Abstract
This study evaluated the susceptibility of pneumococci to cefditoren by agar dilution and microdilution methods (both in air) and by E-test (AB Biodisk, Solna, Sweden) and disk diffusion methods (both in CO2). By the three MIC tests, the MICs at which 50 and 90% of isolates were inhibited (MIC50s and MIC90s) were, respectively, as follows (in micrograms per milliliter): for the 65 penicillin-susceptible strains tested, 0.016 and 0.03 (by agar dilution), 0.016 and 0.03 (by microdilution), and 0.016 and 0.03 (by E test); for the 68 penicillin-intermediate strains tested, 0.125 and 0.5 (by agar dilution), 0.125 and 0.5 (by microdilution), and 0.25 and 0.5 (by E test); and for the 67 penicillin-resistant strains tested, 1.0 and 1.0 (by agar dilution), 0.5 and 1.0 (by microdilution), and 1.0 and 1.0 (by E test). With tentative cefditoren breakpoints (in micrograms per milliliter) of ≤2.0 (susceptible), 4.0 (intermediate), and ≥8.0 (resistant), all strains were susceptible to cefditoren by agar, microdilution, and E-test results; with breakpoints of ≤1.0, 2.0, and ≥4.0 μg/ml, 97% of strains were cefditoren susceptible by agar dilution results, 98% were susceptible by microdilution results, and 99% were susceptible by E-test results. When microdilution and E-test results were compared to those from the reference agar dilution method, 191 (95.5%) and 183 (91.5%) of strains gave essential agreement (±1 log2 dilution); 8 (2.7%) minor discrepancies were found for both methods with a breakpoint of ≤1.0 μg/ml, and no discrepancies were found with a breakpoint of ≤2.0 μg/ml. Disk test results (breakpoint, ≤1.0 μg/ml) produced 2 major and 30 minor errors, with corresponding zone diameters (in millimeters) of ≥20 (susceptible), 17 to 19 (intermediate), and ≤16 (resistant); a ≤2.0-μg/ml breakpoint yielded zone diameters of ≥16 mm (susceptible). All three methods for testing the MIC of cefditoren showed excellent correlation.
Infections caused by pneumococci with increased MICs of penicillin G and other β-lactam and non-β-lactam antibiotics have become a problem, both throughout the United States and elsewhere (1, 5, 6). Previous studies have documented a rate of penicillin resistance of >30% in cases of complicated otitis media (5, 6). The problem of drug-resistant pneumococci is complicated by the ability of this organism to spread from country to country, and from continent to continent (12).
Cefditoren is an oral cephalosporin with excellent activity against penicillin-susceptible, -intermediate and -resistant pneumococci. Because of its excellent concomitant activity against Haemophilus influenzae and Moraxella catarrhalis (2, 4, 7, 10, 15, 17–20), this compound shows great promise for empiric treatment of otitis media and other respiratory tract infections and has been successfully used in Japan for this purpose for several years (17).
Methods used in the routine clinical laboratory to test the activity of antimicrobials against pathogens, including pneumococci, comprise agar dilution, broth microdilution, E test (AB Biodisk, Solna, Sweden), and disk diffusion. The E test, consisting of a continuous stable gradient of antimicrobial agent corresponding to 15 twofold dilutions on a strip, has gained wide acceptance as an accurate, routine method for MIC determination; this has been confirmed by a recently completed chloramphenicol-based study (8). The technique is less labor-intensive than standard agar and microdilution methods (3).
In the present study, we tested the activity of cefditoren against 200 penicillin-susceptible, -intermediate, and -resistant pneumococci by agar dilution, broth microdilution, E-test, and disk diffusion methods.
MATERIALS AND METHODS
Bacteria and antimicrobials.
Organisms tested comprised 65 penicillin-susceptible (MICs of ≤0.06 μg/ml), 68 penicillin-intermediate (MICs of 0.125 to 1.0 μg/ml), and 67 penicillin-resistant (MICs of ≥2.0 μg/ml) strains, stored in double-strength litmus milk (Difco Laboratories, Detroit, Mich.) at −70°C prior to testing. Because all strains were stock isolates subcultured many times, all grew well in air, without needing any additional CO2 in the incubation atmosphere. Cefditoren powder was obtained from Meiji Seika Kaisha Laboratories, Tokyo, Japan. No other drug from the same class as cefditoren was included as an additional quality control.
Interpretive breakpoints.
Cefditoren MICs were interpreted at tentative susceptible breakpoints of ≤1 and ≤2 μg/ml, intermediate breakpoints of 2 and 4 μg/ml, and resistant breakpoints of ≥4 and ≥8 μg/ml, respectively. Tentative breakpoints of ≤2 μg/ml (susceptible), 4 μg/ml (intermediate), and ≥8 μg/ml (resistant) were suggested to us by the manufacturer, based upon the fact that following a 200- or 400-mg dose of cefditoren as cefditoren pivoxil, maximal cefditoren concentrations in plasma are achieved at 2.5 h after the dose, with maximum concentrations in serum of 2.8 and 4.8 μg/ml, respectively. Additionally, with either of these doses, cefditoren concentrations in plasma can be expected to exceed 0.5 μg/ml for 5 to 8 h and to exceed 1.0 μg/ml for 4 to 6 h. The drug has a terminal elimination half-life in plasma of 1.5 to 3 h (11). However, because no definite breakpoints have been recommended yet for cefditoren by the National Committee for Clinical Laboratory Standards (NCCLS), we decided to also analyze data based upon breakpoints 1 dilution lower than the ones suggested.
Agar dilution MICs.
Agar dilution MIC tests were performed according to standard methods (6). Media consisted of Mueller-Hinton agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.) (supplemented with 5% sheep blood), with cefditoren incorporated at concentrations from 0.002 to 16 μg/ml in doubling dilutions. Inocula were prepared by suspending growth from overnight cultures in Mueller-Hinton broth (BBL) to a turbidity of a 0.5 McFarland standard. Final inocula contained 104 organisms/spot. Plates were inoculated with a Steers replicator with 3-mm inoculating pins and incubated overnight at 35°C in air. The lowest concentration of antibiotic showing no growth was read as the MIC. Quality control organisms included Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, and Streptococcus pneumoniae ATCC 49619 and were included in each run. Antimicrobial-containing plates were prepared in-house, stored at 4°C, and used within 7 days of preparation. All strains were tested concomitantly for penicillin susceptibility with each run. The stability of the plates was proven by performing selected tests on the day of preparation and 1 week later. In all cases, identical results were obtained.
Microdilution MICs.
MICs were determined by the broth microdilution method recommended by the NCCLS (13), using cation-adjusted Mueller-Hinton broth (BBL) supplemented with 5% lysed horse blood for pneumococci. Trays were prepared freshly in-house, frozen at −70°C if necessary, and used within 2 days of preparation. For MIC determinations, suspensions with a turbidity equivalent to that of a 0.5 McFarland standard were prepared by suspending growth from blood agar plates in 2 ml of sterile saline. Suspensions were further diluted 1:10 to obtain a final inoculum of 5 × 105 CFU/ml. Trays were incubated for 20 to 24 h in ambient air at 35°C. Standard quality control strains (as described above) were included in each run. The stability of trays after storage was tested and confirmed as described above.
E-test (AB Biodisk) MICs.
Mueller-Hinton plates supplemented with 5% sheep blood were inoculated with a 0.5 McFarland standard of suspension harvested from plates, and cefditoren E-test strips (0.016 to 256 μg/ml) were placed on each. The MIC was determined from the intersection of the ellipse of growth inhibition with the strip (3). Where colonies occurred within the inhibition ellipse, the higher value was used as the MIC. E-test plates were incubated under 5 to 10% CO2 at 35°C for 20 to 24 h.
Disk diffusion testing.
Disk diffusion testing was by standard NCCLS methods (14) using Mueller-Hinton plates supplemented with sheep blood and inoculated with a 0.5 McFarland standard. Cefditoren disks (5 μg; Becton Dickinson Microbiology Systems) were applied. After overnight incubation at 35°C, zone diameters were read with the aid of calipers. Disk diffusion test disks were incubated under 5 to 10% CO2, as recommended by the NCCLS (14).
Interpretation of results.
A MIC within ±1 log2 dilution compared to the MIC determined by the agar dilution reference method was interpreted as essential agreement. Very major discrepancies occurred when the reference method showed resistance and the comparative method showed susceptibility; major discrepancies occurred when the reference method showed susceptibility and the comparative method showed resistance (3). Minor discrepancies occurred when an intermediate result was obtained with one method and a resistant or susceptible result was obtained with the other. Cefditoren quality control values were those recommended by the manufacturer and validated by previous studies in our laboratory (18, 19). Values with S. pneumoniae ATCC 49619 were 0.03 to 0.06 μg/ml (by agar dilution), 0.03 to 0.06 μg/ml (by microdilution), 0.06 μg/ml (by E test), and 25 to 28 mm (disk diffusion).
RESULTS
The MICs (in micrograms per milliliter) at which 50% and 90% of isolates were inhibited (MIC50s and MIC90s) for penicillin G were, respectively, 0.03 and 0.06 for penicillin-susceptible strains, 0.5 and 1.0 for penicillin-intermediate strains, and 2.0 and 4.0 for penicillin-resistant strains. Cefditoren MIC50s and MIC90s were, respectively, very similar by all three methods (Table 1). For penicillin-susceptible strains, MIC50s and MIC90s (in micrograms per milliliter) were 0.016 and 0.03 (by agar dilution), 0.016 and 0.03 (by microdilution), and ≤0.016 and 0.03 (by E test); corresponding values for penicillin-intermediate strains were 0.125 and 0.5 (by agar dilution), 0.125 and 0.5 (by microdilution), and 0.25 and 0.5 (by E test); and those for penicillin-resistant organisms were 1.0 and 1.0 (by agar dilution), 0.5 and 1.0 (by microdilution), and 1.0 and 1.0 (by E test).
TABLE 1.
Cefditoren MICs determined by the three methods tested
| Strain typea (no. tested) | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Agar dilution
|
Microdilution
|
E test
|
||||
| 50% | 90% | 50% | 90% | 50% | 90% | |
| Penicillin S (65) | 0.016 | 0.03 | 0.016 | 0.03 | 0.016 | 0.03 |
| Penicillin I (68) | 0.125 | 0.5 | 0.125 | 0.5 | 0.25 | 0.5 |
| Penicillin R (67) | 1.0 | 1.0 | 0.5 | 1.0 | 1.0 | 1.0 |
| All (200) | 0.125 | 1.0 | 0.125 | 1.0 | 0.25 | 1.0 |
S, susceptible; I, intermediate; R, resistant.
With breakpoint concentrations (in micrograms per milliliter), based upon pharmacokinetic and pharmacodynamic criteria (see above) of ≤2 (susceptible), 4 (intermediate), and ≥8 (resistant), all strains were susceptible to cefditoren by agar, broth microdilution, and E test methods; with breakpoints of ≤1, 2, and ≥4, 97% of strains were cefditoren susceptible and 3% of strains were intermediate by agar dilution; 98% were susceptible and 2% were intermediate by microdilution, and 99% were susceptible and 1% were intermediate by E test (Table 2).
TABLE 2.
Number of strains determined to be susceptible, intermediate, or resistant by the three MIC methods
| S, I, and R breakpointsa (μg/ml) | No. (%) of strains S, I, and R by:
|
||
|---|---|---|---|
| Agar dilution | Microdilution | E test | |
| ≤1, 2, ≥4 | 194 (97%), 6 (3%), 0 | 196 (98%), 4 (2%), 0 | 198 (99%), 2 (1%), 0 |
| ≤2, 4, ≥8 | 200 (100%), 0, 0 | 200 (100%), 0, 0 | 200 (100%), 0, 0 |
S, susceptible; I, intermediate; R, resistant.
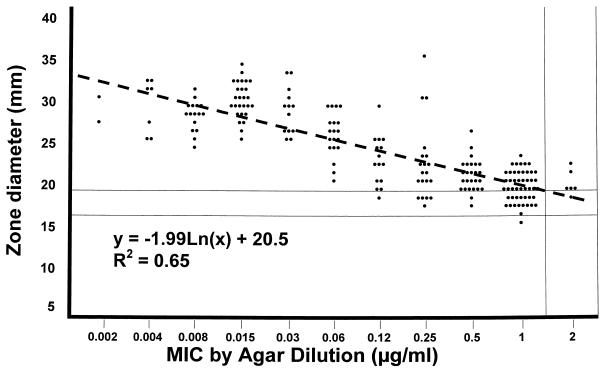
Disk diffusion testing with a ≤1-μg/ml cefditoren-susceptible breakpoint yielded 2 major errors (1%) and 30 minor errors (15%), applying corresponding zone diameters (in millimeters) of ≥20 (susceptible), 17 to 19 (intermediate), and ≤16 (resistant) (Fig. 1). At a ≤2-μg/ml susceptible breakpoint, all strains were susceptible and had zone diameters of ≥16 mm (Fig. 1). Although 30 minor errors is excessive, none of them would significantly impact the clinical situation. Strains for which cefditoren MICs were ≤0.03 μg/ml produced zone diameters of 25 to 35 mm, those for which MICs were 0.06 to 0.5 μg/ml had zone diameters of 18 to 36 mm, and those for which MICs were ≥1.0 μg/ml had zone diameters of 16 to 24 mm (Fig. 1).
FIG. 1.
Scatterplot of cefditoren MICs by agar dilution versus disk diffusion zone diameters. Each symbol indicates one isolate. Dashed line shows regression line. Disk diffusion breakpoints for cefditoren at MIC breakpoints of ≤1, 2, and ≥4 μg/ml are shown and are ≥20, 17 to 19, and ≤16 mm, respectively.
When broth microdilution and E-test results were compared to reference agar dilution results, 191 (95.5%) and 183 (91.5%) of the strains showed essential agreement; eight (2.7%) minor discrepancies were found for both methods with a breakpoint of ≤1 μg/ml. Because no cefditoren MICs of >2 μg/ml were observed, discrepancies for intermediate and resistant strains could not be assessed (Table 3).
TABLE 3.
Comparison of MIC methods using agar dilution as the reference method
| Method | No. of strains with log2 ratio of reference to test MIC of:
|
No. (%) of strains with MIC within ± 1 log2 dilution | No. of categorical discrepanciesa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥3 | 2 | 1 | 0 | −1 | −2 | −3 | Major | Very major | Minor | ||
| Microdilution | 0 | 3 | 73 | 86 | 32 | 6 | 0 | 191 (95.5%) | 0 | 0 | 8 |
| E test | 0 | 0 | 23 | 110 | 50 | 14 | 3 | 183 (91.5%) | 0 | 0 | 8 |
Categorical discrepancies were identified at susceptible, intermediate, and resistant breakpoints of ≤1, 2, and ≥4, respectively. No discrepancies were found at a susceptible breakpoint of ≤2 μg/ml.
DISCUSSION
Previous studies have documented excellent antipneumococcal activity of cefditoren, compared with that of other oral β-lactams, with arithmetic mean MIC90s of ≤0.06, 0.5, and 1 μg/ml against penicillin-susceptible, -intermediate, and -resistant pneumococci, respectively (2, 4, 7, 10, 18–20). Bactericidal activity (99.9% killing) occurred with cefditoren against all pneumococcal strains tested at ≤0.5 μg/ml after 24 h, irrespective of their penicillin susceptibility. Additionally, cefditoren showed 99% killing of all strains after 6 h at test concentrations of ≥4 times the MIC (19). Cefditoren MICs are significantly lower and its kill kinetics are slightly more rapid than those for other oral cephalosporins (9, 16, 18, 19).
Results of this study confirm the excellent activity of cefditoren against penicillin-susceptible, -intermediate, and -resistant pneumococci and show a very good correlation among agar dilution, broth microdilution, and E-test results for all strains. Incubation of E-test plates in CO2, compared with that in air for agar and microdilution, did not significantly influence results.
ACKNOWLEDGMENT
This study was supported by a grant from TAP Holdings, Inc., Deerfield, Ill.
REFERENCES
- 1.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 2.Canton R, Liñares J, Martinez-Beltran J, Tubau F, Almaraz F, Alcaide F, Baquero F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Bactericidal activity of cefditoren (ME-1206) on Streptococcus pneumoniae and Streptococcus of the viridans group, abstr. E11; p. 28. [Google Scholar]
- 3.Clark C L, Jacobs M R, Appelbaum P C. Antipneumococcal activities of levofloxacin and clarithromycin as determined by agar dilution, microdilution, E-test, and disk diffusion methodologies. J Clin Microbiol. 1998;36:3579–3584. doi: 10.1128/jcm.36.12.3579-3584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fremaux A, Sissia G, Brumpt I, Geslin P. Program and abstracts of the 18th International Congress of Chemotherapy. 1993. Cefditoren (ME-1206), a new cephalosporin: in vitro activity against penicillin susceptible and resistant pneumococci, abstr. 998; p. 289. [Google Scholar]
- 5.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs M R, Appelbaum P C. Antibiotic-resistant pneumococci. Rev Med Microbiol. 1995;6:77–93. [Google Scholar]
- 7.Jones R N, Biedenbach D J, Croco M A T, Barrett M S. In vitro evaluation of a novel orally administered cephalosporin (cefditoren) tested against 1249 recent clinical isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1998;31:573–578. doi: 10.1016/s0732-8893(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., and M. A. Pfaller. Unpublished data.
- 9.Liñares J, Alonso T, Pérez J L, Ayats J, Domínguez M A, Pallarés R, Martín R. Decreased susceptibility of penicillin-resistant pneumococci to twenty-four β-lactam antibiotics. J Antimicrob Chemother. 1992;30:279–288. doi: 10.1093/jac/30.3.279. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki S, Miyazaki Y, Tsuji A, Nishida M, Goto S. In vitro antibacterial activity of ME1207, a new oral cephalosporin. Antimicrob Agents Chemother. 1991;35:1691–1694. doi: 10.1128/aac.35.8.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulford, D. (TAP Holdings, Inc.). 1999. Personal communication.
- 12.Munoz R, Musser J M, Crain M, Briles D E, Marton A, Parkinson A J, Sorensen U, Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992;15:112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility testing. NCCLS publication no. M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Negri M C, Morosini M I, Loza E, Baquero F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. In vitro selective concentrations of cefditoren for penicillin resistant Streptococcus pneumoniae populations, abstr. E15; p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson C T, Mason E O, Jr, Kaplan S L. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585–589. doi: 10.1097/00006454-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Shimada K, Shinkai S, Komiya I, Matsumoto T. Program and abstracts of the 29th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1989. Phase-I clinical studies on a novel orally active cephem antibiotic, ME1207, abstr. 366; p. 162. [Google Scholar]
- 18.Spangler S K, Jacobs M R, Appelbaum P C. Activities of RPR 106972 (a new oral streptogramin), cefditoren (a new oral cephalosporin), two new oxazolidinones (U-100592 and U-100766), and other oral and parenteral agents against 203 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40:481–484. doi: 10.1128/aac.40.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spangler S K, Jacobs M R, Appelbaum P C. Time-kill studies on susceptibility of nine penicillin-susceptible and -resistant pneumococci to cefditoren compared with nine other β-lactams. J Antimicrob Chemother. 1997;39:141–148. doi: 10.1093/jac/39.2.141. [DOI] [PubMed] [Google Scholar]
- 20.Tamura A, Okamoto R, Yoshida T, Yamamoto H, Kondo S, Inoue M, Mitsuhashi S. In vitro and in vivo antibacterial activities of ME1207, a new oral cephalosporin. Antimicrob Agents Chemother. 1988;32:1421–1426. doi: 10.1128/aac.32.9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]