Abstract
Background
Pneumonia has a high incidence rate and is a major cause of mortality in children, mostly community-acquired pneumonia (CAP). Human bocavirus (HBoV), since it first identified in 2005, has been repeatedly associated with respiratory tract infections. Nevertheless, the role and related information of HBoV as a pathogen of CAP has not been fulfilled. Here our study is to assess the epidemiological and clinical features in HBoV-positive children with CAP.
Methods
A total of 878 secretions of lower respiratory samples were obtained, multiplex PCR was used to detect HBoV and other respiratory viruses.
Results
Of all cases, HBoV was detected in 10.0%, with a peak incidence of infection among children < 2 year old, and predominantly noted in autumn and winter. Only 8 patients were HBoV single infection. Co-infection with other respiratory viruses was observed in 86.4%. Moreover, co-infection with bacteria occurred in 27.3% and with Mycoplasma pneumoniae (MP) in 33.0% of HBoV-positive patients. Among all HBoV-positive samples co-infected with bacteria, 87.5% are gram negative bacteria. Compared with HBoV-negative group, age (P = 0.048), wheezing (P = 0.015), tachypnea (P = 0.016), lactate dehydrogenase (P = 0.026) and severe pneumonia (P = 0.023) were statistically significant in HBoV-positive patients. Furthermore, HBoV-positive patients less than 1 year old were more likely to have co-infection with bacteria (P = 0.007).
Conclusions
HBoV can be detected alone in respiratory samples of children with CAP, maybe it is one of the causes of CAP in infants. The high incidence of severe pneumonia was found in HBoV-positive patients compared with HBoV-negative cases may indicate a relationship between severe pneumonia and HBoV.
Keywords: Community acquired pneumonia, Human bocavirus, Co-infection, Bacteria, Mycoplasma pneumoniae
Background
Pneumonia is a frequently diagnosed disease, mostly CAP, for hospitalization and a major cause of mortality in infants and children worldwide, especially in developing countries [1, 2]. Multiple pathogens are capable of causing CAP, viruses are the main pathogens for it. Previous investigations of CAP in children have shown that respiratory syncytial virus (RSV), influenza virus (IFV), human rhinovirus (HRV) were identified as common causes of viral CAP [3–5]. Since HBoV first identified in 2005 [6], it has been repeatedly detected in respiratory tract infections [7]. Nevertheless, the process that HBoV talks with host cells as a pathogen of CAP is unclear, mainly due to the lack of specific cell lines for virus culture or experimental animal models [8].
HBoV is small non-enveloped single-stranded DNA viruses of the Parvoviridae family with four HBoV genotypes, HBoV-1 has been mainly identified in respiratory samples, whereas genotypes 2 to 4 of HBoV are principally detected in intestinal infection [9]. It has been reported with frequencies ranging from 1.9 to 24.6% in respiratory samples [10], mostly from children with acute respiratory tract infection, however, HBoV can also be detected in asymptomatic people [8], Probably because HBoV-1 has been shown to stay in the nasopharynx for weeks and even months after acute infection, thereby posing a challenge to diagnosis of acute HBoV-1 infection [11]. HBoV can exist alone or be detected with other pathogens, however latter is more common [12]. Interestingly, recent data suggest that HBoV as the single causative agent also caused severe acute respiratory tract infection [4], and our research also support this conclusion. By now, the information about the epidemiological and clinical characteristics of CAP caused by HBoV in infants and young children is limited. In this study, we described the prevalence of HBoV in infants and children who presented at the hospital with CAP, the clinical features of the infected children, and a phylogenetic analysis of HBoV was also carried out.
Materials and methods
Patients and clinical assessment
A total of 878 children who were hospitalized with CAP and less than 14 years of age at pediatric department of Yinchuan Women and Children Healthcare Hospital in 2018 and 2019 were enrolled in study. All patients were diagnosed according to WHO’s clinical criteria of CAP including respiratory symptoms and chest radiographic findings as evaluated by attending physicians. As part of the research, study questionnaire with the following variables: age, sex, month of admission, diagnosis, clinical symptoms, physical examination and laboratory examinations on admission were recorded.
Specimen collection
Secretions of lower respiratory tract were obtained by suction using a fine, flexible plastic catheter, and then immediately diluted (the ratio of 1 to 1) with virus protection solution and stored at − 80 °C before use. One sample was collected from each patient.
Detection of HBoV and other viruses
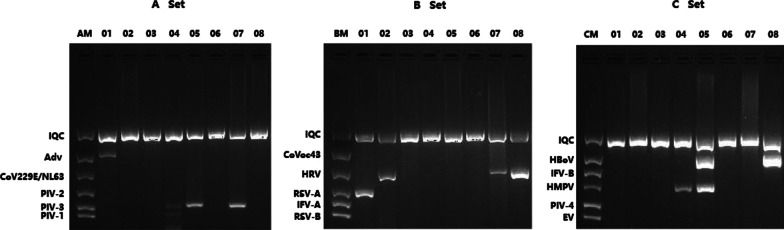
The detection of viruses involves the following steps, extraction of DNA/RNA from samples, reverse transcription and multiplex PCR. Viral nucleic acid was extracted from 100ul sample solution using a Quick DNA/RNA Viral Kit (Zymo Research, America) according to the manufacturer’s instructions, this kit enables simultaneous extraction of viral RNA and DNA. Then, cDNA was synthesized by reverse transcription kit (Transgen, China). We detected viruses using multiplex PCR kit (Seegene, Korea).There are three groups of kit (Fig. 1). A set includes adenovirus (AdV), human coronavirus (HCoV) 229E/NL63 and PIV-1 to 3 (parainfluenza virus types 1, 2 and 3). B set includes human CoVoc43, RSV A/B, HRV and IFV-A. Human metapneumovirus (HMPV), IFV-B (Influenza B virus), PIV-4 (parainfluenza virus types 4), HBoV and Enterovirus (EV) are included in C set. The products of PCR were identified by gel electrophoresis.
Fig. 1.
Results of PCR products electrophoresis. There are 8 samples, A Set, B Set and C Set showed the detection of viruses in 8 samples. No. 01 were positive for AdV and RSV-A; No. 02 was positive for HRV; No. 03 was negative for viruses; No. 04 were positive for PIV-1, PIV-3 and HMPV; No. 05 were positive for HBoV, PIV-3 and HMPV; No. 06 shows no virus; No. 07 were positive for HRV and PIV-3; No. 08 were positive for HBoV and HRV. AM, A marker; BM, B marker; CM, C marker; IQC, internal quality control
Detection of respiratory bacteria
All lower respiratory tract specimens from patients were routinely submitted to the clinical laboratory of the pediatric department of Yinchuan Women and Children Healthcare Hospital. Specimens were planted on blood-, chocolate- (with vancomycin), MacConkey-agar plate for bacteria cultures.
Detection of MP
Venous blood was collected from children, and centrifuged to separate the serum, and the antibody against MP was detected by passive particle agglutination kit (SERODIA®-MYCO II), all specimens were tested in accordance with the methods specified in the kit, single serum antibody titer ≧ 1:40 indicates positive infection.
Phylogenetic analysis
We selected eight HBoV-positive samples and sequenced partial fragments of NS1 (600 bp). The nucleotide sequences of the NS1 gene were compared with other sequences available in GenBank (including HBoV reference strains BLR/Minsk/11/14, BLR/Minsk/10/14, ST1, ST2, JX2239, YOK/08/104, CU6, BLR/Gomel/285/15, BLR/Mogilev/241/14, USA/UNM-0238, USA/TCNP-0070, Eg/BSU-1, Eg/BSU-2, Eg/BSU-3, Mty117, BRA/TO-40, BRA/TO-57, BRA/TO-243, BRA/TO-237, Canine minute virus and Human parvovirus B19). Phylogenetic tree with 1,000 bootstrap replicates was generated using the maximum likelihood method with MEGA 7.0 software.
Statistical analysis
Analyses were conducted using SPSS 22.0. Measurement data do not accorded with normal distribution were expressed as median (IQR), and data were compared by rank sum test. Categorical variables were expressed as numbers or percentages, proportion were compared by chi-squared.Two-sided P values < 0.05 were considered statistically significant.
Results
Characteristics of patients
We collected a total of 878 samples of the respiratory tract secretions from children with CAP. The male (n = 510) to female (n = 368) ratio was 1.4:1, and the median age was 30 months (age range = less than 1 month to 13 years 5 months). 52.6% of patient were aged < 2, 33.8% aged 2 -5 and 13.6% aged ≧ 5 years.
Detection of pathogens
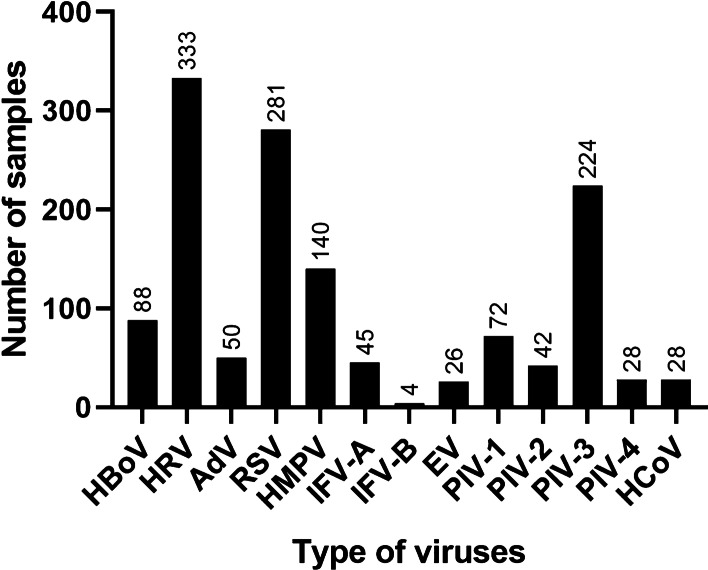
Of the 878 patients with CAP, virus was positive in 749 (85.3%) patients, bacteria in 217 (24.7%) patients, and MP in 184 (21%) patients (Table 1). HBoV was detected in 88 samples (10.0%), and detection rate of other viruses (in descending order) were as follows (Fig. 2): HRV (n = 333, 37.9%), RSV (n = 281, 32.0%), PIV-3 (n = 224, 25.5%), HMPV (n = 140, 15.9%), PIV-1 (n = 72, 8.2%), AdV (n = 50, 5.7%), IFV-A (n = 45, 5.1%), PIV-2 (n = 42, 4.8%), PIV-4 (n = 28, 3.2%) and HCoV (n = 28, 3.2%), EV (n = 26, 3.0%), IFV-B (n = 4, 0.5%).
Table 1.
The detection of pathogen in all samples
| Variable | n (%) |
|---|---|
| n = 878 | |
| Virus-positive | 749 (85.3%) |
| Bacterial-positive | 217 (24.7%) |
| Mycoplasma-positive | 184 (21.0%) |
| HBoV-positive | 88 (10.0%) |
| n = 88 | |
| HBoV only | 8 (9.1%) |
| HBoV + other viruses | 76 (86.4%) |
| HBoV + bacteria | 24 (27.3%) |
| HBoV + MP | 29 (33.0%) |
Fig. 2.
The detection of respiratory viruses
Viral seasonal distribution of HBoV
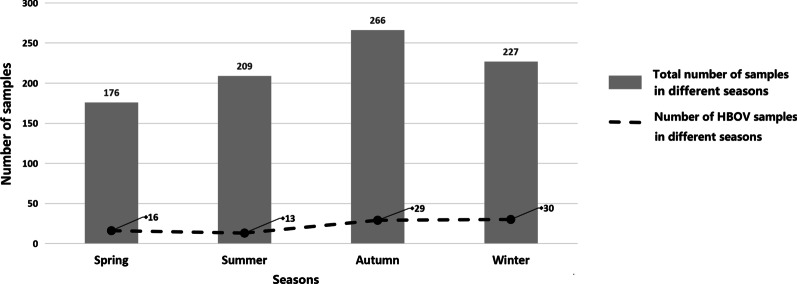
The seasonal distributions of all patients with HBoV infections in 2018 and 2019 were shown in Fig. 3, HBoV infection were detected in each season with a peak incidence in winter (34.1%), followed by autumn (33.0%).
Fig. 3.
Viral seasonal distribution of HBoV. Spring: February to April; Summer: May to July; Autumn: August to October; Winter: November to January
Clinical characteristics of patients with or without HBoV
Patients were divided into two groups (Table 2). Among the 88 HBoV-positive patients and 790 HBoV-negative patients, 57 (64.8%) versus 453 (57.3%) were male and 31 (35.2%) versus 337 (42.7%) were female, no significant difference in gender between two groups (P = 0.180).
Table 2.
Clinical characteristics of HBoV-positive and HBoV-negative patients
| Variable | HBoV-positive (n = 88) | HBoV-negative (n = 790) | X2/Z | P |
|---|---|---|---|---|
| Gender | 1.796 | 0.180 | ||
| Male | 57 (64.8%) | 453 (57.3%) | ||
| Female | 31 (35.2%) | 337 (42.7%) | ||
| Age | 6.059 | 0.048* | ||
| < 2 | 56 (63.6%) | 406 (51.4%) | ||
| 2–5 | 26 (29.5%) | 271 (34.3%) | ||
| ≧ 5 | 6 (6.8%) | 113 (14.3%) | ||
| Clinical features | ||||
| Cough | 85 (96.6%) | 759 (96.1%) | 0.056 | 1.000 |
| Wheezing | 25 (28.4%) | 140 (17.7%) | 5.926 | 0.015* |
| Tachypnea | 15 (17.0%) | 71 (9.0%) | 5.819 | 0.016* |
| Pharyngeal hyperaemia | 65 (73.9%) | 613 (77.6%) | 0.627 | 0.429 |
| Rales | 30 (34.1%) | 214 (27.1%) | 1.935 | 0.164 |
| Fever | 58 (65.9%) | 506 (64.1%) | 0.119 | 0.730 |
| WBC, 109/L | 9.2 (6.9, 11.5) | 8.5 (6.8, 10.8) | − 1.302 | 0.193 |
| HGB, g/L | 128.0 (116.0, 137.0) | 127.0 (118.0, 137.0) | − 0.012 | 0.990 |
| LDH, U/L | 306.5 (271.8, 344.3) | 291.0 (254.5, 335.0) | − 2.231 | 0.026* |
| AST, U/L | 34.6 (28.0, 39.0) | 32.2 (25.6, 39.1) | − 1.384 | 0.178 |
| ALT, U/L | 13.9 (9.9, 20.1) | 14.1 (10.1, 21.9) | − 0.558 | 0.577 |
| Hospitalization days | 7.0 (5.0, 8.8) | 6.0 (5.0, 8.0) | − 0.770 | 0.441 |
| Severe pneumonia | 10 (11.4%) | 39 (4.9%) | 6.207 | 0.023* |
Measurement data were expressed as median (IQR), and data were compared by rank sum test; Categorical variables were expressed as number and percentage, proportion were compared by chi-squared
Fever: T ≧ 37.5℃ (axillary temperature)
Reference levels: WBC (4.4–11.9)109/L, HGB (112–149) g/L, LDH (120–250) U/L, AST (13–35) U/L, ALT (7–40) U/L
*Age, Wheezing, Tachypnea, LDH and Severe pneumonia were statistically significant between the two groups (P < 0.05)
HBoV was detected at a significantly higher rate in patients aged < 2 years (63.6%), followed by aged 2–5 (29.5%) and aged ≧ 5 years (6.8%) (P = 0.048). Cough, pharyngeal hyperaemia and fever were the most frequent symptoms in HBoV positive children (P > 0.05). In addition, significantly more patients in HBoV positive groups developed wheezing (P = 0.015) and tachypnea (P = 0.016). Of interest, we observed that lactate dehydrogenase (LDH) in HBoV positive patients was higher than that in negative patients for laboratory test, the difference was statistically significant (P = 0.026). However, no significant difference between groups was noted for rales, hospitalization days, white blood cells count (WBC), hemoglobin (HGB), alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Meanwhile, for those patients with CAP infected with HBoV, significantly more developed severe pneumonia than in those without HBoV (P = 0.023).
Co-infection with other viruses and bacterium
Among the patients who were positive for HBoV, HBoV alone was detected in 8 (9.1%), co-infection with two or more viruses in 76 (86.4%), with bacteria in 24 (27.3%), and co-infection with MP in 29 (33.0%), shown in Table 1. Of all 76 samples co-infected with other viruses, 47 samples with one type of virus detected, 24 samples with two type of viruses, 4 samples with 3 types of viruses, only 1 sample with four types of viruses (Table 3).The viruses most frequently co-infected with HBoV was HRV (n = 28), followed by RSV (n = 27), other viruses were shown in Fig. 4. In addition, there are 24 HBoV-positive samples co-infected with bacteria, as was showed in Table 4, 87.5% are gram negative bacteria, Escherichia coli were detected in 7 (29.2%) samples, which was dominant bacteria, Klebsiella pneumoniae was subordinate and in 5 (20.8%) samples.
Table 3.
The type of HBoV co-infected with other viruses
| Type of co-infection | n (%) | |
|---|---|---|
|
Two types of viruses (n = 47, 53.4%) |
HBoV + IFV-A | 1 (1.1%) |
| HBoV + RSV | 15 (17.0%) | |
| HBoV + HRV | 18 (20.5%) | |
| HBoV + HMPV | 2 (2.3%) | |
| HBoV + PIV-1 | 2 (2.3%) | |
| HBoV + PIV-3 | 7 (8.0%) | |
| HBoV + EV | 1 (1.1%) | |
| HBoV + AdV | 1 (1.1%) | |
|
Three types of viruses (n = 24, 27.3%) |
HBoV + HCoV + HMPV | 1 (1.1%) |
| HBoV + PIV-3 + RSV | 4 (4.5%) | |
| HBoV + PIV-4 + EV | 1 (1.1%) | |
| HBoV + RSV + IFV-A | 1 (1.1%) | |
| HBoV + HCoV + RSV | 1 (1.1%) | |
| HBoV + HRV + PIV-3 | 3 (3.4%) | |
| HBoV + AdV + PIV-3 | 1 (1.1%) | |
| HBoV + HRV + EV | 2 (2.3%) | |
| HBoV + AdV + HMPV | 1 (1.1%) | |
| HBoV + AdV + HRV | 1 (1.1%) | |
| HBoV + RSV + HMPV | 1 (1.1%) | |
| HBoV + IFV-A + HMPV | 1 (1.1%) | |
| HBoV + IFV-A + RSV | 1 (1.1%) | |
| HBoV + PIV-1 + RSV | 2 (2.3%) | |
| HBoV + PIV-1 + HRV | 1 (1.1%) | |
| HBoV + EV + PIV-3 | 1 (1.1%) | |
| HBoV + HMPV + PIV-3 | 1 (1.1%) | |
|
Four types of viruses (n = 4, 4.5%) |
HBoV + AdV + HRV + PIV-1 | 1 (1.1%) |
| HBoV + PIV-2 + PIV-3 + HRV | 1 (1.1%) | |
| HBoV + PIV-1 + PIV-3 + RSV | 1 (1.1%) | |
| HBoV + HMPV + EV + HRV | 1 (1.1%) | |
|
Five types of viruses (n = 1, 1.1%) |
HBoV + PIV-1 + PIV-3 + RSV + HMPV | 1 (1.1%) |
The percentage in the table represent the proportion of the subjects to the number of HBoV-positive cases (n = 88)
Fig. 4.
Co-infection distribution with HBoV
Table 4.
Co-infection of HBoV with bacteria
| Bacteria (n = 24) | n (%) |
|---|---|
| Escherichia coli | 7 (29.2%) |
| Klebsiella pneumoniae | 5 (20.8%) |
| Enterobacter cloacae | 4 (4.5%) |
| Streptococcal pneumonia | 2 (2.3%) |
| Pseudomonas aeruginosa | 2 (2.3%) |
| Bordetella bronchiseptica | 1 (1.1%) |
| Staphylococcus aureus | 1 (1.1%) |
| Enterobacter aerogenes | 2 (2.3%) |
| Klebsiella oxytoca | 1 (1.1%) |
| Stenotrophomonas maltophilia | 1 (1.1%) |
Clinical characteristics of HBoV-positive patients with or without bacteria
As was showed in Table 5, HBoV-positive patients less than 1 year old are more likely to co-infection with bacteria (P = 0.007), with the ratio of 66.7%. while there were no significant difference in the frequency and numerical value of other clinical features (gender, cough, wheezing, pharyngeal hyperaemia, rales, T≧38.5℃, severe pneumonia, WBC, HGB, AST, ALT, LDH, C-reactive protein, procalcitonin, neutrophilic granulocyte percentage and hospitalization duration) between the two group.
Table 5.
Clinical characteristics of HBoV-positive patients with or without bacteria
| Variable | With bacteria (n = 24) | Without bacteria (n = 64) | X2/Z | P |
|---|---|---|---|---|
| Gender | 1.513 | 0.219 | ||
| Male | 18 (75.0%) | 39 (60.9%) | ||
| Female | 6 (25.0%) | 25 (39.1%) | ||
| Age | 14.245 | 0.007* | ||
| < 1 | 16 (66.7%) | 16 (25.0%) | ||
| 1–2 | 5 (20.8%) | 19 (29.7%) | ||
| 2–3 | 1 (4.2%) | 9 (14.1%) | ||
| 3–5 | 1 (4.2%) | 15 (23.4%) | ||
| ≧ 5 | 1 (4.2%) | 5 (7.8%) | ||
| Clinical Features | ||||
| Cough | 22 (91.7%) | 63 (98.4%) | 2.430 | 0.179 |
| Wheezing | 9 (40.9%) | 16 (27.6%) | 1.318 | 0.251 |
| Tachypnea | 4 (16.7%) | 11 (17.2%) | 0.003 | 1.000 |
| Pharyngeal hyperaemia | 20 (83.3%) | 45 (71.4%) | 1.304 | 0.408 |
| Rales | 11 (45.8%) | 19 (31.1%) | 1.627 | 0.202 |
| T ≧ 38.5℃ | 7 (29.2%) | 25 (39.1%) | 0.739 | 0.390 |
| WBC, 109/L | 9.1 (7.1, 13.7) | 9.2 (6.8, 11.4) | − 0.773 | 0.440 |
| NEUT, % | 46.4 (19.4, 66.5) | 38.8 (30.3, 61.5) | − 0.473 | 0.636 |
| LDH, U/L | 312.5 (284.3, 363.0) | 304.0 (270.3, 342.5) | − 0.947 | 0.344 |
| AST, U/L | 36.9 (29.8, 43.6) | 33.8 (27.5, 38.1) | − 1.474 | 0.140 |
| ALT, U/L | 18.3 (10.9, 24.9) | 13.5 (9.2, 17.9) | − 1.964 | 0.050 |
| CRP, mg/L | 6.0 (1.0, 25.3) | 3.5 (1.1, 8.7) | − 1.010 | 0.312 |
| PCT, ng/mL | 0.29 (0.1, 3.7) | 0.31 (0.11, 5.8) | − 0.972 | 0.331 |
| Hospitalization days | 7.0 (5.0, 9.0) | 6.0 (5.0–8.0) | − 0.826 | 0.409 |
| Severe pneumonia | 4 (16.7%) | 6 (9.4%) | 0.921 | 0.451 |
Measurement data were expressed as median (IQR), and data were compared by rank sum test; Categorical variables were expressed as number and percentage, proportion were compared by chi-squared
CRP, C-reactive protein; PCT, procalcitonin
Reference levels: WBC (4.4–11.9)109/L, NEUT (40–75) %, LDH (120–250) U/L, AST (13–35) U/L, ALT (7–40) U/L, CRP (0–10) mg/L, PCT (0–0.046) ng/mL
*Age was statistically significant between the two groups (P < 0.05)
Clinical characteristics of HBoV-positive patients with or without MP
Of all HBoV-positive patients, 29 (33.0%) co-infected with MP. Rales and fever were the more frequent in HBoV-positive patients co-infected with MP than without MP, but there were no statistical significance difference. Higher level of AST was found in HBoV-positive patients without MP (P = 0.004). In Table 6, data were compared between two groups, we found that the difference had no statistical for other clinical features.
Table 6.
Clinical characteristics of HBoV-positive patients with or without MP
| Variable | With MP (n = 29) | Without MP (n = 59) | X2/Z | P |
|---|---|---|---|---|
| Gender | 1.107 | 0.293 | ||
| Male | 21 (72.4%) | 36 (61.0%) | ||
| Female | 8 (27.6%) | 23 (39.0%) | ||
| Age | 3.313 | 0.191 | ||
| < 2 | 17 (58.6%) | 39 (66.1%) | ||
| 2–5 | 8 (27.6%) | 18 (30.5%) | ||
| ≧ 5 | 4 (13.8%) | 2 (3.4%) | ||
| Clinical features | ||||
| Cough | 27 (93.1%) | 58 (98.3%) | 1.598 | 0.252 |
| Wheezing | 5 (20.8%) | 20 (35.7%) | 1.732 | 0.292 |
| Tachypnea | 4 (13.8%) | 11 (18.6%) | 0.324 | 0.765 |
| Rales | 14 (48.3%) | 16 (28.6%) | 3.248 | 0.071 |
| Fever | 18 (72.0%) | 35 (60.3%) | 1.028 | 0.455 |
| WBC, 109/L | 7.6 (6.6, 10.5) | 10.0 (7.4, 12.0) | − 1.633 | 0.102 |
| CRP, mg/L | 3.8 (1.8, 16.5) | 3.9 (0.94, 11.2) | − 0.284 | 0.776 |
| LDH, U/L | 302.0 (270.0, 338.0) | 311.0 (273.0, 345.0) | − 0.705 | 0.480 |
| AST, U/L | 31.3 (26.5, 35.3) | 36.1 (30.0, 40.8) | − 2.857 | 0.004* |
| ALT, U/L | 12.8 (8.7, 16.7) | 15.6 (10.4, 21.2) | − 1.786 | 0.074 |
| Hospitalization days | 7.0 (5.0, 8.8) | 6.0 (5.0, 8.0) | − 0.171 | 0.864 |
| Severe pneumonia | 2 (6.9%) | 8 (13.8%) | 0.857 | 0.487 |
Measurement data were expressed as median (IQR), and data were compared by rank sum test; Categorical variables were expressed as number and percentage, proportion were compared by chi-squared
Reference levels: WBC (4.4–11.9)109/L, CRP (0–10) mg/L, LDH (120–250) U/L, AST (13–35) U/L, ALT (7–40) U/L
*AST was statistically significant between the two groups (P < 0.05)
Phylogenetic analysis
The partial fragments of NS1 (600 bp) of 8 positive specimens for HBoV were selected and sequenced, we found that the sequences of those specimens were consistent. HBoV strains used in the phylogenetic analysis included the strains obtained in our study in Ningxia, representative strains of HBoV-1, 2 and 3, canine minute virus and human parvovirus B19. Based on phylogenetic analysis (Fig. 5), HBoV strains in this research clustered with the HBoV-1 genotype, and sequences were genetically related (nucleotide identity was 100%) to previous strains detected in Sweden (DQ000495, DQ000496), China (MN887276), Japan (AB551032), Thailand (EF203920), United States (MF374981, MF374982) and other strains showed in Fig. 5.
Fig. 5.
Phylogenetic analysis of the partial NS1 nucleotide sequences of HBoV. Phylogenetic tree with 1,000 bootstrap replicates was generated using the maximum likelihood method with MEGA 7.0 software. The number adjacent to the node represents the bootstrap value. HBoV sequences marked with pentagram was generated from the present study, and other reference sequences were obtained from GenBank
Discussion
In this study, we used multiplex PCR to detect HBoV and other viruses in hospitalized CAP children under 14 years old in Ningxia, China. In all 878 cases, virus was detected in the highest rate, which is consistent with previous studies [1, 13, 14], followed by bacteria, and the lowest was MP, certainly, different age groups have different susceptibility to pathogens. The number of HBoV-positive cases were 88 (10.0%) in our study, and the detection rate of HBoV varies in different countries, which is related to different regions, genetic and detection methods. As previously reported [15], the worldwide average prevalence of HBoV in respiratory tract samples ranged from 1.0% (CI 0.0–2.0) to 56.8% (CI 46.9–66.8), and the total prevalence estimates in respiratory infections was 6.3% (CI 6.2–6.4).The other common viruses in our research are HRV and RSV, this is in line with previous studies [16, 17].
The seasonal peaks of HBoV infections vary among countries because of differences in climatic, geographic factors and population enrolled. In this study, HBoV activity peaked in winter, followed by autumn, it was possibly due to the dry, cold weather, and in agreement with the previous report [18, 19]. In contrast, the seasonal prevalence of HBoV in Changsha and Guangzhou peaked in summer [10, 20], perhaps, hot and humid weather was responsible for that.
In our study, HBoV infected children aged less than 1 month to 13 years 5 months with CAP, the most typical age group was less than 2-years-old, which indicated that infants with lower immunity are more likely to be infected with HBoV. Other studies have also reported HBoV infection is more common in children under two years old, and only rarely has been found in adults and the elderly age [21, 22]. With respect to the clinical features of HBoV infection, our research demonstrated that cough, pharyngeal hyperaemia and fever were the most frequently observed, which were also commonly seen among CAP without HBoV. Thus, clinically, it is impossible to differentiate patients with pneumonia caused by HBoV infection or other pathogens from those symptoms. Interestingly, patients infected with HBoV are more likely to cause wheezing, and it is already known that viral infection could trigger airway hyperresponsiveness and inflammatory immune response, which leading to wheezing, these reactions may be more susceptible to occur after HBoV infection, association of it in respiratory samples and wheezing has been also described in other researches [23–25]. Furthermore, significantly more patients in HBoV positive groups developed tachypnea, this was possibly due to the high incidence of wheezing. We also observed LDH was higher in HBoV-positive patients, which was universally elevated in many pulmonary diseases and has been reported in several studies to be associated with disease severity [26, 27]. Indeed, our data suggest that HBoV-positive patients with CAP were more likely to progress into severe pneumonia, the result was consistent with other studies [28, 29]. Researcher have pointed out that HBoV-1 infection in polarized primary human airway epithelia (HAE) caused airway epithelial damage, including disruption of the tight junction barrier, loss of cilia, and epithelial cell hypertrophy [30]. And among all the HBoV-positive patients, 8 cases were infected alone. Taken together, HBoV may be one of the pathogens for infants and young children with CAP. Other published reports also implied that there is a significant association between HBoV infection and CAP in children [31–33].
HBoV mono-infection is rare, co-infection was observed frequently. There were no significant differences in clinical characteristics between patients with HBoV infection alone and patients with co-infection in our study. Of all the cases infected with the HBoV, 90.9% were co-infections with other pathogen. By far, few published reports demonstrated detailed type of co-infection of HBoV with other viruses and bacterium for CAP in children, in this study, we counted all the types of viruses and bacteria co-infected with HBoV, so as to provide more reference information for clinical work. 86.4% of the HBoV-positive patients co-infected with other respiratory viruses, as showed in Table 3, double infection was the most common, accounting for 53.4%, we even detected four types of viruses co-infected with HBoV in respiratory secretions. Of all co-infected viruses, the top three were HRV, RSV and PIV-3, previous reports showed the most frequently co-infected were RSV, HRV, HMPV and PIV-3 [31, 34, 35], our data were in line with it. Bacteria was detected in 27.3% of HBoV-positive patients, and Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae were principal types of bacteria detected in HBoV-positive patients,87.5% are gram negative bacteria, and the bacterium spectrum was consistent with those in bacterial pneumonia in children. HBoV has been detected in children with or without respiratory symptoms [36–39], and it was found in some asymptomatic children undergoing elective surgery and tonsillectomy [40, 41], which suggest that there is a latent or persistent infections of HBoV in respiratory tract, and a study demonstrated HBoV-1 is often detectable with other viruses in asymptomatic patients, promoting the reactivation of a latent virus by a super-infection [42], all of the above may explain the high prevalence of co-infections.
In our study, dozens of HBoV-positive cases co-infected with bacteria, so we analyzed the clinical characteristics of HBoV-positive patients cases with or without bacteria. In consideration of the age distribution between two groups, HBoV-positive patients less than 1 year old are more susceptible to bacterial infection. In agreement with previous study [43], no significant differences were found in term of frequencies of cough, wheezing and number of hospitalization days. Besides, other clinical features in Table 5 with no statistical significance. Higher proportion of patients with wheezing, rales and severe pneumonia, and higher levels of LDH, AST, ALT and CRP were found in patients with HBoV co-infected with bacteria. Though their statistical significance might had been limited by small sample size, still these might suggest bacterial co-infection would trigger exacerbation of pneumonia.
In recent years, MP showed a yearly upward trend in the detection of pneumonia, this study reported the frequency of MP in pediatric patients diagnosed with CAP was identified in 21.0%, which was similar to several reports [44, 45], however, other studies report lower frequencies [46, 47]. 33.0% of HBoV-positive patients co-infected with MP in our research, compared with the other group who did not co-infected with MP in HBoV infection children, lower level of AST was found, but a study showed that Mycoplasma pneumoniae pneumonia patients in the acute phase had higher levels of AST than those of the patients in the recovery patients and healthy children [48]. It was also reported that AST level of patients with Chlamydia pneumonia was higher than that of Mycoplasma pneumonia [49]. In our study, MP is not the single pathogenic agent of CAP, and viral infection can also increase AST level. In addition, existing laboratory testing techniques are difficult to distinguish between MP carrier and infection status, so the basis for diagnosis of MP infection is still controversial [50], and the results of serum antibody may be false positive or false negative. So, we still need a lot of experiments to confirm this result. For other indicators in Table 6, there was no statistically significant differences between the two groups. These findings revealed that to distinguish CAP patients with HBoV co-infected with MP or not can rarely be established based on the clinical presentation alone.
We selected eight HBoV-positive samples, sequenced partial fragments of NS1, and found that the sequences of those specimens were consistent, which showed a high nucleotide identity between HBoV sequences, and phylogenetic analysis demonstrated that they belonged to HBoV-1. Similar results were revealed in previous studies [32, 51, 52].
This study has the following limitations. Firstly, we may have missed additional viruses not detected by current panels of PCR in our study. Secondly, all our study subjects were hospitalized patients with CAP, the results are not necessarily generalizable to outpatient clinics.
Conclusions
Our study described the epidemiological and clinical features in HBoV-positive infants and children with CAP, and the findings highlighted the importance of HBoV infection in infants and children with CAP. The high incidence of severe pneumonia was found in HBoV-positive patients compared with HBoV-negetive cases may indicate a relationship between severe pneumonia and HBoV. Undeniably, there is a limited population of enrolled patients in this study, therefore further studies are required to investigate the relationship between HBoV infection and CAP in children.
Acknowledgements
We thank the consent of patients and their families, and staff members at the Yinchuan Women and Children Healthcare Hospital for their assistance in the samples and data collection.
Abbreviations
- CAP
Community-acquired pneumonia
- HBoV
Human bocavirus
- MP
Mycoplasma pneumoniae
- RSV
Respiratory syncytial virus
- IFV
Influenza virus
- HRV
Human rhinovirus
- AdV
Adenoviruses
- HCoV
Human coronaviruses
- PIV
Parainfluenza viruses
- HMPV
Human metapneumovirus
- EV
Enterovirus
- WBC
White blood cells count
- HGB
Hemoglobin
- AIL
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CRP
C-reactive protein
- PCT
Procalcitonin
Authors' contributions
KJ, JS, FL, and YS contributed to the design of experiments. LH, KJ, YY, XH contributed to the collection of samples. KJ, JS, FL, YS, YY, JG, AM, and XH contributed to the conduction of experiments. KJ, YY, LH, JG, AM, and XH contributed to the Collection of clinical data. KJ, JS and YY contributed to the analyses of the data. KJ, JS, FL, and YS contributed to the writing the paper. KJ and JS contributed to the editing the paper. All authors read and approved the final manuscript.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (81560340, 31760041).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The process obtained families’ informed consent. The study was approved by the Ethics Committee of the Yinchuan Women and Children Healthcare Hospital and Ningxia Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kai Ji and Jinhan Sun have contributed equally to this work
Contributor Information
Fang Li, Email: fangli2008@live.com.
Yuning Sun, Email: Sunraining_2008@163.com.
References
- 1.Liu WK, Liu Q, Chen DH, Liang HX, Chen XK, Chen MX, et al. Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study. PLoS ONE. 2014;9(5):e96674. doi: 10.1371/journal.pone.0096674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider AC, Frazee BW. Community-acquired pneumonia. Emerg Med Clin North Am. 2018;36(4):665–683. doi: 10.1016/j.emc.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang G, Yu D, Mao N, Zhu Z, Zhang H, Jiang Z, et al. Viral etiology of acute respiratory infection in Gansu Province, China, 2011. PLoS ONE. 2013;8(5):e64254. doi: 10.1371/journal.pone.0064254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moesker FM, van Kampen JJ, van der Eijk AA, van Rossum AM, de Hoog M, Schutten M, et al. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect. 2015;21(10):964.e1–8. doi: 10.1016/j.cmi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S. Epidemiology of viral pneumonia. Clin Chest Med. 2017;38(1):1–9. doi: 10.1016/j.ccm.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Söderlund-Venermo M, Young NS. Human parvoviruses. Clin Microbiol Rev. 2017;30(1):43–113. doi: 10.1128/CMR.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol. 2012;22(1):46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 9.Broccolo F, Falcone V, Esposito S, Toniolo A. Human bocaviruses: Possible etiologic role in respiratory infection. J Clin Virol. 2015;72:75–81. doi: 10.1016/j.jcv.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhou JY, Peng Y, Peng XY, Gao HC, Sun YP, Xie LY, et al. Human bocavirus and human metapneumovirus in hospitalized children with lower respiratory tract illness in Changsha, China. Influenza Other Respir Viruses. 2018;12(2):279–286. doi: 10.1111/irv.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziemele I, Xu M, Vilmane A, Rasa-Dzelzkaleja S, Hedman L, Hedman K, et al. Acute human bocavirus 1 infection in child with life-threatening bilateral bronchiolitis and right-sided pneumonia: a case report. J Med Case Rep. 2019;13(1):290. doi: 10.1186/s13256-019-2222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeke V, Reynders M, Floré K, Vandewal W, Debulpaep S, Sauer K, et al. Human bocavirus infection in Belgian children with respiratory tract disease. Arch Virol. 2019;164(12):2919–2930. doi: 10.1007/s00705-019-04396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengelle C, Mansuy JM, Pierre A, Claudet I, Grouteau E, Micheau P, et al. The use of a multiplex real-time PCR assay for diagnosing acute respiratory viral infections in children attending an emergency unit. J Clin Virol. 2014;61(3):411–417. doi: 10.1016/j.jcv.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouédraogo S, Traoré B, Nene Bi ZA, Yonli FT, Kima D, Bonané P, et al. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso) PLoS ONE. 2014;9(10):e110435. doi: 10.1371/journal.pone.0110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guido M, Tumolo MR, Verri T, Romano A, Serio F, De Giorgi M, et al. Human bocavirus: Current knowledge and future challenges. World J Gastroenterol. 2016;22(39):8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finianos M, Issa R, Curran MD, Afif C, Rajab M, Irani J, et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J Med Virol. 2016;88(11):1874–1881. doi: 10.1002/jmv.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eifan SA, Hanif A, AlJohani SM, Atif M. Respiratory tract viral infections and coinfections identified by Anyplex™ II RV16 detection kit in pediatric patients at a Riyadh Tertiary Care Hospital. Biomed Res Int. 2017;2017:1928795. doi: 10.1155/2017/1928795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadi M, Yavarian J, Karbasizade V, Moghim S, Esfahani BN, Hosseini NS. Phylogenetic analysis of human bocavirus in children with acute respiratory infections in Iran. Acta Microbiol Immunol Hung. 2019;66(4):485–497. doi: 10.1556/030.66.2019.017. [DOI] [PubMed] [Google Scholar]
- 19.Petrarca L, Nenna R, Frassanito A, Pierangeli A, Di Mattia G, Scagnolari C, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World J Pediatr. 2020;16(3):293–298. doi: 10.1007/s12519-019-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu WK, Chen DH, Liu Q, Liang HX, Yang ZF, Qin S, et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis. 2011;11:345. doi: 10.1186/1471-2334-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human bocavirus 1 primary infection and shedding in infants. J Infect Dis. 2015;212(4):516–524. doi: 10.1093/infdis/jiv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang W, Yin F, Zhou W, Yan Y, Ji W. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep. 2016;6:20246. doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.do Amaral de Leon C, Amantea SL, Pilger DA, Cantarelli V. Clinical and epidemiologic profile of lower respiratory tract infections associated with human bocavirus. Pediatr Pulmonol. 2013;48(11):1112–1118. doi: 10.1002/ppul.22732. [DOI] [PubMed] [Google Scholar]
- 24.Calvo C, García-García ML, Pozo F, Carballo D, Martínez-Monteserín E, Casas I. Infections and coinfections by respiratory human bocavirus during eight seasons in hospitalized children. J Med Virol. 2016;88(12):2052–2058. doi: 10.1002/jmv.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Zheng S, Xiao Q, Ren L, Xie X, Luo J, et al. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect Dis. 2014;14:424. doi: 10.1186/1471-2334-14-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiumefreddo R, Zaborsky R, Haeuptle J, Christ-Crain M, Trampuz A, Steffen I, et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med. 2009;9:4. doi: 10.1186/1471-2466-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edner N, Castillo-Rodas P, Falk L, Hedman K, Söderlund-Venermo M, Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol. 2012;50(2):531–532. doi: 10.1128/JCM.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jula A, Waris M, Kantola K, Peltola V, Söderlund-Venermo M, Hedman K, et al. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis. 2013;19(8):1328–1331. doi: 10.3201/eid.1908.130074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, Yan Z, Luo Y, Xu J, Cheng F, Li Y, et al. In vitro modeling of human bocavirus 1 infection of polarized primary human airway epithelia. J Virol. 2013;87(7):4097–4102. doi: 10.1128/JVI.03132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran DN, Nguyen TQ, Nguyen TA, Hayakawa S, Mizuguchi M, Ushijima H. Human bocavirus in children with acute respiratory infections in Vietnam. J Med Virol. 2014;86(6):988–994. doi: 10.1002/jmv.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn JG, Choi SY, Kim DS, Kim KH. Human bocavirus isolated from children with acute respiratory tract infections in Korea, 2010–2011. J Med Virol. 2014;86(12):2011–2018. doi: 10.1002/jmv.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin ET, Taylor J, Kuypers J, Magaret A, Wald A, Zerr D, et al. Detection of bocavirus in saliva of children with and without respiratory illness. J Clin Microbiol. 2009;47(12):4131–4132. doi: 10.1128/JCM.01508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9:247. doi: 10.1186/1743-422X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghietto LM, Cámara A, Zhou Y, Pedranti M, Ferreyra S, Frey T, et al. High prevalence of human bocavirus 1 in infants with lower acute respiratory tract disease in Argentina, 2007–2009. Braz J Infect Dis. 2012;16(1):38–44. doi: 10.1016/S1413-8670(12)70272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chonmaitree T, Alvarez-Fernandez P, Jennings K, Trujillo R, Marom T, Loeffelholz MJ, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis. 2015;60(1):1–9. doi: 10.1093/cid/ciu714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207(6):982–989. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byington CL, Ampofo K, Stockmann C, Adler FR, Herbener A, Miller T, et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) Study. Clin Infect Dis. 2015;61(8):1217–1224. doi: 10.1093/cid/civ486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storch GA. Editorial commentary: plethora of respiratory viruses and respiratory virus data. Clin Infect Dis. 2015;61(8):1225–1227. doi: 10.1093/cid/civ487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longtin J, Bastien M, Gilca R, Leblanc E, de Serres G, Bergeron MG, et al. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis. 2008;14(2):217–221. doi: 10.3201/eid1402.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X, Gooding LR, Erdman DD. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis. 2008;14(8):1332–1334. doi: 10.3201/eid1408.080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peltola V, Söderlund-Venermo M, Jartti T. Human bocavirus infections. Pediatr Infect Dis J. 2013;32(2):178–179. doi: 10.1097/INF.0b013e31827fef67. [DOI] [PubMed] [Google Scholar]
- 43.Deng Y, Gu X, Zhao X, Luo J, Luo Z, Wang L, et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS ONE. 2012;7(3):e34353. doi: 10.1371/journal.pone.0034353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma YJ, Wang SM, Cho YH, Shen CF, Liu CC, Chi H, et al. Clinical and epidemiological characteristics in children with community-acquired mycoplasma pneumonia in Taiwan: a nationwide surveillance. J Microbiol Immunol Infect. 2015;48(6):632–638. doi: 10.1016/j.jmii.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Gao J, Yue B, Li H, Chen R, Wu C, Xiao M. Epidemiology and clinical features of segmental/lobar pattern Mycoplasma pneumoniae pneumonia: A ten-year retrospective clinical study. Exp Ther Med. 2015;10(6):2337–2344. doi: 10.3892/etm.2015.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonnalagadda S, Rodríguez O, Estrella B, Sabin LL, Sempértegui F, Hamer DH. Etiology of severe pneumonia in Ecuadorian children. PLoS ONE. 2017;12(2):e0171687. doi: 10.1371/journal.pone.0171687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi X, Sun X, Li X, Kong D, Zhao L. Significance changes in the levels of myocardial enzyme in the child patients with Mycoplasma Pneumoniae pneumonia. Cell Mol Biol (Noisy-le-grand) 2020;66(6):41–45. [PubMed] [Google Scholar]
- 49.Puljiz I, Kuzman I, Dakovic-Rode O, Schönwald N, Mise B. Chlamydia pneumoniae and Mycoplasma pneumoniae pneumonia: comparison of clinical, epidemiological characteristics and laboratory profiles. Epidemiol Infect. 2006;134(3):548–555. doi: 10.1017/S0950268805005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishaba T. Community-acquired pneumonia caused by mycoplasma pneumoniae: how physical and radiological examination contribute to successful diagnosis. Front Med (Lausanne) 2016;3:28. doi: 10.3389/fmed.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neske F, Blessing K, Tollmann F, Schubert J, Rethwilm A, Kreth HW, et al. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol. 2007;45(7):2116–2122. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madi NM, Al-Adwani A. Human bocavirus (HBoV) in Kuwait: molecular epidemiology and clinical outcome of the virus among patients with respiratory diseases. J Med Microbiol. 2020;69(7):1005–1012. doi: 10.1099/jmm.0.001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.