Abstract
Background and Objective
To investigate the clinical relevance of CSF myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) testing in a large multicenter cohort.
Methods
In this multicenter cohort study, paired serum-CSF samples from 474 patients with suspected inflammatory demyelinating disease (IDD) from 11 referral hospitals were included. After serum screening, patients were grouped into seropositive myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD, 31), aquaporin-4-IgG-positive neuromyelitis optica spectrum disorder (AQP4-IgG + NMOSD, 60), other IDDs (217), multiple sclerosis (MS, 45), and non-IDDs (121). We then screened CSF for MOG-IgG and compared the clinical and serologic characteristics of patients uniquely positive for MOG-IgG in the CSF to seropositive patients with MOGAD.
Results
Nineteen patients with seropositive MOGAD (61.3%), 9 with other IDDs (CSF MOG + IDD, 4.1%), 4 with MS (8.9%), but none with AQP4-IgG + NMOSD nor with non-IDDs tested positive in the CSF for MOG-IgG. The clinical, pathologic, and prognostic features of patients uniquely positive for CSF MOG-IgG, with a non-MS phenotype, were comparable with those of seropositive MOGAD. Intrathecal MOG-IgG synthesis, observed from the onset of disease, was shown in 12 patients: 4 of 28 who were seropositive and 8 who were uniquely CSF positive, all of whom had involvement of either brain or spinal cord. Both CSF MOG-IgG titer and corrected CSF/serum MOG-IgG index, but not serum MOG-IgG titer, were associated with disability, CSF pleocytosis, and level of CSF proteins.
Discussion
CSF MOG-IgG is found in IDD other than MS and also in MS. In IDD other than MS, the CSF MOG-IgG positivity can support the diagnosis of MOGAD. The synthesis of MOG-IgG in the CNS of patients with MOGAD can be detected from the onset of the disease and is associated with the severity of the disease.
Classification of Evidence
This study provides Class II evidence that the presence of CSF MOG-IgG can improve the diagnosis of MOGAD in the absence of an MS phenotype, and intrathecal synthesis of MOG-IgG was associated with increased disability.
Myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) associated disease (MOGAD) has been regarded as a distinct disease of inflammatory demyelinating diseases (IDDs) involving CNS.1,2 Serologic assays for MOG-IgG, using the full-length human MOG, has been widely accepted as an assay of choice to identify clinically relevant MOG-IgG in patient sera.3,4 A recent cohort study identified 3 patients who only had MOG-IgG in their CSF5; the clinical utility of CSF MOG-IgG detection is not clear.6,7
Herein, we aimed to investigate the clinical utility of CSF testing for MOG-IgG in patients with suspected IDD and examine any clinical, pathologic, or prognostic implications of intrathecal MOG-IgG synthesis, using time-matched serum-CSF sample pairs.
Methods
Study Population and Data Collection
Paired serum and CSF from patients with suspected IDD were included from the prospectively collated database of 11 referral hospitals in Korea, from October 2011 to March 2020. Demographics, clinical, radiologic, and pathologic features of patients were obtained by case report form and medical record review. Disability at the time of sampling was measured by the Kurtzke Expanded Disability Status scale.8
Serologic Assay for MOG-IgG and AQP4-IgG
Assays for serum aquaporin-4 IgG (AQP4-IgG) were performed by an in-house flow cytometric assay using live cells expressing human M23 AQP4, as reported.9,10
Assay for serum MOG-IgG were performed either by cell based assay at the John Radcliffe Hospital, Oxford, UK (103 samples, collected from October 2011 to November 2013),3 or by in-house flow cytometric assay (414 samples) with minor modifications of our previous methods11,12 using full-length human MOG and anti-human IgG1 secondary antibody (Alexa Fluor 488 goat anti-human IgG1, 1:100 dilution; A10631, Invitrogen; Supplementary data 1, links.lww.com/NXI/A642).
Subgroups of Patients With IDD
Participants were divided into 5 subgroups based on published clinical criteria and serologic assay results for MOG-IgG/AQP4-IgG: (1) seropositive MOGAD,4,13 (2) AQP4-IgG positive neuromyelitis optica spectrum disorder (AQP4-IgG + NMOSD),14 (3) other IDDs group including seronegative NMOSD (defined as negative for serum AQP4-IgG and MOG-IgG),14 idiopathic optic neuritis,15 idiopathic acute transvers myelitis (iATM),16 acute disseminated encephalomyelitis (ADEM),17 clinical isolated syndrome (CIS) of the brain,18 and Balo concentric sclerosis,19 (4) multiple sclerosis (MS),18 and (5) neurologic diseases other than IDDs group (non-IDDs, supplementary data 2 for detailed diagnosis, links.lww.com/NXI/A642) (Figure 1A). Patients with other IDDs who tested positive for MOG-IgG only in the CSF were further defined as CSF MOG + IDD.
Figure 1. Diagnostic Flow and CSF MOG-IgG Assay Results.
(A) The flowchart of study participants. (B) Examples of CSF assay for MOG-IgG. (C) The MFI ratio of CSF MOG-IgG assays in all participants. Among 474 patients, 19 seropositive MOGAD, 9 other IDDs, and 4 patients with MS were positive for CSF MOG-IgG. None with AQP4-IgG + NMOSD or with non-IDDs had positive results for CSF MOG-IgG. Red dots indicate samples obtained at the time of first attack, and black dots indicate those at relapsing attacks. The MFIr are plotted in logarithmic axis. AQP4-IgG + NMOSD = aquaporin-4 immunoglobulin G positive neuromyelitis optica spectrum disorder; AQP4+ = aquaporin-4 antibody positive; CIS = clinically isolated syndrome; IDD = inflammatory demyelinating disease; IgG = immunoglobulin G; MFIr = mean fluorescence intensity ratio; MOG = myelin oligodendrocyte glycoprotein; MOGAD = myelin oligodendrocyte glycoprotein antibody associated disease; MS = multiple sclerosis.
CSF MOG-IgG Assay and Cutoff Value
CSF draw and serum sampling were performed within 24 hours in 96.3% of the total CSF/serum pairs, and 99.2% were performed within 1 week. All CSF samples were stored at −80°C until required. The in-house flow cytometric assay for CSF MOG-IgG was performed in SNUH Neuroimmunology Laboratory using HEK - 293 T cells transfected with the full-length human MOG with a minor modification from our assay for serum MOG-IgG.11,12 Briefly, cells were incubated with CSF (dilution 1:2 for 1 hour at 4°C), and anti-IgG Fc secondary antibodies (Alexa Fluor 488 goat antihuman IgG, 1:200 dilution, Jackson) were used.5 The mean fluorescence intensity ratio (MFIr) of the patients' samples were determined using the following formula:
MFIr = green MFI of patient's antibody binding to the dsRed-tagged MOG transfected cells (upper gate on red channel)/green MFI of the same gate with healthy control sera (Figure 1B).
Patients with AQP4-IgG + NMOSD (n = 60),14 relapsing-remitting MS (RRMS, n = 40),18 and non-IDDs (n = 121; supplementary data 2 for diagnoses, links.lww.com/NXI/A642) were classified as the MOG-IgG (−) controls group (n = 221). The cutoff for positivity of the CSF MOG-IgG assay (MFIr: 1.96) was set at 4 SD (0.260) above the mean MFIr values (0.923) of the MOG-IgG (−) control group. If the MFIr values were positive but lower than mean +8 SD (3.00) of the MOG-IgG (−) controls, those were considered as low positive results. All AQP4-IgG + NMOSD, non-IDDs, and most patients with MS (41/45) were negative for CSF MOG-IgG (specificity = 98.2%, 95% CI, 95.53–99.52). CSF positive samples with sufficient volume were confirmed in Oxford.
Corrected CSF/Serum MOG-IgG Index
The MOG-IgG endpoint titers were obtained in any sample positive in the serum or CSF. Serum was titrated in doubling dilutions from 1:10 (range from 1:10 to 1:10,240), and CSF was titrated similarly from 1:2 (range from 1:2 to 1:512). The highest dilution with a positive result was considered the endpoint. To measure the same MOG-IgG isotypes in CSF and serum, the same secondary antibody against anti-IgG Fc (1:200, Jackson) was used for both titrations (supplementary data 3, links.lww.com/NXI/A642).3,20,21
The albumin quotient (Qalb = CSF/serum total albumin level), IgG quotient (QIgG = CSF/serum total IgG level), and IgG index (QIgG/Qalb) were calculated. Using Qalb, the Qlim(IgG) was calculated as previous reported. Considering the possibility of blood-CSF barrier compromise, the corrected CSF/serum MOG-IgG index was calculated for the following 2 conditions, respectively.22,23
For QIgG < Qlim(IgG), the corrected CSF/serum MOG-IgG index = [CSF/serum MOG-IgG titer]/QIgG.
For QIgG > Qlim(IgG), the corrected CSF/serum MOG-IgG index = [CSF/serum MOG-IgG titer]/Qlim(IgG).
The “intrathecal MOG-IgG synthesis” is defined as corrected CSF/serum MOG-IgG indices exceeding 4 or MOG-IgG detected uniquely in the CSF, using IgG Fc as the secondary antibody.24
Independent Confirmation of the CSF MOG-IgG Assay
Among CSF samples tested for MOG-IgG in SNUH Neuroimmunology Laboratory, 45 non-IDD samples (all CSF MOG-IgG (−), consecutively sampled between 2015 and August 2017) and 34 IDD samples (22 seropositive MOGAD, 8 other IDD with unique CSF MOG-IgG, and 4 MS with CSF MOG-IgG), with sufficient volume, were tested for MOG-IgG at the Oxford Autoimmune Neurology Diagnostic Laboratory, Oxford, UK by cell-based assay at a starting dilution of 1:2.3
Statistical Methods
The comparisons between subgroups were performed using Fisher exact test for categorical variables and also Kruskal Wallis test with post hoc analysis (Mann-Whitney U test) for continuous variables. The Spearman rank correlation test was used to evaluate correlations between the serum or CSF parameters and clinical data. A Wilcoxon signed rank test was used to analyze the difference of MFIr in longitudinal CSF data. Statistical analyses were performed using the SPSS software (version 23 for Windows; IBM, Chicago, IL) or the GraphPad Prism software (version 5.0; GraphPad Software Inc., La Jolla, CA).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the SNUH Institutional Review Board (approval number: SNUH [H-1005-023-317, H-1902-083-1,010], Seoul National University Bundang Hospital [B-1007/105–401]). All patients provided written informed consent before participating.
Data Availability
Individual participant data will not be made publicly available due to potential confidentiality concerns related to the rarity of the condition and the small study population. Further information about the datasets is available from the corresponding author upon reasonable request.
Results
Participants
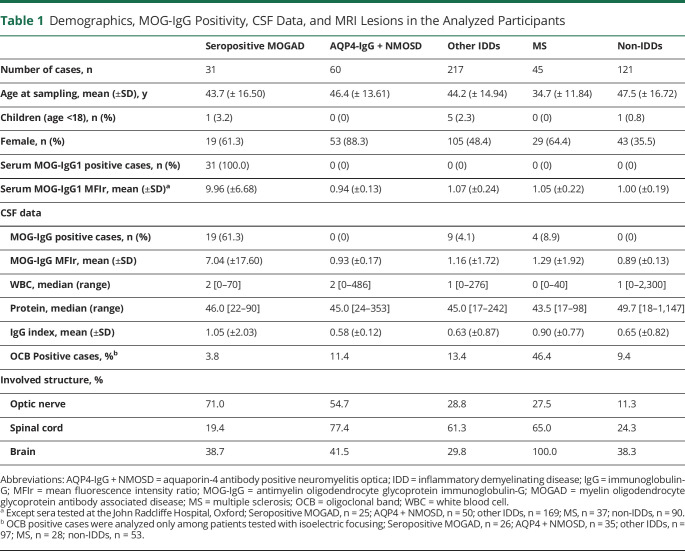
In total, 510 CSF/serum pairs from 474 patients with suspected IDD and complete clinical records were included. After serum screening for MOG-IgG and AQP4-IgG, and applying clinical criteria, our cohort included 31 seropositive MOGAD,4,13 60 AQP4-IgG + NMOSD,14 217 other IDDs (12 seronegative NMOSD,14 49 iON,15,123 iATM,16 8 ADEM,17 24 CIS,18 1 Balo concentric sclerosis19), 45 MS (40 RRMS, 3 secondary progressive MS, 2 primary progressive MS),18 and 121 non-IDDs (Supplementary data 2 for detailed diagnosis) patients (Figure 1). Clinical characteristics and laboratory findings of patients are in Table 1.
Table 1.
Demographics, MOG-IgG Positivity, CSF Data, and MRI Lesions in the Analyzed Participants
CSF MOG-IgG Positivity
Among 474 patients, 19 seropositive MOGAD (61.3%), 9 other IDDs (defined as CSF MOG + IDD, 4.1%), and 4 patients with MS (8.9%) tested positive for CSF MOG-IgG. Neither patients with AQP4-IgG + NMOSD nor with non-IDDs tested positive for CSF MOG-IgG (Figure 1C).
CSF MOG + IDD: Clinical/Radiologic Features and Comparison With Seropositive MOGAD
In the other IDDs group, all of whom were seronegative for MOG-IgG, 9 patients (eTable 1, links.lww.com/NXI/A642, case 1–9 [5 ADEM, 1 CIS, 1 iATM, and 2 ON]) tested positive for CSF MOG-IgG (Figure 1C) and were defined as CSF MOG + IDD. Among them, 6 (66.7%) had relapsing disease courses, 6 (66.7%) were men, and 7 (77.8%) had a good response to steroid therapy for their acute attack. Four relapsing patients did not experience further relapses after long-term maintenance with oral prednisolone (case 2), with rituximab (case 3 and 5), or with monthly infusion of IV immunoglobulin (case 9). All CSF MOG + patients with IDD had brain and/or spinal cord lesions.
The brain and spinal MRI of the CSF MOG + IDD group revealed gray matter lesions involving basal ganglia and thalamus, large confluent lesions, unilateral cerebral cortical lesions, and brainstem lesions, all which have been proposed as typical MRI findings of MOGAD.1,4,25,26. No typical MS lesions such as ovoid lesions adjacent to the body of lateral ventricles or Dawson finger-like lesions were found in the CSF MOG + IDD group. One of our patients had severe cerebral atrophy as a sequelae of severe ADEM-like attacks, as was recently reported in patients with MOGAD (Figure 2).27,28
Figure 2. Radiologic Findings of CSF MOG + Patients With IDD.
(A) Case 1: The brain MRI shows T2 HSI lesions at the right basal ganglia and external capsules in patients who presented with seizure. (B) Case 3: Multiple T2 HSI lesions in the cortex and deep gray matters in the brain MRI of a patient with headache and tetraparesis. (C) After treatment with interferon beta for 5 months, the patient relapsed with multiple cortical and deep gray matter lesions. Her treatment was switched to rituximab, and she had been relapse-free for 3.3 years (D) Case 5: Large, confluent, and disseminated cortical-subcortical lesions and a lesion in the left cerebral peduncle in the brain MRI of a patient who presented with headache, dysarthria, and cognitive Impairment. (E) Her symptoms improved after combined treatment with steroids, intravenous immunoglobulin, and plasmapheresis, and oral steroid maintenance was administered for 5 months 10 days after the cessation of oral steroid treatment. The patient experienced left facial palsy, and her MRI revealed a new lesion involving the right thalamus and internal capsule. She began treatment with rituximab and had been relapse-free for 7 years (F) An MRI taken 7 years after disease onset reveals severe brain atrophy that is more severe in the areas involved in previous attacks. (G) Case 6: Multiple lesions in the pons, unilateral cortex (arrow head), subcortex, medulla, and spinal cord in a patient with visual disturbance, dysarthria, dysphagia, and quadriparesis. (H) Case 2; Lesions involving the unilateral cerebral cortex (arrow head), right thalamus, and cerebral peduncle in a patient with seizures. (I) Case 8: Left optic nerve lesion with asymptomatic cervical spinal cord lesion (arrow head) in a patient with left optic neuritis. (J) Case 9: Right optic nerve lesion with asymptomatic parietal subcortical lesion (arrow head) in a patient with right optic neuritis. HSI = high signal intensity; MOGAD = myelin oligodendrocyte glycoprotein antibody associated disease.
Next, we compared the characteristics of the CSF MOG + IDD group with the seropositive MOGAD group. Although the patients with CSF MOG + IDD group were slightly younger (mean [range], 29.7 [14–47] vs 43.7 [17–70]), and had more brain/spinal cord involvement (100% vs 45.2%), other features including sex, proportion of relapsing patients, and laboratory CSF findings were similar between 2 groups (Table 2).
Table 2.
Comparative Demographics and Clinical Features of Seropositive Patients With MOGAD and CSF MOG + IDD
Pathologic Features of CSF MOG + IDD
Two of the CSF MOG + patients with IDD (case 1 and 5, eTable 1, links.lww.com/NXI/A642) who had recurrent ADEM underwent brain biopsy. In both cases, histopathologic findings showed MOG-dominant myelin loss, infiltration of T cells, with a few CD20 + B cells only in the perivascular area, and the preservation of both axon and AQP4, all of which were consistent with very recent reports of pathologic features in MOGAD.29-31 Activated complement components (C9neo) are present on surface of reactive astrocytes (case 1) and on infiltrating macrophages (case 5).
MS Patients Positive for CSF MOG-IgG
Four patients with MS with CSF MOG-IgG are described in eTable 2. One patient (case 10) with secondary progressive MS had distinctive brain MRI patterns including bilateral inferior temporal lobe lesion and Dawson fingers sign, typical MRI features that distinguish MS from MOGAD in previous studies.25,32 She was also positive for oligoclonal band (OCB) in CSF. A second patient (case 11) had brain MRI lesions involving periventricular white matter and brainstem, experienced a relapse, and has subsequently been relapse free for 2.8 years with glatiramer acetate, a disease modifying treatment for MS. The third patient (case 12) with RRMS was in the early stage of secondary progression at the time of CSF sampling, after 4 years of follow-up despite of interferon beta treatment. The CSF OCBs became positive at follow-up. The last patient with MS with low positive CSF MOG-IgG (case 13) had multiple ovoid lesions in the subcortical white matter and upper cervical spinal cord. She has been relapse free for 2.7 years with interferon beta. These clinical and radiologic findings of our patients with MS support the diagnosis of MS in these patients and suggest that CSF MOG-IgG assay can yield positive test results in some patients with MS (Figure 3).
Figure 3. Histopathologic Findings of 2 CSF MOG + IDD (Cases 1 and 5).
Case 1: (A) The lesion shows marked macrophage infiltration and focal mild perivascular lymphocytic infiltration with reactive astrocytes (arrows). There are some Creutzfeldt-Jakob cells (inlet). (B) Markedly demyelinated lesions with myelin fragment-laden macrophages (LFB) are observed, but (C) axons are relatively preserved (NF). (D) CD4-positive T-cells and (E) CD8-positive T-cells are found in perivascular area. (F) AQP4 immunostain is positive in the membrane of reactive astrocytes, whereas (G) loss of MOG staining is observed in the demyelinated area. (H) Activated complement components (C9neo) are negative for infiltrating macrophages (most negative cells in this figure), but positive in the reactive astrocytes (arrows). Case 5: (I) The lesion also shows marked macrophage infiltration and focal perivascular lymphocytic infiltration. (J) Marked demyelination with myelin fragment-laden macrophages (LFB) but (K) relatively preserved axons (NF) are shown. (L) CD4-positive T-cells are numerous in the perivascular area and scattered in the brain parenchyma, but (M) CD8-positive T-cells are rare. (N) AQP4 is preserved in the membrane of reactive astrocytes, but (O) loss of MOG is predominant. (P) In the infiltrating macrophages and reactive astrocytes (arrows), activated complement components are observed (C9neo). AQP4 = aquaporin-4; CD = cluster of differentiation; H&E = hematoxylin and eosin; LFB = Luxol fast blue; MOG = myelin oligodendrocyte glycoprotein; MOGAD = myelin oligodendrocyte glycoprotein antibody associated disease; NF = neurofilament.
Intrathecal MOG-IgG Synthesis: When and for Whom?
We measured the MOG-IgG titer in both serum and CSF of 36 patients with MOG-IgG (18 seropositive only, 10 dual CSF and serum positive, and 8 CSF positive only) with sufficient volume and also calculated the corrected CSF/serum MOG-IgG index. All but 1 patient, a 14-year-old, were adults (aged ≥18). Twelve patients with MOGAD (4 dual positive and 8 CSF positive only) were considered to have intrathecal synthesis, either a corrected CSF/serum MOG-IgG index exceeding 4 in the dual positive individuals or MOG-IgG detected only in CSF.24 Among these patients with intrathecal synthesis for MOG-IgG, 7 patients had their samples collected during the acute phase of the first attack (red dot in Figure 4A, mean 19 days after disease onset), which suggests that intrathecal synthesis of MOG-IgG can occur early in the disease course of MOGAD. Of note, both the presence of CSF-MOG IgG and intrathecal MOG-IgG synthesis were associated with the involvement of the brain or spinal cord (Figure 4B and C). In our intrathecal MOG-IgG synthesis (+) group, all patients had brain/spinal cord involvement, and most manifested clinically as ADEM (7/12). In contrast, the intrathecal MOG-IgG synthesis (−) group predominantly presented as isolated optic neuritis (75%; p < 0.001). In addition to the difference in lesion location and clinical manifestations, the intrathecal MOG-IgG synthesis (+) group were younger and had higher number of white blood cells in the CSF (eTable 3, links.lww.com/NXI/A642).
Figure 4. Corrected CSF/Serum MOG-IgG Index, Lesion Location, and Clinical Diagnosis in the MOGAD Group.
MOG-IgG titers were measured in both the serum and CSF of 36 patients with MOGAD (28 seropositive MOGAD and 8 CSF MOG + IDD) with a sufficient volume of samples, and the corrected CSF/serum MOG-IgG index was calculated. (A) The corrected CSF/serum MOG-IgG index according to the MOG-IgG titer in the serum and the CSF. Twelve patients with MOGAD (4 seropositive and 8 CSF positive only) have a corrected CSF/serum MOG-IgG index exceeding 4 or MOG-IgG in CSF only and are thereby determined to have intrathecal MOG-IgG synthesis. The CSF/serum MOG-IgG indices could not be calculated because 3 patients were negative for serum MOG-IgG (red box). (B–C) Patients with brain/spinal cord lesions or only optic nerve involvement are significantly different in (B) MOG-IgG positivity in the serum and/or CSF, and (C) intrathecal MOG-IgG synthesis. (D) Clinical diagnoses of patients with MOGAD based on intrathecal MOG-IgG synthesis. All patients with intrathecal MOG-IgG synthesis (+) had involvement in the brain/spinal cord; most (58.3%) manifested with a clinical diagnosis of ADEM. In contrast, in the intrathecal MOG-IgG synthesis (−) group, iON was the most common (75.0%) diagnosis. ADEM = acute disseminated encephalomyelitis; CIS = clinically isolated syndrome; iATM = idiopathic acute transverse myelitis; iON = idiopathic optic neuritis; MOG-IgG = myelin oligodendrocyte glycoprotein immunoglobulin G; MOGAD = myelin oligodendrocyte glycoprotein antibody associated disease; NMOSD = neuromyelitis optica spectrum disorder; SC = spinal cord.
MOG-IgG Titer in the CSF: Association With Disability and CSF Pleocytosis
The degree of disability, number of white blood cells, and level of protein in the CSF of patients with MOGAD were associated with the MOG-IgG titer in the CSF but not in the serum. Moreover, they were also associated with the corrected CSF/Serum MOG-IgG index, representing the degree of intrathecal MOG-IgG synthesis (Figure 5).
Figure 5. Correlation Between MOG-IgG Titers and Clinical Severity.
The MOG-IgG titer and its correlation with the degree of disability, number of white blood cells in the CSF, and level of protein in patients with MOGAD are associated with the MOG-IgG titer (A) in the CSF, but (B) not in the serum. (C) They are also associated with the corrected CSF/serum MOG-IgG index. EDSS = Kurtzke expanded disability status scale; MOG-IgG = myelin oligodendrocyte glycoprotein immunoglobulin G; MOGAD = myelin oligodendrocyte glycoprotein antibody associated disease; WBC = white blood cell.
Longitudinal Data of CSF MOG-IgG
The CSF had been collected more than twice in 28 patients (4 MOGAD, 10 AQP4+ NMOSD, 7 other IDDs, 4 MS, and 3 non-IDDs) because of clinical relapses. Four of these patients (2 MOGAD and 2 MS) tested positive for CSF MOG-IgG in their initial samples, and the mean interval between the first and last sampling was 15.9 months (range, 1–51 months). The CSF MOG-IgG assay results did not differ between initial and follow-up samples in most patients (96.4%, 27/28), but 1 seropositive patient with MOGAD with chronic relapsing inflammatory optic neuropathy converted from low positive (MFIr 2.48) to negative (MFIr 1.48) after 32 months. Furthermore, in 1 seropositive MOGAD patient, 5 serial paired samples of sera and CSF at relapses were all consistently positive for MOG-IgG during 28 months of follow-up (eFigure 2, links.lww.com/NXI/A642).
Independent Confirmation of the CSF MOG-IgG Assay Accuracy
In total, 79 CSF samples were retested at the Oxford Autoimmune Neurology Diagnostic Laboratory, Oxford, UK, for an independent confirmation of CSF MOG-IgG positivity. All CSF samples tested positive for MOG-IgG in Seoul were also positive in Oxford, and the test results of these 2 centers were identical in 77/79 (97.5%) samples. Two additional MOGAD CSF samples that tested positive in Oxford. There was very good correlation between the flow cytometry in Seoul and the CBA score in Oxford (r = 0.7043, p < 0.001; eFigure 1, links.lww.com/NXI/A642).
Discussion
The presence of MOG-IgG, detected on a live test, in the sera of patients with IDD confer a diagnosis of MOGAD.1,4,13 The clinical utility of CSF testing for MOG-IgG is unclear. A recent single center cohort study identified 3 patients with a MOGAD phenotype who had MOG-IgG only in their CSF. A second study identified 4 children with a diagnosis of MS with CSF unique MOG-IgG.5,6 We sought to investigate the clinical relevance of CSF MOG-IgG testing in 474 consecutive patients from 11 tertiary referral hospitals.
In total, we identified 32 individuals with MOG-IgG in their CSF. Among these, 19 of 31 (61%) were seropositive patients with MOGAD and 13 of 262 (5%) were uniquely positive in the CSF: 5 of 8 (62.5%) ADEM, 1 of 24 CIS (4.2%), 1 of 123 (0.8%) iATM, 2 of 49 (4.1%) ON, and 4 of 45 (8.9%) patients with MS. All patients with AQP4-IgG + NMOSD or with non-IDDs were negative.
These data confirm recent reports suggesting both MOGAD and MS cases are positive for MOG-IgG in the CSF. Further work on CSF titres may be required to improve disease specificity in CSF testing as has been necessary for serum testing.
The non-MS, CSF-uniquely positive individuals (n = 9) added another 29% to the serum antibody positive MOGAD group (n = 31). They had comparable clinical, radiologic, pathologic, and prognostic features to those of the seropositive patients with MOGAD. These data suggest that CSF testing in patients with a clinical syndrome compatible with MOGAD will significantly improve the capture of patients with MOGAD, particularly in seronegative patients with ADEM. Of note, 67% (6/9) of these patients had a relapsing disease and 78% (7/9) were untreated. Immunosuppression was associated with a relapse-free follow-up (eTable 1, links.lww.com/NXI/A642). Immunotherapy33 will likely be useful for patients without MS with CSF MOG-IgG, most of whom relapsed.
Although the CSF MOG-IgG assay improves the diagnostic yield for MOGAD, a unique CSF positive result should be interpreted in the clinical context as 4/45 (8.9%) patients with MS were positive. Individuals with MS are rarely seropositive, and when positive is most often borderline as these assays were set-up to rule out typical MS. A similar titration of CSF samples may be needed to improve CSF test specificity. Alternatively, a better understanding of the pathology associated with MOG-IgG may help our interpretation of the presence of these CSF unique MOG-IgG, particularly in MS patients where they are currently not thought to be clinically relevant.
Several studies have shown that patients with MS have intrathecal production of polyspecific antibodies against diverse CNS debris34 or antigens, including MOG.35 These were more evident in secondary progressive MS where ectopic meningeal lymphoid follicles have been identified.36,37 In this study, 1 of 3 patients with secondary progressive MS and a patient with RRMS just before secondary progression were uniquely positive in the CSF. Of note, some of us recently reported the presence of a secondary progressive disease course as an unusual manifestation of seropositive MOGAD.28
Until recently, there has been little evidence for intrathecal synthesis of MOG-IgG.22,38 In our patients with MOGAD, 12 (4 seropositive and 8 CSF positive only) had intrathecal MOG-IgG synthesis. Intrathecal MOG-IgG synthesis was found in samples collected at the time of first attack in 7, which suggested that MOG-IgG synthesis in the CNS is present at disease onset. It can persist throughout the disease course. In addition, both the CSF MOG-IgG titer and corrected CSF/serum MOG-IgG index, but not the serum MOG-IgG titer, correlated with higher disability at the time of attack, more brain and spinal cord involvement, and a higher number of inflammatory cells in the CSF of MOGAD. Finally, in an additional 29% patients with MOGAD, MOG-IgG was only detectable in the CSF. These findings seem to challenge the current dogma that B cells producing CNS-targeting antibodies are produced peripherally and subsequently gain access to the CNS. Rather, we speculate that this finding could imply that the synthesis of MOG-IgG in the CNS can play a role in MOGAD, at least among patients with intrathecal synthesis of MOG-IgG. Nevertheless, further studies, including the experimental model for MOGAD, are required to elucidate the exact role of intrathecal synthesis of MOG-IgG in the pathogenesis of MOGAD.
In the MOGAD group, the CSF MOG-IgG assay yielded a positive result in 61% patients, similar to previous studies (62%–71%).5,6,38 However, we were unable to detect CSF MOG-IgG in most patients with an iON. The one-way flow of CSF from the intracranial subarachnoid space (SAS) to the orbital SAS39 may explain this lack of detection in patients who are sampled via lumbar puncture.
Of note, recent report has demonstrated that the prevalence of CSF MOG-IgG among pediatric encephalitis patients is approximately 1.2%.6,40 In our study, 3 CSF MOG + IDD patients diagnosed with ADEM also had features of seizure/encephalitis.
In conclusion, in the correct clinical context CSF testing for MOG-IgG can improve the capture of MOGAD patients. Intrathecal synthesis of MOG-IgG can be detected in MOGAD from the onset of the disease and is associated with more severe disability at attack, higher number of inflammatory cells in the CSF, and more brain and spinal cord involvement. Further work is required to understand the pathobiology associated with intrathecally produced MOG-IgG to help explain why it seems clinically relevant in non-MS encephalomyelitis and yet seems irrelevant in patients with clinically definite MS.
Acknowledgment
Some of the biospecimens for this study were provided by the Seoul National University Hospital Human Biobank, a member of the Korea Biobank Network, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Glossary
- ADEM
acute disseminated encephalomyelitis
- AQP4+
aquaporin-4 antibody positive
- AQP4-IgG + NMOSD
aquaporin-4 immunoglobulin G positive neuromyelitis optica spectrum disorder
- CIS
clinically isolated syndrome
- iATM
idiopathic acute transvers myelitis
- IDD
inflammatory demyelinating disease
- IgG
immunoglobulin G
- MFIr
mean fluorescence intensity ratio
- MOG
myelin oligodendrocyte glycoprotein
- MOGAD
myelin oligodendrocyte glycoprotein antibody associated disease
- MS
multiple sclerosis
- OCB
oligoclonal band
- RRMS
relapsing-remitting MS
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Contributor Information
Young Nam Kwon, Email: oriman777@gmail.com.
Boram Kim, Email: boram217@gmail.com.
Jun-Soon Kim, Email: bigai300@gmail.com.
Heejung Mo, Email: hjungmo@gmail.com.
Kyomin Choi, Email: libralibrary@daum.net.
Seong-il Oh, Email: seongil.oh@gmail.com.
Jee-Eun Kim, Email: junenr@gmail.com.
Tai-Seung Nam, Email: nts0022@hanmail.net.
Eun Hee Sohn, Email: seh337@daum.net.
Sung Hyuk Heo, Email: shheo73@hanmail.net.
Sang Beom Kim, Email: sbkim@khu.ac.kr.
Key-Chung Park, Email: kcpark@khu.ac.kr.
Sung Sang Yoon, Email: hsyoon96@khu.ac.kr.
Jeeyoung Oh, Email: serein1002@naver.com.
Seol-Hee Baek, Email: virgo3318@gmail.com.
Byung-Jo Kim, Email: nukbj@korea.ac.kr.
Kyung Seok Park, Email: kpark78@naver.com.
Jung-Joon Sung, Email: jjsaint@snu.ac.kr.
Jae Ho Jung, Email: jaeho0130@naver.com.
Seong-Joon Kim, Email: ophjun@snu.ac.kr.
Sung-Hye Park, Email: shparknp@snu.ac.kr.
Patrick Waters, Email: paddy.waters@ndcn.ox.ac.uk.
Study Funding
This work was supported by grant no. 2019M3C7A1031779 and 2020R1C1C1012255 from the National Research Foundation of Korea.
Disclosure
S.-M. Kim has lectured, consulted, and received honoraria from Bayer Schering Pharma, Genzyme, Merck Serono, and UCB; received a grant from the National Research Foundation of Korea and the Korea Health Industry Development Institute Research; is an associate editor of the Journal of Clinical Neurology. S.-M. Kim and Seoul National University Hospital have transferred the technology of flow cytometric autoantibody assay to EONE Laboratory, Korea. The other authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Kim SM, Woodhall MR, Kim JS, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. [DOI] [PubMed] [Google Scholar]
- 3.Waters P, Woodhall M, O'Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotto S, Gajofatto A, Batzu L, et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology. 2019;93:e1867-e1872. [DOI] [PubMed] [Google Scholar]
- 6.Armangue T, Olive-Cirera G, Martinez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19(3):234-246. [DOI] [PubMed] [Google Scholar]
- 7.Waters P, Vincent A. Myelin oligodendrocyte glycoprotein CSF testing needs testing. Neurology. 2019;93(20):871-872. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. [DOI] [PubMed] [Google Scholar]
- 9.Kim SM, Waters P, Woodhall M, et al. Gender effect on neuromyelitis optica spectrum disorder with aquaporin4-immunoglobulin G. Mult Scler. 2017;23(8):1104-1111. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Kim SM, Kim Y-J, et al. Accuracy of the fluorescence-activated cell sorting assay for the aquaporin-4 antibody (AQP4-Ab): comparison with the commercial AQP4-ab assay kit. PLoS One. 2016;12(6):e0180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon YN, Kim B, Ahn S, et al. Serum level of IL-1β in patients with inflammatory demyelinating disease: marked upregulation in the early acute phase of MOG antibody associated disease (MOGAD). J Neuroimmunol. 2020;348:577361. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Kim B, Waters P, et al. Chronic relapsing inflammatory optic neuropathy (CRION): a manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 2018;15(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG–associated disorders. JAMA Neurol. 2018;75(11):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2)177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009;16(3):82-89. [PubMed] [Google Scholar]
- 16.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4)499-505. [DOI] [PubMed] [Google Scholar]
- 17.Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 suppl 2):S23-S36. [DOI] [PubMed] [Google Scholar]
- 18.Thompson A, Banwell B, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 19.Balo J. Encephalitis periaxials concentrica. Arch NeurPsych. 1928;19:242-264. [Google Scholar]
- 20.Gastaldi M, Scaranzin S, Jarius S, et al. Cell-based assays for the detection of MOG antibodies: a comparative study. J Neurol. 2020:267(12):1-10. [DOI] [PubMed] [Google Scholar]
- 21.Waters PJ, Komorowski L, Woodhall M, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92(11):e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akaishi T, Takahashi T, Misu T, et al. Difference in the source of anti-AQP4-IgG and anti-MOG-IgG antibodies in CSF in patients with neuromyelitis optica spectrum disorder. Neurology. 2021;97(1):e1-e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarius S, Franciotta D, Paul F, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 2010;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37(7):1153-1160. [PubMed] [Google Scholar]
- 25.Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140(3):617-627. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody–positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buciuc M, Sechi E, Flanagan EP, Lopez-Chiriboga AS. Unfavorable outcome in highly relapsing MOGAD encephalitis. J Neurol Sci. 2020;418:117088. [DOI] [PubMed] [Google Scholar]
- 28.Kwon YN, Jung JH, Kim S-J, Sung J-J, Kim S-M. Progressive brain atrophy and white matter changes in MOG encephalomyelitis. Neurology. 2020;95(9):402-403. [DOI] [PubMed] [Google Scholar]
- 29.Kwon YN, Waters P, Kim M, et al. Peripherally derived macrophages as major phagocytes in MOG encephalomyelitis. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höftberger R, Guo Y, Flanagan EP, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139(5):875-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takai Y, Misu T, Kaneko K, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. 2020;143(5):1431-1446. [DOI] [PubMed] [Google Scholar]
- 32.Jurynczyk M, Tackley G, Kong Y, et al. Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J Neurol Neurosurg Psychiatry. 2017;88(2):132-136. [DOI] [PubMed] [Google Scholar]
- 33.John JC, Eoin PF, Jiraporn J, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95(2):e111-e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winger RC, Zamvil SS. Antibodies in multiple sclerosis oligoclonal bands target debris. Proc Natl Acad Sci U S A. 2016;113(28):7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecker M, Fitzner B, Wendt M, et al. High-density peptide microarray analysis of IgG autoantibody reactivities in serum and cerebrospinal fluid of multiple sclerosis patients. Mol Cel Proteomics. 2016;15(4):1360-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klawiter E, Piccio L, Lyons J, Mikesell R, O'Connor K, Cross A. Elevated intrathecal myelin oligodendrocyte glycoprotein antibodies in multiple sclerosis. Arch Neurol. 2010;67(9):1102-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089-1104. [DOI] [PubMed] [Google Scholar]
- 38.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killer H, Jaggi G, Flammer J, Miller N, Huber A, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130(pt 2):514. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto D, Uchiyama T, Ohashi T, Iizuka T. Case of steroid-responsive unilateral encephalitis with anti-myelin oligodendrocyte glycoprotein antibodies. Neurol Clin Neurosci. 2017;5:101-102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will not be made publicly available due to potential confidentiality concerns related to the rarity of the condition and the small study population. Further information about the datasets is available from the corresponding author upon reasonable request.