncertainties hinder the clinical application of MSCs.
4.2. Perspectives on current research in MSC‐based therapy
4.2.1. Preclinical trials
Several preclinical studies based on MSCs have demonstrated the enormous potential of MSCs as treatment for acute respiratory diseases, such as ALI, and chronic respiratory diseases, such as asthma, COPDs, and IPF. 26 , 131 , 133 , 142 In these studies, the choice of source and animal model has a significant influence on the experimental results. In addition to efficacy, the safety issue is also key to determine if the method can be clinically used on a large scale. Therefore, in this section, we will discuss the sources, animal model, and safety in preclinical trials.
4.2.2. Sources
MSCs can be obtained from several sources, such as BM‐MSC, AT‐MSC, UC‐MSC, menstrual blood (MB‐MSC), and UC tissue, that is, Wharton's Jelly‐MSC, and each MSC type has its own characteristics. For instance, BM‐MSCs have excellent properties, and are currently the most widely used MSCs. 143 However, they require invasive acquisition and have relatively limited applicability. Meanwhile, AT‐MSCs are also promising for anti‐inflammatory and regeneration purposes, 144 and these cells are easily obtained. Importantly, AT‐MSCs can achieve auto‐transplantation. 145 UC‐MSCs‐based therapy has no risk for ethical issues and is isolated in a noninvasive method. Regarding physiological properties, UC‐MSCs show lower immune rejection and significantly higher growth kinetics and clonal index than those of other MSCs. 146 , 147
As mentioned earlier, MSCs often cause controversy in clinical trial results, which have a certain degree of relationship with the MSC source. Although all MSCs have similar general characteristics, cells from different sources may show significant differences in anti‐inflammatory or regenerative potential, depending on the specific injury at a specific location. 148 Several studies have analyzed the differences among cells from different sources regarding their mechanisms, 146 , 149 , 150 but clinical data are still lacking. Therefore, there is an urgent need to evaluate the therapeutic effects of MSCs from different sources for different diseases. For example, Antunes et al. 10 tested the effects of three MSC sources and different administration routes on lung inflammation and remodeling in experimental emphysema. They found that MSCs from different sources variably improved elastase‐induced lung damage, but only IV administration of BM‐MSCs resulted in better cardiovascular function. Additional preclinical and clinical research on MSCs from different sources will help us standardize the research procedure and treat them symptomatically.
In addition, age and health issues of MSC donors also need to be considered. For example, AT‐MSCs from young mice rather than old mice can prevent bleomycin‐induced pulmonary fibrosis in old mice. 151
4.2.3. Safety
Manoj M Lalu et al. 152 used meta‐analysis to evaluate immediate adverse reactions, including acute injection toxicity, fever, and adverse events in multiple organ systems, to first‐generation MSC product clinical trial security, and showed acceptable safety of MSC‐based therapy. However, the mechanism of action and clinical efficacy of MSC‐based therapy is still poorly understood, 138 , 153 , 154 and safety studies need to be precise at the disease level. In vitro experiments have observed genetic instability in the passage of MSCs, 155 , 156 which has also caused concern about the risk of malignant proliferation and differentiation of MSCs. Therefore, we need high‐resolution genetic analysis to determine the possibility of MSC transformation after long‐term cultivation, including single‐nucleotide polymorphism, copy number variation, and insertion/deletion changes. It is also necessary to consider mutations without survival and growth advantages, which may be difficult to detect. 157 Although MSC‐based therapy is generally safe, large‐scale animal experiments and clinical experiments are still needed to tackle the safety issues of MSCs‐based therapy.
4.2.4. Animal model
Our understanding of the mechanisms of action of MSCs mainly comes from animal models represented by mice. Although several peer‐reviewed scientific reports have demonstrated the adoptive transfer of MSCs in preclinical mouse disease models, MSCs impact on mouse outcomes has not yet been converted to an equivalent in human phase III clinical trials. The inconsistency between mouse experimental results and human clinical results may be explained by (1) immunocompatibility, (2) administration, and (3) significant fitness differences in cultured adapted MSCs. 138 Whether the mouse models themselves can effectively and accurately fit the disease model also remains a problem. For example, bleomycin‐induced pulmonary fibrosis is still considered to be the best animal model available in preclinical testing for IPF. 158 , 159 However, the treatment given in the first 7 days after exposure to bleomycin may work mainly by preventing the inflammatory cascade rather than reversing fibrosis, thereby limiting its applicability to human IPF. 160 In addition, current research lacks an animal model that can represent chronic IPF, 158 , 161 which adds additional obstacles to IPF research. Moreover, the mice modeling method brings uncertainty to the experimental results. For example, lung injury has several models, such as the acid injury model, E. coli endotoxin‐induced, ventilator‐induced, and hypoxia‐induced lung injury model, systemic sepsis model, and respiratory virus infection model. 120 , 123 , 124 , 125 , 126 , 127 , 162 The performance of MSCs‐based therapies differs among these models, indicating that a single animal model cannot be used to represent a class of diseases. Thus, animal models need to be further standardized to the disease, with as much detail as possible, that is, we need more accurate and standardized mouse models to help us build acute and chronic disease models with different stages and different pathogenic factors, and tightly integrate animal models with clinical experiments to improve preclinical studies.
In the past decade, the exploration of MSC‐based therapies has gradually evolved into a standardized development, and an increasing number of preclinical research results have advanced to clinical research. Therefore, it is particularly critical to reassess the safety and effectiveness of preclinical studies of MSCs‐based therapy through regulatory means, 153 , 163 considering details, such as cell expansion conditions, medium composition, cell treatment protocols, administration dosage, MSC source, and animal model.
4.2.5. Clinical trials
As MSCs‐based therapies have made considerable breakthroughs in preclinical research, several of them have progressed to clinical studies with more than 800 registered clinical studies being conducted on MSCs‐based treatment approaches worldwide, 153 according to Clinical Trails Data Bank at the National Institutes of Health clinical trials (Table 6).
TABLE 6.
The clinic trials of inflammatory diseases in the respiratory system over last decade
| Diseases | Time | Cell source | Study | Route | Status | Outcome | NCT number |
|---|---|---|---|---|---|---|---|
| IPF | 2013 | PD‐ MSC |
A Study to Evaluate the Potential Role of Mesenchymal Stem Cells in the Treatment of Idiopathic Pulmonary Fibrosis (MSC in IPF) |
i.v. | Completed | No severe negative effect | NCT01385644 |
| 2014 | AT‐MSC | Evaluate Safety and Efficacy of Intravenous Autologous AT‐MSCs for Treatment of Idiopathic Pulmonary Fibrosis | i.v. | UN | NYR | NCT02135380 | |
| 2017 | BM‐MSC | Role of Stem Cell Therapy in Interstitial Pulmonary Fibrosis | i.v. | Completed | NYR | NCT03187431 | |
| 2018 | BM‐MSC | Safety and Efficacy of Allogeneic Mesenchymal Stem Cells in Patients With Rapidly Progressive Interstitial Lung Disease | i.v. | NYR | NYR | NCT02594839 | |
| 2018 | BM‐MSC | Study of Autologous Mesenchymal Stem Cells to Treat Idiopathic Pulmonary Fibrosis (CMM/FPI) | v.FOB | UN | NYR | NCT01919827 | |
| 2019 | UC‐MSC | A Study on Radiation‐induced Pulmonary Fibrosis Treated With Clinical Grade Umbilical Cord Mesenchymal Stem Cells | v.FOB | Completed | NYR | NCT02277145 | |
| ALI | 2014 | AT‐MSC | Adipose‐derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome | i.v. | UN | NYR | NCT01902082 |
| 2014 | Mens‐MSC | Using Human Menstrual Blood Cells to Treat Acute Lung Injury Caused by H7N9 Bird Flu Virus Infection | i.v. | ongoing | NYR | NCT02095444 | |
| 2014 | BM‐MSC | Treatment of Severe Acute Respiratory Distress Syndrome With Allogeneic Bone Marrow‐derived Mesenchymal Stromal Cells | NR | UN | NYR | NCT02215811 | |
| 2015 | UC‐MSC | Human Umbilical‐Cord‐Derived Mesenchymal Stem Cell Therapy in Acute Lung Injury (UCMSC‐ALI) | i.v. | UN | NYR | NCT02444455 | |
| 2015 | BM‐MSC | Human Mesenchymal Stem Cells For Acute Respiratory Distress Syndrome (START) | i.v. | completed | Ref | NCT01775774 | |
| 2016 | BM‐MSC | Mesenchymal Stem Cell in Patients With Acute Severe Respiratory Failure (STELLAR) | i.v. | UN | NYR | NCT02112500 | |
| 2019 | BM‐MSC | Human Mesenchymal Stromal Cells For Acute Respiratory Distress Syndrome (START) (START) | i.v. | completed | Ref | NCT02097641 | |
| 2019 | Multiple stem cells | A Phase 1/2 Study to Assess MultiStem® Therapy in Acute Respiratory Distress Syndrome (MUST‐ARDS) | NR | completed | NYR | NCT02611609 | |
| 2019 | UC‐MSC | Mesenchymal Stem Cells for Multiple Organ Dysfuntion Syndrome After Surgical Repair of Acute Type A Aortic Dissection | i.v. | recruiting | NYR | NCT03552848 | |
| 2019 | UC‐MSC | Human Umbilical Cord Mesenchymal Stem Cells (MSCs) Therapy in ARDS (ARDS) | i.v. | recruiting | NYR | NCT03608592 | |
| 2020 | BM‐MSC | Mesenchymal Stromal Cells For Acute Respiratory Distress Syndrome (STAT) | i.v. | recruiting | NYR | NCT03818854 | |
| 2020 | BM‐MSC | Mesenchymal Stem Cells (MSCs) for Treatment of Acute Respiratory Distress Syndrome (ARD) in Patients With Malignancies | i.v. | completed | NYR | NCT02804945 | |
| 2020 | UC‐MSC | Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID‐19) (REALIST) | i.v. | recruiting | NYR | NCT03042143 | |
| Asthma | 2020 | hMSC | Allogeneic Human Cells (hMSC) Via Intravenous Delivery in Patients With Mild Asthma | i.v. | Active, not recruiting | NYR | NCT03137199 |
| 2018 | UC‐MSC | Safety and Feasibility Study of Intranasal Mesenchymal Trophic Factor (MTF) for Treatment of Asthma | i.v. | Active, not recruiting | NYR | NCT02192736 | |
| COPD | 2020 | MSC | Mesenchymal Stem Cells in the Treatment of Subjects With Advance Chronic Obstructive Pulmonary Disease (COPD) | i.v. | Recruiting | NYR | NCT04047810 |
| 2020 | UC‐MSC | Mesenchymal Stem Cells for The Treatment of Chronic Obstructive Pulmonary Disease | i.v. | Active, not recruiting | NYR | NCT04047810 | |
| 2020 | BM‐MSC (PROCHYMAL) | PROCHYMAL™ (Human Adult Stem Cells) for the Treatment of Moderate to Severe Chronic Obstructive Pulmonary Disease (COPD) | i.v. | Completed |
no infusional toxicities, no deaths or severity, no significant adverse effect, no significant differences in PFTs or quality‐of‐life indicators; CRP decrease in early stage |
NCT00683722 | |
| 2020 | ATSC | Safety, Tolerability and Preliminary Efficacy of Adipose Derive Stem Cells for Patients With COPD | i.v. | Terminated | NYR | NCT02161744 | |
| 2019 | BM‐MSC | Cell Therapy Associated With Endobronchial Valve | i.v. | Not yet recruiting | NYR | NCT04018729 | |
| 2017 | ATSC | Safety and Efficacy of Adipose Derived Stem Cells for Chronic Obstructive Pulmonary Disease | i.v. | Completed | NYR | NCT02216630 | |
| 2016 | AT‐MSC (hADAS) | Adipose Derived Stem Cells Transplantation for Chronic Obstructive Pulmonary Disease | i.v. | UN | NYR | NCT02645305 |
i.v.: Intravenous; NR: not reported; UN: Unknown; NYR: Not yet reported; NCT number: The national clinical trial number; AT‐MSC: Adipose tissue derived mesenchymal stem cells; BM‐MSC: Bone marrow mesenchymal stromal cells; UC‐MSC: Umbilical cord mesenchymal stem cells; PD‐MSC: Placenta‐derived MSC.
Owing to the difference between the animal model and the actual clinical scenario, 138 while conducting clinical research based on preclinical studies, regulatory issues, such as cell source, dosage, and disease stage, should be considered, since these factors can directly lead to different results under the same therapy regimen. For example, a large part of IPF research has explored the therapeutic effects of MSC‐based therapy during the early stages of inflammation rather than the late stage of fibrosis, which implies that MSC administration only prevents IPF and cannot be used as a late‐stage treatment. 164 Given the limitations of current clinical research regarding the sample size and variable control, more high‐quality clinical research studies are warranted that are based on large samples, are randomized and double‐blinded, and control variables to help clinical frontline workers understand MSC‐based therapy and improve its efficacy in the future. Moreover, we need to classify and stage the disease in patients, so that they can receive targeted and precise treatment. Unfortunately, MSCs‐based therapy is not devoid of ethical issues, and double‐blinded clinical research may be difficult. However, we advocate the introduction of optimized procedures to standardize the variables in MSCs‐based therapies, such as cell culture conditions and MSC sources.
4.3. Improvement of MSCs‐based therapy
4.3.1. Combination therapy
To maximize the therapeutic effect, the combination of MSC and traditional drug therapy to produce a synergistic effect is a frequently used solution in clinical practice. For example, for IPF treatment, the two pharmacological targets approved by the US Food and Drug Administration are TGF‐β (pirfenidone) and PDGF/VEGF/FGF (nintedanib). The efficacy of pirfenidone is impressive. Compared with those in the placebo group, pirfenidone can significantly reduce inflammation and prolong survival, but cannot reproduce the normal structure of lung tissue. 165 MSCs‐based therapy has the function of tissue repair, which can make up for the defects of targeted drug therapy to a certain extent. In addition, because the pathogenesis of these lung diseases, especially chronic diseases, involves multiple coactivation pathways, targeted therapy is unlikely to be effective in isolation. 166 Combining traditional and MSCs‐based therapy may improve therapy efficacy. 167 , 168 However, there are still insufficient clinical data to support the effectiveness of this therapy.
4.3.2. Improve the effectiveness of MSCs
Modifying MSC homing, migration, and regenerative properties through biochemical means, gene‐editing combined with computational modeling, or with material science can improve MSCs‐based therapy efficiency. In clinical applications, MSCs may have different administration modes: systemic administration (IV or intra‐arterial [IA] injection) or local administration (intracoronary [IC] injection or direct injection to the target tissue). Among these, IV injection is the most widely used because it has a minimally invasive effect, is easy to repeat infusion, and the cells will remain close to oxygen‐rich and nutrient‐rich blood vessels after infiltrating the target tissue. 169 Although MSCs have homing ability, this process does not seem to be very efficient. Therefore, it is vital to deliver MSCs to target tissues accurately. A large number of preclinical studies have shown that the transplantation rate is very low, close to 5%, 117 which is attributed to the low expression level of homing molecules, loss of expression of such molecules during amplification, and MSC heterogeneity. 170 Several research teams are trying to improve the homing efficiency of MSCs, including changing the method of administration, changing MSC culture conditions to optimize the expression of homing molecules, modifying cell surface receptors, and modifying target tissues to enhance MSC attraction. Regarding administration method, Sakar D et al. 169 combined MSC administration with the use of heparin, significantly reducing the capture of MSCs in the lung and increasing the capture rate of targeted sites. Considering culture methods, as MSCs downregulate the expression of homing molecules during amplification, adding cytokines to the amplification system can increase CXCR4 expression on the membrane, thereby upregulating the expression of homing molecules. These cytokines include, but are not limited to, flt3 ligand, stem cell factor, hepatocyte growth factor, IGF‐1, TNF‐α, IL‐1β, and IFNγ. 171 , 172 , 173 , 174 , 175 Additionally, hypoxic conditions can also increase the expression of CXCR4. 176
To improve homing efficiency, as MSCs are heterogeneous cell populations, a single‐cell sequencing method can be used to find specific subsets with a robust homing ability for separation. Cell surface engineering, that is, the transient modification of the cell surface, also improves the homing ability of MSCs, 177 and several research groups have developed different techniques for MSC surface modification. 178 , 179 , 180 In addition, the target tissue can be modified by chemical, magnetic field, or radiotherapy to improve the homing efficiency of MSCs. 181 , 182
Regarding the migration and localization of MSCs in vivo, current research is mainly based on experimental measurements and clinical observations. These experiments attempt to elucidate the processes involved in migration from statistical results, including cell signaling and protein interactions. 183 However, this process is complicated, mainly owing to the low frequency of stem cells and the lack of quantitative tracking experimental techniques for stem cell migration in vivo. Different mechanical/physical stimuli (such as shear flow, compressive force, stiffness, tension, and geometry) and various biochemical factors (such as the concentration and distribution of chemokines) are difficult to study through controlled variables using experimental methods.
In recent years, computational modeling has become a compelling method, making up for the lack of experimental methods, to understand the migration process of stem cells and help them accurately migrate to target tissues. When applying computational modeling to MSC research, the model should address the differences in migration kinetics between stem cells and other cells, and those between MSCs and other stem cells, considering whether they differentiate or not and the diversity of stem cells, respectively. Simulations of MSC migration kinetics in the local microenvironment in vitro 184 , 185 showed how numerical simulation can help us obtain unknown cell behaviors and tissue functions of small samples, study the process of MSCs to target damage sites, and design mechanisms to increase the number of cells targeted to damaged tissues. 186 While the migration process of a single cell is relatively easy to understand, in a real situation, the migration of multiple cells should be considered. Understanding the protein expression and signaling network in migrating cells will help to model and analyze collective migration.
In general, the development of computational modeling is still insufficient, mainly owing to data scarcity and uncertainty about the MSC mechanism caused by the lack of physical and biochemical sensor technologies and basic research, respectively. Stem cell models are roughly divided into deterministic models, stochastic models, and hybrid models. Deterministic models are mainly based on the concept that stem cells respond to differentiation stimuli in a dose‐dependent manner and therefore have deterministic behaviors. Contrastingly, stochastic methods use probability functions to describe events, such as differentiation and diffusion. 187 Therefore, understanding the MSC mechanism can help improve deterministic models, and data collection can help improve random and hybrid models. Meanwhile, the explosion of data and the improvement of computing power can also bring about essential changes in these models based on the concept of big data. We believe that in the near future, with the continuous collection of MSC in vivo data and increased understanding of MSC‐related mechanisms, computational modeling will be significantly strengthened and improved to assist us enhancing MSC migration activities.
In addition to strengthening the homing and migration functions of MSCs, it is also necessary to regulate their differentiation and regeneration ability and immunological characteristics. Under normal culture conditions, MSCs usually proliferate through cell division, unless induced by special differentiation conditions. Moreover, through high‐density inoculation of MSCs, the expression of some genes related to immunosuppressive properties can be increased. 188 , 189 Combined with the rapid development of gene‐editing technology in recent years, we can now easily genetically modify MSCs. Using CRISPR‐Cas9 technology, 190 , 191 additional available MSC editing technologies have been developed. For example, by combining nanotechnology, we can design an exosome‐liposome hybrid nanoparticle to easily provide the CRISPR/Cas9 system in MSCs. 192 Furthermore, with the development of gene therapy, the combination of genetic modification and MSC‐based therapy has become a trend. For example, ALI can be treated by UC‐MSCs with a modified Angiopoietin‐1 gene. 193 Besides, the combination of MSC‐based therapy and tissue engineering technology can also enhance MSC repair and regeneration functions. For example, Yang et al. combined MSCs with allogeneic cartilage tissue engineering to discuss the proliferation, differentiation, and secretion of various soluble factor functions of MSCs in a three‐dimensional environment to inspire scaffold design and achieve the required tissue regeneration. 194 Moreover, gene‐editing technology can also be used to adapt the existing two‐dimensional environment‐adapted MSCs to the three‐dimensional environment, promote tissue generation, and reduce immune rejection. 195 Additionally, modeling the lungs of patients and implement in vitro 3D‐tissue culture is a promising strategy for some diseases with severe lung necrosis that require transplantation, such as advanced IPF. Hence, it is necessary to establish a highly integrated 3D network of different lung cell types, including mesenchymal, epithelial, fibroblast, endothelial, inflammatory, and neuronal cells.
4.3.3. Cell‐free therapy
In clinical trials, MSCs‐based therapies usually display a low migration rate and low survival rate of MSCs in the recipient tissues. 140 However, this does not seem to have a significant impact on MSC function, which supports the hypothesis that their secretions may mediate the regenerative capacity. As mentioned above, MSC paracrine effects have been widely studied; their secretions are mainly responsible for the interaction between MSCs and target cells. 196 , 197 On this basis, research on MSC‐CM and EV has gradually received attention. Several studies have used different animal models to prove that CM can mimic the anti‐inflammatory mechanism of parental MSCs. For example, in acute and chronic asthma, intranasal infusion of BM‐MSC‐CM can reduce the levels of inflammatory cytokines IL‐4 and IL‐13 in the lungs and increase the level of IL‐10. 198 Shen and colleagues studied the protective effect of BM‐MSC‐CM on bleomycin‐induced lung injury and fibrosis in vitro (A549 alveolar epithelial cells) and in vivo (rat model). After treatment with MSC‐CM, A549 cells were significantly protected from bleomycin‐induced apoptosis, while lung inflammation, fibrosis scores, collagen deposition, and apoptosis in rats challenged with bleomycin were reduced. 199 In a rat model of COPD, MSC‐CM administration can reduce emphysema caused by CS and increase the number of small pulmonary vessels and MSCs. 200 However, the main limitation of MSCs‐CM is the relatively rapid degradation of biologically active molecules. Additionally, as MSC‐based therapies, it lacks standard procedures for regulations. 201
EVs provide another popular cell‐free therapy. They can stably transport biologically active substances without being hydrolyzed or mutated, and several teams have demonstrated the anti‐inflammatory effects of EVs in ALI, 26 asthma, 202 IPF, 28 , 203 and other diseases. In the neonatal rat model, MSC‐derived EVs are as effective as parental MSCs, which can confer VEGF‐mediated protection against activated macrophages, proinflammatory cytokines, and increased cell death. 204 In the neonatal rat model, EVs derived from MSCs are as effective as parental MSCs. This protective effect is mainly mediated by the transfer of VEGF contained within EVs. 204 Recent evidence shows that EVs derived from MSCs can carry miRNAs with antiapoptotic properties and can promote the reduction of lung inflammation, 205 , 206 thus being a promising method to reduce inflammation. Compared with traditional MSCs‐based therapies, EVs are more stable, easily enter the systemic circulation, and maintain long‐lasting high concentrations. 207 Moreover, cell‐free therapy also reduces the risk of tumor formation and has advantages similar to those of traditional medicine in terms of transportation, storage, and standardization. However, EVs have a high degree of heterogeneity, and the content of stored bioactive compounds varies greatly, which adds to the challenge of their dosage. In general, cell‐free therapy is safer, and has advantages over cell therapies in variable control and standardization, with a strong potential for immunomodulation and anti‐inflammatory effects in lung diseases.
5. CONCLUSION
All in all, the effectiveness of MSC‐based therapy for respiratory diseases has been proven by preclinical studies and clinical trials. However, limitations of related research need to be worked out to explore their clinical applications. In conclusion, MSC‐based therapy has provided a promising strategy for the treatment of respiratory diseases.
CONFLICTS OF INTERESTS
The authors have declared that no competing interest exists.
AUTHOR'S CONTRIBUTIONS
MY Wang, TY Zhou, and ZD Zhang contributed equally to the review, and should be viewed as cofirst authors, they drafted and revised the manuscript, and did the bulk of literature search and categorization of references. HY Liu and ZY Zheng contributed by summarizing related researches guiding the framework, placing the points in context, and constantly giving invaluable feedback. HQ Xie provided assistance and guidance during manuscript writing. All authors read and approved the final manuscript.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31771065), Sichuan Provincial Science and Technology Program (Grant No. 2019JDRC0020), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC18002).
Wang M‐y, Zhou T‐y, Zhang Z‐d, Liu H‐y, Zheng Z‐y, Xie H‐q. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm. 2021;2:351–380. 10.1002/mco2.74
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreira A, Kahlenberg S, Hornsby P. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol. 2017;59(3):R109–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Blanc K, Davies LC. MSCs‐cells with many sides. Cytotherapy. 2018;20(3):273–278. [DOI] [PubMed] [Google Scholar]
- 4. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med. 2004;10(8):858–864. [DOI] [PubMed] [Google Scholar]
- 5. Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin‐receptor‐expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cerrada A, de la Torre P, Grande J, Haller T, Flores AI, Perez‐Gil J. Human decidua‐derived mesenchymal stem cells differentiate into functional alveolar type II‐like cells that synthesize and secrete pulmonary surfactant complexes. PLoS One. 2014;9(10):e110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu A, Chen S, Cai S, et al. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One. 2014;9(3):e90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1):e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Worthington EN, Hagood JS. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int J Mol Sci. 2020;21(7):2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antunes MA, Abreu SC, Cruz FF, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Oliveira HG, Cruz FF, Antunes MA, et al. Combined bone marrow‐derived mesenchymal stromal cell therapy and one‐way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl Med. 2017;6(3):962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armitage J, Tan DBA, Troedson R, et al. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur Respir J. 2018;51(3):1702369. [DOI] [PubMed] [Google Scholar]
- 13. Rahbarghazi R, Keyhanmanesh R, Aslani MR, Hassanpour M, Ahmadi M. Bone marrow mesenchymal stem cells and condition media diminish inflammatory adhesion molecules of pulmonary endothelial cells in an ovalbumin‐induced asthmatic rat model. Microvasc Res. 2019;121(1):63–70. [DOI] [PubMed] [Google Scholar]
- 14. Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Effect of mesenchymal stem cells on inhibiting airway remodeling and airway inflammation in chronic asthma. J Cell Biochem. 2013;114(7):1595–1605. [DOI] [PubMed] [Google Scholar]
- 15. Srour N, Thébaud B. Stem cells in animal asthma models: a systematic review. Cytotherapy. 2014;16(12):1629–1642. [DOI] [PubMed] [Google Scholar]
- 16. Dai R, Yu Y, Yan G, Hou X, Ni Y, Shi G. Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm Med. 2018;18(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol ‐ Lung Cell Mol Physiol. 2010;299(6):760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavoie JR, Rosu‐Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95(12):2212–2221. [DOI] [PubMed] [Google Scholar]
- 20. Broekman W, Amatngalim GD, de Mooij‐Eijk Y, et al. TNF‐aα and IL‐1β‐activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia‐induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou X, Zhang G, Cheng Z, et al. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia‐reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline‐induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110(3):319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaubey S, Thueson S, Ponnalagu D, et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome‐associated factor TSG‐6. Stem Cell Res Ther. 2018;9(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. [DOI] [PubMed] [Google Scholar]
- 27. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell‐derived extracellular vesicle‐based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7(10):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beninson LA, Fleshner M. Exosomes: an emerging factor in stress‐induced immunomodulation. Semin Immunol. 2014;26(5):394–401. [DOI] [PubMed] [Google Scholar]
- 32. Harrell CR, Miloradovic D, Sadikot R, et al. Molecular and cellular mechanisms responsible for beneficial effects of mesenchymal stem cell‐derived product “exo‐d‐MAPPS” in attenuation of chronic airway inflammation. Anal Cell Pathol. 2010;285(34):26211–26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29(12):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med. 2009;15(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self‐activated to produce prostaglandin E2 that directs stimulated macrophages into an anti‐inflammatory phenotype. Stem Cells. 2012;30(10):2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti‐inflammatory protein TSG‐6 secreted by activated MSCs attenuates zymosan‐induced mouse peritonitis by decreasing TLR2/NF‐kappaB signaling in resident macrophages. Blood. 2011;118(2):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mittal M, Tiruppathi C, Nepal S, et al. TNFalpha‐stimulated gene‐6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc Natl Acad Sci U S A. 2016;113(50):E8151–E8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yagi H, Soto‐Gutierrez A, Navarro‐Alvarez N, et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18(10):1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bottcher M, Hofmann AD, Bruns H, et al. Mesenchymal stromal cells disrupt mTOR‐signaling and aerobic glycolysis during T‐cell activation. Stem Cells. 2016;34(2):516–521. [DOI] [PubMed] [Google Scholar]
- 40. Jitschin R, Bottcher M, Saul D, et al. Inflammation‐induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia. 2019;33(7):1783–1796. [DOI] [PubMed] [Google Scholar]
- 41. Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged‐1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO‐independent suppression of T cell effector function by IFN‐gamma‐licensed human mesenchymal stromal cells. J Immunol. 2014;192(4):1491–1501. [DOI] [PubMed] [Google Scholar]
- 44. Ni K, Liu M, Zheng J, et al. PD‐1/PD‐L1 pathway mediates the alleviation of pulmonary fibrosis by human mesenchymal stem cells in humanized mice. Am J Respir Cell Mol Biol. 2018;58(6):684–695. [DOI] [PubMed] [Google Scholar]
- 45. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. [DOI] [PubMed] [Google Scholar]
- 46. Shi Y, Du L, Lin L, Wang Y. Tumour‐associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35–52. [DOI] [PubMed] [Google Scholar]
- 47. Zhang E, Yang Y, Zhang J, et al. Efficacy of bone marrow mesenchymal stem cell transplantation in animal models of pulmonary fibrosis after exposure to bleomycin: a meta‐analysis. Exp Ther Med. 2019;17(3):2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Prim. 2017;3(1):1–19. [DOI] [PubMed] [Google Scholar]
- 49. Raghu G, Chen S, Yeh W, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir. 2014;2(7):566–572. [DOI] [PubMed] [Google Scholar]
- 50. Sontake V, Gajjala PR, Kasam RK, Madala SK. New therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin Ther Targets. 2019;23(1):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spagnolo P, Rossi G, Cavazza A. Pathogenesis of idiopathic pulmonary fibrosis and its clinical implications. Expert Rev Clin Immunol. 2014;10(8):1005–1017. [DOI] [PubMed] [Google Scholar]
- 52. Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial–mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018;71‐72(2017):112–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. [DOI] [PubMed] [Google Scholar]
- 54. Pan LH, Yamauchi K, Uzuki M, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17(6):1220–1227. [DOI] [PubMed] [Google Scholar]
- 55. Cahill EF, Kennelly H, Carty F, Mahon BP, English K. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin‐induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5(10):1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang C, Yin X, Zhang J, Ao Q, Gu Y, Liu Y. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe idiopathic pulmonary fibrosis: a case report. Exp Ther Med. 2017;13(5):1922–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin‐induced lung injury. Am J Pathol. 2009;175(1):303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Banerjee E, Laflamme M. Human embryonic stem cells differentiated to lung lineage‐specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. Eur Respir J. 2018;51(3):1702369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Averyanov A, Koroleva I, Sotnikova A, et al. Safety and efficacy of transplantation of high cumulative dose allogeneic bone marrow‐derived mesenchymal stem cells in patients with idiopathic pulmonary fibrosis and rapid lung function decline. Adv ild Ther. 2019;1: A2640–A2640. [Google Scholar]
- 60. Horie S, Masterson C, Devaney J. Stem cell therapy for acute respiratory distress syndrome: a promising future? Curr Opin Crit Care. 2016;22(1):14–20. [DOI] [PubMed] [Google Scholar]
- 61. Khemani R, Smith L, Zimmerman J. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5_suppl):S23–S40. [DOI] [PubMed] [Google Scholar]
- 62. Jaitovich A, Jourd'heuil D. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol. 2017;967:105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cardenes N, Aranda‐Valderrama P, Carney JP, et al. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6(1):e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Trivedi A, Miyazawa B, Gibb S, et al. Bone marrow donor selection and characterization of MSCs is critical for pre‐clinical and clinical cell dose production. J Transl Med. 2019;17(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID‐19 treatment. Engineering. 2020;6(10):1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC‐MSCs) as a therapeutic strategy in managing critically Ill COVID‐19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 67. Lopes‐Pacheco M, Robba C, Rocco PRM, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2020;36(1):83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berg K, Wright JL. The pathology of chronic obstructive pulmonary disease: progress in the 20th and 21st centuries. Arch Pathol Lab Med. 2016;140(12):1423–1428. [DOI] [PubMed] [Google Scholar]
- 69. Li X, Wang J, Cao J, Ma L, Xu J. Immunoregulation of bone marrow‐derived mesenchymal stem cells on the chronic cigarette smoking‐induced lung inflammation in rats. Biomed Res Int. 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boulet LP, Fitzgerald JM, Reddel HK. The revised 2014 GINA strategy report: opportunities for change. Curr Opin Pulm Med. 2015;21(1):1–7. [DOI] [PubMed] [Google Scholar]
- 71. Behnke J, Kremer S, Shahzad T, et al. MSC based therapies—new perspectives for the injured lung. J Clin Med. 2020;9(3):682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen QH, Liu AR, Qiu HB, Yang Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res Ther. 2015;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hu S, Li J, Xu X, et al. The hepatocyte growth factor‐expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther. 2016;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guan XJ, Song L, Han FF, et al. Mesenchymal stem cells protect cigarette smoke‐damaged lung and pulmonary function partly via VEGF–VEGF receptors. J Cell Biochem. 2013;114(2):323–335. [DOI] [PubMed] [Google Scholar]
- 75. Wang G, Cao K, Liu K, et al. Kynurenic acid, an IDO metabolite, controls TSG‐6‐mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25(7):1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maacha S, Sidahmed H, Jacob S, et al. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhen G, Xue Z, Zhao J, et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain‐induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12(5):605–614. [DOI] [PubMed] [Google Scholar]
- 78. Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO‐dependent mechanism. J Immunol. 2002;168(2):689–695. [DOI] [PubMed] [Google Scholar]
- 79. Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell‐mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. [DOI] [PubMed] [Google Scholar]
- 80. Bartosh TJ, Ylostalo JH, Bazhanov N, Kuhlman J, Prockop DJ. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self‐activates caspase‐dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells. 2013;31(11):2443–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sala E, Genua M, Petti L, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163–176. [DOI] [PubMed] [Google Scholar]
- 82. Liu M, Zeng X, Wang J, et al. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease‐associated lung fibrosis. Stem Cell Res Ther. 2016;7(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–L977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nemeth K, Keane‐Myers A, Brown JM, et al. Bone marrow stromal cells use TGF‐beta to suppress allergic responses in a mouse model of ragweed‐induced asthma. Proc Natl Acad Sci U S A. 2010;107(12):5652–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell‐induced IL‐10+IFN‐gamma+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190(5):2372–2380. [DOI] [PubMed] [Google Scholar]
- 86. Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase‐1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–3694. [DOI] [PubMed] [Google Scholar]
- 87. Ono M, Ohkouchi S, Kanehira M, et al. Mesenchymal stem cells correct inappropriate epithelial–mesenchyme relation in pulmonary fibrosis using stanniocalcin‐1. Mol Ther. 2015;23(3):549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reddy M, Fonseca L, Gowda S, Chougule B, Hari A, Totey S. Human adipose‐derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with pirfenidone. Int J Stem Cells. 2016;9(2):192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen X, Wu S, Tang L, et al. Mesenchymal stem cells overexpressing heme oxygenase‐1 ameliorate lipopolysaccharide‐induced acute lung injury in rats. J Cell Physiol. 2019;234(5):7301–7319. [DOI] [PubMed] [Google Scholar]
- 90. Zhao F, Liu W, Yue S, et al. Pretreatment with G‐CSF could enhance the antifibrotic effect of BM‐MSCs on pulmonary fibrosis. Stem Cells Int. 2019;2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cárdenes N, Álvarez D, Sellarés J, et al. Senescence of bone marrow‐derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther. 2018;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL‐37. Stem Cells. 2010;28(12):2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu L, Bao L, Deng W, et al. Novel avian‐origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. J Infect Dis. 2014;209(4):551–556. [DOI] [PubMed] [Google Scholar]
- 94. Gao R, Cao B, Hu Y, et al. Human infection with a novel avian‐origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–1897. [DOI] [PubMed] [Google Scholar]
- 95. Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381(9881):1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu Y, Peng R, Zhang W, et al. Avian‐to‐human receptor‐binding adaptation of avian H7N9 influenza virus hemagglutinin. Cell Rep. 2019;29(8):2217–2228. [DOI] [PubMed] [Google Scholar]
- 97. Gao H‐N, Lu H‐Z, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277–2285. [DOI] [PubMed] [Google Scholar]
- 98. Zhou J, Wang D, Gao R, et al. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499(7459):500–503. [DOI] [PubMed] [Google Scholar]
- 99. Husain M. Avian influenza A (H7N9) virus infection in humans: epidemiology, evolution, and pathogenesis. Infect Genet Evol. 2014;28:304–312. [DOI] [PubMed] [Google Scholar]
- 100. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT. Interaction of severe acute respiratory syndrome‐associated coronavirus with dendritic cells. J Gen Virol. 2006;87(7):1953–1960. [DOI] [PubMed] [Google Scholar]
- 102. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bey E, Prat M, Duhamel P, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow‐derived stem cell administrations. Wound Repair Regen. 2010;18(1):50–58. [DOI] [PubMed] [Google Scholar]
- 108. Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID‐19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore). 2020;99(31):e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Glenn JD. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6(5):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2‐mesenchymal stem cells improves the outcome of patients with covid‐19 pneumonia. Aging Dis. 2020;11(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang Y, Ding J, Ren S, et al. Intravenous infusion of human umbilical cord Wharton's jelly‐derived mesenchymal stem cells as a potential treatment for patients with COVID‐19 pneumonia. Stem Cell Res Ther. 2020;11(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shi L, Huang H, Lu X, et al. Effect of human umbilical cord‐derived mesenchymal stem cells on lung damage in severe COVID‐19 patients: a randomized, double‐blind, placebo‐controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lands LC, Stanojevic S. Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2019;9(1465–1858):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cheng K, Ashby D, Smyth RL. Oral steroids for long‐term use in cystic fibrosis. Cochrane Database Syst Rev. 2015;2015(12):CD000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Akkoc T. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4(2):119–124. [DOI] [PubMed] [Google Scholar]
- 116. Fong ELS, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32(2):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14(9):2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mei SHJ, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. [DOI] [PubMed] [Google Scholar]
- 119. Curley GF, Ansari B, Hayes M, et al. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator‐induced lung injury. Anesthesiology. 2013;118(4):924–933. [DOI] [PubMed] [Google Scholar]
- 120. Islam D, Huang Y, Fanelli V, et al. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am J Respir Crit Care Med. 2019;199(10):1214–1224. [DOI] [PubMed] [Google Scholar]
- 121. Zhang L, Li Q, Liu W, Liu Z, Shen H, Zhao M. Mesenchymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med Sci Monit. 2019;25:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stone ML, Zhao Y, Robert Smith J, et al. Mesenchymal stromal cell‐derived extracellular vesicles attenuate lung ischemia‐reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rojas M, Cárdenes N, Kocyildirim E, et al. Human adult bone marrow‐derived stem cells decrease severity of lipopolysaccharide‐induced acute respiratory distress syndrome in sheep. Stem Cell Res Ther. 2014;5(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hayes M, Masterson C, Devaney J, et al. Therapeutic efficacy of human mesenchymal stromal cells in the repair of established ventilator‐induced lung injury in the rat. Surv Anesthesiol. 2015;59(6):259–260. [DOI] [PubMed] [Google Scholar]
- 125. Shologu N, Scully M, Laffey JG, O'toole D. Human mesenchymal stem cell secretome from bone marrow or adipose‐derived tissue sources for treatment of hypoxia‐induced pulmonary epithelial injury. Int J Mol Sci. 2018;19(10):2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin‐induced systemic response by bone marrow‐derived mesenchymal stem cells in mice. Am J Physiol ‐ Lung Cell Mol Physiol. 2007;293(1):131–141. [DOI] [PubMed] [Google Scholar]
- 127. Chan MCW, Kuok DIT, Leung CYH, et al. Human mesenchymal stromal cells reduce influenza A H5N1‐associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113(13):3621–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Antoniou KM, Margaritopoulos GA, Proklou A, et al. Investigation of telomerase/telomeres system in bone marrow mesenchymal stem cells derived from IPF and RA‐UIP. J Inflamm (United Kingdom). 2012;9(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fomby P, Cherlin AJ, Hadjizadeh A, et al. Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow‐derived mesenchymal stem cells. J Tissue Eng Regen Med. 2009;3(3):163–174. [DOI] [PubMed] [Google Scholar]
- 130. Haack‐Sorensen M, Friis T, Bindslev L, Mortensen S, Johnsen HE, Kastrup J. Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scand J Clin Lab Invest. 2008;68(3):192–203. [DOI] [PubMed] [Google Scholar]
- 131. Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase‐induced emphysema model. Mol Ther. 2011;19(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Stessuk T, Ruiz MA, Greco OT, Bilaqui A, Ribeiro‐Paes MJde O, Ribeiro‐Paes JT. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow‐up of up to 3 years. Rev Bras Hematol Hemoter. 2013;35(5):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14(1):31–39. [DOI] [PubMed] [Google Scholar]
- 134. Wang LT, Ting CH, Yen ML, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune‐ and inflammation‐mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abreu SC, Antunes MA, Xisto DG, et al. Bone marrow, adipose, and lung tissue‐derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Transl Med. 2017;6(6):1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kitoko JZ, Castro LL de, Nascimento AP, et al. Therapeutic administration of bone marrow‐derived mesenchymal stromal cells reduces airway inflammation without up‐regulating Tregs in experimental asthma. Clin Exp Allergy. 2017;48(2):205–216. [DOI] [PubMed] [Google Scholar]
- 137. Galleu A, Riffo‐Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient‐mediated immunomodulation. Sci Transl Med. 2017;9(416):1–12. [DOI] [PubMed] [Google Scholar]
- 138. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Capasso S, Alessio N, Squillaro T, et al. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget. 2015;6(37):39457–39468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Haque N, Abu Kasim NH, Rahman MT. Optimization of pre‐transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci. 2015;11(3):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gu W, Song L, Li XM, Wang D, Guo XJ, Xu WG. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down‐regulation of cyclooxygenase‐2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 144. Rajashekhar G, Traktuev DO, Roell WC, et al. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 2008;26(10):2674–2681. [DOI] [PubMed] [Google Scholar]
- 145. Antunes MA, Laffey JG, Pelosi P, Rocco PRM. Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem. 2014;115(6):1023–1032. [DOI] [PubMed] [Google Scholar]
- 146. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Reinisch A, Bartmann C, Rohde E, et al. Humanized system to propagate cord blood‐derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2007;2(4):371–382. [DOI] [PubMed] [Google Scholar]
- 148. Moodley Y, Vaghjiani V, Chan J, et al. Anti‐inflammatory effects of adult stem cells in sustained lung injury: a comparative study. PLoS One. 2013;8(8):e69299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ricciardi M, Malpeli G, Bifari F, et al. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One. 2012;7(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Di Benedetto P, Panzera N, Cipriani P, et al. Mesenchymal stem cells of systemic sclerosis patients, derived from different sources, show a profibrotic microRNA profiling. Sci Rep. 2019;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tashiro J, Elliot SJ, Gerth DJ, et al. Therapeutic benefits of young, but not old, adipose‐derived mesenchymal stem cells in a chronic mouse model of bleomycin‐induced pulmonary fibrosis. Transl Res. 2015;166(6):554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta‐analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Moll G, Ankrum JA, Kamhieh‐Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–163. [DOI] [PubMed] [Google Scholar]
- 154. Martin I, Galipeau J, Kessler C, Blanc KL, Dazzi F. Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019;11(480):1–4. [DOI] [PubMed] [Google Scholar]
- 155. Kim M, Rhee JK, Choi H, et al. Passage‐dependent accumulation of somatic mutations in mesenchymal stromal cells during in vitro culture revealed by whole genome sequencing. Sci Rep. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nikitina V, Astrelina T, Nugis V, et al. Clonal chromosomal and genomic instability during human multipotent mesenchymal stromal cells long‐term culture. PLoS One. 2018;13(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tashiro J, Rubio GA, Limper AH, et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front Med. 2017;4(7):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jenkins RG, Moore BB, Chambers RC, et al. An official American Thoracic Society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;56(5):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Srour N, Thebaud B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: a systematic review. STEMCELLS Transl Med. 2015;4(12):1500–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang T, Jia Y, Ma Y, Cao L, Chen X, Qiao B. Comparative proteomic analysis of bleomycin‐induced pulmonary fibrosis based on isobaric tag for quantitation. Am J Med Sci. 2017;353(1):49–58. [DOI] [PubMed] [Google Scholar]
- 162. Loy H, Kuok DIT, Hui KPY, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A/H5N1‐associated acute lung injury. J Infect Dis. 2019;219(2):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Marks PW, Witten CM, Califf RM. Clarifying stem‐cell therapy's benefits and risks. N Engl J Med. 2017;376(11):1007–1009. [DOI] [PubMed] [Google Scholar]
- 164. Li X, Yue S, Luo Z. Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget. 2017;8(60):102600–102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nathan SD, Albera C, Bradford WZ, et al. Effect of pirfenidone on mortality: pooled analyses and meta‐analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41. [DOI] [PubMed] [Google Scholar]
- 166. Wuyts WA, Antoniou KM, Borensztajn K, et al. Combination therapy: the future of management for idiopathic pulmonary fibrosis? Lancet Respir Med. 2014;2(11):933–942. [DOI] [PubMed] [Google Scholar]
- 167. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. [DOI] [PubMed] [Google Scholar]
- 168. Chuang HM, Shih TE, Lu KY, Tsai SF, Harn HJ, Ho LI. Mesenchymal stem cell therapy of pulmonary fibrosis: improvement with target combination. Cell Transplant. 2018;27(11):1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sarkar D, Spencer JA, Phillips JA, et al. Engineered cell homing. Blood. 2011;118(25):e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. De Becker A, Van Riet I. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8(3):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. [DOI] [PubMed] [Google Scholar]
- 172. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. [DOI] [PubMed] [Google Scholar]
- 173. Li Y, Yu XY, Lin SG, Li XH, Zhang S, Song YH. Insulin‐like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356(3):780–784. [DOI] [PubMed] [Google Scholar]
- 174. Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon‐γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29(10):1549–1558. [DOI] [PubMed] [Google Scholar]
- 175. Fan H, Zhao G, Liu L, et al. Pre‐treatment with IL‐1β enhances the efficacy of MSC transplantation in DSS‐induced colitis. Cell Mol Immunol. 2012;9(6):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Vertelov G, Kharazi L, Muralidhar MG, Sanati G, Tankovich T, Kharazi A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther. 2013;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Teo GSL, Ankrum JA, Martinelli R, et al. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor‐α‐activated endothelial cells via both leukocyte‐like and novel mechanisms. Stem Cells. 2012;30(11):2472–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14(2):181–187. [DOI] [PubMed] [Google Scholar]
- 179. Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF‐1 gradient. Biomaterials. 2014;35(21):5627–5635. [DOI] [PubMed] [Google Scholar]
- 180. Cheng H, Byrska‐Bishop M, Zhang CT, et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell–selectin interaction kinetics. Biomaterials. 2012;33(20):5004–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhao Z, Watt C, Karystinou A, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur Cells Mater. 2011;22(0):344–358. [DOI] [PubMed] [Google Scholar]
- 182. Li L, Wu S, Liu Z, et al. Ultrasound‐targeted microbubble destruction improves the migration and homing of mesenchymal stem cells after myocardial infarction by upregulating SDF‐1/CXCR4: a pilot study. Stem Cells Int. 2015;2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adhes Migr. 2012;6(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34‐like protein PODXL and ά6‐integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113(4):816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Liu AR, Liu L, Chen S, et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow‐derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013;228(6):1270–1283. [DOI] [PubMed] [Google Scholar]
- 186. Shi C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology. 2012;136(2):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wu J, Rostami MR, Tzanakakis ES. Stem cell modeling: from gene networks to cell populations. Curr Opin Chem Eng. 2013;2(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kim DS, Lee MW, Yoo KH, et al. Gene expression profiles of human adipose tissue‐derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–166. [DOI] [PubMed] [Google Scholar]
- 190. Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR‐Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR‐Cas9 system. Nat Protoc. 2013;8(11):2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Lin Y, Wu J, Gu W, et al. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Huang ZW, Liu N, Li D, et al. Angiopoietin‐1 modified human umbilical cord mesenchymal stem cell therapy for endotoxin‐induced acute lung injury in rats. Yonsei Med J. 2017;58(1):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yang J, Chen X, Yuan T, Yang X, Fan Y, Zhang X. Regulation of the secretion of immunoregulatory factors of mesenchymal stem cells (MSCs) by collagen‐based scaffolds during chondrogenesis. Mater Sci Eng C. 2017;70:983–991. [DOI] [PubMed] [Google Scholar]
- 195. Adkar SS, Wu CL, Willard VP, et al. Step‐wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR‐Cas9 genome editing. Stem Cells. 2019;37(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Konala VBR, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell‐free regeneration. Cytotherapy. 2016;18(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell‐derived extracellular vesicles. Proteomics. 2013;13(10‐11):1637–1653. [DOI] [PubMed] [Google Scholar]
- 198. Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic‐adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Shen Q, Chen B, Xiao Z, et al. Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol Med Rep. 2015;11(4):2831–2837. [DOI] [PubMed] [Google Scholar]
- 200. Huh JW, Kim SY, Lee JH, et al. Bone marrow cells repair cigarette smoke‐induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L255–L266. [DOI] [PubMed] [Google Scholar]
- 201. Gomzikova MO, Zhuravleva MN, Miftakhova RR, et al. Cytochalasin B‐induced membrane vesicles convey angiogenic activity of parental cells. Oncotarget. 2017;8(41):70496–70507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Cruz FF, Borg ZD, Goodwin M, et al. Systemic administration of human bone marrow‐derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract‐induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4(11):1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Willis GR, Fernandez‐Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197(1):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ahn SY, Park WS, Kim YE, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell‐derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Yi X, Wei X, Lv H, et al. Exosomes derived from microRNA‐30b‐3p‐overexpressing mesenchymal stem cells protect against lipopolysaccharide‐induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383(2):111454. [DOI] [PubMed] [Google Scholar]
- 206. Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells‐derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti‐apoptotic miR‐21‐5p. Eur J Pharmacol. 2019;852(12):68–76. [DOI] [PubMed] [Google Scholar]
- 207. Phinney DG, Pittenger MF. Concise review: MSC‐derived exosomes for cell‐free therapy. Stem Cells. 2017;35(4):851–858. [DOI] [PubMed] [Google Scholar]
- 208. Nakamura A, Mori Y, Hagiwara K, et al. Increased susceptibility to LPS‐induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)‐deficient mice. J Exp Med. 2003;197(5):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Li J, Huang S, Zhang J, et al. Mesenchymal stem cells ameliorate inflammatory cytokine‐induced impairment of AT‐II cells through a keratinocyte growth factor‐dependent PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2016;13(5):3755–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin‐induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Oki H, Yazawa T, Baba Y, et al. Adenovirus vector expressing keratinocyte growth factor using CAG promoter impairs pulmonary function of mice with elastase‐induced emphysema. Microbiol Immunol. 2017;61(7):264–271. [DOI] [PubMed] [Google Scholar]
- 212. Takeoka M, Ward WF, Pollack H, Kamp DW, Panos RJ. KGF facilitates repair of radiation‐induced DNA damage in alveolar epithelial cells. Am J Physiol. 1997;272(6):L1174–L1180. [DOI] [PubMed] [Google Scholar]
- 213. Yen BL, Yen ML, Hsu PJ, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid‐derived suppressor cells via hepatocyte growth factor/c‐met and STAT3. Stem Cell Rep. 2013;1(2):139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Chen PM, Liu KJ, Hsu PJ, et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell‐derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96(2):295–303. [DOI] [PubMed] [Google Scholar]
- 215. Lee SH, Jang AS, Kim YE, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin‐1. J Biol Chem. 2010;285(34):26211–26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Danchuk S, Ylostalo JH, Hossain F, et al. Human multipotent stromal cells attenuate lipopolysaccharide‐induced acute lung injury in mice via secretion of tumor necrosis factor‐α‐induced protein 6. Stem Cell Res Ther. 2011;2(3):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta‐defensin‐2 via toll‐like receptor 4 signalling. Cell Microbiol. 2016;18(3):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Sun J, Han ZB, Liao W, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4CD25 + forkhead Boxp3 (FOXP3) + regulatory T cells and balancing anti‐ and pro‐inflammatory factors. Cell Physiol Biochem. 2011;27(5):587–596. [DOI] [PubMed] [Google Scholar]
- 220. Dos Santos CC, Murthy S, Hu P, et al. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. Am J Pathol. 2012;181(5):1681–1692. [DOI] [PubMed] [Google Scholar]
- 221. Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS‐induced acute lung injury in rats. J Inflamm (United Kingdom). 2012;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram‐negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol ‐ Lung Cell Mol Physiol. 2012;302(10):L1003–L1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zhang X, Wang H, Shi Y, et al. Role of bone marrow‐derived mesenchymal stem cells in the prevention of hyperoxia‐induced lung injury in newborn mice. Cell Biol Int. 2012;36(6):589–594. [DOI] [PubMed] [Google Scholar]
- 225. Maron‐Gutierrez T, Silva JD, Asensi KD, et al. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med. 2013;41(11):319–333. [DOI] [PubMed] [Google Scholar]
- 226. Zhao Y, Yang C, Wang H, et al. Therapeutic effects of bone marrow‐derived mesenchymal stem cells on pulmonary impact injury complicated with endotoxemia in rats. Int Immunopharmacol. 2013;15(2):246–253. [DOI] [PubMed] [Google Scholar]
- 227. Martínez‐González I, Roca O, Masclans JR, et al. Human mesenchymal stem cells overexpressing the IL‐33 antagonist soluble IL‐1 receptor‐like‐1 attenuate endotoxin‐induced acute lung injury. Am J Respir Cell Mol Biol. 2013;49(4):552–562. [DOI] [PubMed] [Google Scholar]
- 228. Asmussen S, Ito H, Traber DL, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69(9):819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chang YS, Ahn SY, Jeon HB, et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51(3):391–399. [DOI] [PubMed] [Google Scholar]
- 230. Chao YH, Wu HP, Wu KH, et al. An increase in CD3+CD4+CD25 + regulatory T cells after administration of umbilical cord‐derived mesenchymal stem cells during sepsis e110338. PLoS One. 2014;9(10):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Pedrazza L, Lunardelli A, Luft C. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res. 2014;63(9):719–728. [DOI] [PubMed] [Google Scholar]
- 232. Carlos Sepúlveda J, Tomé M, Eugenia Fernández M, et al. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells. 2014;32(7):1865–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zhao X, Liu D, Gong W, et al. The toll‐like receptor 3 Ligand, Poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR‐143. Stem Cells. 2014;32(2):521–533. [DOI] [PubMed] [Google Scholar]
- 234. Alcayaga‐Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Devaney J, Horie S, Masterson C, et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70(7):625–635. [DOI] [PubMed] [Google Scholar]
- 236. Güldner A, Maron‐Gutierrez T, Abreu SC, et al. Expanded endothelial progenitor cells mitigate lung injury in septic mice. Stem Cell Res Ther. 2015;6(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hayes M, Curley GF, Masterson C, Devaney J, O'Toole D, Laffey JG. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator‐induced lung injury. Intensive Care Med Exp. 2015;3(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wang Y, Tan L, Jin J, et al. Non‐cultured dermal‐derived mesenchymal cells attenuate sepsis induced by cecal ligation and puncture in mice. Sci Rep. 2015;5(10):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lan YW, Choo KB, Chen CM, et al. Hypoxia‐preconditioned mesenchymal stem cells attenuate bleomycin‐induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Jackson M V., Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Yang Y, Hu S, Xu X, et al. The vascular endothelial growth factors‐expressing character of mesenchymal stem cells plays a positive role in treatment of acute lung injury in vivo. Mediators Inflamm. 2016;2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lee FY, Chen KH, Wallace CG, et al. Xenogeneic human umbilical cord‐derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget. 2017;8(28):45626–45642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Pedrazza L, Cunha AA, Luft C, et al. Mesenchymal stem cells improves survival in LPS‐induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017;232(12):3552–3564. [DOI] [PubMed] [Google Scholar]
- 244. Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of menstrual blood‐derived mesenchymal stem cells promotes the repair of LPS‐induced acute lung injury. Int J Mol Sci. 2017;18(4):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Zhang Z, Li W, Heng Z, Zheng J, Li P, Yuan X. Combination therapy of human umbilical cord mesenchymal stem cells and FTY720 attenuates acute lung injury induced by lipopolysaccharide in a murine model. Oncotarget. 2017;8(44):77407–77414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Huang Z, Liu H, Zhang X, et al. Transcriptomic analysis of lung tissues after hUC‐MSCs and FTY720 treatment of lipopolysaccharide‐induced acute lung injury in mouse models. Int Immunopharmacol. 2018;63(June):26–34. [DOI] [PubMed] [Google Scholar]
- 247. Silva JD, Lopes‐Pacheco M, Paz AHR, et al. Mesenchymal stem cells from bone marrow, adipose tissue, and lung tissue differentially mitigate lung and distal organ damage in experimental acute respiratory distress syndrome. Crit Care Med. 2018;46(2):e132–e140. [DOI] [PubMed] [Google Scholar]
- 248. Mokhber Dezfouli MR, Jabbari Fakhr M, Sadeghian Chaleshtori S, Dehghan MM, Vajhi A, Mokhtari R. Intrapulmonary autologous transplant of bone marrow‐derived mesenchymal stromal cells improves lipopolysaccharide‐induced acute respiratory distress syndrome in rabbit. Crit Care. 2018;22(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wang C, Lv D, Zhang X, Ni ZA, Sun X, Zhu C. Interleukin‐10‐overexpressing mesenchymal stromal cells induce a series of regulatory effects in the inflammatory system and promote the survival of endotoxin‐induced acute lung injury in mice model. DNA Cell Biol. 2018;37(1):53–61. [DOI] [PubMed] [Google Scholar]
- 250. Zhang S, Jiang W, Ma L, Liu Y, Zhang X, Wang S. Nrf2 transfection enhances the efficacy of human amniotic mesenchymal stem cells to repair lung injury induced by lipopolysaccharide. J Cell Biochem. 2018;119(2):1627–1636. [DOI] [PubMed] [Google Scholar]
- 251. Wang L, Shi M, Tong L, et al. Lung‐resident mesenchymal stem cells promote repair of LPS‐induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation. 2019;42(1):199–210. [DOI] [PubMed] [Google Scholar]
- 252. Jerkic M, Masterson C, Ormesher L, et al. Overexpression of IL‐10 enhances the efficacy of human umbilical‐cord‐derived mesenchymal stromal cells in E. coli pneumosepsis. J Clin Med. 2019;8(6):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Li L, Dong L, Zhang J, Gao F, Hui J, Yan J. Mesenchymal stem cells with downregulated Hippo signaling attenuate lung injury in mice with lipopolysaccharide‐induced acute respiratory distress syndrome. Int J Mol Med. 2019;43(3):1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Silva JD, Lopes‐Pacheco M, De Castro LL, et al. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue‐derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res Ther. 2019;10(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Silva JD, De Castro LL, Braga CL, et al. Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells Int. 2019;2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Zhang X, Chen J, Xue M, et al. Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells in LPS‐induced ARDS mice. Stem Cell Res Ther. 2019;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 257. Li L, Jin S, Zhang Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin‐induced acute lung injury in mice through secretion of exosome. Int J Clin Exp Med. 2015;8(3):3825–3832. [PMC free article] [PubMed] [Google Scholar]
- 258. Kennelly H, Mahon BP, English K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre‐clinical model of COPD. Sci Rep. 2016;6(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kim Y, Kokturk N, Kim J, et al. Gene profiles in a smoke‐induced COPD mouse lung model following treatment with mesenchymal stem cells. Mol Cells. 2016;39(10):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Li X, Zhang Y, Liang Y, et al. IPSC‐derived mesenchymal stem cells exert SCF‐dependent recovery of cigarette smoke‐induced apoptosis/proliferation imbalance in airway cells. J Cell Mol Med. 2016;21(2):265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Cappetta D, De Angelis A, Spaziano G, et al. Lung mesenchymal stem cells ameliorate elastase‐induced damage in an animal model of emphysema. Stem Cells Int. 2018;2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Poggio HA, Antunes MA, Rocha NN, et al. Impact of one versus two doses of mesenchymal stromal cells on lung and cardiovascular repair in experimental emphysema 11 Medical and Health Sciences 1102 Cardiorespiratory Medicine and Haematology. Stem Cell Res Ther. 2018;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Woo J, Sung K, Young J. Effects of Wharton ’ s jelly‐derived mesenchymal stem cells on chronic obstructive pulmonary disease. Regen Ther. 2019;11:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Chen MIN, Huang Z, Bi H, et al. Effects of bone marrow‐derived mesenchymal stem cell transplantation on chronic obstructive pulmonary disease/obstructive sleep apnea overlap syndrome in rats. Mol Med Rep. 2019;20(5):4665–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Sun YQ, Deng MX, He J, et al. Human pluripotent stem cell‐derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30(12):2692–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Duong KM, Arikkatt J, Ullah MA, et al. Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite‐induced airway inflammation in mice. Am J Respir Cell Mol Biol. 2015;53(5):615–624. [DOI] [PubMed] [Google Scholar]
- 267. Zeng SL, Wang LH, Li P, Wang W, Yang J. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol Med Rep. 2015;12(2):2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Mohammadian M, Reza H, Iraj S, Kashani R. Evaluation of simvastatin and bone marrow‐derived mesenchymal stem cell combination therapy on airway remodeling in a mouse asthma model. Lung. 2016;194(5):777–785. [DOI] [PubMed] [Google Scholar]
- 269. Braza F, Dirou S, Forest V, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836–1845. [DOI] [PubMed] [Google Scholar]
- 270. Kang SY, Park DE, Song WJ, et al. Immunologic regulatory effects of human umbilical cord blood‐derived mesenchymal stem cells in a murine ovalbumin asthma model. Clin Exp Allergy. 2017;47(7):937–945. [DOI] [PubMed] [Google Scholar]
- 271. Li Y, Qu T, Tian L, Han T, Jin Y, Wang Y. Human placenta mesenchymal stem cells suppress airway inflammation in asthmatic rats by modulating Notch signaling. Mol Med Rep. 2018;17(4):5336–5343. [DOI] [PubMed] [Google Scholar]
- 272. Mohammadian M, Sadeghipour HR, Jahromi GP, et al. Simvastatin and bone marrow‐derived mesenchymal stem cells (BMSCs) affects serum IgE and lung cytokines levels in sensitized mice. Cytokine. 2019;113:83–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


