Abstract
We report the controlled synthesis of thin films of prototypical zirconium metal–organic frameworks [Zr6O4(OH)4(benzene-1,4-dicarboxylate-2-X)6] (X = H, UiO-66 and X = NH2, UiO-66-NH2) over the external surface of shaped carbonized substrates (spheres and textile fabrics) using a layer-by-layer method. The resulting composite materials contain metal–organic framework (MOF) crystals homogeneously distributed over the external surface of the porous shaped bodies, which are able to capture an organophosphate nerve agent simulant (diisopropylfluorophosphate, DIFP) in competition with moisture (very fast) and hydrolyze the P–F bond (slow). This behavior confers the composite material self-cleaning properties, which are useful for blocking secondary emission problems of classical protective equipment based on activated carbon.
Keywords: composite, thin film, chemical warfare agent, filter, protective garments, heterogeneous catalysis
Introduction
Despite the international prohibition of the use and stockpiling of chemical warfare agents (CWAs), there is a high threat of exposure to highly toxic chemicals, as a consequence of destabilizing group actions. This problem has increased the demand for CWA detection and protective equipment.1 CWA toxicity is related to lipophilicity and the reactive nature of X–Y bonds (X = C, P; Y = F, Cl, N, S) typically found in the structure of these systems. These features lead to facile permeation through biological tissues and irreversibly binding to highly relevant biomolecules, namely, deoxyribonucleic acid (DNA) and acetylcholinesterase (AChE) for vesicant and nerve agents, respectively (Scheme 1).2,3 The ideal protective systems should be able to efficiently capture and decompose these extremely toxic chemicals before they come into contact with their biological target.
Scheme 1. Toxicity Mechanism of Sarin (Nerve Agent) and Sulfur Mustard (Vesicant Agent).

Current protective systems against CWAs take advantage of high porosity, hydrophobicity and easy shaping of activated carbon (AC) adsorbents.4,5 However, AC-based protective systems lack safety, as a small fraction of the adsorbed contaminant molecule can desorb from the pore surface (a phenomenon called secondary emission) and subsequently contaminate the user. In this regard, a variety of porous metal–organic frameworks (MOFs), constructed from metal clusters connected by multitopic organic ligands,6 behave as efficient catalysts for decontamination of nerve agents.7−10 However, as MOF materials are usually obtained in a powder form, they need to be shaped11 to allow their integration onto protective systems like suits, cartridge filters, or ventilation equipment. Recent efforts have been focused on the development of advanced protective materials based on catalytically active MOFs integrated onto textile fabrics12−17 combining the lightness and air permeation of the fibers. Still, it will be advantageous that the MOF support would have complementary adsorptive properties to increase the filter protection ability. In this regard, AC materials possess a broad range of pore sizes and are easily shaped in the form of spheres and textile fabrics suitable as filters. Nevertheless, MOF integration onto AC materials has been poorly explored.18−21
Herein, we report the controlled synthesis of two prototypical zirconium-based MOFs [Zr6O4(OH)4(benzene-1,4-dicarboxylate-2-X)6] (X = H, UiO-66; X = NH2, UiO-66-NH2) over the surface of activated carbon substrates in the form of fabrics (suited for the fabrication of protective suits) and spheres (suited as fillers on mask cartridges). We show that a layer-by-layer (LBL) synthesis is more advantageous than the in situ MOF synthesis with the LBL yielding systems containing Zr-MOF thin films homogeneously dispersed over the AC shaped bodies. Moreover, the resulting MOF@AC composites behave as self-cleaning materials in the capture and degradation of the organophosphate nerve agent simulant diisopropyl fluorophosphate (DIFP) blocking the secondary emission problems of traditional AC-based protective filters.
Results and Discussion
Scheme 2 shows a general synthetic procedure for the layer-by-layer (LBL) MOF thin-film growth over the shaped carbonized substrates. In the first step, the carbonized substrate, in the form of fabrics and spheres, was treated with an oxidant to increase the concentration of oxygen groups (i.e., hydroxyl, carboxylate) on its surface to provide suitable metal-ion-anchoring sites. Temptative studies with nitric acid (HNO3) and hydrogen peroxide (H2O2) lead to the selection of the latter as it does not damage the AC porosity, as proven by N2 adsorption measurements (see below). This oxidative treatment led to an increase in oxygen groups on the surface as confirmed by X-ray photoelectron spectroscopy (XPS) analysis (see the Supporting Information, SI).
Scheme 2. Synthesis of UiO-66(UiO-66-NH2)@AC (Fabrics, Spheres) Composites in a Layer-by-Layer Approach.

In the second step, two separate solutions containing the MOF precursors were prepared: (a) a preformed Zr6 oxocluster [Zr6O4(OH)4(AcO)12] solution in dimethylformamide (DMF)/AcOH22 and (b) a DMF solution of the organic spacer (4-benzenedicarboxylic acid (H2bdc) or amino-1,4-benzenedicarboxylic acid (H2bdc-NH2)) (see the SI). The LBL crystallization process consisted of the alternate immersion of the AC substrate in the zirconium cluster and linker solutions (Scheme 2). A washing step with DMF was carried out between transfers of the AC material into the different solutions to remove any unreacted MOF precursor, thus avoiding MOF crystallization outside of the carbon shaped bodies. In this manner, strongly attached Zr-MOF films were constructed for all materials. The resulting composites were labeled UiO-66@AC(fabrics, spheres) and UiO-66-NH2@AC(fabrics, spheres).
The MOF thin-film growth process was carried out using 1 h immersion at 130 °C in each component solution. A combined study of powder X-ray diffraction (PXRD), inductively coupled plasma-mass spectrometry (ICP-MS) (Figure 1a–d), and thermogravimetric analysis (TGA) (Figures S7 and S8) over 15 growth cycles shows the formation of a crystalline UiO-66 phase on the surface of AC materials after approximately three cycles. The MOF content in the materials increases with growth cycles. Similarly, Fourier transform infrared spectroscopy (FTIR) results (Figure S6) showed that vibrational bands associated with the bdc ligand (1100–1800 cm–1 region) increase with the increasing number of growth cycles. The amount of deposited UiO-66 thin films, after nine cycles, was estimated to reach 0.422 and 0.496 wt % MOF content for AC fabrics and spheres, respectively.
Figure 1.
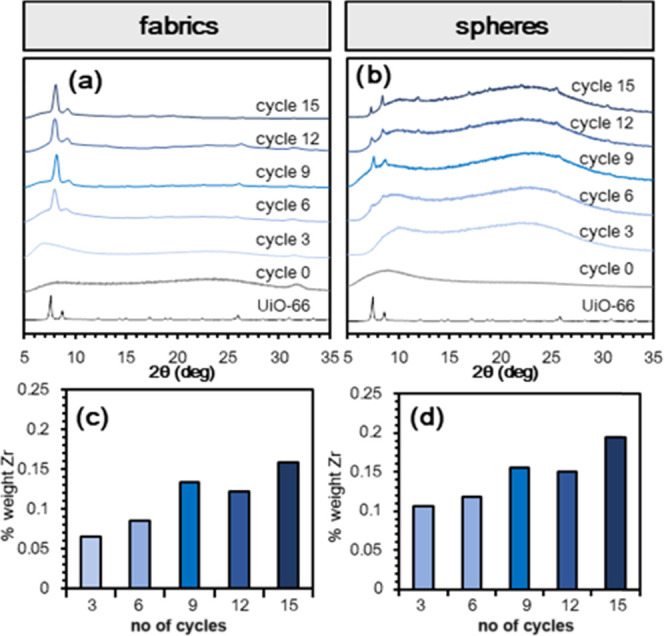
Study of UiO-66 growth over shaped activated carbon (AC) substrates. PXRD patterns for UiO-66@AC composite materials after different number of cycles for (a) fabrics and (b) spheres. Zr weight percentage after different number of cycles for (c) fabrics and (d) spheres, as determined by ICP-MS.
In a parallel study, we also addressed the UiO-66 typical solvothermal synthesis in the presence of shaped carbon materials. With this aim, the MOF precursors were mixed in a solvothermal reactor and heated at an elevated temperature (220 °C) and pressure overnight. This process was repeated during nine growth cycles leading to the formation of microcrystalline UiO-66 both attached to the shaped AC together with a large excess of loose MOF materials. Scanning electron microscopy (SEM) images show a heterogeneous distribution of UiO-66 microcrystals on the external surface of the AC spheres and fibers giving rise to terraces and granules (Figure 2a–c). The observed behavior for the solvothermal-prepared UiO-66@AC composites is in clear contrast to a homogeneous film formation observed for the LBL-prepared composites (Figures 2e−h and 3).
Figure 2.

Comparison between solvothermal and LBL syntheses. SEM images at three different magnifications for (a–d) solvothermally prepared and (e–h) LBL-prepared (cycle 10) composites (2 h cycles at 130 °C). For comparison, Figure S9 shows SEM images for pristine fabrics and spheres.
Figure 3.
Characterization of Zr-MOF@AC composites (nine cycles, 130 °C, 30 min each cycle). PXRD patterns for (a) fabrics and (g) spheres and nitrogen adsorption isotherms for (b) fabrics and (h) spheres. SEM–energy dispersive X-ray analysis (EDX) study of (c–f) UiO-66@AC(fabrics) and (i–l) UiO-66@AC(spheres). Color code: pristine carbon (black), H2O2-mediated oxidized carbon (gray), UiO-66@CA (green), and UiO-66-NH2@CA (purple).
In a second stage in this work, we prepared a new set of MOF@AC composite materials of UiO-66 and UiO-66-NH2 networks, reducing the time length of each growth cycle from 2 h to 30 min over nine cycles. The results show the successful preparation of UiO-66@AC(fabrics, spheres) and UiO-66-NH2@AC(fabrics, spheres) composites. PXRD patterns (Figure 3a,g) show the presence of crystalline MOF over the surface of all composites. TGA analysis (Figures S7 and S8) shows an increased pyrolytic residue for the MOF@AC composite materials indicative of the presence of ZrO2 from the Zr-MOF layer. ICP analysis (Table 1) showed Zr wt % values ranging from 0.022 to 0.051 corresponding to 0.07–0.16% MOF incorporation on the MOF@AC composites (Table 1). SEM–EDX mapping analyses (Figures 3c–f,i–l and S10) show a homogeneous dispersion of Zr and MOF crystals on the external surface of the activated carbon shaped bodies. A cross-sectional elemental analysis of the fabric fiber showed a Zr signal only on the outer surface of the fibers with a layer thickness of approximately 300 nm (Figure S10). This is indicative of the preferential formation of the MOF thin-film layer at the hydrophilic external surface (oxygen groups) of AC shaped bodies with no MOF formation onto the inner hydrophobic AC pore surface. Nitrogen adsorption isotherms indicated that both the MOF and AC porous structures are accessible in the MOF@AC composites (Figure 3b,h and Table 1). A drop in the adsorption capacity of the activated carbon material of 19% for AC fabric and 9% for AC spheres is noticed upon the oxidation treatment, which can be related to the partial degradation of materials. A further adsorption capacity drop is also noticed after the MOF layer deposition of around 5 and 15% for the fabric and spheres, respectively, which might be the related to hindered diffusion of N2 molecules through the microporous MOF layer and/or adsorbed organic linkers, thereby reducing their associated porosity. Slightly lower adsorption capacities for UiO-66-NH2@AC composites are attributed to the steric effects of the NH2 group.
Table 1. Textural and Compositional Characteristics of MOF@AC Materials.
| material | Vp (cm3 g–1) | SBET (m2 g–1) | Zr wt % | estimated MOF wt % | estimated MOF in the catalyst (mg) |
|---|---|---|---|---|---|
| carbon fabrics | 0.50 | 1070 | |||
| oxidized carbon fabrics | 0.42 | 1040 | |||
| UiO-66@AC(fabrics) | 0.40 | 840 | 0.051 | 0.16 | 0.06 |
| UiO-66-NH2@AC(fabrics) | 0.34 | 745 | 0.048 | 0.15 | 0.06 |
| carbon spheres | 0.87 | 2045 | |||
| oxidized carbon spheres | 0.80 | 1880 | |||
| UiO-66@AC(spheres) | 0.73 | 1520 | 0.041 | 0.12 | |
| UiO-66-NH2@AC(spheres) | 0.72 | 1495 | 0.022 | 0.07 |
Once we structurally characterized the MOF@AC composites, we proceeded to evaluate their possible functionality for detoxification of CWAs, as a proof of concept for self-cleaning materials. With this aim, we have evaluated the activity of UiO-66(UiO-66-NH2)@AC(fabrics) in the catalytic hydrolysis of the nerve agent simulant diisopropyl fluorophosphate (DIFP) (Figure 4a). For this reaction, 40 mg of the MOF@AC catalyst was impregnated with 10 μL of H2O and 1.25 μL of DIFP (MOF/DIFP ratio of approximately 1:5) in a closed vial. Subsequently, the vials were incubated at 60 °C for increasing time periods at 1, 10 min, 1, 5, and 24 h, followed by the extraction with CH2Cl2 (0.5 mL) and analysis by GC. The results are indicative of self-cleaning behavior for UiO-66@AC(fabric) and UiO-66-NH2@AC(fabric) composites with respective 80 and 40% DIFP detoxification after 24 h (Figures 4c and S7). Figure 4b shows the plausible mechanism of the three-step model nerve agent detoxification process. In the first step, fast and strong adsorption of DIFP on the hydrophobic activated carbon pore structure takes place, accounting for the traditional protective behavior of activated carbon materials. In the second step, a slow equilibrium takes place between the physisorbed contaminant (secondary emission) and the MOF particles that are distributed over the external surface of the activated carbon fibers. Finally, DIFP is hydrolyzed on the Zr-MOF pore structure, as shown in previous works.23−25 The observed lower performance of UiO-66-NH2@AC(fabric) might be related to a pH effect of unbuffered media as recently found by Cohen and co-workers.26 We assume the secondary emission diffusion process as the rate-limiting step in this detoxification process. In this regard, DIFP secondary emission is only appreciable above room temperature, as demonstrated previously by our group for AC Blücher spheres.27 It should be noted that the pristine AC fabrics do not give rise to any DIFP degradation under similar conditions, which indicated the benefit of the incorporation of the MOF layer on the AC fabrics to yield self-cleaning adsorbent materials.
Figure 4.

Blocking of the secondary emission by MOF@AC materials. (a) DIFP hydrolytic reaction. (b) Proposed three-step mechanism for the synergistic detoxification of DIFP on the composite surface. (c) DIFP hydrolysis kinetic study. Reaction conditions: 40 mg of the MOF@AC catalyst, 1.25 μL of DIFP, 10 μL of H2O, MOF/DIFP ratio of approximately 1:5, 60 °C. Extraction with CH2Cl2 (0.5 mL) at 1, 10 min, 1, 5, and 24 h.
Nevertheless, a similar study on the UiO-66@AC(spheres) composites is indicative of no improvement in the detoxification properties upon incorporation of the MOF layer (Figure S13), which can be attributed to the strong competition of the adsorption process on the spheres pore structure with the detoxification properties on the UiO-66 layer. This result correlates with the much higher adsorption capacity and Brunauer–Emmett–Teller (BET) surface of AC spheres in comparison to AC fabrics.
Conclusions
The controlled layer-by-layer growth of crystalline MOF thin films over AC shaped substrates can be regarded as a suitable approach for the fabrication of advanced self-cleaning porous materials. The characterization results demonstrated the high dispersion of Zr-MOF crystals, and catalysis results showed their efficiency in the blocking of the secondary emission problem of classical CWA protective garments. We can conclude that the combination of two porous materials of different characteristics (porous hydrophobic carbon and catalytically active hydrophilic MOF) is a suitable way of improving the features of activated carbon traditional protective filters. Further work needs to be carried out to find a suitable balance between physisorption of CWA on a hydrophobic adsorbent and hydrolytic detoxification on a hydrophilic MOF to bring these composites to real applications.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.1c12095.
Details of experimental procedures, composite preparation, and characterization (PDF)
Author Present Address
† Faculty of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50-383 Wrocław, Poland
Author Present Address
‡ Chemistry Department & CICECO, University of Aveiro, 3810-193 Aveiro, Portugal.
Author Contributions
R.G.-S.-M. and P.D. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This research was funded by the Directorate for Planning, Technology, and Innovation (SDG PLATIN) from the Directorate General of Armaments and Material (DGAM) of the Spanish Ministry of Defense, COINCIDENTE Program exp. 1003219007500—NBQD2. The authors also acknowledge EU Feder funding, MINECO (CTQ2017-84692-R and PID2020-113608RB-I00), Universidad de Granada (Plan Propio de Investigación), and Junta de Andalucia (P18-RT-612). Funding for open access charge: Universidad de Granada/CBUA.
The authors declare no competing financial interest.
Supplementary Material
References
- Giannakoudakis D. A.; Bandosz T. J.. Detoxification of Chemical Warfare Agents: From WWI to Multifunctional Nanocomposite Approaches; Springer International Publishing, 2018. [Google Scholar]
- Mercey G.; Verdelet T.; Renou J.; Kliachyna M.; Baati R.; Nachon F.; Jean L.; Renard P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Acc. Chem. Res. 2012, 45, 756–766. 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- Chauhan S.; Chauhan S.; D’Cruz R.; Faruqi S.; Singh K. K.; Varma S.; Singh M.; Karthik V. Chemical Warfare Agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. 10.1016/j.etap.2008.03.003. [DOI] [PubMed] [Google Scholar]
- DeCoste J. B.; Peterson G. W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. 10.1021/cr4006473. [DOI] [PubMed] [Google Scholar]
- Giannakoudakis D. A.; Barczak M.; Florent M.; Bandosz T. J. Analysis of Interactions of Mustard Gas Surrogate Vapors with Porous Carbon Textiles. Chem. Eng. J. 2019, 362, 758–766. 10.1016/j.cej.2019.01.064. [DOI] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Mondloch J. E.; Katz M. J.; Isley Iii W. C.; Ghosh P.; Liao P.; Bury W.; Wagner G. W.; Hall M. G.; DeCoste J. B.; Peterson G. W.; Snurr R. Q.; Cramer C. J.; Hupp J. T.; Farha O. K. Destruction of Chemical Warfare Agents Using Metal–Organic Frameworks. Nat. Mater. 2015, 14, 512–516. 10.1038/nmat4238. [DOI] [PubMed] [Google Scholar]
- Bobbitt N. S.; Mendonca M. L.; Howarth A. J.; Islamoglu T.; Hupp J. T.; Farha O. K.; Snurr R. Q. Metal–Organic Frameworks for the Removal of Toxic Industrial Chemicals and Chemical Warfare Agents. Chem. Soc. Rev. 2017, 46, 3357–3385. 10.1039/C7CS00108H. [DOI] [PubMed] [Google Scholar]
- Jabbour C. R.; Parker L. A.; Hutter E. M.; Weckhuysen B. M. Chemical Targets to Deactivate Biological and Chemical Toxins Using Surfaces and Fabrics. Nat. Rev. Chem. 2021, 5, 370–387. 10.1038/s41570-021-00275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells-Gil J.; M Padial N.; Almora-Barrios N.; Gil-San-Millán R.; Romero-Ángel M.; Torres V.; da Silva I.; Vieira B. C. J.; Waerenborgh J. C.; Jagiello J.; Navarro J. A. R.; Tatay S.; Martí-Gastaldo C. Heterometallic Titanium-Organic Frameworks as Dual-Metal Catalysts for Synergistic Non-Buffered Hydrolysis of Nerve Agent Simulants. Chem 2020, 6, 3118–3131. 10.1016/j.chempr.2020.09.002. [DOI] [Google Scholar]
- Tian T.; Zeng Z.; Vulpe D.; Casco M. E.; Divitini G.; Midgley P. A.; Silvestre-Albero J.; Tan J.-C.; Moghadam P. Z.; Fairen-Jimenez D. A Sol–Gel Monolithic Metal–Organic Framework with Enhanced Methane Uptake. Nat. Mater. 2018, 17, 174–179. 10.1038/nmat5050. [DOI] [PubMed] [Google Scholar]
- López-Maya E.; Montoro C.; Rodríguez-Albelo L. M.; Aznar Cervantes S. D.; Lozano-Pérez A. A.; Cenís J. L.; Barea E.; Navarro J. A. R. Textile/Metal–Organic-Framework Composites as Self-Detoxifying Filters for Chemical-Warfare Agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. 10.1002/anie.201502094. [DOI] [PubMed] [Google Scholar]
- Lu A. X.; McEntee M.; Browe M. A.; Hall M. G.; DeCoste J. B.; Peterson G. W. MOFabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. 10.1021/acsami.7b01621. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Ma K.; Mahle J. J.; Wang H.; Syed Z. H.; Atilgan A.; Chen Y.; Xin J. H.; Islamoglu T.; Peterson G. W.; Farha O. K. Integration of Metal–Organic Frameworks on Protective Layers for Destruction of Nerve Agents under Relevant Conditions. J. Am. Chem. Soc. 2019, 141, 20016–20021. 10.1021/jacs.9b11172. [DOI] [PubMed] [Google Scholar]
- Lee D. T.; Jamir J. D.; Peterson G. W.; Parsons G. N. Protective Fabrics: Metal-Organic Framework Textiles for Rapid Photocatalytic Sulfur Mustard Simulant Detoxification. Matter 2020, 2, 404–415. 10.1016/j.matt.2019.11.005. [DOI] [Google Scholar]
- Lee D. T.; Zhao J.; Peterson G. W.; Parsons G. N. Catalytic “MOF-Cloth” Formed via Directed Supramolecular Assembly of UiO-66-NH2 Crystals on Atomic Layer Deposition-Coated Textiles for Rapid Degradation of Chemical Warfare Agent Simulants. Chem. Mater. 2017, 29, 4894–4903. 10.1021/acs.chemmater.7b00949. [DOI] [Google Scholar]
- Phadatare A.; Kandasubramanian B. Metal Organic Framework Functionalized Fabrics for Detoxification of Chemical Warfare Agents. Ind. Eng. Chem. Res. 2020, 59, 569–586. 10.1021/acs.iecr.9b06695. [DOI] [Google Scholar]
- Soleimanpour A.; Farsi M.; Keshavarz P.; Zeinali S. Modification of Activated Carbon by MIL-53(Al) MOF to Develop a Composite Framework Adsorbent for CO2 Capturing. Environ. Sci. Pollut. Res. 2021, 28, 37929–37939. 10.1007/s11356-021-13382-y. [DOI] [PubMed] [Google Scholar]
- Muñoz-Senmache J. C.; Kim S.; Arrieta-Pérez R. R.; Park C. M.; Yoon Y.; Hernández-Maldonado A. J. Activated Carbon–Metal Organic Framework Composite for the Adsorption of Contaminants of Emerging Concern from Water. ACS Appl. Nano Mater. 2020, 3, 2928–2940. 10.1021/acsanm.0c00190. [DOI] [Google Scholar]
- McHugh L. N.; Terracina A.; Wheatley P. S.; Buscarino G.; Smith M. W.; Morris R. E. Metal–Organic Framework-Activated Carbon Composite Materials for the Removal of Ammonia from Contaminated Airstreams. Angew. Chem., Int. Ed. 2019, 58, 11747–11751. 10.1002/anie.201905779. [DOI] [PubMed] [Google Scholar]
- de Oliveira C. A. F.; da Silva F. F.; Jimenez G. C.; Neto J. F. D. S.; de Souza D. M. B.; de Souza I. A.; Júnior S. A. MOF@activated Carbon: A New Material for Adsorption of Aldicarb in Biological Systems. Chem. Commun. 2013, 49, 6486–6488. 10.1039/C3CC40449H. [DOI] [PubMed] [Google Scholar]
- DeStefano M. R.; Islamoglu T.; Garibay S. J.; Hupp J. T.; Farha O. K. Room-Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361. 10.1021/acs.chemmater.6b05115. [DOI] [Google Scholar]
- Gil-San-Millan R.; López-Maya E.; Hall M.; Padial N. M.; Peterson G. W.; DeCoste J. B.; Rodríguez-Albelo L. M.; Oltra J. E.; Barea E.; Navarro J. A. R. Chemical Warfare Agents Detoxification Properties of Zirconium Metal–Organic Frameworks by Synergistic Incorporation of Nucleophilic and Basic Sites. ACS Appl. Mater. Interfaces 2017, 9, 23967–23973. 10.1021/acsami.7b06341. [DOI] [PubMed] [Google Scholar]
- Gil-San-Millan R.; López-Maya E.; Platero-Prats A. E.; Torres-Pérez V.; Delgado P.; Augustyniak A. W.; Kim M. K.; Lee H. W.; Ryu S. G.; Navarro J. A. R. Magnesium Exchanged Zirconium Metal–Organic Frameworks with Improved Detoxification Properties of Nerve Agents. J. Am. Chem. Soc. 2019, 141, 11801–11805. 10.1021/jacs.9b05571. [DOI] [PubMed] [Google Scholar]
- Moon S.-Y.; Wagner G. W.; Mondloch J. E.; Peterson G. W.; DeCoste J. B.; Hupp J. T.; Farha O. K. Effective, Facile, and Selective Hydrolysis of the Chemical Warfare Agent VX Using Zr6-Based Metal–Organic Frameworks. Inorg. Chem. 2015, 54, 10829–10833. 10.1021/acs.inorgchem.5b01813. [DOI] [PubMed] [Google Scholar]
- Palomba J. M.; Credille C. V.; Kalaj M.; De Coste J. B.; Peterson G. W.; Tovar T. M.; Cohen S. M. High-throughput screening of solid-state catalysts for nerve agent degradation. Chem. Commun. 2018, 54, 5768–5771. 10.1039/C8CC03255F. [DOI] [PubMed] [Google Scholar]
- Montoro C.; Linares F.; Quartapelle Procopio E.; Senkovska I.; Kaskel S.; Galli S.; Masciocchi N.; Barea E.; Navarro J. A. R. Capture of Nerve Agents and Mustard Gas Analogues by Hydrophobic Robust MOF-5 Type Metal–Organic Frameworks. J. Am. Chem. Soc. 2011, 133, 11888–11891. 10.1021/ja2042113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




