Abstract
The efficient ATP production in mitochondria relies on the highly specific organization of its double membrane. Notably, the inner mitochondrial membrane (IMM) displays a massive surface extension through its folding into cristae, along which concentrate respiratory complexes and oligomers of the ATP synthase. Evidence has accumulated to highlight the importance of a specific phospholipid composition of the IMM to support mitochondrial oxidative phosphorylation. Contribution of specific phospholipids to mitochondrial ATP production is classically studied by modulating the activity of enzymes involved in their synthesis, but the interconnection of phospholipid synthesis pathways often impedes the determination of the precise role of each phospholipid. Here, we describe a protocol to specifically enrich mitochondrial membranes with cardiolipin or phosphatidylcholine, as well as a fluorescence-based method to quantify phospholipid enrichment. This method, based on the fusion of lipid vesicles with isolated mitochondria, may further allow a precise evaluation of phospholipid contribution to mitochondrial functions.
Keywords: Cardiolipin, Phosphatidylcholine, Vesicles, Acridine Orange 10-Nonyl Bromide, NAO, TopFluor CL, Mitotracker
Background
Membranes in living cells are more than just barriers; they are also the sites of many important biological processes. Phospholipids are key membrane components, and by controlling membrane fluidity, permeability, rigidity, and morphology, they can modulate important functions. In eukaryote cells, mitochondria are at the crossroad of many cellular processes, including ATP production and apoptosis, and are altered in different diseases. Two membranes with different levels of permeability and unique phospholipid composition constitute their envelope. In particular, the inner mitochondrial membrane (IMM) is characterized by the presence of long invaginations called “cristae” that are enriched in cardiolipin (CL), a specific mitochondrial phospholipid. CL is established as a crucial actor of mitochondrial architecture and ATP production ( Paradies et al., 2014 ; Ikon and Ryan 2017a; Schlame et al., 2017 ; Pennington et al., 2019 ), and alteration of CL synthesis or content may result in the development of the Barth syndrome, a rare, life-threatening cardiomyopathy ( Barth et al., 1983 ; Ikon et al., 2017b ), or skeletal muscle weakness ( Prola et al., 2021 ). More recently, a tight regulation of other phospholipids such as phosphatidic acid or phosphatidylethanolamine has been shown to be essential for mitochondrial function ( Adachi et al., 2016 ; Heden et al., 2019 ). However, the roles of phospholipids in mitochondrial function have mostly been identified through the mutation of genes involved in their synthesis, and this indirect approach might lead to biases. For example, knockout of the tafazzin gene to study the role of CL is not only associated with a drop in mature CL content, but also with a strong enrichment of unmatured CL (monolyso- and dilyso-CL) that impairs mitochondrial function. Thus, complementary methods to study the role of phospholipids in mitochondrial function are needed. Previous publications used different methods to enrich mitochondria with CL, phosphatidylserine, or phosphatidylglycerol, but the validation of the enrichment and a complete description of the protocols used are lacking ( Bobyleva et al., 1997 ; Piccotti et al., 2002 ). Here, we describe a new method that we used to demonstrate the direct, key role of CL in the control of mitochondrial coupling in skeletal muscle ( Prola et al., 2021 ). This method relies on the fusion of lipid vesicles with isolated mitochondria to enrich mitochondrial membranes with a single phospholipid. Below, we will discuss details of the protocol set up in our laboratory and also present our post-experimental data analysis and quality control workflow.
Materials and Reagents
Cell strainer (100 µm, Greiner Bio-One)
50 ml centrifuge tube (Falcon)
Strengthened centrifuge 15 ml glass tube (Kimble Chase, catalog number: 45500)
Glass Pasteur pipette (Dutscher, catalog number: 065421)
1.5 ml and 2 ml Microtubes (Eppendorf)
Pipette tips (Star Lab)
96-well plate (black, µclear flat bottom; Greiner, catalog number: 655096)
Acridine Orange 10-Nonyl Bromide (Sigma, catalog number: A7847)
Adenosine 5′-diphosphate monopotassium salt dihydrate (Sigma, catalog number: A5285)
Bicinchoninic acid assay (Pierce, Invitrogen, catalog number: 23225)
Bovine serum albumin (fatty acid free; Sigma, catalog number: A7030)
Cardiolipin sodium salt from bovine Heart (chloroform solution; Avanti Polar Lipids, catalog number: 840012C)
Chloroform (Sigma, catalog number: 288306)
Digitonin (Sigma, catalog number: D5628)
Dimethyl sulfoxide (Sigma, catalog number: D8418)
Ethylene-bis(oxyethylenenitrilo)tetraacetic acid (EGTA) (Sigma, catalog number: 03777)
Ethylenediaminetetraacetic acid tetrasodium salt dihydrate (EDTA) (Sigma, catalog number: E6511)
Formaldehyde solution 36% (Sigma, catalog number: 47608)
Glutamate (Sigma, catalog number: G1501)
HEPES (Sigma, catalog number: H3375)
Magnesium Chloride (MgCl2 1 M solution; Sigma, catalog number: 63069)
Malate (Sigma, catalog number: M6413)
Mannitol (Sigma, catalog number: M8429)
Methanol (Sigma, catalog number: 34860)
MitoTrackerTM Red CMXRos (Invitrogen, catalog number: M7512)
PBS (Sigma, catalog number: P4417)
L-α-phosphatidylcholine (95%) (Egg, Chicken, chloroform solution) (Sigma, catalog number: 131601C)
Potassium Phosphate monobasic (KH2PO4; Sigma, catalog number: P5655)
Pyruvate (Sigma, catalog number: P2256)
Sucrose (Sigma, catalog number: S0389)
Texas RedTM 1,2-Dihexadecanoyl-sn-Glycero-3-Phosphoethanolamine, Triethylammonium Salt (Thermofischer, catalog number: T1395MP)
TopFluor® Cardiolipin (Avanti Polar Lipids, catalog number: 810286)
Tris-HCl (Sigma, catalog number: T5941)
Trypsin EDTA (10×) 0.5%/0.2% in DPBS (PAA Laboratories, catalog number: L11-003)
Mouse anti-COXIV antibody (Abcam, catalog number: ab14744)
Rabbit anti-β-tubulin antibody (Cell Signaling, catalog number: 2128)
Rabbit anti-Calnexin antibody (Sigma, catalog number: C4731)
Mouse anti-eIF2α antibody (Cell Signaling, catalog number: 2103)
Rabbit anti-Insulin Receptor β antibody (Cell signaling, catalog number: 3025)
Rabbit anti-MFF antibody (Abcam, catalog number: ab129075)
Rabbit anti-TBP antibody (Cell Signaling, catalog number: 8515)
Rabbit anti-VDAC antibody (Custom made, a kind gift from Dr. C. Lemaire, Inserm U1180)
Mitochondria Homogenization Buffer (MHB) (see Recipes)
Mitochondria Isolation Buffer (MIB) (see Recipes)
ADP solution (see Recipes)
Fusion buffer (see Recipes)
10× BSA solution (see Recipes)
Digitonin solution (see Recipes)
Mitoplast Preparation Buffer (MPB) (see Recipes)
Hypotonic Mitoplast Preparation Buffer (HMPB) (see Recipes)
Mitotracker Red 1 mM stock solution (see Recipes)
Formaldehyde 2% (see Recipes)
Nonyl-acridine orange 35 mM stock solution (see Recipes)
Equipment
Centrifuge (Eppendorf, model: 5810R with rotor FA-45-6-30)
Dounce, 2 ml (Kimble, Type A, catalog number: 885300-0002)
Hamilton syringe 500 µl (ThermoFischer, catalog number: 10570042)
Nitrogen or argon gas bottle, or medical gas installation with pressure regulator
Non-CO2 incubator (Memmert, catalog number: D06057, Model 100)
MACSMIX Tube rotator (Miltenyi, catalog number: 130-090-753)
Spectrofluorimeter (TECAN, model: Infinite M200)
Sonicator bath (Branson, catalog number: 3510)
Zeiss LSM800 confocal (Zeiss, model: LSM800)
Water bath
Software
Prism 8 (GraphPad Software, www.graphpad.com)
Software Magellan (TECAN)
Zen (Zeiss)
Procedure
-
Isolation of mitochondria
The objective is to isolate mitochondria from the cells or tissues of choice. The protocol presented here was optimized for the isolation of mitochondria from mouse skeletal muscle ( Prola et al., 2021 ), with a protocol adapted from Frezza et al. (2007) . It may need to be adapted to the specificity of the cells or tissue of choice.
Notes:
It is crucial to obtain pure and functional mitochondria prior to the phospholipid enrichment experiment. To preserve mitochondria properties throughout the procedure, it is important to i) work with freshly isolated mitochondria, ii) reduce at its minimum the time between the different steps of the procedure, and iii) keep mitochondria on ice or at 4°C all throughout the procedure.
Precool Dounce in an ice-bath 5 min before starting the procedure and equilibrate the centrifuge at 4°C.
-
Kill the mouse by cervical dislocation. Using a scalpel, rapidly remove the skeletal muscles of interest and immerse them in a small beaker containing 5 ml of ice-cold PBS supplemented with 10 mM EDTA.
Note: Local and national regulations on animal care and handling vary. Ensure that you hold the appropriate authorization to perform animal experiments.
In the same beaker, mince the muscles into small pieces using scissors and remove visible fat, ligaments, and connective tissue.
Wash the minced muscles twice or thrice with 1 ml of ice-cold PBS supplemented with 10 mM EDTA.
Transfer the minced muscles using a large opening pipette tip in ice-cold PBS supplemented with 10 mM EDTA and 0.05% trypsin-0.02% EDTA (approximately 1 ml of solution in a 2 ml tube per 100 mg of muscle). Mince with scissors in very small pieces (need 2/3 min for each tube), and incubate for 30 min at 4°C.
Centrifuge at 200 × g for 5 min at 4°C and discard the supernatant.
Resuspend the pellet in ice-cold Mitochondria Homogenization Buffer (MHB; see Recipes). The optimal ratio between tissue and isolation buffer ranges between 1:10 and 1:30 (w:v) (the following protocol corresponds to 2.5 ml for 100 mg of muscle).
Homogenize muscles using a Dounce (100 up and down strokes with pestle A). Homogenization, as well as the following steps, must be performed at 4°C.
Pass the homogenate through a 100 µm cell strainer into a 50 ml polypropylene Falcon tube.
Centrifuge at 700 × g for 10 min at 4°C.
-
Carefully transfer the supernatant (by reverting the tube) into a glass centrifuge tube and centrifuge at 8,000 × g for 10 min at 4°C.
Note: Up to 200 µl of the homogenate obtained in Step A9 may be used for bicinchoninic acid quantitation and western blot analysis. To ensure the purity of mitochondria preparation, avoid transferring any visible material from the pellet. Glass tubes are preferred because (i) they are better thermic insulators to preserve mitochondria and (ii) they cause reduced binding of mitochondria compared to plastic, which avoids material loss, allowing the preparation of a smaller and more concentrated pellet compared to plastic tubes.
Discard the supernatant and resuspend the pellet in 5 ml of ice-cold Mitochondria Isolation Buffer (MIB).
Centrifuge at 8,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet containing mitochondria in the small amount of buffer that remains after discarding the supernatant (around 100 µl, avoid adding more MIB). Mitochondria are now ready to be used.
-
Use 1 µl of the mitochondrial suspension to quantify mitochondrial protein content using bicinchoninic acid (BCA) assay (He, 2011).
Notes:
Hereafter, mitochondria quantity will refer to the mitochondrial protein content as quantified here.
Functionality of mitochondria can be checked by measuring mitochondrial coupling as previously described (Mourier et al., 2014). Purity of mitochondria can be checked by western blot, as shown in Figure 1. One hundred milligrams of muscle typically yields 500-1,000 µg of mitochondria. Following steps for fusion with phospholipid vesicles must be done on freshly isolated mitochondria (the same day). For other applications, such as western blot, mitochondria could be aliquoted and snap frozen in liquid nitrogen.
-
Production of pure phospholipid vesicles (PV)
Note: Manipulate lipids using stainless steel, glass, or Teflon. Do not use plastic tubes or pipette tips. See Figure 2 for pictures taken during different steps of the protocol.
-
Using a Hamilton syringe, put 2 mg of phospholipids (200 µl from 10mg/ml chloroform stock solution) into a glass tube and evaporate the chloroform using nitrogen or argon gas propelled at 0.2 bar. Rotate the tube during evaporation to form a thin lipid film on the tube (see Video 1).
Note: For the production of fluorescent PV, put 0.2 mg of fluorescent phospholipids and 1.8 mg of non-fluorescent phospholipids. Protect from light throughout the procedure. Tubes may be placed for one hour in a vacuum pump to evaporate the chloroform.
Add 1 ml of MIB (pre-equilibrated at room temperature) in the tube, incubate for one hour at room temperature, and then vortex during 30 s. The solution should appear cloudy. Final PV concentration are around 1.38 mM for CL (MW = 1449.9 g/mol) and 2.60 mM (MW = 770.1 g/mol) for PC.
-
Sonicate the phospholipid solution for 25 min in a water bath sonicator (40 kHz) filled with ice. Repeat this step until obtaining a clear solution.
Note: Tubes are held on a plastic rack placed in the water bath sonicator. A probe sonicator may also be used.
-
Use immediately or store the solution at 4°C for one week. Re-sonicate prior to each experiment under the same conditions.
Note: Once produced, PV can be manipulated using classical plastic tips.
-
-
Fusion of PV with isolated mitochondria
-
Put 500 µg of freshly isolated mitochondria (according to the BCA assay) into a 2 ml microtube, add 2.5 mM ADP (5 µl from the 500 mM ADP solution; see Recipes), 15 nmol of PV (10.9 µl of 1.38 mM CL stock solution or 5.8 µl of 2.60 mM PC stock solution), and complete to 1 ml with Fusion buffer pre-heated at 30°C.
Note: Add reagents in the following order: mitochondria, ADP solution, fusion buffer, and PV.
-
Incubate for 20 min at 30°C under constant stirring agitation (12 rotations per minute).
Note: Incubation time and temperature were optimized here for CL and PC incorporation into muscle mitochondria. They may need to be optimized for other phospholipid species or sources of mitochondria. Increasing temperature and incubation duration increases phospholipid enrichment. Over-enrichment should be avoided as it hampers mitochondrial function.
-
Add 1 ml of ice-cold MIB to stop fusion.
Note: Cooling the buffer increases membrane rigidity, which in combination with the dilution of ADP, PV, and mitochondria results in inhibition of fusion.
-
Centrifuge at 10,000 × g for 10 min at 4°C to pellet mitochondria.
Note: Over-enriched mitochondria may not pellet and may remain in the supernatant.
Discard all the supernatant and resuspend the pellet in 100 µl of ice-cold MIB and layer on an ice-cold sucrose gradient (1 ml of 0.6 M sucrose in a 2 ml microtube) to remove unfused PV and overfused mitochondria.
Centrifuge at 10,000 × g for 10 min at 4°C to pellet mitochondria.
Discard all the supernatant and wash the pellet with 1 ml of ice-cold MIB.
Centrifuge at 10,000 × g for 10 min at 4°C to pellet mitochondria.
-
Discard all the supernatant and resuspend enriched mitochondria with the buffer of your choice. Enriched mitochondria are now ready to be used.
Notes:
Enriched mitochondria are highly sensitive to mechanical stress, so pipet and manipulate them carefully (no vortex, no up and down, aspirate and eject softly with a 200 µl-pipette). Perform the experiment quickly after the enrichment.
Appropriate controls for these experiments could be (i) mitochondria submitted to the same protocol but in the presence of vehicle only (MIB) instead of PV or (ii) mitochondria fused with PV made of another control lipid.
-
-
Mitoplast preparation
To validate the enrichment of IMM with phospholipids, the outer mitochondrial membrane (OMM) can be stripped away by permeabilization combined with osmotic shock to produce purified objects called mitoplasts (IMM plus matrix), using the following procedure (adapted from Chazotte and Hackenbrock, 1988):
Note: Precool the Dounce in an ice-bath 5 min before starting the procedure.
-
Prepare the OMM permeabilization solution: 100 µl of 10× BSA solution (see Recipes), mitochondria suspension (from A13), digitonin (0.12 µg of digitonin per µg of mitochondrial proteins, e.g., for 100 µg of mitochondria, add 12 µl of digitonin solution at 1 mg/ml; see Recipes) and complete to 1 ml with Mitoplast Preparation Buffer (MPB; see Recipes).
Note: Add reagents in the following order: MPB, mitochondria, 10× BSA, and then digitonin. One ml is sufficient for up to 500 µg of mitochondria; for a higher amount, the volume should be increased.
Incubate for 15 min at 4°C under constant stirring agitation (12 rotations per minute).
Centrifuge at 8,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet in 1 ml of ice-cold Hypotonic Mitoplast Preparation Buffer (HMPB; see Recipes) and incubate for 15 min at 4°C with shaking.
Homogenize mitochondria using a Dounce (100 up and down strokes with pestle A).
Centrifuge at 8,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet in 1 ml of ice-cold HMPB and centrifuge at 8,000 × g for 10 min at 4°C.
-
Discard the supernatant and resuspend the pellet with the buffer of your choice. Mitoplasts are now ready to use.
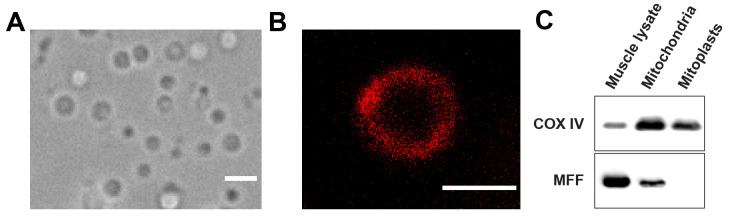
Note: When placed in hypotonic buffer, mitoplasts are devoid of cristae and form large vesicles around 1 µm in diameter. Efficiency of the purification can be verified by microscopic observation (Figure 3A). Mitoplasts can be stained by resuspending the pellet with a buffer containing Mitotracker (dilute the 1 mM stock solution to 100 nM in the buffer of your choice). After 10 min of incubation at room temperature, centrifuge at 8,000 × g for 10 min, discard the supernatant, and resuspend the pellet in the buffer of your choice (e.g., HMPB) to distinguish them from pure lipid vesicles (Figure 3B). Absence of contamination with proteins from the OMM can be checked by western blot (Figure 3C).
-
-
Validation of phospholipid enrichment
The use of fluorescent lipids allows validating the enrichment of mitochondria with phospholipids. For CL, the enrichment can also be validated using the Acridine Orange 10-Nonyl Bromide (NAO) probe, which binds to CL (Garcia Fernandez et al., 2004 ).
-
Fluorescent lipids
Use the protocol described in Procedure C to fuse mitochondria with PV containing fluorescent lipids (see the note in B1).
Mitochondria enrichment with phospholipids can then be checked by measuring fluorescence with a spectrofluorimeter or through the observation of fluorescence by microscopy according to fluorescence properties of the lipid used (Figure 4).
If incorporation in the IMM is expected, mitoplasts can be prepared from enriched mitochondria (see Procedure D) and checked as in Figure 3.
-
NAO quantification
Note: Experimental controls with mitochondria not stained with NAO must be included for quantification (see Data analysis).
-
After enrichment with CL as described in Procedure C, fix isolated mitochondria or mitoplasts (100 µg of proteins) with formaldehyde 2% (see Recipes) for 15 min, at 4°C.
Note: Fixation before NAO staining is recommended to dissipate mitochondrial membrane potential that interferes with NAO fluorescence.
Centrifuge at 10,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet with 1 ml of ice-cold PBS.
Centrifuge at 10,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet in 100 µl of PBS supplemented with 35 µM of NAO (from a 35 mM stock solution in DMSO; see Recipes).
Incubate for 10 min at room temperature.
Centrifuge at 10,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet with 1 ml of ice-cold PBS.
Centrifuge at 10,000 × g for 10 min at 4°C.
Discard the supernatant and resuspend the pellet in 100 µl of PBS and transfer in 96-well plate (black, µclear flat bottom).
Measure fluorescence (λ excitation: 495 nm, λ emission: 620 nm) with a spectrofluorimeter (Figure 5).
-
-
Figure 1. Representative western blot with crude muscle lysate or purified mitochondria.
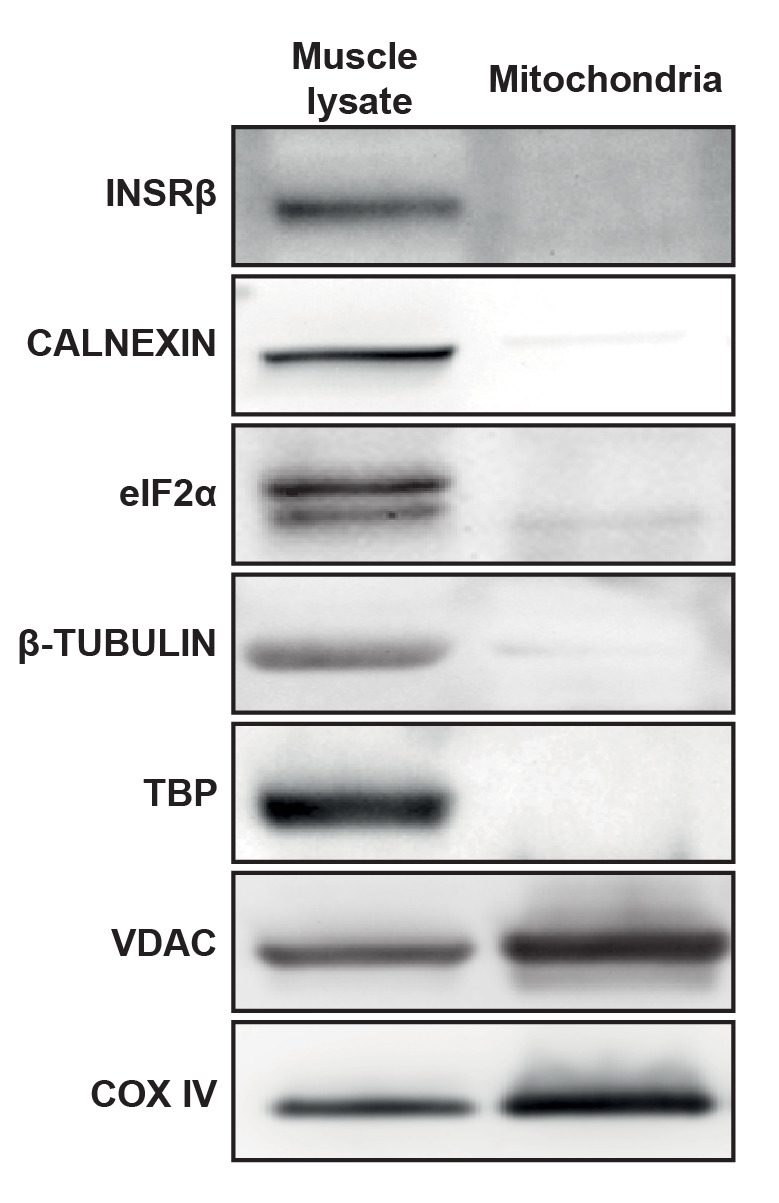
INSRβ: Insulin receptor β (plasma membrane), CALNEXIN (endoplasmic reticulum), eIF2α (cytoplasm), β-TUBULIN (cytoskeleton), TBP (nucleus), VDAC (outer mitochondrial membrane), and COX IV (inner mitochondrial membrane). These blots confirmed the enrichment in mitochondria as well as the absence of remnants from other subcellular compartments after the isolation procedure.
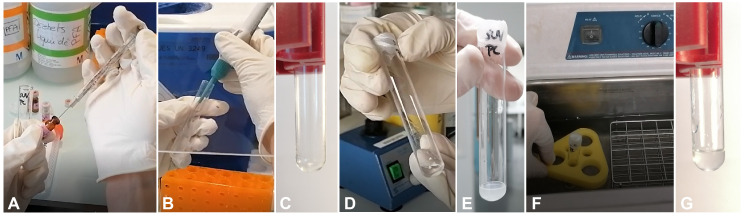
Figure 2. Workflow for the production of phospholipid vesicles.
A. Put phospholipids in a glass tube using a Hamilton syringe. B. Evaporate chloroform using argon or nitrogen gas. C. Evaporated phospholipids form a thin film on the tube. D. Add 1 ml of MIB to the tube and incubate for one hour. E. Vortex for 30 s, the solution should then appear cloudy. F. Sonicate for 25 min in a water bath sonicator. G. After sonication, the solution should be clear. Repeat sonication if it is not the case.
Video 1. Evaporation of chloroform using argon gas.
Figure 3. Validation of mitoplast preparation.
A. Representative mitoplast shape and size in HMPB buffer. Scale bar, 2 µm. B. Mitoplasts stained with MitoTracker Red. Scale bar, 1 µm. C. MFF (outer mitochondrial membrane) and COX IV (inner mitochondrial membrane) protein content in total muscle lysate, purified mitochondria, and mitoplasts. These blots confirmed the enriched proportion of mitochondrial proteins in purified mitochondria as well as the absence of remnants from the outer mitochondrial membrane in mitoplasts.
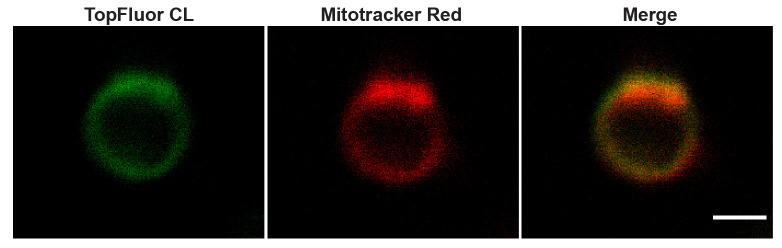
Figure 4. Cardiolipin enrichment and MitoTracker Red staining.
Representative images of mitoplasts made from mitochondria after fusion with TopFluor-Cardiolipin (CL) vesicles and stained with Mitotracker Red. Scale bar: 1 µm.
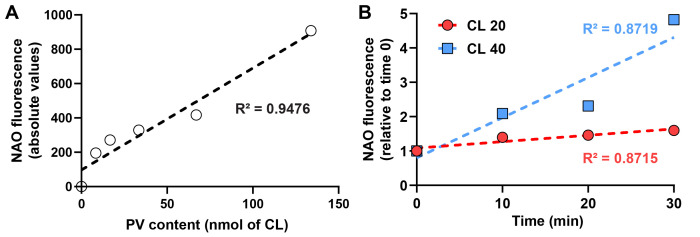
Figure 5. Cardiolipin enrichment and NAO quantification.
A. NAO fluorescence in the presence of varying amounts of PV made of CL. Linear regression is shown in dotted line with respective R fitting. B. NAO fluorescence after fusion of 500 µg of mitochondria with 20 (red) or 40 (blue) nmol of CL vesicles during 0 (control), 10, 20, and 30 min at 30°C. Results are shown relative to the control set to 1.0. Linear regression is shown in dotted line with respective R2 fitting.
Data analysis
For fluorescence analysis on a spectrophotometer (fluorescent lipids or NAO experiments described in procedure E), the values obtained were corrected from blank (mitochondria with no fluorescent lipids or not stained with NAO) and normalized on mitochondrial protein content. Values are then expressed as relative to control set to 1.0.
Notes
This protocol has been optimized for the enrichment of mitochondrial membranes with CL and PC. Some adjustments may be required for the enrichment with other phospholipids and validated using fluorescent lipids.
While the ATP/O ratio is not impacted by the enrichment with CL, the absolute rate of respiration and ATP production are both decreased, as previously reported ( Prola et al., 2021 ). This may be due to respiratory chain complex dilution within the membrane ( Schneider et al., 1980 ) or to an impairment in lipid arrangement following phospholipid enrichment. PC enrichment impairs both the absolute rate of respiration and ATP/O ratio.
This multi-step procedure induces a loss of material, and thus, a large quantity of mitochondria is mandatory at the start (depending on the application, use at least 300 µg of mitochondria).
Recipes
-
Mitochondria Homogenization Buffer (MHB)
67 mM sucrose
50 mM KCl
10 mM EDTA
0.2% BSA
50 mM Tris-HCl
pH 7.40 (buffered with 1 M KOH solution)
Store at -20°C for several months without BSA.
Ready to use solution with BSA can be stored for one week at 4°C.
-
Mitochondria Isolation Buffer (MIB)
250 mM sucrose
3 mM EGTA
10 mM Tris-HCl
pH 7.40 (buffered with 1 M KOH solution)
Store at -20°C for several months or one week at 4°C.
Note: For Recipes 1 and 2, see Frezza et al. (2007) for more detailed instructions.
-
ADP solution
500 mM ADP
150 mM MgCl2
Aliquots can be stored at -20°C.
-
Fusion buffer
220 mM mannitol
70 mM sucrose
2 mM HEPES
10 mM KH2PO4
5 mM MgCl2
1 mM EGTA
10 mM glutamate
2 mM malate
10 mM pyruvate
pH 6.50 (buffered with 1 M KOH solution)
Store at -20°C for several months or one week at 4°C.
-
10× BSA solution
Dilute 5 mg of fatty acid free BSA in 1 ml of MPB. Use the same day.
-
Digitonin solution
Dilute 1 mg of digitonin in 1ml of MPB, heat at 95°C for 5 min, and cool on ice before use.
Use the same day.
-
Mitoplast Preparation Buffer (MPB)
70 mM sucrose
220 mM mannitol
2 mM HEPES
pH 7.40 (buffered with 1 M KOH solution)
Store at -20°C for several months or one week at 4°C.
-
Hypotonic Mitoplast Preparation Buffer (HMPB)
Dilute MPB 7.5 times in deionized H2O (e.g., 6 ml of MPB and 39 ml of deionized H2O for 45 ml of HMPB). Use the same day.
-
Mitotracker Red 1 mM stock solution
Add 94 µl of DMSO to 50 µg vial. Aliquots and store at -20°C.
-
Formaldehyde 2%
Dilute formaldehyde stock solution in PBS.
-
Nonyl-acridine orange 35 mM stock solution
Dilute 10 mg of NAO in 605 µl of DMSO. Aliquots can be stored at -20°C.
Acknowledgments
This work was primarily supported by the “Association Française contre les Myopathies” (AFM 16143, Translamuscle I #19507 and Translamuscle II #22946).
This method was originally used in Prola et al. (2021) for Science Advances, DOI: 10.1126/sciadv.abd6322.
Competing interests
The authors declare that they have no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Adachi Y., Itoh K., Yamada T., Cerveny K. L., Suzuki T. L., Macdonald P., Frohman M. A., Ramachandran R., Iijima M. and Sesaki H.(2016). Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division. Mol Cell 63(6): 1034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barth P. G., Scholte H. R., Berden J. A., Van der Klei-Van Moorsel J. M., Luyt-Houwen I. E., Van't Veer-Korthof E. T., Van der Harten J. J. and Sobotka-Plojhar M. A.(1983). An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci 62(1-3): 327-355. [DOI] [PubMed] [Google Scholar]
- 3. Bobyleva V., Bellei M., Pazienza T. L. and Muscatello U.(1997). Effect of cardiolipin on functional properties of isolated rat liver mitochondria. Biochem Mol Biol Int 41(3): 469-480. [DOI] [PubMed] [Google Scholar]
- 4. Chazotte B. and Hackenbrock C. R.(1988). The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem 263(28): 14359-14367. [PubMed] [Google Scholar]
- 5. Frezza C., Cipolat S. and Scorrano L.(2007). Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2(2): 287-295. [DOI] [PubMed] [Google Scholar]
- 6. Garcia Fernandez M. I., Ceccarelli D. and Muscatello U.(2004). Use of the fluorescent dye 10-N-nonyl acridine orange in quantitative and location assays of cardiolipin: a study on different experimental models. Anal Biochem 328(2):174-180. [DOI] [PubMed]
- 7. He F.(2011). BCA(Bicinchoninic Acid) Protein Assay. Bio-101: e44. [Google Scholar]
- 8. Heden T. D., Johnson J. M., Ferrara P. J., Eshima H., Verkerke A. R. P., Wentzler E. J., Siripoksup P., Narowski T. M., Coleman C. B., Lin C. T., et al.(2019). Mitochondrial PE potentiates respiratory enzymes to amplify skeletal muscle aerobic capacity. Sci Adv 5(9): eaax8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikon N. and Ryan R. O.(2017). Cardiolipin and mitochondrial cristae organization. Biochim Biophys Acta Biomembr 1859(6): 1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikon N. and Ryan R. O.(2017). Barth Syndrome: Connecting Cardiolipin to Cardiomyopathy. Lipids 52(2): 99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mourier A., Ruzzenente B., Brandt T., Kuhlbrandt W. and Larsson N. G.(2014). Loss of LRPPRC causes ATP synthase deficiency. Hum Mol Genet 23(10): 2580-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paradies G., Paradies V., De Benedictis V., Ruggiero F. M. and Petrosillo G.(2014). Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837(4): 408-417. [DOI] [PubMed] [Google Scholar]
- 13. Pennington E. R., Funai K., Brown D. A. and Shaikh S. R.(2019). The role of cardiolipin concentration and acyl chain composition on mitochondrial inner membrane molecular organization and function. Biochim Biophys Acta Mol Cell Biol Lipids 1864(7): 1039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piccotti L., Marchetti C., Migliorati G., Roberti R. and Corazzi L.(2002). Exogenous phospholipids specifically affect transmembrane potential of brain mitochondria and cytochrome C release. J Biol Chem 277(14): 12075-12081. [DOI] [PubMed] [Google Scholar]
- 15. Prola A., Blondelle J., Vandestienne A., Piquereau J., Denis R. G. P., Guyot S., Chauvin H., Mourier A., Maurer M., Henry C., et al.(2021). Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci Adv 7(1): eabd6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlame M., Xu Y. and Ren M.(2017). The Basis for Acyl Specificity in the Tafazzin Reaction. J Biol Chem 292(13): 5499-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider H., Lemasters J. J., Hochli M. and Hackenbrock C. R.(1980). Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J Biol Chem 255(8): 3748-3756. [PubMed] [Google Scholar]