Abstract
Purpose
The diagnostic criteria of allergic fungal rhinosinusitis focus on characteristic clinical, radiographic, histopathologic findings and immunologic characteristics of the disease. None of these are useful for a prompt outpatient diagnosis of the condition. No clear endoscopic signs (pathognomonic) of polyps in allergic fungal rhinosinusitis are mentioned in the literature.
Objective
The objective of this study is to describe and evaluate the sensitivity and specificity of an endoscopic sign the intrapolypoidal white particles for the diagnosis of allergic fungal rhinosinusitis in outpatient setting.
Methodology
In a descriptive, cross-sectional study, 46 chronic rhinosinusitis patients were examined by endoscope in the outpatient clinic. The endoscopic images of the nasal polypi were captured preoperatively. During endoscopic surgery, a sample of nasal polypi was taken for fungal staining and culture. Results of histopathology were compared to the impression of rhinologist on the images of nasal polypi captured preoperatively.
Results
The most common endoscopic features were the expansion of sinus (24, 52.2%) and intrapolypoidal white particles (50%). Intrapolypoidal white particles were calculated to have 85.71% sensitivity, 65.63% specificity, 52.17% positive predictive value, 91.3% negative predictive value and 71.74% diagnostic accuracy.
Conclusion
This study offers a new endoscopic sign, intrapolypoidal white particles for diagnosing allergic fungal rhinosinusitis.
Keywords: Allergic fungal rhinosinusitis, AFRS, Nasal polypi, Endoscopic sign
Introduction
Allergic fungal rhinosinusitis (AFRS) is a noninvasive variant of recurrent chronic rhinosinusitis with nasal polyposis. It is considered to be an immunologically mediated inflammation in the presence of an extramucosal fungal antigen within the sinus cavity [1–7]. AFRS comprises approximately 5–10% of chronic rhinosinusitis (CRS) patients [8]. Patients commonly present with chronic rhinosinusitis with nasal polyps, inhalant atopy, elevated total serum immunoglobulin E (IgE) [1, 9]. The involved sinuses are usually obstructed with inspissated brown or greenish black allergic mucin. The extramucosal “peanut buttery” allergic mucin is laden with intact and degenerating eosinophils, Charcot–Leyden crystals, cellular debris and sparse fungal hyphae [1–3, 10, 11]. Dematiaceous fungi such as Bipolaris spicifera or Curvularia lunata, or Aspergillus species such as A. Fumigatus, flavus or niger are pathogen most frequently isolated from the allergic mucin [1, 7, 8]. However, up to 13% of AFRS fungus is not detected in culture despite histopathologic confirmation [7]. AFRS is highly destructive to the bone of the nose and paranasal sinuses.
Over the years, AFS has been an underdiagnosed clinical entity. The diagnostic criteria of allergic fungal rhinosinusitis (AFRS) focus on the combination of characteristic clinical, radiographic, histopathologic findings and immunologic characteristics of the disease [12–14]. This makes it rather difficult to diagnose AFRS in the outpatient setting. Endoscopic examination which is part of routine physical examination in outpatient setting can provide an early means to diagnose allergic fungal rhinosinusitis.
AFRS is a common disease in Saudi Arabia. In our long-term clinical experience, we have noticed the presence of multiple white particles on the nasal polyps of patients who were later diagnosed to have AFRS. The aim of this study is to determine the sensitivity and specificity of these particles for the diagnosis of AFRS.
We did extensive literature search by the keywords “Allergic Fungal Rhinosinusitis (AFRS)” and “allergic fungal sinusitis (AFS)” focusing on these white particles, but we did not find this sign mentioned in any of the papers. We anticipate that these intrapolypoidal white particles (IWP) could provide a basis for future endoscopic diagnostic criteria for early and prompt diagnosis of AFRS in the outpatient setting.
Methods
A descriptive, cross-sectional study was conducted in the Department of Otorhinolaryngology, Head and Neck Surgery. Research was approved by the institutional review board. A total of 46 clinically diagnosed chronic rhinosinusitis patients were recruited who fulfilled the following inclusion criteria: nasal polyposis, not responding to medical therapy and potential candidate for functional endoscopic sinus surgery (FESS) and no history of oral steroid 1 moth prior surgery, no history of previous endoscopic sinus surgery. Non-probability, purposive sampling technique was used in the current study.
Informed consent was obtained from all patients. Examination and illustration of nasal polyps were performed, and images of the nasal polypi were captured preoperatively. Hadley’s clinical scoring system of nasal polyposis was applied to grade the nasal polypi according to the criteria mentioned below:
Grade 1: smallest size polyps within the middle meatus not reaching the inferior edge of the middle turbinate.
Grade 2: polyps within the middle meatus reaching the inferior border of the middle turbinate.
Grade 3: polyps extending into the nasal cavity below the edge of the middle turbinate but not below the inferior edge of the inferior turbinate.
Grade 4: polyps filling up the nasal cavity.
During the endoscopic surgery, a sample of nasal polypi were taken for both the fungal staining and culture in normal saline and for the histopathologic assessment in 10% formalin. All the samples were delivered to the laboratory within the first hour of the surgery. Special fungal staining technique using Grocott methenamine silver (GMS) was used to identify the fungal evidence. Results of fungal staining, culture and histopathology were compared to the impression of rhinologist on the images of nasal polypi captured preoperatively. Images were reported as fungal or non-fungal rhinosinusitis on the basis of the presence of the intrapolypoidal white particles (IWP). To reduce the bias, the images were sent randomly to the rhinologist. His decisions were compared to the final histopathology and fungal stain and the presence of mucin. The final preoperative and postoperative results were analyzed.
Information about history, preoperative examination and illustration of nasal polyps was recorded in the questionnaire. Decision of rhinologist on the images and reports of fungal staining, culture and histopathology were also documented.
The data were analyzed with Statistical Package for Social Sciences (SPSS) version 19. The frequency and percentages were calculated for nominal and categorical variables, whereas mean ± standard deviation (SD) was calculated for numerical or continuous variables. Tests of statistical significance were not required for the study. To calculate the sensitivity, specificity and other characteristics of IWP as diagnostic test the results were tabulated in a 2 × 2 table format using SPSS (Table 1).
Table 1.
Performance of intrapolypoidal white particles as a diagnostic sign for AFRS 2 × 2 table
| Intrapolypoidal white particles | AFRS (Fungal stain) | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Present | 12 | 11 | 23 |
| Absent | 2 | 21 | 23 |
| Total | 14 | 32 | 46 |
The sensitivity was defined as percentage of patients with AFRS who had IWP in preoperative endoscopic images. The specificity was defined as the percentage of patients who did not carry a clinical diagnosis of AFRS along with the absence of IWP in preoperative endoscopic images. Positive predictive value was defined as the percentage of patients with IWP who actually had AFRS. Negative predictive value was defined as the percentage of patients who neither had IWP nor AFRS.
Results
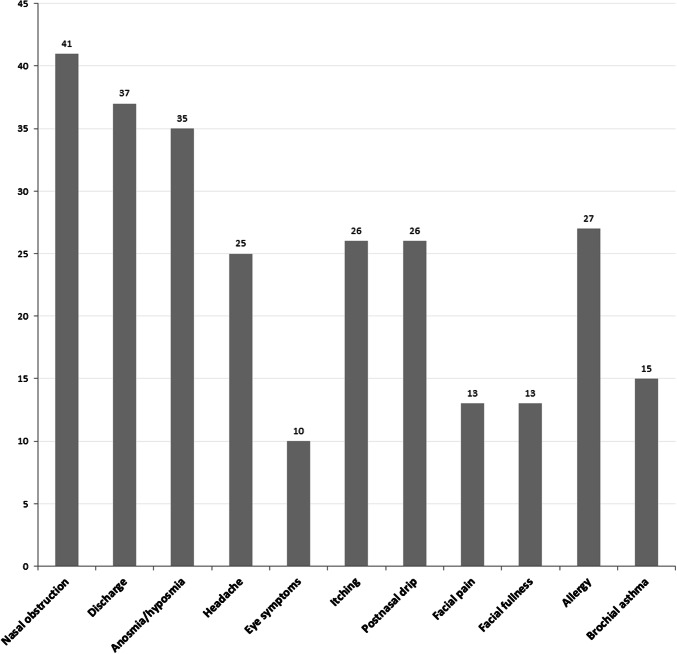
All of the 46 patients had nasal polyposis with chronic rhinosinusitis (32, 69.6%) or allergic fungal rhinosinusitis (14, 30.4%). AFRS was diagnosed based on the Bent and Khun criteria (8), the presence of nasal polyposis, CT characteristics, the presence of mucin and positive GMS stain for fungi. Table 2 describes the baseline characteristics of the study participants. Briefly, the mean age was 33.91 ± 14.71 (95% CI = 29.44–38.38) years and majority were males 28 (60.86%). Nasal obstruction was the most common symptom present in 93.2% patient followed by discharge (84.1%) and anosmia/hyposmia (79.5%) as shown in Fig. 1. Twenty-six patients (56.5%) presented with bilateral nasal polyps. According to Hadley’s clinical scoring system, half (23, 50%) of the patients had grade II nasal polypi.
Table 2.
Demographic and pathological characteristic of study population
| Age-group (years) | Frequency (%) |
|---|---|
| 11–20 | 07 (15.2) |
| 21–30 | 20 (43.5) |
| 31–40 | 05 (10.9) |
| 41–50 | 06 (13.0) |
| ≥ 51 | 08 (17.4) |
| Gender | |
| Male | 28 (60.86) |
| Female | 18 (39.78) |
| Pathological tests | |
| Fungal stain | 14 (30.4%) |
| Culture | 11 (23.9%) |
| Histopathology | 17 (37.0%) |
Fig. 1.
Symptoms of AFRS in study population
The most common endoscopic findings observed in study population were intrapolypoidal white particles (IWP) (23, 50.0%) as presented in Table 3. The most common abnormality observed in CT scan was the expansion of sinus (24, 52.2%) and heterogeneous opacifications (19, 41.3%).
Table 3.
Endoscopic and CT findings in patients with AFRS
| Endoscopic findings | Number (%) |
|---|---|
| Intrapolypoidal white particles | 23 (50%) |
| Mucin | 16 (34.8%) |
| CT findings | |
| Expansion of sinus | 24 (52.2%) |
| Heterogeneous opacifications | 19 (41.3%) |
| Asymmetric sinus | 16 (34.8%) |
| Bone erosion | 15 (32.6%) |
| Displacement of adjacent structures | 09 (19.5%) |
Fungus was visualized by staining in 14 (30.4%) of patient specimen, 11 (23.9%) were culture positive and 17 (37.0%) demonstrated characteristic histopathologic features. The remaining patients showed negative results on all three laboratory modalities (Table 2).
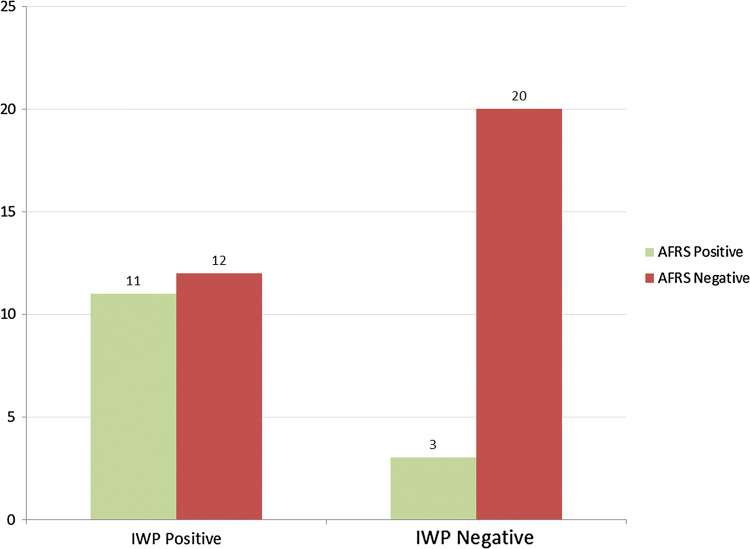
IWP were seen in 23 (50%) of the patients out of which 12 (26.1%) were diagnosed as AFRS on a positive GMS stain and 11 (23.9%) were diagnosed as chronic rhinosinusitis (CRS) on a negative GMS stain. IWP were absent in 23 (50%) of the patients out of which only 2 (4.3%) were diagnosed as AFRS, whereas 21 (45.7%) were diagnosed as CRS as shown in Fig. 2.
Fig. 2.
Relationship between the presence and absence of intrapolypoidal white particles and diagnosis of AFRS. IWP—intrapolypoidal white particles, AFRS—allergic fungal rhinosinusitis
During the endoscopic visualization white patches on the polyps were observed by the consultant. The presence of IWP was 85.71% sensitive and 65.63% specific for the diagnosis of AFRS. The positive predictive value (PPV) of IWP for diagnosing AFRS was 52.17%, and negative predictive value (NPV) was 91.3%. We reached 71.74% diagnostic accuracy. Likelihood ratio of a positive test (LPT) was 2.49, likelihood ratio of negative test (LNT) was 0.22 and diagnostic odds were 11.45 (Table 4).
Table 4.
Sensitivity, specificity, false positive rate and false negative rate of intrapolypoidal white particles for the diagnosis of AFRS
| Sensitivity (95% CI) = 85.71% (57.19–98.22%) |
| Specificity (95% CI) = 65.63% (46.81–81.43%) |
| Positive predictive value (95% CI) = 52.17% (39.24–64.82%) |
| Negative predictive value (95% CI) = 91.3% (56.54–84.01%) |
| Diagnostic accuracy = 71.74% |
Discussion
IWP appear to be promising as an endoscopic diagnostic sign of AFRS. This study shows that the presence of IWP is highly sensitive but moderately specific for clinical diagnosis of AFRS. This implies that it has the ability to correctly identify the presence of AFRS and moderate ability to correctly identify the absence of AFRS.
AFRS is a relatively novel disease, recognized as a definitive entity within the last 40 years only. As the disease is still incompletely understood and new information is still emerging, underdiagnosis or misdiagnosis is not very uncommon, leading to considerable delay in the management. Although the classic Bent and Kuhn criteria are helpful to distinguish between different types of rhinosinusitis, the requirement of the CT findings and fungal stain during surgery impedes the diagnosis of AFRS in the outpatient setting. The major criteria include characteristic computed tomography (CT) scan findings, which is costly as well as time-consuming as the wait for a CT appointment is often long in some centers. Furthermore, CT scan facility might not be available in primary healthcare centers all over the world. Also a radiological staging system has been developed specifically for AFRS based on the degree of bony erosion and expansion [20]. Endoscopic diagnosis will pave the way for early diagnosis in the outpatient setting. No clear endoscopic signs (pathognomonic) of polyps in allergic fungal rhinosinusitis are mentioned in the literature. We focused on the endoscopic findings of AFRS, thereby trying to establish alternative diagnostic criteria that will help to diagnose acute fungal rhinosinusitis in outpatient setting. Early and prompt diagnosis of AFRS will help developing countries where cost of investigations is a confounding factor in terms of health provisions. Cost-effective diagnosis will help in decreasing the burden of disease caused by AFRS.
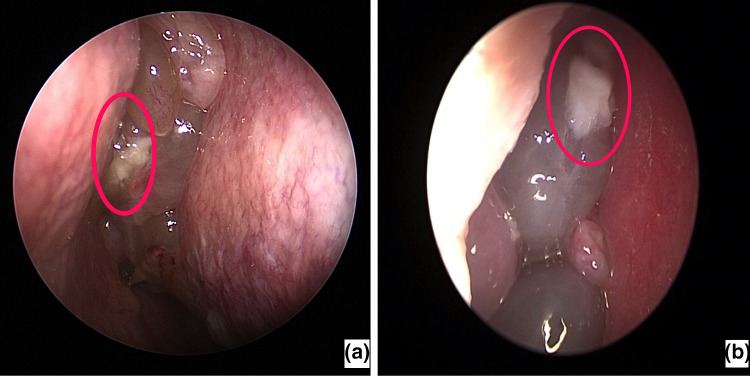
Endoscopy revealed multiple unilateral or bilateral glistening semitransparent nasal polyps. We noticed smooth white patches on the surface of the polyps, which were 3-6 mm in size, irregular in shape resembling tiny pieces of cotton wool, mostly multiple and in some cases single (Fig. 3a, b). These particles demonstrated high sensitivity and moderate specificity.
Fig. 3.
a, b Endoscopic image of intrapolypoidal white particles: single smooth white patch resembling tiny pieces of cotton wool, on the surface of glistening semitransparent nasal polyp
Multiple sets of criteria have been proposed over the time for the diagnosis of AFRS. They are all used with some national and regional variations. The generally accepted criteria, the one by Bent and Kuhn [15] proposed for diagnoses of AFS, consist of: (1) nasal polyposis, (2) allergic fungal mucin, (3) CT scan characteristic finding, (4) positive fungal histopathology and/or culture and (5) type 1 hypersensitivity (atopy). Deshazo et al. [16] presented cases of AFS without atopy, and only two-thirds of patients had positive skin test for fungi. Cody et al. [17] has diagnosed AFS on the basis of allergic mucin containing hyphae with no evidence of tissue invasion and positive culture for fungi. Ponikau [18] proposed the following set of criteria for the diagnosis of AFS: 1. CRS, 2. mucin containing hyphae, 3. atopy with or without polyps and 4. characteristic CT scan.
The CT presentation of AFRS is unique and has high sensitivity (70%) and very high specificity (90%) when used in combination with the presence of nasal polyps and Aspergillus-specific IgE [19]. Several studies reported the asymmetric involvement of the sinuses [12, 15, 20–22] apparent in CT scan of up to 78% of patients [6]. Bent and Kuhn [15] and Manning et al. [12] included asymmetry in the characteristic CT findings [12, 15] of the diagnostic criteria. Expansion of sinus and bone erosion is the characteristic CT scan findings of the disease [1, 3, 5, 6, 12] and is encountered in about 20% of the patients [22] along with heterogeneous opacities [1, 3, 6, 15]. The displacement of adjacent structures by the expanded sinus is frequently encountered in AFRS and visualized in CT scan [21, 22].
In a retrospective study conducted by Tariq and Rotan [23], they examined 700 patients of CRS and found that AFRS was present in 13% of the cases, whereas culture was positive in 43%, and histopathologic results were positive in 57% for fungal hyphae and allergic mucin. The most common causative organism was Aspergillus flavus. In a similar study conducted in Saudi Arabia, Al-Dousary [24] noted that out of 406 patients of CRS, fungal cultures were positive in 16.9% cases of CRS. Based on radiological features, histopathologic findings and culture results, AFS was diagnosed in 14.5% cases. Nasal polyposis was present in 94.9% cases. Our study showed that only 31% cases had positive fungal staining, culture and histopathologic findings. This indicates that acute fungal rhinosinusitis can be clinically evident in culture negative patients.
AFRS is often misdiagnosed or diagnosed late. Based on the findings reported here, the new IWP sign could serve a valuable role in diagnosing AFRS in the outpatient setting and therefore hasten the initiation of treatment. The relatively small sample size is a limitation of this study. Further studies are necessary to establish the relationship of clinical and endoscopically diagnosed AFRS.
Conclusion
Endoscopic diagnosis criteria for acute fungal rhinosinusitis have not yet been developed, and there is no literature available on endoscopic findings in AFRS to the best of our knowledge. This study reports that the presence of IWP in the endoscopic examination indicates AFRS with high sensitivity but moderate specificity. The results of this study are promising in that they demonstrate that the IWP could be a good candidate for reliable endoscopic sign for the diagnosis of AFRS in outpatient settings. Identifying an endoscopic sign will lead to prompt and efficient diagnosis and initiation of treatment in an outpatient setting.
Funding
None
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoyt AE, Borish L, Gurrola J, Payne S. Allergic fungal rhinosinusitis. J Allergy Clin Immunol Pract. 2016;4:599–604. doi: 10.1016/j.jaip.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Brescia G, Franz L, Alessandrini L, Parrino D, Barion U, Marioni G. Prognostic role of blood eosinophil and basophil levels in allergic fungal rhinosinusitis (AFRS) Am J Otolaryngol. 2019;41(1):102301. doi: 10.1016/j.amjoto.2019.102301. [DOI] [PubMed] [Google Scholar]
- 3.Dykewicz MS, Rodrigues JM, Slavin RG. Allergic fungal rhinosinusitis. J Allergy Clin Immunol. 2018;142:341–351. doi: 10.1016/j.jaci.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Rai G, Das S, Ansari MA, Singh PK, Gupta N, Sharma S, et al. Phenotypic and functional profile of Th17 and Treg cells in allergic fungal sinusitis. Int Immunopharmacol. 2018;57:55–61. doi: 10.1016/j.intimp.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Singh V. Fungal rhinosinusitis: unravelling the disease spectrum. J Maxillofac Oral Surg. 2019;18:164–179. doi: 10.1007/s12663-018-01182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marple BF. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope. 2001;111(6):1006–1019. doi: 10.1097/00005537-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Azar S, Mansour B, Parivash K, Babak B. Fungal rhinosinusitis in hospitalized patients in Khorramabad, Iran. MEJSR. 2011;7:387–391. [Google Scholar]
- 8.Glass D, Amedee RG. Allergic fungal rhinosinusitis: a review. Ochsner J. 2011;11:271–275. [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A, Farman A, Din S, Khan N, Dawar A. Frequency of allergic fungal chronic rhinosinusitis. Pak J Otolaryngol. 2011;27:12–14. [Google Scholar]
- 10.Kamal MS, Ahmed KU, Humayun P, Atiq T, Hossain A, Rasel MA. Association between allergic rhinitis and sino-nasal polyposis. Bangladesh J Otorhinolaryngol. 2011;17:117–120. doi: 10.3329/bjo.v17i2.8851. [DOI] [Google Scholar]
- 11.Marcus S, Roland LT, DelGaudio JM, Wise SK. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2018;4(1):13–17. doi: 10.1002/lio2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107:170–176. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 14.Fandino M, Macdonald KI, Lee J, Witterick IJ. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27:e146–e157. doi: 10.2500/ajra.2013.27.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bent JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 16.de Shazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 17.Cody DT, Neel HB, Ferreiro JA, Roberts GD. Allergic fungal sinusitis: the Mayo Clinic experience. Laryngoscope. 1994;104:1074–1079. doi: 10.1288/00005537-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 19.Dhiwakar M, Thakar A, Bahadur S, Sarkar C, Banerji U, Handa KK, et al. Preoperative diagnosis of allergic fungal sinusitis. Laryngoscope. 2003;113:688–694. doi: 10.1097/00005537-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Wise SK, Rogers GA, Ghegan MD, et al. Radiologic staging system for allergic fungal rhinosinusitis (AFRS) Otolaryngol Head Neck Surg. 2009;140:735–740. doi: 10.1016/j.otohns.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Dutre T, Al Dousary S, Zhang N, Bachert C. Allergic fungal rhinosinusitis-more than a fungal disease? J Allergy Clin Immunol. 2013;132(2):4879.e1. doi: 10.1016/j.jaci.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Marple BF. Allergic fungal rhinosinusitis: a review of clinical manifestations and current treatment strategies. Med Mycol. 2006;44(Supplementary 1):S277–S284. doi: 10.1080/13693780600778650. [DOI] [PubMed] [Google Scholar]
- 23.Ashour TA, Ashour R. Prevalence of allergic fungal sinusitis in refractory chronic rhinosinusitis in Saudi Arabia. PAJR. 2014;4:57. [Google Scholar]
- 24.Al-Dousary SH. Allergic fungal sinusitis: radiological and microbiological features of 59 cases. Ann Saudi Med. 2008;28:17–21. doi: 10.5144/0256-4947.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]