Abstract
Gastric cancer (GC) is the most common malignant tumor of digestive tract. Taraxasterol (TAX), a kind of phytosterol, has been proved to exert anti-tumor functions in GC. Herein, the current work was carried out to identify the biological role of TAX and molecular mechanisms underlying TAX in the progression of GC. In the present study, CCK-8 assay, Colony formation assay, EDU staining and TUNEL staining were performed to evaluate the malignant behaviors of GC cells. Levels of proliferation and apoptosis-associated proteins were assessed using western blotting analysis. Besides, GPD2 expression in GC cells was presented on CCLE database and the interaction between TAX and GPD2 was obtained from STRING database. The glucose uptake, lactate production, LDH activity, ATP and expressions of glycolysis-associated enzymes were measured to evaluate glycolysis level. Results of the present research revealed that TAX suppressed the proliferative and clone-forming abilities of GC cells and boosted the apoptosis of GC cells. TAX reduced GPD2 expression in GC cells. Furthermore, overexpression of GPD2 reversed the inhibitory effects of TAX on the proliferative and clone-forming abilities of GC cells as well as abolished the promoting effects of TAX on the apoptosis of GC cells. Besides, upregulation of GPD2 abrogated the inhibition of TAX on glycolysis. To conclude, TAX could suppress GC progression via inhibiting GPD2-mediated glycolysis, which helps to develop a promising molecular target for GC therapies.
Keywords: Taraxasterol, GPD2, Glycolysis, Gastric cancer
Introduction
Gastric Cancer (GC) is one of the most common gastrointestinal malignancy, which generally derives from gastric mucosal epithelial cells (Hong et al. 2019). According to the data released by the global cancer statistics, the incidence rate of GC ranks fifth and the mortality rate of GC ranks fourth in malignant tumors (Sung et al. 2021). Surgery is the main therapeutics for GC. Body mass index (BMI) of GC patients after gastrectomy significantly decreased, so postoperative nutritional nursing is of great significance to improve the prognosis of GC patients. According to statistic, 90% patients with early GC can achieve a 5-year survival. However, due to the lack of early screening, invasion and metastasis have occurred in most GC patients at the time of consultation (Zhou and Li 2015). Thus, it is of great significance to deeply investigate the pathogenesis of GC and discover novel molecular markers and therapeutic targets.
Unlike normal cells, tumor cells tend to rely more on aerobic glycolysis to produce energy and obtain intermediate metabolites, which are then directed to other biosynthetic pathways that promote tumor growth (Arundhathi et al. 2021). Therefore, triggering glycolysis dysfunction is one of the strategies to kill tumor cells (Fang et al. 2021). Glycerol 3-phosphate dehydrogenase (GPD2) is a mitochondrial gene that encodes the mitochondrial glycerophosphate dehydrogenase (mGPDH) (Pecinová et al. 2020). It is an integral component of the mammalian respiratory chain and glycerophosphate (GP)-shuttle which connects mitochondrial and cytosolic processes, and has a vital catalytic role in glycolysis (Singh 2014; Wu et al. 2020). Recently, it has been confirmed that GPD2 is closely associated with several malignancies. GPD2 inhibitors can effectively inhibit the proliferation of prostate cancer cells (Mikeli et al. 2020). Meanwhile, downregulation of GPD2 blocks the survival and growth of CD133-positive HuH-7 cells by disrupting the homeostasis of energy metabolism (Gachumi et al. 2021). Importantly, it has been reported that GPD2 gene may be related to the progression and prognosis of GC (Wei et al. 2021).
Phytosterol has a similar structure and biological function with cholesterol (Vezza et al. 2020). It possesses protective effects on many diseases, such as cardiovascular diseases, liver diseases and cancers (Yang et al. 2021). Taraxasterol (TAX), a kind of phytosterol, has higher content in the root of taraxacum mongolicum (Bao et al. 2018). Literature has verified that TAX treatment can suppress the migration, invasion and epithelial-to-mesenchymal transition (EMT) of papillary thyroid cancer cells (Chen et al. 2020). Besides, TAX exerts anti-tumor activity against the development of GC (Zhu et al. 2021). However, are yet to be fully elucidated.
Herein, the current work was carried out to identify the biological role of TAX and molecular mechanisms underlying TAX in the progression of GC through a series of functional experiments. What’s more, the regulatory functions of TAX on GPD2-mediated glycolysis were fully elucidated in vitro.
Materials and methods
Cell culture
Human gastric cancer cell line HGC-27 and human gastric mucosal epithelial cell line GES-1 were obtained from the Cell Research Institute of Shanghai Academy of Sciences (Shanghai, China). All cells were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Gibco, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, NY, USA) and 1% penicillin/streptomycin solution in an incubator with 5% CO2 at 37 °C.
Cell transfection
The pcDNA-GPD2 (Ov-GPD2) and the corresponding negative control (Ov-NC) were designed and synthesized from GenePharma (Shanghai, China). For in vitro transfection, cells were transfected with Ov-GPD2 or Ov-NC using Lipofectamine® 2000 (Invitrogen, CA, USA) according to the manufacturer's protocol.
Cell treatment
HGC-27 and GES-1 cells were incubated with 0, 5, 10, 15, 20 μM TAX (Yuan-Ye Bio-Technology, Shanghai, China) for 48 h.
Cell viability assay
Cells were seeded into 96-well plates at a density of 5 × 103 cells/well and further incubated at 37 °C for 48 h. Next, cell counting kit-8 (CCK-8) solution (Beyotime, Shanghai, China) was added to each well. After 2 h of incubation, the light absorption at 450 nm was measured using a microplate reader (Bio-Rad, CA, USA).
Colony formation assay
Cells (500 cells/well) were incubated in 1640 medium containing 10% FBS at 37 °C. Two weeks later, cells were fixed and stained with 0.1% crystal violet (Beyotime, Shanghai, China). The numbers of visible colonies were counted manually.
EDU (5-Ethynyl-2′-deoxyuridine) staining
Cells were fixed with 4% paraformaldehyde for 30 min and permeabilization was promoted with 0.5% Triton X-100 for 20 min. Then, the cell-light EDU apollo567 in vitro kit (Ribobio, Guangzhou, China) was employed for EDU detection following the manufacturer’s instructions. DAPI (Solarbio, Beijing, China) was applied to re-stain nuclei. A fluorescence microscopy (Nikon, Tokyo, Japan) was used to visualize the cells.
TUNEL staining
In brief, cells (5 × 103 cells/well) were washed with PBS and then fixed with 4% paraformaldehyde for 10 min at 4 °C. Next, cells were treated with 1% Triton-100 for 30 min at room temperature and TUNEL reaction buffer (Roche, Basel, Switzerland) for 1 h at 37 °C in the dark. After washing twice with PBS, cells were incubated with DAPI for 10 min at room temperature. TUNEL-positive cells were observed and imaged using a fluorescence microscope (Nikon, Tokyo, Japan).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Thermo Fisher Scientific, MA, USA) following the manufacturer's instructions. Then, 1 µg RNA was reverse-transcribed into complementary DNA (cDNA) using a PrimeScript RT kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. Next, the collected cDNA was amplified using SYBR Master Mixture (Takara, Tokyo, Japan) on the ABI 7500 quantitative PCR instrument (ABI/Perkin Elmer, CA, USA). The primer sequences were as follows: GPD2 (forward primer: 5′- GTGGCCAAAATGGCAAGTGT -3′, reverse primer: 5′-CCACAGCAGTGCAGGCATAC-3′); Ki67 (forward primer: 5′-CTTTGGGTGCGACTTGACGA-3′, reverse primer: 5′-TTCTGCCATTACGTCCAGCG-3′); PCNA (forward primer: 5′-CTGAGAGCGGAGGACAATGC-3′, reverse primer: 5′-GGAATTCCAAGCTGCTCCAC-3′); GAPDH (forward primer: 5′-GAAGGTCGGAGTCAACGGATTT-3′, reverse primer: 5′-CTGGAAGATGGTGATGGGATTTC-3′). The reaction was performed under the following thermocycling conditions: Initial activation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 50 s, and a final extension at 72 °C for 30 s. GAPDH served as an endogenous control. The relative expression of mRNA was calculated using the 2–ΔΔCt method (Livak and Schmittgen 2001).
Western blotting
Cells were lysed in Radioimmunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China) and the total proteins were quantified using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Then, the proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Solarbio, Beijing, China). Next, the membranes were blocked with 5% BSA for 1.5 h at room temperature and then incubated overnight at 4 °C with primary antibodies against: Ki67 (Abcam, 1:5000, ab92742), PCNA (Abcam, 1:5000, ab92552), Bcl-2 (Abcam, 1:1000, ab32124), Bax (Abcam, 1:2000, ab182733), Cleaved caspase-3 (Abcam, 1:500, ab2302), Caspase-3 (Abcam, 1:5000, ab32351), GPD2 (Abcam, 1:5000, ab188585), HK2 (Abcam, 1:1000, ab209847), LDHA (Abcam, 1:5000, ab52488), PFKM (Abcam, 1:5000, ab154804) and GAPDH (Abcam, 1:1000, ab181602). On the following day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, 1:20,000, ab205718) for 1 h at room temperature. The membranes were developed using an enhanced chemiluminescence (ECL) detection (Beyotime, Shanghai, China). The protein blots were visualized and analyzed using Bio-Rad imaging system (Bio-Rad, CA, USA).
Glycolysis analysis
Briefly, the supernatants of cell culture medium were collected. Then, the activity of intracellular Lactate dehydrogenase (LDH) and the levels of glucose and lactate were analyzed using a LDH or glucose or lactate assay kit (Sigma, MO, USA) following the manufacturer's instructions.
The detection of ATP level in lysate of cells was performed using an Enhanced ATP assay kit (Beyotime, Shanghai, China) according to the manufacturer's recommendations.
Statistical analysis
Data were expressed as mean values ± SD of three independent experiments. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Statistical differences between two groups were determined using unpaired Student’s t-test. SPSS 17.0 software (SPSS Inc., Chicago, USA) was used for statistical analysis. P < 0.05 indicated a statistically significant difference. *p < 0.05, **p < 0.01, ***p < 0.001.
Results
TAX suppressed the proliferative and clone-forming abilities of GC cells
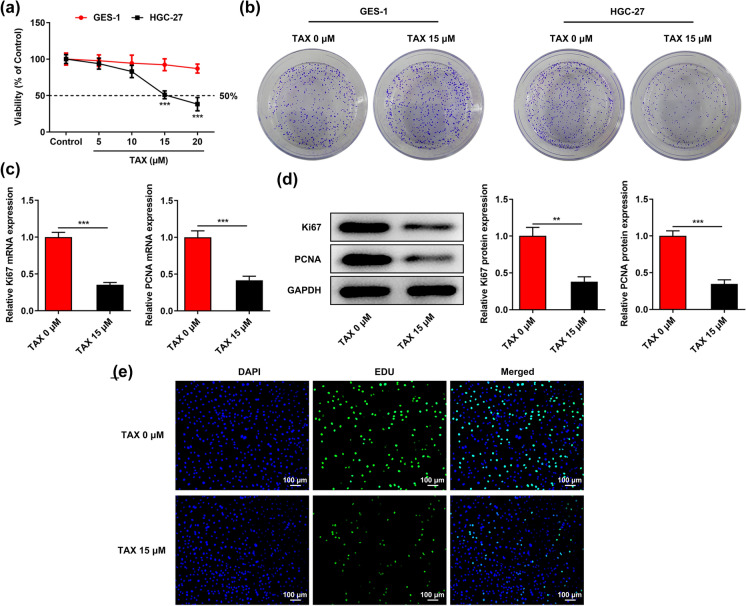
Human gastric cancer cell line HGC-27 and human gastric mucosal epithelial cell line GES-1 were treated with 0, 5, 10, 15, 20 μM TAX for 48 h and cell viabilities were assessed using CCK-8 assay. TAX inhibited the proliferation of HGC-27 cells in a dose-dependent manner while had no obvious influence on the viability of GES-1 cells (Fig. 1a). Viability of HGC-27 cells treated with 15 μM TAX reached IC50, and then TAX at a dose of 15 μM was chosen for subsequent experiments. Colony formation assay also revealed that treatment with TAX (15 μM) repressed the clone-forming ability of HGC-27 cells while had no influence on that of GES-1 cells (Fig. 1b). Additionally, TAX treatment significantly inhibited the mRNA and protein expressions of proliferation-associated factors (Ki67 and PCNA) in HGC-27 cells (Fig. 1c, d). Furthermore, the inhibitory effect of TAX on the proliferation of HGC-27 cells was further confirmed by EDU staining. The proliferation of HGC-27 cells was significantly decreased in the presence of TAX, as indicated by the reduced green staining (Fig. 1e).
Fig. 1.
TAX suppressed the proliferative and clone-forming abilities of GC cells. a After treatment with 0, 5, 10, 15, 20 μM TAX for 48 h, viabilities of human gastric cancer cell line HGC-27 and human gastric mucosal epithelial cell line GES-1 were assessed using CCK-8 assay. b After treatment with 0, 15 μM TAX, the cloning formation of HGC-27 and GES-1 cells was examined by colony formation assay. c qRT-PCR was performed to detect the mRNA levels of Ki67 and PCNA in HGC-27 cells after treatment with 0, 15 μM TAX. d Western blotting analysis was applied to detect the expressions of proliferation-associated proteins (Ki67 and PCNA) in HGC-27 cells after treatment with 0, 15 μM TAX. e EDU staining was employed to detect the proliferation of HGC-27 cells after treatment with 0, 15 μM TAX. **p < 0.01, ***p < 0.001
TAX boosted the apoptosis of GC cells
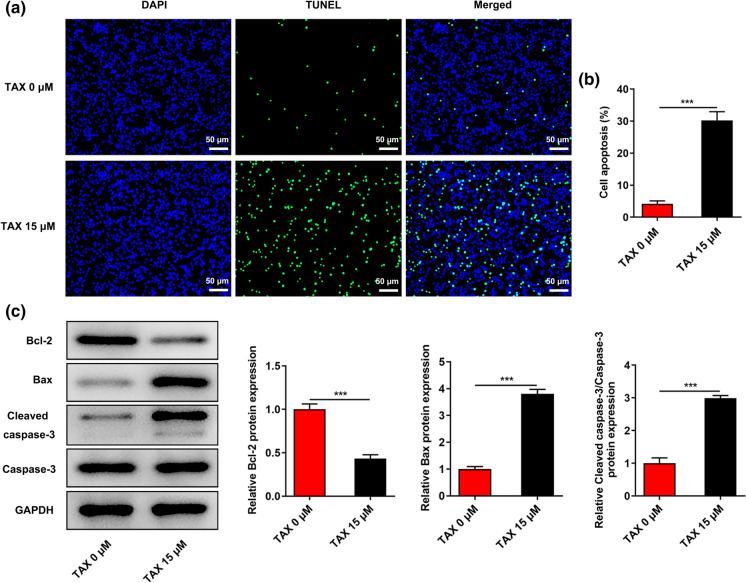
TUNEL staining was employed to evaluate the effects of TAX on the apoptosis of HGC-27 cells. In comparison with the control, TAX treatment significantly facilitated the apoptosis of HGC-27 cells (Fig. 2a, b). Moreover, decreased Bcl-2 expression and increased Bax and Cleaved caspase-3 expression in HGC-27 cells caused by TAX treatment (15 μM) further evidenced the above finding (Fig. 2c).
Fig. 2.
TAX boosted the apoptosis of GC cells. a and b TUNEL staining was employed to detect the apoptosis of HGC-27 cells after treatment with 0, 15 μM TAX. c Western blotting analysis was conducted to detect the expressions of apoptotic-associated proteins (Bcl-2, Bax, Cleaved caspase-3, Caspase-3) in HGC-27 cells after treatment with 0, 15 μM TAX. ***p < 0.001
TAX reduced GPD2 expression in GC cells
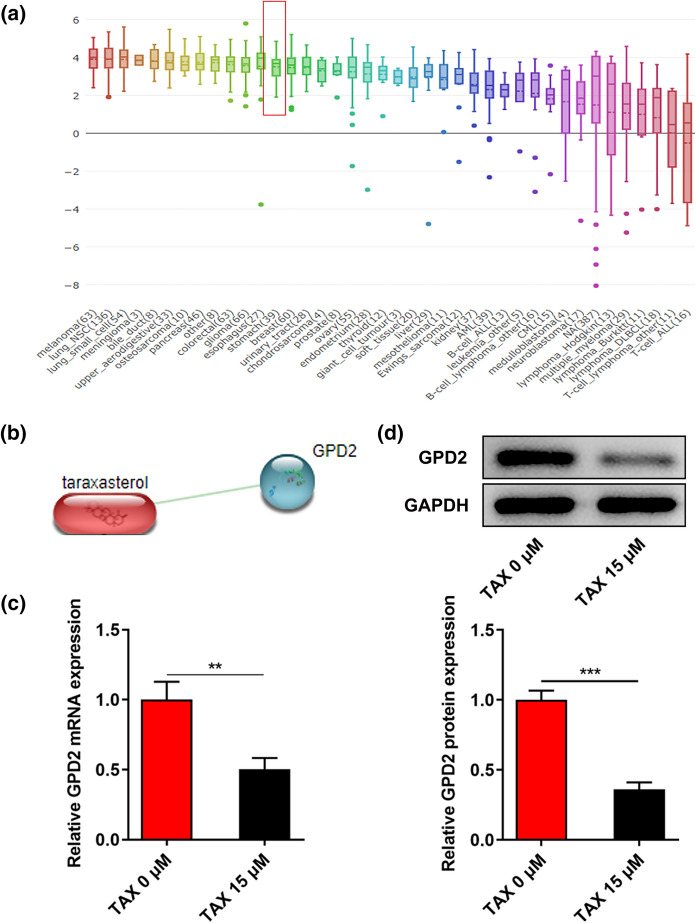
Distinctly increased GPD2 expression in GC cell lines was obtained from Broad Institute Cancer Cell Line Encyclopedia database (Fig. 3a). Subsequently, the interaction between TAX and GPD2 was analyzed and obtained from the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (Fig. 3b). It was observed that TAX treatment markedly inhibited GPD2 mRNA and protein expression in HGC-27 cells (Fig. 3c, d).
Fig. 3.
TAX reduced GPD2 expression in GC cells. a Broad Institute Cancer Cell Line Encyclopedia (CCLE) database presented GPD2 expression in GC cell lines. b Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database was employed to assess the interaction between TAX and GPD2. c qRT-PCR was performed to detect the mRNA level of GPD2 in HGC-27 cells after treatment with 0, 15 μM TAX. d Western blotting analysis was applied to detect the protein expression of GPD2 in HGC-27 cells after treatment with 0, 15 μM TAX. **p < 0.01, ***p < 0.001
Overexpression of GPD2 reversed the inhibitory effects of TAX on the proliferative and clone-forming abilities of GC cells
In order to identify the biological role of GPD2 in GC, HGC-27 cells were transfected with Ov-GPD2 and the overexpression efficiency was validated by performing qRT-PCR. In comparison with transfection with Ov-NC, an obvious increase in GPD2 expression was observed in HGC-27 cells transfected with Ov-GPD2 (Fig. 4a). Then, colony formation assay confirmed that TAX suppressed the clone-forming ability of HGC-27 cells, which was abolished by GPD2 overexpression (Fig. 4b). Moreover, elevated mRNA and protein levels of Ki67 and PCNA (Fig. 4c, d) and increased green staining in EDU assay (Fig. 4e) supported that the inhibition of TAX on the proliferation of HGC-27 cells was partially reversed upon upregulation of GPD2.
Fig. 4.
Overexpression of GPD2 reversed the inhibitory effects of TAX on the proliferative and clone-forming abilities of GC cells. a qRT-PCR was performed to detect the mRNA level of GPD2 in HGC-27 cells transfected with Ov-GPD2. b Colony formation assay was employed to examine the influence of GPD2 overexpression on the inhibitory effect of TAX on the clone-forming ability of HGC-27 cells. c qRT-PCR was performed to detect the influence of GPD2 overexpression on the regulatory effects of TAX on the mRNA levels of Ki67 and PCNA in HGC-27 cells. d Western blotting analysis was applied to detect the influence of GPD2 overexpression on the regulatory effects of TAX on the protein levels of Ki67 and PCNA in HGC-27 cells. e EDU staining was employed to assess the influence of GPD2 overexpression on the inhibitory effect of TAX on the proliferative ability of HGC-27 cells. ***p < 0.001
Overexpression of GPD2 reversed the promoting effects of TAX on the apoptosis of GC cells
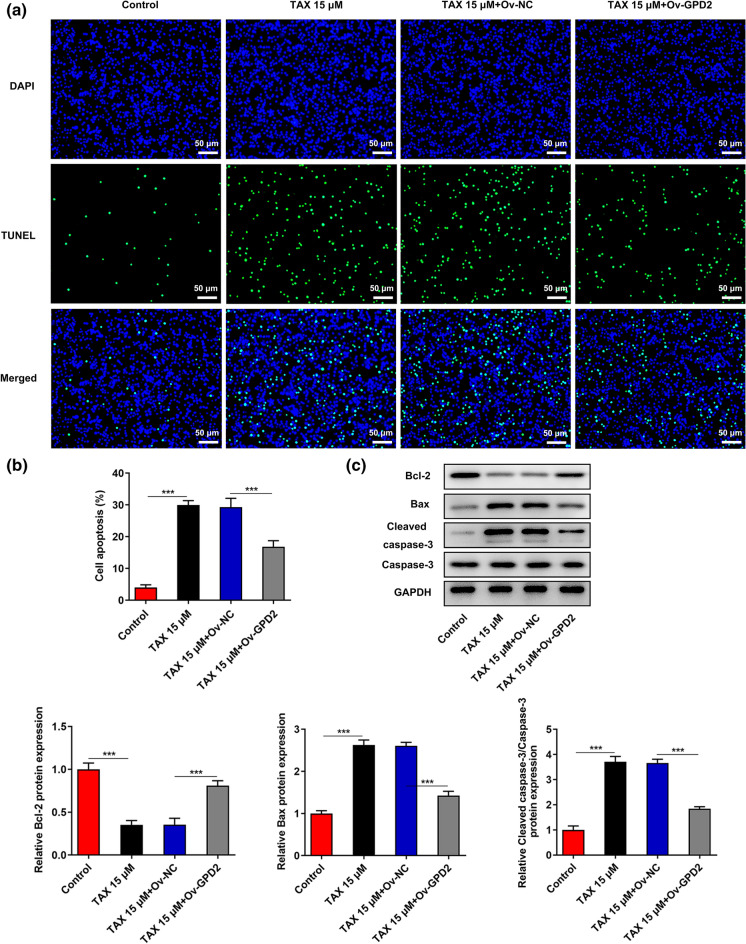
TUNEL staining revealed that overexpression of GPD2 partly abrogated TAX-mediated pro-apoptotic effects on HGC-27 cells (Fig. 5a, b). In addition, elevated Bcl-2 expression and reduced Bax and Cleaved caspase-3 expression caused by GPD2 overexpression further confirmed that the promoting effects of TAX on the apoptosis of HGC-27 cells were abolished upon upregulation of GPD2 (Fig. 5c).
Fig. 5.
Overexpression of GPD2 reversed the promoting effects of TAX on the apoptosis of GC cells. a and b TUNEL staining was employed to examine the influence of GPD2 overexpression on the promoting effect of TAX on the apoptosis of HGC-27 cells. c Western blotting analysis was conducted to detect the influence of GPD2 overexpression on the regulatory effects of TAX on the protein levels of Bcl-2, Bax, Cleaved caspase-3 and Caspase-3 in HGC-27 cells. ***p < 0.001
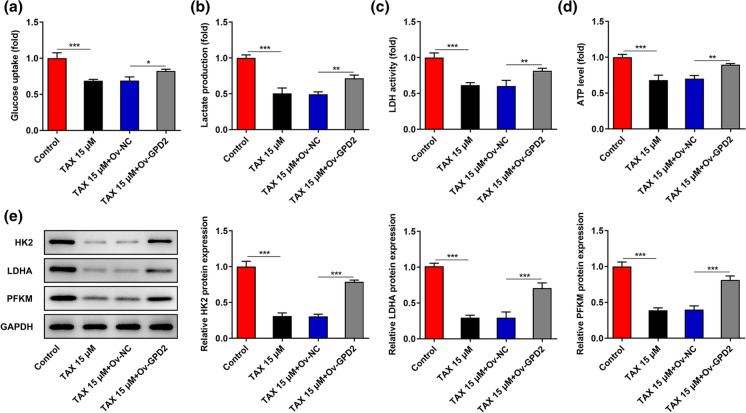
TAX inhibited GPD2-mediated glycolysis in GC cells
To deeply probe into the molecular mechanism underlying the participation of TAX and GPD2 in GC, the levels of glycolysis in HGC-27 cells were measured. TAX significantly suppressed the glucose uptake, lactate production, LDH activity and ATP level in HGC-27 cells, while overexpression of GPD2 partly reversed the inhibitory effect of TAX on glycolysis (Fig. 6a–d). Moreover, levels of glycolysis-associated enzymes were assessed by western blotting analysis. Upregulation of GPD2 partially abolished the suppressing effects of TAX on the expressions of HK2, LDHA and PFKM (Fig. 6e). To sum up, TAX could repress GPD2-mediated glycolysis in GC cells.
Fig. 6.
TAX inhibited GPD2-mediated glycolysis in GC cells. a–d The glucose uptake, lactate production, LDH activity and ATP level in HGC-27 cells were measured to evaluate the effects of TAX and GPD2 on glycolysis in GC cells. e Western blotting analysis was conducted to detect the effects of TAX and GPD2 on the expression levels of glycolysis-associated enzymes (HK2, LDHA and PFKM) in HGC-27 cells. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
GC is a common malignant tumor with high incidence and poor prognosis, greatly threating the public's health and aggravating the global burden (Hong et al. 2019). Under reasonable medical measures, patients with early GC can achieve a better survival. However, most of the GC patients are diagnosed in the middle and late stages (Zhou and Li 2015). Besides, although it has been confirmed that the occurrence of GC is closely related to helicobacter pylori infection, diet, smoking and drinking, etc., its exact pathogenesis remains to be further elucidated (Cheng et al. 2016; Raei et al. 2016).
Taraxacum mongolicum is a traditional Chinese medicine which has a long history in China (Ge et al. 2021). TAX, a pentacyclic-triterpene, is the main active ingredient of taraxacum mongolicum (Yang et al. 2021). Accumulating studies have revealed that taraxacum mongolicum extracts, including TAX, play vital roles in the development and progression of multiple malignancies (Menke et al. 2018; Chen et al. 2020). Deng et al. (2021) indicated that taraxacum mongolicum extract could suppress the proliferation, migration and invasion of triple-negative breast cancer cells. Importantly, Bao et al. (2018) reported that TAX treatment markedly suppressed cell growth, promoted cell cycle arrest at G0/G1 phase and boosted cell apoptosis in liver cancer cells. Additionally, TAX administration could suppress the growth of GC xenografts in nude mice (Zhu et al. 2021). Here, this current work demonstrated that TAX could inhibit the proliferation and clonal growth and facilitate the apoptosis of GC cells.
Aberrant glucose metabolism is one of the most important characteristics of tumor cells. As the dominant metabolic pathway of tumor cells, high-speed aerobic glycolysis provides the possibility for tumor growth, invasion and metastasis (Fang et al. 2021). Lactic acid, as the main end product of aerobic glycolysis, can produce an acidic environment that is in favor of tumor invasion and inhibition of anti-cancer immune effects (Fan et al. 2021). Results of the present research discovered that TAX suppressed glycolysis in GC cells.
GPD2 is a flavin-linked respiratory chain dehydrogenase that oxidizes glycerol 3-phosphate (G3P) to dihydroxyacetone phosphate (DHAP) with concurrent transferring of cytosolic reduction equivalents into the mitochondrial for oxidation (Morales-Sánchez et al. 2017; Wu et al. 2020). GPD2 increases during glycolysis and plays a vital role in the occurrence and development of a variety of malignant tumors (Mikeli et al. 2020). Wu et al. (2020) verified that esculetin can affect glycolysis in cancer cells by binding to glycolytic proteins including GPD2, thus exerting anti-tumor effects. Besides, literature reported that blocking the phosphorylation of GPD2 at threonine 10 could suppress the catalytic rate of glycolysis and attenuate the proliferation of glioma cells (Lu et al. 2020). Furthermore, a study established a prognostic model based on the expressions of 19 lipid metabolism-related genes (including GPD2) in GC patients, which could be a prognostic and therapeutic target of GC (Wei et al. 2021). Herein, the present study revealed that TAX treatment reduced GPD2 expression in GC cells and the suppressing effects of TAX on the malignant behaviors of GC cells and glycolysis were abolished by upregulation of GPD2.
Conclusion
Taken together, the current study validated that TAX could suppress GC cell proliferation and boost GC cell apoptosis by inhibiting GPD2-mediated glycolysis. In spite of above achievement, this work still lacks in vivo research. What’s more, clinical analysis should be conducted to confirm these findings and excavate the predictive values of TAX and GPD2 in the future.
Funding
Shaanxi Provincial Key Research and Development Program (Grant No. 2020SF-278).
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
No conflict of interest exists in the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arundhathi J, Mathur SR, Gogia A, Deo S, Mohapatra P, Prasad CP. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol Biol Rep. 2021;48:4733–4745. doi: 10.1007/s11033-021-06414-w. [DOI] [PubMed] [Google Scholar]
- Bao T, Ke Y, Wang Y, Wang W, Li Y, Wang Y, Kui X, Zhou Q, Zhou H, Zhang C, Zhou D, Wang L, Xiao C. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J Mol Med (berl) 2018;96:661–672. doi: 10.1007/s00109-018-1652-7. [DOI] [PubMed] [Google Scholar]
- Chen W, Li J, Li C, Fan HN, Zhang J, Zhu JS. Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer. Int J Immunopathol Pharmacol. 2020;34:2058738420933107. doi: 10.1177/2058738420933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors. 2016;3:25–36. doi: 10.1159/000443995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XX, Jiao YN, Hao HF, Xue D, Bai CC, Han SY. Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 / STAT3 / PD-L1 signaling pathways. J Ethnopharmacol. 2021;274:113978. doi: 10.1016/j.jep.2021.113978. [DOI] [PubMed] [Google Scholar]
- Fan Y, Hu D, Li D, Ma C, Tang Y, Tao Q, Deng L, Tang D. UCHL3 promotes aerobic glycolysis of pancreatic cancer through upregulating LDHA expression. Clin Transl Oncol. 2021;23:1637–1645. doi: 10.1007/s12094-021-02565-1. [DOI] [PubMed] [Google Scholar]
- Fang Z, Sun Q, Yang H, Zheng J. SDHB suppresses the tumorigenesis and development of ccRCC by inhibiting glycolysis. Front Oncol. 2021;11:639408. doi: 10.3389/fonc.2021.639408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachumi G, Poudel A, Wasan KM, El-Aneed A. Analytical strategies to analyze the oxidation products of phytosterols, and formulation-based approaches to reduce their generation. Pharmaceutics. 2021;13:268. doi: 10.3390/pharmaceutics13020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge BJ, Zhao P, Li HT, Sang R, Wang M, Zhou HY, Zhang XM. Taraxacum mongolicum protects against Staphylococcus aureus-infected mastitis by exerting anti-inflammatory role via TLR2-NF-κB/MAPKs pathways in mice. J Ethnopharmacol. 2021;268:113595. doi: 10.1016/j.jep.2020.113595. [DOI] [PubMed] [Google Scholar]
- Hong W, Guo F, Yang M, Xu D, Zhuang Z, Niu B, Bai Q, Li X. Hydroxysteroid sulfotransferase 2B1 affects gastric epithelial function and carcinogenesis induced by a carcinogenic agent. Lipids Health Dis. 2019;18:203. doi: 10.1186/s12944-019-1149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, Xu Z, Duan H, Ji H, Zhen Z, Li B, Wang H, Tang H, Zhou J, Guo T, Wu B, Wang D, Liu Y, Niu Y, Zhang R. Tumor-associated macrophage interleukin-β promotes glycerol-3-phosphate dehydrogenase activation, glycolysis and tumorigenesis in glioma cells. Cancer Sci. 2020;111:1979–1990. doi: 10.1111/cas.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke K, Schwermer M, Felenda J, Beckmann C, Stintzing F, Schramm A, Zuzak TJ. Taraxacum officinale extract shows antitumor effects on pediatric cancer cells and enhance mistletoe therapy. Complement Ther Med. 2018;40:158–164. doi: 10.1016/j.ctim.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Mikeli M, Fujikawa M, Nagahisa K, Yasuda S, Yamada N, Tanabe T. Contribution of GPD2/mGPDH to an alternative respiratory chain of the mitochondrial energy metabolism and the stemness in CD133-positive HuH-7 cells. Genes Cells. 2020;25:139–148. doi: 10.1111/gtc.12744. [DOI] [PubMed] [Google Scholar]
- Morales-Sánchez D, Kim Y, Terng EL, Peterson L, Cerutti H. A multidomain enzyme, with glycerol-3-phosphate dehydrogenase and phosphatase activities, is involved in a chloroplastic pathway for glycerol synthesis in Chlamydomonas reinhardtii. Plant J. 2017;90:1079–1092. doi: 10.1111/tpj.13530. [DOI] [PubMed] [Google Scholar]
- Pecinová A, Alán L, Brázdová A, Vrbacký M, Pecina P, Drahota Z, Houštěk J, Mráček T. Role of mitochondrial glycerol-3-phosphate dehydrogenase in metabolic adaptations of prostate cancer. Cells. 2020;9:1764. doi: 10.3390/cells9081764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raei N, Behrouz B, Zahri S, Latifi-Navid S. Helicobacter pylori infection and dietary factors act synergistically to promote gastric cancer. Asian Pac J Cancer Prev. 2016;17:917–921. doi: 10.7314/APJCP.2016.17.3.917. [DOI] [PubMed] [Google Scholar]
- Singh G. Mitochondrial FAD-linked glycerol-3-phosphate dehydrogenase: a target for cancer therapeutics. Pharmaceuticals (basel) 2014;7:192–206. doi: 10.3390/ph7020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Vezza T, Canet F, de Marañón AM, Bañuls C, Rocha M, Víctor VM. Phytosterols: nutritional health players in the management of obesity and its related disorders. Antioxidants (basel) 2020;9:1266. doi: 10.3390/antiox9121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XL, Luo TQ, Li JN, Xue ZC, Wang Y, Zhang Y, Chen YB, Peng C. Development and validation of a prognostic classifier based on lipid metabolism-related genes in gastric cancer. Front Mol Biosci. 2021;8:691143. doi: 10.3389/fmolb.2021.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ST, Liu B, Ai ZZ, Hong ZC, You PT, Wu HZ, Yang YF. Esculetin inhibits cancer cell glycolysis by binding tumor PGK2, GPD2, and GPI. Front Pharmacol. 2020;11:379. doi: 10.3389/fphar.2020.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Ye XJ, Chen MY, Li HC, Wang YF, Zhong MY, Zhong CS, Zeng B, Xu LH, He XH, Ouyang DY. Inhibition of NLRP3 inflammasome activation and pyroptosis in macrophages by taraxasterol is associated with its regulation on mTOR signaling. Front Immunol. 2021;12:632606. doi: 10.3389/fimmu.2021.632606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li XB. Endoscopic prediction of tumor margin and invasive depth in early gastric cancer. J Dig Dis. 2015;16:303–310. doi: 10.1111/1751-2980.12251. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li X, Zhang S, Liu J, Yao X, Zhao Q, Kou B, Han P, Wang X, Bai Y, Zheng Z, Xu C. Taraxasterol inhibits TGF-β1-induced epithelial-to-mesenchymal transition in papillary thyroid cancer cells through regulating the Wnt/β-catenin signaling. Hum Exp Toxicol. 2021;5:9603271211023792. doi: 10.1177/09603271211023792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.