Abstract
The aim of the study was to provide accurate information regarding live-born infant survival after diagnosis of fatal fetal anomaly (FFA) to aid decision-making in respect of pregnancy management, and to ascertain the natural history of live-born infants with FFAs via a retrospective analysis of death records (2006–2018), from the National Paediatric Mortality Registry (source Central Statistics Office 2019). Diagnoses and survival times were ascertained from narrative records with further ascertainment and reconciliation of trisomies 13 and 18 cases by review of cytogenetic test records, the National Death Events Register and National Perinatal Epidemiology Centre data. During the study period, termination of pregnancy was not permitted under the Irish Constitution. Patients are live-born babies with fatal fetal anomalies whose deaths were registered in the Republic of Ireland. The main outcome measure was construction of anomaly-specific survival curves, or survival time range and median for those anomalies with rare occurrence. Survival curves for anencephaly, bilateral renal agenesis, thanatophoric dysplasia, trisomy 13, and trisomy 18 show that 90% (n = 95), 93% (n = 60), 100% (n = 14), 37% (n = 92) and 33% (n = 162), respectively, were deceased by 24 h and 98%, 100%, 100%, 73%, and 53%, respectively, by 1 week post-delivery. Survival time range and median were calculated for triploidy (3.5 h–20 days; 10.5 days), whose occurrence was rare. Anhydramnios, craniorachischisis, hydranencephaly and severe hydrocephalus were extremely rare and all deaths were neonatal deaths. Our results provide 13 years of national natural history data of live birth FFA survival. This provides objective information to aid obstetric counselling of couples upon diagnosis of an FFA.
Keywords: Fatal fetal abnormality, Live-born survival, Thanatophoric dysplasia, Trisomy 13, Trisomy 18, Anencephaly, Renal agenesis
Introduction
Decision-making following an antenatal diagnosis of a fatal fetal anomaly (FFA) is anguishing. Objective information on all possible outcomes, including live birth prognosis, is required to aid couples to make an informed choice (Pergament and Pergament 2012). Termination of pregnancy is now legalised in the Republic of Ireland (ROI) (Royal College of Physicians 2019) and Northern Ireland (UK Public General Acts 2019) with termination past 12 weeks of pregnancy permitted for ‘a condition affecting the fetus that is likely to lead to the death of the fetus either before, or within 28 days of, birth’ (Royal College of Physicians 2019). It is important to provide detailed information about longevity in live births diagnosed with a FFA for families; we therefore present national data to aid counselling.
Prior to 2019, termination of pregnancy was not permitted under the Irish Constitution. Irish couples electing to terminate a pregnancy affected by a FFA had to self-fund travel abroad. Practical, financial and cultural considerations have led to a high percentage of couples opting to continue with their pregnancy after FFA diagnosis. During the study period, supportive management and palliative care for FFAs have been and continue to be the norm in the Republic of Ireland. Similarly, in Northern Ireland prior to 2020, termination of pregnancy was only available if continuing the pregnancy put the health of the mother at risk, all other women seeking abortion services had to travel (Department of Health, Social Services and Public Safety 2016).
In a national review of Irish maternity ultrasound providers in 2016, Hayes-Ryan et al. (2017) found that 64% of women receive a 2nd trimester anomaly scan and 47% receive a first trimester ultrasound, with significant regional variation. Their findings were consistent with similar studies in 2007 and 2012, which coincides with our study period. A variety of independent national data sources (Irish National Paediatric Mortality Registry 2020; National Paediatric Epidemiology Centre 2020) capture national data on live-born babies, their diagnosis and survival. It is thus possible to establish estimates of birth prevalence and survival in live-borns with FFAs in a relatively unbiased population sample.
The introduction of new termination legislation highlights the requirement for national data to enable couples in their decision-making. This study addresses the question ‘If my baby with a fatal fetal anomaly is born alive, how long are they likely to live?’.
Methods
Data sources were reviewed to obtain live-born survival information on all FFAs listed in the ‘Interim clinical guidance on pathway for management of fatal fetal anomalies and/or life-limiting conditions during pregnancy’ (Royal College of Physicians 2019): diagnoses considered to be fatal are listed in Table 1, and comparable internationally (Wilkinson et al. 2012). The National Paediatric Mortality Register was used as our primary data source as it provides more accurate data for survival time analysis of early neonatal deaths.
Table 1.
Frequency and birth prevalence of live-born fatal fetal abnormalities (2006–2018) from all data sources. Fetal anomalies are those listed as ‘fatal fetal anomalies’ in Interim clinical guide on pathway for management of fatal fetal anomalies and/or life-limiting conditions during pregnancy: Termination of Pregnancy2
| Diagnosis | N | Birth prevalence (per 10,000) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Anencephaly | 95 | 1.06 | 0.87 | 1.30 |
| Trisomy 13 | 119 | 1.33 | 1.11 | 1.59 |
| Trisomy 18 | 173 | 1.93 | 1.66 | 2.24 |
| Renal agenesis (Potters) | 60 | 0.67 | 0.52 | 0.86 |
| Anhydramnios caused by multicystic kidneys | 10 | 0.11 | 0.06 | 0.21 |
| Dysplastic kidneys or infantile polycystic kidney disease | 18 | 0.20 | 0.13 | 0.32 |
| Thanatophoric dysplasia | 14 | 0.16 | 0.09 | 0.26 |
| Hydrancephaly | < 5 | 0.04 | 0.02 | 0.12 |
| Triploidy | < 5 | 0.04 | 0.02 | 0.12 |
| Craniorachischisis/exencephaly/iniencephaly | < 5 | 0.01 | 0.00 | 0.08 |
| Congenital severe hydrocephalus with absent or minimal brain growth | < 5 | 0.03 | 0.01 | 0.10 |
| Severe skeletal dysplasia and severe osteogenesis imperfecta | 14 | 0.16 | 0.09 | 0.26 |
| Non-immune hydrops with major cardiac defect | 15 | 0.17 | 0.10 | 0.28 |
| Inoperable conjoined twins | < 5 | 0.04 | 0.02 | 0.12 |
| Total | 534 | 5.95 | 5.47 | 6.48 |
Data sources
National Paediatric Mortality Register (NPMR)
This was the primary data source used for all FFAs. Complete annual data from 2006 to 2018 was available for analysis. Details of deaths in children, registered in Republic of Ireland, were obtained by the NPMR CHI at Temple Street. Data is provided to the NPMR from the Central Statistics Office (CSO) (2019) on a quarterly basis in the form of encrypted microdata files. The data fields reviewed for each case included date of death, survival time (in minutes), hospital, ICD code and cause of death narrative text from death certificate.
Other data sources used for trisomy 13 and trisomy 18 reconciliation
Other data sources (cytogenetics reports, death records, National Pediatric Epidemiology Centre) were used to capture cases of trisomy 13 and trisomy 18 as live-born babies with these conditions are discharged into the community where the death registration narrative may not always capture the underlying diagnosis accurately.
Cytogenetic test reports
All karyotypes on neonates born in the ROI are analysed at the cytogenetics department of Children’s Health Ireland (CHI) at Crumlin. The cytogenetic database was queried for all results in the study period matching for ‘ + 18’ or ‘ + 13’. A list of trisomy 13 and trisomy 18 was compiled; cases with mosaicism were excluded from this study to ensure consistency, as mosaic aneuploidy may lead to a milder phenotype which would not be consistent with a fatal fetal anomaly. The data fields used for each case included name, date of birth, date of death (where recorded), hospital, date sample received, date report produced, report result text and home address. Survival was calculated as the number of days between date of birth and date of death.
Death Events Search of the General Registration Office (GRO)
Registration details of all deaths in Ireland are collected by the GRO on behalf of the Minister for Health. This database was used to confirm date of deaths for trisomies 13 and 18 cases identified from cytogenetics and NPMR records. The data fields used for each case included name, date of birth, date of death, hospital and home address. Survival time was calculated as the number of days between date of birth and date of death.
National Paediatric Epidemiology Centre (NPEC)
A report of causes of death in perinatal mortality (up to 28 days) in the Irish Republic is generated annually from anonymized data from all 19 maternity units on deaths arising from births occurring > 500 g birthweight or at > 24+0 weeks’ gestation. Complete data for 2011–2015 was provided for this study. Data fields used for each case included date of birth, date of death, hospital, cytogenetic test performed, chromosomal disorder and cause of death narrative text. Survival time was calculated as the number of days between date of birth and date of death.
For the few cases where no death records were found in our data sources, additional enquiries were made to assess the child’s status. The referring neonatologists of record were asked to review the hospital notes for evidence of documentation of follow-up in hospital of birth or transfer to paediatric care. Hospital information systems in the two participating tertiary paediatric hospitals were searched to look for recent or scheduled episodes of care.
Analysis
Survival curves for five common FFA were constructed using survival times (in minutes) captured by NPMR.
Following further review of additional data sources for cases of trisomies 13 and 18, totals for each FFA over the 13-year period were reported, birth prevalence per 10,000 livebirths and 95% confidence intervals were calculated. Numeric values with counts of less than 5 are not given, in order to avoid disclosure and as a requirement of GDPR. For the five common FFA, median survival time and percentage survivors, with 95% Kaplan–Meier confidence intervals, were calculated at 1, 7, 28, 90, 180 and 365 days.
Results
During the study period of January 1, 2006, to December 31, 2018, 896,888 livebirths were recorded in the ROI by the CSO. A total of 534 unique cases of FFA were recorded during this time period. The number and birth prevalence of each FFA are shown in Table 1.
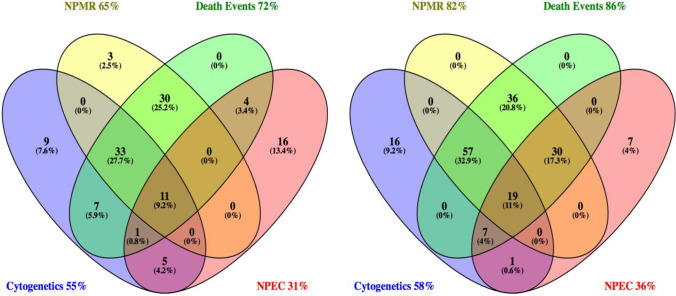
The most complete source of ascertainment for trisomies 13 and 18 was the CSO Death Events Search, confirming death of 72% of trisomy 13 and 91% of trisomy 18 cases during the study period, as shown in Fig. 1. The contribution of each dataset to the total cases illustrated by Venn diagrams shows that only 9–11% of cases were found in all four datasets, and that the dataset with the lowest yield of cases—NPEC—still independently detected 13.4% of trisomy 13 cases and 4% of trisomy 18 cases.
Fig. 1.
Ascertainment sources for trisomies 13 and 18. NPMR National Paediatric Mortality Register; Death Events, Death Events Search of the General Registration Office; Cytogenetics, cytogenetic laboratory results; NPEC, National Paediatric Epidemiology Centre. a Trisomy 13 N=119. b Trisomy 18 N=173
Survival curves were constructed for trisomy 13, trisomy 18, anencephaly, bilateral renal agenesis and thanatophoric dysplasia for those cases with survival data from NPMR, as shown in Fig. 2a–e. Inclusion of only cases with survival time derived from NPMR data, with a survival time in minutes, allowed the most accurate portrayal of death events occurring at less than 24 h.
Fig. 2.
Survival curves for infants live-born with anencephaly, renal agenesis, trisomy 13, trisomy 18 and thanatophoric dysplasia, 2006–2018, using data collected from the National Paediatric Mortality Register. a Anencephaly, N = 95. b Renal agenesis, N = 60. c Thanatophoric dysplasia, N = 14. d Trisomy 13, N = 92. e Trisomy 18, N = 162. f Trisomy 18 N = 170, trisomy 13 N = 119, full survival. g Survival curves by type of fatal fetal anomaly, truncated at 28 days
An additional survival curve with more information regarding longer-term survival (Fig. 2f) for trisomies 13 and 18 cases was constructed using all datasets, with early survival recorded in days or parts thereof rather than minutes. Included in this analysis were four trisomy 13 cases with no date of death, known to have survived > 1 year (range 1.5–13 years) from medical records. Three cases of trisomy 18 had no date of death in any data source, and their information was reviewed. One had died by the age of 5 months but no precise date of death was known. Two had been discharged home to palliative care in the newborn period; for these, the diagnosis of trisomy 18 was clinically apparent at birth and subsequently confirmed by cytogenetics. For the two discharged home with no confirmation of death, there were no records of further neonatal or paediatric hospital care, or transfer to other hospitals, in contrast to long-term survivors where episodes of hospital care were captured. These three cases were excluded from the survival analysis, but included in the calculation of birth prevalence. Survival analysis calculated with data from all sources, by intervals, from data in Fig. 2 is shown in Table 2. A summary curve (Fig. 2g) of survival to 28 days was calculated using all data. The survival rate of live-born infants with an FFA to 28 days is 21.6% (C.I. 14.9–27.0%) for trisomy 18, 20.2% (C.I. 13.5–27.8%) for trisomy 13 and 0% for anencephaly, renal agenesis and thanatophoric dysplasia.
Table 2.
Kaplan–Meier survival estimate of mortality in the first year of life by type of birth defect, Republic of Ireland, 2006–2018. For data initially reported in minutes or hours, ‘1 day’ was considered survival to 24 h, or 1440 min. This data represents only those infants live-born and does not include cases antenatally diagnosed, or diagnoses made in miscarriages or stillborn infants
| Survival estimates (%, Kaplan–Meier 95% confidence interval) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of birth defect | N | Median survival | 1 day | CI | 7 days | CI | 28 days | CI | 90 days | CI | 180 days | CI | 1 year | CI |
| Trisomy 18 | 170 | 3 days | 61.2 | 53.4–68.0 | 38.2 | 31.0–45.5 | 20.6 | 14.9–27.0 | 5.9 | 3.0–10.1 | 2.4 | 0.8–5.5 | 0.6 | 0.05–2.3 |
| Trisomy 13 | 119 | 3 days | 63.0 | 53.7–71.0 | 30.3 | 22.3–38.6 | 20.2 | 13.5–27.8 | 12.6 | 7.4–19.2 | 8.4 | 4.3–14.2 | 5.9 | 2.6–11.1 |
| Anencephaly | 95 | 107 min | 10.5 | 5.4–17.6 | 2.1 | 0.4–6.7 | 0 | – | – | – | – | – | – | – |
| Renal agenesis | 60 | 101 min | 6.6 | 2.1–14.8 | 0 | – | – | – | – | – | – | – | – | – |
| Thanatophoric dysplasia | 14 | 90 min | 0 | – | – | – | – | – | – | – | – | – | – | – |
Discussion
The review of lethal congenital malformations from Wilkinson et al. (2012) is one of the data sources referenced for the published Irish guidance (Royal College of Physicians 2019). Their published prevalence of renal agenesis, anencephaly, thanatophoric dysplasia, trisomies 13 and 18, holoprosencephaly and hydrancephaly (Wilkinson et al. 2012) are in line with our data presented in Table 1.
It is recognized from previous Irish studies that the risk of fetal loss during a pregnancy and delivery after diagnosis of FFA is high, 44% (Murphy et al. 2020), 46% (Barry et al. 2015) and 54% (Houlihan and O’Donoghue 2013) for trisomy 13; 52% (Houlihan and O’Donoghue 2013) and 57% (Murphy et al. 2020) for trisomy 18; 58% for anencephaly (Obeidi et al. 2010). Burke et al. (2013) reported gestational time-dependent fetal loss in trisomy 18, with an 88% versus 44% fetal loss rate depending on diagnosis made prior to, or after, 20 weeks’ gestation. Our study, unlike other smaller Irish studies (Barry et al. 2015; Burke et al. 2013; Houlihan and O’Donoghue 2013; Murphy et al. 2020; Obeidi et al. 2010), did not collect fetal loss data as this was not available on a national basis.
Our live-born survival data cannot be used in isolation to estimate whether a fetal anomaly should be classified as a fatal fetal anomaly, as shown in our extrapolations in Table 2, it is essential to consider fetal loss aspect of these disorders. Cavadino and Morris (2017) calculate that, when diagnosed at 12–14 weeks (excluding terminations of pregnancy), 49% of pregnancies with trisomy 13 and 70% with trisomy 18 will undergo fetal demise. If these percentages are applied to our live birth data, it would suggest that, for pregnancies diagnosed at 12–14 weeks, the chance of survival to 28 days and 1 year would be 6.2% and 0.2% for trisomy 18, and 10.3% and 3.0% for trisomy 13 respectively. This derived survival from early pregnancy to 28 days and 1 year is much lower than the 20% and 0.6% (trisomy 18) and 20% and 5.9% (trisomy 13) that we predicted by looking at only the cohort of live-born babies (Table 2), and would be much more in line with survival of other conditions considered at fatal fetal abnormalities. It is known that despite the definition of fatal fetal anomalies causing death by 28 days, it is recognized that for some of the FFAs, survival beyond 28 days can occur (Wilkinson et al. 2012).
However, our study does provide valuable data to answer the question ‘If my baby is born alive, how long are they likely to live’. Management of some of the FFA is more active in a number of jurisdictions and this will be reflected in potentially different survival data. Currently, and throughout the study period, supportive management for babies with FFA is the norm in the Republic of Ireland. Populations where termination of pregnancy for FFA is prevalent can be limited in their ability to capture a sufficient number of live-born rare-event diagnoses to make any predictions on longevity of live-born FFAs. Also, case reports of live-born children with fatal fetal abnormalities can be biased towards long survivors, limiting their generalizability.
Obeidi et al. (2010) give a median survival time of 55 min (range 10 min to 8 days) for babies with anencephaly in their regional Irish study. This smaller sample size is consistent with our finding of 107 min, with the longest survivor living 13 days. Nguyen et al. (2018) report a similar median survival of 1 day.
Sawai et al. (2019) report survival in infants with thanatophoric dysplasia: 100% of 25 infants without respiratory intervention survived ≤ 2 days, and 66% of 24 infants with mechanical ventilation survived > 1 year: our results would be comparable to the non-interventional group in this report.
Nguyen et al. (2018) report a median survival of 1 day and a 1 month survival of 21% for infants with renal agenesis, in contrast to our study’s median survival of 101 min and 0% survival at 1 week. However, Nguyen’s cohort was actively managed (including peritoneal dialysis) compared with conservative management in our cohort. Nguyen et al. (2018) also acknowledge that their dataset under-ascertained deaths occurring in the first day of life, skewing their results towards long survival.
A large-scale, active surveillance of births (Vendola et al 2010) reported trisomy 18 survival of 52% at 1 week, 30% at one month and 3% at 1 year, with a median survival of 7 days. For trisomy 13, they report survival of 58% at 1 week, 20% at 1 month and 3% at 1 year with a median survival of 4.5 days. These survival figures are longer than we report in Table 2; however, the authors report skewed results towards longer survivors due to missing data on very short-term survivors.
The large-scale study of Nguyen et al. (2018) also recognized underreporting of very short-term survivors in their reporting: trisomy 18 survival of 64% at 1 week, 46% at 1 month and 17% at 1 year, with a median survival of 20 days. For trisomy 13, they report survival of 59% at 1 week, 37% at 1 month and 18% at 1 year with a median survival of 12 days. They also ascribe their longer survival rates to case selection by ICD9 coding which would include mosaic aneuploidy in the analysis.
Meyer et al. (2015) reported American registry data yielding trisomy 18 survival of 52% at 1 week, 37% at 1 month and 12% at 1 year, with a median survival of 8 days. For trisomy 13, they report survival of 43.6% at 1 week, 26.0% at 1 month and 10% at 1 year with a median survival of 5 days. Their figures are higher than those in other publications or than we report, but they suggest that the bias to long-term survivors was due to the inclusion of infants with mosaic aneuploidy and a study group more likely to terminate pregnancies with more severe phenotypes.
Studies with multisource ascertainment in one region of England in the 1990s estimated the prevalence of trisomy 18 at 1.2 per 10,000 live births (Embleton et al. 1996) at 0.49 per 10,000 live births for trisomy 13 (Wylie et al 1994). These are significantly lower than those obtained from our study of 1.8 (C.I. 1.6–2.1) and 1.0 (C.I. 0.8–1.3) respectively. This could be attributable both to differing aneuploidy risks due to maternal age differences, as well as differing legal status of terminations of pregnancy in the jurisdictions.
Our data give an overall minimum estimate of 5.5 (C.I. 5.0–6.0) live-born babies with a fatal fetal anomaly per 10,000 live births equating to approximately 33 per year in Ireland. There are additional cases with potentially life-limiting conditions (PLLC) diagnosed antenatally that would be considered on a case-by-case basis. These babies and their families require adequate resources for a supportive approach to decision-making and palliation (O’Donoghue 2019).
Strengths and limitations
One limitation of this study is the risk of missing cases, highlighted in the discrepancies in number of cases detected by different national data sources for cases of trisomy 13 and trisomy 18. We are also aware, of babies with FFA diagnoses incorrectly coded, from a publication during our study interval (McCarthy and O’Donoghue 2014). The need for ascertainment of rare disease cases through multiple data sources in Irish hospital systems has specifically been highlighted by Lefter et al. (2014). The independence of hospital outpatient records, lack of a unique health identifier and lack of centralised electronic health care records are contributing factors and should serve as a caution to other researchers to not be reliant on a single source of data.
With respect to possible unrecognized longevity of cases (as we have sourced our data through death records), our ongoing 18-year national cohort study of paediatric rare diseases with multisource ascertainment has not identified any infants with thanatophoric dysplasia, renal agenesis surviving past 1 month and identified only one outlier case of anencephaly, surviving until 6 weeks (E. Gunne, personal communication). Nor have we found other long-term survivors with any other fatal fetal anomalies in the 13 years of NPMR mortality data in this study.
Our initial analysis of trisomies 13 and 18 (Fig. 2d–e) solely based on NPMR data biases our study towards earlier mortality for trisomies 13 and 18. Whilst this analysis compromises our sample size, it yields more accurate data under 48 h of life, with capture of survival time in minutes, which has not been possible in some other studies. This study bias was the premise for redoing the analysis with data from all sources (Fig. 2f).
This analysis is not intended to present evidence for refining the definition of what conditions constitute a fatal fetal abnormality within the Irish guidance, as clinical information at the time of diagnosis (both antenatally and postnatally) plays a central part in defining the outlook for the pregnancy (Power et al 2021) For example, it is recognized internationally that whilst the majority of babies with trisomy 13 or trisomy 18 will not survive to birth, but from conception to soon after birth, there are a minority that survive long term. Survival curves such as those we have constructed, along with clinical information, can help inform decision-making when couples are faced with a potential FFA diagnosis in pregnancy. Whilst this study does not provide information about the chance that a couple will have a live-born baby following a prenatal diagnosis of a FFA, it does provide information about the likelihood of how long a baby may live if they are live-born. It is also important to recognize that parents go home with babies with trisomies 13 and 18 and are told to expect the baby will die within days/weeks and whilst bereavement services are in place, long-term medical follow-up and services are often not planned. However, our figures show that a minority of babies can survive for > 1 year and those parents can feel very isolated and a care plan may need to be put in place (Boland 2016).
Generalizability of the birth prevalence and survival data from this study to other health services will depend on access to antenatal diagnostics, and legislative and societal acceptance of termination of pregnancy for fetal anomalies.
The strength of the study remains the consistency of the national practices across the study population throughout the entire study period: access rates for antenatal ultrasound, inaccessibility of legal abortion services within the Republic of Ireland and the conservative management of live-born babies with fatal fetal anomalies. All data sources were consistent across the study period, resulting in comparable data over time. Our data on a larger series provides detailed survival times which should help couples make an informed decision should a FFA be diagnosed in a pregnancy.
Conclusion
The historic cultural environment that existed in Ireland has allowed us to produce this data. Overall, in Ireland, 6 live-born infants per 10,000 have fatal fetal anomalies, and resources should be identified for palliative support for these infants and their families. There are very rare long-term survivors of non-mosaic trisomy 13 and trisomy 18. Our results provide detailed natural history data of fatal fetal anomaly live-born survival in the ROI over a 13-year period, which will aid obstetric counselling.
Acknowledgements
We would like to thank Dr. Jan Miletan, Dr. Breda Hayes, Dr. Niamh Ni Shuibne, Mr. Paul Corcoran, Ms. Mary Cunniffe and Ms. Rita Kavanagh for their help in this study. We thank the patients and staff of the maternity units in Ireland who have contributed to the National Perinatal Epidemiology Centre. We are grateful to the individual units for their participation in the NPEC national clinical audits and the data coordinators at unit level who facilitate data submission. We thank the NPEC, the NPEC Governance Committee and the NPEC Perinatal Mortality Group which facilitate this work.
Author contribution
All authors made substantial contributions to this work.
EAG: study design, data collection, interpretation and analysis and manuscript preparation.
SAL: study design, data interpretation and analysis and manuscript preparation.
CMcG: data collection and manuscript preparation.
KH: data collection and manuscript preparation.
DML: study design, data interpretation and analysis and manuscript preparation.
Funding
This study was part of a larger study for which we received grant support (RPAC 17–06) from the Temple Street Foundation, Dublin, Ireland. The funder had no role in the study design, execution, analysis or manuscript preparation. The research was undertaken at Temple Street Children’s University Hospital, Dublin.
Data availability
Original data is not available as it captures rare patient events that might be identifiable despite being anonymised.
Declarations
Ethics approval
Permission for this project was obtained as a health researcher in adherence with the Statistics Act, 1993, which allows access to more detailed vital statistics to those engaged in medical or social research.
Conflict of interest
Emer Gunne, Sally Ann Lynch, Cliona McGarvey, Karina Hamilton and Deborah Lambert have no competing interests to declare. ‘Results are based on analysis of strictly controlled Research Microdata Files provided by the Central Statistics Office (CSO). The CSO does not take any responsibility for the views expressed or the outputs generated from this research’.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barry SC, Walsh CA, Burke AL, McParland P, McAuliffe FM, Morrison JJ. Natural history of fetal trisomy 13 after prenatal diagnosis. Am J Med Genet A. 2015;167A:147–150. doi: 10.1002/ajmg.a.36824. [DOI] [PubMed] [Google Scholar]
- Boland R (2016) Caring for our disabled daughter: I fear for our marriage. https://www.irishtimes.com/life-and-style/health-family/caring-for-our-disabled-daughter-i-fear-for-our-marriage-1.2880564. Accessed October 2020
- Burke AL, Field K, Morrison J. Natural history of fetal trisomy 18 after prenatal diagnosis. Arch Dis Child Fetal Neonatal Ed. 2013;98:F152–F154. doi: 10.1136/archdischild-2011-301589. [DOI] [PubMed] [Google Scholar]
- Cavadino A, Morris JK. Revised estimates of the risk of fetal loss following a prenatal diagnosis of trisomy 13 or trisomy 18. Am J Med Genet. 2017;173:953–958. doi: 10.1002/ajmg.a.38123. [DOI] [PubMed] [Google Scholar]
- Central Statistics Office https://www.cso.ie. Accessed May 2019)
- Department of Health, Social Services and Public Safety (2016) Guidance for HSC professionals on termination of pregnancy in Northern Ireland, March 2016. https://www.health-ni.gov.uk/publications/guidance-hsc-professionals-termination-pregnancy-northern-ireland. Accessed March 2020
- Embleton ND, Wyllie JP, Wright MJ, Burn J, Hunter S. Natural history of trisomy 18. Arch Dis Child. 1996;75:F38–F41. doi: 10.1136/fn.75.1.F38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes-Ryan D, McNamara K, Russell N et al (2017) Maternity ultrasound in the Republic of Ireland 2016: a review. Irish Med J 110(7). https://www.lenus.ie/handle/10147/622508. Accessed January 2020 [PubMed]
- Houlihan OA, O’Donoghue K. The natural history of pregnancies with a diagnosis of trisomy 18 or trisomy 13; a retrospective case series. BMC Pregnancy Childbirth. 2013;13:209. doi: 10.1186/1471-2393-13-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish National Paediatric Mortality Registry: Available on https://www.hiqa.ie/areas-we-work/health-information/data-collections/national-paediatric-mortality-register. Accessed January 2020
- Lefter S, Hardiman O, Ryan A. Methodology and design of a national epidemiological study on adult neuromuscular disease. Methods Neuroepidemiol. 2014;43:123–130. doi: 10.1159/000367634. [DOI] [PubMed] [Google Scholar]
- McCarthy CM, O’Donoghue K. Conjoined twins: experience in an Irish tertiary centre. J Obstet Gynaecol. 2014;34(3):225–228. doi: 10.3109/01443615.2013.835305. [DOI] [PubMed] [Google Scholar]
- Meyer ER, Liu G, Gilboa S, Ethen MK, et al. Survival of children with trisomy 13 and trisomy 18: a multi-state population based study. Am J Med Genet A. 2015;170A:825–837. doi: 10.1002/ajmg.a.37495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NC, Dunne H, Flood K. Prenatally diagnosed fetal aneuploidy: natural history and subsequent management. Ir Med J. 2020;113(3):34–40. [PubMed] [Google Scholar]
- National Paediatric Epidemiology Centre. https://www.ucc.ie/en/npec/. Accessed February 2020
- Nguyen JE, Salemi JL, Tanner JP, Kirby RS, et al. Survival and healthcare utilization of infants diagnosed with lethal congenital malformations. J Perinatol. 2018;38:1674–1684. doi: 10.1038/s41372-018-0227-3. [DOI] [PubMed] [Google Scholar]
- Obeidi N, Russell N, Higgins JR, O’Donoghue K. The natural history of anencephaly. Prenat Diagn. 2010;30(4):357–360. doi: 10.1002/pd.2490. [DOI] [PubMed] [Google Scholar]
- O’Donoghue K (2019) Pathway for management of fatal fetal anomalies and/or life-limiting conditions diagnosed during pregnancy – perinatal palliative care http://52.164.250.231/eng/about/who/acute-hospitals-division/woman-infants/bereavement-care/pathway-for-perinatal-palliative-care.pdf. Accessed March 2020
- Pergament E, Pergament D. Reproductive decisions after fetal genetic counselling. Best Pract Res Clin ObGyn. 2012;26:517–529. doi: 10.1016/j.bpobgyn.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Power S, Meaney S, O’Donoghue K. Fetal medicine specialist experiences of providing a new service of termination of pregnancy for fatal fetal anomaly: a qualitative study. BJOG. 2021;128(4):676–684. doi: 10.1111/1471-0528.16502. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians of Ireland (2019) Interim clinical guidance on pathway for management of fatal fetal anomalies and/or life-limiting conditions during pregnancy: termination of pregnancy, January 2019. https://rcpi-live-cdn.s3.amazonaws.com/wp-content/uploads/2019/01/IOG-TOPFA-PATHWAY-FINAL-180119.pdf. Accessed November 2019
- Sawai H, Oka K, Ushioda M, et al. National survey of prevalence and prognosis of thanatophoric dysplasia in Japan. Peds Internat. 2019;61:748–753. doi: 10.1111/ped.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Public General Acts (2019) Northern Ireland (Executive Formation etc) Act 2019. https://www.legislation.gov.uk/ukpga/2019/22/enacted. Accessed October 2020
- Vendola C, Canfield M, Daiger SP, et al. Survival of Texan infants born with trisomies 21, 18 and 13. Am J Med Genet A. 2010;152A:360–366. doi: 10.1002/ajmg.a.33156. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Thiele P, Watkins A, De Crespigny L. Fatally flawed? A review and ethical analysis of lethal congenital malformations. British J Obs Gyn. 2012;119:1302–1308. doi: 10.1111/j.1471-0528.2012.03450.x. [DOI] [PubMed] [Google Scholar]
- Wylie JP, Wright J, Burn J, Hunter S. Natural history of trisomy 13. Arch Dis Child. 1994;71:343–345. doi: 10.1136/adc.71.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data is not available as it captures rare patient events that might be identifiable despite being anonymised.