Abstract
The advent of molecular genetic technologies paved a path for the diagnosis of many neurological disorders. Joint evaluation by a neurologist and a medical genetics specialist can potentially increase diagnostic effectiveness by ensuring the exclusion of non-genetic conditions with similar phenotypes and by rationally selecting appropriate genetic diagnostic tools. Therefore, a monthly adult neurogenetics clinic was established. A retrospective review of medical records of all patients who attended the clinic from April 2015 to March 2019 was conducted. Eighty-two patients were evaluated (age: 47.1 ± 15.7, male: 37(45%), 42 (51%) had a positive family history). Disease duration was typically long (11.4 ± 0.9 years). Futile use of diagnostic modalities was very common (45 (55%) had repeated MRI, 28 (34%) hospitalized for observation in neurologic departments, 12 (14%) had a normal metabolic workup, 4 (5%) with a non-conclusive muscle biopsy, 1 with a normal cerebral angiography). Following clinical evaluation, molecular genetic testing was offered to 67 (82%) patients. In the other 15 (18%), routine workup for the exclusion of non-genetic conditions was not complete; obtainable information regarding family members was missing or that a neurogenetic disorder seemed improbable. Twenty-seven (33%) patients received a definitive diagnosis, either a genetic (23, 28%) or non-genetic (4, 5%). Excluding 4 cases of pre-symptomatic diagnosis, the diagnostic yield was 30%. The adherence to genetic testing recommendations was 62%. The reasons for non-adherence were lack of public funding for the required test (52%) and patient decision not to proceed (48%). Given the frequent futile use of diagnostic modalities, referral of non-genetic conditions with similar phenotypes among neurogenetic disorders, and the complexity of clinical genomic data analysis, a multi-disciplinary neurogenetics clinic seems justified.
Keywords: Diagnostic yield, Molecular genetics technologies, Neurogenetics clinic
Introduction
The era of genomic medicine has been transforming the clinical care of patients. The advent of molecular genetic technologies as chromosome microarray analysis (CMA) and next generation sequencing (NGS) paved a path for the diagnosis of many neurological disorders.
As the cost and turnaround time for genetic testing continue to fall, a genome-first approach may be appropriate in many clinical scenarios. Several studies of NGS technology in a variety of neurologic disorders have concluded that when a genetic disorder is suspected, an earlier and comprehensive genetic evaluation enhances the diagnostic yield. Cost per diagnosis is also reduced by avoiding the lengthy diagnostic odyssey. For patients, uncomfortable and sometimes invasive diagnostic procedures may be saved (Stark et al. 2017).
Clinical features of many Mendelian diseases are nonspecific and overlap with non-genetic disorders. For example, multiple sclerosis has well-described Mendelian conditions with similar phenotypes (Weisfeld-Adams et al. 2015). As a result, hypothesis-driven targeted genetic testing may be limited. A joint evaluation by a clinical neurologist and a medical genetics specialist could potentially increase diagnostic effectiveness by rationally selecting appropriate diagnostic tools. By means of multi-disciplinary discussion, the possibility of non-genetic conditions with similar phenotypes is reconsidered and the use of futile diagnostic modalities is reduced. Furthermore, patients can receive at the same visit both state-of-the-art genetics counseling for them and their family members as well as clinical neurologic consultation for their condition.
For these reasons, we have established in April 2015 a monthly adult neurogenetics clinic in our medical center. In this clinic, patients and their families are jointly seen by a neurologist, and a medical genetics specialist.
In this report, we aim to describe the clinical characteristics of patients with a suspected neurogenetic disorder that were referred to our clinic, to elaborate the diagnostic workflow and adherence to diagnostic recommendations. Finally, we aimed at calculating the diagnostic yield of our clinic and to identify potential barriers for reaching a diagnosis.
Methods
This report is based on a retrospective review of the electronic medical records of all the patients who attended our neurogenetics clinic from its establishment in April 2015 until March 2019.
The neurogenetics clinic
Patients were referred to the clinic by community neurologists, family physicians, or by the department of neurology, following diagnostic hospitalization. During clinical encounters, patients were interviewed by both, a clinical neurologist and a medical genetics expert. The neurologist obtained medical history, performed a targeted neurological examination, and reviewed previous diagnostic tests (e.g., MRI, nerve conduction studies, and electromyography). The medical genetics expert obtained a detailed family history and constructed a pedigree chart. Following mutual data acquisition, a multi-disciplinary discussion was held regarding the differential diagnosis. When indicated, family members with similar phenotype were also seen at the clinic and an effort was made to obtain their medical documentation.
When a genetic disease was deemed probable, molecular genetic testing was offered. Family history of a related disorder was not required to proceed with genetic evaluation if the phenotype was suggestive and other possibilities in the differential diagnosis were excluded (it is well known that genetic diseases may appear de novo from new mutations; also, if a genetic disorder is with low penetrance, it may occur in cases with negative family history).
The specific type of test was determined by the presumed clinical diagnosis and the guidelines of the Israeli health basket. During the reported period, sequencing of specific genes and chromosomal microarrays were reimbursed but sequencing of wide gene panels, exome, or genome wide sequencing were not. Therefore, a staged approach to diagnosis was practiced, prioritizing reimbursed tests. For instance, in case of a phenotype reminiscent of a demyelinative hereditary motor and sensory polyneuropathy (HMSN), sequencing of the PMP22 gene was recommended prior to obtaining a gene panel for HMSN. In case of a spinocerebellar ataxia phenotype (SCA), sequencing of ataxin 1 and 3 genes relevant for SCA 1 and 3 (Machado-Joseph disease) was performed first, as they were reimbursed and only if negative, gene panels for hereditary cerebellar ataxias or exome were offered. In case of a non-specific phenotype, e.g., suggestive of hereditary spastic paraplegia, wide gene panel or exome sequencing was offered first; however, patients had to fund these tests by themselves.
Additionally, in cases where non-genetic disorders seemed relevant, recommendation for further neurologic diagnostic testing and follow-up were given. For example, further non-genetic workup was recommended to patients who were referred due to suspected demyelinative hereditary motor and sensory neuropathy but had features suggestive of chronic inflammatory demyelinating polyneuropathy (e.g., positive sensory symptoms or conduction blocks on nerve conduction studies) (Burns and Mauermann 2011).
Upon the return of test results, a second meeting was arranged. In case of a diagnosis of a genetic disorder, comprehensive information was provided by the medical genetics expert to patients and families regarding the personal and familial implications including ways to prevent the transmission of the disorder to the next generation. At the same time, patients received an up-to-date information about available symptomatic and disease-modifying treatments from the attending neurologist.
Data extraction procedure
For the preparation of this report, the following variables were obtained from the electronic medical record of each patient: demographics (age, gender), the referring source (family physician, community neurologist or the department of neurology), time from symptoms onset, previous use of diagnostic modalities, and the type and results of the recommended genetic tests.
The study was approved by the Institutional Review Board (IRB) of the Lady Davis Carmel Medical Center (IRB approval number: 0192–20-CMC, date of approval: February 1st, 2021). Descriptive statistics are mean ± standard deviation or number (percent).
Results
Overall, 82 patients were evaluated. Fifty-three (65%), 16 (20%), and 13 (15%) patients were referred by community neurologists, family physicians, and the department of neurology of our medical center, respectively.
The average age was 47.1 ± 15.7; 37 (47%) patients were male. Forty-two (51%) had a positive family history of a related disorder. Disease duration was typically long (11.4 ± 0.9 years). Futile use of diagnostic modalities was common: 45 (55%) had repeated MRI; 28 (34%) were hospitalized for observation and investigation in neurologic departments; 17 (21%) underwent nonstandard diagnostic tests: 12 (15%) metabolic workup, 4 (5%) a non-conclusive nerve or muscle biopsy, 1 with a normal cerebral angiography, PET scan, and thrombophilia workup.
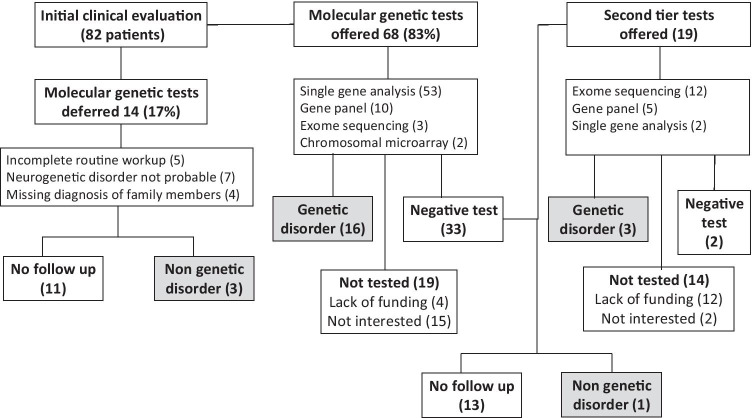
Clinical decisions at the clinic and diagnostic outcome are outlined in Fig. 1. Following clinical evaluation, initial molecular genetic testing was offered to 68 patients (83%). In the remaining 14 (17%), routine workup for the exclusion of non-genetics conditions was incomplete (5 patients), obtainable information regarding family members was missing (4 patients), or a neurogenetic disorder seemed very unlikely (7 patients). Of these, a non-genetic disorder was established in 3 patients. The eleven others were lost to follow up.
Fig. 1.
Clinical decisions and diagnostic outcome
The recommended genetic tests included the following: specific genetic variants, 53 patients (78%); limited or wide gene panels, 10 patients (15%); whole exome sequencing (WES), 3 patients (4%); and CMA, 2 patients (3%). By specific genetic variants, we mean specific gene testing to find a known pathogenic variant (e.g., looking for a duplication of the PMP22 gene upon clinical suspicion of hereditary motor-sensory neuropathy type 1A or looking for expanded trinucleotide repeat in the HTT gene upon clinical suspicion of Huntington’s chorea).
Of the 68 patients who were offered genetic testing, 49 (72%) had performed the recommended test, and 16 genetic disorders were established. Thirty-three tests yielded normal results. Nineteen patients were not tested (4 due to lack of public funding, 15 were not interested to proceed).
Of the 33 patients with normal (negative) results, 19 were offered a second-tier test (12 WES, 5 gene panels, 2 single gene analysis). Of these, 5 patients continued the genetic evaluation with 3 additional diagnoses of genetic disorders. Two patients had normal (negative) results. One non-genetic disorder was diagnosed. Fourteen patients were not tested (12 due to lack of public funding, 2 were not interested to proceed).
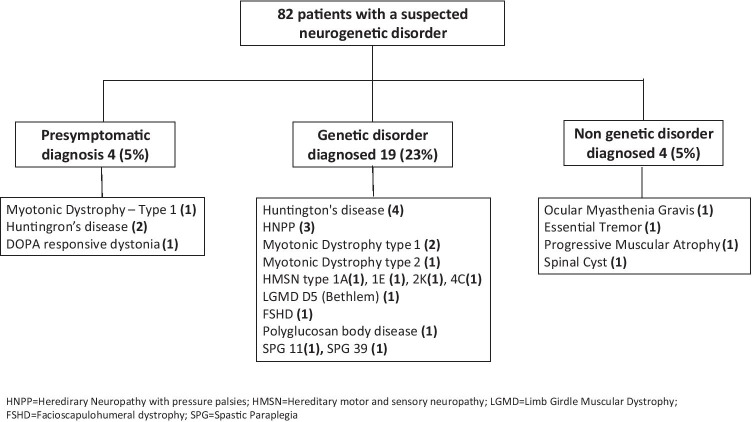
Overall, a definitive diagnosis was established in 27 (33%) patients (Fig. 2), either a genetic (23, 28%) or non-genetic (4, 5%). Four genetic diagnoses were of healthy people who wished to know if they or their offsprings were at risk for future disease after a first-degree relative was diagnosed. Excluding these pre-symptomatic diagnoses, the overall diagnostic yield was 23 of 78 (30%).
Fig. 2.
Diagnostic yield of the neurogenetics clinic. Overall, a definitive diagnosis was established in 27 (33%) patients. Excluding pre-symptomatic diagnoses of healthy individuals, the overall diagnostic yield was 23 of 78 (30%)
Genetic diagnoses were the following: Huntington’s disease, hereditary neuropathy with pressure palsies, myotonic dystrophy types 1 and 2, hereditary motor and sensory neuropathies (types 1A, 1E, 2 K, and 4C), limb girdle muscular dystrophy type D5 (Bethlem), facioscapulohumeral dystrophy (and Klinefelter syndrome in the same patient), polyglucosan body disease, spastic paraplegia 11, and dopa responsive dystonia.
Non-genetic diagnoses were the following: ocular myasthenia gravis, essential tremor, progressive muscular atrophy, and a spinal cyst (Table 1, Fig. 3). Symptoms and genetic tests of the 29 patients who remained undiagnosed despite at least partially following workup recommendations are elaborated in Table 2.
Table 1.
Non-genetic conditions with similar phenotypes of neurogenetic disorders that were encountered at the neurogenetics clinic
| Suspected diagnosis (reason of referral) | Clue for alternative diagnosis | Final diagnosis |
|---|---|---|
| Progressive external ophthalmoplegia | Improved with pyridostigmine, positive acetyl choline receptor antibody | Ocular myasthenia gravis |
| Nieman pick type C | Age: 75, mild postural tremor only without cerebellar, conspicuous extrapyramidal or gaze abnormalities | Essential tremor |
| Spinal muscular atrophy | Onset age:70, no family history, rapid progression over 2 years, proximal and distal weakness, wasting and fasciculations in 4 limbs, no bulbar involvement, electromyographic evidence of acute and chronic denervation as well as reinnervation in 4 limbs, normal sensory nerves conduction | Progressive muscular atrophy (motor neuron disease) |
| Hereditary motor and sensory neuropathy | Normal sural nerve conduction, paraparesis and pyramidal signs | Spinal cyst |
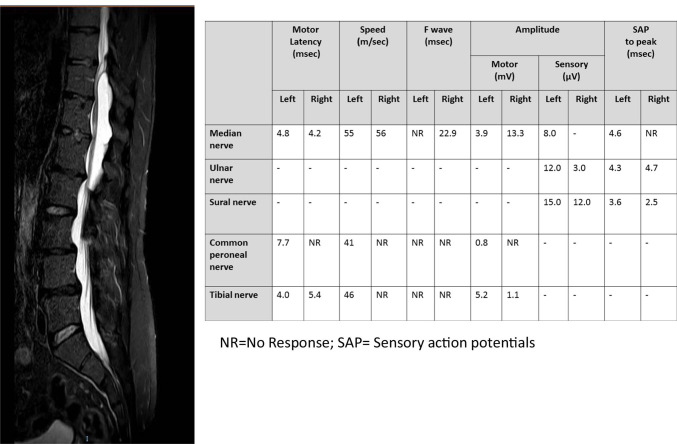
Fig. 3.
An example of a non-genetic mimic encountered at the clinic. This patient was referred by a community neurologist with a clinical diagnosis of hereditary motor and sensory neuropathy (HSMN). However, sensory nerve conductions of both sural nerves were normal. Neurologic examination at the clinic revealed paraparesis and pyramidal signs. Spinal MRI was recommended, rather than molecular genetic tests and a spinal cyst was diagnosed
Table 2.
Workup of patients who remained undiagnosed
| Reason of referral | Age | Gender | Genetic tests performed |
|---|---|---|---|
| Peripheral nervous system diseases | |||
| Demyelinating polyneuropathy |
57 60 62 63 |
Female Female Male Male |
Duplication of the gene encoding peripheral myelin protein-22 (PMP-22, Charcot-Marie-Tooth, type 1A) |
| Axonal polyneuropathy | 50 | Male | Hereditary Motor and Sensory Neuropathy panel (102 genes) |
| Syndactyly and small fiber neuropathy | 31 | Female |
Chromosomal microarray Mutations in the GLA gene, encoding alpha-galactosidase A (Fabry) Mutations in TTR gene, encoding Transthyretin (Hereditary amyloidosis) |
| Small fiber neuropathy | 57 | Female |
Mutations in the GLA gene, encoding alpha-galactosidase A (Fabry) Mutations in TTR gene, encoding Transthyretin (Hereditary amyloidosis) |
| Myopathy, mental retardation, dysmorphism (high forehead, prognathism, macroglossia, pectus excavatum, scoliosis) | 30 | Male |
Fluorescence in situ hybridization (FISH) to rule out Velo-Cario-Facial syndrome Trinucleotide repeat (CGG), in the FMR1 gene (fragile X syndrome) |
| Proximal weakness, myotonia | 38 | Female | Expansion of a CCTG repeat in intron 1 of the zinc finger protein-9 gene (Myotonic dystrophy type 2) |
| Myotonia since age 20 without cataract, endocrine or cardiac complications. Hypertrophic calves | 38 | Female | Mutation in the CLCN1 gene (myotonia congenita) |
| Central nervous system diseases | |||
| Cerebellar ataxia ± pyramidal signs |
46 51 61 73 80 82 |
Female Male Male Female Male Female |
Mini panel of 7 genes: CAG trinucleotide repeat expansions in the genes: ataxin 1, 2, 3, 7 (SCA 1, 2, 3, 7) SCA 6 Dentatorubral-pallidoluysian atrophy (DRPLA) GAA trinucleotide repeat expansion in intron 1 of the frataxin gene (Friedreich’s ataxia) |
| Ataxia and tremor |
53 73 |
Male Male |
Trinucleotide repeat (CGG), in the FMR1 gene (Fragile X associated tremor ataxia syndrome) |
| Spastic paraparesis and mental retardation in two brothers of Iraqi Jewish ancestry |
52 56 |
Male Male |
Trinucleotide repeat (CGG), in the FMR1 gene (Fragile X syndrome) OPA3 gene mutation (Costeff syndrome) |
| Spastic paraparesis with diffuse, peri-ventricular white matter changes on brain MRI | 56 | Female | Exome sequencing |
| Cerebral small vessel disease |
34 48 |
Female Female |
Mutations in the GLA gene, encoding alpha-galactosidase A (Fabry) Mutation in the NOTCH3 gene (CADASIL—cerebral arteriopathy autosomal dominant with subcortical infarcts and leukoencephalopathy) |
| Generalized dystonia |
19 24 |
Male Male |
Mutation in the TOR1A gene, encoding the ATP-binding protein torsin-A, after returned negative: Dystonia gene panel: 38 genes |
| Spastic paraparesis | 33 | Female | Hereditary spastic paraparesis panel (78 genes) |
| Chorea |
54 54 |
Female Male |
Trinucleotide repeat (CAG), in the HTT gene (Huntington's chorea) |
| Spastic paraparesis and peripheral neuropathy | 40 | Female | Exome sequencing |
The diagnostic yield of specific tests was the highest for wide gene panel (4/8, 50%), 40% for WES (2/5) and 33.3% for specific gene changes (13/39). The diagnostic yield was the lowest for the limited gene panel (0/6) and CMA (0/2).
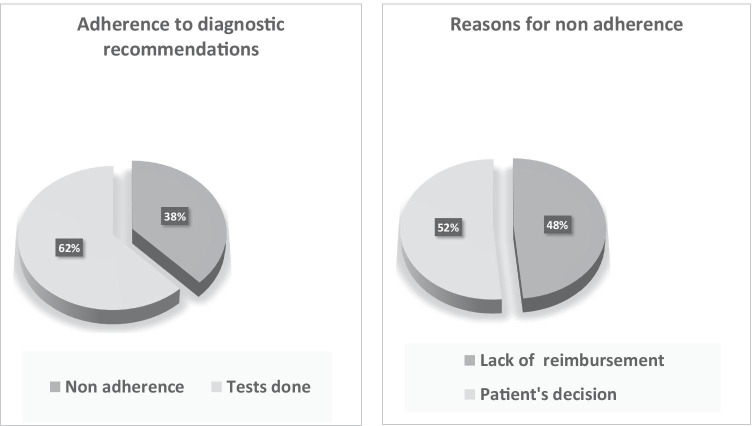
Overall adherence to genetic testing recommendations was 62%. Adherence was higher in the first round of testing (72%) than in the second round (26%), after a negative initial investigation. The reasons for non-adherence were lack of public funding for the required test (48%) and patient decision not to proceed (52%) (Fig. 4).
Fig. 4.
Adherence to diagnostic recommendations. The adherence to genetic testing recommendations is shown. Reasons for non-adherence were lack of public funding for the required test and patient decision not to proceed
Discussion
In this report, we look back at our experience in a multi-disciplinary neurogenetics clinic. The diagnostic yield of the clinic was fair. A definitive diagnosis was established in 30% of referred patients with an undiagnosed neurological syndrome. Importantly, non-genetic conditions with similar phenotypes were also referred to our clinic with a presumed genetic diagnosis and 5% of referred patients ended up with a non-genetic neurological diagnosis, highlighting the importance of a multi-disciplinary approach. Single gene analyses had high diagnostic yield when specific phenotypes were encountered (e.g., Huntington’s disease, Charcot-Marie-Tooth demyelinating neuropathies, myotonic dystrophy). In other cases, comprehensive gene panels and exome sequencing improved the diagnostic yield.
In view of the typically long time that our patients spent without diagnosis (11 years on average), our diagnostic yield of 30% is substantial. The benefits of accurate DNA-based diagnosis are clear and many. Molecular genetic diagnosis ends the diagnostic odyssey (Hartley et al. 2020), which may last years and include repeated futile, sometimes invasive, diagnostic testing. Moreover, genetic diagnosis facilitates therapeutic decisions and help to avoid inappropriate and redundant treatments due to suspected non-genetics conditions. For example, unnecessary and potentially harmful treatment due to presumed multiple sclerosis or cerebral small vessel disease can be prevented by molecular diagnosis of polyglucosan body disease (Hellmann et al. 2015). Timely diagnosis of a genetic disorder enables screening for anticipated complications, e.g., manageable life-threatening cardiac complications in patients with myotonic dystrophy (Wahbi and Furling 2020). Also, accurate diagnosis enables a reliable genetic counseling including pre-symptomatic testing and prenatal diagnosis for family members.
The diagnostic yield of our clinic was like that reported by other multidisciplinary clinics, in which genetics specialists collaborate with clinicians with a specific medical specialty. A similar monthly multidisciplinary clinic in Ireland managed 27 patients over 12 months, of whom 10, 33% were diagnosed with a genetic disorder after a pathogenic mutation was identified (Olszewska et al. 2018). In a related neurogenetics clinic in Argentina, 387 patients were evaluated during a 6-year period, of whom 106 were definitively diagnosed, corresponding to an overall diagnostic yield of 27% (Rodríguez-Quiroga et al. 2015). Remarkably, the same diagnostic yield of 24% to 30% was reported also from neurogenetics clinics in the USA (Edlefsen et al. 2007; Gahl et al. 2012).
The notion of multidisciplinary clinics was successfully implemented in other fields of medicine as well. Zentner et al. have described their multidisciplinary cardiac genetics clinic over 6 years of follow-up (Zentner et al. 2015). A total of 1170 patients were seen; genetic tests were obtained from 32.6% of the patients. The diagnostic yield was 47.6% representing 15.3% of the total population. Mallett et al. described their multidisciplinary renal genetics clinic during 2 years of follow-up (Mallett et al. 2016). A total of 108 patients were seen, of whom 69% underwent genetic testing. The diagnostic yield was 39%. Battista et al. reviewed the literature of genetics in clinical practice in Europe, North America, and Australia (Battista et al. 2011). The authors concluded that “Multidisciplinary specialist clinics and coordinated services appeared to be key to delivering proper care in rare genetic disorders. For oncogenetics, neurogenetics and cardiogenetics, interprofessional collaboration between geneticists and other specialists seemed to be favored.” The same conclusion was reached by others in various fields of medicine (Dunnenberger et al. 2016; Alkanderi et al. 2017).
We found that when specific phenotypes are encountered, such as those of Huntington’s chorea or myotonic dystrophy, single gene analyses have high diagnostic yield. However, in cases of non-specific phenotype (e.g., spastic paraparesis or progressive cerebellar ataxia) wide gene panels or whole exome sequencing has greater diagnostic yield. This finding is in line with previous reports. For epileptic patients of unknown etiology, the diagnostic yield was highest for WES (0.45), followed by panels (0.23) and CMA (0.08) (Fernández et al. 2019). Studies evaluating the efficacy of NGS in diagnosing movement disorders have reported a diagnostic yield of up to 10.1% for familial and 15.7% for early-onset Parkinson disease, 11.7–37.5% for dystonia, 12.1–61.8% for ataxia/spastic paraplegia, and 11.3–28% for combined movement disorders (Gorcenco et al. 2020).
One recognized barrier to our practice was the lack of public funding to WES and wide gene panels during the period of this report. Consequently, our approach was graded, endorsing relevant publicly funded tests first and reserving further testing, privately funded, to a second tier of investigations (Table 2). Unfortunately, many patients did not have funds to continue the exploration or lost interest in the process (Fig. 1). Thus, lack of public funding to the whole gamut of molecular genetics diagnostic modalities may prolong the evaluation process and decrease its yield. Furthermore, this lack of public funding promotes disparity in medicine, as those who could privately fund testing were referred to high yield tests earlier. As of the middle of 2019, multi-gene panels by NGS techniques were added to the Israeli health basket, which may expedite the diagnostic workup, decrease non-adherence, and increase the diagnostic yield.
Notably, 4 patients were diagnosed with non-genetic disorders (Table 1). The presence of a neurologist in the evaluating team enabled reconsideration of the differential diagnosis, rather than routinely following the recommendations of the referring physician. Some non-genetic diagnoses were based on the results of diagnostic tests: myasthenia gravis was supported by the presence of acetyl choline receptor antibodies and spinal pathology was supported by MRI scan together with normal sensory nerve conduction studies (Fig. 3). Although essential tremor is a clinical diagnosis, to the best of our clinical judgement, the hypothesized diagnosis of the referring physician (Nieman pick type C) was very unlikely in a 75-year-old patient with mild postural tremor as the only finding. Pure lower motor neuron presentations of motor neuron disease (MND) in adults are usually sporadic, but late-onset spinal muscular atrophy (SMA) is an important treatable possibility (Maggi et al. 2020). Adult-onset SMA typically presents in the third or fourth decade, it is slowly progressive, bulbar involvement is common, and muscle weakness is more pronounced proximally and on the lower limbs (Garg et al. 2017). In our patient, the disease started in the eighth decade, was rapidly progressive without bulbar signs, and the weakness involved proximal and distal muscles to the same extent, therefore clinically suggestive of sporadic MND. We have nevertheless tested the SMN1 gene, which was found normal.
The multidisciplinary composition of consultants has an added value also in the field of presymptomatic diagnosis: the individual with presymptomatic diagnosis of DOPA responsive dystonia (Fig. 2) wanted to gain insight into the severity and management of this condition, in order to make an informed decision regarding the necessity of testing the mutation in an offspring, during an ongoing pregnancy. Thus, the neurologist in the team can answer clinical management questions while the genetic consultants can answer about means of preventing disease transmission.
In summary, adapting a multidisciplinary approach to the diagnosis of patients with suspected neurogenetic disorders facilitates the exclusion of non-genetic conditions with similar phenotypes along with providing accurate genetic counselling. Given the frequent futile use of diagnostic modalities and the complexity of clinical genomic data analysis, a multidisciplinary clinic in which the expertise of both genetic specialties and neurologists can be harnessed for optimal patient management seems justified.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alkanderi S, Yates LM, Johnson SA, Sayer JA. Lessons learned from a multidisciplinary renal genetics clinic. QJM. 2017;110:453–457. doi: 10.1093/qjmed/hcx030. [DOI] [PubMed] [Google Scholar]
- Battista RN, Blancquaert I, Laberge AM, et al. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2011;15:34–45. doi: 10.1159/000328846. [DOI] [PubMed] [Google Scholar]
- Burns TM, Mauermann ML. The evaluation of polyneuropathies. Neurology. 2011;76(7 Suppl 2):S6–13. doi: 10.1212/WNL.0b013e31820c3622. [DOI] [PubMed] [Google Scholar]
- Dunnenberger HM, Biszewski M, Bell GC, et al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am J Heal Pharm. 2016;73:1956–1966. doi: 10.2146/ajhp160072. [DOI] [PubMed] [Google Scholar]
- Edlefsen KL, Tait JF, Wener MH, Astion M. Utilization and diagnostic yield of neurogenetic testing at a tertiary care facility. Clin Chem. 2007;53:1016–1022. doi: 10.1373/clinchem.2006.083360. [DOI] [PubMed] [Google Scholar]
- Fernández IS, Loddenkemper T, Gaínza-Lein M, et al. Diagnostic yield of genetic tests in epilepsy: a meta-analysis and cost-effectiveness study. Neurology. 2019;92:E418–E428. doi: 10.1212/WNL.0000000000006850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Markello TC, Toro C, et al. The national institutes of health undiagnosed diseases program: insights into rare diseases. Genet Med. 2012;14:51–59. doi: 10.1038/gim.0b013e318232a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndromes. J Neurol Neurosurg Psychiatry. 2017;88:474–483. doi: 10.1136/jnnp-2016-313526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorcenco S, Ilinca A, Almasoudi W, et al. New generation genetic testing entering the clinic. Park Relat Disord. 2020;73:72–84. doi: 10.1016/j.parkreldis.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Hartley T, Lemire G, Kernohan KD, et al. New diagnostic approaches for undiagnosed rare genetic diseases. Annu Rev Genomics Hum Genet. 2020;21:351–372. doi: 10.1146/annurev-genom-083118-015345. [DOI] [PubMed] [Google Scholar]
- Hellmann MA, Kakhlon O, Landau EH, et al. Frequent misdiagnosis of adult polyglucosan body disease. J Neurol. 2015;262:2346–2351. doi: 10.1007/s00415-015-7859-4. [DOI] [PubMed] [Google Scholar]
- Maggi L, Bello L, Bonanno S, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91:1166–1174. doi: 10.1136/jnnp-2020-323822. [DOI] [PubMed] [Google Scholar]
- Mallett A, Fowles LF, McGaughran J, et al. A multidisciplinary renal genetics clinic improves patient diagnosis. Med J Aust. 2016;204:58–59. doi: 10.5694/mja15.01157. [DOI] [PubMed] [Google Scholar]
- Olszewska DA, McVeigh T, Fallon EM, et al. The benefits of a Neurogenetics clinic in an adult Academic Teaching Hospital. Ir J Med Sci. 2018;187:1073–1076. doi: 10.1007/s11845-018-1784-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Quiroga SA, Cordoba M, González-Morón D, et al. Neurogenetics in Argentina: diagnostic yield in a personalized research based clinic. Genet Res (Camb) 2015;97:e10. doi: 10.1017/s0016672315000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark Z, Schofield D, Alam K, et al. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. 2017;19:867–874. doi: 10.1038/gim.2016.221. [DOI] [PubMed] [Google Scholar]
- Wahbi K, Furling D. Cardiovascular manifestations of myotonic dystrophy. Trends Cardiovasc Med. 2020;30:232–238. doi: 10.1016/j.tcm.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Weisfeld-Adams JD, Sand IBK, Honce JM, Lublin FD. Differential diagnosis of Mendelian and mitochondrial disorders in patients with suspected multiple sclerosis. Brain. 2015;138:517–539. doi: 10.1093/brain/awu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner D, Thompson TN, James PA, et al. The cardiac genetics clinic: A model for multidisciplinary genomic medicine. Med J Aust. 2015;203:261.e1–261.e6. doi: 10.5694/mja14.01674. [DOI] [PubMed] [Google Scholar]