Abstract
Per- and polyfluoroalkyl substances (PFAS) are a family of chemicals that are ubiquitous in the environment. Some of these chemicals, such as perfluorooctanesulfonic acid (PFOS), perfluorohexanesulfonate (PFHxS) and perfluorooctanoic acid (PFOA), are found in human sera and have been shown to cause liver steatosis and reduce postnatal survival and growth in rodents. The purpose of this work is to evaluate the impact of diet and PFAS exposure to mouse dam (mus musculus) on the risk to pup liver and metabolism endpoints later in life, as well as evaluate PFAS partitioning to pups. Timed-pregnant dams were fed a standard chow diet or 60% kcal high fat diet (HFD). Dams were administered either vehicle, 1 mg/kg PFOA, 1 mg/kg PFOS, 1 mg/kg PFHxS, or a PFAS mixture (1 mg/kg of each PFOA, PFOS, and PFHxS) daily via oral gavage from gestation day 1 until postnatal day (PND) 20. At PND 21, livers of dams and 2 pups of each sex were evaluated for lipid changes while remaining pups were weaned to the same diet as the dam for an additional 10 weeks. Dam and pup serum at PND 21 and PND 90 were also evaluated for PFAS concentration, alanine aminotransferase (ALT), leptin and adiponectin, and glycosylated hemoglobin A1c. Perinatal exposure to a HFD, as expected, increased pup body weight, maternal liver weight, pup liver triglycerides, pup serum ALT, and pup serum leptin. PFOA and the PFAS mixture increased liver weights, and. treatment with all three compounds increased liver triglycerides. The maternal HFD increased dam and pup serum PFAS levels, however, was protective against PFOA-induced increase in serum ALT and observed increases in liver triglycerides. The PFAS mixture had very distinct effects when compared to single compound treatment, suggesting some cumulative effects, particularly when evaluating PFAS transfer from dam to pup. This data highlights the importance of diet and mixtures when evaluating liver effect of PFAS and PFAS partitioning.
Keywords: Liver, perfluoroalkyl substances (PFAS), perinatal, Mixtures
1.0. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of over 7,000 synthetic chemicals on the market for use in numerous household and consumer products, as well as firefighting foams (Wang et al. 2017; Glüge et al., 2020; US EPA, Chemistry Dashboard, 2020). PFAS have strong carbon-fluorine bonds that allow them to persist in the environment (Mortensen et al. 2011). The most well studied PFAS are the perfluoroalkyl acids (PFAA), such as perfluorooctanesulfonate (PFOS), perfluorooctanoic acid (PFOA), and perfluorohexanesulfonic acid (PFHxS), which will be the focus of this work. A major concern to government regulators regarding many of these compounds are the long serum half-lives in humans; PFOS, PFHxS, and PFOA have mean half-lives of 5.4 years, 8.5 years, and 3.8 years, respectively (Olsen et al. 2007). In 2006, eight companies that were part of the EPA PFOA stewardship program in the US voluntarily agreed to reduced PFOA emissions and content of PFOA by 95 percent by 2010 (US EPA, 2020). Since 1999–2000, serum PFOS and PFHxS concentrations in the U.S. population have been declining, whereas the PFOA concentrations remained constant between 2003–2008 (Kato et al. 2011). PFOA, PFOS, and PFHxS are still widely distributed in humans and the natural environment (Jian et al., 2018).
Many human studies have found significant associations with adverse outcomes and PFAA exposure, such as suppressed immunity, dyslipidemia, and kidney and testicular cancers in areas with extremely high exposures (Barry et al. 2013; Vieira et al. 2013; Grandjean et al. 2017a; Grandjean et al. 2017b; Lin et al. 2019; Sunderland et al. 2019). PFOS and PFOA have been shown to transfer to the fetus and are found in umbilical cord serum and human breast milk and may, consequently, pose a risk for developmental toxicity (Midasch et al. 2007; Tao et al. 2008; von Ehrenstein et al. 2009; Fromme et al. 2010; Ode et al. 2013). Self-reported health outcomes of pregnancies from the C8 Science Panel study population (2000–2006) have reported associations of PFOA with preeclampsia, and of PFOS with preeclampsia and low birth weight (Stein et al. 2009). Studies in rats and mice found exposure to PFOS and PFOA in utero delays development and reduces postnatal survival and growth (Lau et al. 2003; Butenhoff et al. 2004; Luebker et al. 2005; Lau et al. 2006). In rodents and monkeys, liver is known to be a sensitive organ to PFAA exposure. PFOS and PFOA treatment has been shown to decrease body weight, increase liver weight, and cause hepatocellular hypertrophy and lipid vacuolation (Butenhoff et al. 2002; Seacat et al. 2002; Seacat et al. 2003; Son et al. 2008; Qazi et al. 2010; Wan et al. 2012). Human studies have found positive associations between PFAA exposure and biomarkers of liver injury; however, it is unclear if PFAA exposure causes liver steatosis in humans (Lin et al. 2010; Gallo et al. 2012; Gleason et al. 2015; Darrow et al. 2016; Bassler et al. 2019). Perinatal exposure to PFOS and PFOA has been shown to increase body weight gain, as well as effect leptin and insulin later in life (Hines et al. 2009; Wan et al. 2014). The livers of rats and mice exposed to PFOA and PFOS perinatally have been described to have hepatomegaly and altered liver fatty acid metabolism gene expression (Bjork et al, 2008; Lau et al, 2003; Luebker et al, 2005; Abbott et al. 2009; White et al. 2011), and in utero PFOA exposure induced liver lesions in 18-month-old mice (Filgo et al., 2015). In adults, liver effects of PFOS have been shown be modulated with diet and the time in which the diet was administered (Marques et al., 2020; Pfohl et al., 2021; Salter et al., 2021). However, few studies have explored how diet may, effect and modulate liver effects with perinatal exposure to PFOA, PFOS, or PFHxS.
Here, high fat diet (HFD) feeding and perinatal PFAS (specifically PFOA, PFOS and PFHxS) exposure to pregnant CD-1 dams was evaluated as a risk factor of liver steatosis. Timed-pregnant mice were treated with PFAS and a HFD during gestation and lactation. Maternal intake of HFD during gestation and lactation has been shown to predispose adult offspring to hepatic steatosis (Gregorio et al. 2010). We hypothesized that PFAS administration would worsen hepatic steatosis later in life. We have included treatments with PFOA, PFOS and PFHxS, as well as a mixture of the three. Humans are exposed to multiple PFAS, so it is relevant to understand the combined effect. This knowledge could be leveraged to further the current understanding on how maternal diet may play a role in perinatal PFAS exposure and potential adverse outcomes later in life.
2.0. Material and Methods
2.1. Chemicals.
PFAS chemicals were purchased from Sigma Aldrich (St. Louis, MO): PFOA (Perfluorooctanoic acid, CAS# 335–67-1, Catalog# 171468, 95% purity), PFOS, (Heptadecafluorooctanesulfonic acid potassium salt, CAS# 2795–39-3, Catalog# 89374, ≥98.0% purity, ~70% linear and ~30% branched isomers based on LC-MS/MS analysis [data not shown]) and PFHxS (Tridecafluorohexane-1-sulfonic acid potassium salt, CAS# 3871–99-6, Catalog# 50929, ≥98.0% purity). Stable isotope-labeled internal standards were purchased from Wellington Laboratories (Ontario, Canada): 13C4-PFOS (Product code: MPFOS), 13C4-PFOA (Product code: MPFOA), and 13C3-PFHxS (Product code: M3PFHxS). Other chemicals and solvents, if not specified, were obtained from Sigma Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Waltham, MA).
2.2. Dosing Solutions.
PFOS, PFOA, and PFHxS were dissolved in 0.5% Tween 20 in Millipore-treated water. The final dose administered was 1 mg/kg (dosing volume of 10 mL/kg) for each individual compound, and 3 mg/kg total PFAS for the mixture using a 1:1:1 ratio of the same dose as the individual treatments. The rationale for the stacking the dose was to be able to compare effects of the mixture to the same dose of single PFAS treatments to understand the potential cumulative effects. As the present study was focused on mechanistic analysis, the dose of 1 mg/kg/day was selected to be above the lowest observed adverse effect level (LOAEL) of liver enlargement in dams, and to ensure maternal transfer of PFAS to pups, and is not necessarily representative of potential human exposure levels (Lau et al. 2006; Wan et al. 2014).
2.3. Animals and treatments.
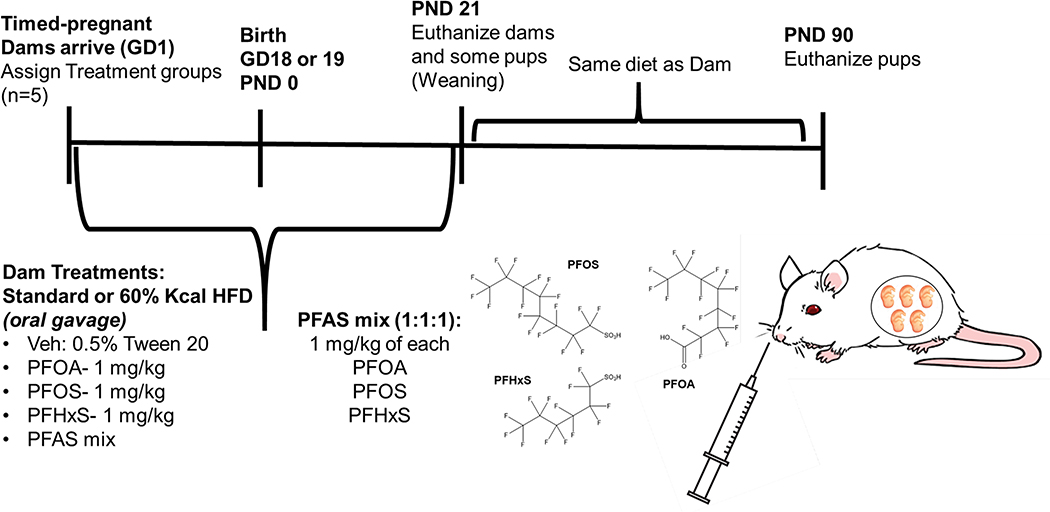
All animal protocols were reviewed and approved by the University of Rhode Island (URI) Institutional Animal Care and Use Committee (IACUC). The overall study timeline is illustrated in Fig. 1. Timed-pregnant CD-1 mice from Charles River Laboratories (Wilmington, MA) were received on gestation day (GD) 1 (day of sperm-positive designated as GD 0). Upon arrival, mice were weighed and randomly distributed to PFAS and diet treatment groups. The mice were housed under a controlled temperature (20–26°C) with relative humidity (30–70%), lighting (12 h, light-dark cycles). Mice received either a standard chow diet (SD; Harlan Teklad Extruded Global Diet, 2020X) or 60% kCal high fat diet (HFD; Research Diets, D12492) ad libitum. Dams were dosed via oral gavage with either vehicle,1 mg/kg PFOA, 1 mg/kg PFOS, 1 mg/kg PFHxS, or a PFAS mixture (1 mg/kg of each PFOS, PFOA, and PFHxS) throughout gestation (GD1-birth [GD18 or 19]) and lactation (birth to postnatal day [PND] 21). Body weights of dams were recorded every 3–4 days and used for dose calculations, with the last dose on PND 20. At PND 5, neonates were weighted, and litters were culled to 10 pups to ensure equal lactational PFAS exposure. At PND 21, dams and 2 pups of each sex were euthanized. Remaining pups were weaned to same diet as dam and continued on study for an additional 10 weeks. Dams and pups were fasted for 4–6 hours prior to euthanasia via cardiac puncture. Liver weights were measured, ~50 mg sections of the left lateral lobe were prepared for histopathology and scoring, and the remaining liver tissues were snap frozen in liquid nitrogen and stored at −70°C until analysis.
Figure 1. Treatment scheme for perinatal PFAS exposure.
PFAS was administered to timed-pregnant CD-1 dams during gestation (gestation day [GD] 1 to birth, or GD 18 or 19) and lactation (GD 18 or 19 to postnatal day [PND] 21). Tissues were collected from dams, as well as some male and female offspring at PND 21. Remaining offspring at PND 21 was weaned and fed the same diet as the dam. Tissues were collected from remaining pups at PND 90.
2.4. Histopathology and scoring.
Liver sections were fixed in 10% buffered formalin for a minimum of 24 h, processed to paraffin, and sectioned to 5 μm thickness. Sections were stained with hematoxylin and eosin (H&E) or post-fixed with osmium tetroxide and periodic acid-Schiff counterstain, and evaluated for lipid content as describe by Gates et al., (2016). All sections were examined/analyzed by a board-certified veterinary pathologist for the surveillance of any treatment related histomorphological changes including lipid content. Osmium-stained sections were scored for subtle lipid accumulation with incidence and semi-quantitative scoring based on the vacuole size number and distribution of lipid. Semiquantitative volume/distribution rankings were roughly as follows: 0 (none), 1 (minimal), 2 (mild), 3 (moderate), 4 (marked) and 5 (severe). Statistical analysis was performed using Kruskal–Wallis test followed by uncorrected Dunn’s test for multiple comparisons using GraphPad Prism v9.1.0 (La Jolla, CA). Significance was considered to be p < 0.05.
2.5. Liver Lipid analysis.
Liver lipids were isolated using the Bligh and Dyer (1959) method. Briefly, tissue was homogenized with ceramic beads in methanol using an Omni Bead Ruptor Elite (Omni International, Kennesaw, GA) for 30 sec at 4 meters/sec. The resulting homogenate was then mixed with 1 mL of water (accounting for 65% water content in liver tissue) and 0.9 mL chloroform. An additional 1 mL water, 0.9 mL chloroform and 1 mL methanol were added and then mixed. The organic layer was isolated by centrifugation (1200 × g for 10 min), and solvent was evaporated. The residue was re-suspended in methanol, and liver lipid content was normalized with exact tissue weight. Triglyceride and total cholesterol concentrations were measured using colorimetric assay kits from Pointe Scientific Inc. (Canton, MI) according to the manufacturer protocols.
2.6. Serum alanine aminotransferase (ALT), glycated hemoglobin A1c (GHbA1c), leptin and adiponectin measurements.
Blood serum collected and isolated at necropsy was used for calorimetric ALT analysis (Pointe Scientific Inc., Canton, MI). Serum GHbA1c was evaluated using murine glycated hemoglobin A1c (GHbA1c) ELISA Kit (BIOTANG Inc. Lexington, MA). Serum leptin and adiponectin were evaluated using sandwich enzyme-linked immunosorbent assay (ELISA) kits with biotin-labelled antibodies (Leptin Mouse/Rat ELISA catalog# D291001200R, Adiponectin Mouse ELISA, catalog# RD293023100R, BioVendor, LLC, Asheville, NC) according to manufacturer instructions. Serum inputs were diluted 5X for leptin and 10,000X for adiponectin, before using 100 μL for analysis.
2.7. Serum and Liver PFAS Extraction and Quantification by LC-MS/MS.
Serum and liver tissues collected at necropsy were prepared according to methods modified from Hansen et al., (2001) and Chang et al., (2017). Briefly, 10, 20, or 60 μL of sera, were spiked with a fixed amount of isotope-labeled internal standards and combined with tetrabutylammonium bisulfate (TBA; adjusted to pH 10) and 400 μL of 0.25 M sodium carbonate in a 15-mL polypropylene tube. 5 mL of methyl tert-butyl ether (MTBE) was added to the solution, and the mixture was placed on a shaker for 20–30 min at room temperature. For frozen liver, ~50 mg of tissue was homogenized in 2 mL Omni Hard Tissue Homogenizing tubes containing 1.4 mm ceramic beads, with 400 μL cold, deionized water spiked with a fixed amount of stable isotope-labeled internal standards. Using an Omni Bead Ruptor Elite (Omni International, Kennesaw, GA), the mixture was homogenized for 30 sec at 4 meters/sec. 250 μL of homogenate was then digested overnight at room temperature in 10% 1N KOH. 100 μL of digested homogenate was further treated with 100 μL of 2N HCl, 500 μL 1N formic acid, 500 μL of saturated ammonium sulfate, and 5 mL methyl tert-butyl ether (MTBE) and shaken for 20–30 min at room temperature. For both liver and sera, the organic layer was separated by centrifugation (2500 × g, 5 min), and an exact volume of MTBE (4.5 mL) was removed from solution, transferred to a new tube, and evaporated. The resulting samples was reconstituted with 0.1, 0.3, 0.5, 2, or 5 mL of acetonitrile and water (1:1), passed through a 0.2 μm polyethersulfone membrane syringe filter (MDI Membrane Technologies, Harrisburg, PA) into an autosampler vial, and vortexed for 30 sec prior to LC-MS/MS analysis.
LC-MS/MS analysis was conducted as previous described in (Marques et al., 2020). Briefly, liquid chromatography was performed on a SHIMADZU Prominence UFLC system (Columbia, MD). Samples and standards were injected (10 μL) on a Waters XBridge C18 column (100 mm X 4.6 mm i.d., 5 μm, Milford, MA) at 40°C. The mobile phase consisted of 0.1% (v/v) formic acid/water (A) and 0.1% (v/v) formic acid/acetonitrile (B). The elution gradient was 70% of B increased to 90% of B over 8 min with a flow rate of 0.600 mL/min; at 8 min the gradient was reverted to original conditions for washing and column re-equilibration. Analytes were measured on a Sciex QTRAP 4500 mass spectrometer (MS) with electrospray ionization (ESI) in MRM (Multiple Reaction Monitoring) mode (SCIEX, Framingham, MA). The MRM ion pairs used for quantification were 412.8/368.9, 498.9/79.8, and 399.0/79.8 (parent ion m/z / fragment ion m/z; for PFOA, PFOS, and PFHxS respectively) in conjunction with a matrix matched calibration curve and an isotope dilution method with mass labeled analogs used as surrogate standards in both, the calibration curve and analyzed samples to determine unknown concentration. Nitrogen was used for collision-induced dissociation of analytes. MS parameters were optimized as follows: negative ionization, IonSpray voltage, −4500; nebulizer gas, 40; auxiliary heater gas, 45; curtain gas, 20; turbo gas temperature, 400; entrance potential, −10; collision cell exit potential, −15, MS parameters were also optimized for each MRM ion pair: declustering potential −5, −60 and −150; collision energy −14, −122, and −37, (for PFOA, PFOS, and PFHxS, respectively). The data were acquired using Analyst 1.6.3 software and processed using MultiQuant 3.0.1 software (SCIEX, Framingham, MA).
2.8. Statistical Analysis.
Dams and/or litters were considered the unit of measurement, therefore, if a dam was described by more than one pup, the values for each pup were averaged. All data are represented as the mean ± SEM. Statistical analysis was performed using one-way or two-way ANOVA followed by Fisher’s least significant difference (LSD) test for multiple comparisons using GraphPad Prism v8.4.0.671 (La Jolla, CA). Further statistical analysis was conducted to qualify marginal effects more explicitly and is presented in a supplementary file. Significance was considered to be p < 0.05.
3.0. Results
3.1. Effect of Diet and PFAS on body weight and liver weight.
As described in Table 1, the number of live births per litter decreased by 26% with PFOA treatment in the HFD as compared to the HFD+Veh group. The maternal body weight at PND 21 was increased by 11% with HFD diet, in the Veh control. Maternal body weight at PND 21 was also increased with HFD in the PFHxS treatment groups by 9%, as compared to SD+Veh control. The maternal body weight of the HFD+PFOS and HFD+PFAS mixture groups were similar to either SD+Veh or HFD+Veh groups, however the SD+PFOA was increased by 13% compared to SD+Veh. The maternal body weight of the HFD+PFOA group was decreased by 9% as compared to HFD+Veh. PFOA and the PFAS mixture enlarged maternal livers: compared to respective Veh diet controls, maternal liver weight was increased by 96% in the SD+PFOA and by 36% in the HFD+PFOA group. Similarly, to PFOA, maternal liver weight was increased by 92% in the SD+PFAS mixture and by 70% the HFD+PFAS mixture group as compared to respective Veh diet controls. After normalizing liver weight to body weight, SD+PFOA and HFD+PFOA increased the liver to body weight ratio by 83% and 44%, respectively as compared to respective Veh diet controls. The liver-to-body weight ratio for the SD+PFAS mixture and HFD+PFAS mixture also increased the liver to body weight ratio by 89% and 65%, respectively as compared to respective Veh diet controls.
Table 1:
Maternal and Offspring Body and Liver Weights.
| Parameter | SD+Veh | HFD+Veh | SD+PFOA | HFD+PFOA | SD+PFOS | HFD+PFOS | SD+PFHxS | HFD+PFHxS | SD+PFAS mix | HFD+PFA mix | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Maternal Endpoints | # of Live Births per litter | 12.2 ± 1.8 | 13.6 ± 1.2 | 14.2 ± 0.6 | 10.0 ± 1.3 #¶ | 11.4 ± 1.0 | 13.0 ± 0.9 | 12.6 ± 1.1 | 14.4 ± 0.7 | 14.3 ± 1.2 | 12.3 ± 1.4 |
| BW at PND 21 (g) | 31.8 ± 1.00 | 35.2 ± 0.68 * | 35.2 ± 1.10 * | 32.1 ± 1.10 #¶ | 31.9 ± 0.41 | 34.3 ± 0.82 | 31.9 ± 0.44 | 34.8 ± 0.78 *¶ | 33.4 ± 1.43 | 34.0 ± 0.70 | |
| Liver Weight (g) | 1.67 ± 0.09 | 1.79 ± 0.06 | 3.28 ± 0.20 * | 2.44 ± 0.19 *#¶ | 1.74 ± 0.19 | 1.53 ± 0.05 | 1.94 ± 0.10 | 1.89 ± 0.13 | 3.20 ± 0.39 *ǂ† | 3.04 ± 0.13 *#§ǂ† | |
| Liver: BW ratio (%) | 5.07 ± 0.12 | 5.08 ± 0.18 | 9.27 ± 0.67 * | 7.32±0.34 *#¶ | 5.46 ± 0.14 | 4.49 ± 0.32 | 6.09 ± 0.21 | 5.42 ± 0.26 | 9.58 ± 1.07 *ǂ† | 8.36 ± 0.09 *#§ǂ† | |
|
| |||||||||||
| Neonatal (PND 5) | BW (g) ** | 3.3 ± 0.2 | 3.6 ± 0.2 | 3.2 ± 0.1 | 3.7 ± 0.2 | 3.5 ± 0.5 | 3.6 ± 0.1 | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.1 ± 0.2 | 4.0 ± 0.2 *¶ |
|
| |||||||||||
| Pup Endpoints at PND 21 | Male BW (g) | 14.6 ± 0.15 | 16.8 ± 0.40 * | 13.1 ± 0.73 | 15.3 ± 0.72 ¶ | 13.7 ± 0.81 | 16.6 ± 0.30 *¶ | 14.0 ± 0.47 | 17.4 ± 0.39 *¶ | 13.6 ± 0.42 | 16.1 ± 0.90 ¶ |
| Female BW (g) | 13.7 ± 0.31 | 16.0 ± 0.33 * | 12.6 ± 0.55 | 14.3 ± 0.73 #¶ | 13.1±0.67 | 15.8±0.34 *¶ | 13.8 ± 0.42 | 16.6 ± 0.33 * | 13.3 ± 0.52 | 15.1 ± 0.91 ¶ | |
| Liver Weight (g) ** | 0.649 ± 0.017 | 0.712 ± 0.029 | 0.711 ± 0.034 | 0.908 ± 0.043 *#¶ | 0.564 ± 0.051 | 0.664 ± 0.027 | 0.593 ± 0.029 | 0.676 ± 0.015 | 0.672 ± 0.036 | 0.897 ± 0.044 *¶#ǂ† | |
| Liver: BW ratio (%) ** | 4.49 ± 0.11 | 4.40 ± 0.12 | 5.50 ± 0.13 * | 5.79 ± 0.21 *# | 4.16 ± 0.17 | 4.03 ± 0.10 * | 4.20 ± 0.09 | 3.99 ± 0.08 *# | 4.92 ± 0.10 §ǂ† | 5.84±0.28 *¶#ǂ† | |
|
| |||||||||||
| Pup Endpoints at PND 90 | Male BW (g) | 42.5 ± 1.06 | 57.3 ± 2.46 * | 41.6 ± 2.09 | 60.0 ± 2.56 *¶ | 43.4 ± 0.37 | 59.5 ± 4.33 *¶ | 40.1 ± 2.97 | 56.9 ± 1.00 *¶ | 40.5 ± 1.07 | 58.7 ± 1.36*¶ |
| Male Liver Weight (g) | 1.78 ± 0.04 | 2.59 ± 0.29 * | 1.79 ± 0.12 | 2.80 ± 0.29 *¶ | 1.72 ± 0.03 | 2.64 ± 0.47 *¶ | 1.44 ± 0.08 | 2.24 ± 0.12 ¶ | 1.64 ± 0.11 | 2.38 ± 0.22 ¶ | |
| Male Liver: BW ratio (%) | 4.19 ± 0.11 | 4.47 ± 0.31 | 4.29 ± 0.23 | 4.62 ± 0.36 | 3.96 ± 0.11 | 4.37 ± 0.51 | 3.63 ± 0.13 | 3.91 ± 0.19 | 4.04 ± 0.17 | 4.03 ± 0.34 | |
| Female BW (g) | 28.5 ± 1.49 | 45.5 ± 3.19 * | 33.2 ± 1.47 | 42.8 ± 2.87 *¶ | 31.2 ± 3.50 | 43.8 ± 5.14 *¶ | 33.5 ± 0.83 | 42.8 ± 1.86 *¶ | 32.7 ± 1.59 | 39.4 ± 7.01* | |
| Female Liver Weight (g) | 1.18 ± 0.11 | 1.52 ± 0.15 | 1.34 ± 0.11 | 1.43 ± 0.14 | 1.20 ± 0.11 | 1.73 ± 0.15 *¶ | 1.34 ± 0.03 | 1.36 ± 0.05 | 1.35 ± 0.13 ǂ | 1.19 ± 0.07 ǂ | |
| Female Liver: BW ratio (%) | 4.13 ± 0.21 | 3.32 ± 0.13 * | 4.19 ± 0.20 | 3.35 ± 0.15 *¶ | 3.90 ± 0.11 | 4.15 ± 0.86 # | 4.00 ± 0.12 | 3.20 ± 0.07 *¶ | 4.12 ± 0.22 | 3.15 ± 0.33 *¶ǂ | |
Timed-pregnant female CD-1 mice were dosed with either vehicle (0.5% Tween 20 in water), 1 mg/kg PFOA, 1 mg/kg PFOS, 1 mg/kg PFHxS, or a PFAS mixture (1 mg/kg of each PFOS, PFOA, and PFHxS) via oral gavage (10 mL/kg) from gestation day (GD) 1 through postnatal day (PND) 21. Maternal body weights (BW) and litter sizes were recorded. At PND 5, neonatal body weights were measured, and litters were culled to 10 pups. At PND 21, dams and some pups from each dam were euthanized, and liver weights were determined. Remaining pups continued the same diet as dam and were euthanized at 13 week of age, and liver weights were determined. Calculations were performed using a two-way ANOVA followed by Fisher’s LSD test.
no differences were observed between male and female pups for this timepoint, and data presented represent the average of both male and female pups per litter. All values are means ± SEM; N = 3–5 dams or litters.
p<0.05 versus SD+Veh
p<0.05 versus HFD+Veh.
p<0.05 SD versus HFD within each treatment (i.e., SD+PFOA vs HFD+PFOA)
p<0.05 versus PFOA and PFAS mix within each diet (i.e., SD+PFOA vs SD+PFAS mix)
p<0.05 versus PFOS and PFAS mix within each diet (i.e., SD+PFOS vs SD+PFAS mix)
p<0.05 versus PFHxS and PFAS mix within each diet (i.e., SD+PFHxS vs SD+PFAS mix).
At PND 5, neonate body weight was increased in the HFD+PFAS mix group by 21% as compared to SD+Veh. At PND 21, male and female pup body weight was increased by ~17% in all treatment groups with HFD, except for HFD+PFOA. In the HFD+PFOA group, female pup body weight was decreased compared to HFD+Veh control by 11%. PFOA and the PFAS mixture enlarged pup livers at PND 21 in the pups exposed to a maternal HFD. Pup liver weight in the HFD+PFOA group was increased 40% and 28% compared to SD+Veh and HFD+Veh respectively, and in the HFD+PFAS mixture group was increased by 38% and 26% compared to SD+Veh and HFD+Veh, respectively. After normalizing liver weight to body weight in the pups, HFD+PFOS lowered liver-to-body weight ratio by 10% compared to SD+Veh, and the HFD+PFHxS group had a lower liver to body weight ratio by 11% compared to SD+Veh and by 9% compared to HFD+Veh. SD+PFOA and HFD+PFOA increased the liver to body weight ratio by 22% and 32%, respectively as compared to respective Veh diet controls. The liver to body weight ratio for the SD+PFAS mixture group was not different as compared to Veh control SD, however it was increase compared to SD+PFOS and SD+PFHxS group by 18% and 17%, respectfully and decreased by 11% as compared to SD+PFOA group. The liver-to-body weight ratio for the HFD+PFAS mixture group was increased by 33% as compared to HFD+Veh.
At PND 90, there were no observed changes in liver weight of pup with maternal PFOA or PFAS mixture treatment. As expected, maternal and pup HFD had the most profound impact on body and liver weights. In male pups, body and liver weights were increased by HFD in the Veh and PFAS treated groups by ~24% and ~42%, respectively.
In female pups, body weight was increased by HFD in the Veh and PFAS treated groups by ~50%, however female pups were resistant to HFD induced liver weight increase. Only the female pups in the HFD+PFOS group had an increased liver weight by ~46% compared to SD+Veh and SD+PFOS groups. Once liver weight was normalized to body weight the HFD+PFOS group, the liver-to-body weight ratio was increased compared by 25% compared to HFD+Veh control pups.
3.2. Diet and PFAS effects on serum ALT and liver lipid content in Dams.
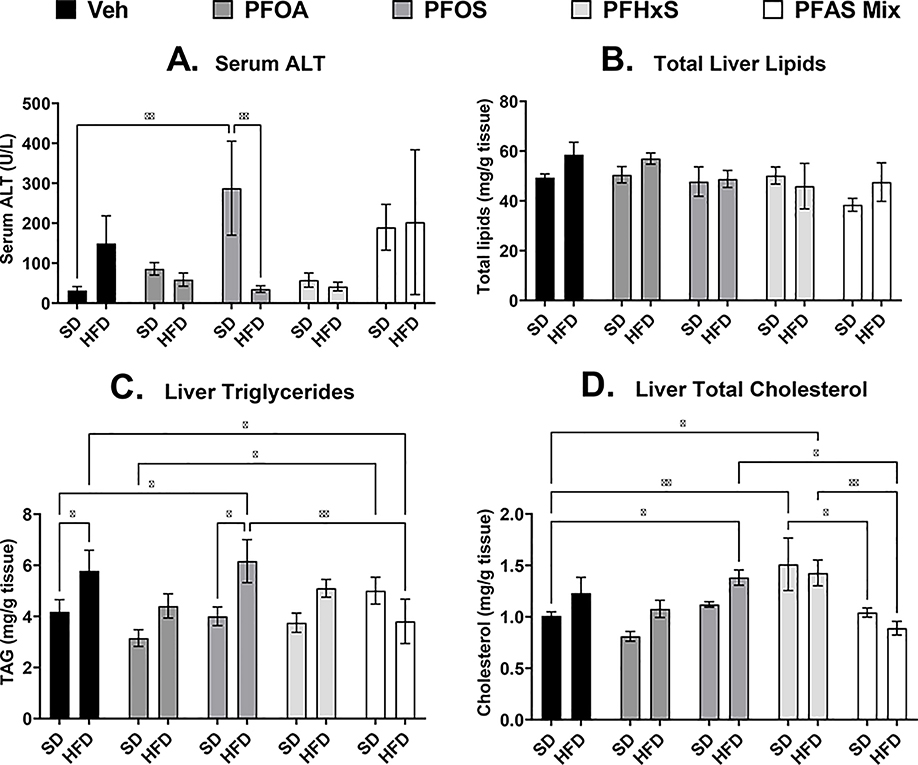
As described in Fig. 2A, serum ALT, a marker of liver damage (Ozer et al., 2008), was increased in the dams exposed to PFOS (SD+PFOS) by 8-fold compared SD+Veh dams, however when combined with HFD, (HFD+PFOS) the serum ALT is reduced by 88% compared to SD+PFOS. Total lipid mass of the dam liver was not changed with PFAS or HFD (Fig. 2B). Liver triglycerides in the dam liver (Fig. 2C) was increased with HFD+Veh by 38% compared to SD+Veh, and liver triglycerides were also increased in the HFD+PFOS group by 48% and 54% compared to SD+Veh, and SD+PFOS groups, respectively. Liver triglycerides in the SD+PFAS mixture group was increased by 59% compared to the SD+PFOA group. The HFD+PFAS mixture decreased liver triglycerides by in 34% compared to the HFD+Veh group and by 38% compared to the HFD+PFOS group. Liver total cholesterol in the dam liver (Fig. 2D) was increased in the HFD+PFOS, SD+PFHxS, and HFD+PFHxS by 37%, 50%, and 41% compared to the SD+Veh group, respectively. Liver cholesterol was decreased in the SD+PFAS mixture compared to the SD+PFHxS group by 31% and liver cholesterol was also decreased in the HFD+PFAS mixture compared to HFD+PFOS and HFD+PFHxS by 36% and 38%, respectively.
Figure 2. Perinatal PFAS and high fat diet affects maternal serum ALT and liver lipid content.
At PND 21, sera and livers of dams exposed to PFAS during gestation and lactation were collected. A) Serum alanine transaminase (ALT) was measured via colorimetric assay, B) Total lipids were isolated and normalized to tissue weight. C) Triglycerides, and B) cholesterol content, and was measured via colorimetric assay and normalized to tissue weight. Calculations were performed using a two-way ANOVA followed by Fisher’s LSD test. All values are means ± SEM; N = 3–5 litters. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
3.3. Diet and PFAS effects on liver lipid content in pups at PND 21.
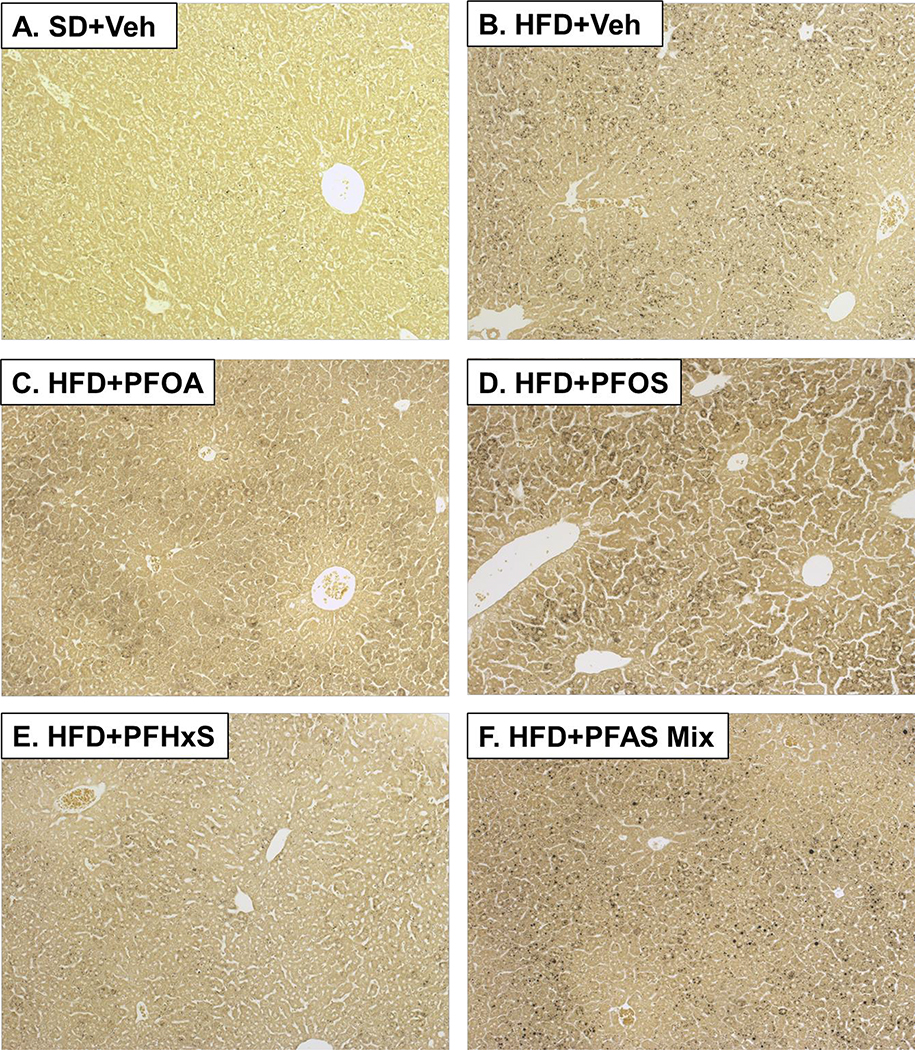
Histological assessment of hepatic tissue sections from pups at PND 21 indicated increased lipid accumulation in the pups exposed to the maternal HFD compared to SD+Veh group. Representative images of male pups from Veh controls and treatments with increased lipid accumulation is presented in Fig.3. Variably sized, black foci are osmium positive-staining of microvesicular lipid droplets. Pups that were exposure to the maternal HFD were characterized by microvesicular, osmium positive lipid droplets throughout the parenchyma centered on portal triads (Fig 3B). In all the HFD and PFAS groups (Fig 3B–F), the incidence and severity of lipid accumulation was increased, and at least 12.5% of livers exposed to a maternal HFD had severity scores of 3 or more (≥3) compared to SD+Veh (Table 2). In the HFD+PFOA group, the severity of lipid accumulation was lower compared to HFD+PFAS mixture by 86%.
Figure 3. Liver histology.
Hepatic tissue sections from pups at PND 21 were formalin fixed, post-fixed with osmium tetroxide, and stained via the periodic acid-Schiff dual-staining technique. The sections were scored for lipid accumulation (Table 2). Representative images of male pups from Veh controls and treatments with increased lipid accumulation, viewed at 100X, are presented. A) SD+Veh, B) HFD+Veh, C) HFD+PFOA, D) HFD+PFOS, E) HFD+PFHxS, and F) HFD+PFAS Mix. Black spots on slides are indicative of osmium positive small microvesicular lipid droplets.
Table 2.
Effect of PFAS and maternal HFD on pup liver steatosis histopathology
| Scores | SD+Veh | HFD+Veh | SD+PFOA | HFD+PFOA | SD+PFOS | HFD+PFOS | SD+PFHxS | HFD+PFHxS | SD+PFAS mix | HFD+PFAS mix |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 0 | 4/7 | 0/8 | 5/8 | 1/8 | 4/8 | 0/8 | 1/8 | 0/8 | 3/6 | 0/8 |
| 1 | 3/7 | 1/8 | 3/8 | 2/8 | 3/8 | 0/8 | 4/8 | 3/8 | 2/6 | 0/8 |
| 2 | 0/7 | 1/8 | 0/8 | 4/8 | 1/8 | 2/8 | 3/8 | 2/8 | 1/6 | 1/8 |
| 3 | 0/7 | 2/8 | 0/8 | 0/8 | 0/8 | 2/8 | 0/8 | 1/8 | 0/6 | 3/8 |
| 4 | 0/7 | 4/8 | 0/8 | 1/8 | 0/8 | 3/8 | 0/8 | 2/8 | 0/6 | 2/8 |
| 5 | 0/7 | 0/8 | 0/8 | 0/8 | 0/8 | 1/8 | 0/8 | 0/8 | 0/6 | 2/8 |
|
| ||||||||||
| ≥3 | 0/7 | 6/8 | 0/8 | 1/8 | 0/8 | 6/8 | 0/8 | 3/8 | 0/6 | 7/8 |
| 0% | 75%* | 0% | 12.5%*¶ | 0% | 75%*¶ | 0% | 37.5%* | 0% | 87.5%*¶§ | |
Formalin fixed hepatic tissue sections were post-fixed with osmium tetroxide and stained via the periodic acid-Schiff dual-staining technique. The sections were scored for lipid accumulation via osmium positive small (microvesicular) lipid droplets throughout liver parenchyma (range from 0 to 5, where 0 is the least and 5 is most severe). Statistical analysis was performed using Kruskal–Wallis test followed by Dunn’s multiple comparison test for multiple comparisons. N=6–8/treatment group.
p< 0.05 versus SD+Veh
p<0.05 versus HFD+Veh.
p<0.05 SD versus HFD within each treatment (i.e., SD+PFOA vs HFD+PFOA)
p<0.05 versus PFOA and PFAS mix within each diet (i.e., SD+PFOA vs SD+PFAS mix)
p<0.05 versus PFOS and PFAS mix within each diet (i.e., SD+PFOS vs SD+PFAS mix)
p<0.05 versus PFHxS and PFAS mix within each diet (i.e., SD+PFHxS vs SD+PFAS mix).
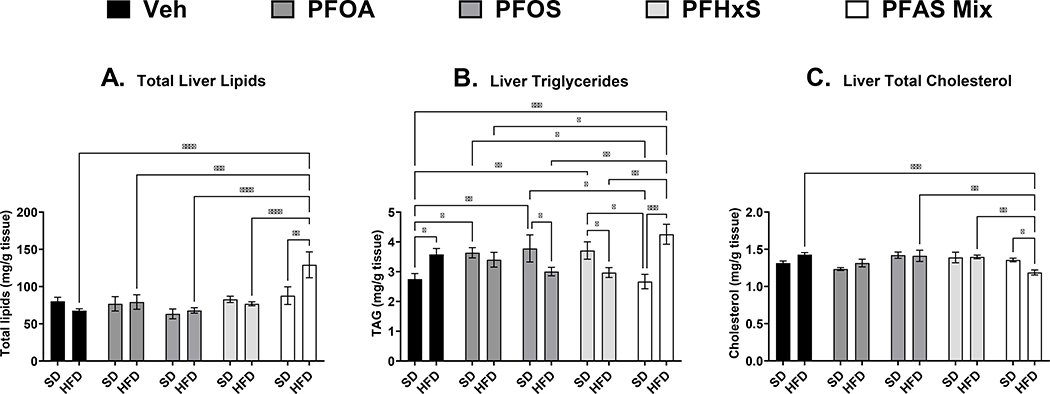
As described in Fig. 4A, total lipid mass from isolated lipids in liver tissues in the pups at PND 21 was only increased in the HFD+PFAS mixture group by 57% compared to SD+Veh. Liver triglycerides (Fig. 4B) was increased with maternal HFD feeding by 30%. An increase in liver triglycerides was not observed in any of the single PFAS treatments with the maternal HFD, however, in all the single PFAS treatments with SD, liver triglycerides were increased in the pups by 32%, 37%, and 35% for PFOA, PFOS, and PFHxS, respectively. The SD+PFAS mixture was not different compared to SD+Veh, however unlike the single PFAS treatments, the HFD+PFAS mixture was increased compared to SD+Veh by 55%. Total cholesterol (Fig. 4C) was significant decreased in the HFD+PFAS mixture group 17% compared to HFD+Veh. HFD+PFAS mixture was also decreased compared PFOS and HFD+PFHxS groups by ~37%.
Figure 4. Perinatal PFAS and high fat diet effects pup liver lipid content At PND 21.
Frozen livers of pups exposed to PFAS during gestation and lactation were collected at PND 21. A) Total lipids were isolated and normalized to tissue weight. B) Triglycerides, and C) cholesterol content, and was measured via colorimetric assay and normalized to tissue weight. No significant differences in lipid content were observed between male and female pups, and data presented represent the average of both male and female pups per litter. Calculations were performed using a two-way ANOVA followed by Fisher’s LSD test. All values are means ± SEM; N = 3–5 litters. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
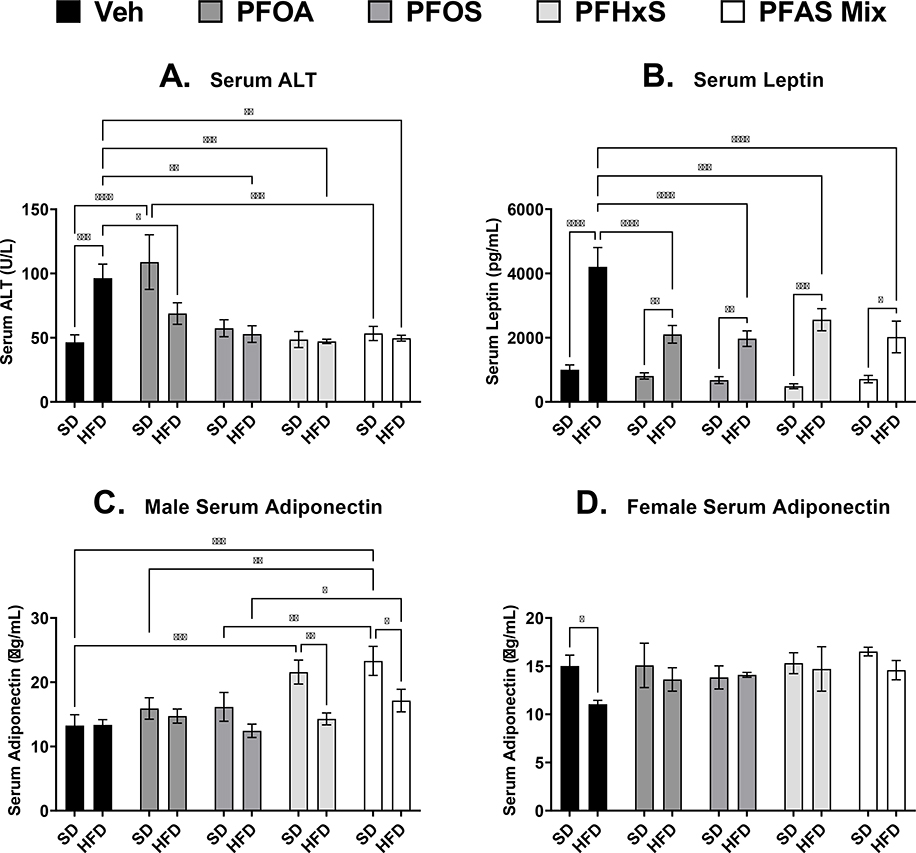
3.4. Diet and PFAS effects on pup serum adipokines at PND 21.
As described in Fig. 5A, serum ALT, a marker of liver damage (Ozer et al. 2008), was increased with the HFD by 108% in Veh controls at PND 21. In all single PFAS (PFOA, PFOS, and PFHxS) treatments and the PFAS mixture with the HFD, there was a decrease compared to HFD+Veh by 29%, 45%, 51%, and 49% respectively. The only PFAS treatment that increased ALT was SD+PFOA by 135% compared to SD+Veh. Leptin and adiponectin are serum hormones secreted by adipose tissue that regulates appetite, energy balance and insulin sensitivity (Park et al. 2004). As expected, HFD more than triples leptin levels compared to the Veh control (Fig. 5B). In all PFAS treatments with HFD, PFOA, PFOS, PFHxS, and the PFAS mixture decrease leptin by 50%, 53%, 39%, and 52% compared to HFD+Veh. Unlike ALT and leptin at PND 21, serum adiponectin also had significant sex differences. In male pups (Fig. 5C), serum adiponectin was increased by 63% and 76% in the SD+PFHxS and SD+PFAS mixture groups respectively, compared to SD+Veh. The HFD+PFAS mixture group was 38% higher compared to HFD+PFOS, but not HFD+Veh. In female pups, the only difference in serum adiponectin levels observed was a decrease with HFD by 26% compared to SD+Veh (Fig. 5D).
Figure 5. Perinatal PFAS and high fat diet effects serum adipokines in pups at PND 21.
Serum from perinatal PFAS-exposed pups were analyzed for A) alanine transaminase (ALT) via colorimetric assay, and B) leptin, and C and D) adiponectin via sandwich enzyme-linked immunosorbent assay (ELISA) kits. No significant differences in ALT and leptin were observed between male and female pups, and data presented represent the average of both male and female pups. Calculations were performed using a two-way ANOVA followed by Fisher’s LSD test. All values are means ± SEM; N = 3–5 litters. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
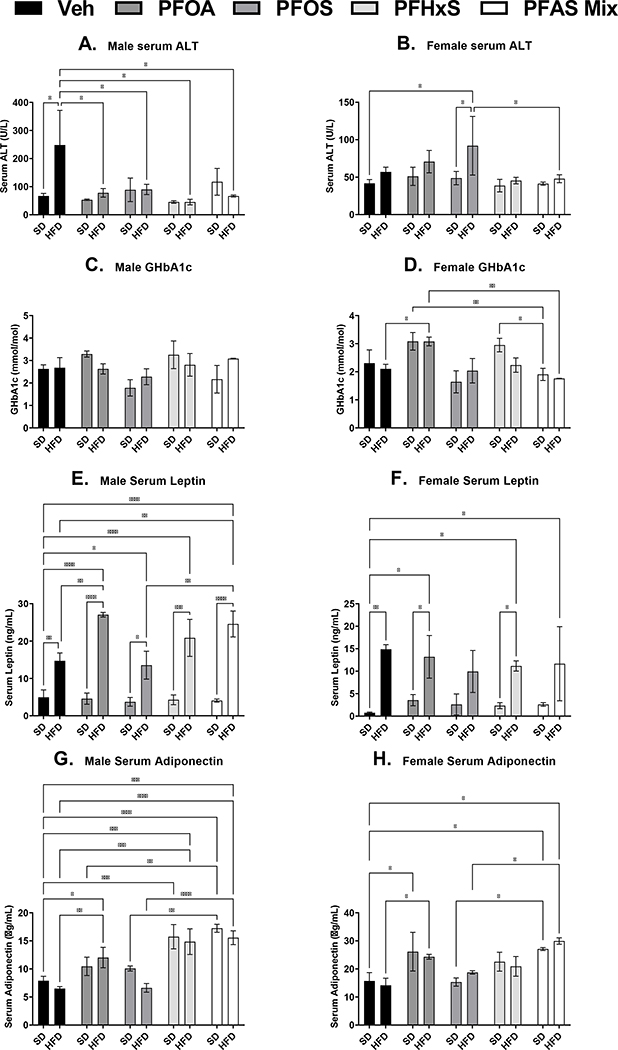
3.5. Diet and PFAS effects on pup serum proteins at PND 90.
As described in Fig. 6A, male serum ALT, had similar to trend as PND 21 timepoint with respect to the HFD. Male serum ALT was increased with the HFD by almost 3-fold in Veh controls at PND 21. In all single PFAS (PFOA, PFOS, and PFHxS) treatments and the PFAS mixture with the HFD, there was a decrease compared to HFD+Veh by 69%, 64%, 82%, and 73% respectively. In female pups at PND 90 (Fig. 6B), only HFD+PFOS, unlike males, was increased by 120% and 89% SD+Veh and SD+PFOS controls, respectively. GHbA1c is a biomarker that indicates the presence of excessive sugar in the bloodstream, is used often used to determine the three-month average blood sugar level (WHO, 2011). In male pups (Fig. 6C), there were only non-significant tends in GHbA1c, similar to female pup GHbA1c levels. Female pups (Fig. 6D) in the HFD+PFOA had increased GHbA1c levels by 46% compared to HFD+Veh controls. Female pups in both the SD+PFOA and SD+PFHxS groups has increased GHbA1c, levels by 61% and 55% respectively, compared to the SD+PFAS mixture group. Female pups also in the HFD+PFOA group has increased GHbA1c levels by 75% compared to the HFD+PFAS mixture group. In both male and female pups at PND 90, HFD increase serum leptin levels, as expected, by ~3-fold and ~10-fold respectively in all treatments compared to the Veh control (Fig. 6E and 6F). Only in male pups (Fig. 6E), leptin is increased HFD+PFOA and HFD+PFAS mixture by 84% and 67% compared to HFD+Veh controls, which differs from observed changes at PND 21. In male and female pups at PND 90 (Fig. 6G and 6H), serum adiponectin levels had no changes with HFD alone. In male pups (Fig. 6G), treatment with PFHxS and the PFAS mixture increased serum adiponectin in both SD and HFD diet groups by ~118% and ~128% respectively. Additionally, HFD+PFOA, HFD+PFHxS, and HFD+PFAS mixture groups were also increased compared to HFD+Veh by 85%, 128%, and 139%. In female pups (Fig. 6H) only PFOA and the PFAS mixture increased serum adiponectin compared to Veh controls. SD+PFOA and SD+PFAS mixture groups, however, was increased by 55% and 72% respectively compared to the SD+Veh controls, and HFD+PFOA and HFD+PFAS mixture was increased by 72% and 111% respectively compared to the HFD+Veh controls.
Figure 6. Perinatal PFAS and high fat diet effects serum adipokines in pups at PND 90.
Serum from perinatal PFAS-exposed pups were analyzed for A and B) alanine transaminase (ALT) via colorimetric assay. C and D) Glycated hemoglobin A1c (GHbA1c) E and F) leptin, and G and H) adiponectin were analyzed via enzyme-linked immunosorbent assay (ELISA) kits. Calculations were performed using a two-way ANOVA followed by Fisher’s LSD test. All values are means ± SEM; N = 3–5 litters. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
3.6. Dam and Pup Serum PFAS concentrations.
As described in Table 3, serum PFAS concentration was measured to understand whether PFAS levels in dams and pups could explain the treatment effects observed. In the dams at PND 21, the 1 mg/kg/day dose of PFOA, PFOS, and PFHxS corresponded to serum concentrations of 115.2 ± 9.23 μg/mL PFOA, 7.32 ± 0.82 μg/mL of PFOS, and 212.9 ± 16.2 μg/mL PFHxS in the SD groups. Serum PFOS levels in the dams were higher with HFD feeding in both the SD and HFD groups by 34% and 61% respectively. Similar trends were also observed in single PFOA and PFHxS treatment, however the trends were not significant. In addition, serum PFOS levels in the dams that received the PFAS mixture was decreased by 42% and 30% in both the SD and HFD groups, respectively.
Table 3:
Dam and Pup Serum PFAS Concentrations.
| SD+Veh | HFD+Veh | SD+PFOA | HFD+PFOA | SD+PFOS | HFD +PFOS | SD+PFHxS | HFD+PFHxS | SD+PFAS mix | HFD+PFAS mix | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Dams | PFOA (μg/mL) | <LLOQ | <LLOQ | 115.2± 9.23 | 139.2± 8.43 | - | - | - | - | 145.9± 18.1 | 114.7± 8.23 |
| PFOS (μg/mL) | <LLOQ | <LLOQ | - | - | 7.32± 0.82 | 9.84± 0.61* | - | - | 4.27± 0.31# | 6.87± 0.82*# | |
| PFHxS (μg/mL) | <LLOQ | <LLOQ | - | - | - | - | 212.9± 16.2 | 255.6± 25.2 | 223.7± 36.2 | 216.6± 15.9 | |
|
| |||||||||||
| Pups at PND 21 ** | PFOA (μg/mL) | <LLOQ | <LLOQ | 11.73± 1.42 | 17.51± 1.41* | - | - | - | - | 11.47± 0.94 | 15.21± 1.35 |
| PFOS (μg/mL) | <LLOQ | <LLOQ | - | - | 0.61± 0.15 | 0.46± 0.04 | - | - | 0.28± 0.01# | 0.39± 0.10 | |
| PFHxS (μg/mL) | <LLOQ | <LLOQ | - | - | - | - | 27.97± 2.09 | 24.10± 1.69 | 24.41± 2.78 | 25.67± 2.74 | |
|
| |||||||||||
| Male Pups at PND 90 | PFOA (μg/mL) | <LLOQ | <LLOQ | 1.08± 0.26 | 1.89± 0.37* | - | - | - | - | 0.37± 0.03 | 1.80± 0.06* |
| PFOS (μg/mL) | <LLOQ | <LLOQ | - | - | 0.12± 0.03 | 0.11± 0.01 | - | - | 0.10± 0.01 | 0.11± 0.01 | |
| PFHxS (μg/mL) | <LLOQ | <LLOQ | - | - | - | - | 3.51± 0.16 | 3.82± 0.97 | 2.32± 0.42 | 3.00± 0.28 | |
|
| |||||||||||
| Female Pups at PND 90 | PFOA (μg/mL) | <LLOQ | <LLOQ | 0.16± 0.04¶ | 2.00± 0.40* | - | - | - | - | 0.18± 0.08 | 1.35± 0.27* |
| PFOS (μg/mL) | <LLOQ | <LLOQ | - | - | 0.11± 0.01 | 0.20± 0.01*¶ | - | - | 0.09± 0.01 | 0.15± 0.02* | |
| PFHxS (μg/mL) | <LLOQ | <LLOQ | - | - | - | - | 3.02± 0.26 | 4.75± 0.46* | 2.22± 0.45 | 4.09± 0.38* | |
Timed-pregnant female CD-1 mice were dosed with either vehicle (0.5% Tween 20 in water), 1 mg/kg PFOA, 1 mg/kg PFOS, 1 mg/kg PFHxS, or a PFAS mix (1 mg/kg of each PFOS, PFOA, and PFHxS) via oral gavage (10 mL/kg) from gestation day (GD) 1 through postnatal day (PND) 21. PFAS were extracted from dams and pup serum collected at PND 21, and pups at PND 90. PFAS were quantified using LC-MS/MS. All mice that were not dosed with PFAS had concentrations below the lower limit of quantification (LLOQ). LLOQ were 5 ng/mL for PFOA, 1 ng/mL for PFOS, and 15 ng/mL for PFHxS. Calculations were performed using a one-way ANOVA followed by Fisher’s LSD test.
no differences were observed between male and female pups for this timepoint, and data presented represent the average of both male and female pups per litter. All values are means ± SEM; N = 3–5 litters.
p<0.05 between diet treatment within the same compound (i.e., SD+PFOS vs PFOS HFD)
p<0.05 between single compound treatment and the PFAS mixture with same diet (i.e., SD+PFOS vs SD+PFAS mix).
p<0.05 verse males of the same treatment group.
In the pups at PND 21, the 1 mg/kg/day dose of PFOA, PFOS, and PFHxS corresponded to serum concentrations of 11.73 ± 1.33 μg/mL PFOA, 0.61 ± 0.15 μg/mL of PFOS, and 27.97 ± 2.09 μg/mL PFHxS in the SD groups. Male and female pups had similar levels at PND 21 (data not shown). Pup PFOA serum concentrations were decreased with HFD feeding to dams by 50%, and PFOS serum concentrations were decreased by 54% in the SD+PFAS mixture compared to SD+PFOS group.
At PND 90, male pups that received perinatal PFOA, PFOS, and PFHxS had serum concentrations of 1.08 ± 0.26 μg/mL PFOA, 0.12 ± 0.03 μg/mL of PFOS, and 3.51 ± 0.16 μg/mL PFHxS in the SD groups, and female pups that received perinatal PFOA, PFOS, and PFHxS had serum concentrations of 0.16 ± 0.04 μg/mL PFOA, 0.11 ± 0.01 μg/mL of PFOS, and 3.02 ± 0.26 μg/mL PFHxS in the SD groups. At PND 90, serum levels of PFOA in the SD diet were 85% lower in female pups compared to male pups. Further, serum PFOA levels were also higher with HFD feeding in both the males and females by 73% and 115% respectively. This was also observed in the PFAS mixture with higher PFOA levels with HFD feeding in both the males and females by 386% and 650%. However, PFOS and PFHxS serum levels were increased with HFD feeding only in the female mice. For the single PFAS treatments, PFOS and PFHxS levels were increased by 82% and 57%, respectively, with HFD feeding and in the PFAS mixture PFOS and PFHxS levels were increased by 67% and 84% respectively with HFD feeding.
3.7. Pup Liver PFAS concentrations.
As liver lipids were evaluated at PND 21, PFAS concentrations in the liver were evaluated at PND 21 (Table 4). PFAS liver concentrations of 26.41 ± 2.07 μg/g tissue PFOA, 1.41 ± 0.09 μg/g tissue of PFOS, and 4.54 ± 0.20 μg/g tissue PFHxS in the SD groups. Similar trends were observed as serum as PND 21; pup PFOA liver concentrations were decreased with HFD feeding to dams by 35%, and PFOS liver concentrations were decreased by 35% in the SD+PFAS mixture compared to SD+PFOS group. The liver to serum ratios of PFOA, PFOS, and PFHxS were 2.3 ± 0.22, 2.3 ± 0.57, and 0.17 ± 0.01 in the SD groups, respectively. Interestingly, in the PFAS mixture the liver to serum ratio of PFHxS concentration was higher than PFHxS alone by 47% in SD groups.
Table 4:
PND 21 Pup Liver PFAS Concentrations and liver to serum ratios.
| SD+Veh | HFD+Veh | SD+PFOA | HFD+PFOA | SD+PFOS | HFD+ PFOS | SD+PFHxS | HFD+PFHxS | SD+PFAS mix | HFD+PFAS mix | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Pups at 3 weeks of age(PND 21) ** | PFOA (μg/g tissue) | <LLOQ | <LLOQ | 26.41 ± 2.07 | 35.73 ± 2.57 * | - | - | - | - | 18.79 ± 3.22 | 33.04 ± 4.00 |
| PFOS (μg/g tissue) | <LLOQ | <LLOQ | - | - | 1.41 ± 0.09 | 1.30 ± 0.18 | - | - | 0.91 ± 0.07 # | 1.11 ± 0.06 | |
| PFHxS (μg/g tissue) | <LLOQ | <LLOQ | - | - | - | - | 4.54 ± 0.20 | 4.79 ± 0.44 | 6.03 ± 0.81 | 5.69 ± 0.59 | |
|
| |||||||||||
| Liver to Serum ratios | PFOA (μg/mL:μg/g tissue) | - | - | 2.3 ± 0.22 | 2.1 ± 0.20 | - | - | - | - | 1.6 ± 0.18 | 2.2 ± 0.28 |
| PFOS (μg/mL:μg/g tissue) | - | - | - | - | 2.3 ± 0.57 | 3.0 ± 0.60 | - | - | 3.2 ± 0.27 | 3.4 ± 0.79 | |
| PFHxS (μg/mL:μg/g tissue) | - | - | - | - | - | - | 0.17 ± 0.011 | 0.20 ± 0.012 | 0.25 ± 0.019 # | 0.22 ± 0.017 | |
Timed-pregnant female CD-1 mice were dosed with either vehicle (0.5% Tween 20 in water), 1 mg/kg PFOA, 1 mg/kg PFOS, 1 mg/kg PFHxS, or a PFAS mix (1 mg/kg of each PFOS, PFOA, and PFHxS) via oral gavage (10 mL/kg) from gestation day (GD) 1 through postnatal day (PND) 21. PFAS were extracted from pup serum collected at PND 21, and PFAS were quantified using LC-MS/MS. All mice that were not dosed with PFAS had concentrations below the lower limit of quantification (LLOQ). LLOQ were 0.14 μg/g for PFOA, 0.14 μg/g for PFOS, and 0.14 μg/g for PFHxS.
no differences were observed between male and female pups for this timepoint, and data presented represent the average of both male and female pups per litter. Calculations were performed using a one-way ANOVA followed by Fisher’s LSD test. All values are means ± SEM; N = 3–5 litters.
p<0.05 between diet treatment within the same compound (i.e., SD+PFOS vs PFOS HFD)
p<0.05 between single compound treatment and the PFAS mixture with same diet (i.e., SD+PFOS vs SD+PFAS mix).
4.0. Discussion
The purpose of this study was to evaluate effect of maternal HFD with perinatal PFAS and PFAS mixture exposure. Liver effects of PFOS in adults have been shown be modulated with diet and the time in which the diet was administered (Marques et al., 2020; Pfohl et al., 2021; Salter et al., 2021). Perinatal exposure to PFOS and PFOA has been shown to hepatomegaly, body weight gain, and effect leptin and insulin levels later in life (Bjork et al. 2008; Hines et al. 2009; Wan et al. 2014), however it is unclear how the liver may be impacted with diet and developmental PFAS exposure. Maternal intake of HFD during gestation and lactation has been shown to predispose adult mouse offspring to hepatic steatosis (Gregorio et al. 2010). Our results for maternal HFD feeding through gestation and lactation were consistent with other studies showing significant increases in maternal liver weight, pup body weight at PND 21, pup liver triglycerides, pup serum ALT, and pup serum leptin (Gregorio et al. 2010; Masuyama and Hiramatsu 2014; Kjaergaard et al. 2017; Zinkhan et al. 2018). Key results are summarized in Table 5. These data suggest that a HFD may increase PFOA partitioning to dam and pups and play a role in PFAS loss over time.
Table 5:
Key Results Summary Table
| Endpoint | PFOA effects | PFOS effects | PFHxS effects | PFAS Mix effects | HFD effects | PFAS+HFD combined effects | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Dams | Litter Size | ↓ w/ PFOA | |||||
| BW (PND 21) | ↑ | ↑ | |||||
| Liver Weight | ↑ | ↑ | |||||
| Serum ALT | ↑ | ↓ w/ PFOA | |||||
| Liver TAG | ↓ | ↑ | |||||
| Liver Cholesterol | ↑ | ||||||
| Serum PFAS | ↓ PFOS | ↑ PFOS | |||||
|
| |||||||
| Pups (PND 21) | Male BW | ↑ | |||||
| Female BW | ↓ | ↑ | |||||
| Liver Weight | ↑ | ↑ | |||||
| Liver Histopathology | ↑ | ||||||
| Total Liver Lipid | ↑ | ||||||
| Liver TAG | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ w/ all PFAS | |
| Liver Cholesterol | ↓ | ||||||
| Serum ALT | ↑ | ↑ | ↓ w/ all PFAS | ||||
| Serum Leptin | ↑ | ↓ w/ all PFAS | |||||
| Male Serum Adiponectin | ↑ | ↑ | |||||
| Female Serum Adiponectin | ↓ | ||||||
| Serum PFAS | ↓ PFOA | ↓ PFOS | |||||
| Liver PFAS | ↓ PFOA | ↓ PFOS | |||||
|
| |||||||
| Pups (PND 90) | Male BW | ↑ | |||||
| Female BW | ↑ | ||||||
| Male Liver Weight | ↑ | ↑ | |||||
| Female Liver Weight | ↑ | ↑ | |||||
| Male Serum ALT | ↑ | ↓ w/ all PFAS | |||||
| Female Serum ALT | ↑ | ||||||
| Female GHbA1c | ↑ | ||||||
| Male Serum Leptin | ↑ | ↑ | ↑ | ||||
| Female Serum Leptin | ↑ | ||||||
| Male Serum Adiponectin | ↑ | ↑ | |||||
| Female Serum Adiponectin | ↑ | ↑ | |||||
| Male Serum PFAS | ↑ PFOA | ||||||
| Female Serum PFAS | ↑ PFOA verse Male | ↑ all PFAS | |||||
Of the PFAS treatments, only PFOA and the PFAS mixture increased liver weights in both dams and pups. Additionally, the SD+PFOA group was also the only PFAS treatment to increase serum ALT levels. Consistent with our results, transient hepatomegaly after developmental PFOA has been observed at dose of 1 mg/kg at PND 22 (Abbott et al. 2009; White et al. 2011). The three PFAS compounds combined with SD increased liver triglyceride content in the pups suggested that, despite no observed increased in liver weight, there may have been an increase in hepatic steatosis-related pathways. It has been suggested that the liver effects of PFOS and PFOA in mice is due to activation of Peroxisome proliferator-activated receptor alpha (PPARα), a transcription factor that controls expression of lipid metabolism genes (Takacs and Abbott 2007; Bjork et al. 2011). Filgo et al. (2015) has also observed latent liver toxicity with gestational exposures to PFOA in PPARα-KO mice aged to 18 months, suggesting PPARα-independent pathways. Further evaluation of the mechanisms related to hepatomegaly and hepatic steatosis is needed to understand the relationship of maternal HFD and liver endpoints.
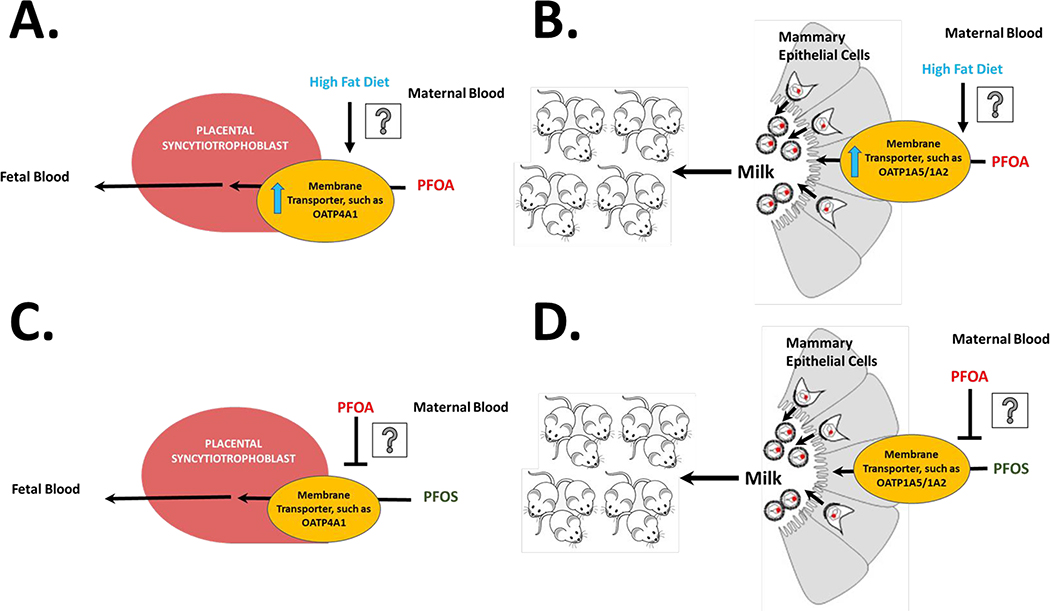
We observed that the increase in liver weight at PND 21 with PFOA treatment was lower with HFD feeding in the dam, which is supported with the dam serum concentrations as PFOA level with HFD feeding were trending down. In contrast, the PFOA-induced increase in liver weight was higher with HFD feeding in the pups. This observation was supported by the measured serum and liver concentrations of PFOA in the pups, where serum and liver PFOA concentration were higher if the dam was fed HFD. This suggests that diet may play a role in PFAS partitioning to, not only the dams, but to the pups as well. Previous work in our laboratory has also demonstrated differences in PFAS partitioning to the liver when combined with HFD feeding (Marques et al., 2020; Pfohl et al., 2020; Pfohl et al., 2021). Oatps and Ntcp have been described to transport PFAS (Zhao et al. 2015; Zhao et al. 2017). The observed difference in PFOA partitioning to pups with a HFD may be explained by upregulation of placenta and mammary epithelium transporters with a HFD as described in Fig. 7A and 7B (Laporta et al. 2013; Mahany et al. 2018; Son et al. 2019). Sex differences in the disposition of PFAS in rats have been observed and are believed to be caused by differential expression of renal transporters (Han et al., 2012). Females have been described to have higher clearance in humans and rodents (Li et al., 2018; Pizzurro et al., 2019). Consistent with previous studies, sex differences were observed herein at PND 90; PFOA concentrations in female pups were lower in the SD diet treatment groups, as expected. Differences in serum PFAS concentrations were also observed at the PND 90 timepoint where PFOA in both males and female achieved higher concentrations combined with HFD feeding. Interestingly, this trend was also observed with PFOS and PFHxS exposure with HFD feeding, however this was only observed in females. This suggests that the female pups may be more sensitive to PFAS effects in combination with a HFD and warrants further investigation.
Figure 7. Hypothesized mechanisms of PFAS partitioning to pups.
PFOA levels in pup serum and liver were increased with HFD compared PFOA treatment alone, it is hypothesized that this may be controlled by upregulation of membrane transporters in A) placental syncytiotrophoblasts and/or B) mammary epithelial cells. PFOS levels in pup serum and liver in the SD group were lower in the PFAS mixture. PFOA may act as an inhibitor of membrane transporter in C) placental syncytiotrophoblasts and/or D) mammary epithelial cells and reduce PFOS partitioning to pups in the mixture.
Diet also influenced serum ALT and liver triglycerides. Perinatal HFD feeding increased pup serum ALT (at PND 21 and in males at PND 90) and pup liver triglycerides, however when combined with PFAS, PFAS treatment ameliorated HFD-induced elevations in serum ALT, and liver triglyceride content. A similar paradoxical protective effect on liver lipid accumulation was observed in adult mice with PFOS treatment of 1 mg/kg/day or 0.24 mg/kg/day with concurrent high fat diet exposure (Huck et al., 2018; Pfohl et al., 2021). Pfohl et al. also observed a reduction in Oatp transporter expression when PFOS was combined with the HFD, which was hypothesized to be due to Pparα activation. Pparα activation can reduce Oatp expression (Cheng and Klaassen, 2008), a known uptake transporter for PFAS. Decreased Oatp expression coupled with increased serum lipids from a HFD diet may increase competition for fatty acid uptake thus reducing hepatic lipids. In addition, Pparα activation may also increase lipid oxidation which may also reduce hepatic lipid accumulation. Activation of Pparα with PFOS and PFOA had been described as a sensitive pathway in rodents (Takacs and Abbott 2007; Bjork et al. 2011). However, human liver PPARα has been shown to have less overall DNA binding activity than mouse PPARα (Palmer et al., 1998), and further study is necessary to understand the role of PPARα activation in the context of human exposure. Future investigations on the role of transporters in PFAS disposition may aid in mechanistic understand of the role of an HFD. At PND 90, serum ALT levels in female pups were not affected by the HFD, however perinatal PFOS exposure when combined with a HFD in female pups increased serum ALT levels. This again suggests that female pups may be more sensitive to PFAS liver effects as demonstrated by increased serum PFAS levels and ALT when combined with a HFD. Elevated ALT was observed with PFOS and HFD in female pups, and not male pups, likewise Attanasio (2019) also found sex differences in liver function enzymes in US adolescents associated with serum levels of PFAA. Females had higher odds of elevated ALT with increased PFOA, and males had decreased odds of elevated ALT with increased PFOA (Attanasio, 2019). This suggested that liver endpoints in females with PFAS exposure, such as ALT, should be further evaluated, especially in models of obesity and diet.
The dose of 1 mg/kg/day was selected to be above LOAEL of liver enlargement in dams, and to ensure maternal transfer of PFAS to pups (Lau et al. 2006; Wan et al. 2014). PFAS serum concentrations in the dam and pups are comparable to concentrations found in studies of perinatal exposure and were within a 10-fold difference depending on differences in dose and timing (Chang et al., 2018; Fenton et al., 2009; Lau et al., 2006; Macon et al., 2011; Thibodeaux et al., 2003; Wan et al., 2014). The doses chosen were not necessarily representative of potential human exposure levels, and the observed PFOA, PFOS, and PFHxS levels in humans are much lower. Mean serum levels of PFOA, PFOS, PFHxS in human mothers sampled 3 weeks after delivery have been reported as 2.8, 14, and 2.2 ng/mL (Gyllenhammar et al., 2018), which is approximately 40,000-fold, 500-fold, and 100,000-fold lower than serum concentrations PND 21 dams in this study. Mean serum levels of PFOA, PFOS, PFHxS in 2–4 month old infants have been reported as 7.7, 8.6, and 2.6 ng/mL (Gyllenhammar et al., 2018), which is approximately 1500-fold, 70-fold, and 10,000-fold lower than serum concentrations in PND 21 pups described this study. Unfortunately, mice possess significantly higher clearance mechanisms for PFOA, PFOS, and PFHxS and results in significantly shorter half-lives of weeks compared to years in humans (Chang et al., 2012; Lau et al., 2007; Olsen et al., 2007; Sundström et al., 2012). The consequence of this difference often increases the doses used in mice.
Studies have shown divergent results for serum leptin depending on timing, and exposure level. Herein, we showed a significant decrease in serum leptin with all the single PFAS treatments when combined with a HFD at an early time point (PND 21) and an increase in serum leptin with PFOA treatment combined with a HFD at a later time point (PND 90). Human studies in the Faroese population have shown that decreases in leptin and adiponectin were associated with PFAS concentrations for 5-year-old children, however there were mostly null associations for PFAS at ages 7 to 13 years (Shelly et al. 2019). Other human developmental studies have found no statistically significant associations between PFAS exposure and serum adipokines, however the PFAS concentrations these studies reported (interquartile ranges for maternal serum PFOS of ~ 3.2 to 18 ng/mL) were much lower than the Faroese population (23.3 to 35.5 ng/mL) (Minatoya et al. 2017; Buck et al. 2018). In mice, developmental low-dose PFOA (0.01–0.1 mg/kg/day) exposure has been shown to increase serum leptin levels in midlife (21 to 33 weeks) (Hines et al. 2009), which does correspond to our results. This suggests that timing and dose may be important to understanding metabolic effects of PFAS on adipokines. In addition, serum adiponectin levels were also influenced by sex differences at both PND 21 and PND 90. In males, serum adiponectin was increased by PFHxS and the PFAS mixture at both time points, however this effect was not conserved in females.
A novel aspect of this work was the inclusion of a mixture of PFAS; most experimental in vivo studies investigate the effects of a single compound. Current work has evaluated PFAS mixtures, in silico or in vitro. Hoover et al. (2019) utilized an in silico model to estimate mixture effects, based on single studies on cytotoxicity in an amphibian fibroblast cell line. Mixtures would be approximately additive with the exception of PFOS and PFOA, which were found to be weakly synergistic (Hoover et al., 2019). Wolf et al. (2014) also found that at low concentrations PFAA were additive with regards to PPARα activation in a luciferase reporter assay. Other studies with mixtures have found synergistic effects of PFAS with regard to endocrine activity and cytotoxicity in vitro (Rosenmai et al. 2018; Ojo et al. 2020). This is the first perinatal exposure study to evaluate a PFAS mixture in vivo. The PFAS mixture had very distinct effects when compared to single compound treatment. With regard to liver weights and liver to body weight ratios increases, the PFAS mixture data were analogous to the effects seen with PFOA treatment. However, unlike PFOA, the serum ALT level, did not increase in the PFAS mixture. In the case of liver lipids, only the PFAS mixture in combination with HFD feeding decreased total cholesterol in the pups and increased total lipid in the pups. However, liver triglycerides were increased with all three single PFAS treatments with the SD, and in treatment with the PFAS mixture with SD, there was no change compared to control. Increased liver triglycerides and lowered liver cholesterol levels have also been described for PFOS, PFOA, and PFHxS administration in adult mice (Bijland et al., 2011; Li et al., 2019; Marques et al., 2020; Pfohl et al., 2020). These results suggest that there are multiple pathways in which PFAS could add, synergize, or antagonize specific effects, and warrants further investigation of dose response data with model predictions of additivity. PFOS levels in pup and dam serum were lower in the PFAS mixture compared to PFOS treatment alone. As mentioned before, these compounds are known substrates for cellular transporters such as Oatp, which are present on placenta and mammary epithelium (St-Pierre et al. 2002; W. Zhao et al. 2015; Zhao et al. 2017; García-Lino et al. 2019). Yang et al. (2009) has also found that PFOA was both a substrate and an inhibitor of rat Oatp1a1, and PFOA could potentially play a role in inhibiting Oatp-mediated transfer to dam and the pup placenta or mammary epithelium as shown in Fig. 7C and 7D.
5.0. Conclusions
The purpose of this work is to evaluate the impact of diet and PFAS exposure to dam on the risk to pup liver and metabolism endpoints later in life, as well as evaluate diet related effects on PFAS partitioning to pups. Herein we have demonstrated that maternal diet can influence how PFAS affects the pup liver at PND 21, and partitions to the mouse pups. Our serum measurement at PND 90 have also suggests that female pups may be more sensitive to perinatal PFAS effects when combined with a HFD and warrants further investigation. To our knowledge, this is the first perinatal study to evaluate a PFAS mixture in vivo. The PFAS mixture had very distinct effects when compared to single compound treatment, suggesting cumulative properties of the mixture, particularly when evaluating PFAS transfer from dam to pup. Further study to evaluate the mechanisms behind the observations made in this body of work is needed to confirm these conclusions.
Supplementary Material
6.0. Acknowledgements
The authors would like to thank the URI Comparative Biology Resources Center (CBRC) Animal Care staff for assistance with monitoring the feed and health of the animals on study, and Giuseppe Tarantola, Yaslin Alicea-Hidrovo, Naomi Pajarillo, and Olga Skende for assistance with mouse dosing and colorimetric assays.
7.0. Funding
This work was supported by National Institute of Health [grant number P42ES027706].
This material is based upon work conducted at a Rhode Island NSF EPSCoR research facility, Molecular Characterization Facility, supported in part by the National Science Foundation EPSCoR Cooperative Agreement # OIA-1655221, and at a Rhode Island Institutional Development Award (IDeA) Network of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- PFAS
Per- and polyfluoroalkyl substances
- PFOS
perfluorooctanesulfonate
- PFOA
perfluorooctanoic acid
- PFHxS
perfluorohexanesulfonic acid
- HFD
high fat diet
Footnotes
8.0 Conflict of Interest
The authors of this manuscript declare no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9.0 References
- Abbott BD, Wolf CJ, Das KP, Zehr RD, Schmid JE, Lindstrom AB, Strynar MJ, Lau C. 2009. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reprod Toxicol. 27(3–4):258–265. doi: 10.1016/j.reprotox.2008.05.061. [DOI] [PubMed] [Google Scholar]
- Attanasio R, 2019. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: Analyses of NHANES 2013–2016. Environmental Pollution 254, 113061. 10.1016/j.envpol.2019.113061 [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 121(11–12):1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, Cave MC. 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 247:1055–1063. doi: 10.1016/j.envpol.2019.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijland S, Rensen PCN, Pieterman EJ, Maas ACE, van der Hoorn JW, van Erk MJ, Havekes LM, Willems van Dijk K, Chang S-C, Ehresman DJ, Butenhoff JL, Princen HMG, 2011. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol. Sci 123, 290–303. 10.1093/toxsci/kfr142 [DOI] [PubMed] [Google Scholar]
- Bjork JA, Lau C, Chang SC, Butenhoff JL, Wallace KB, 2008. Perfluorooctane sulfonate-induced changes in fetal rat liver gene expression. Toxicology 251, 8–20. 10.1016/j.tox.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL, Wallace KB. 2011. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 288(1–3):8–17. doi: 10.1016/j.tox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Buck CO, Eliot MN, Kelsey KT, Calafat AM, Chen A, Ehrlich S, Lanphear BP, Braun JM. 2018. Prenatal exposure to perfluoroalkyl substances and adipocytokines: the HOME Study. Pediatr Res. 84(6):854–860. doi: 10.1038/s41390-018-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, Jung R, Kennedy G, Lieder P, Olsen G, et al. 2002. Toxicity of Ammonium Perfluorooctanoate in Male Cynomolgus Monkeys after Oral Dosing for 6 Months. Toxicol Sci. 69(1):244–257. doi:.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL, Frame SR, O’Connor JC, York RG. 2004. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 196(1–2):95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Chang S-C, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, Butenhoff JL, 2012. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reproductive Toxicology, A Second Special Issue on Recent Advances in Perfluoroalkyl Acid Research 33, 428–440. 10.1016/j.reprotox.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Chang S, Allen BC, Andres KL, Ehresman DJ, Falvo R, Provencher A, Olsen GW, Butenhoff JL, 2017. Evaluation of Serum Lipid, Thyroid, and Hepatic Clinical Chemistries in Association With Serum Perfluorooctanesulfonate (PFOS) in Cynomolgus Monkeys After Oral Dosing With Potassium PFOS. Toxicol Sci 156, 387–401. 10.1093/toxsci/kfw267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB, Seed JG, 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. Reproductive Toxicology 78, 150–168. 10.1016/j.reprotox.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD, 2008. Critical Role of PPAR-α in Perfluorooctanoic Acid– and Perfluorodecanoic Acid–Induced Downregulation of Oatp Uptake Transporters in Mouse Livers. Toxicological Sciences 106, 37–45. 10.1093/toxsci/kfn161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A, Shin H-M, Bartell SM, Steenland K. 2016. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ Health Perspect. 124(8):1227–1233. doi: 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou S-SE, 2009. Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reproductive Toxicology, Recent Advances in Perfluoroalkyl Acid Research 27, 365–372. 10.1016/j.reprotox.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. 2015. Perfluorooctanoic Acid (PFOA)-induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARα Is Not Required. Toxicol Pathol. 43(4):558–568. doi: 10.1177/0192623314558463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczény O, Koletzko B, et al. 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol. 44(18):7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, Lopez-Espinosa M-J, Frisbee SJ, Karlsson L, Ducatman AM, Fletcher T. 2012. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 120(5):655–660. doi: 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lino AM, Álvarez-Fernández I, Blanco-Paniagua E, Merino G, Álvarez AI. 2019. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients. 11(10):2372. doi: 10.3390/nu11102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates L, Adler RR, Elangbam CS, 2016. Osmium tetroxide post-fixation and periodic acid-Schiff dual-staining technique to demonstrate intracellular lipid and glycogen in the mouse liver section – a novel method for co-visualization of intracellular contents in paraffin-embedded tissue. Journal of Histotechnology 39, 2–7. 10.1080/01478885.2015.1106072 [DOI] [Google Scholar]
- Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, Lohmann R, Ng CA, Trier X, Wang Z, 2020. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22, 2345–2373. 10.1039/d0em00291g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JA, Post GB, Fagliano JA. 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environmental Research. 136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. 2017. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ Health Perspect. 125(7):077018. doi: 10.1289/EHP275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, Budtz-Jørgensen E. 2017. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol. 14(1):188–195. doi: 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio BM, Souza-Mello V, Carvalho JJ, Mandarim-de-Lacerda CA, Aguila MB. 2010. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am J Obstet Gynecol. 203(5):495.e1–8. doi: 10.1016/j.ajog.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Benskin JP, Sandblom O, Berger U, Ahrens L, Lignell S, Wiberg K, Glynn A, 2018. Perfluoroalkyl Acids (PFAAs) in Serum from 2–4-Month-Old Infants: Influence of Maternal Serum Concentration, Gestational Age, Breast-Feeding, and Contaminated Drinking Water. Environ Sci Technol 52, 7101–7110. 10.1021/acs.est.8b00770 [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW, 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chem. Res. Toxicol. 25, 35–46. 10.1021/tx200363w [DOI] [PubMed] [Google Scholar]
- Hansen KJ, Clemen LA, Ellefson ME, Johnson HO, 2001. Compound-Specific, Quantitative Characterization of Organic Fluorochemicals in Biological Matrices. Environ. Sci. Technol. 35, 766–770. 10.1021/es001489z [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Molecular and Cellular Endocrinology. 304(1):97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hoover G, Kar S, Guffey S, Leszczynski J, Sepúlveda MS. 2019. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere. 233:25–33. doi: 10.1016/j.chemosphere.2019.05.065. [DOI] [PubMed] [Google Scholar]
- Huck I, Beggs K, Apte U. 2018. Paradoxical Protective Effect of Perfluorooctanesulfonic Acid Against High-Fat Diet-Induced Hepatic Steatosis in Mice. Int J Toxicol 37(5):383–392. doi: 10.1177/1091581818790934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J-M, Chen D, Han F-J, Guo Y, Zeng L, Lu X, Wang F, 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Science of The Total Environment 636, 1058–1069. 10.1016/j.scitotenv.2018.04.380 [DOI] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kjaergaard M, Nilsson C, Secher A, Kildegaard J, Skovgaard T, Nielsen MO, Grove K, Raun K. 2017. Differential hypothalamic leptin sensitivity in obese rat offspring exposed to maternal and postnatal intake of chocolate and soft drink. Nutr Diabetes. 7(1):e242. doi: 10.1038/nutd.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporta J, Peters TL, Merriman KE, Vezina CM, Hernandez LL. 2013. Serotonin (5-HT) Affects Expression of Liver Metabolic Enzymes and Mammary Gland Glucose Transporters during the Transition from Pregnancy to Lactation. PLoS One. 8(2). doi: 10.1371/journal.pone.0057847. [accessed 2020 Apr 28]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3585179/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. 2003. Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. II: Postnatal Evaluation. Toxicol Sci. 74(2):382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J, 2007. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci 99, 366–394. 10.1093/toxsci/kfm128 [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Klaunig JE, 2019. The effects of perfluorooctanoate on high fat diet induced non-alcoholic fatty liver disease in mice. Toxicology 416, 1–14. 10.1016/j.tox.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K, 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75, 46–51. 10.1136/oemed-2017-104651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Lin L-Y, Chiang C-K, Wang W-J, Su Y-N, Hung K-Y, Chen P-C. 2010. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol. 105(6):1354–1363. doi: 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- Lin P-ID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert M-F, Fleisch AF, Calafat AM, Webster TF, Horton ES, et al. 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults—longitudinal analysis of the diabetes prevention program outcomes study. Environment International. 129:343–353. doi: 10.1016/j.envint.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. 2005. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology. 215(1–2):149–169. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, White SS, Helfant L, Fenton SE, 2011. Prenatal Perfluorooctanoic Acid Exposure in CD-1 Mice: Low-Dose Developmental Effects and Internal Dosimetry. Toxicological Sciences 122, 134–145. 10.1093/toxsci/kfr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahany EB, Han X, Borges BC, da Silveira Cruz-Machado S, Allen SJ, Garcia-Galiano D, Hoenerhoff MJ, Bellefontaine NH, Elias CF. 2018. Obesity and High-Fat Diet Induce Distinct Changes in Placental Gene Expression and Pregnancy Outcome. Endocrinology. 159(4):1718–1733. doi: 10.1210/en.2017-03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques E, Pfohl M, Auclair A, Jamwal R, Barlock BJ, Sammoura FM, Goedken M, Akhlaghi F, Slitt AL, 2020. Perfluorooctanesulfonic acid (PFOS) administration shifts the hepatic proteome and augments dietary outcomes related to hepatic steatosis in mice. Toxicology and Applied Pharmacology 408, 115250. 10.1016/j.taap.2020.115250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y. 2014. Additive Effects of Maternal High Fat Diet during Lactation on Mouse Offspring. PLOS ONE. 9(3):e92805. doi: 10.1371/journal.pone.0092805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. 2007. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health. 80(7):643–648. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- Minatoya M, Itoh S, Miyashita C, Araki A, Sasaki S, Miura R, Goudarzi H, Iwasaki Y, Kishi R. 2017. Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines and birth size: The Hokkaido Study on environment and children’s health. Environmental Research. 156:175–182. doi: 10.1016/j.envres.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Mortensen AS, Letcher RJ, Cangialosi MV, Chu S, Arukwe A. 2011. Tissue bioaccumulation patterns, xenobiotic biotransformation and steroid hormone levels in Atlantic salmon (Salmo salar) fed a diet containing perfluoroactane sulfonic or perfluorooctane carboxylic acids. Chemosphere. 83(8):1035–1044. doi: 10.1016/j.chemosphere.2011.01.067. [DOI] [PubMed] [Google Scholar]
- Ode A, Rylander L, Lindh CH, Källén K, Jönsson BAG, Gustafsson P, Olofsson P, Ivarsson SA, Rignell-Hydbom A. 2013. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ Sci Pollut Res. 20(11):7970–7978. doi: 10.1007/s11356-013-1573-5. [DOI] [PubMed] [Google Scholar]
- Ojo AF, Peng C, Ng JC. 2020. February 15. Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Environmental Pollution:114182. doi: 10.1016/j.envpol.2020.114182. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. 2007. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ Health Perspect. 115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S, 2008. The current state of serum biomarkers of hepatotoxicity. Toxicology, Biomarkers of Toxicity 245, 194–205. 10.1016/j.tox.2007.11.021 [DOI] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF, 1998. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol. Pharmacol. 53, 14–22. [PubMed] [Google Scholar]
- Park K-G, Park KS, Kim M-J, Kim H-S, Suh Y-S, Ahn JD, Park K-K, Chang Y-C, Lee I-K. 2004. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Research and Clinical Practice. 63(2):135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Pfohl M, Ingram L, Marques E, Auclair A, Barlock B, Jamwal R, Anderson D, Cummings BS, Slitt AL, 2020. Perfluorooctanesulfonic Acid and Perfluorohexanesulfonic Acid Alter the Blood Lipidome and the Hepatic Proteome in a Murine Model of Diet-Induced Obesity. Toxicol Sci 178, 311–324. 10.1093/toxsci/kfaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl M, Marques E, Auclair A, Barlock B, Jamwal R, Goedken M, Akhlaghi F, Slitt AL, 2021. An ‘Omics Approach to Unraveling the Paradoxical Effect of Diet on Perfluorooctanesulfonic Acid (PFOS) and Perfluorononanoic Acid (PFNA)-Induced Hepatic Steatosis. Toxicol Sci. 10.1093/toxsci/kfaa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzurro DM, Seeley M, Kerper LE, Beck BD, 2019. Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regulatory Toxicology and Pharmacology 106, 239–250. 10.1016/j.yrtph.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. 2010. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol. 10(11):1420–1427. doi: 10.1016/j.intimp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Ahrens L, le Godec T, Lundqvist J, Oskarsson A. 2018. Relationship between peroxisome proliferator-activated receptor alpha activity and cellular concentration of 14 perfluoroalkyl substances in HepG2 cells. Journal of Applied Toxicology. 38(2):219–226. doi: 10.1002/jat.3515. [DOI] [PubMed] [Google Scholar]
- Salter DM, Wei W, Nahar PP, Marques E, Slitt AL, 2021. Perfluorooctanesulfonic acid (PFOS) thwarts the beneficial effects of calorie restriction and Metformin. Toxicol Sci. 10.1093/toxsci/kfab043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL. 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol Sci. 68(1):249–264. [DOI] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, Butenhoff JL. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology. 183(1–3):117–131. doi: 10.1016/S0300-483X(02)00511-5. [DOI] [PubMed] [Google Scholar]
- Shelly C, Grandjean P, Oulhote Y, Plomgaard P, Frikke-Schmidt R, Nielsen F, Zmirou-Navier D, Weihe P, Valvi D. 2019. Early Life Exposures to Perfluoroalkyl Substances in Relation to Adipokine Hormone Levels at Birth and During Childhood. J Clin Endocrinol Metab. 104(11):5338–5348. doi: 10.1210/jc.2019-00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H-Y, Kim S-H, Shin H-I, Bae HI, Yang J-H. 2008. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol. 82(4):239–246. doi: 10.1007/s00204-007-0246-x. [DOI] [PubMed] [Google Scholar]
- Son JS, Liu X, Tian Q, Zhao L, Chen Y, Hu Y, Chae SA, de Avila JM, Zhu M-J, Du M 2019. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. The Journal of Physiology. 597(13):3333–3347. doi: 10.1113/JP277698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Dougan M. 2009. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol. 170(7):837–846. doi: 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. 2002. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab. 87(4):1856–1863. doi: 10.1210/jcem.87.4.8431. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J Expo Sci Environ Epidemiol. 29(2):131–147. doi: 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström M, Chang S-C, Noker PE, Gorman GS, Hart JA, Ehresman DJ, Bergman Å, Butenhoff JL, 2012. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reproductive Toxicology, A Second Special Issue on Recent Advances in Perfluoroalkyl Acid Research 33, 441–451. 10.1016/j.reprotox.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. 2007. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci. 95(1):108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL. 2008. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ Sci Technol. 42(8):3096–3101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C, 2003. Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. I: Maternal and Prenatal Evaluations. Toxicological Sciences 74, 369–381. 10.1093/toxsci/kfg121 [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, Chemistry Dashboard | PFASMASTER Chemicals. [accessed 2020 Apr 23]. https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster.
- United States Environmental Protection Agency, PFOA Stewardship Program. [accessed 2020 Apr 23]. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfas#tab-3. [Google Scholar]
- Vieira VM, Hoffman K, Shin H-M, Weinberg JM, Webster TF, Fletcher T. 2013. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 121(3):318–323. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP. 2009. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod Toxicol. 27(3–4):239–245. doi: 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CKC. 2012. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim Biophys Acta. 1820(7):1092–1101. doi: 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Leung PY, Wong CKC. 2014. Perinatal Exposure to Perfluorooctane Sulfonate Affects Glucose Metabolism in Adult Offspring. PLOS ONE. 9(1):e87137. doi: 10.1371/journal.pone.0087137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ Sci Technol. 51(5):2508–2518. doi: 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. 2011. Gestational and Chronic Low-Dose PFOA Exposures and Mammary Gland Growth and Differentiation in Three Generations of CD-1 Mice. Environmental Health Perspectives. 119(8):1070–1076. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, N.C. for B., 2011. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes, Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. World Health Organization. [PubMed] [Google Scholar]
- Wolf CJ, Rider CV, Lau C, Abbott BD. 2014. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology. 316:43–54. doi: 10.1016/j.tox.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Yang C-H, Glover KP, Han X. 2009. Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicology Letters. 190(2):163–171. doi: 10.1016/j.toxlet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhong S, Qu H, Xie Y, Cao Z, Li Q, Yang P, Varghese Z, Moorhead JF, Chen Y, et al. 2015. Chronic inflammation aggravates metabolic disorders of hepatic fatty acids in high-fat diet-induced obese mice. Scientific Reports. 5:10222. doi: 10.1038/srep10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Ehresman DJ, Chang S-C, Butenhoff JL, Forster J, Hagenbuch B. 2015. Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium-Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats. Toxicol Sci. 146(2):363–373. doi: 10.1093/toxsci/kfv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Weaver Y, Ehresman DJ, Chang S-C, Butenhoff JL, Hagenbuch B. 2017. Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicol Sci. 156(1):84–95. doi: 10.1093/toxsci/kfw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkhan EK, Yu B, Schlegel A. 2018. Prenatal Exposure to a Maternal High Fat Diet Increases Hepatic Cholesterol Accumulation in Intrauterine Growth Restricted Rats in Part Through MicroRNA-122 Inhibition of Cyp7a1. Front Physiol. 9. doi: 10.3389/fphys.2018.00645. [accessed 2020 Mar 7]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.