Abstract
Kaposiform lymphangiomatosis (KLA) is a rare, life-threatening congenital lymphatic malformation. Diagnosis is often delayed due to complex indistinct symptoms. Blood angiopoietin-2 (ANG2) levels are elevated in KLA and may be useful as a biomarker to monitor disease status. We report a 7-year-old male child with easy bruising, inguinal swelling, and consumptive coagulopathy, diagnosed with KLA. A multimodal treatment regimen of prednisone, sirolimus, vincristine, and adjunctive zoledronate was used. Plasma ANG2 levels were highly elevated at diagnosis but decreased during treatment. The patient showed significant clinical improvement over a 38-month period and normalization of ANG2 levels correlated with resolution of the coagulopathy.
Keywords: angiogenesis, vascular malformations, vincristine
1 |. INTRODUCTION
Kaposiform lymphangiomatosis (KLA) is a rare life-threatening disorder with a 51% 5-year mortality rate and mean interval between diagnosis and death of 2.75 years.1 KLA patients often develop serious complications including consumptive coagulopathy, pericardial and/or pulmonary effusions, ascites, infections, and bone destruction.1,2 KLA is characterized histologically by abnormal lymphangiogenesis, thin-walled lymphatic channels with flattened endothelial cells, and focal areas of spindled endothelial cells.1 Diagnosis of KLA can be challenging as there is a spectrum of disease severity and misdiagnosis is common. Imaging features can be similar to generalized lymphatic anomaly (GLA) and central collecting lymphatic anomalies leading to further misdiagnosis. Tissue biopsy may be necessary for diagnosis but difficult because of coagulopathy and severity of illness. Reliable biomarkers that facilitate earlier diagnosis and monitor disease status could be very helpful. Elevated blood levels of angiogenic factors in KLA patients, including ANG2 and soluble vascular endothelial growth factor receptor 3 (sVEGFR3), have recently been reported.3,4 ANG2 levels were increased in KLA patients and patients with kaposiform hemangioendothelioma and Kasabach-Merritt phenomenon (KHE+KMP), but not generalized lymphatic anomaly.3 ANG2 levels decreased with sirolimus in most KLA and KHE+KMP patients showing a clinical response to treatment.5 Angiopoietin-1 (ANG1) and ANG2 are ligands for endothelial-specific receptor tyrosine kinase Tie2.6 ANG1 promotes blood vessel maturation/stability, while ANG2 can be antagonistic to ANG1 resulting in vessel destabilization, although in certain conditions ANG2 can act as a Tie2 agonist. While ANG2 may play a role in lymphangiogenesis7 and other human diseases,8 why it is elevated in KLA and its role are unclear.
Treatment of KLA is aimed at stabilizing lymphatic overgrowth with partial responses to the mammalian target of rapamycin (mTOR) inhibitor, that is, sirolimus.9,10 Zolendronate is a bisphosphonate used in lymphatic malformation syndromes with osteolysis.11,12 Outcomes with zoledronate have not been described in KLA. We report a male child with KLA and his treatments, clinical responses, and results from a blood biomarker study over 38 months.
2 |. PRESENTATION AND DIAGNOSIS
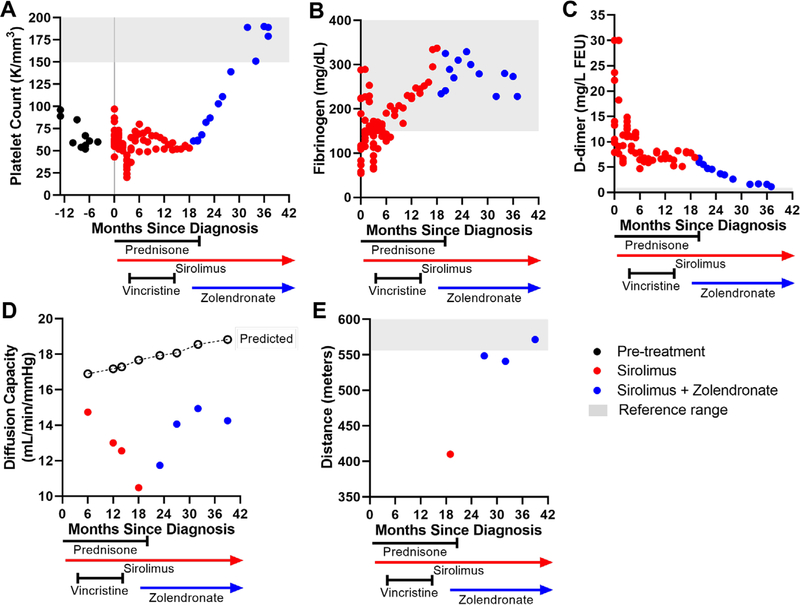
A 7-year-old male child patient, with a history of thrombocytopenia presenting 9 months prior (negative prior work-up from age 6 are presented in Supporting Information as early case presentation), developed a significant cephalohematoma after minor head trauma. Blood work indicated disseminated intravascular coagulopathy (low hemoglobin 9.3 g/dL, platelet count 63 000/mm3, fibrinogen 81 mg/dL, markedly elevated D-dimer > 30 mg/L FEU) (Figure 1A) requiring an infusion of cryoprecipitate. Total body magnetic resonance imaging (MRI) revealed an extensive, infiltrative T2 hyperintense soft tissue lesion involving the entirety of the mesentery and retroperitoneum with encasement of the solid abdominal organs, mesenteric vasculature, and rectal wall. Microcystic lymphatic malformations were also noted in the soft tissues of the lower neck and mediastinum as well as a small superficial soft tissue involvement in the lower aspect of the left upper arm, consistent with a diffuse lymphatic malformation (Figure S1). There was evidence of postcontrast enhancement of the lesion, most notably within the osseous components in the sacrum, right greater than left iliac bone, right posterior acetabulum, and L4 and L5 vertebral bodies indicating bone involvement. Biopsy of the paraspinous muscle revealed multiple thin-walled lymphatic vessels positive for D2–40 (podoplanin) suggestive of KLA diagnosis, although no spindle-shaped cells were noted. The diagnosis of KLA was made secondary to the coagulopathy, radiologic appearance, and pathology.
FIGURE 1.
Hematological, pulmonary, and physical function assessments of response to treatment. Panels (A)-(C) show platelet count (A), fibrinogen (B), and d-dimer (C) measurements in months relative to diagnosis (time 0) and over treatment course. Panel (D) shows pulmonary function test results of diffusion capacity of the lung for carbon monoxide relative to predicted (open circles). Panel (E) shows distance walked in meters during 6-min walk test. Initiation and duration of medications are denoted along x-axis. Normal reference ranges are indicated by gray shading
3 |. TREATMENTS
The patient enrolled in multiple research studies, approved by Institutional Review Boards at the participating institutions. The time course of multimodal treatments is shown in Figures 1 and 2. The standard of care for high-risk KLA (coagulopathy, significant disease-soft tissue, bone disease) is steroids and sirolimus,9 which were started when the diagnosis of KLA was made in this patient. These treatments are used to decrease lymphatic endothelial proliferation, stabilize the lymphatic endothelium, and reduce inflammation. Sirolimus was initiated at the time of KLA diagnosis and titrated based on pharmacokinetic modeling, targeting a trough level of 10–13 ng/mL. Intravenous high-dose methylprednisolone (750 mg) was administered daily for 3 days, then transitioned to 50 mg oral prednisone daily and tapered down to 10 mg daily over 3 months. Platelet count declined below 20 000/mm3 as prednisone was weaned below 10 mg (Figure 1A), therefore vincristine was initiated (weekly for eight doses, biweekly for six doses and every 3 weeks for eight doses). Vincristine can be effective in treating refractory thrombocytopenia13,14 in KHE.15 Prednisone was decreased to 2.5 mg daily with a stable platelet count of 50–60 000/mm3. Attempts to wean prednisone further failed with worsening of thrombocytopenia. Therefore, additional medical regimens were discussed. Bisphosphonates in combination with sirolimus have suggested a synergistic effect when utilized in complicated lymphatic anomalies of the bone (GLA and Gorham Stout Disease)11,12 and in the treatment of malignancies with metastatic bone disease.16 Given his bone involvement and continued thrombocytopenia, around 18 months after diagnosis the patient was initiated on a trial of zoledronate (0.025 mg/kg intravenously monthly for 6 months, then every 3 months for 1 year). The patient was able to discontinue prednisone 1 month after beginning zoledronate. Currently, the patient continues on sirolimus 2.5 mg/twice daily (trough levels 9.4–14.8 ng/mL) and zoledronate (0.025 mg/kg every 6 months).
FIGURE 2.
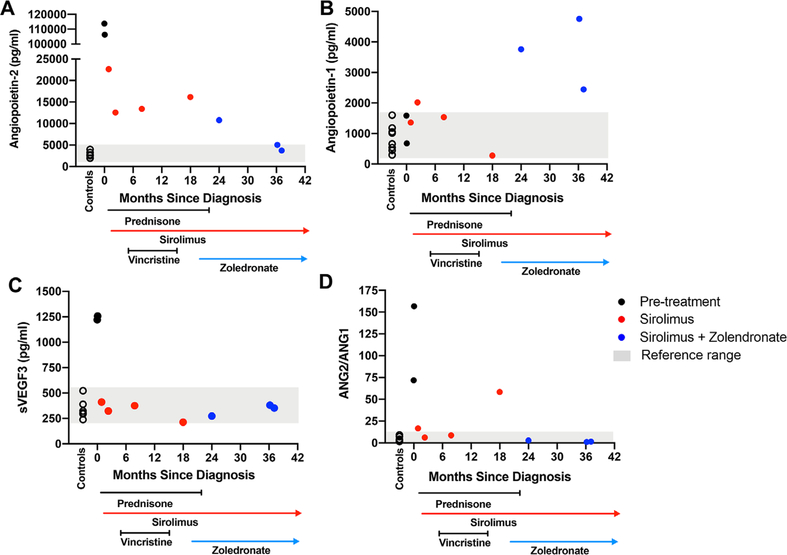
Plasma angiogenic biomarkers before and on treatment. Angiopoietin-2 (ANG2) levels (A), angiopoietin-1 (ANG1) (B), soluble vascular endothelial growth factor receptor 3 (sVEGFR3) (C), and ratio of ANG2/ANG1 (D) were measured by multiplex in plasma from age and sec-matched controls (open circles) and the KLA patient at diagnosis (black circles) and over the treatment course as indicated along x-axis. Normal reference ranges are indicated by gray shading
4 |. CLINICAL AND LABORATORY RESPONSES
Lymphatic malformations were assessed by serial full-body MRI. Partial response of disease was noted on MRI (size and enhancement) after eight weekly doses of vincristine and then stabilized (Figure S1B). During the period when the vincristine frequency and prednisone dose were reduced, the fibrinogen levels continued to increase, while the patient’s platelet count and D-dimer remained abnormal, but stable (Figure 1A–C). With the addition of zoledronate, hematologic parameters progressively improved with decrease in D-dimer and increase in platelets, normalizing 13 months after starting zoledronate (Figure 1A–C).
5 |. PULMONARY RESPONSE
Pulmonary involvement and pleural effusions can be a major contributor to KLA morbidity and mortality.1,17 Computed tomography (CT) scan results showed patchy opacities and no pneumonitis. While we were not clear of the etiology of the findings on CT, we did not think it was sirolimus-induced pneumonitis but most likely due to the KLA disease. Pulmonary function was assessed every 6 months by measuring diffusion capacity (Figure 1D). A minor reduction in diffusion capacity was detected, which worsened during the first year after diagnosis, but improved with the addition of zoledronate. Fatigue and low endurance were evaluated by 6-min walk test, which improved to low normal range at the most recent follow-up (Figure 1E). Oxygen saturations were measured during the walk test and were above 95% on room air. No pulmonary or pericardial effusions developed.
6 |. ORTHOPEDIC RESPONSE
Skeletal health was monitored throughout zoledronate treatment (Figure S2). X-rays of the wrists and knees demonstrated zebra stripes18 but no Erlenmeyer flask deformities, indicating a lack of severe skeletal side effects from the protracted bisphosphonate therapy.19 Bone mineral density showed increased height-adjusted z-scores at the spine, hip, and total body less head, although all remained less than the mean for age. Bone resorption marker, carboxyterminal cross-linking telopeptide (c-telopeptide) of bone collagen, was initially elevated and decreased to normal with zoledronate. Bone formation marker, osteocalcin, remained normal throughout the treatment course, with sirolimus plus zoledronate only mildly suppressing osteocalcin. Bone involvement improved modestly with vincristine and has remained stable. Thoracic scoliosis was managed with bracing and remained stable throughout treatment (Figure S2).
7 |. BIOMARKER STUDY AND RESULTS
Plasma samples before treatment, at the time of diagnosis (T = 0), and during the 38-month treatment period were used for the biomarker study (Figure 2). Control plasma samples were taken from eight males (5–13 years old) with no significant health issues. ANG2, ANG1, sVEGFR3, and tenascin C in the plasma samples was measured by multiplex assay.4 At diagnosis, plasma ANG2 and sVEGFR3 levels were highly elevated (Figure 2A,B). Tenascin C was slightly above controls (Figure S3). ANG2, sVEGFR3, and tenascin C decreased with sirolimus and vincristine. sVEGFR3 and tenascin C normalized with sirolimus and did not change further. ANG2 decreased further during combination treatment, correlating with normalization of platelet counts and D-dimer reductions. ANG2 normalization may be due to the combination therapy or prolonged effects of sirolimus alone. ANG1 levels (Figure 2C) were similar to controls before treatment and on sirolimus but increased when zoledronate was added. The ratio of ANG2/ANG1 was the lowest and most stable during the sirolimus and zoledronate treatment period (Figure 2D). Circulating DNA was purified from plasma obtained before treatment and digital droplet PCR was used to evaluate if the patient had a neuroblastoma RAS mutation (NRAS) p.Q61R reported in some KLA cases.20 DNA from melanoma cells with NRAS p.Q61R was used as a positive control. p.Q61R NRAS was not detected in circulating DNA (Figure S4). This may be due to low abundance of the mutant DNA in the circulation or due to the presence of a different mutation.
8 |. CONCLUSIONS
The KLA patient had an excellent outcome with multimodal treatment, including normalization of platelet counts after the addition of zoledronate and resumption of normal activities. While causality cannot be definitively proven, the pattern of relatively rapid improvement is compelling and suggests an impact from the treatments. The efficacy of this multimodal treatment protocol needs to be tested in more patients in the future. The underlying disease mechanism in KLA is not well understood, although clinical responses to sirolimus9 indicate a role for mTOR signaling in the pathophysiology. Reductions in ANG2 correlated with clinical responses and suggest that this biomarker may be useful in monitoring disease status. The contribution of ANG2 to the pathophysiology of KLA remains unclear but is suspected given that ANG2 can promote lymphangiogenesis and vascular dysfunction.21–27 Further studies in additional KLA patients are needed to validate use of these blood biomarkers in monitoring disease status.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patient and the patient’s family for their consent to participate in the study and supporting publication of this report. The proactive approach by the family in seeking out interdisciplinary expertise led to this synergistic collaboration between physicians and scientists at five major medical centers. They also thank Tricia Pastura for assistance with the biomarker assays. Funding from the Lymphatic Malformation Institute is also acknowledged.
Abbreviations:
- ANG
angiopoietin
- CT
computed tomography
- GLA
generalized lymphatic anomaly
- KHE+KMP
kaposiform hemangioendothelioma and Kasabach-Merritt phenomenon
- KLA
kaposiform lymphangiomatosis
- MRI
magnetic resonance imaging
- mTOR
mammalian target of rapamycin
- NRAS
neuroblastoma RAS mutation
- sVEGFR3
soluble vascular endothelial growth factor receptor 3
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ETHICS STATEMENT
Informed consent was obtained from the patient and family to use photographs of the patient.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Croteau SE, Kozakewich HP, Perez-Atayde AR, et al. 3rd. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safi F, Gupta A, Adams D, Anandan V, McCormack FX, Assaly R. Kaposiform lymphangiomatosis, a newly characterized vascular anomaly presenting with hemoptysis in an adult woman. Ann Am Thorac Soc. 2014;11:92–95. [DOI] [PubMed] [Google Scholar]
- 3.Le Cras TD, Mobberley-Schuman PS, Broering M, Fei L, Trenor CC 3rd, Adams DM. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. 2017;20:163–173. [DOI] [PubMed] [Google Scholar]
- 4.Ozeki M, Nozawa A, Kawamoto N, Fujino A, Hirakawa S, Fukao T. Potential biomarkers of kaposiform lymphangiomatosis. Pediatr Blood Cancer. 2019;66:e27878. [DOI] [PubMed] [Google Scholar]
- 5.LeCras TD, Mobberley-Schumann P, Broering M, Fei L, Trenor III CC, Adams DM. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. 2017;20:163–173. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. [DOI] [PubMed] [Google Scholar]
- 7.Karpanen T, Makinen T. Regulation of lymphangiogenesis—from cell fate determination to vessel remodeling. Exp Cell Res. 2006;312:575–583. [DOI] [PubMed] [Google Scholar]
- 8.Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2:a006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams DM, Trenor CC 3rd, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Successful treatment of kaposiform lymphangiomatosis with sirolimus. Pediatr Blood Cancer. 2015;62:1291–1293. [DOI] [PubMed] [Google Scholar]
- 11.Posadas MD, Viejo Stuart S, Romano O, Giamello A. Gorham-Stout syndrome: a case report. Eur Rev Med Pharmacol Sci. 2014;18: 81–83. [PubMed] [Google Scholar]
- 12.Cramer SL, Wei S, Merrow AC, Pressey JG. Gorham-Stout disease successfully treated with sirolimus and zoledronic acid therapy. J Pediatr Hematol/Oncol. 2016;38:e129–e132. [DOI] [PubMed] [Google Scholar]
- 13.Stirnemann J, Kaddouri N, Khellaf M, et al. Vincristine efficacy and safety in treating immune thrombocytopenia: a retrospective study of 35 patients. Eur J Haematol. 2016;96:269–275. [DOI] [PubMed] [Google Scholar]
- 14.Ganti AK, Landmark JD, Kessinger A, Smith LM, Tarantolo SR. Vincristine-laden platelet transfusion for patients with refractory thrombocytopenia. In Vivo. 2006;20:559–563. [PubMed] [Google Scholar]
- 15.Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Steroid-resistant kaposiform hemangioendothelioma: a retrospective study of 37 patients treated with vincristine and long-term follow-up. Pediatr Blood Cancer. 2015;62:577–580. [DOI] [PubMed] [Google Scholar]
- 16.Mercatali L, Spadazzi C, Miserocchi G, et al. The effect of everolimus in an in vitro model of triple negative breast cancer and osteoclasts. Int J Mol Sci. 2016;17:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes VM, Fargo JH, Saini S, et al. Kaposiform lymphangiomatosis: unifying features of a heterogeneous disorder. Pediatr Blood Cancer. 2015;62:901–904. [DOI] [PubMed] [Google Scholar]
- 18.Al Muderis M, Azzopardi T, Cundy P. Zebra lines of pamidronate therapy in children. J Bone Joint Surg Am. 2007;89:1511–1516. [DOI] [PubMed] [Google Scholar]
- 19.Otero JE, Gottesman GS, McAlister WH, et al. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res. 2013;28:419–430. [DOI] [PubMed] [Google Scholar]
- 20.Ozeki M, Aoki Y, Nozawa A, et al. Detection of NRAS mutation in cell-free DNA biological fluids from patients with kaposiform lymphangiomatosis. Orphanet J Rare Dis. 2019;14:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veikkola T, Alitalo K. Dual role of ANG2 in postnatal angiogenesis and lymphangiogenesis. Dev. Cell. 2002;3:302–304. [DOI] [PubMed] [Google Scholar]
- 22.Yuen D, Grimaldo S, Sessa R, et al. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014;55:3320–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spicer A, Calfee CS. Fixing the leak: targeting the vascular endothelium in sepsis. Crit Care. 2012;16:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazquez C, Casanova ML, Planas A, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–531. [DOI] [PubMed] [Google Scholar]
- 26.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reilly JP, Wang F, Jones TK, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.