Abstract
Purpose
End-stage temporomandibular joint (TMJ) disease are not uncommon and affects quality of life. Multiple surgical procedures have been mentioned in literature for management of TMJ disease which ranges from conservative management to aggressive resection of involved joint and replacement with alloplastic total joint prosthesis. The purpose of the present paper was to provide an overview of the role of alloplastic total joint prosthesis in TMJ replacement.
Methods and results
Alloplastic total joint prosthesis is nowadays considered as a standard of care in the adult patients who require TMJ replacement. The requirement of alloplastic total prosthesis has increased in present era with the improvement in design and material of implants, surgical skills and reported victorious outcome along with improved quality of life after its use. It provides restoration of form and functions, improvement in quality of life, reduction in pain and maintenance of ramal height. Additionally, in TMJ ankylosis it reduces chances of re-ankylosis and allows facial asymmetry correction. Currently, enough evidence is however not available for replacement in skeletally immature patient.
Conclusion
The authors conclude that the total joint replacement is a standard procedure for end-stage TMJ disease. Every maxillofacial surgeon should be well-acquainted with TMJ replacement.
Keywords: Alloplastic temporomandibular joint, Temporomandibular joint ankylosis, End-stage temporomandibular disease
Introduction
Replacement of the temporomandibular joint (TMJ) is a complex procedure as TMJ is among the most complicated articulations in the body. End-stage TMJ disease can lead to severe disability in mastication and speech affecting the physical, social, psychological, and overall quality of life [1–3]. It may also affect the esthetics and reduce the airway space. Alloplastic replacement is the mainstay in treatment for end-stage TMJ disease. Historically, the failure of TMJ prosthesis has been well documented with device failure [4]. Initially, alloplastic total joint replacement (TJR) was used for trauma, severe joint disease, multiple failed open joint surgery, and joint reconstruction after ablative surgery [5]. The pathologies of TMJ can hamper the growth and function of the mandible. The temporomandibular joint ankylosis (TMJA) can cause immobility of joint and shortening of the ramus and corpus of the mandible with facial asymmetry. This, however, depends on at what stage of growth of the individual the joint was affected. The end-stage TMJ disease causes pain, reduced mouth opening, the collapse of the joint, and severely hampered quality of life. Both resorption and growth restriction can lead to backward positioning of the mandible leading to obstructive sleep apnea (OSA). With the increased availability of evidence, the pendulum is now swinging more and more toward the use of alloplastic replacements in adult TMJ ankylosis and end-stage TMJ disease [6].
Alloplastic joint prostheses can fulfill all the goals of TMJ reconstruction. The use of TJR in adults has been adequately researched and documented, but its use in skeletally immature patients lacks significant evidence. Only some sporadic, short-term successful reports of alloplastic replacement in skeletally immature patients are available [7, 8]. The historical perspective, goals of TMJ replacement, indications and contraindications, advantages and disadvantages, the difference between normal and alloplastic joint, presurgical consideration, custom, and stock prosthesis, preoperative planning, and surgical technique of TMJ replacement and documented complications have been discussed here.
Background
For severe joint disease, surgical excision was the preferred treatment between 1536 and 1840 [9, 10]. In 1840, John Murray Carnochan interposed a block of wood between surfaces after resection of the diseased part of condyle [11]. In 1890, Gluck reported total joint arthroplasties with ivory prosthetic TMJ [12]. Risdon used gold foil as interpositional material in TMJA patients after gap arthroplasty. The first total TMJ prosthesis was designed by Christensen in 1965 [13]. This device had two components: ramal/condylar component which articulates with a metal fossa. The condylar head component was made up of polymethylmethacrylate (PMMA) with cast Vitallium ramal component fitting into as a post. Later, he abandoned the use of PMMA because of potential side effects of particulation under function. In 1997, Christensen invented a metal-on-metal cast Vitallium prosthesis using a stereolithographic model [14]. It was approved as a CE-certified medical device in 1998 and 2001 by European Union and the US Food and Drug Administration (FDA), respectively. The metal-on-metal device, however, resulted in early metal wear debris causing lymphocyte type rejection and foreign body response [15]. In 1995, Mercuri et al. used patient-fitted TJR (TMJ Concept, Ventura, CA) [16]. TMJ Concept Prosthesis was approved by FDA in 1999. The TMJ concepts prosthesis has a fossa component made of ultra-high molecular weight polyethylene (UHMWPE). The surface facing the temporal bone/fossa has a pure titanium mesh. The ramus component has a titanium alloy shaft including a chromium–cobalt molybdenum (Cr–Co–Mo) condylar articular head. The prosthesis is said to provide maximum host-prosthesis contact despite difficult anatomical form. In the year 2000, Quinn introduced a stock TMJ replacement device (Zimmer Biomet, Jacksonville, FL) [5]. The Biomet (formerly Lorenz) total TMJ prosthesis combines a prosthetic shaft with a sphere-shaped head of cobalt–chromium or titanium and UHMWPE fossa component. The Biomet stock prosthesis has three fossa baseplate sizes and five different ramus components along with an offset ramus variant in all five sizes. Both stock and custom-made prostheses have shown satisfactory outcomes in the long-term studies [17, 18].
Both safety and effectiveness are required from a material to be biocompatible. Along with its biocompatible nature, the material should be able to bear the functional load of TMJ and should be stable in the implanted site. Currently, FDA-approved material for alloplastic TMJ TJR includes cobalt–chromium alloys, commercially pure titanium (cpTi), alloyed titanium (Ti6Al4V), and ultra-high molecular weight polyethylene (UHMWPE).
Goals of TMJ Replacement
The goals of TMJ replacement include restoration of form and functions of TMJ as close as possible to the natural joint; to improve quality of life; to maintain ramal height and prevent facial asymmetry; to provide pain-free maximal incisal opening (MIO); prevention of recurrence like in TMJA cases; concomitant orthognathic surgery for facial correction; to limit excessive treatment and cost; and to prevent further morbidity.
Indications and Contraindications of TMJ TJR
Indications for alloplastic TMJ replacement include [5]: severe inflammatory arthritis involving the TMJ not responsive to other treatment modalities; recurrent fibrous or bony ankylosis especially in cases where joint is anatomically mutilated; failed autogenous graft and alloplastic devices; destruction of graft tissue by pathology; loss of ramus height and condylar resorption; connective tissue and autoimmune disease (juvenile idiopathic arthritis, ankylosing spondylitis, scleroderma).
Relative contraindications of alloplastic total TMJ replacement are [5]: the age of the patient (the use of TJR in skeletally immature patients is still not proven as alloplastic prosthesis does not have growth potential); uncontrolled systemic disease; active infection at the surgical site; allergy to the implant material.
Advantages and Disadvantages
Advantages of alloplastic prosthesis are no second surgical site, thereby avoiding the donor site morbidity and reduction in the surgical time as autogenous graft harvesting is not required. Alloplastic total joint prosthesis resembles the anatomy of the natural joint resulting in biomechanically better adaptation. Physiotherapy exercise can begin immediately when the chances of heterotopic bone formation are greatest; a concomitant orthognathic surgery can be carried out for facial asymmetry correction. Cost of the prosthesis; material wear and failure; potential allergic reaction; long-term stability and inability to provide growth required in skeletally immature patients are potential disadvantages.
Difference Between Normal Joint and Alloplastic TMJ TJR
TMJ is a bilateral ginglyoathroidal joint that is divided into two compartments (superior and inferior). The superior compartment is responsible for translation, and the inferior joint space is for rotation or hinge movements. After TMJ TJR, only a single space exists where only pure rotational movements are possible. In natural TMJ, there are muscles and ligaments which are responsible for functions of TMJ. There is no role of ligaments in TJR. In TJR, the absence of translation is due to detachment of lateral pterygoid muscle. Some translation, however, can happen after TJR by recruiting the suprahyoid, masseter, and medial pterygoid muscles or the design of the fossa which may allow pseudotranslation. (Zimmer Biomet). After TJR, the kinematics is different because of the geometry of the bearing surfaces of the device components and the loss of bony and soft tissue components that are responsible for normal movements. In the natural joint, a concave articular disk is present between the condyle and the glenoid fossa. This allows the bony components of the joint to remain congruent during wide mandibular movements. In TJR, this congruency depends on the design of the implant.
Stock versus Custom-Made Alloplastic Prosthesis
The stock and custom-made prosthesis have different articular surface geometries and material compositions. In the case of stock, prosthesis bone fits the prosthesis, and custom devices are manufactured/printed from the preoperative CT scan data of anatomy of the lateral surface of mandible and the fossa so that it fits the lateral surface of mandible and fossa without much preparation. Advantages of the stock prosthesis are immediate availability, size flexibility, and lower cost than a custom joint. Disadvantages are questionable fit of the joint especially in a long-standing disease-causing warping of the mandible [19], longer intraoperative time as the bony surface has to be prepared to fit the prosthesis, limited potential for anterior-inferior movement of the mandible, and surgical experience is required to manage the variability of fit. Imperfections in fit can cause material fatigue, and subsequent micromotions eventually lead to prosthesis failure [20–24].
Custom-made TJR can adapt easily; no alteration of patient's bone is required. It also addresses the issue of distorted anatomy like warping of the mandible. It requires less operative time, excessive anterior-inferior movement is possible, and the total contact surface between prosthesis and bone allows improved osseointegration and stability [20]. Custom TJR allows controlled occlusal correction and proper mastication without simultaneous orthognathic surgery. The posterior stop present in the fossa design reduces the chances of dislocation. In case of severe hypoplasia of joint as in hemifacial microsomia, the extended mandibular and fossa component can act as a substitute for the missing bone [25]. Similarly, custom TJR can be used for defects due to trauma, osteomyelitis, pathological resection [25, 26]. Screw position and length can be determined preoperatively to prevent damage to the inferior alveolar nerve [27, 28]. For simple cases, the custom-made prosthesis can be inserted through a mini-retromandibular incision, decreasing the risk of damage to the marginal mandibular branch of the facial nerve [29]. Disadvantages of custom joints are longer fabrication time, need for a significant amount of planning by the surgeon and technical team, excessive cost, and limited flexibility. Many authors have used custom-made prostheses in cases with severe anatomical abnormalities [27, 30–33]. Wolford et al. [34] compared MIO after stock and custom prostheses. They have reported adequate MIO after the use of both types of joints. Zhao et al. [24] calculated the amount of bone required to trim from skull base and mandible to fit stock prosthesis. Their calculation suggests that bone trimming required was 150–300 mm3 in 46% of the skull base and more than 300 mm3 in 33% of cases. Medium to large amounts (27% and 29%) of bone trimming were required for the mandible. Due to extreme variability in mandibular anatomy and angulation of fit of the condylar head to the fossa, a custom joint is preferred in TMJ.
One-Stage versus Two-Stage Protocol for Alloplastic TMJ Replacement
Both one-stage or two-stage treatment protocols have been used for TMJ replacement [35–38]. Indications of the one-stage protocol are when the patient can maintain occlusion in CT, when aesthetics are satisfactory, and when fossa anatomy is easily adjusted with minor corrections at planned surgery. Indication of two-stage alloplastic replacement is fossa or condyle anatomy that requires significant modifications or resection, significant bony ankylosis, when significant occlusal alterations are necessary, resection of large tumors of the TMJ region with associated hard tissue defects, and removal of failed alloplastic hardware. Due to reduction in cumulative operative and anesthetic risk and decrease in duration and number of hospital admission and stay, one stage is preferred. It is straightforward to expose the TMJ and avoid nerve injury in a one-stage protocol as compared to the two-stage approach. Two-stage protocol, however, has the advantage of confirming successful arthroplasty with no heterotopic bone formation. This also allows for orthodontic preparation for concomitant TJR and orthognathic surgery. In the two-stage protocol, osteoarthrectomy/condylectomy along with interposition of medical-grade silastic as a spacer is performed at stage one, and in the second stage reconstruction of TMJ with alloplastic joint is done.
Preoperative Planning
It is always prudent to preoperatively plan the TJR on a virtual platform by importing the CT DICOM (Digital Imaging and Communications in Medicine) data from a recent CT scan acquired with an interslice thickness of 1 mm.
Custom 3D Printed Prosthesis
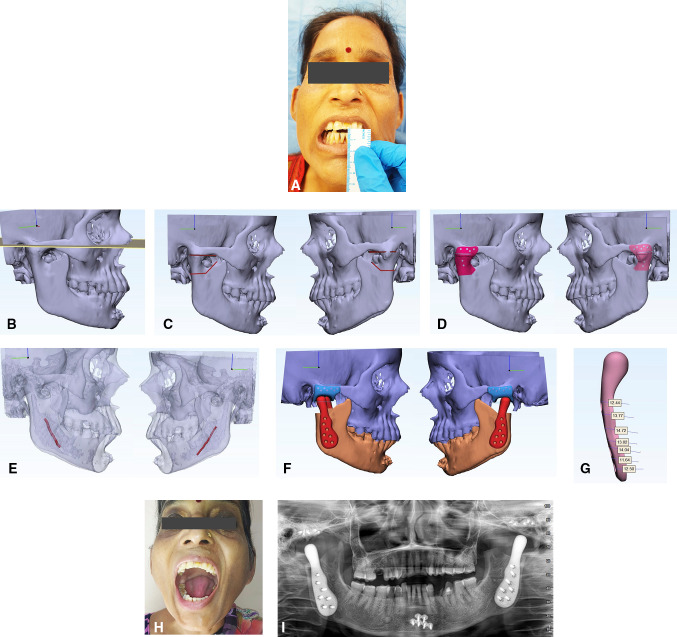
Custom devices are manufactured to fit the patient's mandible and zygomatic arch. Custom prosthesis requires considerable time for planning the design of the device. DICOM data are imported in MIMICS Materialize software 22.0 (Belgium). A virtual condylectomy is performed. The engineer in consultation with the surgeon jointly designs the fossa and the mandibular component for a snug fit according to the available anatomy. After 360-degree visualization of the fit of the designed fossa and the mandibular implant and satisfactory fit, a 3D model can be printed for handheld visualization and further modifications in design. The custom joint is ordered for manufacturing after the surgeon and engineering team are satisfied. The manufacturing can be done by computer numerical control (CNC) machining or by 3D printing. Planning for an ankylosed joint is slightly more cumbersome as there is a component of a superior bone cut and inferior bone cut. There is also precision required for the transfer of the virtually planned cuts to the operating table. Superior osteotomy is planned parallel to FH plane without encroaching the auditory canal and inferior osteotomy should be planned such that the inferior alveolar canal is not violated. During the planning of the inferior osteotomy cut, it should be kept in mind that the complete ankylotic mass should be removed and inferior osteotomy should be below the level of ankylotic mass. Cutting or positioning guides to help to transfer exact virtual planning to operating table [39–46]. Cutting guides are mostly used for custom-fitted joints. The cutting guide allows the subcondylar bone cut to be performed at the exact site planned virtually. Various types of cutting guides have been used. Some are fixed to the lateral surface or the posterior border and used to mark the areas of subcondylar osteotomy. We prefer to use fossa-based cutting guides, as the superior and inferior osteotomy cuts can be exactly transferred to the operative site with its use. A cutting guide with pre-drilled holes that coincides with the final fossa drill hole position is used. We print the guide in titanium with channels for piezo cutting exactly as planned in virtual surgery and ordered for the printing of the prosthesis. Removing the cutting guide after osteotomy allows the fossa to be fixed in the same holes that were used for guide fixation. Nerve mapping (to avoid injury to neurovascular bundle), screw mapping (to know the length of the screw), finite element analysis (to know the area of stress and strain) should be done in each case. A complete case of the custom-made prosthesis with planning is shown in Fig. 1a–i.
Fig. 1.
a Clinical preoperative picture showing 4-mm preoperative maximal incisal opening. b Drawing of Frankfort horizontal plane (the patient had maxillofacial trauma 5 years back, fixation was done at symphysis with two plates). c Planning of superior and inferior osteotomy on the right and left side. d A cutting guide for right and left side. e Nerve mapping on right and left side to avoid neurovascular injury. f Checking of fit and accuracy of the implant on a 3D virtual model on right and left side. g Screw mapping to place bicortical screws and to avoid neurovascular injury. h Follow-up maximal incisal opening. i Follow-up orthopantomogram showing bilateral total joint prosthesis in place
Stock Prosthesis
Checking the fit feasibility of the stock prosthesis on the virtual platform before the operation saves the embarrassment of non-fit intraoperatively. The stock implants available have limited sizes that may not be suitable for the Indian population. After importing the CT DICOM data of the patient to MIMICS Materialize 22.0 (Belgium), 3D reconstruction is done. Checking of fit feasibility of stock joint and fossa is the next step. If there is no ankylosis, a virtual condylectomy gap of 1.5-2 cm should be provided for the condylar head of the implant to be accommodated. The fossa would be, however, prepared intraoperative for a flat or tripod stability. Previously scanned and saved stereolithography (STL) file of standard Zimmer Biomet® prosthesis is imported to assess for a virtual fit and feasibility of the stock TMJ TJR. The imported STL file of the mandibular component is then virtually implanted on the patient mandible such that it is parallel to the posterior border of the ramus, in maximum contact with the ramus of the mandible and at least five fixation screws avoiding injury to the neurovascular bundle. The virtual image is rotated and viewed from all angles to see for superior-inferior, mediolateral fit. The areas, which would require intraoperative lateral ramus surface reshaping, should be noted. The surgeon should reconfirm the fit feasibility of the prosthesis from all angles. A complete case of a stock joint prosthesis is shown in Fig. 2a–g.
Fig. 2.
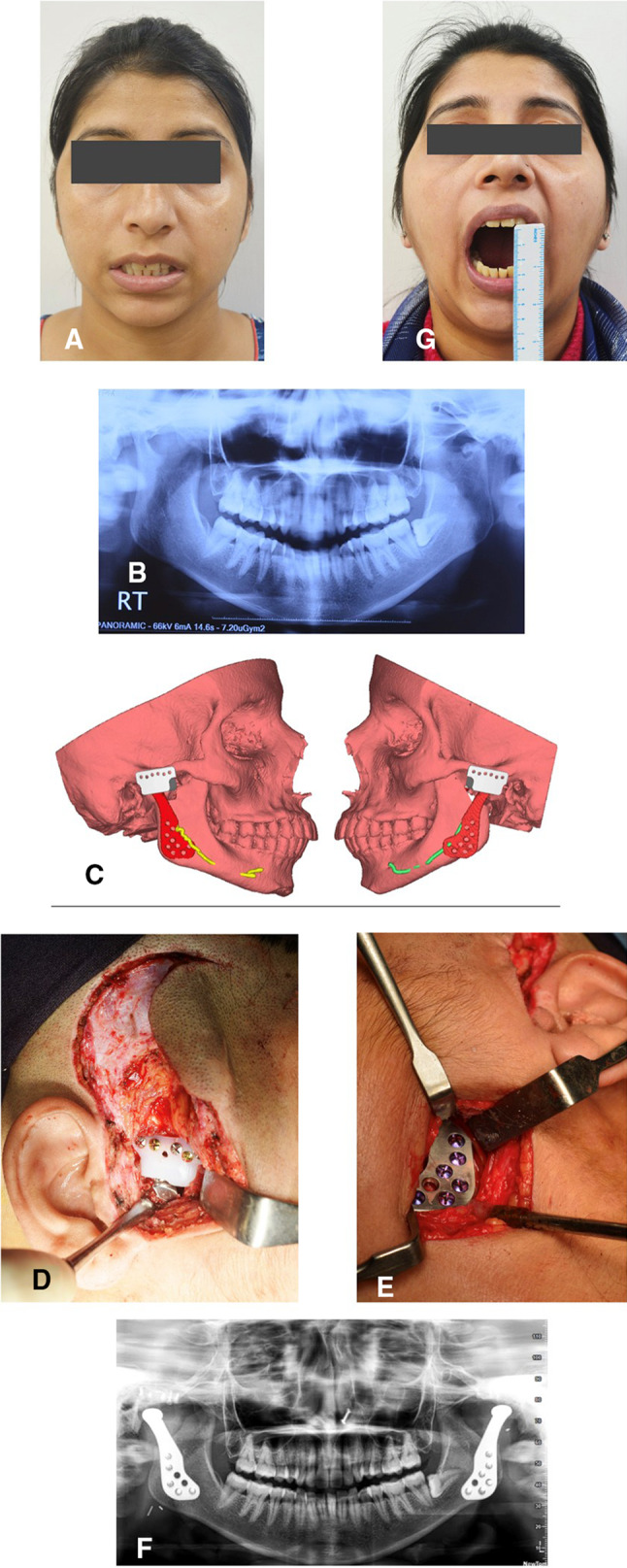
a Clinical preoperative picture showing nil mouth opening. b Preoperative orthopantomogram showing degenerative changes in the bilateral joint. c Checking of fit and accuracy of the implant. d Intraoperative picture showing placement of condyle in posterior-most of fossa on the right side. e Intraoperative picture showing the retromandibular approach to access ramus and fixation of ramal component on the left side. f Follow-up clinical picture showing adequate mouth opening. g Follow-up orthopantomogram showing bilateral total joint prosthesis in place
Surgical Consideration
Maintaining a strict aseptic surgical field is mandatory to avoid contamination. The authors advise isolation of eyes, mouth, and nares with iodine-impregnated incision drape (Fig. 3), vancomycin-soaked gauze in the ear, meticulous iodine preparation of oral cavity (if, intraoral coronoidectomy or concomitant TJR and orthognathic surgery is planned), change of surgeon scrub, and gloves every time the intraoral and extraoral site is approached. Though preauricular with extended temporal incision (Fig. 2d) is used to access the diseased condyle for osteoarthrectomy/condylectomy and preparation of the temporal bone for fossa component fit, a standard preauricular endaural incision (Fig. 4) should be used for improved cosmesis. Retromandibular/submandibular incision is used to expose the ramus and fixation of the mandibular component. The mastoid process, sternocleidomastoid muscle, and the posterior border of the mandible is marked. A 3-4-cm-long incision is placed halfway the distance of the anterior border of the sternocleidomastoid and posterior border of the mandible. The scant fibers of platysma are cut. This should enable entry to the fairly avascular zone between the anterior border of the sternocleidomastoid and the parotid gland. Further dissection should expose the posterior belly of the digastric muscle. This is the limit of the medial dissection. The terminal branch of the external carotid lies beneath the digastric and can be quickly accessed should any torrential hemorrhage is encountered during osteoarthrectomy. One may encounter the retromandibular vein or facial vein and artery. This should be adequately ligated. Placing a large retractor and lifting the posterior parotid will expose the pterygomassetric sling. This should be released to expose the lateral surface of the mandible, right up to the osteotomy from the lower border. This is enough to have a footplate of the mandibular component. The stock prosthesis will, however, need further bone flattening at places but the custom prosthesis can easily slip into position and be fixed using screws. Preservation of branches of the facial nerve is important during dissection. Conventionally fissure bur/saw is used for condylectomy, but the authors prefer to use the piezoelectric saw as it reduces the chances of heterotopic bone formation. Additionally, in an ankylosed joint, the piezo cutting results in a flat surface where fossa can be fixed without rocking. Where the anatomy of the fossa is maintained, a thick diamond bur is used to flattened the temporal bone to obtain tripod stability. When bur is used, copious saline irrigation is done to remove all the bone chips and slurry. This is an important step to reduce heterotopic bone formation [47, 48]. The fossa component should be parallel to FH plane with the anterior portion slightly inferior to the posterior to avoid anterior dislocation of the prosthesis. All the sharp bone, if any, should be removed. The occlusion of the patient should be secured with maxillomandibular fixation. During fixation of mandibular component, it should be kept in mind that the head of the condyle should be posterior in the fossa (allows some amount of pseudotranslation) [49]. Also, anterior positioning of the condyle in the fossa can lead to anterior dislocation of the condyle. Fossa should be secured with a 2.0-mm screw at zygomatic bone with preferably 4-5 screws. For the ramus component, the most proximal screw adjacent to ramal osteotomy is important. The mandibular component should be fixed using at least five bicortical screws. When using the stock prosthesis, care should be exercised to have the best possible fit of the mandibular component after reshaping the lateral ramus. Fat grafting should always be performed by mobilizing the nearby available buccal fat pad [50, 51] or abdominal fat pad [52–54]. The authors, however, prefer abdominal fat due its resistance to shrinkage. A rigorous postoperative mouth opening exercise for a period of at least 6 months is advised.
Fig. 3.
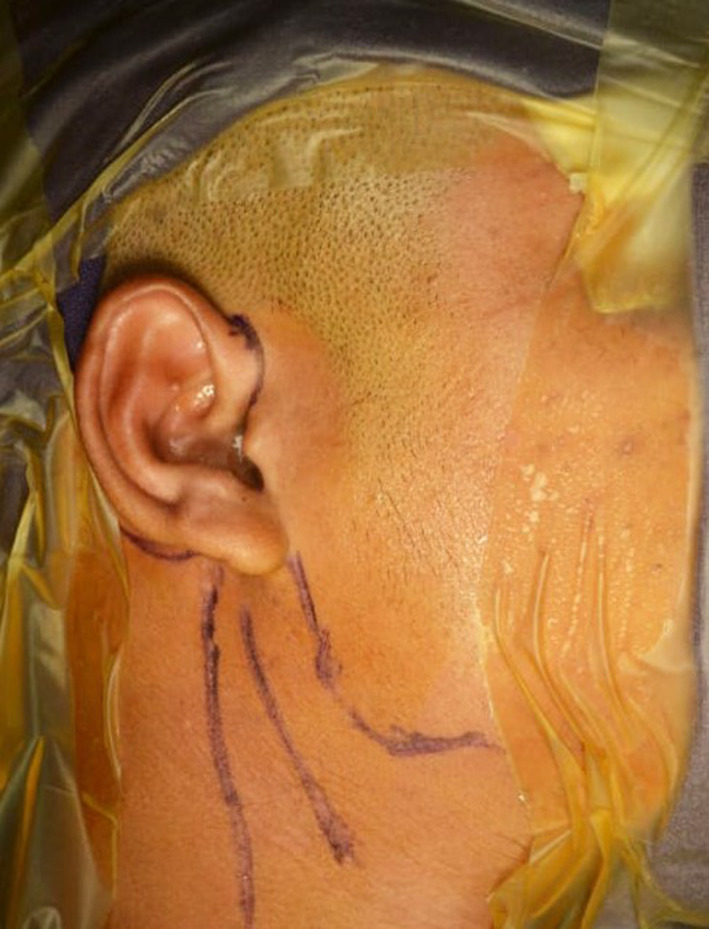
Iodine-based surgical drape for isolation of the surgical field essential to prevent periprosthetic joint infection
Fig. 4.
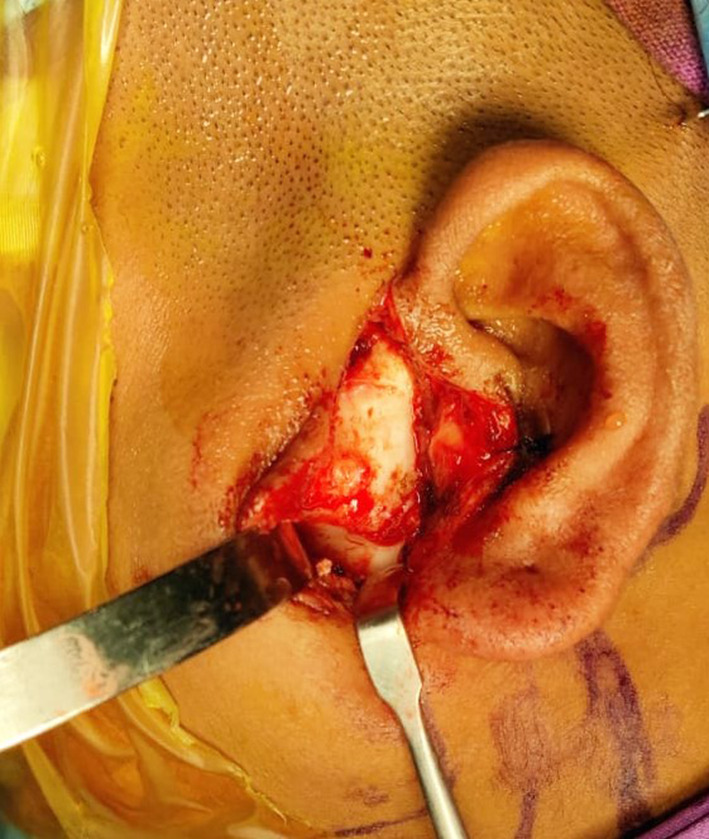
Preauricular endaural incision to expose temporomandibular joint for improved cosmesis
Discussion
Alloplastic joint replacement is well documented in orthopedics [55–57]. Approximately 330,000 hip and 720,000 knee replacements were performed in 2010 in the USA [58]. TMJ like all other joints like knee and hip can be affected by joint disorders making the replacement of joint a necessity. Alloplastic TMJ TJR can be considered as a definitive treatment protocol in an adult patient with end-stage TMJ disease [17]. Our clinical experience has shown promising results with the use of alloplastic TMJ TJR in TMJ replacement. The major advantage of alloplastic TJR is its resemblance to TMJ anatomy. It also allows immediate postoperative physiotherapy, when the chances of heterotopic bone formation are maximum. Alloplastic total joint prosthesis has been shown to yield better results when autogenous reconstruction of TMJ was compared with alloplastic prosthesis [59, 60].
A disease affecting the TMJ can lead to restricted mouth opening either by the formation of heterotopic bone in the joint area or affecting masticatory muscle thereby causing pain leading to inability to open mouth or chew normally. Adequate mouth opening is one of the important outcome factors when measuring the success of TMJ replacement. Successful results with adequate mouth opening have been reported following the use of alloplastic joint replacement [61–63]. One of the indications for alloplastic TMJ TJR is a recurrent disease as scar tissue does not allow capillaries to penetrate in multiple times operated cases leading to failure of autogenous graft [64]. Significant pain problems can be encountered related to fibrosis, calcifications, cervical neuropathy, residual inflammatory disease, or immunological problems to alloplastic material in multiply operated patients. Inflammatory disease of TMJ can lead to pain and abnormal sensation. Significant reduction in pain has been reported after the use of alloplastic total joint prosthesis [22, 65]. The cause of the pain, however, should be from the joint per se and not from the muscles for the replacement to be effective in alleviating pain.
End-stage TMJ disorder can result in structural changes that can affect all the functions of the jaw and esthetic of the patient [66]. As discussed, improvement in mouth opening is one of the most discussed parameters in disease affecting TMJ. Recently, attention has been paid to the importance of the reattachment of lateral pterygoid muscle [67]. Collin et al. [68] compared stock prosthesis joints with or without lateral pterygoid muscle reattachment and found improved protrusive movement in the joint where muscle reattachment was performed. Similar results were obtained by Mommaerts in three patients [69]. Recently Edward Zebovitz stated that restoring the function of lateral pterygoid can improve functions of the jaw along with a reduction in pain with the use of custom-made devices [67]. Zou et al. [70] have come out with a novel design of TMJ prosthesis for lateral pterygoid muscle attachment. They concluded that porous titanium scaffold structure in the condylar neck area can help in lateral pterygoid muscle re-attachment.
A significant advantage of alloplastic TJR lies in the fact that simultaneous orthognathic surgery can be performed. Concomitant orthognathic surgery may be required in patients with facial asymmetry, in patients with occlusal cant, or patients with marked mandibular undergrowth, and in OSA patients. The disadvantage of performing simultaneous TJR and orthognathic surgery is that it requires additional planning with greater surgical and technical expertise. In some TMJ diseases like TMJ ankylosis, restricted mouth opening can hinder the fabrication of splints. Splint-less orthognathic surgery with 3D-printed plates can be performed in these cases. Performing only orthognathic surgical procedures, in existing TMJ pathology, may lead to exacerbation of preexisting pathology. In the patients requiring both the surgical procedures, it can be staged. Both one-stage and two-stage TMJ TJR have their advantages and disadvantages. It is easy to use concomitant orthognathic surgery in unilateral cases. In bilateral cases, a large gap and significant advancement may jeopardize fixation with an adequate number of screws, especially in a stock prosthesis. In these patients, custom joints should be the preferred prosthesis. In cases, where simultaneous maxillary surgery and alloplastic TJR is required, the mandibular position should be determined first as the prosthesis cannot be adjusted to fit once the maxilla is moved, whereas minor adjustment of the maxilla is possible once the prosthesis is in situ.
Alloplastic replacement has been used in orthopedics in skeletally immature patients with successful outcome [71, 72]. The use of alloplastic TMJ TJR may hamper the growth of the mandible in skeletally immature patients and may require future revision surgery. Limited evidence is there in literature for alloplastic TMJ replacement in skeletally immature patients [7, 8, 73]. Recently, Sinn et al. have found encouraging outcomes of the mandibular function after TJR in growing patients. The authors are convinced that there will be no future harm to the growth of the mandible after TJR in the growing patient as their initial findings do demonstrate some growth [74].
Yadav et al. [47] in a recent publication explained strategies to reduce the chances of heterotopic bone formation in TMJ ankylosis patients. Recurrence can hamper the quality of life of patients [75]. Numerous evidence exists in the literature to support the use of autogenous fat graft around the alloplastic joint [2, 3, 47, 52–54, 62]. The authors prefer to measure the ankylotic mass through preoperative CT scan, complete removal of ankylotic mass by the use of a piezoelectric scalpel, parallel and inferior osteotomy at the narrowest part, copious irrigation with saline to remove all the bone slurry and chips, abdominal or buccal pad fat grafting around the alloplastic joint, use of vacuum drain and aggressive physiotherapy. Other methods reported in the literature to prevent heterotopic bone formation are the use of non-steroidal anti-inflammatory drugs (NSAIDs) and radiation. These methods have limited evidence and are associated with significant disadvantages [76, 77]. Selbong et al. [78] reported heterotopic bone formation in three patients (out of 3) requiring removal and reimplantation. On contrary to this, Sahdev et al. [79] reported heterotopic bone formation only in only 2.8% of patients.
Other complications of alloplastic stock and custom joint are derangement of occlusion, dislocation of the condyle, material hypersensitivity, foreign body reaction, periprosthetic joint infection, temporary or permanent facial nerve paralysis, component and/or fixation loosening, device failure, etc. Pain has been reported as the most common complication after 2 years in 40% of the patients after TMJ TJR [80]. Although rare, derangement of occlusion has been reported in 1% of the patient from UK data collected between 1994 and 2012 [81]. Our experience over 7 years also suggests minimal occlusal derangement even when concomitant orthognathic surgery with TJR is performed.
Along with the mechanical response of alloplastic prosthesis, biological response of the prosthesis like hypersensitivity to metal should be considered. Metal hypersensitivity can be a potential reason for pain after alloplastic TJR [82]. Although rare but material hypersensitivity with alloplastic TMJ replacement material can occur [83, 84]. Routine testing is not common currently. Different possible mechanisms have been discussed for metal hypersensitivity [85–94]. Diagnosis of metal hypersensitivity is considered as a diagnosis of exclusion [95]. Common causes of all symptomatic joints should be ruled out. In vivo skin patch test and in vitro lymphocyte transformation test have been advised in literature. History of intolerance to jewelry or any prior allergic reaction should always be asked [95]. Mercuri and Caicedo gave a protocol for the management of material hypersensitivity [95]. They advised to take an appropriate previous history of allergy to any metal preoperatively, and if reported, then allergen-free prosthesis should be considered. Postoperatively, if symptoms appear, then rule out all the other possible reason, and if metal hypersensitivity detected then observation and lymphocyte transformation test is advised. If positive, then the explantation is advised. There are fewer chances of wear and particulation after TMJ TJR as the functional load on TMJ is less than knee and hip joint. Westermark has reported that modern TMJ prosthesis (with condyle made up of Co–Cr–Mo and fossa made up of UHMWPE) function without foreign body reaction [96].
Coronoidectomy is usually performed in TMJ replacement. This can lead to dislocation of prosthesis especially where bilateral coronoidectomy has been performed [97, 98]. To avoid dislocation of prosthesis head, accurate alignment of the head of mandibular component is important particularly when simultaneous orthognathic surgery has been performed [99]. Mustafa and Sidebottom advised trying to dislocate the joint prosthesis intraoperatively; if the dislocation is noted, then the patient should be kept on light elastics for one week to prevent postoperative dislocation [100]. Relocation of the joint prosthesis is easier in these patients as compared to normal patients.
Prevention of periprosthetic joint infections (PJIs) is very important as the management of PJIs is a difficult task. PJIs have been reported after the use of alloplastic TJR [101]. Measures to prevent PJI [2] include preoperative 1 gm ceftriaxone and 600 mg clindamycin 1 h before the procedure, vancomycin-soaked gauze in ear, isolation of eyes, mouth, and nose by iodine-based drapes, meticulous iodine preparation of oral cavity if intraoral contralateral coronoidectomy or simultaneous orthognathic surgery is planned, change of gloves and scrub by the surgeon when sites (intraoral and extraoral) are changed and soaking of the implant in antibiotic solution.
Wolford et al. [17] found that the alloplastic TJR continued to function well after a follow-up of 21 years. Leandro et al. [18] concluded that alloplastic TJR is a safe and effective option for the reconstruction of TMJ. Onoriobe et al. [102] calculated the number of TMJ devices that will be required in 2030, based on data provided by one TMJ TJR manufacturer. They predicted a 65% increase in TMJ TJR device production. The maxillofacial surgeons should be well prepared in the coming years. TMJ TJR should be performed by a surgeon who is experienced in open joint surgery as TJR can be very unforgiving if not done correctly. From all the results in the literature and our experience, the authors can state that alloplastic total joint prosthesis can provide long-term stability, improvement in mandibular form and function, TMJ pain relief, and enhanced quality of life in end-stage TMJ diseases.
Conclusion
The authors conclude that alloplastic TMJ replacement is a successful and effective surgical option for the management of end-stage TMJ diseases. Though the custom joints have made the fit smooth and easy, thorough knowledge and experience in open joint surgery and the ability to interact with the technical team cannot be overstated. If replacement is performed with proper surgical and technical expertise, it results in an improvement in mouth opening and other mandibular functions, decrease in pain, correction in facial asymmetry, and improvement in the quality of life of patients. The knowledge of indication and surgical procedure of TMJ replacement is necessary for all surgeons dealing with the temporomandibular joint.
Key Points
TMJ replacement is an effective surgical option for the management of end-stage TMJ diseases in the hands of an experienced surgeon.
Patient selection, presurgical virtual planning ensures a proper fit and is essential for a successful outcome.
Strategies to prevent periprosthetic joint infection should be strictly followed.
The use of cutting guides helps in the exact transfer of virtual planning of cuts and position of prosthesis onto the operative table.
The stock prosthesis requires bone preparation for fitting, whereas custom prosthesis requires minimal host bone preparation.
The fossa and mandibular component should be fixed with five screws each.
Postoperative physiotherapy is essential for lasting success.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elgazzar RF, Abdelhady AI, Saad KA, Elshaal MA, Hussain MM, Abdelal SE, Sadakah AA. Treatment modalities of TMJ ankylosis: experience in Delta Nile. Egypt Int J Oral Maxillofac Surg. 2010;39(4):333–342. doi: 10.1016/j.ijom.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Roychoudhury A, Yadav P, Alagarsamy R, Bhutia O, Goswami D. Outcome of stock total joint replacement with fat grafting in adult temporomandibular joint ankylosis patients. J Oral Maxillofac Surg. 2021;79(1):75–87. doi: 10.1016/j.joms.2020.07.214. [DOI] [PubMed] [Google Scholar]
- 3.Roychoudhury A, Yadav P, Bhutia O, Mane R, Yadav R, Goswami D, Jose A. Alloplastic total joint replacement in the management of temporomandibular joint ankylosis. J Oral Biol Craniofac Res. 2021 doi: 10.1016/j.jobcr.2021.05.006(inpress). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercuri LG. Alloplastic temporomandibular joint reconstruction. Oral Surg Oral Med Oral Pathol. 1988;85:631–637. doi: 10.1016/S1079-2104(98)90028-2. [DOI] [PubMed] [Google Scholar]
- 5.Quinn PD (2000) Lorenz prosthesis. In: Donlon WC (ed) Total temporomandibular joint reconstruction. Oral Maxillofac Surg Clin N Am 12:93–104
- 6.Giannakopoulos HE, Sinn DP, Quinn PD. Biomet Microfixation Temporomandibular Joint Replacement System: a 3-year follow-up study of patients treated during 1995 to 2005. J Oral Maxillofac Surg. 2012;70(4):787–794. doi: 10.1016/j.joms.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri LG, Swift JQ. Considerations for the use of alloplastic temporomandibular joint replacement in the growing patient. J Oral Maxillofac Surg. 2009;67(9):1979–1990. doi: 10.1016/j.joms.2009.05.430. [DOI] [PubMed] [Google Scholar]
- 8.Keyser BR, Banda AK, Mercuri LG, Warburton G, Sullivan SM. Alloplastic total temporomandibular joint replacement in skeletally immature patients: a pilot survey. Int J Oral Maxillofac Surg. 2020;49(9):1202–1209. doi: 10.1016/j.ijom.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Swanson SAV, Freeman MAR. The scientific basis of joint replacement. New York: Wiley and sons; 1977. [Google Scholar]
- 10.Dumbleton JH. Tribology of natural and artificial joints. New York: Elsevier; 1981. [Google Scholar]
- 11.Carnochan JM (1860) Mobilizing a patient’s ankylosed jaw by placing a block of wood between the raw bony surfaces after resection. Archiv de Medicin. pp 284–286
- 12.Gluck T. Referat Uber die Durch das Moderne Chirurgische Experiment Gewonnenen Positiven Resultate, Betreffenddie Naht und den Ersatz von Defecten Hoerer Gewebe, Sowie Uber die Verwerthung Resorbirbarer und Bebendiger Tampons in der Chirgurie. Arch Klin Chir. 1891;4:186. [Google Scholar]
- 13.Christensen RW. The correction of mandibular ankylosis by arthroplasty and insertion of a cast vitallium glenoid fossa. Am J Orthop. 1963;48:16–24. [PubMed] [Google Scholar]
- 14.Saha S, Campbell CE, Sarma A, Saha S, Christensen RW. A biomechanical evaluation of the Christensen temporomandibular joint implant. Crit Rev Biomed Eng. 2000;28:399–403. doi: 10.1615/CritRevBiomedEng.v28.i34.320. [DOI] [PubMed] [Google Scholar]
- 15.Sidebottom AJ, Speculand B, Hensher R. Foreign body response around total prosthetic metal-on-metal replacements of the temporomandibular joint in the UK. Br J Oral Maxillofac Surg. 2008;46(4):288–292. doi: 10.1016/j.bjoms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri LG, Wolford LM, Sanders B, White RD, Hurder A, et al. Custom CAD/CAM total temporomandibular joint reconstruction system: preliminary multicenter report. J Oral Maxillofac Surg. 1995;53:106–115. doi: 10.1016/0278-2391(95)90381-X. [DOI] [PubMed] [Google Scholar]
- 17.Wolford LM, Mercuri LG, Schneiderman ED, Movahed R, Allen W. Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ concepts device. J Oral Maxillofac Surg. 2015;73:952–960. doi: 10.1016/j.joms.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Leandro LF, Ono HY, Loureiro CC, Marinho K, Guevara HA. A ten-year experience and follow-up of three hundred patients fitted with the Biomet/Lorenz Microfixation TMJ replacement system. Int J Oral Maxillofac Surg. 2013;42:1007–1013. doi: 10.1016/j.ijom.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Alagarsamy R, Roychoudhury A, Bhutia O, Lal B, Yadav R, Bhalla AS. Evaluation of fit feasibility of stock total joint replacement in temporomandibular joint ankylosis patients. Brit J Oral Maxillofac Surg. 2020 doi: 10.1016/j.bjoms.2020.11.016(inpress). [DOI] [PubMed] [Google Scholar]
- 20.De Meurechy N, Braem A, Mommaerts MY. Biomaterials in temporomandibular joint replacement: current status and future perspectives—a narrative review. Int J Oral Maxillofac Surg. 2017;47(4):518–533. doi: 10.1016/j.ijom.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Mercuri LG, Wolford LM, Giobbie-hurder A. Long-term follow-up of the CAD/CAM patient fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2002;60(12):1440–1448. doi: 10.1053/joms.2002.36103. [DOI] [PubMed] [Google Scholar]
- 22.Mercuri LG, Edibam NR, Giobbie-Hurder A. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2007;65(6):1140–1148. doi: 10.1016/j.joms.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Kanatas AN, Needs C, Smith AB, Moran A, Jenkins G, Worrall SF. Short-term outcomes using the Christensen patient-specific temporomandibular joint implant system: a prospective study. Br J Oral Maxillofac Surg. 2011;50(2):149–153. doi: 10.1016/j.bjoms.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Zou L, He D, Ellis E. Comparison of bone adaptation after modification in Biomet standard alloplastic temporomandibular joint prostheses. J Cranio-Maxillofacial Surg. 2018;46(10):1707–1711. doi: 10.1016/j.jcms.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Westermark A, Heden P, Aagaard E, Cornelius CP. The use of TMJ Concepts prostheses to reconstruct patients with major temporomandibular joint and mandibular defects. Int J Oral Maxillofac Surg. 2011;40(5):487–496. doi: 10.1016/j.ijom.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Elledge R, Mercuri L, Speculand B. Extended total temporomandibular joint replacements: a classification system. Br J Oral Maxillofac Surg. 2018;56(7):578–581. doi: 10.1016/j.bjoms.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Mercuri LG. Alloplastic temporomandibular joint replacement: rationale for the use of custom devices. Int J Oral Maxillofac Surg. 2012;41(9):1033–1040. doi: 10.1016/j.ijom.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Abramowicz S, Barbick M, Rose SP, Dolwick MF. Adaptability of stock TMJ prosthesis to joints that were previously treated with custom joint prosthesis. Int J Oral Maxillofac Surg. 2012;41(4):518–520. doi: 10.1016/j.ijom.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Biglioli F, Colletti G. Mini-retromandibular approach to condylar fractures. J Craniomaxillofac Surg. 2008;36(7):378–383. doi: 10.1016/j.jcms.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Johnson NR, Roberts MJ, Doi SA, Batstone MD. Total temporomandibular joint replacement prostheses: a systematic review and bias-adjusted meta-analysis. Int J Oral Maxillofac Surg. 2016;46(1):1–7. doi: 10.1016/j.ijom.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Saeed NR, Hensher R, McLeod NMH, Kent JN. Reconstruction of the temporomandibular joint autogenous compared with alloplastic. Br J Oral Maxillofac Surg. 2002;40(4):296–299. doi: 10.1016/S0266-4356(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 32.Elledge R, Attard A, Green J, et al. UK temporomandibular joint replacement database: a report on one-year outcomes. Br J Oral Maxillofac Surg. 2017;55(9):927–931. doi: 10.1016/j.bjoms.2017.08.361. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Perez LM, Gonzalez-Perez-Somarriba B, Centeno G, Vallellano C, Montes-Carmona JF. Evaluation of total alloplastic temporo-mandibular joint replacement with two different types of prostheses: a three-year prospective study. Med Oral Patol Oral Cir Bucal. 2016;21(6):e766–e775. doi: 10.4317/medoral.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolford LM, Dingwerth DJ, Talwar RM, Pitta MC. Comparison of 2 temporomandibular joint total joint prosthesis systems. J Oral Maxillofac Surg. 2003;61(6):685–690. doi: 10.1053/joms.2003.50112. [DOI] [PubMed] [Google Scholar]
- 35.Gerbino G, Zavattero E, Berrone S, Ramieri G. One stage treatment of temporomandibular joint complete bony ankylosis using total joint replacement. J Craniomaxillofac Surg. 2016;44(4):487–492. doi: 10.1016/j.jcms.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Haq J, Patel N, Weimer K, Matthews NS. Single stage treatment of ankylosis of the temporomandibular joint using patient-specific total joint replacement and virtual surgical planning. Br J Oral Maxillofac Surg. 2014;52(4):350–355. doi: 10.1016/j.bjoms.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Brown E, Wilson MH, Revington P. Single-stage temporomandibular joint arthroplasty in a patient with complete bony ankylosis and previous extradural haematoma. BMJ Case Rep. 2016 doi: 10.1136/bcr-2015-213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egemen O, Ozkaya O, Filinte GT, Uscetin I, Akan M. Two-stage total prosthetic reconstruction of temporomandibular joint in severe and recurrent ankylosis. J Craniofac Surg. 2012;23(5):e520–e524. doi: 10.1097/SCS.0b013e31825b5afd. [DOI] [PubMed] [Google Scholar]
- 39.Kraeima J, Merema BJ, Witjes MJH, Spijkervet FKL. Development of a patient- specific temporomandibular joint prosthesis according to the Groningen principle through a cadaver test series. J Cranio-Maxillofac Surg. 2018;46(5):779–784. doi: 10.1016/j.jcms.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Sembronio S, Tel A, Costa F, Isola M, Robiony M. Accuracy of custom-fitted temporomandibular joint alloplastic reconstruction and virtual surgical planning. Int J Oral Maxillofac Surg. 2019;48(8):1077–1083. doi: 10.1016/j.ijom.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Ma H, Jin S. One-stage treatment of giant condylar osteoma: alloplastic total temporomandibular joint replacement aided by digital templates. J Craniofac Surg. 2018;29(3):636–639. doi: 10.1097/SCS.0000000000004097. [DOI] [PubMed] [Google Scholar]
- 42.Patel A, Otterburn D, Saadeh P, Levine J, Hirsch DL. 3D volume assessment techniques and computer-aided design and manufacturing for preoperative fabrication of implants in head and neck reconstruction. Facial Plast Surg Clin North Am. 2011;19(4):683–709. doi: 10.1016/j.fsc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Jones R. The use of virtual planning and navigation in the treatment of temporomandibular joint ankylosis. Aust Dent J. 2013;58(3):358–367. doi: 10.1111/adj.12086. [DOI] [PubMed] [Google Scholar]
- 44.Bai G, He D, Yang C, Chen M, Yuan J, Wilson JJ. Application of digital templates to guide total alloplastic joint replacement surgery with biomet standard replacement system. J Oral Maxillofac Surg. 2014;72(12):2440–2452. doi: 10.1016/j.joms.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Bai G, Yang C, He D, Zhang X, Abdelrehem A. Application of fossa bone graft to stabilize stock total joint prosthesis in temporomandibular joint surgery. J Craniomaxillofac Surg. 2015;43(8):1392–1397. doi: 10.1016/j.jcms.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 46.Rhee SH, Baek SH, Park SH, Kim JC, Jeong CG, Choi JY. Total joint reconstruction using computer-assisted surgery with stock prostheses for a patient with bilateral TMJ ankylosis. Maxillofac Plast Reconstr Surg. 2019;41(1):41. doi: 10.1186/s40902-019-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav P, Roychoudhury A, Bhutia O. Strategies to reduce re-ankylosis in temporomandibular joint ankylosis patients. Brit J Oral Maxillofac Surg. 2021 doi: 10.1016/j.bjoms.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Jose A, Nagori SA, Virkhare A, Bhatt K, Bhutia O, Roychoudhury A. Piezoelectric osteoarthrectomy for management of ankylosis of the temporomandibular joint. Br J Oral Maxillofac Surg. 2014;52(7):624–628. doi: 10.1016/j.bjoms.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Voiner J, Yu J, Deitrich P, Chafin C, Giannakopoulos H. Analysis of mandibular motion following unilateral and bilateral alloplastic TMJ reconstruction. Int J Oral Maxillofac Surg. 2011;40(6):569–571. doi: 10.1016/j.ijom.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Ibikunle AA, James O, Adeyemo WL. Buccal Fat Pad for Interpositional Arthroplasty in Temporomandibular Joint Ankylosis. J Maxillofac Oral Surg. 2019;18(3):382–387. doi: 10.1007/s12663-018-1130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rattan V. A simple technique for use of buccal pad of fat in temporomandibular joint reconstruction. J Oral Maxillofac Surg. 2006;64(9):1447–1451. doi: 10.1016/j.joms.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Wolford LM, Karras SC. Autologous fat transplantation around temporomandibular joint total joint prostheses: preliminary treatment outcomes. J Oral Maxillofac Surg. 1997;55(3):245–252. doi: 10.1016/S0278-2391(97)90535-8. [DOI] [PubMed] [Google Scholar]
- 53.Wolford LM, Morales-Ryan CA, Morales PG, Cassano DS. Autologous fat grafts placed around temporomandibular joint total joint prostheses to prevent heterotopic bone formation. Proc (Bayl Univ Med Cent) 2008;21(3):248–254. doi: 10.1080/08998280.2008.11928404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roychoudhury A, Acharya S, Bhutia O, Seith Bhalla A, Manchanda S, Pandey RM. Is there a difference in volumetric change and effectiveness comparing pedicled buccal fat pad and abdominal fat when used as interpositional arthroplasty in the treatment of temporomandibular joint ankylosis? J Oral Maxillofac Surg. 2020;78(7):1100–1110. doi: 10.1016/j.joms.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 56.NICE . Total hip replacement and resurfacing arthroplasty for end stage arthritis of the hip. NICE technology appraisal guidance 304. London: National Institute for Health and Care Excellence; 2014. [Google Scholar]
- 57.Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647–654. doi: 10.1016/S0140-6736(18)31665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Hospital Discharge Survey (2010) www.cdc.gov/nchs/fastats/inpatientsurgery.html
- 59.Mercuri LG. Costochondral graft versus total alloplastic joint for temporomandibular joint reconstruction. Oral Maxillofac Surg Clin North Am. 2018;30(3):335–342. doi: 10.1016/j.coms.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Dimitroulis G. Temporomandibular joint surgery: what does it mean to India? J Maxillofac Oral Surg. 2012;11(3):249–257. doi: 10.1007/s12663-012-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolford L, Movahed R, Teschke M, Fimmers R, Havard D, Schneiderman E. Temporomandibular joint ankylosis can be successfully treated with TMJ concepts patient-fitted total joint prosthesis and autogenous fat grafts. J Oral Maxillofac Surg. 2016;74(6):1215–1227. doi: 10.1016/j.joms.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 62.Sidebottom AJ, Gruber E. One-year prospective outcome analysis and complications following total replacement of the temporomandibular joint with the TMJ Concepts system. Br J Oral Maxillofac Surg. 2013;51(7):620–624. doi: 10.1016/j.bjoms.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Mercuri LG, Ali FA, Woolson R. Outcomes of total alloplastic replacement with periarticular autogenous fat grafting for management of re-ankylosis of the temporomandibular joint. J Oral Maxillofac Surg. 2008;66(9):1794–1803. doi: 10.1016/j.joms.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Mercuri LG. Total joint reconstruction–autologous or alloplastic. Oral Maxillofac Surg Clin North Am. 2006;18(3):399–410. doi: 10.1016/j.coms.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Wolford LM, Mehra P. Custom-made total joint prostheses for temporomandibular joint reconstruction. Proc (Bayl Univ Med Cent) 2000;13(2):135–138. doi: 10.1080/08998280.2000.11927656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou L, He D, Ellis E. A comparison of clinical Follow-Up of Different total temporomandibular joint replacement prostheses: A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg. 2018;76:294. doi: 10.1016/j.joms.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 67.Zebovitz E. Total Temporomandibular Joint Prosthetic Reconstruction: The Importance of Lateral Pterygoid Muscle Reattachment to Lateral Excursive and Protrusive Mandibular Movement. J Oral Maxillofac Surg. 2021;79(6):1191–1194. doi: 10.1016/j.joms.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Collins CP, Wilson KJ, Collins PC. Lateral pterygoid myotomy with reattachment to the condylar neck: An adjunct to restore function after total joint reconstruction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:672. doi: 10.1067/moe.2003.153. [DOI] [PubMed] [Google Scholar]
- 69.Mommaerts MY. On the reinsertion of the lateral pterygoid tendon in total temporomandibular joint replacement surgery. J Craniomaxillofac Surg. 2019;47:1913. doi: 10.1016/j.jcms.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Zou L, Zhong Y, Xiong Y, He D, Li X, Lu C, Zhu H. A Novel Design of Temporomandibular Joint Prosthesis for Lateral Pterygoid Muscle Attachment: A Preliminary Study. Front Bioeng Biotechnol. 2021;8:630983. doi: 10.3389/fbioe.2020.630983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bessette BJ, Fassier F, Tanzer M, Brooks CE. Total hip arthroplasty in patients younger than 21 years: a minimum, 10-year follow- up. Can J Surg. 2003;46:257–262. [PMC free article] [PubMed] [Google Scholar]
- 72.Wroblewski BM, Purbach B, Siney PD, Fleming PA. Charnley low-friction arthroplasty in teenage patients: the ultimate challenge. J Bone Joint Surg Br. 2010;92:486–488. doi: 10.1302/0301-620X.92B4.23477. [DOI] [PubMed] [Google Scholar]
- 73.Sidebottom AJ. Alloplastic or autogenous reconstruction of the TMJ. J Oral Biol Craniofac Res. 2013;3:135–139. doi: 10.1016/j.jobcr.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sinn DP, Tandon R, Tiwana PS. Can alloplastic total temporomandibular joint reconstruction be used in the growing patient? A preliminary report. J Oral Maxillofac Surg. 2021 doi: 10.1016/j.joms.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 75.Lindqvist C, Soderholm AL, Hallikainen D, Sjövall L. Erosion and heterotopic bone formation after alloplastic temporomandibular joint reconstruction. J Oral Maxillofac Surg. 1992;50:942–950. doi: 10.1016/0278-2391(92)90051-Z. [DOI] [PubMed] [Google Scholar]
- 76.Bhatt K, Pandey S, Bhutia O, Roychoudhury A. Use of indomethacin as an adjuvant to surgery for recurrent temporomandibular joint ankylosis in adults. Natl J Maxillofac Surg. 2014;5(2):198–201. doi: 10.4103/0975-5950.154836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60(3):888–895. doi: 10.1016/j.ijrobp.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Sahdev R, Wu BW, Anderson N, Khawaja SN, Kim S, Keith DA. A Retrospective Study of Patient Outcomes After Temporomandibular Joint Replacement With Alloplastic Total Joint Prosthesis at Massachusetts General Hospital. J Oral Maxillofac Surg. 2019;77(2):280–288. doi: 10.1016/j.joms.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Selbong U, Rashidi R, Sidebottom A. Management of recurrent heterotopic ossification around total alloplastic temporomandibular joint replacement. Int J Oral Maxillofac Surg. 2016;45(10):1234–1236. doi: 10.1016/j.ijom.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 80.Machoň V, Levorová J, Hirjak D, Drahoš M, Brizman E, Beňo M, Foltán R. Evaluation of complications following stock replacement of the temporomandibular joint performed between the years 2006 and 2015: a retrospective study. Oral Maxillofac Surg. 2020;24(3):373–379. doi: 10.1007/s10006-020-00840-z. [DOI] [PubMed] [Google Scholar]
- 81.Idle MR, Lowe D, Rogers SN, et al. UK temporomandibular joint replacement database: Report on baseline data. Br J Oral Maxillofac Surg. 2014;52:203. doi: 10.1016/j.bjoms.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Hallab N. Metal sensitivity in patients with orthopaedic im- plants. J Clin Rheumatol. 2001;7:215. doi: 10.1097/00124743-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Foussereau J, Laugier P. Allergic eczemas from metallic foreign bodies. Trans St Johns Hosp Dermatol Soc. 1966;52:220. [PubMed] [Google Scholar]
- 84.Basketter DA, Briatico-Vangosa G, Kaestner W, Lally C, Bontinck WJ. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis? Contact Dermatitis. 1993;28(1):15–25. doi: 10.1111/j.1600-0536.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 85.Cadosch D, Chan E, Gautschi OP, Simmen HP, Filgueira L. Bio-corrosion of stainless steel by osteoclasts–in vitro evidence. J Orthop Res. 2009;27(7):841–846. doi: 10.1002/jor.20831. [DOI] [PubMed] [Google Scholar]
- 86.Arnholt CM, MacDonald DW, Malkani AL, Klein GR, Rimnac CM, Kurtz SM; Implant Research Center Writing Committee, Kocagoz SB, Gilbert JL (2016) Corrosion damage and wear mechanisms in long-term retrieved CoCr femoral components for total knee arthroplasty. J Arthroplasty. 31(12): 2900–2906 [DOI] [PMC free article] [PubMed]
- 87.Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010;134(6):886–893. doi: 10.1309/AJCPLTNEUAH8XI4W. [DOI] [PubMed] [Google Scholar]
- 88.Mercuri LG, Urban RM, Hall DJ, Mathew MT. Adverse Local Tissue Responses to Failed Temporomandibular Joint Implants. J Oral Maxillofac Surg. 2017;75(10):2076–2084. doi: 10.1016/j.joms.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 89.Thomas P, Summer B, Sander CA, Przybilla B, Thomas M, Naumann T. Intolerance of osteosynthesis material: evidence of dichromate contact allergy with concomitant oligoclonal T-cell infiltrate and TH1-type cytokine expression in the peri-implantar tissue. Allergy. 2000;55(10):969–972. doi: 10.1034/j.1398-9995.2000.00619.x. [DOI] [PubMed] [Google Scholar]
- 90.Summer B, Paul C, Mazoochian F, et al. Nickel (Ni) allergic patients with complications to Ni containing joint replacement show preferential IL-17 type reactivity to Ni. Contact Dermatitis. 2010;63(1):15–22. doi: 10.1111/j.1600-0536.2010.01744.x. [DOI] [PubMed] [Google Scholar]
- 91.Nich C, Takakubo Y, Pajarinen J, et al. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101(10):3033–3045. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nich C, Goodman SB. Role of macrophages in the biological reaction to wear debris from joint replacements. J Long Term Eff Med Implants. 2014;24(4):259–265. doi: 10.1615/JLongTermEffMedImplants.2014010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013;31(10):1633–1642. doi: 10.1002/jor.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumazawa R, Watari F, Takashi N, Tanimura Y, Uo M, Totsuka Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials. 2002;23(17):3757–3764. doi: 10.1016/S0142-9612(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 95.Mercuri LG, Caicedo MS. Material hypersensitivity and alloplastic temporomandibular joint replacement. J Oral Maxillofac Surg. 2019;77(7):1371–1376. doi: 10.1016/j.joms.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 96.Westermark A. Total reconstruction of the temporomandibular joint. Up to 8 years of follow-up of patients treated with Biomet® total joint prostheses. Int J Oral Maxillofac Surg. 2010;39(10):951–955. doi: 10.1016/j.ijom.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Gruber EA, McCullough J, Sidebottom AJ. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg. 2015;53(5):412–415. doi: 10.1016/j.bjoms.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Murdoch B, Buchanan J, Cliff J. Temporomandibular joint replacement: a New Zealand perspective. Int J Oral Maxillofac Surg. 2014;43(5):595–599. doi: 10.1016/j.ijom.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Mercuri GL. The role of custom-made prosthesis for temporomandibular joint replacement. Revista Española de Cirugía Oral y Maxilofacial. 2013;35(1):1–10. doi: 10.1016/j.maxilo.2012.02.003. [DOI] [Google Scholar]
- 100.el Mustafa M, Sidebottom A. Risk factors for intraoperative dislocation of the total temporomandibular joint replacement and its management. Br J Oral Maxillofac Surg. 2014;52(2):190–192. doi: 10.1016/j.bjoms.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 101.Mercuri LG, Psutka D. Perioperative, postoperative, and prophylactic use of antibiotics in alloplastic total temporomandibular joint replacement surgery: a survey and preliminary guidelines. J Oral Maxillofac Surg. 2011;69(8):2106–2111. doi: 10.1016/j.joms.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Onoriobe U, Miloro M, Sukotjo C, Mercuri LG, Lotesto A, Eke R. How many temporomandibular joint total joint alloplastic implants will be placed in the United States in 2030? J Oral Maxillofac Surg. 2016;74(8):1531–1538. doi: 10.1016/j.joms.2016.04.011. [DOI] [PubMed] [Google Scholar]