Abstract
Lymphatic vessels are abundant in the skin where they regulate interstitial fluid uptake and immune surveillance. Defects in dermal lymphatic vessels, such as fewer vessels and abnormal lymphatic vessel coverage with mural cells, are frequently associated with lymphedema and other lymphatic disorders. Whole-mount immunohistochemistry allows the visualization of dermal lymphatic vessels and identifies morphogenetic defects. Most dermal lymphatic vessels start growing during embryogenesis from lymph sacs that are located close to the axilla towards the dorsal and ventral midlines. Here, we present an approach that we have developed to permeabilize, immunolabel, clear, and visualize the lymphatic vessels. These simple and inexpensive techniques reproducibly generate images of dermal lymphatic vessels with great clarity.
Keywords: Lymphatic vessels, Lymphatic valves, Imaging, Whole-mount immunohistochemistry, iDISCO
Background
Lymphatic vessels are 3-dimensional structures that regulate interstitial fluid homeostasis, lipid absorption, and immune cell trafficking. Defects in lymphatic vessels are associated with diseases such as lymphedema and lymphatic malformations and could result in tissue swelling and inflammation. The growth and patterning of dermal lymphatic vessels can be visualized by whole-mount immunohistochemistry. This approach also identifies defects in lymphatic vessels such as fewer branch points, lack of lymphatic valves, and abnormal recruitment of mural cells. Nevertheless, whole-mount immunohistochemistry is a challenging procedure as skin is a cell-dense tissue with plenty of fat and extracellular matrix proteins such as collagen. To overcome this limitation, we have combined the existing approaches to harvest and visualize dermal lymphatic vessels with the immunolabeling-enabled three-dimensional imaging of solvent-cleared organs (iDISCO) protocol to obtain superior antibody permeabilization and tissue clearing ( James et al., 2013 ; Renier et al., 2014 ; Betterman et al., 2018 ). This inexpensive approach is simple and easy to perform. High quality images can be obtained within a week of starting the procedure. Here, we present the step-by-step protocol to analyze the dermal lymphatic vessels of mouse embryos (embryonic day (E) 14.5-18.5). However, this protocol can be used with minimal modifications to visualize blood and lymphatic vessels in other organs, such as the embryonic and postnatal mesentery and adult ear ( Cha et al., 2016 , 2018 and 2020; Geng et al., 2016 and 2020; Mahamud et al., 2019 ).
Materials and Reagents
12-well plate
6-well plate
24-well plate
Sylgard 184 silicone elastomer kit (Fisher Scientific, catalog number: NC9285739)
Insect pin (Fine Science Tools, catalog number: 26002-20)
Heparin (Millipore Sigma, catalog number: H3393): resuspend in water at 50 mg/ml, aliquot, and store at -20°C)
Sodium deoxycholate (VWR, catalog number: 0613-100g): to make 10% stock solution dissolve 5 g in 30 ml water, add water to 50 ml, and store at room temperature protected from light.
Microscope slides (Denville Scientific INC, catalog number: M1021)
Wax pen (Vector, catalog number: H-4000)
Microscope cover glass (Fisher Scientific, catalog number: 12-545-M)
Mounting medium (Electron Microscopy Sciences, catalog number: 17985-01-02-03)
Goat anti mVEGFR3 (R&D System, catalog number: AF743)
Rat anti mCD31 (BD Pharmingen, catalog number: 553370)
Rabbit anti PROX1 (AngioBio Co. catalog number: 11-002)
Rat anti mNRP2 (R&D System, catalog number: AF567)
Goat anti hPROX1 (R&D System, catalog number: AF2727)
Goat anti mVEGFR2 (R&D System, catalog number: AF644)
Goat anti mLYVE-1 (R&D System, catalog number: AF2125)
Sheep anti mFOXC2 (R&D System, catalog number: AF6989)
Rabbit anti mClaudin 5 (ThernoFisher, catalog number: 34-1600)
CY3-conjugated Mouse anti-Rabbit (Jackson ImmunoResearch Laboratories, catalog number: 711-165-152)
Alexa488-conjugated Donkey anti-Goat (Jackson ImmunoResearch Laboratories, catalog number: 705-545-147)
CY5-conjugated Donkey anti-Rat (Jackson ImmunoResearch Laboratories, catalog number: 712-175-150)
CY3-conjugated Donkey anti-Sheep (Jackson ImmunoResearch Laboratories, catalog number: 713-165-147)
Rat anti mCD144 (BD Pharmingen, catalog number: 550548)
20× PBS (see Recipes)
20% paraformaldehyde (PFA) (see Recipes)
10% Triton X-100 (see Recipes)
PBST (see Recipes)
PBST/20% DMSO (see Recipes)
10% Tween 20 (see Recipes)
10% NP40 (see Recipes)
PTTDND (see Recipes)
1 M glycine (see Recipes)
PTDG (see Recipes)
Blocking buffer (see Recipes)
PTWH (see Recipes)
PTWH/5% DMSO/3% donkey serum (see Recipes)
PTWH/3% donkey serum (see Recipes)
Equipment
Forceps (Fine Science Tools, catalog number: 11252-00)
Benchtop rocker
Spring scissors (Fine Science Tools, catalog number: 15000-08))
Nikon microscope
Zeiss confocal microscope
Procedure
-
Sample preparation
Prepare sylgard plate per manufacturer’s instruction. Once the sylgard plate is made, it can be used indefinitely.
Dissect E14.5-E18.5 embryos from the uterus and remove extra embryonic membranes in ice-cold 1× PBS with forceps.
Open the abdomen of the embryos and fix them in 1% PFA in a 12-well (E14.5 to E16.5) or 6-well (E17.5 to E18.5) plate overnight at 4°C.
Wash embryos three times with 1× PBS on ice, 30 min each time (Figure 1A).
Make incisions with spring scissors from the ears to just under the forelimbs (Figure 1B).
Remove the skin carefully with forceps and try to take as little underlying tissue as possible (Figure 1C).
Pin the skin on the sylgard plate and remove the connective tissue (arrows) with forceps (Figures 1D-1G).
Remove the skin from sylgard plate and place in a 24-well plate for whole-mount immunostaining.
-
Whole-mount immunohistochemistry
Wash the samples twice with 1 ml PBST, 30 min each time.
Incubate samples in 1 ml PBST with 20% DMSO for 4 h.
Incubate samples in 1 ml PTTDND overnight.
Wash samples twice with 1 ml PBST, 30 min each time.
Incubate samples in 1 ml 1 PTDG for 4 h.
Block in 300 µl blocking buffer overnight.
Wash twice with 1 ml PTWH, 30 min each time.
Incubate with primary antibodies in 300 µl PTWH with 5% DMSO and 3% donkey serum overnight.
Wash four times with 1 ml PTWH, 30 min each time.
Incubate with secondary antibodies in 300 µl PTWH with 3% donkey serum overnight.
Wash four times with 1 ml PTWH, 30 min each time.
Wash twice with 1 ml 1× PBS, 10 min each time.
Draw squares on the microscope slides with wax pen (Figures 2A and 2B).
Add sufficient mounting media to the middle of the square (Figure 2B) and place the skin in the mounting media (Figure 2C). Let the inner side of the skin face up and position the anterior end (ears) of the dorsal skin towards the anterior end of the slide.
Remove ears from the skin with spring scissors (Figure 2D).
Overlay the sample with cover glass and gently press the skin by using the back of a forceps to smoothen out the wrinkles (Figure 2E).
Seal the edges of the slides with nail polish and image under a fluorescent microscope (Figures 2F and 2G).
Figure 1. Dermal skin preparation.
A. E16.5 embryo fixed in PFA and ready for dorsal skin dissection. B. Incisions on the dorsal skin outlining the region that will be dissected out. C. Embryo after the removal of the dorsal skin. Notice that the underlying tissue was not significantly damaged by dissection. Subsequent cleaning steps are minimized when the skin is dissected out with the least amount of attached tissues. D. The skin sample was pinned onto the sylgard plate with the inside facing up. The arrows point to the collagen fibers that are attached to the skin. E-G. The same skin sample from which the connective tissue (arrows) is removed by peeling without damaging the underlying skin. The arrow in G indicates the connective tissue that can be discarded.
Figure 2. Imaging the dermal skin.
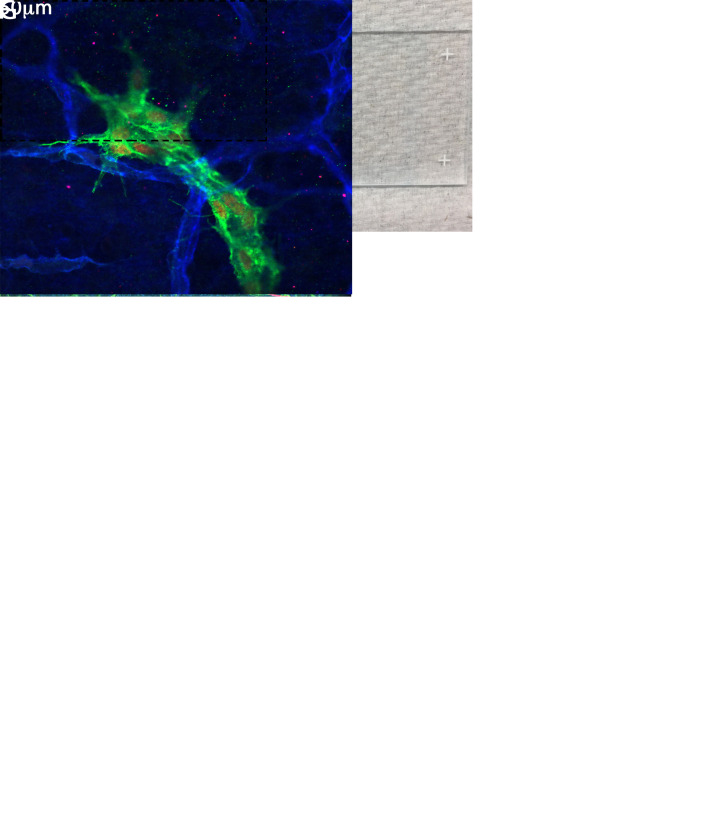
A. The dashed line on the slide indicates the approximate location of the chambers that will be made by outlining with the wax pen. B. After the outline is made with the wax pen, a drop of mounting media is placed in the middle of the chamber. C. Immunostained dorsal skin immersed in the mounting media with the inside of the skin facing up. D. Sample with ears carefully removed. E. Sample overlaid with a cover slip and gently pressed to remove air bubbles and wrinkles. F-G. Lower and higher magnification images of samples processed according to the described protocol. The VEGFR3+CD31+ (F) and VEGFR3+CD31+Prox1+ (G) structures are the lymphatic vessels. The SMA+ CD31+ vessels in F are the arteries and veins. The CD31+ vessels in G are the blood capillaries.
Notes
All washing and incubation steps are performed at room temperature using a benchtop rocker.
Step B2 can be shortened to 4 h.
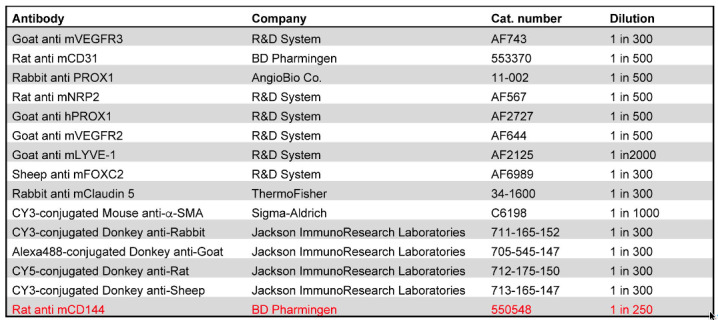
This procedure may not be suitable for all antibodies. For example, the high concentration of detergents could disrupt the epitopes of some cell junction molecules. The user will have to perform preliminary experiments to determine the appropriateness of this protocol for their needs. Information about the antibodies that we have used is provided in Table 1. The antibody that did not work well with the iDISCO protocol is highlighted in red.
Table 1. List of antibodies and conditions that can or cannot be used with the iDISCO protocol.
The antibody that did not provide good signal when used with the described protocol is highlighted in red. The rest of the antibodies provide excellent results.
|
Recipes
-
20× PBS
Weigh 160 g NaCl, 4 g KCl, 28.8 g Na2HPO4, and 4.8 g KH2PO4. Transfer to a 1 L glass beaker. Add water to a volume of 900 ml. Mix and adjust pH to 7.4 with NaOH. Make up to 1 L with water. Autoclave.
-
20% paraformaldehyde (PFA)
Weigh 200 g PFA and transfer to a 1 L glass beaker. Add 500 ml water and stir with a magnetic stirrer on a hot plate in a fume hood. Heat the PFA suspension to 55-60°C (never over 60°C). Add 5 N NaOH in a drop wise manner until the suspension turns clear. Cool down the solution to room temperature. Add 50 ml of 20× PBS and adjust pH to 7.4 by adding 5 N NaOH in a drop wise manner. Make up to 1 L with water. Aliquot the solution with 50 ml falcon tubes and store at -20°C. Add 2.5 ml 20% PFA to 47.5 ml PBS to make 1% PFA.
-
10% Triton X-100
Mix 10 ml Triton X-100 with 90 ml water.
-
PBST (1× PBS with 0.2% Triton X-100)
Mix 50 ml 20× PBS and 20 ml 10% Triton X-100 in a 1 L cylinder. Make up to 1 L with water.
-
PBST with 20% DMSO
Mix 5 ml 20× PBS, 2 ml 10% Triton X-100, and 20 ml DMSO in 100 ml cylinder. Make up to 100 ml with water. Store in an amber glass bottle for up to 4 weeks.
-
10% Tween 20
Mix 10 ml Tween 20 with 90 ml water.
-
10% NP40
Mix 10 ml NP40 with 90 ml water.
-
PTTDND (1× PBS with 0.1% Tween-20, 0.1% TritonX-100, 0.1% Deoxycholate, 0.1% NP40, and 20% DMSO)
Mix 5 ml 20× PBS, 20 ml DMSO, 1 ml 10% Triton 100, 1 ml 10% Tween 20, 1 ml 10% NP40, and 1 ml 10% sodium deoxycholate in 100 ml cylinder. Make up to 100 ml with water. Store in an amber glass bottle for up to 4 weeks.
-
1 M glycine
Dissolve 75 g in 800 ml water, add water to 1 L, and filter with 0.45 μm filter.
-
PTDG (1× PBS with 0.2% Triton X-100, 20% DMSO, and 0.3 M glycine)
Mix 5 ml 20× PBS, 2 ml 10% Triton 100, 20 ml DMSO, and 30 ml 1 M glycine in 100 ml cylinder. Make up to 100 ml with water. Store in an amber glass bottle for up to 4 weeks.
-
Blocking buffer (1× PBS with 0.2% Triton X-100, 10% DMSO, and 6% donkey serum)
To make 5 ml solution by mixing 4.1 ml 1× PBS, 0.1 ml 10% Triton 100, 0.5 ml DMSO, and 0.3 ml donkey serum. Make fresh each time.
-
PTWH (1× PBS with 0.2% Tween-20 and 10 µg/ml heparin)
Mix 25 ml 20× PBS and 10 ml 10% Tween 20 in a 500 ml cylinder. Make up to 500 ml with water. Add 0.1 ml 50 mg/ml Heparin. Mix well and store in a glass bottle for up to 4 weeks.
-
PTWH with 5% DMSO and 3% donkey serum
To make 5 ml solution, mix 0.15 ml donkey serum, 0.25 ml DMSO with 4.6 ml PTWH. Make fresh each time.
-
PTWH with 3% donkey serum
To make 5 ml solution, mix 0.15 ml donkey serum with 4.85 ml PTWH. Make fresh each time.
Acknowledgments
This work is supported by NIH/NHLBI (R01HL131652 and R01HL133216 to RSS), NIH/NIGMS COBRE (P20 GM103441 to XG; PI: Dr. Rodger McEver), and Oklahoma Center for Adult Stem Cell Research (4340 to RSS) grants.
Competing interests
The authors declare no competing interests.
Ethics
Mice were handled in accordance with OMRF’s IACUC approval.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Betterman K. L. and Harvey N. L.(2018). Histological and morphological characterization of developing dermal lymphatic vessels. Methods Mol Biol 1846:19-35. [DOI] [PubMed] [Google Scholar]
- 2. Cha B., Geng X., Mahamud M. R., Fu J., Mukherjee A., Kim Y., Jho E. H., Kim T. H., Kahn M. L., Xia L., Dixon J. B., Chen H. and Srinivasan R. S.(2016). Mechanotransduction activates canonical wnt/beta-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev 30(12): 1454-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cha B., Geng X., Mahamud M. R., Zhang J. Y., Chen L., Kim W., Jho E. H., Kim Y., Choi D., Dixon J. B., Chen H., Hong Y. K., Olson L., Kim T. H., Merrill B. J., Davis M. J. and Srinivasan R. S.(2018). Complementary Wnt Sources Regulate Lymphatic Vascular Development via PROX1-Dependent Wnt/beta-Catenin Signaling. Cell Rep 25(3): 571-584 e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cha B., Ho Y. C., Geng X., Mahamud M. R., Chen L., Kim Y., Choi D., Kim T. H., Randolph G. J., Cao X., Chen H. and Srinivasan R. S.(2020). YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Development 147(23): dev195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geng X., Cha B., Mahamud M. R., Lim K. C., Silasi-Mansat R., Uddin M. K. M., Miura N., Xia L., Simon A. M., Engel J. D., Chen H., Lupu F. and Srinivasan R. S.(2016). Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev Biol 409(1): 218-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geng X., Yanagida K., Akwii R. G., Choi D., Chen L., Ho Y., Cha B., Mahamud M. R., Berman de Ruiz K., Ichise H., Chen H., Wythe J. D., Mikelis C. M., Hla T. and Srinivasan R. S.(2020). S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight 5(14): e137652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. James J. M., Nalbandian A. and Mukouyama Y. S.(2013). Tgfbeta signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development. 140(18): 3903-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahamud M. R., Geng X., Ho Y. C., Cha B., Kim Y., Ma J., Chen L., Myers G., Camper S., Mustacich D., Witte M., Choi D., Hong Y. K., Chen H., Varshney G., Engel J. D., Wang S., Kim T. H., Lim K. C. and Srinivasan R. S.(2019). GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development 146(21): dev184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renier N., Wu Z., Simon D. J., Yang J., Ariel P. and Tessier-Lavigne M.(2014). Idisco: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159(4): 896-910. [DOI] [PubMed] [Google Scholar]