Abstract
Color detection is used by animals of diverse phyla to navigate colorful natural environments and is thought to require evolutionarily conserved opsin photoreceptor genes. We report that Caenorhabditis elegans roundworms can discriminate among colors despite lacking eyes or opsins. Specifically, we found that white light guides C. elegans foraging decisions away from a blue pigment toxin secreted by harmful bacteria. These foraging decisions are guided by specific blue-to-amber ratios of light. The color specificity of color-dependent foraging varies strikingly among wild C. elegans strains, indicating that color discrimination is ecologically important. We identified two evolutionarily conserved cellular stress-response genes required for opsin-independent color-dependent foraging by C. elegans and speculate that cellular stress response pathways can more generally mediate spectral discrimination by photosensitive cells and organisms, even those lacking opsins.
One-sentence summary:
Despite lacking opsins, the worm Caenorhabditis elegans can discriminate among colors in environmental light when making foraging decisions.
The roundworm C. elegans lives in decomposing organic matter like compost heaps, where it feeds on microorganisms (1, 2), some of which secrete colorful pigments (3–5). C. elegans lacks the specialized eyes, photoreceptor cells, and opsin genes underlying canonical visual system functions (6–8). Nevertheless, C. elegans can detect and respond to short-wavelength light, including blue light, using the LITE-1 and GUR-3 proteins, which are similar to insect gustatory chemoreceptors (9–14). While visible light can influence C. elegans physiology (15) and behavior (9–14), whether microbial pigments affect C. elegans foraging has not been addressed.
We asked if white light alters the avoidance by C. elegans strain N2 of pathogenic Pseudomonas aeruginosa PA14 bacterial lawns secreting the blue pigment pyocyanin, a reactive oxygen species (ROS)-generating toxin (Fig. 1A, fig. S1A) (16–23). White light dramatically potentiated gradual avoidance of PA14 but not of non-toxic E. coli strain OP50 (Fig. 1B). lite-1 null-mutant worms also avoided PA14, but their avoidance was unaffected by white light (fig. S1B). We examined avoidance of a P. aeruginosa mutant strain, PA14ΔphzM, that cannot synthesize pyocyanin but still synthesizes other non-blue ROS-generating toxins (19). PA14ΔphzM cultures were not blue (Fig. 1C), and light only minimally affected avoidance of PA14ΔphzM by wild-type or lite-1 null-mutant worms (Fig. 1D, fig. S1C). These results demonstrate that light-dependent potentiation of PA14 avoidance requires both lite-1 and pyocyanin.
Figure 1. Blue pigment toxin pyocyanin underlies light-potentiated avoidance of P. aeruginosa.
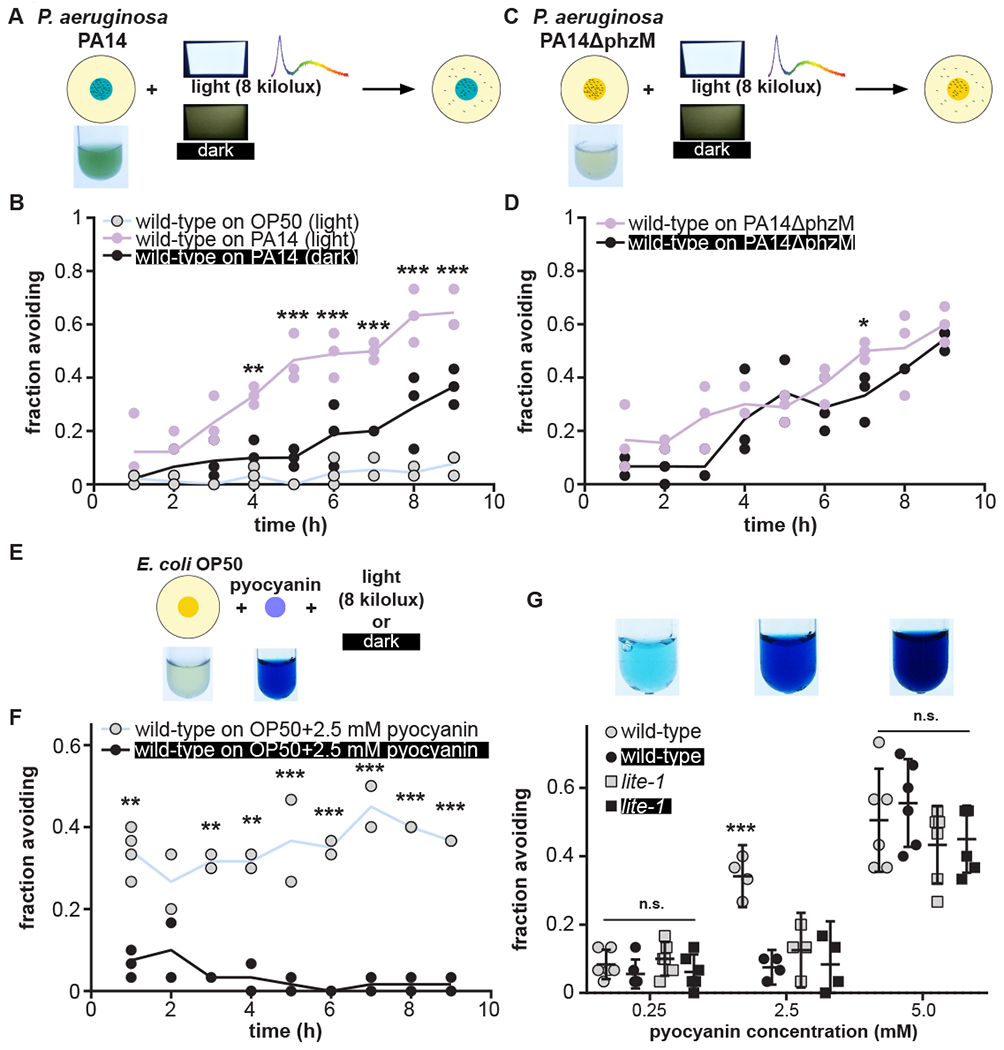
(A) Schematic depicting worms (n = 30/assay) on a lawn of P. aeruginosa strain PA14 in the absence (dark) or presence (light) of 8 kilolux white light. Lines represent the average of assays individually depicted by data points. (B) Time-course of wild-type worm avoidance of lawns of PA14 in the light (purple) and dark (black) or E. coli OP50 in the light (gray). n=3. (C,D) Time-course of wild-type worm avoidance of PA14ΔphzM lawns in light (purple) and dark (black). PA14ΔphzM is incapable of synthesizing pyocyanin (note that these cultures are not blue). n = 3. (E,F) Time-course of avoidance by wild-type worms of E. coli OP50 lawns supplemented with 2.5 mM pyocyanin in the presence (gray) or absence (black) of light. n = 4 for measurement at 1 hr, n = 2 for measurements 2-9 hrs. (G) One-hour avoidance of OP50 lawns supplemented with 0.25 mM, 2.5 mM, or 5 mM pyocyanin by wild-type (circles) and lite-1 null-mutant (squares) worms in the presence (gray) or absence (black) of light. Data for wild-type avoidance of lawns supplemented with 2.5 mM pyocyanin in panels F and G were from the same experiments. (All statistical comparisons for time-course experiments were by two-way ANOVA with time as a repeated measure and post-hoc Bonferroni tests. p and F values, see Table S1. Experiments are single time-point (1 hr) unless otherwise indicated. Statistical analyses were performed by one-way ANOVA with post-hoc Tukey-Kramer for pairwise comparisons or Dunnett or Bonferroni tests as appropriate for comparisons with control; error bars denote 95% C.I.; and * indicates p < 0.05, ** indicates p ≤ 0.01, and *** indicates p ≤ 0.001.)
By supplementing lawns of non-toxic OP50 with pyocyanin (Fig. 1E), we found that pyocyanin is sufficient to confer light- and lite-1-dependent avoidance to otherwise innocuous bacteria (Fig. 1F,G). We tested whether a chemically inert blue dye spectrally matched (fig. S2A) to pyocyanin coupled with the colorless ROS-generating toxin paraquat (fig. S2B) would support light-potentiated avoidance. Light potentiated wild-type but not lite-1 avoidance of OP50 supplemented with both blue dye and paraquat, but not with either independently (Fig. 2A,B,C,D, fig. S2C,D,E,F). This observation indicates that avoidance of pyocyanin-containing lawns relies on both pyocyanin’s chemical and spectral properties.
Figure 2. Spectral content potentiates avoidance of toxic bacterial lawns.
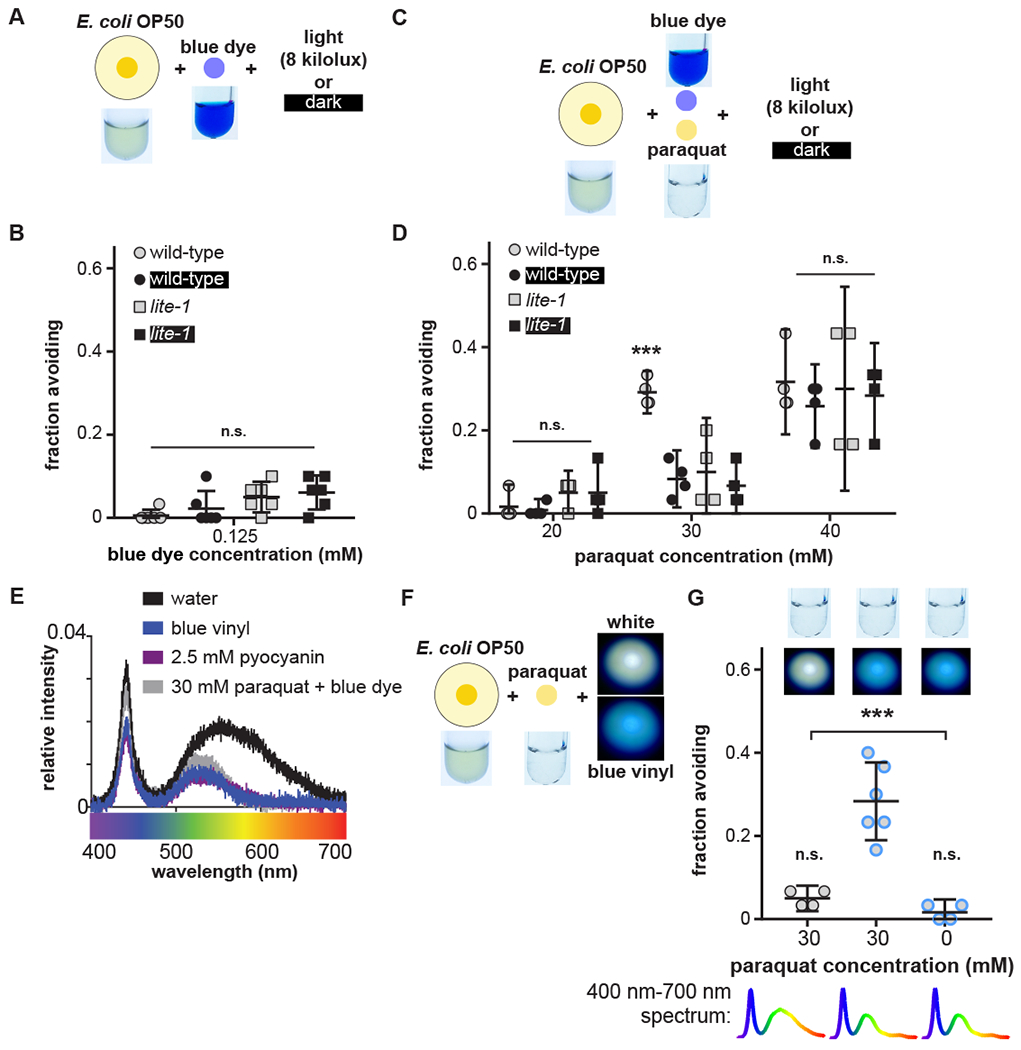
(A,B) Avoidance of OP50 lawns supplemented with 0.125 mM blue dye. (C,D) Avoidance of OP50 lawns supplemented with 0.125 mM blue dye and specified concentrations of paraquat. (E) Comparison of spectra of white light filtered with water, blue vinyl filter, 2.5 mM pyocyanin solution, or 30 mM paraquat and blue dye solution. (F, G) Avoidance of OP50 lawns supplemented with or without paraquat in the presence (gray with blue borders) or absence (gray) of a blue vinyl filter modifying the color of the white light.
Using optical filters (fig. S3A,B), we found that eliminating short-wavelength blue or long-wavelength amber light disrupted light-potentiated avoidance (fig. S3C). By contrast, directly filtering incident white light through a “blue vinyl” filter that increased the blue-to-amber ratio to match the spectral properties of pyocyanin potentiated avoidance of OP50 supplemented with paraquat without blue pigment (Fig. 2E,F,G). These results indicate that blue pigments enhance avoidance by changing the spectrum of light in the worm’s environment (also see fig. S4). Blue vinyl-filtered light also potentiated avoidance of OP50 lawns in the presence of the aversive odorant 1-octanol (24), suggesting that color might generally influence avoidance of aversive stimuli (Fig. 3A,B, fig. S5A).
Figure 3. Foraging is guided by the relative intensities of blue and amber light.

(A, B) Avoidance of OP50 lawns in the presence of the specified dilutions of 1-octanol and in the presence or absence of the blue vinyl filter. (C, D) Avoidance of OP50 lawns in the presence of 5% 1-octanol and incident light of different colors composed of the specified ratios of narrow-band blue and amber light.
To analyze spectral influences on foraging, we tested combinations of monochromatic blue and amber light sources for potentiation of OP50 lawn avoidance in the presence of 1-octanol (Fig. 3C). While neither pure blue nor pure amber light potentiated lawn avoidance, mixed colors differentially potentiated avoidance, depending on the blue-to-amber ratio (Fig. 3D). This observation indicates that the relative intensities of blue and amber visible light guide foraging decisions and establishes that C. elegans can discriminate colors.
Next we asked if C. elegans strains independently isolated from the wild and presumably adapted to diverse ecological niches (25–27) exhibit variation in color-dependent foraging. We detected substantial variation in 4:1 and 1:2 blue-to-amber spectrum-dependent foraging among 59 wild strains (Fig. 4A). Interestingly, 4:1 and 1:2 blue-to-amber sensitivity were uncorrelated (Fig. 4B), suggesting that complex, potentially distinct mechanisms underlie color-specific sensitivities. For example, compared to N2, strains CX11276 and JU830 exhibited relatively heightened sensitivity to 4:1 blue-to-amber light and 1:2 blue-to-amber light, respectively, while NIC2 maximally avoided the lawn regardless of color (Fig. 4A). To dissect how spectral discrimination and 1-octanol avoidance contribute to color-dependent foraging, we tested avoidance of lawns illuminated with 4:1 and 1:2 blue-to-amber light, with and without octanol, by these strains. CX11276 and JU830 were sensitive to specific colors even without 1-octanol, whereas NIC2 avoided lawns with 1-octanol even without light (Fig. 4C). Thus, whereas color-dependent lawn avoidance by N2, which is relatively insensitive to colors, requires the presence of an additional aversive stimulus, for more color-sensitive strains like CX11276, color-specific illumination is sufficient. These results demonstrate that naturally varying color and odorant sensitivities drive strain differences in foraging.
Figure 4. Evolutionarily conserved genes jkk-1 and lec-3 are required for naturally varying color-dependent foraging.

(A) Avoidance of OP50 lawns by fifty-nine wild C. elegans strains in the presence of 5% 1-octanol and 4:1 or 1:2 blue-to-amber light. N2, CX11276, JU830, and NIC2 strains are indicated in red. n = 2. (B) Correlation of lawn avoidance in the presence of 5% 1-octanol and 4:1 and 1:2 blue-to-amber light by each wild strain. Pearson’s r(57) = 0.24, p = 0.063. (C) Avoidance of OP50 lawns by N2, CX11276, JU830, and NIC2 strains with or without 5% 1-octanol and 4:1 or 1:2 blue-to-amber light as specified. (D) Statistical genetics analysis with highest-scoring polymorphisms (indicated in red) and neighboring high-confidence polymorphisms suggested candidate genes chtl-1 and jkk-1 (blue box) and F52H3.6 and lec-3 (orange box). (E) Avoidance of OP50 lawns in the presence of 5% 1-octanol and 4:1 or 1:2 blue-to-amber light by chtl-1, jkk-1, F52H3.6, and lec-3 null-mutant worms. (F) Blue pigment absorbs long-wavelength light and thereby alters the spectral composition of light detected. The integration of color and chemical information guides worms’ foraging decision to stay on or leave bacterial lawns.
Using approaches adapted from statistical genetics (27), we determined that no single genomic polymorphism could causally account for the observed variation in color-dependent foraging (Fig. 4D). However, by considering together multiple neighboring SNPs within a given genomic region (28), we identified two sets of two genes - chtl-1 and jkk-1, and F52H3.6 and lec-3 – in the regions of the two highest-scoring polymorphisms contributing to variation in avoidance of lawns under 4:1 and 1:2 blue-to-amber light, respectively (Fig. 4E, fig. S6). Color-dependent avoidance was abolished in two independent null-mutant strains of each of jkk-1 and lec-3, while color-dependent foraging by chtl-1 and F52H3.6 null-mutant worms was unaffected (Fig. 4E). Loss of jkk-1 or lec-3 function did not impair avoidance responses to brighter blue light or higher 1-octanol concentrations (fig. S7). These results revealed that jkk-1 and lec-3 contribute to color-dependent foraging.
In short, we established that C. elegans discriminates colors and identified two genes required for color-dependent foraging. Mammalian homologs of jkk-1 and lec-3 – MKK7, an activator of c-Jun N-terminal Kinases (JNKs) (29), and galectin, a member of a protein family that binds beta-galactoside sugars (30), respectively – can interact in mediating cellular responses to stressors, including ultraviolet light (31–37). Genes like jkk-1 and lec-3 might function in opsin-independent spectrally-sensitive stress response pathways to guide C. elegans foraging decisions on food sources varying in color and toxicity (Fig. 4F). The functions of microbial pigmentation are poorly understood (38). We suggest that pigmentation contributes to evolving interactions between pathways underlying the synthesis and secretion of pigmented factors by microbes and the responses to these pigments by foraging hosts like C. elegans.
Supplementary Material
Acknowledgments:
We thank S. Xu, E. Andersen, S. Mitani, and the Caenorhabditis Genetics Center for C. elegans strains, F. Ausubel and L. Dietrich for P. aeruginosa strains, and members of the Nitabach, Horvitz, and Kazmierczak labs for technical support, advice, and comments. D.D.G. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. Work in the laboratory of H.R.H. was supported by National Institute of General Medical Sciences (NIGMS), NIH (R01GM024663). H.R.H. is an investigator of the Howard Hughes Medical Institute. Work in the laboratory of M.N.N. is supported in part by NIGMS, NIH (R01GM098931).
Footnotes
Competing Financial Interests:
The authors declare no competing financial interests.
Data and materials availability:
All data are available in the manuscript or the supplementary material.
References:
- 1.Frezal L, Felix MA, Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel BS, Rowedder H, Braendle C, Felix MA, Ruvkun G, Proc Natl Acad Sci U S A, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradel E et al. , Proc Natl Acad Sci U S A 104, 2295–2300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burlinson P et al. , ISME J 7, 1126–1138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page AP et al. , BMC Biol 17, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichaud F, Briscoe A, Desplan C, Curr Opin Neurobiol 9, 622–627 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Longden KD, Curr Biol 26, R981–R988 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Cuthill IC et al. , Science 357, (2017). [DOI] [PubMed] [Google Scholar]
- 9.Edwards SL et al. , PLoS Biol 6, e198 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward A, Liu J, Feng Z, Xu XZ, Nat Neurosci 11, 916–922 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J et al. , Nat Neurosci 13, 715–722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatla N, Horvitz HR, Neuron 85, 804–818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatla N, Droste R, Sando SR, Huang A, Horvitz HR, Curr Biol 25, 2075–2089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong J et al. , Cell 167, 1252–1263 e1210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Magalhaes Filho CD et al. , Nat Commun 9, 927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM, Cell 96, 47–56 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Tan MW, Mahajan-Miklos S, Ausubel FM, Proc Natl Acad Sci U S A 96, 715–720 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM, Proc Natl Acad Sci U S A 96, 2408–2413 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cezairliyan B et al. , PLoS Pathog 9, e1003101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Lu H, Bargmann CI, Nature 438, 179–184 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Meisel JD, Kim DH, Trends Immunol 35, 465–470 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH, Cell 159, 267–280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh J, Aballay A, Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bargmann CI, Hartwieg E, Horvitz HR, Cell 74, 515–527 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Barrière A, Fèlix MA, WormBook, 1–19 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans KS et al. , G3 (Bethesda) 7, 289–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook DE, Zdraljevic S, Roberts JP, Andersen EC, Nucleic Acids Res 45, D650–D657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J et al. , Nat Genet 44, 369–375, S361–363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawasaki M et al. , EMBO J 18, 3604–3615 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemoto-Sasaki Y et al. , Biochim Biophys Acta 1780, 1131–1142 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Foltz IN et al. , J Biol Chem 273, 9344–9351 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Bernerd F, Sarasin A, Magnaldo T, Proc Natl Acad Sci U S A 96, 11329–11334 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tournier C et al. , Science 288, 870–874 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Tournier C et al. , Genes Dev 15, 1419–1426 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwabara I et al. , J Biol Chem 277, 3487–3497 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Saegusa J et al. , J Invest Dermatol 128, 2403–2411 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt B, Abou-Eladab EF, Tiedge M, Walzel H, Cell Death Dis 1, e23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu GY, Nizet V, Trends Microbiol 17, 406–413 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI, Nature 472, 313–318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reference Solar Spectral Irradiance: ASTM G-173.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript or the supplementary material.


