Abstract
Background and aim
The BRCA 1 and BRCA 2 genes are associated with an inherited susceptibility to breast cancer with a cumulative risk of 60% in BRCA 1 mutation carriers and of 30% in BRCA 2 mutation carriers. Several lifestyle factors could play a role in determining an individual’s risk of breast cancer. Obesity, changes in body size or unhealthy lifestyle habits such as smoking, alcohol consumption and physical inactivity have been evaluated as possible determinants of breast cancer risk. The aim of this study was to explore the current understanding of the role of harmful lifestyle and obesity or weight change in the development of breast cancer in female carriers of BRCA 1/2 mutations.
Methods
Articles were identified from MEDLINE in October 2020 utilizing related keywords; they were then read and notes, study participants, measures, data analysis and results were used to write this review.
Results
Studies with very large case series have been carried out but only few of them have shown consistent results. Additional research would be beneficial to better determine the actual role and impact of such factors.
Keywords: BRCA mutation, Lifestyle, Obesity, BRCA-associated cancer
Introduction
Breast cancer (BC) is the most common female malignancy worldwide. Approximately 5–10% of breast cancer cases are hereditary and arise from autosomal dominant mutations in specific cancer genes, including the two breast cancer susceptibility genes BRCA 1 and BRCA 2. Women who carry these mutations have up to an 80% risk of developing breast cancer [1–3]. Identifying modifiable exposures is very important in BRCA 1/2 mutation carriers. The onset of breast cancer in these women may be influenced by genetic factors such as AdipoQ gene polymorphism associated to alterations in adipokines [4–6]. Evidence suggests that additional modifying factors influence cancer penetrance in BRCA 1/2 mutations carriers. Exposure to environmental factors and unhealthy lifestyle factors, including obesity, change in body size, smoking, alcohol consumption and physical inactivity, have been suggested to increase breast cancer (BC) risk in BRCA 1/2 mutation carriers [7–9]. An association of these factors has been widely reported to enhance the risk of developing cancer [10]. In a previous study of 2020 (Bruno et al.), we examined the relationships between selected lifestyle, metabolic exposures and BRCA related cancer in 502 women with BRCA mutations and found that increased fat mass and dysmetabolism were significantly associated with BC risk and had a greater effect in BRCA 2 positive women [11]. Obesity may increase BC risk through multiple mechanisms including insulin-resistance, metabolic syndrome, increased production of sex hormones and insulin-like growth factor-1 (IGF-1). In 2020, Pasanisi et al. reported that a Mediterranean diet with protein restriction is effective in reducing potential modulators of BRCA penetrance [12].
Given the high penetrance of BRCA 1 and BRCA 2 mutations, prevention and lifestyle changes have an extremely important risk-reducing role in women who have a higher risk of developing breast cancer.
Methods
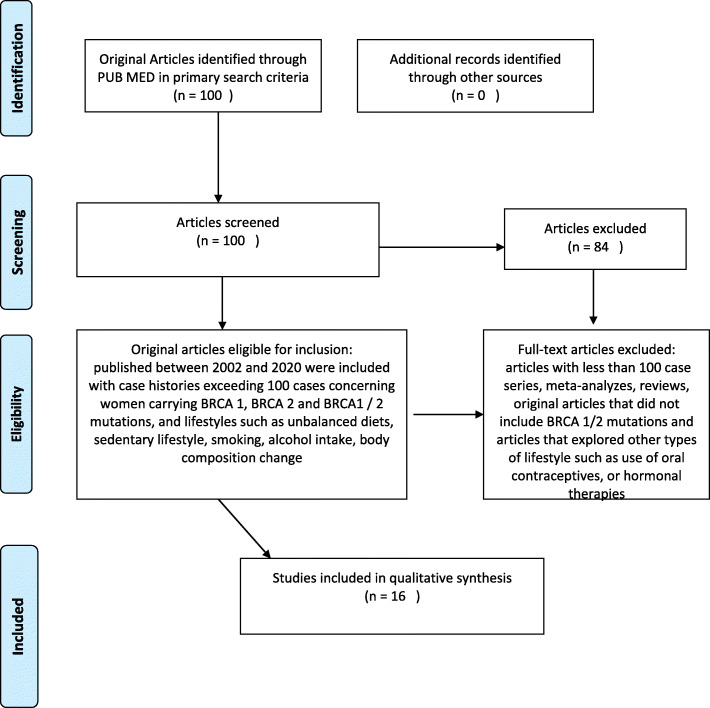
A broad review of the literature was carried out using MEDLINE (via PubMed) and sixteen articles published from 2002 to 2020, were selected from a total of one hundred. Search terms included keywords, combining the conditions (BRCA 1, BRCA 2, mutations, carriers, breast cancer risk), obesity, change in body weight and lifestyle (alcohol, smoking, physical inactivity). We included only original peer-reviewed articles on large prospective, retrospective and cohort studies that investigated obesity and unhealthy lifestyle habits as probable risk factors for the development of breast cancer. The selected articles concerned BRCA1 / 2 mutant women and investigated whether this status could increase the risk of breast cancer in relation to exposure to certain lifestyles. Studies that considered women without BRCA 1/2 mutations (in the case or control group), those with analyses that incorporated untested individuals or tested negative women, series with fewer than 100 patients enrolled, meta-analyses and reviews were excluded from this review (Fig. 1). One reviewer screened the titles and abstracts to select the studies and reviewed the full-text publications to confirm their eligibility and extract the relevant information from the included trials. A predefined spreadsheet (Excel 2007, Microsoft Corporation®) was used for data extraction. The most significant articles for lifestyles considered in this review are listed in Tables 1, 2, and 3.
Fig. 1.
Strategy used to identify literature for review
Table 1.
Article list used to analyzed cancer penetrance associated to weight gain and change body composition
| Authors/years | Reference | Study Design | Population | Sample Size | Control Group | Outcomes Measures | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Bruno O. 2020 | Clinical Breast Cancer | Cohort-Trial -Study | BRCA1/2 mutation carriers | 502,0 | yes | Change in body weight and composition and lifestyle characterisics | Higher fat mass associated with ncrease BC risk with greater effects in BRCA-2 |
| Qian F. 2019 | J Natl Cancer Inst | Randomized study | BRCA1/2 mutation carriers | 11,451 | yes | Anthropometric measures | Height and BMI associated to BC risk |
| Kim SJ. 2018 | Int J of Epidem | Cohort-Study | BRCA1/2 mutation carriers | 3734 | yes | Changes in body weight | No association with breast cancer risk |
| Manders P. 2011 | Br Can Res Treat | Cohort-Study | BRCA1/2 mutation carriers | 719,0 | yes | Anthropometric measures | Overweight and weight gain increase risk of postmenopausal breast cancer |
| Kotsopoulos J. 2005 | Br Can Res | Multicenter study | BRCA1/2 mutation carriers | 3291 | yes | Changes in body weight | Weigh loss within 30 yrs reduces breast cancer risk while no association later in life |
Table 2.
Article list used to analyzed cancer penetrance associated to harmful lifestyle habit
| Author/years | Reference | Study Design | Population | Sample Size | Control Group | Outcomes Measures | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Li H. 2020 | Cancer Epidemiol Biomarkers Prev | Cohort-study | BRCA1/2 mutation carriers | 13,118 | no | Alcohol consumption, smoking, | Smoking associated to BC risk for beginning to smoke more than 5 yers before full term pregnancy, no association with alcohol consumption |
| Kehm RD. 2020 | Cancer Res | Cohort-study | BRCA1/2 mutation carriers | 15,550 | yes | Physical activity | Physical inactivity associated to 20% reduction BC risk |
| Ko KP. 2018 | Int J Cancer | Longitudinal cohort study | BRCA1/2 mutation carriers | 7195 | no | Tobacco smoking | Tobacco smoking is associated with a modest increase of breast and ovarian cancer |
| van Erkelens A. 2017 | J Genet Counsel | Cohort-Study | BRCA1/2 mutation carriers | 268,0 | no | Physical inactivity, smoking and alcohol consumption around childbearing age | Higher breast cancer risk in overweight women who drink alcohol, smoke and do not exercise |
| Dennis J. 2010 | Breast | Case-control study | BRCA1/2 mutation carriers | 1925 | yes | Alcohol consumption | No association between alcohol consumption and BC risk |
| Ginsburg O. 2009 | Breast Can Res Treat | Case-control study | BRCA1/2 mutation carriers | 2538 | yes | Smoking | Increase BC risk in BRCA 1 carriers with a past history of smoking |
| Mc Guire V. 2006 | Cancer Epidemiol Biomarkers Prev | Case-control study | BRCA1/2 mutation carriers | 323,0 | yes | Alcohol consumption | No association between alcohol intake and BC risk |
| Ghardirian P. 2004 | Int J Cancer | Case-control study | BRCA1/2 mutation carriers | 1097 | yes | Smoking | Smoking is not a risk factor for BC |
Table 3.
Article list used to analyzed cancer penetrance associated to oral contraceptive use
| Authors/years) | Reference | Study Design | Population | Sample Size | Control Group | Outcomes Measures | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Schrijver LH. 2018 | JNCI Canc Spectr | Cohort Study | BRCA1/2 mutation carriers | 9839 | yes | Oral contraceptive use | No association between this use and BC risk |
| Lee E. 2008 | Canc Epi Biom Prev | Population based study | BRCA1/2 mutation carriers | 1469 | yes | Oral contraceptive use | No association between this use and BC risk |
| Narod SA. 2002 | J Cell Biol | Case control study | BRCA1/2 mutation carriers | 1311 | yes | Oral contraceptive use | Increase BC risk only in BRCA 1 women who used oral contraceptive before 30 years. |
Results
Inheritance of a BRCA 1 or 2 mutation is associated with an increased lifetime risk of breast cancer [13]. The relationship between anthropometric characteristics such as weight gain and /or BMI and breast cancer risk has been examined extensively [14]. In 2014, a meta analysis selected 44 articles, according to established quality criteria, considering smoking and alcohol consumption as a risk factor for the onset of BC. The authors found that subjects who smoked for more than 4 years were at greater BC risk than those who had never smoked (ES = 1.97; 95% CI = 1.43 to 2.72) while no correlation was highlighted in works that investigated the habitual intake of alcohol vs total abstemia (ES = 0.87; 95% CI = 0.50 to 1.23) [15].
Cancer penetrance associated to weight gain and changes in body composition
Weight gain and unfavorable changes in body composition with a significant increase in percentage body fat and decreased lean body mass are risk factors for breast cancer [16–19]. The relationship of anthropometric parameters and body weight changes with breast cancer risk has been examined extensively among women in the general population and several studies have investigated the impact of weight gain and cancer risk in women with a BRCA 1 or BRCA 2 mutation.
In a recent article of 2019, Qian F et al. investigated whether height or body mass index (BMI) could change the risk of developing breast cancer in 11,451 cases of breast cancer in BRCA 1/2 mutation carriers. These authors found that height was positively associated with breast cancer risk (per 10 cm increase HR = 1.9, 95% CI = 1.0 to 1.17; p = 1.17) while BMI was inversely associated with breast cancer risk (per 5 kg/ m2 increase HR = 0.94, 95% CI = 0.90 to 0.98; p = 0.007) [20].
In 2005, a multicenter study by Kotsopoulos J et al. investigated body weight changes and breast cancer risk in a total of 3291 women who carried BRCA 1 or BRCA 2 mutations and provided information on weight at ages 18, 30 and 40 showing that a weight loss of at least 4.5 kg between ages 18 and 30 was associated with a significant reduction in breast cancer risk (34%) thereafter (OR = 0.66; 95% CI 0.46–0.93). Weight gain later in life was not associated with increased risk [21]. In a multicenter longitudinal cohort study of 2018, Kim SJ et al. investigated the relationship between body size and breast cancer risk in 3734 BRCA mutation carriers and found no association between height, BMI and weight change and breast cancer risk [22]. In a retrospective cohort study published in 2011, Manders P. et al. investigated the association between anthropomentric measures and BC risk in 719,0 women with BRCA1 or BRCA2 mutation in pre and post menopause. The results reported in the work showed a decrease in risk in relation to BMI at 18 years while in postmenopausal age there was an increased risk respect to weight and in particular a higher BC risk in women weighing> 72 kg compared to those with weight < 72 kg, suggesting that postmenopausal mutated women should pay close attention to maintaining their body weight [23]. The summary list of these works is shown in Table 1
Cancer penetrance associated to harmful lifestyle habits
Smoking, drinking alcoholic beverages [24, 25] and physical inactivity [26] are well-known lifestyle risk factors for pre- and postmenopausal breast cancer in the general female population. The association between breast cancer risk and these unhealthy lifestyle choices has been also investigated in BRCA 1/2 mutation carriers.
In a recent retrospective and prospective cohort study of 2020, Li H et al. investigated the association between smoking and alcohol consumption and the risk of developing breast cancer in 13,118 BRCA 1/2 mutation carriers and found that the only variable associated with the risk of BC for both carrier groups it was for mutated women who had smoked for at least 5 years before their first pregnancy compared to first-time mothers who had never smoked before. The results, found no correlation between BC cancer risk and alcohol intake in in both groups [27].
In 2020, Kehm RD et al. carried out a prospective cohort study on 15,550 women who had a familial breast cancer risk and investigated the association between recreational physical activity and decreased risk in adult women. The authors tested interactions of physical activity with predicted absolute familial BC risk based on BRCA 1 and BRCA 2 mutation status and concluded that physical activity can reduce the risk of getting BC by about 20% even women with high penetrance due to their genetic family history or medical history [28].
van Erkelens A et al. in a cohort study in 2017, investigated the correlation of unhealthy lifestyles (alcohol intake, smoking and low physical activity) in 268,0 women with BRCa 1 and 2 mutation reporting that 38% of the participants had at least 2 high risk factors for BC, plus age the diagnosis of the mutation correlated with a decrease in physical activity (OR = 0.93/year, 95% CI = 0.86–0.99) and a prevalence of overweight (OR = 1.07/year, 95% CI =1.02–1.13) [29].
Dennis J et al. in 2010 conducted a case-control study of 1925 premenopausal women who carried a BRCA 1 or BRCA 2 mutation to investigate alcohol consumption and the risk of breast cancer, reporting an inversely proportional association between alcohol intake and increased risk of developing BC only in BRCA1-mutated women, while no association was found in BRCA2-mutated women (OR = 0.82; 95% CI 0.70–0.96) vs OR = 1.00; CI 0.71–1.41) [30].
In 2004, Ghadirian P et al. studied the correlation between smoking and the risk of breast cancer in a large cohort of BRCA 1 and BRCA 2 mutation carriers [31]. The authors, in a case-control study conducted on 1097 BRCA1 and 2 mutated women vs healthy women, found no significant association between the 2 groups considered (mutated vs healthy women) whether they were smokers or ex smokers or the age at which smoking began within 5 years of menarche (OR = 1.03;95% CI = 0.90 to 1.33) or before the first pregnancy concluding that smoking could not be a breast cancer risk in carriers of BRCA mutations.
Two other eligible studies by Ko KP et al. in 2018 and Ginsburg O et al. in 2009 investigated the association between smoking and increased breast cancer risk. The first was a cohort study of 7195 women that demonstrated an increased risk of breast and ovarian cancer in women smokers with a BRCA 1 or BRCA 2 mutation (HR = 1.17; 95% CI 1.01–1.37), [32] and the second was a case control study of 2538 BRCA 1/2 carriers that showed a modest, but significant increase BC risk in BRCA 1 carriers with a past history of smoking (OR = 1.27; 95% CI 1.06–1.50) [33]. McGuire V et al. in 2006 investigated the association between alcohol consumption and increased risk of breast cancerin 323,0 women suggesting no positive association between alcohol intake and breast cancer risk in BRCA 1 and BRCA 2 mutation carriers aged [34]. The summary list of these works is shown in Table 2
Cancer penetrance associated to oral contraceptive use
In the general population, multiparity and breastfeeding are among the protective factors of the risk of developing breast cancer, while the use of oral contraceptives could represent a predisposing factor; the data in the literature on the use of contraceptives in mutated women are still discordant but a possible role of estrogens on carcinogenesis has its foundation [35, 36]. The BRCA 1 /2 genes are involved in several functions including DNA damage and repair so the cancer-promoting effects of estrogen can be stronger in mutated BRCA 1 or BRCA 2 genes [37].
In 2018, Schrijver LH et al. investigated the association between the use of oral contraceptives and breast cancer risk in BRCA 1 and BRCA 2 mutation carriers in a retrospective and prospective cohort study of 9839 cases. They found no association between the use of oral contraceptives and BC risk in women with a BRCA 1 mutation (HR: =1.08, 95% CI = 0.75 to 1.56) while for BRCA 2 mutation carriers, the authors highlighted an increased risk of developing breast cancer in mutated women taking oral contraceptives (HR: =1.75, 95% CI = 1.03 to 2.97) and this risk was also related to the duration of treatment particularly in the period prior to the first full-term pregnancy [38].
In a 2008 population-based study on 1469 women and 444 control subjects Lee E et al. investigated reproductive factors and oral contraceptive use in BRCA 1/2 mutations carriers and non-carriers and reported no association between oral contraceptive use and BC risk in women carrying the mutations [39].
In 2002, in a matched case-control study on 1311 pairs of women with a known BRCA 1/2 mutation Narod SA et al. found an increased risk of breast cancer in women with a BRCA 1 mutation who first used oral contraceptives before age 30, or who used them for more than 5 years, while a similar risk did not appear in BRCA 2 mutation carriers [40]. The summary list of these works is shown in Table 3
Discussion
The presence of BRCA1 and 2 mutations may predispose to a higher risk of breast cancer in the percentage of 60 and 30% respectively. It is important to underline that not all mutated women will certainly develop cancer during their lifetime, but knowing the genetic or environmental risk factors that can increase the subjective predisposition to cancer is of fundamental importance. It has been proposed that several lifestyle factors such as smoking, alcohol consumption, poor nutrition or sedentariness may be potential modulators of BRCA penetrance, but the data reported in the literature are still conflicting and incomplete. The BRCA 1 gene and the BRCA 2 gene located on chromosome 17 and on chromosome 13, respectively (Fig. 2), are tumor suppressors capable of regulating cell proliferation and repairing any damage in DNA replication. It is therefore plausible that carcinogens that are contained, for example, in cigarette smoke or in some food may increase the risk of BC in female carriers of BRCA 1/2 mutations. The carcinogens present in the cigarette have the ability to infiltrate the pulmonary alveolus [41] and the bloodstream flowing into the breast by means of plasma lipoproteins [42, 43]. They are lipophilic, tobacco-related carcinogens can be stored in breast adipose tissue [33, 34] and then metabolized and activated by human mammary epithelial cells [44]. Many authors have reported that cigarette smoke contains various substances harmful to the breast parenchyma highlighting the presence of p53 mutation in the breast parenchyma of smokers compared to non-smokers [45]. On the others hand, heterocyclic amines and acrylamides, foods rich in starch and cooked at high temperatures (e.g., grilled or overcooked meat) or rich in animal protein or milk [11] may be potentially more likely to promote the development of breast cancer and favor BRCA penetrance. Although several studies have largely reported concordant results about the correlation between unhealthy lifestyle habits and sustained weight gain over time and breast cancer risk in the general population, few studies with large series have been conducted on women carrying the BRCA 1 and 2 mutation. This mini review considered 16 studies (12 prospective and 4 retrospective) that highlighted a discrepancy between the effects of some unhealthy lifestyle factors in increasing the risk of breast cancer. Alcohol consumption was not observed to have a key role in the onset of breast cancer while smoking, weight gain and physical inactivity, especially in postmenopausal age, seem to increase the risk of breast cancer.
Fig. 2.
Chromosomal localization and structure of BRCA 1 and BRCA 2 genes. Image (idea from Narod SA et al. 2008)
Conclusion
Numerous factors have been reported to modify breast cancer risk. Our review of the specific literature has highlighted that there are few consistent results across different studies and that additional research would be beneficial to better determine the actual role and impact of such factors.
Acknowledgements
We thank Athina Papa for the language editing.
Authors’ contributions
Antonella Daniele and Angelo Virgilio Paradiso creators of the review and wrote the paper; Carla Minoia, Miriam Dellino and Salvatore Pisconti parteciped in data collection, Patrizia Pasanisi, Margherita Patruno and Maria Digennaro parteciped in critical revision of manuscript, Rosa Divella, Porzia Casamassima and Eufemia Savino parteciped in study design. All authors approved final version of manuscript.
Funding
Not applicable for that section.
Availability of data and materials
Not applicable for that section.
Declarations
Ethics approval and consent to participate
Not applicable for that section.
Consent for publication
All authors agree to the publication.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA 1 and BRCA 2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 2.Nkondjock A, Ghadirian P. Epidemiology of breast cancer among BRCA mutation carriers: an overview. Cancer Lett. 2004;205(1):1–8. doi: 10.1016/j.canlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Pruthi S, Gostout BS, Lindor NM. Identification and Management of Women with BRCA mutations or hereditary predisposition for breast and ovarian Cancer. Mayo Clin Proc. 2010;85(12):1111–1120. doi: 10.4065/mcp.2010.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniele A, Paradiso AV, Divella R, Digennaro M, Patruno M, Tommasi S, Pilato B, Tufaro A, Barone M, Minoia C, Colangelo D, Savino E, Casamassima P, Bruno E, Oliverio A, Pasanisi P. The Role of Circulating Adiponectin and SNP276G>T at ADIPOQ Gene in BRCA-mutant Women. Cancer Genomics Proteomics. 2020;17(3):301–307. doi: 10.21873/cgp.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, AJM d C, Delgado G, Dimitriou M, ASF D, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, PKE M, Mahajan A, WL MA, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Study LLC, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, SA MC, AJ MK, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, JRB P, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ‘t Hooft FM, AAE V, Westra HJ, Zheng W, Zondervan KT, ADIPOGen Consortium. AGEN-BMI Working Group. CARDIOGRAMplusC4D Consortium. CKDGen Consortium. GLGC. ICBP; MAGIC Investigators. MuTHER Consortium. MIGen Consortium. PAGE Consortium. ReproGen Consortium. GENIE Consortium. International Endogene Consortium. Heath AC, Arveiler D, SJL B, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, JJP K, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, CA MK, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, PAF M, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, PEH S, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, PIW d B, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, CNA P, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O'Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, MI MC, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, RJF L, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, Estrada K, Luan J, Kutalik Z, Amin N, Buchkovich ML, Croteau-Chonka DC, Day FR, Duan Y, Fall T, Fehrmann R, Ferreira T, Jackson AU, Karjalainen J, Lo KS, Locke AE, Mägi R, Mihailov E, Porcu E, Randall JC, Scherag A, Vinkhuyzen AA, Westra HJ, Winkler TW, Workalemahu T, Zhao JH, Absher D, Albrecht E, Anderson D, Baron J, Beekman M, Demirkan A, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Fraser RM, Goel A, Gong J, Justice AE, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Lui JC, Mangino M, Mateo Leach I, Medina-Gomez C, Nalls MA, Nyholt DR, Palmer CD, Pasko D, Pechlivanis S, Prokopenko I, Ried JS, Ripke S, Shungin D, Stancáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Afzal U, Arnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Bolton JL, Böttcher Y, Boyd HA, Bruinenberg M, Buckley BM, Buyske S, Caspersen IH, Chines PS, Clarke R, Claudi-Boehm S, Cooper M, Daw EW, De Jong PA, Deelen J, Delgado G, Denny JC, Dhonukshe-Rutten R, Dimitriou M, Doney AS, Dörr M, Eklund N, Eury E, Folkersen L, Garcia ME, Geller F, Giedraitis V, Go AS, Grallert H, Grammer TB, Gräßler J, Grönberg H, de Groot LC, Groves CJ, Haessler J, Hall P, Haller T, Hallmans G, Hannemann A, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hemani G, Henders AK, Hillege HL, Hlatky MA, Hoffmann W, Hoffmann P, Holmen O, Houwing-Duistermaat JJ, Illig T, Isaacs A, James AL, Jeff J, Johansen B, Johansson Å, Jolley J, Juliusdottir T, Junttila J, Kho AN, Kinnunen L, Klopp N, Kocher T, Kratzer W, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Lu Y, Lyssenko V, Magnusson PK, Mahajan A, Maillard M, McArdle WL, McKenzie CA, McLachlan S, McLaren PJ, Menni C, Merger S, Milani L, Moayyeri A, Monda KL, Morken MA, Müller G, Müller-Nurasyid M, Musk AW, Narisu N, Nauck M, Nolte IM, Nöthen MM, Oozageer L, Pilz S, Rayner NW, Renstrom F, Robertson NR, Rose LM, Roussel R, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Schunkert H, Scott RA, Sehmi J, Seufferlein T, Shi J, Silventoinen K, Smit JH, Smith AV, Smolonska J, Stanton AV, Stirrups K, Stott DJ, Stringham HM, Sundström J, Swertz MA, Syvänen AC, Tayo BO, Thorleifsson G, Tyrer JP, van Dijk S, van Schoor NM, van der Velde N, van Heemst D, van Oort FV, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Waldenberger M, Wennauer R, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Bergmann S, Biffar R, Blangero J, Boomsma DI, Bornstein SR, Bovet P, Brambilla P, Brown MJ, Campbell H, Caulfield MJ, Chakravarti A, Collins R, Collins FS, Crawford DC, Cupples LA, Danesh J, de Faire U, den Ruijter HM, Erbel R, Erdmann J, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Gansevoort RT, Gejman PV, Gieger C, Golay A, Gottesman O, Gudnason V, Gyllensten U, Haas DW, Hall AS, Harris TB, Hattersley AT, Heath AC, Hengstenberg C, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Jacobs KB, Jarvelin MR, Jousilahti P, Jula AM, Kaprio J, Kastelein JJ, Kayser M, Kee F, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kooner JS, Kooperberg C, Koskinen S, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lupoli S, Madden PA, Männistö S, Manunta P, Marette A, Matise TC, McKnight B, Meitinger T, Moll FL, Montgomery GW, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Ouwehand WH, Pasterkamp G, Peters A, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ritchie M, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Sebert S, Sever P, Shuldiner AR, Sinisalo J, Steinthorsdottir V, Stolk RP, Tardif JC, Tönjes A, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Records EM, Genomics (eMEMERGEGE) Consortium. MIGen Consortium. PAGEGE Consortium. LifeLines Cohort Study. Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hayes MG, Hui J, Hunter DJ, Hveem K, Jukema JW, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Powell JE, Power C, Quertermous T, Rauramaa R, Reinmaa E, Ridker PM, Rivadeneira F, Rotter JI, Saaristo TE, Saleheen D, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Strauch K, Stumvoll M, Tuomilehto J, Uusitupa M, van der Harst P, Völzke H, Walker M, Wareham NJ, Watkins H, Wichmann HE, Wilson JF, Zanen P, Deloukas P, Heid IM, Lindgren CM, Mohlke KL, Speliotes EK, Thorsteinsdottir U, Barroso I, Fox CS, North KE, Strachan DP, Beckmann JS, Berndt SI, Boehnke M, Borecki IB, MI MC, Metspalu A, Stefansson K, Uitterlinden AG, van Duijn CM, Franke L, Willer CJ, Price AL, Lettre G, Loos RJ, Weedon MN, Ingelsson E, O'Connell JR, Abecasis GR, Chasman DI, Goddard ME, Visscher PM, Hirschhorn JN, Frayling TM. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund / American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of Cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 8.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4(3):157–173. doi: 10.1046/j.1467-789X.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 9.Pichard C, Plu-Bureau G, Neves ECM, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60(1):19–30. doi: 10.1016/j.maturitas.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Narod SA. Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer. 2002;2(2):113–123. doi: 10.1038/nrc726. [DOI] [PubMed] [Google Scholar]
- 11.Bruno E, Oliverio A, Paradiso A, Daniele A, Tommasi S, Terribile DA, Filippone A, Digennaro M, Pilato B, Danza K, Guarino D, Rossi C, Rossi MM, Venturelli E, Giussani M, Peissel B, Pasanisi P. Lifestyle Characteristics in Women Carriers of BRCA Mutations: Results From an Italian Trial Cohort. Clin Breast Cancer. 2020;S1526–8209(20):30273–30271. doi: 10.1016/j.clbc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Pasanisi P, Bruno E, Venturelli E, Morelli D, Oliverio A, Baldassari I, Rovera F, Iula G, Taborelli M, Peissel B, Azzolini J, Manoukian S. A Dietary Intervention to Lower Serum Levels of IGF-I in BRCA Mutation Carriers. Cancers (Basel) 2018;10(9):309. doi: 10.3390/cancers10090309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno E, Oliverio A, Paradiso AV, Daniele A, Tommasi S, Tufaro A, Terribile DA, Magno S, Filippone A, Venturelli E, Morelli D, Baldassari I, Cravana ML, Manoukian S, Pasanisi P. A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers (Basel) 2020;12(12):3732. doi: 10.3390/cancers12123732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 15.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA 1 and BRCA 2 mutation carriers: systematic review and meta-analysis. J National Cancer Institute. 2004;106(6):dju091. doi: 10.1093/jnci/dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang JS, Cai H, Wang CY, Zhang J, Zhang MX. Body weight changes in breast cancer patients following adjuvant chemotherapy and contributing factors. Mol Clin Oncol. 2014;2(1):105–110. doi: 10.3892/mco.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin Cancer Res. 2017;23(14):3537–3543. doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trentham-Dietz A, Newcomb PA, Storer BE, Longnecker MP, Baron J, Greenberg ER, Willett WC. Body size and risk of breast cancer. Am J Epidemiol. 1997;145(11):1011–1019. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- 20.Qian F, Wang S, Mitchell J, McGuffog L, Barrowdale D, Leslie G, Oosterwijk JC, Chung WK, Evans DG, Engel C, Kast K, Aalfs CM, Adank MA, Adlard J, Agnarsson BA, Aittomäki K, Alducci E, Andrulis IL, Arun BK, MGEM A, Azzollini J, Barouk-Simonet E, Barwell J, Belotti M, Benitez J, Berger A, Borg A, Bradbury AR, Brunet J, Buys SS, Caldes T, Caligo MA, Campbell I, Caputo SM, Chiquette J, KBM C, Margriet Collée J, Couch FJ, Coupier I, Daly MB, Davidson R, Diez O, Domchek SM, Donaldson A, Dorfling CM, Eeles R, Feliubadaló L, Foretova L, Fowler J, Friedman E, Frost D, Ganz PA, Garber J, Garcia-Barberan V, Glendon G, Godwin AK, Gómez Garcia EB, Gronwald J, Hahnen E, Hamann U, Henderson A, Hendricks CB, Hopper JL, Hulick PJ, Imyanitov EN, Isaacs C, Izatt L, Izquierdo Á, Jakubowska A, Kaczmarek K, Kang E, Karlan BY, Kets CM, Kim SW, Kim Z, Kwong A, Laitman Y, Lasset C, Hyuk Lee M, Won Lee J, Lee J, Lester J, Lesueur F, Loud JT, Lubinski J, Mebirouk N, HEJ M-H, Meindl A, Miller A, Montagna M, Mooij TM, Morrison PJ, Mouret-Fourme E, Nathanson KL, Neuhausen SL, Nevanlinna H, Niederacher D, Nielsen FC, Nussbaum RL, Offit K, Olah E, Ong KR, Ottini L, Park SK, Peterlongo P, Pfeiler G, Phelan CM, Poppe B, Pradhan N, Radice P, Ramus SJ, Rantala J, Robson M, Rodriguez GC, Schmutzler RK, Hutten Selkirk CG, Shah PD, Simard J, Singer CF, Sokolowska J, Stoppa-Lyonnet D, Sutter C, Yen Tan Y, Teixeira RM, Teo SH, Terry MB, Thomassen M, Tischkowitz M, Toland AE, Tucker KM, Tung N, van Asperen CJ, van Engelen K, van Rensburg EJ, Wang-Gohrke S, Wappenschmidt B, Weitzel JN, Yannoukakos D, GEMO Study Collaborators. HEBON. EMBRACE. Greene MH, Rookus MA, Easton DF, Chenevix-Trench G, Antoniou AC, Goldgar DE, Olopade OI, Rebbeck TR, Huo D. Height and Body Mass Index as Modifiers of Breast Cancer Risk in BRCA 1/2 Mutation Carriers: A Mendelian Randomization Study. J Natl Cancer Inst. 2019;111(4):350–364. doi: 10.1093/jnci/djy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotsopoulos J, Olopado OI, Ghadirian P, Lubinski J, Lynch HT, Isaacs C, Weber B, Kim-Sing C, Ainsworth P, Foulkes WD, Eisen A, Sun P, Narod SA. Changes in body weight and the risk of breast cancer in BRCA 1 and BRCA 2 mutation carriers. Breast Cancer Res. 2005;7(5):R833–R843. doi: 10.1186/bcr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Huzarski T, Gronwald J, Singer CF, Møller P, Lynch HT, Armel S, Karlan BY, Foulkes WD, Neuhausen SL, Senter L, Eisen A, Eng C, Panchal S, Pal T, Olopade O, Zakalik D, Lubinski J, Narod SA, Kotsopoulos J, Hereditary Breast Cancer Clinical Study Group Prospective evaluation of body size and breast cancer risk among BRCA 1 and BRCA 2 mutation carriers. Int J Epidemiol. 2018;47(3):987–997. doi: 10.1093/ije/dyy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, van Asperen CJ, Meijers-Heijboer H, Ausems MG, van Os TA, Gomez-Garcia EB, Brohet RM, HEBON, van Leeuwen FE, Rookus MA. Body weight and risk of breast cancer in BRCA 1/2 mutation carriers. Breast Cancer Res Treat. 2011;126(1):193–202. doi: 10.1007/s10549-010-1120-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women’s health study. Am J Epidemiol. 2007;165(6):667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 25.Bennicke K, Conrad C, Sabroe S, Sørensen HT. Cigarette smoking and breast cancer. BMJ. 1995;310(6992):1431–1433. doi: 10.1136/bmj.310.6992.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narod SA. Modifiers of risk of hereditary breast cancer. Oncogene. 2006;25(43):5832–5836. doi: 10.1038/sj.onc.1209870. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Terry MB, Antoniou AC, Phillips KA, Kast K, Mooij TM, Engel C, Noguès C, Stoppa-Lyonnet D, Lasset C, Berthet P, Mari V, Caron O, GENEPSO study. Barrowdale D, Frost D, Brewer C, Evans DG, Izatt L, Side L, Walker L, Tischkowitz M, Rogers MT, Porteous ME, Snape K, EMBRACE study. HEJ M-H, JJP G, Blok MJ, Hoogerbrugge N, HEBON Investigators. Daly MB, Andrulis IL, Buys SS, John EM, SA ML, Friedlander M, kConFab Investigators. Tan YY, Osorio A, Caldes T, Jakubowska A, Simard J, Singer CF, Olah E, Navratilova M, Foretova L, Gerdes AM, Roos-Blom MJ, Arver B, Olsson H, Schmutzler RK, Hopper JL, Milne RL, Easton DF, Van Leeuwen FE, Rookus MA, Andrieu N, Goldgar DE. Alcohol Consumption, Cigarette Smoking, and Risk of Breast Cancer for BRCA 1 and BRCA 2 Mutation Carriers: Results from The BRCA 1 and BRCA 2 Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2020;29(2):368–378. doi: 10.1158/1055-9965.EPI-19-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehm RD, Genkinger JM, MacInnis RJ, John EM, Phillips KA, Dite GS, Milne RL, Zeinomar N, Liao Y, Knight JA, Southey MC, Chung WK, Giles GG, McLachlan SA, Whitaker KD, Friedlander M, Weideman PC, Glendon G, Nesci S, Investigators K, Andrulis IL, Buys SS, Daly MB, Hopper JL, Terry MB. Recreational Physical Activity Is Associated with Reduced Breast Cancer Risk in Adult Women at High Risk for Breast Cancer: A Cohort Study of Women Selected for Familial and Genetic Risk. Cancer Res. 2020;80(1):116–125. doi: 10.1158/0008-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Erkelens A, Derks L, Sie AS, Egbers L, Woldringh G, Prins JB, Manders P, Hoogerbrugge N. Lifestyle Risk Factors for Breast Cancer in BRCA 1/2-Mutation Carriers Around Childbearing Age. J Genet Couns. 2017;26(4):785–791. doi: 10.1007/s10897-016-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis J, Krewski D, Côté FS, Fafard E, Little J, Ghadirian P. Breast cancer risk in relation to alcohol consumption and BRCA gene mutations--a case-only study of gene-environment interaction. Breast J. 2011;17(5):477–484. doi: 10.1111/j.1524-4741.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghadirian P, Lubinski J, Lynch H, Neuhausen SL, Weber B, Isaacs C, Baruch RG, Randall S, Ainsworth P, Friedman E, Horsman D, Tonin P, Foulkes WD, Tung N, Sun P, Narod SA. Smoking and the risk of breast cancer among carriers of BRCA mutations. Int J Cancer. 2004;110(3):413–416. doi: 10.1002/ijc.20106. [DOI] [PubMed] [Google Scholar]
- 32.Ko KP, Kim SJ, Huzarski T, Gronwald J, Lubinski J, Lynch HT, Armel S, Park SK, Karlan B, Singer CF, Neuhausen SL, Narod SA, Kotsopoulos J. Hereditary Breast Cancer Clinical Study Group. The association between smoking and cancer incidence in BRCA 1 and BRCA 2 mutation carriers. Int J Cancer. 2018;142(11):2263–2272. doi: 10.1002/ijc.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginsburg O, Ghadirian P, Lubinski J, Cybulski C, Lynch H, Neuhausen S, Kim-Sing C, Robson M, Domchek S, Isaacs C, Klijn J, Armel S, Foulkes WD, Tung N, Moller P, Sun P, Narod SA. Hereditary Breast Cancer Clinical Study Group. Smoking and the risk of breast cancer in BRCA 1 and BRCA 2 carriers: an update. Breast Cancer Res Treat. 2009;114(1):127–135. doi: 10.1007/s10549-008-9977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire V, John EM, Felberg A, Haile RW, Boyd NF, Thomas DC, Jenkins MA, Milne RL, Daly MB, Ward J, Terry MB, Andrulis IL, Knight JA, Godwin AK, Giles GG, Southey M, West DW, Hopper JL. Whittemore AS; kConFab Investigators. No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA 1 and BRCA 2 mutations ages <50 years. Cancer Epidemiol Biomark Prev. 2006;15(8):1565–1567. doi: 10.1158/1055-9965.EPI-06-0323. [DOI] [PubMed] [Google Scholar]
- 35.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 36.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27(27):75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida K, Miki Y. Role of BRCA 1 and BRCA 2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95(11):866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrijver LH, Olsson H, Phillips KA, Terry MB, Goldgar DE, Kast K, Engel C, Mooij TM, Adlard J, Barrowdale D, Davidson R, Eeles R, Ellis S, Evans DG, Frost D, Izatt L, Porteous ME, Side LE, Walker L, Berthet P, Bonadona V, Leroux D, Mouret-Fourme E, Venat-Bouvet L, Buys SS, Southey MC, John EM, Chung WK, Daly MB, Bane A, van Asperen CJ, Gómez Garcia EB, MJE M, TAM v O, Roos-Blom MJ, Friedlander ML, SA ML, Singer CF, Tan YY, Foretova L, Navratilova M, Gerdes AM, Caldes T, Simard J, Olah E, Jakubowska A, Arver B, Osorio A, Noguès C, Andrieu N, Easton DF, van Leeuwen FE, Hopper JL, Milne RL, Antoniou AC, Rookus MA, EMBRACE. GENEPSO. BCFR. HEBON. kConFab. IBCCS Oral Contraceptive Use and Breast Cancer Risk: Retrospective and Prospective Analyses From a BRCA 1 and BRCA 2 Mutation Carrier Cohort Study. JNCI Cancer Spectr. 2018;2(2):pky023. doi: 10.1093/jncics/pky023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee E, Ma H, McKean-Cowdin R, Van Den Berg D, Bernstein L, Henderson BE, Ursin G. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA 1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomark Prev. 2008;17(11):3170–3178. doi: 10.1158/1055-9965.EPI-08-0396. [DOI] [PubMed] [Google Scholar]
- 40.Plant AL, Benson DM, Smith LC. Cellular uptake and intracellular localization of benzo(a)pyrene by digital fluorescence imaging microscopy. J Cell Biol. 1985;100(4):1295–1308. doi: 10.1083/jcb.100.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu HP, Bymun EN. Systemic excretion of benzo(a)pyrene in the control and microsomally induced rat: the influence of plasma lipoproteins and albumin as carrier molecules. Cancer Res. 1983;43(2):485–490. [PubMed] [Google Scholar]
- 42.Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39(2-3):119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- 43.Morris JJ, Seifter E. The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses. 1992;38(3):177–184. doi: 10.1016/0306-9877(92)90090-Y. [DOI] [PubMed] [Google Scholar]
- 44.MacNicoll AD, Easty GC, Neville AM, Grover PL, Sims P. Metabolism and activation of carcinogenic polycyclic hydrocarbons by human mammary cells. Biochem Biophys Res Commun. 1980;95(4):1599–1606. doi: 10.1016/S0006-291X(80)80081-7. [DOI] [PubMed] [Google Scholar]
- 45.el-Bayoumy K. Environmental carcinogens that may be involved in human breast cancer etiology. Chem Res Toxicol. 1992;5:585–590. doi: 10.1021/tx00029a001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable for that section.