Abstract
Objective
To investigate the association between sleep duration and cognitive frailty among older adults dwelling in western China.
Methods
We used the baseline data from West China Health and Aging Trend (WCHAT) study. Sleep duration was classified as short sleep duration (< 6 h), normal sleep duration (6–8 h) and long sleep duration (≥ 9 h). Fried frailty criteria and Short Portable Mental Status Questionnaire were used to measure cognitive frailty. Multinomial logistic regression was conducted to estimate odds ratio (OR) and 95% confidence interval (CI).
Results
A total of 4093 older adults (age = 67.8 ± 5.9 years, 1708 males and 2385 females) were included in the analysis. The prevalence of cognitive frailty was 11.8% among older adults in western China. Approximately 11.9% participants had short sleep duration (< 6 h); 22.2% had a long sleep duration (≥ 9 h). After adjusting for covariates, only long sleep duration was significantly associated with high risk of cognitive frailty (OR = 2.07, 95%CI = 1.60–2.68, P < 0.001) in western China older adults compared to normal sleep duration.
Conclusions
Long sleep duration was significantly related to cognitive frailty in older adults. Intervention for long sleep duration may be helpful to prevent cognitive frailty.
Trial registration
Chinese Clinical Trial Registry: ChiCTR1800018895.
Keywords: Cognitive frailty, Older adults, Sleep duration
Introduction
Physical frailty and cognitive impairment are two common and pervasive medical conditions among older adults. The prevalence of physical frailty in older adults ranged from 4.0 to 75.6% [1, 2] and the prevalence of cognitive impairment was estimated to be 3–42% [3]. Recent studies suggested that 70% of physically frail older adults coexisted cognitive impairment [4], and half of older adults with cognitive impairment were also physical frail [5]. Both physical frailty and cognitive impairment were associated with a range of deleterious outcomes in older adults such as lower quality of life (QOL) [6, 7], increased use of health care services, risk of morbidity and mortality [8, 9]. Based on the evidence revealing the relationship between physical frailty and cognitive impairment, the concept of “cognitive frailty” was first proposed in 2013 to describe a clinical condition characterized by the co-existing physical frailty and cognitive impairment but no dementia (CIND) [10]. Previous studies have demonstrated that physical frailty was a significant predictor of Alzheimer disease (AD) among older adults [11]. Compared with older adults with physical frailty or cognitive impairment alone, those with cognitive frailty were considered to have a higher risk of AD, disability and mortality [12, 13]. Therefore, public health efforts are deserved to identify the risk factors of cognitive frailty among older adults.
Sleep, a biobehavioral phenomenon regulated by circadian, homeostatic, and neurohormonal processes [14], is critical for maintaining physical performance and cognitive function [15]. Sleep disorder is very common, and a decrease in total sleep duration often occurs among older adults [16]. Previous studies have found that physical frailty and cognitive impairment was significantly associated with sleep duration among older adults, respectively [17, 18]. However, to our knowledge, the association between sleep duration and cognitive frailty in older adults has not been investigated.
To fill this gap, the aims of this study are as follows: 1) to determine the prevalence of cognitive frailty among community-dwelling older adults in the western China; 2) to explore the association between cognitive frailty and sleep duration.
Methods
Study design and sample selection
Data were based on the baseline of West China Health and Aging Trend (WCHAT) study, a population-based longitudinal study of aging and health of community-dwelling Chinese aged 50 and older in western China [19]. The cohort study began in 2018 and was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445) and was conducted in accordance with the 2013 version of the Declaration of Helsinki. The study registered at the Chinese Clinical Trial Registry under number ChiCTR1800018895. Trained interviewers collected the baseline data through face-to-face interviews and physical examination. A written informed consent was obtained from all participants (or their legal proxies for those who were unable to sign their names).
A total of 7536 participants from 18 ethnic groups in Sichuan, Yunnan, Guizhou and Xinjiang province were included in the WCHAT study. To better analyze the association between sleep duration and cognitive frailty, we only included the participants aged 60 and older with relevant data in the present study. We eventually included 4093 participants in the current analysis, after excluding 3022 participants under 60 years old, 370 with missing data on physical frailty phenotype, 41 with missing data on cognitive function, 3 diagnosed with dementia, and 7 with missing data on the sleep duration.
Physical frailty assessment
The modified Fried frailty phenotype was used to assess physical frailty in this study [20]. The detailed criteria include:
Shrinking: self-reported unintentional weight loss of 4.5 kg in last year or body mass index (BMI) < 18.5 kg/m2.
Weakness: maximum grip strength in dominant hand ≤20% of the population distribution, adjusted for gender and body mass index (BMI).
Exhaustion: self-reported lack of energy (energy score was no more than 3, when 10 represents the most powerful condition); or self-reported excessively fatigue or weak for most of the time.
Slowness: walking time/4-m ≤ 20% of the population distribution, adjusted for gender and height.
Low physical activity level: energy consumption (kcal/week) spend on commonly performed physical activities ≤20% of the population distribution, adjusted for gender. Physical activities were measured by using a validated China Leisure Time Physical Activity Questionnaire (CLTPAQ) [21], which was a modified version of the Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ) [22] based on the Chinese lifestyle and cultural background.
According to previously established cut-points [20], we specified that a phenotype of physical frailty was indicated by the presence of three or more of the items, pre-physical frailty was indicated by the presence of one or two of the items and robust was the absence of all items.
Cognitive assessment
We used the 10-point Short Portable Mental Status Questionnaire (SPMSQ) to assess cognitive function of the participants [23]. A score greater than 4 in participants with primary school education and lower, or a score of greater than 2 in participants with high school education and higher are defined as cognitive impairment [24, 25].
Definition of cognitive frailty
As in previous studies [26, 27], we divided our participants into five groups according to physical frailty status and cognitive function: 1) robust group, robust older adults who had no physical frailty and cognitive impairment, 2) pre-physical frailty group, physically pre-frail older adults without cognitive impairment, 3) physical frailty group, physically frail older adults without cognitive impairment, 4) cognitive impairment group, non-physically pre-frail/frail older adults but with cognitive impairment, and 5) cognitive frailty group, physically pre-frailty/frailty older adults with cognitive impairment.
Sleep duration
We used the following question, “During the past month, how many hours of actual sleep did you get at night (average hours for one night)” to collect the sleep duration from the participants. Based on the recommendations of sleep duration from the American Academy of Sleep Medicine [28], we divided our participants into three groups. Short sleep duration was < 6 h, normal sleep duration was 6–8 h, and long sleep duration ≥9 h, and normal sleep duration was chosen as the referent categories.
Covariates
We collected the following information through face-to-face interviews: age, gender, education (illiteracy/primary school/secondary school or advanced), ethnicity (Han/Qiang/Tibetan/other minority ethnic groups) and marital status (married/single), smoking history (yes/no), alcohol drinking history (yes/no) and number of chronic diseases (0/1/≥ 2). We defined smoking and drinking history as a period of regular use of smoking or drinking (i.e. former or current smoker/drinker: drinking/smoking more than half of a week; never smoker/drinker: dinking/smoking less than half of a week). In addition, we evaluated the depression of the participants via 15-item Geriatric Depression Scale (GDS-15) [29]. Depression was defined as GDS-15 score ≥ 8 [29].
Statistical analysis
Analyses were conducted by using Stata software, version 15.1 (Stata Corp, College Station, TX, USA). Means ± standard deviation (SD) and count (percentage) were used to summarize continuous and categorical variables, respectively. Difference of groups were tested by ANOVA for continuous variables and the chi square test for categorical variables among 5 groups: “robust”, “pre-physical frailty”, “physical frailty”, “cognitive impairment” and “cognitive frailty”. When p < 0.05, LSD post-hoc test (continuous variables) or partitions of chi-square (categorical variables) was applied to determine the significant differences among subgroups. Multinomial logistic regression was conducted to find the relationship between cognitive frailty and sleep duration. The multivariable adjusted model included age, gender, ethnicity, education level, marital status, smoking history, drinking history, number of chronic diseases and depression as covariates. Odds ratio (OR) and 95% confidence intervals (CI) were conducted and P<0.05 was used to determine whether the effect was significant.
Results
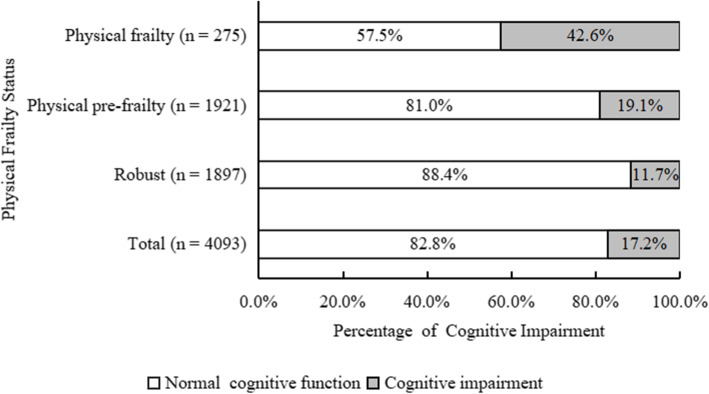
Overall, we included 4093 participants (1708 males and 2385 females) in our study. The mean age of the participants was 67.8 ± 5.9 years (ranging from 60 to 95). The percentage of physically frail participants was 6.7% (n = 275), while 46.9% (n = 1921) were physically pre-frail by the modified Fried frailty phenotype. The prevalence of cognitive impairment was 17.2% (n = 704) in our participants. A total of 38.0% (n = 1555) participants had pre-physical frailty without cognitive impairment, 3.9% (n = 158) had physical frailty without cognitive impairment, 5.4% (n = 221) had only cognitive impairment and 11.8% (n = 483) had cognitive frailty (Fig. 1). According to the recommendation of sleep duration, 11.9% participants (n = 485) had short sleep duration, while 22.2% participants (n = 910) had long sleep duration in our study (Table 1).
Fig. 1.
Percentage of participants with cognitive impairment by physical frailty status
Table 1.
Characteristics of the participants according to cognitive frailty status
| Characteristics | All subjects (n = 4093) | Robust (n = 1676) | Physical pre-frailty (n = 1555) | Physical frailty (n = 158) | Cognitive impairment (n = 221) | Cognitive frailty (n = 483) | P-value |
|---|---|---|---|---|---|---|---|
| Age (years)* | 67.8 ± 5.9 | 66.4 ± 4.9 | 68.1 ± 6.1a | 71.9 ± 6.4b | 67.1 ± 5.6 | 70.6 ± 6.9d | < 0.001 |
| Age (years) (n, %) | < 0.001 | ||||||
| 60–69 | 2700 (66.0) | 1279 (76.3) | 990 (63.7)a | 60 (38.0)b | 146 (66.1)c | 225 (46.6)d | |
| 70–79 | 1205 (29.4) | 375 (22.4) | 485 (31.2)a | 74 (47.0)b | 66 (29.9)c | 205 (42.4)d | |
| ≥ 80 | 188 (4.6) | 22 (1.3) | 80 (5.1)a | 24 (15.2)b | 9 (4.1)c | 53 (11.0)d | |
| Gender (n, %) | < 0.001 | ||||||
| Males | 1708 (41.7) | 728 (43.4) | 723 (46.5) | 83 (52.5)b | 51 (23.1)c | 123 (25.5)d | |
| Females | 2385 (58.3) | 948 (56.6) | 832 (53.5) | 75 (47.5)b | 170 (76.9)c | 360 (74.5)d | |
| Ethnicity (n, %) | < 0.001 | ||||||
| Han | 1546 (37.8) | 699 (41.7) | 621 (39.9) | 55 (34.8) | 51 (23.1)c | 120 (24.8)d | |
| Tibetan | 691 (16.9) | 243 (14.50) | 262 (16.9) | 42 (26.6)b | 35 (15.8) | 109 (22.6)d | |
| Qiang | 862 (21.1) | 449 (26.8) | 264 (17.0)a | 6 (3.8)b | 69 (31.2) | 74 (15.3)d | |
| Others | 994 (24.3) | 285 (17.0) | 408 (26.2)a | 55 (34.8)b | 66 (29.9)c | 180 (37.3)d | |
| Education (n, %) | < 0.001 | ||||||
| Illiterate | 1381 (33.7) | 409 (24.4) | 479 (30.8)a | 54 (34.2) | 140 (63.4)c | 299 (61.1)d | |
| Primary school | 1563 (38.2) | 684 (40.8) | 655 (42.1)a | 55 (34.8) | 54 (24.4)c | 115 (23.9)d | |
| Secondary school and above | 1149 (28.1) | 583 (34.8) | 421 (27.1) | 49 (31.0) | 27 (12.2)c | 69 (14.3)d | |
| Marital status (n, %) | < 0.001 | ||||||
| Married | 3223 (78.7) | 1398 (83.4) | 1224 (78.7)a | 106 (67.1)b | 175 (79.2) | 320 (66.3)d | |
| single | 870 (21.3) | 278 (16.6) | 331 (21.3)a | 52 (32.9)b | 46 (20.8) | 163 (33.7)d | |
| Smoking history (n, %) | < 0.001 | ||||||
| No | 3209 (78.8) | 1290 (77.2) | 1191 (77.1) | 123 (78.3) | 192 (87.3)c | 413 (86.0)d | |
| Yes | 863 (21.3) | 381 (22.8) | 353 (22.9) | 34 (21.7) | 28 (12.7)c | 67 (14.0)d | |
| Drinking history (n, %) | < 0.001 | ||||||
| No | 2973 (72.7) | 1160 (69.2) | 1129 (72.7)a | 127 (80.4)b | 163 (73.8) | 394 (81.6)d | |
| Yes | 1119 (27.4) | 516 (30.8) | 425 (27.3)a | 31 (19.6)b | 58 (26.2) | 89 (18.4)d | |
| Number of chronic disease (n, %) | < 0.001 | ||||||
| 0 | 2099 (51.3) | 922 (55.0) | 750 (48.2)a | 67 (42.4)b | 120 (54.3) | 240 (49.7)d | |
| 1 | 897 (21.9) | 386 (23.0) | 342 (22.0) | 27 (17.1) | 50 (22.6) | 92 (19.1) | |
| ≥ 2 | 1097 (26.8) | 368 (22.0) | 463 (30.0)a | 64 (40.5)b | 51 (23.1) | 151 (31.3)d | |
| Depression (n, %) | < 0.001 | ||||||
| No | 3292 (80.4) | 1449 (86.5) | 1257 (80.8)a | 114 (72.2)b | 170 (76.9)c | 302 (62.5)d | |
| Yes | 800 (19.6) | 226 (13.5) | 298 (19.2)a | 44 (27.8)b | 51 (23.1)c | 181 (37.5)d | |
| Sleep duration (n, %) | < 0.001 | ||||||
| < 6 h | 485 (11.9) | 189 (11.2) | 209 (13.4) | 20 (12.7) | 20 (9.1) | 47 (9.7) | |
| 6–8 h | 2698 (66.0) | 1175 (70.1) | 1014 (65.2)a | 93 (58.9)b | 142 (64.3) | 274 (56.7)d | |
| ≥ 9 h | 910 (22.2) | 312 (18.6) | 332 (21.4) | 45 (28.5)b | 59 (26.7)c | 162 (33.5)d |
* Data are presented as the mean ± standard deviation (SD)
a P values were < 0.05 between robust group and physical pre-frailty group
b P values were < 0.05 between robust group and physical frailty group
c P values were < 0.05 between robust group and cognitive impairment group
d P values were < 0.05 between robust group and cognitive frailty group
Table 1 presents the characteristics of the study participants by physical frailty and cognitive impairment status. Significant differences were observed among the 5 groups regarding age, gender, ethnicity, education level, marital status, smoking history, drinking history, number of chronic diseases, depression and sleep duration (P < 0.001). Compared to other groups, cognitive frailty participants were more likely to be older (> 80 years), Tibetan, illiterate and single. The cognitive frail group contained a smaller proportion of participants with drinking history than other 4 groups. The proportion of participants who had high number of chronic disease (≥ 2), depression and long sleep duration (≥ 9 h) increased with the following order: robust, physically pre-frailty, physically frailty, cognitive impairment and cognitive frailty.
Table 2 shows the results of logistic regression analysis examining the association between frailty, cognitive function and sleep duration. It showed that long sleep duration (≥ 9 h) were significantly associated with an increased prevalence of pre-physical frailty (OR = 1.23, 95%CI = 1.03–1.47, P = 0.02), physical frailty (OR = 1.82, 95%CI = 1.25–2.66, P = 0.002), cognitive impairment (OR = 1.56, 95%CI = 1.13–2.17, P = 0.008) and cognitive frailty (OR = 2.23, 95%CI = 1.77–2.80, P < 0.001). Multivariate adjustment for age, sex, race, marital status, education level, smoking history, drinking history, number of chronic diseases and depression only resulted in small change to estimated OR in long sleep duration (≥ 9 h). Long sleep duration was independently associated with the increased odds of pre-physical frailty (OR = 1.20, 95%CI = 1.0–1.44, P = 0.049), physical frailty (OR = 1.70, 95%CI = 1.14–2.54, P = 0.009), cognitive impairment (OR = 1.45, 95%CI = 1.03–2.04, P = 0.032) and cognitive frailty (OR = 2.07, 95%CI = 1.60–2.68, P < 0.001) than the control group. Opposed to long sleep duration, the associations between short sleep duration (< 6 h) and cognitive frailty were not observed in either unadjusted or adjusted model.
Table 2.
Associations between sleep duration and cognitive frailty according to unadjusted and adjusted logistic regression models (n = 4093)
| Robust | Physical pre-frailty OR [95%CI], P-value | Physical frailty OR [95%CI], P-value | Cognitive impairment OR [95%CI], P-value | Cognitive frailty OR [95%CI], P-value | |
|---|---|---|---|---|---|
| Unadjusted model | |||||
| Sleep duration < 6 h | Reference | 1.28 [1.03, 1.59], P = 0.023 | 1.33 [0.81, 2.22], P = 0.262 | 0.88 [0.54, 1.43], P = 0.597 | 1.07 [0.75, 1.51], P = 0.715 |
| Sleep duration 6–8 h | Reference | Reference | Reference | Reference | Reference |
| Sleep duration ≥9 h | Reference | 1.23 [1.03, 1.47], P = 0.020 | 1.82 [1.25, 2.66], P = 0.002 | 1.56 [1.13, 2.17], P = 0.008 | 2.23 [1.77, 2.80], P < 0.001 |
| Adjusted modela | |||||
| Sleep duration < 6 h | Reference | 1.19 [0.95, 1.48], P = 0.137 | 1.06 [0.62, 1.82], P = 0.824 | 0.82 [0.49, 1.36], P = 0.438 | 0.88 [0.60, 1.28], P = 0.499 |
| Sleep duration 6–8 h | Reference | Reference | Reference | Reference | Reference |
| Sleep duration ≥9 h | Reference | 1.20 [1.0, 1.44], P = 0.049 | 1.70 [1.14, 2.54], P = 0.009 | 1.45 [1.03, 2.04], P = 0.032 | 2.07 [1.60, 2.68], P < 0.001 |
a Model adjusted for age, gender, ethnicity, marital status, education, smoking history, drinking history, number of chronic diseases, depression. OR odds ratio; CI confidence intervals
Discussion
This is the first study to explore the association between cognitive frailty and sleep duration in a relatively large sample size of older adults in western China. Our results revealed that long sleep duration was associated with 2.07 times increased odds of cognitive frailty.
The overall prevalence of cognitive frailty in older adults dwelling in western China communities was 11.8%, which was generally consistent with the previous study conducted by Feng et al. [4]. They reported that the prevalence of pre-physical frailty and physical frailty concurrent with cognitive impairment was 10.7% in 2375 Chinese Singaporeans aged 55 years and older [4]. It is worth noting that the co-occurrence of pre-physical frailty, physical frailty and cognitive impairment were very common in our study. We found that 19.05% of physically pre-frail and 42.55% of physically frail participants had impaired cognitive function. The results were in line with previous studies that showed a higher prevalence of cognitive impairment in the older individuals with physical frailty [30–32]. Meanwhile, 51.99 and 16.62% of cognitive impaired participants had pre-physical frailty and physical frailty, respectively.
Results from our study indicated that long sleep duration was positively associated with pre-physical frailty, physical frailty, cognitive impairment and cognitive frailty. What’s more, higher odds risk of cognitive frailty was observed in the participants with long sleep duration among 5 groups. Although there is no direct research about the association between cognitive frailty and long sleep, several studies have found that physical frailty and cognitive impairment were related to long sleep separately, leading support to the link between cognitive frailty and long sleep duration. Sun et al. found that sleep time 9 h was associated with higher odds of physical frailty and pre-physical frailty in community-dwelling older adults aged 70–87 years [33]. Suh et al. demonstrated that older adults with long sleep duration (≥ 7.95 h) at baseline were related to the risk of cognitive decline at 4-year follow-up [34]. In addition, a prospective study focusing on frail elderly participants who were followed up for 18 months found that longer nighttime in bed increased the incidence of cognitive decline. These findings implied that long sleep duration can not only impair the physiological reserves of multiple systems in older adults, but also impair their cognitive function, thus ultimately leads to cognitive frailty.
Although exact mechanisms remain unclear, there are several similar underlying mechanisms between frailty and cognitive impairment and long sleep duration. Firstly, long sleep is known to be associated with cardiovascular disease, stroke, obesity and depression [35, 36]. It suggested that long sleep may be markers for poor health which increases the likelihood of frailty [37–40] and cognitive impairment [41–43]. Although we adjusted the number of diseases covariates and depression symptoms in this study, we could not ignore the possibility. Secondly, increased levels of pro-inflammatory factors C-reactive protein (CRP) and interleukin-6 (IL-6) were reported in participants with long sleep duration [44]. Meanwhile, the meta-analysis conducted by Soysal et al. indicated a positive association between physical frailty and pro-inflammatory factors, such as CRP and IL-6 [45], which was also found to be elevated in the participants with cognitive impairment [46, 47]. Therefore, inflammation is considered as one of the pathogenesis for physical frailty and cognition and long sleep duration. Furthermore, the atrophy of superior frontal gyrus (SFG) could be another possible underlying mechanism. Previous studies reported that SFG was involved in a variety of cognitive and motor control tasks [48–51]. A longitudinal study found those reporting > 7 h of sleep had higher rates of thinning in the SFG of the left hemisphere during an approximately 8-year follow-up period compared to those reporting 7 h of sleep [52]. These findings suggest the atrophy of SFG may increase the vulnerability of older adults for both physical and cognitive disorders through long sleep.
The present study did not observe the relationship between short sleep duration and frailty as well as cognitive impairment in older adults. Yet, previous studies have investigated the relationship among short sleep duration, frailty and cognitive function with inconsistent results. Similar to our results, both Sun et al. and Baniak et al. found no association between short sleep duration and frailty in either Americans or Chinese older adults [33, 53]. Tamayo et al. found sleeping less than 5 h could promote frailty in Mexican older women [17], while Ensrud et al. found no significant association between short sleep duration (< 5 h) and greater frailty status in American older men [54]. Meanwhile, one study in Korea demonstrated that short sleep duration (< 6 h) was not associated with frailty among older adults of both sexes [55]. Besides, a recent system review including 32 observational studies found that more studies suggested that long (rather than short) sleep duration were related to worse cognition [56]. The inconsistent results from different studies may be due to the differences of sleep duration’s definition (Night-time sleep duration or 24-Hour sleep duration) and short sleep duration’s classification (i.e., short sleep duration defined as < 4 h, < 5 h or < 6 h or < 7 h) [56]. The inconclusive results between studies may also raise the question of whether the older adults in different countries or regions have different sleep need.
The recurrent findings might provide a new approach for the prevention of cognitive frailty in older adults. Comparing to other risk factors, long sleep duration can be easily targeted in interventions by the geriatricians. Therefore, it is critical to evaluate sleep duration in the participants with cognitive frailty. Meanwhile, more intervention studies are needed to verify whether improving sleep duration is helpful for preventing or delaying cognitive frailty in the future. Nevertheless, some limitations of the present study need to be mentioned. First, similar to other cross-sectional studies, the causal association between sleep duration and cognitive frailty could not be established. In future research, longitudinal research should be conducted to verify this relationship. Second, sleep duration was only measured with self-reported questionnaire in this study. Although the application of self-reported questionnaire to assess participants’ sleep duration is common in routine clinical practice, self-reported sleep duration may introduce bias, especially when participants have severe cognitive impairment. Furthermore, there remains residual confounding factors in our study although we adjusted with many confounders in our study.
Conclusions
In summary, our findings indicated that long sleep duration was an independent associated factor for cognitive frailty in older adults. As sleep duration is potentially modifiable, interventions directed to improve sleep may provide new strategies for the prevention of cognitive frailty in the future.
Acknowledgements
We thank all the participants for their contribution in the WCHAT study.
Abbreviations
- AD
Alzheimer disease
- BMI
Body mass index
- CI
Confidence intervals
- CIND
Cognitive impairment but no dementia
- CLTPAQ
China leisure time physical activity questionnaire
- CRP
C-Reactive protein
- GDS-15
15-Item geriatric depression scale
- IL-6
Interleukin-6
- IQR
Interquartile range
- MLTPAQ
Minnesota leisure time physical activity questionnaire
- OR
Odds ratio
- QOL
Quality of life
- SD
Standard deviation
- SFG
Superior frontal gyrus
- SPMSQ
Short portable mental status questionnaire
- WCHAT
West China health and aging trend study
Authors’ contributions
YZ formulated the research question, designed the study, analyzed the data, and drafted the paper. YL, WZ, YW, MG, LZ, JY, QH designed the study, analyzed the data, and revised the paper. BD assisted with formulating the research question, interpretation of data, supervising the quality of the paper. All authors reviewed, provided feedback to, and confirmed the final version of the manuscript.
Funding
This research was funded by “Chengdu Science and Technology Bureau Major Science and Technology Application Demonstration Project (2019YF0900083SN)”, “Sichuan Science and Technology Program (2019YFS0277)”, “Postdoctoral Fund of West China Hospital (2019HXBH054)” and “the National Natural Science Foundation of China (81901411)”. The funders have no role in the study design, data collection and analysis, preparation of manuscript and decision of the publication.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445). All participants gave a written informed consent before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 2.Kojima G. Prevalence of frailty in nursing homes: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2015;16(11):940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8(1):14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Feng L, Zin Nyunt MS, Gao Q, Feng L, Yap KB, Ng TP. Cognitive frailty and adverse health outcomes: findings from the Singapore longitudinal ageing studies (SLAS) J Am Med Dir Assoc. 2017;18(3):252–258. doi: 10.1016/j.jamda.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Fougère B, Daumas M, Lilamand M, Sourdet S, Delrieu J, Vellas B, Abellan van Kan G. Association between frailty and cognitive impairment: cross-sectional data from toulouse frailty day hospital. J Am Med Dir Assoc. 2017;18(11):990.e1–990.e5. doi: 10.1016/j.jamda.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):716–721. doi: 10.1136/jech-2015-206717. [DOI] [PubMed] [Google Scholar]
- 7.Gorgoraptis N, Zaw-Linn J, Feeney C, Tenorio-Jimenez C, Niemi M, Malik A, Ham T, Goldstone AP, Sharp DJ. Cognitive impairment and health-related quality of life following traumatic brain injury. NeuroRehabilitation. 2019;44(3):321–331. doi: 10.3233/NRE-182618. [DOI] [PubMed] [Google Scholar]
- 8.Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell M, Teo K, Gao P, Anderson C, Sleight P, Dans A, Marzona I, Bosch J, Probstfield J, Yusuf S. Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J. 2012;33(14):1777–1786. doi: 10.1093/eurheartj/ehs053. [DOI] [PubMed] [Google Scholar]
- 10.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.a.N.a./I.a.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 11.Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and Meta-analysis. J Am Med Dir Assoc. 2016;17(10):881–888. doi: 10.1016/j.jamda.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Bu Z, Huang A, Xue M, Li Q, Bai Y, Xu G. Cognitive frailty as a predictor of adverse outcomes among older adults: a systematic review and meta-analysis. Brain Behav. 2021;11(1):e01926. doi: 10.1002/brb3.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumimoto K, Doi T, Nakakubo S, Kim M, Kurita S, Ishii H, Shimada H. Cognitive frailty as a risk factor for incident disability during late life: a 24-month follow-up longitudinal study. J Nutr Health Aging. 2020;24(5):494–499. doi: 10.1007/s12603-020-1365-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Duffy JF. Circadian rhythm sleep-wake disorders in older adults. Sleep Med Clin. 2018;13(1):39–50. doi: 10.1016/j.jsmc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Halson SL, Juliff LE. Sleep, sport, and the brain. Prog Brain Res. 2017;234:13–31. doi: 10.1016/bs.pbr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176(9):1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-Tamayo K, Manrique-Espinoza B, Ortiz-Barrios LB, Cárdenas-Bahena Á, Ramírez-García E, Sánchez-García S. Insomnia, low sleep quality, and sleeping little are associated with frailty in Mexican women. Maturitas. 2020;136:7–12. doi: 10.1016/j.maturitas.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Chen JC, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, Phillips LS, Robinson JG, Kotchen JM, Johnson KC, Manson JAE, Stefanick ML, Sarto GE, Mysiw WJ. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016;12(1):21–33. doi: 10.1016/j.jalz.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou L, Liu X, Zhang Y, Zhao W, Xia X, Chen X, Lin X, Yue J, Ge N, Dong B. Cohort profile: West China health and aging trend (WCHAT) J Nutr Health Aging. 2021;25(3):302–310. doi: 10.1007/s12603-020-1530-1. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Wang YY, Deng CY, Ding D, Song Y, Taiping L, Jirong Y, et al. Development and validation of the china leisure time physical activity questionnaire in the elderly. Pract Geriatr. 2019;33(03):229–233. [Google Scholar]
- 22.Conway JM, Irwin ML, Ainsworth BE. Estimating energy expenditure from the Minnesota leisure time physical activity and Tecumseh occupational activity questionnaires - a doubly labeled water validation. J Clin Epidemiol. 2002;55(4):392–399. doi: 10.1016/s0895-4356(01)00497-8. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Whitson HE, Malhotra R, Chan A, Matchar DB, Østbye T. Comorbid visual and cognitive impairment: relationship with disability status and self-rated health among older Singaporeans. Asia Pac J Public Health. 2014;26(3):310–319. doi: 10.1177/1010539512443698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazir A, LaMantia M, Chodosh J, Khan B, Campbell N, Hui S, Boustani M. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963. doi: 10.1111/jgs.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panza F, Lozupone M, Solfrizzi V, Sardone R, Dibello V, Di Lena L, et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis. 2018;62(3):993–1012. doi: 10.3233/JAD-170963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto T, Sakurai T, Ono R, Kimura A, Saji N, Niida S, Toba K, Chen LK, Arai H. Epidemiological and clinical significance of cognitive frailty: a mini review. Ageing Res Rev. 2018;44:1–7. doi: 10.1016/j.arr.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan S, Tasali E. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of sleep medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim PP, Ng LL, Chiam PC, Ong PS, Ngui FT, Sahadevan S. Validation and comparison of three brief depression scales in an elderly Chinese population. Int J Geriatr Psychiatry. 2000;15(9):824–830. doi: 10.1002/1099-1166(200009)15:9<824::aid-gps207>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, Carrière I, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 31.Macuco CR, Batistoni SS, Lopes A, Cachioni M, da Silva Falcão DV, Neri AL, et al. Mini-mental state examination performance in frail, pre-frail, and non-frail community dwelling older adults in Ermelino Matarazzo, São Paulo, Brazil. Int Psychogeriatr. 2012;24(11):1725–1731. doi: 10.1017/S1041610212000907. [DOI] [PubMed] [Google Scholar]
- 32.Feng L, Nyunt MS, Gao Q, Feng L, Lee TS, Tsoi T, et al. Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the Singapore longitudinal ageing studies. J Gerontol A Biol Sci Med Sci. 2017;72(3):369–375. doi: 10.1093/gerona/glw050. [DOI] [PubMed] [Google Scholar]
- 33.Sun XH, Ma T, Yao S, Chen ZK, Xu WD, Jiang XY, Wang XF. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 2020;20(1):9. doi: 10.1186/s12877-019-1407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH, Lee KH, Han G, Hong JW, Kwak KP, Kim BJ, Kim SG, Kim JL, Kim TH, Ryu SH, Moon SW, Park JH, Seo J, Youn JC, Lee DY, Lee DW, Lee SB, Lee JJ, Jhoo JH, Kim KW. Sleep and cognitive decline: a prospective nondemented elderly cohort study. Ann Neurol. 2018;83(3):472–482. doi: 10.1002/ana.25166. [DOI] [PubMed] [Google Scholar]
- 35.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. doi: 10.1016/j.smrv.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–670. doi: 10.1002/da.22386. [DOI] [PubMed] [Google Scholar]
- 37.Wong TY, Massa MS, O'Halloran AM, Kenny RA, Clarke R. Cardiovascular risk factors and frailty in a cross-sectional study of older people: implications for prevention. Age Ageing. 2018;47(5):714–720. doi: 10.1093/ageing/afy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer K, Vetrano DL, Padua L, Romano V, Rivoiro C, Scelfo B, Marengoni A, Bernabei R, onder G. Frailty syndromes in persons with cerebrovascular disease: a systematic review and Meta-analysis. Front Neurol. 2019;10:1255. doi: 10.3389/fneur.2019.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021:afab039. 10.1093/ageing/afab039. [DOI] [PubMed]
- 40.Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, Lopez Samaniego L, Rodríguez-Mañas L, Bernabei R, onder G, Joint Action ADVANTAGE WP4 Group Frailty and multimorbidity: a systematic review and Meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(5):659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 41.Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, et al. Cognitive impairment and heart failure: systematic review and Meta-analysis. J Card Fail. 2017;23(6):464–475. doi: 10.1016/j.cardfail.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Makin SD, Turpin S, Dennis MS, Wardlaw JM. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry. 2013;84(8):893–900. doi: 10.1136/jnnp-2012-303645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feinkohl I, Winterer G, Pischon T. Obesity and post-operative cognitive dysfunction: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2016;32(6):643–651. doi: 10.1002/dmrr.2786. [DOI] [PubMed] [Google Scholar]
- 44.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and Meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Mangiafico RA, Sarnataro F, Mangiafico M, Fiore CE. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to C-reactive protein and D-dimer levels. Age Ageing. 2006;35(1):60–65. doi: 10.1093/ageing/afi219. [DOI] [PubMed] [Google Scholar]
- 47.Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: the northern Manhattan study. J Stroke Cerebrovasc Dis. 2006;15(1):34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Russo F, Berchicci M, Bianco V, Perri RL, Pitzalis S, Quinzi F, Spinelli D. Normative event-related potentials from sensory and cognitive tasks reveal occipital and frontal activities prior and following visual events. Neuroimage. 2019;196:173–187. doi: 10.1016/j.neuroimage.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 50.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.du Boisgueheneuc F, Levy R, Volle E, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129(Pt 12):3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 52.Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, Ferrucci L, Resnick SM. Sleep duration and subsequent cortical thinning in cognitively Normal older adults. Sleep. 2016;39(5):1121–1128. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baniak LM, Yang K, Choi J, Chasens ER. Long sleep duration is associated with increased frailty risk in older community-dwelling adults. J Aging Health. 2020;32(1):42–51. doi: 10.1177/0898264318803470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ensrud KE, Blackwell TL, Ancoli-Israel S, Redline S, Cawthon PM, Paudel ML, Dam TTL, Stone KL. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. doi: 10.1016/j.sleep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang I, Kim S, Kim BS, Yoo J, Kim M, Won CW. Sleep latency in men and sleep duration in women can be frailty markers in community-dwelling older adults: the Korean frailty and aging cohort study (KFACS) J Nutr Health Aging. 2019;23(1):63–67. doi: 10.1007/s12603-018-1109-2. [DOI] [PubMed] [Google Scholar]
- 56.Devore EE, Grodstein F, Schernhammer ES. Sleep duration in relation to cognitive function among older adults: a systematic review of observational studies. Neuroepidemiology. 2016;46(1):57–78. doi: 10.1159/000442418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.