Abstract
Parkinson's disease (PD) is an adult-onset neurodegenerative condition caused by oxidative stress and mitochondrial malfunction. In this study, the neuroprotective and neuritogenic activity of water fraction (Sw-fr) containing sorbicillin-like active metabolites of halotolerant P. flavigenum isolated from Salt Lake in Konya, Turkey were investigated on a 6-hydroxydopamine (6-OHDA)-induced PD in vitro PC-12 Adh cell model. Firstly, Sw-fr containing sorbicillin-like active metabolites were extracted from P. flavigenum and was compared with a sorbicillin standard by liquid chromatography-mass spectrometry (LC–MS). Then, the effects of non-cytotoxic concentrations of Sw-fr on the 6-OHDA-induced PD cell model were investigated via real-time cell proliferation analysis using the RTCA DP instrument. The effects of these concentrations on mitochondrial membrane integrity, caspase-3 were investigated by flow cytometry. Neurite outgrowth analysis and immunofluorescence staining were used to explore the neuritogenic effects of neuroprotective doses. By improving PC-12 Adh cell viability, decreasing reactive oxygen species production, and reducing apoptotic cell death, 1 and 10 μg/mL Sw-fr and sorbicillin standard proved neuroprotective against 6-OHDA-induced neurotoxicity. Furthermore, 1 and 10 µg/mL Sw-fr significantly induced neurite outgrowth. As a result, sorbicillin-like active metabolites containing Sw-fr were found to have neuroprotective and neuritogenic effects. Sorbicillin-like metabolites obtained from fungi may be novel natural medicines for neurodegenerative diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-021-00498-9.
Keywords: Neuritogenic, Neuroprotection, Parkinson’s disease, Penicillium flavigenum, Sorbicillin
Introduction
Mitochondrial deterioration caused by increased electron release-related oxidative stress and the induction of reactive oxygen species (ROS) production is involved in the pathogenesis of neurodegenerative diseases such as Alzheimer's, Huntington's and Parkinson's disease (PD) (Andersen 2004). Excessive free radical formation caused by electron transfer in the electron transport chain during dopamine and energy metabolism leads to oxidative stress. Notably, this event causes mitochondrial dysfunction and subsequent processes that lead to impaired brain function. Reducing oxidative stress and excitotoxicity is one of the most important purposes of neuroprotection and involves preventing or slowing down neuronal damage (Potashkin and Seidl 2011).
Some compounds that serve an important role in the adaptation of halophilic and halotolerant fungi to high salt concentrations by helping to balance cellular redox potential are unique sources of natural antioxidants (Frisvad and Filtenborg 1990). In biotechnology, microfungi have important potential for obtaining secondary metabolites with fermentative processes (Dominguete and Takahashi 2018). Moreover, fungal secondary metabolites are rich sources of novel bioactive compounds. Many metabolites such as fumaric acid, kojic acid, penicillic acid, quinones, statins, cyclosporin, fumagillin, and mycophenolic acid are isolated from fungi (Grijseels et al. 2017). However, few studies have examined the screening of fungal neuroprotective compounds (Dominguete and Takahashi 2018).
ROS formation is one of the most important factors in the pathogenesis of neurodegenerative disorders, while imbalances between antioxidant enzyme activities contribute to the brain damage process (Chu et al. 2012). Due to their strong antioxidant and anti-inflammatory properties, microfungi have drawn attention as novel sources (Gunawardena et al. 2014). Besides penicillin, roquefortine and sorbicillin are the most common active metabolites of Penicillium chrysogenum, which is one of the most common fungal species (Nicolaou et al. 1999). These compounds are diketopiperazine alkaloids with antibiotic and neurotrophic properties derived from tryptophan and histidine (Bringmann et al. 2005).
Cellular signal transduction initiates with the binding of a ligand—such as nerve growth factor (NGF) or neurotransmitters—to a specific receptor on the cell. This event systematically activates signal cascades that initiate responses such as cell differentiation and neurite outgrowth (Phan et al. 2015). NGF is a polypeptide that acts as a neurotrophic factor prototype, which is required for the development of neurons in the peripheral nervous system as well as in the central nervous system. However, NGF cannot cross the blood–brain barrier due to its high molecular weight. Therefore, the use of NGF as a medicine is not convenient due to the need for intraventricular administration to patients. To overcome this challenge, low molecular weight compounds that exhibit neurotrophic effects or strengthen the effects of exogenic NGF secreted from brain cells are very important in developing a therapeutic agent against neuronal cell death (Eguchi et al. 2000). To date, various NGF-inducing fungi-derived bioactive compounds have been reported. Fungi from halotolerant environments, where conditions are extreme and competition to exploit resources is low, evolve peculiar antioxidant capabilities to adapt to the environment; therefore, their secondary metabolites might have the potential to induce or mimic neurotrophic activity (Phan et al. 2017).
Sorbicillinoids are metabolites isolated from marine and terrestrial fungal sources (Du et al. 2009). These metabolites represent strong candidates for the development of new pharmaceutical agents due to their special structural features, and many biological activity studies have especially focused on their antioxidant and anticancer activity (Harned and Volp 2011). The free radical scavenging (Washida et al. 2007), anticancer (Wang et al. 2015), antimicrobial (Maskey et al. 2005) and antiviral activity (Bringmann et al. 2003) of sorbicillinoids have previously been reported. In addition to these effects, sorbicillinoids are potent tumour necrosis-alfa (TNF-α) inhibitors. Therefore, they may hold potential for the treatment of diseases such as arthritis, Alzheimer's, cancer and depression, which are defined by the abnormal regulation of TNF-α—a cytokine that plays a key role in inflammation and immune response (Warr et al. 1996).
Tuz Lake is a salt lake (salinity above 30% NaCl) located in the Central Anatolia Region, Konya, Turkey. Until recently, it was thought that only prokaryotic organisms could survive in this lake’s extreme hypersaline environment. However, fungi that can tolerate hypersalinity and low water activity conditions were recently discovered. Additionally, due to their adaptations to this extreme environment, the secondary metabolites of these fungi have remarkably high antioxidant and anti-inflammatory features, which makes them interesting natural drug candidates (Dikmen et al. 2017a). In this study, the neuritogenic and neuroprotective potential of sorbicillin-like active metabolites containing water fraction (Sw-fr) extracted from halotolerant P. flavigenum isolated from Tuz Lake were investigated.
Materials and methods
Extraction of Sorbicillin-like active metabolites
P. flavigenum was isolated from Tuz Lake water samples, and classical and molecular identification was previously completed and stocked (Canturk et al. 2017). The stock culture represents part of the Anadolu University Pharmaceutical Microbiology Laboratory Culture Collection. The activated culture was produced in a liquid medium within an incubator at 27 °C, and was incubated for 7 days (Malpure et al. 2006). Additionally, 15% of salt brought from Tuz Lake was added to the medium. After the incubation period, the isolates were filtered and separated from micelles. The collected supernatant was extracted three times with 1:1 hexane and three times with 1:1 ethyl acetate. The remaining water fraction from the fermentation liquid was extracted with two different organic solvents and lyophilised.
Liquid chromatography/mass spectrometry (LC–MS)
The standard sorbicillin (catalogue no.: 79950-85-9, Hengda Biotech, China) and P. flavigenum culture liquid water fraction were analysed using LC–MS (Agilent Technologies 1290 series UPLC (Ultra Performance Liquid Chromatography) with 6460 Triple Quad LC/MS (Agilent, Waldbronn, Germany) at the Anadolu University Plant, Medicine and Scientific Research Centre (BİBAM). The mobile phase consists of two solvent systems: (A) 0.2% formic acid/water and (B) 0.2% formic acid/acetonitrile. Gradient elution was then performed, followed by separation in a Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 µm particle size). Gradient elution conditions were as follows: 0 min, 100% A, 0% B; 0–15 min, 0% A, 100% B. Moreover, a 0.2% formic acid–water mixture was used as a solvent with a flow rate of 0.2 mL/min and an injection volume of 2 µL. The column temperature was kept constant at 30 °C throughout the analysis.
Cell culture and differentiation of PC-12 Adh cells
The PC-12 Adh (CRL-1721.1™) cell line was obtained from the American Type Culture Collection (ATCC). Cells were maintained as undifferentiated at 37 °C in a humidified incubator with 5% CO2 in a growth medium consisting of Dulbecco′s Modified Eagle Medium (DMEM) containing 10% horse serum, 5% fetal bovine serum and 1% penicillin/streptomycin. Cell differentiation into the neuronal phenotype was induced with the replacement of growth medium with 100 nM NGF, 1% fetal bovine serum and 1% penicillin–streptomycin containing DMEM, and the cells were incubated for 5 days. Cell differentiation was visualised under a light microscope after the incubation period (Mustafa et al. 2016).
Determination of non-cytotoxic concentrations by WST-1 assay
The non-cytotoxic concentrations of the samples on the PC-12 Adh cells were determined by WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt) assay (Roche, Mannheim, Germany) (Lian et al. 2003). Previously differentiated PC-12 Adh cells were seeded at a 1 × 104 density per well onto 96-well plates. After the 24 h incubation period, cells were treated with 400, 100, 10, 1 and 0.1 µg/mL concentrations of sorbicillin standard and Sw-fr in fresh medium. After 24 h, WST-1 reagent was added to wells and incubated for 3 h. The absorbance values of the wells were measured at 420 nm after incubation by using a Cytation 3 Cell Imaging Multi-Mode Reader (BioTek, Vermont, USA). The absorbance values obtained from the wells were directly correlated to the number of viable cells that were expressed as a percentage of the control group.
Determination of 6-OHDA toxicity on PC-12 Adh cells using a real-time cell analyser
The RTCA DP instrument (Roche, Mannheim, Germany) measures changes in impedance as cells attached with a read out given as cell index (CI) values and monitors cell proliferation (Mustafa et al. 2016). PC-12 Adh cells were added to the 96-well E-plates at a 1 × 104 density per well and incubated until they reached the log phase after 24 h. PC-12 Adh cell viability was monitored in real time after applying 25, 50, 100, 200 and 400 µM 6-OHDA to determine the concentration to be used in the in vitro PD model. Dose–response curves at 24 h were generated to determine IC50 values during the incubation period using RTCA DP 1.2.1 software.
Investigation of the neuroprotective effects using a real-time cell analyser
The neuroprotective potentials of standart sorbicillin and Sw-fr were determined by an in vitro 6-OHDA-induced neurotoxicity PD model. PC-12 cells were plated onto 96-well E-plates in 100 μM differentiation medium at a 1 × 104 density per well and incubated for 24 h. After incubation, cells were treated with 1, 10 ve 100 µg/mL concentrations of sorbicillin and Sw-fr (non-cytotoxic concentrations based on WST-1 assay). After 6 h, the medium containing sorbicillin and Sw-fr was removed from wells. Then the cells were treated with 150 µM 6-OHDA (IC50 value: Supplemental Fig. 1) for 24 h, and the results were analysed using RTCA DP (Liu et al. 2015). Notably, the 100 µg/mL concentration group was removed from subsequent studies because of the cytotoxicity determined in real time analyse.
Determination of mitochondrial membrane potential and caspase-3 activity
The loss of mitochondrial membrane potential and caspase-3 level of cells were determined with Flow Cytometry Mitochondrial Membrane Potential Detection and Caspase-3 Apoptosis Kits (BD Pharmingen, CA,USA). Differentiated PC-12 Adh cells were plated on six-well plates at a 1 × 105 density per well for 24 h and then incubated with 1 and 10 µg/mL sorbicillin and Sw-fr for 6 h. To induce neurodegeneration, the medium was replaced with fresh medium including 150 µM 6-OHDA and then cells were incubated for 24 h. Finally, methods were conducted according to the kit’s procedure and results were analysed on a BD Accuri™ C6 Flow Cytometry (Engür et al. 2016).
Determination of reactive oxygen species accumulation
The total amount of ROS of groups was measured using a Total ROS/Superoxide Detection Kit (Enzo Life Science, Germany). Differentiated PC-12 Adh cells were plated on 96-well plates at a density of 2 × 104 per well and incubated for 24 h. Then, the medium was replaced with fresh medium containing 1 and 10 µg/mL sorbicillin and Sw-fr, followed by incubation for 6 h. After the incubation period, cells were treated with 200 µM ROS inducer (pyocyanin) and 150 µM 6-OHDA for 24 h. Consequently, 5 mM ROS inhibitor (N-acetyl cysteine (NAC)) was applied as negative control and the experimental procedure was continued in accordance with the kit manufacturer’s instructions. Fluorescence measuremets were performed using a Cytation 3 Cell Multi-Mode Reader (BioTek). ROS accumulation was expressed as a percentage of the control.
Neurite outgrowth analysis and immunofluorescence staining
To determine the neurite outgrowth, cells were seeded in differentiation medium at a density of 2 × 103 cells per well onto 96-well plates coated with collagen IV (BD BioCoat™ Collagen IV Cellware, BD Biosciences, Germany). Then, cells were incubated with 1 and 10 µg/mL sorbicillin and Sw-fr as well as 50 nM positive control NGF for 5 days. Morphometric analysis was performed on 5th day using images of live cells taken with a Leica DM 300 inverted microscope. Neurite outgrowth analysis was performed as previously described (Kaya-Tilki et al. 2016; Mustafa et al. 2016). Briefly, the longest neurite length and (if present) branch length were measured by manually tracing the images using the Leica LAS Image Analysis program (measurements in μm). A total of 50 neurites were randomly chosen from five different photograph for each group and neurites were measured. The average neurite length per group was determined by dividing the total length by 50. The statistical significance of the results was calculated according to the NGF positive control group with GraphPad Prism 6 (GraphPad Campany, San Diego).
After the neurite outgrowth analysis experiment was performed, immunofluorescence staining was carried out. Cells were fixed and then permeabilised with 0.1% Tween 20 containing PBS for 20 min and washed twice. After the cells were incubated to block solution, cells were treated with 100 µL of anti-beta III tubulin antibody [2G10] (Abcam, catalogue no.: ab78078) in the blocking solution at + 4 °C overnight. Then, cells were washed twice with PBS and treated with goat anti-mouse IgG H and L (Alexa Fluor® 488) (Abcam, catalogue no: ab150113) secondary antibody and stored at room temperature for 1 h. Cell nuclei were stained with Hoechst 33258(sigma) for 10 min before imaging with a Cytation 3 Cell Imaging Multi-Mode Reader (Bio-Tek, USA) (Kaya-Tilki et al. 2016; Dikmen et al. 2017b).
Statistical analysis
Statistical analysis of the results was performed using one-way ANOVA and Tukey’s post hoc test via GraphPad Prism 6.0 software (GraphPad Campany, San Diego). The results are the means of three independent experiments and expressed as mean ± standard deviation. p values represent the significance of the results compared to the control group (n.s; p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
Results
Evaluation of LC–MS analysis
According to the LC–MS analysis results, sorbicillin and Sw-fr had similar peaks of 233 Da molecular weight. Moreover, standart sorbicillin and Sw-fr peaks coincide completely (Fig. 1A–C).
Fig. 1.
A Liquid chromatography/mass spectrometry (LC–MS) for Standard (Sorbicillin), B Liquid chromatography/mass spectrometry (LC–MS) for Sample (Sw-fr), C Comparison of sorbicillin (red) and Sw-fr (green) with liquid chromatography/mass spectrometry (LC–MS)
Evaluation of non-cytotoxic Sw-fr and Sorbicillin concentrations by WST-1 assay
According to WST-1 assay results, the non-cytotoxic concentrations of sorbicillin and Sw-fr on PC-12 Adh cells were determined (Fig. 2). Notably, 400 µg/mL sorbicillin, as well as 100 and 400 µg/mL Sw-fr concentrations, significantly decreased PC-12 Adh cell viability. Therefore, further neuroprotective activity analyses were conducted with 100, 10 and 1 µg/mL concentrations.
Fig. 2.
Effects of sorbicillin and Sw-fr concentrations on the cell viability of the PC-12 Adh cell line. Cells were treated with sorbicillin for 24 h (mean ± standard deviation) (n = 8) (p < 0.0001****)
Determination of neurotoxic 6-OHDA concentration using a real-time cell analyser
To determine the neurotoxic concentration of 6-OHDA to induce neurodegeneration on the in vitro PD model with PC-12 Adh cells, IC50 value was calculated as 150 µM at 24 h according to the CI values presented in (Supplemental Fig.1A, B).
Evaluation of the neuroprotective effects
Since evaluation of the neuroprotective effects of sorbicillin and Sw-fr agains to 6-OHDA-induced neurotoxicity, PC-12 cell viability was recorded and analysed using RTCA DP 1.2.1 software (Fig. 3A). The slope of cell viability was calculated according to the CI values presented in Fig. 3B. In this study, 1 and 10 µg/mL sorbicillin and Sw-fr showed to neuroprotective effects on 6-OHDA-induced neurotoxicity. Especially, it was determined that, the neuroprotective effects of Sw-fr were slightly higher than standart sorbicillin.
Fig. 3.
A Evaluation of the neuroprotective effects of sorbicillin and Sw-fr on PC-12 Adh cells using a real-time cell analyser (Arrow: Sw-fr and sorbicillin treatment period. Following this incubation period, the medium was replaced with 150 µM of 6-OHDA-containing medium to induce oxidative stress and determined the neuroprotective effects of the samples). B Slope graph of the neuroprotective effects of sorbicillin and Sw-fr concentrations against 6-OHDA-induced neurodegeneration on PC-12 Adh cells after a 24 h incubation period (n = 5, p < 0.1*, p < 0.01**, p < 0.001***)
The effects of Sw-fr and Sorbicillin on mitochondrial membrane potential and caspase-3 activation
According to the mitochondrial membrane potential analysis results (Table 1, Suplemental Fig.2 and Fig. 4A), mitochondrial depolarisation increased by approximately sevenfold with 6-OHDA treatment (34.4%), which is a demonstration of proper cellular damage, according to the control group (5.5%). The mitochondrial depolarisation levels of the cells treated with sorbicillin and Sw-fr without 6-OHDA treatment were similar to the control group. But, pre-treatment of cells with 1 and 10 µg/mL sorbicillin and Sw-fr before 6-OHDA administration significantly decreased mitochondrial depolarisation levels by 13.8, 13.5 and 10.4, 15.3%, respectively (p < 0.0001****).
Table 1.
The effects of sorbicillin and Sw-fr on the mitochondrial membrane potential and the caspase-3 enzyme activation levels of PC-12 Adh cells were determined using flow cytometry
| Concentration groups | JC-1-negative cells (%) | JC-1-positive cells (%) | Caspase-3-negative cells (%) | Caspase-3-positive cells (%) |
|---|---|---|---|---|
| Control | 94.4 | 5.5 | 95.3 | 4.7 |
| 6-OHDA | 64.1 | 34.4 | 72.6 | 27.4 |
| Sorbicillin 1 µg/mL | 92 | 7.4 | 92 | 8 |
| Sorbicillin 10 µg/mL | 95 | 4.9 | 90.8 | 9.2 |
| Sw-fr 1 µg/mL | 94.8 | 5.2 | 92.8 | 7.2 |
| Sw-fr 10 µg/mL | 93.9 | 5.4 | 95.8 | 4.2 |
| Sorbicillin 1 µg/mL + 6-OHDA | 86.1 | 13.8 | 86.2 | 13.8 |
| Sorbicillin 10 µg/mL + 6-OHDA | 85.4 | 13.5 | 86 | 14 |
| Sw-fr 1 µg/mL + 6-OHDA | 89.2 | 10.4 | 93.7 | 6.3 |
| Sw-fr 10 µg/mL + 6-OHDA | 84.3 | 15.3 | 93.2 | 6.8 |
Fig. 4.
Effects of sorbicillin and Sw-fr on the mitochondrial membrane potential levels (A) and caspase-3 enzyme activation levels (B) of the PC-12 Adh cells (shown as a percentage value graph based on the JC-1 analysis (A) and caspase-3-positive cells (B) results) (n = 3, p < 0.0001****)
As the caspase-3 analysis results (Table 1, Fig. 4B and Suplemental Fig. 3), caspase-3 enzyme activation increased by approximately sixfold with 6-OHDA treatment (27.4%) according to the control group (4.4%). The caspase-3-positive levels of the cells treated with sorbicillin and Sw-fr without 6-OHDA treatment were similar to those of the control group. In parallel with the mitochondrial membrane potential results, pre-treatment with 1 and 10 µg/mL sorbicillin and Sw-fr before 6-OHDA administration significantly decreased caspase 3 positive cells by 13.8, 14 and 6.3, 6.8%, respectively (p < 0.0001****). Notably, 1 µg/mL Sw-fr was determined as the most effective concentration on the mitochondrial membrane potential and caspase-3 activity.
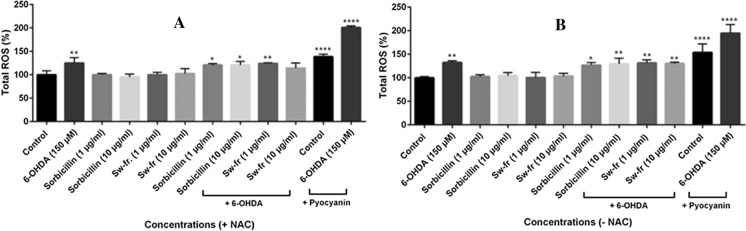
Evaluation of the effects of Sw-fr and Sorbicillin on total ROS levels
According to the total ROS level results presented in Fig. 5A and B, 6-OHDA increased ROS production by 50% (p < 0.01**) compared to the control group with or without the presence of ROS inducer. The total ROS levels of the PC-12 cells treated with sorbicillin or Sw-fr were not significantly altered. However, the induction of ROS levels with 6-OHDA decreased with the 10 µg/mL Sw-fr treatment in the presence of the ROS inhibitor, and also decreased with the 1 µg/mL sorbicillin treatment without the ROS inhibitor. These results indicate that sorbicillin and Sw-fr can potentially protect neurons against 6-OHDA damage by reducing the intracellular production of ROS.
Fig. 5.
A Effects of sorbicillin and Sw-fr on the total ROS levels of the PC-12 Adh cells (shown as a percentage value graph based on the results obtained using the ELISA method with the presence of the ROS inhibitor 5 mM N-acetylcysteine (NAC) and, B without the presence of the ROS inhibitor NAC. (n = 3, p < 0.5*, p < 0.01**, p < 0.001***, p < 0.0001**** relative to the control group)
The effects of Sw-fr and Sorbicillin on neurite outgrowth
After 5-day incubation period, the effects of the neuritogenic activity on PC-12 cells of sorbicillin and Sw-fr concentrations was observed by immunofluorescence staining (Fig. 6A–F). At the 1 and 10 µg/mL sorbicillin and Sw-fr concentrations, mean neurite lengths were calculated as 63.26, 67.25, 68.07, 87.06 and 84.46 µm, respectively. According to the neurite outgrowth analysis results (Fig. 6G), a significant increase in neurite lengths at 1 and 10 µg/mL of Sw-fr produced compared to the NGF group (p < 0.0001****). Sorbicillin concentrations also resulted in slightly increased neurite lengths according to the NGF group; however, this increase was not statistically significant. The highest effects on neuronal differentiation and neurite elongation of PC-12 Adh were obtained with 1 and 10 µg/mL Sw-fr concentrations.
Fig. 6.
Neuritogenic activity of NGF, sorbicillin and Sw-fr on PC-12 Adh cells. PC-12 Adh cell images were taken at the end of a 5-day incubation period with the following treatments: A control (0.1% DMSO); B positive control (NGF, 50 nM); C sorbicillin (1 µg/ml); D sorbicillin (10 µg/ml); E Sw-fr (1 µg/ml); F Sw-fr (10 µg/ml). Cell nuclei (stained blue with Hoechst 33,258) and tubulins (neurofilaments, stained green with Alexa Fluor® 488). Images were taken using a Cytation 3 Cell Imaging Multi-Mode Reader, 20 × objective). G Morphometric analysis was performed on 5th day by manually tracing live-cell images using the Leica LAS Image Analysis program (measurements in μm). A total of 50 neurites were randomly chosen for each group. The measured and mean neurite length values were obtained by averaging the length of 50 neurites (p < 0.0001**** relative to the NGF)
Discussion
Parkinson's disease is one of the most common neurodegenerative disorders in humans, and its prevalence increases with age. Although the exact mechanisms underlying neuronal damage in PD remain to be fully elucidated, oxidative stress and impaired mitochondrial function are known to serve important roles in PD development (Lin et al. 2015). The results of various studies that have elucidated the ability of fungi-derived natural compounds to overcome challenging disorders such as PD have demonstrated the potential for using fungal secondary metabolites in the development of effective new compounds. Sorbicillin compounds are pharmaceutically valuable metabolites that exhibit bioactivity. Sorbicillin is a metabolite that belongs to a large group of hexaketides known as sorbicillinoids. Currently, more than 10 sorbicillinoid analogues have been isolated from P. chrysogenum (Meng et al. 2016). In addition to producing sorbicillin, P. flavigenum produce metabolites such as penicillins, penitrem A, roquefortine C and meleagrin (Frisvad and Samson 2004). Notably, the active metabolites of P. flavigenum from Tuz Lake have previously shown common antimicrobial and anticancer effects (Canturk et al. 2017). Therefore, the present study was conducted to discover novel neuritogenic and neuroprotective effects of sorbicillin-like secondary metabolites of halotolerant P. flavigenum isolated from Tuz Lake in Turkey.
To obtain sorbicillin-like active metabolites of P. flavigenum culture, pre-solvent fractionation was performed with hexane and ethyl acetate. The obtained Sw-fr was lyophilized and LC–MS analysis was performed by coinciding with a sorbicillin standard. According to the LC–MS results, the similar peaks of 233 Da molecular weight of sorbicillin and Sw-fr were shown. In a similar study, P. chrysogenum mycelia were separated from a fermentation liquid by centrifugation and analysed using LC–MS after filtering without solvent fractionation (Salo et al. 2016). The genus Penicillium currently includes more than 354 accepted species (Visagie et al. 2014), many of which are capable of producing a wide variety of secondary metabolites, including the most well-known secondary metabolite—the antibiotics penicillin and griseofulvin (Oxford et al. 1939). A previous study demonstrated that using low-molecular weight compounds (NG-011 and NG-012) of P. verrucosum in microbial fermentation liquid can mimic or potentiate NGF following peripheral administration (Ito et al. 1992). In another study using sorbicillactone A (a member of the sorbicillinoid family), low cytotoxic effects on Hela S3 and PC-12 cells, but higher cytotoxic activity on L5178y leukaemia cells, were reported (Bringmann et al. 2005). To date, the bioactivity of fungal secondary metabolites has been studied in a wide variety of pathways. However, studies involving the screening of fungi-derived compounds according to their neuroprotective features remain rare in the literature. In this study, we first time evaluated the neuroprotective effects of Sw-fr, a 6-OHDA-induced neurotoxicity model of PD. According to our results, 1 and 10 µg/mL Sw-fr and sorbicillin standard were determined to be significantly neuroprotective when compared to the 6-OHDA group. Additionally, the effects of Sw-fr and sorbicillin on mitochondrial membrane potential were also investigated and espicially 1 and 10 µg/mL Sw-fr and sorbicillin reduced mitochondrial depolarisation, which increased approximately sevenfold with 6-OHDA compared to the control. Caspases are a family of cysteinyl aspartate-specific proteases that serve an important role in regulating and performing apoptotic cell death. Caspase-3 is an important effector in most studied apoptotic protein and apoptosis pathways, which strengthens the signal from the initiator caspases (such as caspase-8) and triggers apoptotic breakdown (Iijima 2006). Since caspase-3 is known to be a major contributor to the apoptotic machinery in many cell types (including neurons), potent caspase-3 inhibitors have emerged as a therapeutic target for developing neuroprotective compounds. According to our results, caspase-3 levels increased with 6-OHDA treatment by approximately sixfold compared to the control, while caspase-3 levels decreased significantly with both Sw-fr and sorbicillin concentrations. But, 1 and 10 μg/mL Sw-fr was determined to be more neuroprotective than standart sorbicillin in both flow cytometry experiments. To determine whether Sw-fr protects against 6-OHDA toxicity by reducing ROS production, ROS quantities were measured after 24 h of treatment. Notably, 6-OHDA increased ROS production by 50% when compared to the control group with or without the ROS inducer. ROS formation caused by 6-OHDA was slightly suppressed with both pre- or post-treatment with Sw-fr and sorbicillin concentrations. This indicates that Sw-fr and sorbicillin can potentially protect neurons against 6-OHDA damage by reducing the intracellular production of ROS. In this context, similar results have been reported from various studies conducted with primary neurons and neuronal cell lines, in which 6-OHDA-induced cell death was associated with increased ROS formation. Sensitivity to 6-OHDA-induced apoptosis has been reported in various cell types, including primary rat striatal and ventral mesencephalic neurons, PC-12 Adh pheochromocytoma cells and other human neuroblastoma strains. However, the mechanisms that trigger and maintain 6-OHDA-induced neurotoxicity remain unclear. 6-OHDA is an oxidative toxic metabolite of dopamine, while PD pathogenesis involves exposure to 6-OHDA. Moreover, 6-OHDA has been identified as endogenous in humans and clinical suitability was supported by detecting high concentrations of the compound in urine and brain samples of PD patients treated with L-DOPA (Gliyazova and Ibeanu 2016). This is the first study to investigate the effects of sorbicillin-like compounds on ROS formation in neuronal cell culture. In parallel with our findings, a study investigating the effects of 6-OHDA caused toxicity on SH-SY5Y cells, 6-OHDA reduced in vitro cell viability and increased the level of intracellular ROS production (Jaisin et al. 2018). Also, 6-OHDA-induced ROS initiates the phosphorylation of p38 MAPK, which is a key apoptotic event that leads to the induction of cytochrome c release and the cleavage of caspase-8 and caspase-9. In response to 6-OHDA-induced apoptosis, p38 MAPK phosphorylation, caspase and Bax are activated (Choi et al. 2004). Moreover, it was reported that HT22 cell death induced by 6-OHDA as a murine neuronal cell model initiates through a pathway including the mitochondria by increasing ROS levels, raising intracellular calcium ([Ca2+]i), enhancing the release of cytochrome c to the cytosol, and promoting activation of stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) signalling (Kim et al. 2019). According to our results, 6-OHDA causes cell death by increasing ROS levels by increasing the release of cytochrome c to the cytosol via the mitochondrial route in PC-12 cells. PC-12 cells are protected by sorbicillin and Sw-fr against 6-OHDA-induced neuronal cell death via ROS production reducing and the inhibition of mitochondrial depolarisation and caspase-3 activation.
According to our results, neurite length significantly increased with 1 and 10 µg/mL Sw-fr concentrations when compared to the positive control NGF. In parallel with our neurite length analysis results, the highest neurite differentiation and elongation was also visualised with immunofluorescence staining. As far as we know, this is the first study that evaluates the significant effects of sorbicillin-like fungal metabolites derived from P. flavigenum on neurite prolongation and differentiation.
Conclusions
In conclusion, PD is an incurable disease that significantly reduces patients’ quality of life. Sorbicillin-like secondary metabolites of halotolerant P. flavigenum isolated from Tuz Lake may have clinical potential, with their neuroprotective and neuritogenic effects potentially serving as a new treatment strategy for neurodegenerative diseases such as PD. Such active compounds with natural origins, especially from extreme environments, might represent a new therapeutic approach that should be investigated further.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Scientific and Technological Research Council of Turkey (Project Number: 116R006).
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Declarations
Conflict of interest
None of the authors had any conflict of interest that could affect the performance of the work or the interpretation of the data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Lang G, Mühlbacher J, et al. Sponges (Porifera) New York: Springer; 2003. Sorbicillactone A: a structurally unprecedented bioactive novel-type alkaloid from a sponge-derived fungus; pp. 231–253. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Lang G, Gulder TAM, et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron. 2005;61:7252–7265. doi: 10.1016/j.tet.2005.05.026. [DOI] [Google Scholar]
- Canturk Z, Kocabiyik E, Ozturk N, İlhan S. Evaluation of antioxidant and antiproliferative metabolites of Penicillium flavigenum isolated from hypersaline environment: Tuz (Salt) Lake by Xcelligence technology. Microbiology. 2017;86:346–354. doi: 10.1134/S0026261717030055. [DOI] [Google Scholar]
- Choi W-S, Eom D-S, Han BS, et al. Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8-and-9-mediated apoptotic pathways in dopaminergic neurons. J Biol Chem. 2004;279:20451–20460. doi: 10.1074/jbc.M311164200. [DOI] [PubMed] [Google Scholar]
- Chu Y-F, Chang W-H, Black RM, et al. Crude caffeine reduces memory impairment and amyloid β1–42 levels in an Alzheimer’s mouse model. Food Chem. 2012;135:2095–2102. doi: 10.1016/j.foodchem.2012.04.148. [DOI] [PubMed] [Google Scholar]
- Dikmen M, Cantürk Z, Kaya Tilki E, Engür S. Evaluation of antiangiogenic and antimetastatic effects of Penicillium chrysogenum secondary metabolites. Indian J Pharm Sci. 2017;79:49–57. doi: 10.4172/pharmaceutical-sciences.1000200. [DOI] [Google Scholar]
- Dikmen M, Kaya-Tilki E, Engur S, Ozturk Y. Neuritogenic activity of epigallocatechin gallate and curcumin combination on rat adrenal pheochromocytoma cells. Fresenius Environ Bull. 2017;26:4726. [Google Scholar]
- Dominguete L, Takahashi JA. Filamentous fungi as source of biotechnologically useful metabolites and natural supplements for neurodegenerative diseases treatment. Chem Eng Trans. 2018;64:295–300. [Google Scholar]
- Du L, Zhu T, Li L, et al. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem Pharm Bull. 2009;57:220–223. doi: 10.1248/cpb.57.220. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Kanai S, Kakinuma K, et al. Synthesis of NG-061 and its analogs, and their biological evaluation as an enhancer of nerve growth factor. Chem Pharm Bull. 2000;48:1470–1473. doi: 10.1248/cpb.48.1470. [DOI] [PubMed] [Google Scholar]
- Engür S, Dikmen M, Öztürk Y. Comparison of antiproliferative and apoptotic effects of a novel proteasome inhibitor MLN2238 with bortezomib on K562 chronic myeloid leukemia cells. Immunopharmacol Immunotoxicol. 2016;38:87–97. doi: 10.3109/08923973.2015.1122616. [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O. Modern concepts in Penicillium and Aspergillus classification. New York: Springer; 1990. Secondary metabolites as consistent criteria in Penicillium taxonomy and a synoptic key to Penicillium subgenus Penicillium; pp. 373–384. [Google Scholar]
- Frisvad JC, Samson RA. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol. 2004;49:1–174. [Google Scholar]
- Gliyazova NS, Ibeanu GC. The chemical molecule B355252 is neuroprotective in an in vitro model of Parkinson’s disease. Cell Mol Neurobiol. 2016;36:1109–1122. doi: 10.1007/s10571-015-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijseels S, Nielsen JC, Nielsen J, et al. Physiological characterization of secondary metabolite producing Penicillium cell factories. Fungal Biol Biotechnol. 2017;4:1–12. doi: 10.1186/s40694-017-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena D, Shanmugam K, Low M, et al. Determination of anti-inflammatory activities of standardised preparations of plant-and mushroom-based foods. Eur J Nutr. 2014;53:335–343. doi: 10.1007/s00394-013-0531-9. [DOI] [PubMed] [Google Scholar]
- Harned AM, Volp KA. The sorbicillinoid family of natural products: isolation, biosynthesis, and synthetic studies. Nat Prod Rep. 2011;28:1790–1810. doi: 10.1039/c1np00039j. [DOI] [PubMed] [Google Scholar]
- Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res. 2006;55:234–243. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ito M, Tsuchida Y, Mizoue K, Hanada K. NG-011 AND NG-012, novel potentiators of nerve growth factor II. The structure determination of NG-011 AND NG-012. J Antibiot (tokyo) 1992;45:1566–1572. doi: 10.7164/antibiotics.45.1566. [DOI] [PubMed] [Google Scholar]
- Jaisin Y, Ratanachamnong P, Kuanpradit C, et al. Protective effects of γ-mangostin on 6-OHDA-induced toxicity in SH-SY5Y cells. Neurosci Lett. 2018;665:229–235. doi: 10.1016/j.neulet.2017.11.059. [DOI] [PubMed] [Google Scholar]
- Kaya-Tilki E, Dikmen M, Öztürk Y. Effects of DNMT and HDAC inhibitors (RG108 and trichostatin a) on NGF-induced Neurite outgrowth and cellular migration. Int J Pharmacol. 2016;12:351. doi: 10.3923/ijp.2016.351.360. [DOI] [Google Scholar]
- Kim KY, Hwang S-K, Park SY, et al. l-Serine protects mouse hippocampal neuronal HT22 cells against oxidative stress-mediated mitochondrial damage and apoptotic cell death. Free Radic Biol Med. 2019;141:447–460. doi: 10.1016/j.freeradbiomed.2019.07.018. [DOI] [PubMed] [Google Scholar]
- Lian Z, Niwa K, Gao J, et al. Association of cellular apoptosis with anti-tumor effects of the Chinese herbal complex in endocrine-resistant cancer cell line. Cancer Detect Prev. 2003;27:147–154. doi: 10.1016/S0361-090X(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Lin C-M, Lin Y-T, Lin R-D, et al. Neurocytoprotective effects of aliphatic hydroxamates from lovastatin, a secondary metabolite from monascus-fermented red mold rice, in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. ACS Chem Neurosci. 2015;6:716–724. doi: 10.1021/cn500275k. [DOI] [PubMed] [Google Scholar]
- Liu H, Mao P, Wang J, et al. Allicin protects PC12 cells against 6-OHDA-induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell Physiol Biochem. 2015;36:966–979. doi: 10.1159/000430271. [DOI] [PubMed] [Google Scholar]
- Malpure PP, Shah AS, Juvekar AR (2006) Antioxidant and anti-inflammatory activity of extract obtained from Aspergillus candidus MTCC 2202 broth filtrate [PubMed]
- Maskey RP, Grün-Wollny I, Laatsch H. Sorbicillin analogues and related dimeric compounds from Penicillium n otatum. J Nat Prod. 2005;68:865–870. doi: 10.1021/np040137t. [DOI] [PubMed] [Google Scholar]
- Meng J, Wang X, Xu D, et al. Sorbicillinoids from fungi and their bioactivities. Molecules. 2016;21:715. doi: 10.3390/molecules21060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AM, Maggi F, Papa F, et al. Isofuranodiene: a neuritogenic compound isolated from wild celery (Smyrnium olusatrum L., Apiaceae) Food Chem. 2016;192:782. doi: 10.1016/j.foodchem.2015.07.079. [DOI] [PubMed] [Google Scholar]
- Nicolaou KC, Simonsen KB, Vassilikogiannakis G, et al. Biomimetic explorations towards the bisorbicillinoids: total synthesis of bisorbicillinol, bisorbibutenolide, and trichodimerol. Angew Chem Int Ed. 1999;38:3555–3559. doi: 10.1002/(SICI)1521-3773(19991203)38:23<3555::AID-ANIE3555>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Oxford AE, Raistrick H, Simonart P. Studies in the biochemistry of micro-organisms: griseofulvin, C17H17O6Cl, a metabolic product of Penicillium griseo-fulvum Dierckx. Biochem J. 1939;33:240. doi: 10.1042/bj0330240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan C-W, David P, Naidu M, et al. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: diversity, metabolite, and mechanism. Crit Rev Biotechnol. 2015;35:355–368. doi: 10.3109/07388551.2014.887649. [DOI] [PubMed] [Google Scholar]
- Phan C-W, David P, Sabaratnam V. Edible and medicinal mushrooms: emerging brain food for the mitigation of neurodegenerative diseases. J Med Food. 2017;20:1–10. doi: 10.1089/jmf.2016.3740. [DOI] [PubMed] [Google Scholar]
- Potashkin J, Seidl SE. The promise of neuroprotective agents in Parkinson’s disease. Front Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo O, Guzmán-Chávez F, Ries MI, et al. Identification of a polyketide synthase involved in sorbicillin biosynthesis by Penicillium chrysogenum. Appl Environ Microbiol. 2016;82:3971–3978. doi: 10.1128/AEM.00350-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. Identification and nomenclature of the genus Penicillium. Stud Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-T, Xue Y-R, Liu C-H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar Drugs. 2015;13:4594–4616. doi: 10.3390/md13084594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr GA, Veitch JA, Walsh ANNW, et al. BMS-182123, a fungal metabolite that inhibits the production of TNF-α by macrophages and monocytes. J Antibiot (tokyo) 1996;49:234–240. doi: 10.7164/antibiotics.49.234. [DOI] [PubMed] [Google Scholar]
- Washida K, Abe N, Sugiyama Y, Hirota A. Novel DPPH radical scavengers, demethylbisorbibutenolide and trichopyrone, from a fungus. Biosci Biotechnol Biochem. 2007;71:1052–1057. doi: 10.1271/bbb.60711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).