Abstract
The human circulatory system is a marvelous fluidic system, which is very sensitive to biophysical and biochemical cues. The current animal and cell culture models do not recapitulate the functional properties of the human circulatory system, limiting our ability to fully understand the complex biological processes underlying the dysfunction of this multifaceted system. In this review, we discuss the unique ability of microfluidic systems to recapitulate the biophysical, biochemical, and functional properties of the human circulatory system. We also describe the remarkable capacity of microfluidic technologies for exploring the complex mechanobiology of the cardiovascular system, mechanistic studying of cardiovascular diseases, and screening cardiovascular drugs with the additional benefit of reducing the need for animal models. We also discuss opportunities for further advancement in this exciting field.
Keywords: Microfluidics, Organ-on-a-chip, Human circulatory system, Cardiovascular diseases, Mechanobiology
Introduction
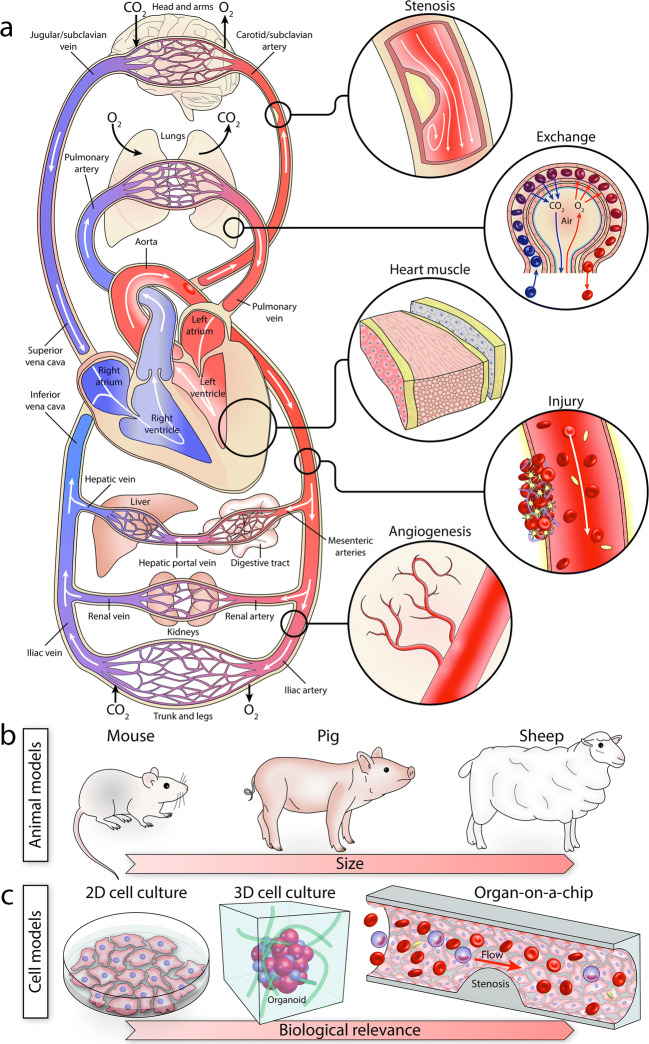
The human circulatory system is a complex, multi-component organ system, composed of the heart at its center and a fascinating network of large to small vessels spanning throughout the body (Fig. 1a). It is dynamic and highly responsive to biophysical and biochemical cues (Gimbrone et al. 2000). Changes in the geometry, composition, and stiffness of the vessels and heart valves due to chronic deposition of fat, cholesterol, calcium, aging or genetic disorders can change the physiological flow patterns and lead to a cascade of molecular and cellular events that disrupt their function (Paneni et al. 2017; Wootton and Ku 1999).
Fig. 1.
a Schematic of the human circulatory system, consisting of the heart at the center and a complex network of vessels which interact with other organs. b Animal models are extensively used for studying the human circulatory system. c The complexity, cost, and inherent differences between the human and animal models have led to the evolution of cell models, ranging from simple 2D models (Petri dish, flasks) to more complex 3D models (hydrogel, spheroids, organoids) and more physiologically relevant microfluidic models
Animal models are widely used for studying cardiovascular diseases, and have been instrumental in advancing our understanding of the human circulatory system (Zaragoza et al. 2011) (Fig. 1b). Large animals (e.g., pigs and sheep) closely mirror the human circulatory system, as they exhibit size, anatomy, physiology, and hemodynamics comparable to humans (Tsang et al. 2016). However, large animal models are costly, require substantial housing and caring resources, and are subject to ethical considerations (Tsang et al. 2016). In comparison, small animals (e.g., mice and rats) are relatively cost-effective, easy to maintain, and readily available; can be tested in large numbers; and importantly, can be genetically modified to represent cardiovascular disorders seen in patients (Camacho et al. 2016). Despite these advantages, small-animal models are inherently limited due to significant differences in size, cardiovascular anatomy, physiology, pathology, and heart function (e.g., the heart rate of mice is 400–600 bpm) (Santos et al. 2015) compared to humans. These parameters have imposed challenges and resulted in the failure of many clinical drug trials despite successfully conducted prior animal studies (Editorial 2013; Hay et al. 2014).
In comparison, cell-based assays enable understanding the fundamental biology of the human circulatory system in a simple, fully controllable, time- and cost-effective manner (Fig. 1c). Traditional 2D cell culture models facilitate growing cells in a Petri dish or flask chambers to analyze cellular response to drugs or pathogens. More advanced 3D cell models, based on hydrogel scaffolds, spheroids, and organoids, facilitate growing cells in a more physiological environment, which better mimic the structural and functional properties of the native organ, and thus promote cell-cell and cell-extracellular matrix (ECM) interactions (Duval et al. 2017; Grebenyuk and Ranga 2019; Jensen and Teng 2020; Salehi et al. 2020). Alternative cell-based models that can better mimic the physiology and pathology of the human circulatory system are pivotal to better understand the fundamental biological processes underlying this complex system.
Recent advances in microfabrication technologies have facilitated the evolution of various microfluidic models (Convery and Gadegaard 2019) (Fig. 1c). These models can mimic the biophysical and biochemical properties of the human circulatory system under customized, fully controllable physiological and pathological conditions. This has enabled mechanistic studies of complex, multi-step biological processes, which can be employed for the modeling of diseases and the development of new drugs and treatment strategies (Bersini et al. 2016; Regnault et al. 2018). The adoption of microfluidic models can substantially reduce the time and costs associated with evaluating the efficacy of new drugs and facilitates the development of personalized, patient-specific therapeutics (Tay et al. 2016).
Here, we review the recent microfluidic technologies engineered for studying the human cardiovascular system. We present the evolution of complex, multilayered, deformable vessel structures, which closely mimic the biophysical and biochemical properties of human vessels. We highlight the unique ability for establishing deformable microfluidic elements to recapitulate the rhythmic contraction of cardiac muscles and valve leaflets. We describe the suitability of microfluidic systems for exploring the complex mechanobiology of the cardiovascular system and modeling various stages of cardiovascular diseases under a highly controlled microenvironment, which can be employed for discovering novel therapeutic targets and screening cardiovascular drugs. We also present multi-layered, multi-component microfluidic platforms to enable studying the exchange of various biomolecules, ions, and gasses between the vessel walls and surrounding body organs/tissues. Finally, we provide suggestions to further advance this rapidly growing field.
An overview of microfluidic models of the human cardiovascular system
A variety of microfluidic models have been developed for recapitulating the human cardiovascular system. To provide an overview of these advancements, in this section, we discuss (i) microfluidic vessel models with an emphasis on the geometry, anatomy, materials, and fabrication techniques; (ii) stretchable and deformable elements to recapitulate soft vessels, contracting cardiac muscles, and valve leaflets; (iii) microfluidic systems for modeling various cardiovascular diseases or pathological conditions; and (iv) multi-layered microfluidic systems suitable for studying the exchange of various molecules, ions, and materials between the vessel walls and surrounding body organs/tissues, as presented below.
Microfluidic vessel structures
The human circulatory system consists of a network of arteries, arterioles, capillaries, venules, and veins to facilitate the recirculation of blood throughout the body (Walter Boron 2016). A variety of microfluidic systems have been developed for mimicking the human circulatory system. The geometry of the vessels varies from simple, straight configurations (Mandy et al. 2011; Nguyen et al. 2018; Polacheck et al. 2019; Wang and Shuler 2018) to more complex configurations, involving curved, bifurcated, or branched architectures (Bertassoni et al. 2014; Fenech et al. 2019; Tsvirkun et al. 2017). Microfluidic vessel models provide unique tools for studying the fundamental aspects of vascular endothelial cell biology under customized physiological and pathological shear stress levels (Baratchi et al. 2017; Hahn and Schwartz 2009; Lai et al. 2021; Thurgood et al. 2019) as well as tailored flow dynamics driven by static, oscillatory, or pulsatile flows (Mohammed et al. 2019a, 2019b; Shao et al. 2009). When fabricating a microfluidic vessel, two major parameters need to be considered. This includes the cross-sectional profile of the microfluidic vessel and the arrangement of biological layers across the vessel wall, which are briefly discussed in the following sections.
Cross-sectional profile of microfluidic vessels
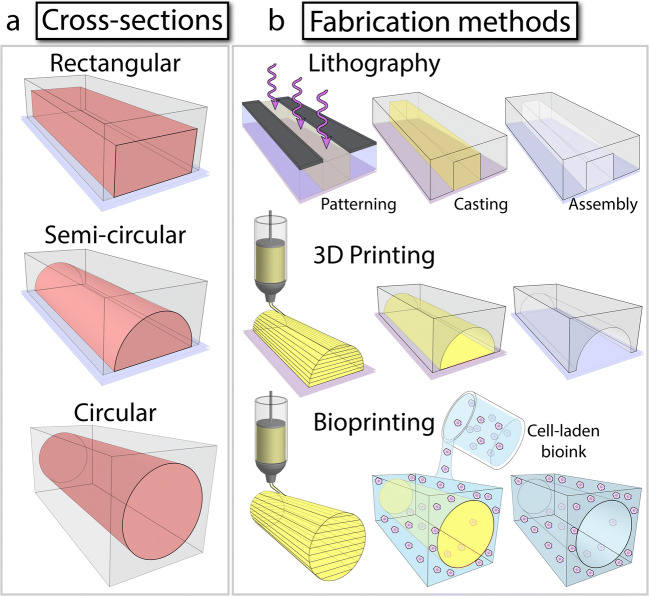
Microfluidic vessel models are provided in rectangular, semi-circular, and circular cross sections (Fig. 2). The choice of the cross section is generally determined by the technique used for fabricating the microfluidic vessel, as outlined in Table 1, and described in this section.
Fig. 2.
Methods for fabricating microfluidic vessel structures: a Microfluidic vessel structures with rectangular, semi-circular, and circular cross sections which can be fabricated using, b lithography, 3D printing, and bioprinting techniques
Table 1.
Common techniques for the fabrication of microfluidic vessel structures
| Techniques | Features | References |
|---|---|---|
| Lithography |
• Well established • Requires microfabrication facilities • Suitable for planar structures (channels with rectangular cross sections) • Suitable for serial fabrication |
(Akbari et al. 2018; Alsmadi et al. 2017; Brown et al. 2014; Fenech et al. 2019; Ha and Lee 2013; Hansen et al. 2013; Herbig and Diamond 2017; Jain et al. 2016; Kwon et al. 2020; Mandy et al. 2011; Mathur et al. 2019; Menon et al. 2017; Muthard and Diamond 2013; Seo et al. 2017; Thomas et al. 2016; Tovar-Lopez et al. 2019; Tovar-Lopez et al. 2013; Tovar-Lopez et al. 2010; Tsvirkun et al. 2017; Westein et al. 2013; Zheng et al. 2015) |
| 3D printing |
• Suitable for 3D complex structures • Rapid prototyping • Rapidly evolving • Ability to print multiple materials • Limited resolution |
(Costa et al. 2017; Hernández Vera et al. 2019) |
| Bioprinting |
• Suitable for 3D complex vascularized structures • Multilayer tissues and organs can be printed |
(Abudupataer et al. 2019; Bertassoni et al. 2014; Kolesky et al. 2016; Kolesky et al. 2014; Shimizu et al. 2020; Zhang et al. 2013) |
| Laser cutting |
• Rapid prototyping • Low cost • Suitable for fabrication of thermoplastic devices • Limited resolution • Rough surfaces |
(Wang and Shuler 2018) |
| Micromilling |
• Rapid prototyping • Low cost • Suitable for high-aspect-ratio structures • Suitable for fabrication of thermoplastic devices • Limited resolution • Rough surfaces |
(Li et al. 2012; van Dijk et al. 2020) |
| Injection molding |
• Suitable for mass production • Suitable for fabrication of thermoplastic devices |
(Mohammed et al. 2019a, 2019b; Zhu et al. 2019) |
| Unconventional |
• Rapid prototyping • Enables 3D complex structures • Does not rely on microfabrication facilities or equipment • Does not require knowledge and expertise in microfabrication |
(Chiu et al. 2003; Mannino et al. 2015; Nguyen et al. 2018; Parekh et al. 2016; Polacheck et al. 2019) |
| Biomimetic |
• Suitable for replication of microvascular network • Suitable for studying vasculogenesis and angiogenesis processes |
(Kim et al. 2015; Kim et al. 2016a, 2016b; Kim et al. 2013; Li et al. 2019; Michelle et al. 2017; Nguyen et al. 2013; Oh et al. 2017; Ryu et al. 2015; Sobrino et al. 2016) |
Lithography
Vessel structures with a rectangular cross section can be readily fabricated using conventional lithography techniques (Fig. 2a) (Baratchi et al. 2014; Tsvirkun et al. 2017). The height of these channels is generally in the order of 100 μm while the width of the channels can reach hundreds of microns. The flat surface of the channel facilitates the high-resolution microscopy of endothelial cells cultured onto the channel. Lithographically made structures (known as master) are generally templated in biocompatible elastomers such as polydimethylsiloxane (PDMS) (Baratchi et al. 2014; Tsvirkun et al. 2017). Alternatively, master structures can be templated in more biologically relevant, permeable, and softer materials such as hydrogels that are commonly used in tissue engineering (Akbari et al. 2018; Brown et al. 2014; Cheng et al. 2007; Gutierrez et al. 2008; Ling et al. 2007; Menon et al. 2017; Shin et al. 2019), to better resemble the mechanical properties of extracellular matrix (ECM), as comprehensively reviewed elsewhere (Muehleder et al. 2014).
3D printing
More physiologic vessel structures, involving semi-circular or circular cross sections, can be fabricated using 3D printing technologies (Fig. 2b) (Costa et al. 2017; Hernández Vera et al. 2019). The application of washable (fugitive) inks enables the vessel structures to be fully embedded in PDMS or hydrogel. Remarkably, this approach facilitates the fabrication of patient-specific vessel models based on computed tomography angiography imaging data (Costa et al. 2017).
Bioprinting
An alternative strategy for the fabrication of vessel-like structures is based on direct printing of fugitive inks in hydrogels (Fig. 2b). This has been proven to be a versatile method for making multilayered vascularized tissues (Kolesky et al. 2016; Kolesky et al. 2014). An elegant example is the fabrication of vessel-like architectures by printing of agarose fibers in gelatin methacryloyl (GelMA) hydrogels (Bertassoni et al. 2014). This method enables the fabrication of complex, interconnected structures, including bifurcating networks. Fluorescent microscopy confirmed the formation of a confluent monolayer of endothelial cells with developed intercellular junctions along the inner surface of the channels. This method was also shown to be effective in improving the diffusion, viability, and differentiation of osteogenic cells embedded in cell-laden GelMA hydrogels.
Other techniques
Methods such as laser cutting (Wang and Shuler 2018), micromilling (Li et al. 2012; van Dijk et al. 2020), and injection molding (Mohammed, et al., 2019; Zhu et al. 2019) have also been used for the fabrication of microfluidic vessel structures. Among these methods, laser cutting and micromilling enable the direct engraving of channels in plastic or acrylic materials. Such methods facilitate the rapid prototyping of microfluidic structures in the absence of lithography and 3D printing facilities. In comparison, injection molding is suitable for the mass production of commercially available thermoplastic microfluidic devices (Mohammed, et al., 2019; Zhu et al. 2019).
Unconventional
Several innovative methods have also been proposed for the rapid prototyping of vessel-like structures with semi-circular or circular cross sections without having access to conventional fabrication facilities, as discussed above. One example includes the pasta templating method, which involves the deposition of pasta structures on a temperature-sensitive colloid such as petroleum jelly that undergoes a liquid-to-gel phase transition (Nguyen et al. 2018). This approach enabled the fabrication of semi-circular structures, in which the circularity and height of the channel can be readily modulated during the deposition of pasta structures. Circular vessel structures have also been made by templating optical fibers (Mannino et al. 2015), steel needles (Polacheck et al. 2019), reeling of core-shell microfibers (Sun et al. 2020), and liquid metal alloys (Nguyen et al. 2019; Parekh et al. 2016; Yan et al. 2018).
Biomimetic
The replication of a microvascular network is rather challenging using conventional fabrication techniques due to the geometrical diversity and complexity of the vessels (Fenech et al. 2019). This has inspired the researchers to harness the biochemical and biomechanical principles used in our body to facilitate the formation of new vessels (vasculogenesis) or growing vessels from existing vessels (angiogenesis) (Chen and Kaji 2017; Laschke and Menger 2016; Semenza 2007). Angiogenic sprouting has also been recapitulated by patterning two parallel microfluidic channels in a collagen matrix (Nguyen et al. 2013). One of the channels was seeded with endothelial cells while the other channel was perfused with angiogenic factors. This established a gradient across the collagen matrix, causing the endothelial cells to migrate into the matrix, thus facilitating the development of filopodial protrusions toward the angiogenic channel. The release of angiogenic factors can also be achieved by seeding human lung fibroblasts seeded onto the microfluidic channel (Kim et al. 2013). This method has been shown effective for the creation of a large microvascular network across a microfluidic channel filled with collagen matrix within 5 days. Immunofluorescent imaging confirmed the structural integrity of the engineered microvascular network, characterized by the formation of complex interconnected, bifurcated vessels and robust cell-cell junctions. A similar strategy has been adopted to recapitulate the formation of new lymphatic vessels (Kim, et al., 2016).
Arrangement of biological layers across the vessel wall
Vessels are multilayered and composed of endothelial cells and smooth muscle cells separated by an extracellular matrix (also known as subendothelial layer) containing collagen and elastane (Charalambos Vlachopoulos and Nichols 2011). Therefore, to ensure microfluidic vessels recapitulate the biophysical properties of human vessels, they should ideally contain the above three layers (Fig. 3). Endothelialization of glass slides, involving the direct seeding of vascular endothelial cells onto glass or polymer substrates, is very common for understanding the mechanobiology of endothelial cells under various shear stress levels (Baratchi et al. 2014; Mandy et al. 2011; Mohammed et al. 2019a, 2019b). The glass slides can also be coated with collagen or other hydrogels to better mimic the biomechanical properties of the ECM (Shin et al. 2019). Alternatively, the glass slides can be coated with cell adhesion molecules (P-selectin, E-selectin, or ICAM-1) to promote the adhesion of leukocytes to the endothelial cells, mimicking the inflamed endothelium (Kwon et al. 2020). Multilayer microfluidic vessels coated with three layers, including endothelial cells, smooth muscle cells, and fibroblasts, have also been reported for studying the transmigration and accumulation of monocytes in the early stages of atherosclerosis (Zhang et al. 2020).
Fig. 3.
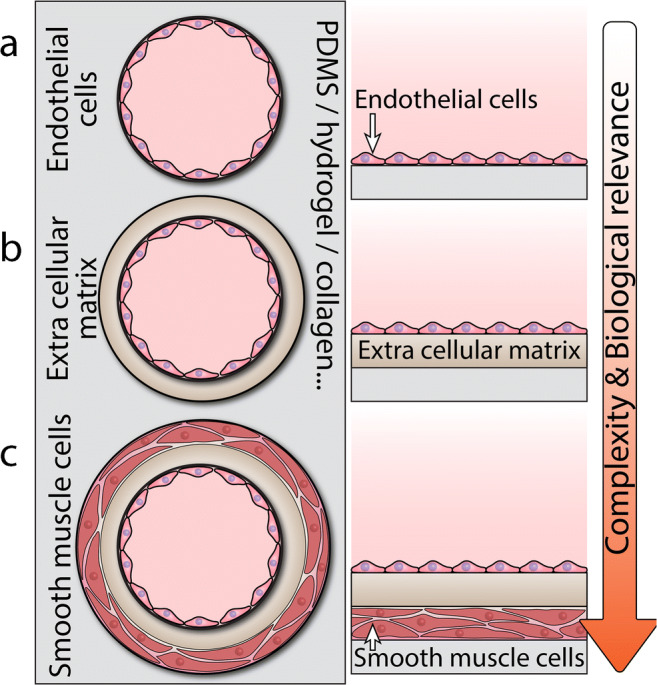
Methods for coating the surface of microfluidic vessel structures: a single layer involving vascular endothelial cells directly coated onto the surface, b double layer involving collagen coated onto the surface to serve as an extracellular matrix for vascular endothelial cells, and c triple layer involving smooth muscle cells, collagen, and endothelial cells coated onto the surface
Stretchable and deformable vessel and cardiac models
The pulsation of cardiac muscles leads to the periodic opening and closing of heart valves and the consequent recirculation of blood through the vessels. Blood flow exerts both tangential (shear stress) and normal (pressure) loads on the vessel walls (Hahn and Schwartz 2009). Given the soft nature of the vessels, the pulsatile pressure leads to cyclic stretch of the vessels, which plays important roles in their physiology, development, and remodeling (Hahn and Schwartz 2009). Microfluidic platforms incorporating movable, deformable elements enable recapitulating the dynamic environment of stretchable blood vessels, cardiac muscles, and valve leaflets, as outlined in Table 2 and described in this section.
Table 2.
Deformable microfluidic models for recapitulating mechanical stress/strain in the human cardiovascular system
| Configuration | References |
|---|---|
| Stretchable flow-free devices | (Kamble et al. 2017; Mann et al. 2012; Shao et al. 2014; Sinha et al. 2016; Sniadecki et al. 2007) |
| Stretchable flow-through vessels | (Estrada et al. 2011; Jin et al. 2020; Michielin et al. 2015; Moraes et al. 2013; Ribas et al. 2017; Sato et al. 2019; Shimizu et al. 2020; Tan et al. 2008; Zheng et al. 2012; Zhou and Niklason 2012) |
| Cardiac muscle tissues | (Agarwal et al. 2013; Aung et al. 2016; Feinberg et al. 2007; Grosberg et al. 2011; Liu et al. 2020a, 2020b; Marsano et al. 2016; Mathur et al. 2015; McAleer et al. 2019; Ong et al. 2015; Zhang et al. 2016) |
| Cardiac valves | (Baratchi et al. 2020; Duan et al. 2014; Flanagan et al. 2007; Grigoryan et al. 2019; Hockaday et al. 2012; Hu et al. 2020; Lee et al. 2019) |
Stretchable flow-free devices
Vessel walls are subjected to shear stress caused by the blood flow and cyclic stretch caused by the pulsatile blood pressure (Hahn and Schwartz 2009). Shear stress mainly affects the endothelial cells, which are in direct contact with the blood flow, whereas the cyclic stretch mainly affects the smooth muscle cells (Qiu et al. 2014). Microfabricated cell stretching devices have enabled studying the mechano-transduction of vascular endothelial cells (Shao et al. 2014; Sinha et al. 2016), smooth muscle cells (Mann et al. 2012), and fibroblasts (Kamble et al. 2017; Sniadecki et al. 2007) under cyclic stretch. These devices take advantage of the flexibility of PDMS membranes or pillar arrays to stretch cells in a static (flow-free) condition.
The incorporation of advanced techniques such as atomic force microscopy (Krieg et al. 2019), magnetic tweezers (Aermes et al. 2020), optical tweezers (Arbore et al. 2019), Förster resonance energy transfer (FRET) sensors (Liu, et al., 2020), and scanning ion conductance microscopy (Swiatlowska et al. 2020) has enabled the high-resolution measurement of cell biomechanical properties in response to physical stimuli, as comprehensively reviewed by others (Bajpai et al. 2021; Iskratsch et al. 2014; Liu, et al., 2020; Mohammed, et al., 2019).
Stretchable vessels
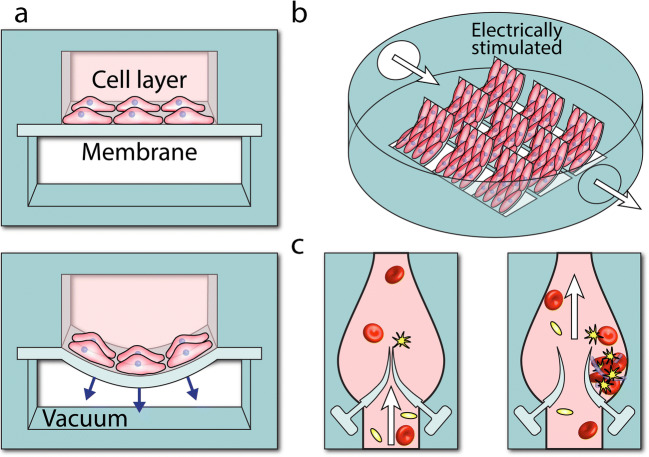
Multilayered microfluidic systems, pioneered for developing lung-on-chip devices (Huh et al. 2010), provide unique opportunities for studying the mechanobiology of vascular endothelial and smooth muscle cells under the combined effect of shear stress and cyclic stretch (Sato et al. 2019; van Engeland et al. 2018; Zheng et al. 2012; Zhou and Niklason 2012), and therefore can better mimic the complex biomechanics of vessels. An elegant example is a microfluidic system devised to study the effect of cyclic stretch on vascular aging (Ribas et al. 2017) (Fig. 4a). The system consists of two parallel microchannels separated by a PDMS membrane. The top fluidic channel was seeded with primary smooth muscle cells (SMCs) derived from healthy and Hutchinson-Gilford progeria syndrome (HGPS) donors experiencing accelerated aging. The bottom channel was connected to a vacuum line. The membrane stretch was proportional to the vacuum pressure, enabling the system to stimulate the cells under both physiological (9% strain) and pathological (16% strain) conditions. Physiological strain led to the cytoskeletal reorientation of both healthy and HGPS cells. However, the pathological strain triggered inflammatory and vascular injury responses in HGPS cells, as evidenced by an exacerbated increase in the expression of inflammatory and injury genes.
Fig. 4.
Microfluidic structures with movable elements: a simultaneous mechanical and shear stress stimulation of cells using a multilayered microfluidic system (Ribas et al. 2017), b harmonically deflecting cantilever beams to mimic cardiac muscles (Agarwal et al. 2013), and c leaflet-like barriers to mimic the complex flow dynamics of heart valves (Hu et al. 2020)
Multilayer microfluidic systems also provide unique platforms for direct measurement of endothelial barrier function through assessing the electrical resistance (van der Helm et al. 2016) or electrochemical permeability (Wong and Simmons 2019) across the endothelial monolayer cultured on the membrane. The incorporation of electrochemical sensors in the PDMS membrane has also been demonstrated for real-time monitoring of transient electrochemical signals generated by endothelial cells during the cyclic stretch (Jin et al. 2020).
The heart
The heart is a muscular pump that continuously recirculates blood through the circulatory system. The rhythmic contracting and relaxing of the heart are facilitated by cardiac muscle tissue, also known as the myocardium, which is surrounded by thin layers of epicardium and endocardium (Walter Boron 2016). Microfluidic technologies facilitate the development of unique in vitro models for studying the biomechanics, pathology, and drug sensitivity of cardiac muscle tissues. Stem cells are increasingly used for engineering microfluidic-based cardiac muscle tissues (Jastrzebska et al. 2016; Mathur et al. 2015). Multilayered microfluidic systems, incorporating stretchable membranes, can recapitulate the biomechanics of cardiac cells during the systolic and diastolic cycles (Marsano et al. 2016). Experiments revealed that the cyclic stretch of rat and human induced pluripotent stem cells (iPSC) improves the proliferation and expression of cell-cell junctions in the engineered cardiac tissue.
Bioinspired platforms utilizing thin PDMS cantilevers coated with cardiac myocytes have been also developed to mimic the cyclic contraction of the cardiac muscle (Agarwal et al. 2013) (Fig. 4b). These cantilevers can be harmonically deflected upon electrical field stimulation, and thus serve as programmable live robotic actuators (Feinberg et al. 2007). Fluorescent microscopy confirmed the formation of a confluent monolayer of cardiac cells on the cantilevers. The contractile stress exerted on the cells was calculated based on the change of curvature in contracting cantilevers, which could be monitored in real time. This feature was used to evaluate the effect of isoproterenol on electrically stimulated cardiomyocytes covering the cantilevers. Microfluidic systems capable of real-time monitoring of contractile stresses and electrophysiological activities of cardiac cells under physiological and pathological conditions have been also reported (Aung et al. 2016; Liu, et al., 2020).
Cardiac valves
Microfluidic platforms, incorporating leaflet-like barriers, facilitate the study of the mechanobiology of aortic endothelial cells under tailored flow conditions (Baratchi et al. 2020). Dynamic venous valve models, incorporating a pair of flexible valve leaflets along a microfluidic channel, have been recently reported (Hu et al. 2020) (Fig. 4c). The leaflets were fabricated by polymerizing a UV curable polymer solution with the elastic modulus of the leaflets being set to 100 kPa. The contraction-relaxation of the heart muscle was mimicked by utilizing a motor-controlled clamp between the two valves. The clamp facilitated the deformation of the PDMS channel and the anchored valves, causing one valve to open while the other valve to close. The periodic opening and closing of the valves altered the flow profile within the microfluidic channel. The opening of the downstream valve formed a high-velocity jet flow between the two valve leaflets while a low-velocity vortical flow between the leaflets and the channel (also known as valve pocket). This produced a backward flow in the upstream valve, which was close. Fluorescent microscopy revealed the formation of fibrin networks at the valve pocket induced by low shear vortical flows. The periodic backward flow enabled the removal of the fibrin networks from the valve pocket, thus inhibiting the growth of thrombus. Disrupting the regular backward flow led to the formation of dense fibrin networks.
Modeling cardiovascular diseases and pathological conditions
Microfluidic platforms provide unique models for modeling various cardiovascular diseases. This can be achieved by varying the physical properties (size, contraction, or materials) or operating conditions (flow rate or flow dynamics) of the artificial vessels to trigger target pathologies within the cultured cells. Compared to animal models, which need to undergo surgical procedures or genetic modifications to create various cardiovascular pathologies (Tsang et al. 2016; Zaragoza et al. 2011), this process is easier, faster, and more controllable using microfluidic models. This strategy has been used for modeling a variety of cardiovascular pathologies. This includes chronic diseases such as atherosclerosis, vessel and aortic valve stenosis, acute cases caused by vessel injury as well as general diseases such as cancer, in which the tumors take advantage of blood vessels to invade the neighboring organs, as outlined in Table 3, and briefly discussed in this section.
Table 3.
Microfluidic systems for studying various cardiovascular diseases
| Pathological condition/disease | References |
|---|---|
| Endothelial dysfunction | (Akbari et al. 2018; Mathur et al. 2019; Seo et al. 2017; Shin et al. 2019; Thomas et al. 2016; van Dijk et al. 2020; Zheng et al. 2012) |
| Atherosclerosis | (Chen et al. 2018; Kim et al. 2014; Menon et al. 2018; Menon et al. 2017; Park et al. 2019; Salminen et al. 2019; Shin et al. 2019; Zhang et al. 2020; Zheng et al. 2016) |
| Vessel stenosis (high shear stress) | (Alsmadi et al. 2017; De Lizarrondo et al. 2017; Ha and Lee 2013; Hansen et al. 2013; Jain et al. 2016; Jung and Yeom 2017; Li et al. 2012; Mannino et al. 2015; Menon et al. 2018; Menon et al. 2017; Szydzik et al. 2018; Tovar-Lopez et al. 2013; Tovar-Lopez et al. 2010; Westein et al. 2013; Xu et al. 2020; Yazdani and Karniadakis 2016) |
| Valve leaflet stenosis | (Osnabrugge et al. 2013) |
| Reversed flow (low shear stress) | (Balaguru et al. 2016; Chiu et al. 2003; Sei et al. 2017; Tovar-Lopez et al. 2019; Zarins et al. 1983) |
| Vessel injury | (Brown et al. 2014; de Witt et al. 2014; Gutierrez et al. 2008; Hansen et al. 2013; Herbig and Diamond 2017; Jain et al. 2016; Muthard and Diamond 2013) |
| Tumor growth | (Cross et al. 2010; Kim et al. 2015; Lee et al. 2014; Michelle et al. 2017; Oh et al. 2017; Sobrino et al. 2016; Zervantonakis et al. 2012) |
Atherosclerosis
Endothelial dysfunction in response to low shear stress levels has been investigated in several works (Seo et al. 2017; Thomas et al. 2016; Zheng et al. 2012). Particularly, microfluidic systems have been utilized for recapitulating various stages of atherosclerosis (Shelton and Kamm 2020). This includes the early stages of atherosclerosis, characterized by an increased endothelial permeability and disruption of endothelial cell junctions (Shin et al. 2019) to the later stages of atherosclerosis, associated with leukocyte-endothelial adhesion (Menon et al. 2018), trans-endothelial migration of leukocytes (Chen et al. 2018; Salminen et al. 2019), and foam cell formation (Zhang et al. 2020).
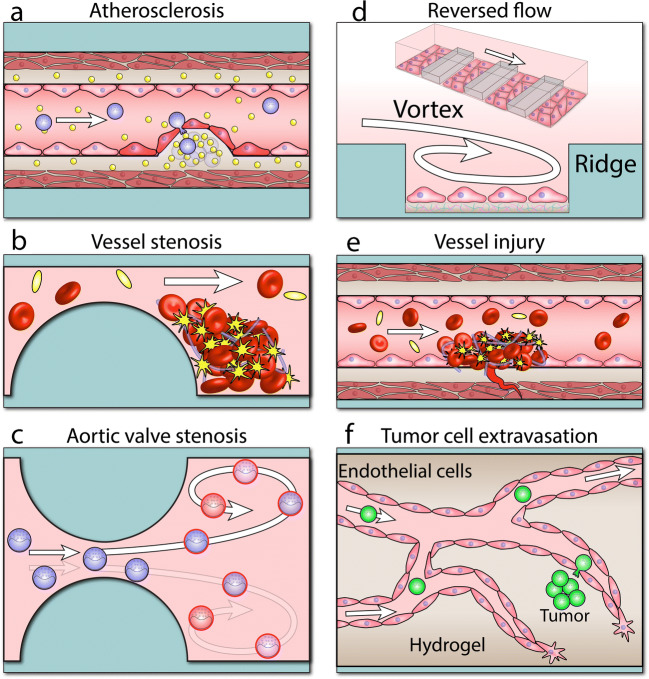
An elegant example is the arteriole-scale vessel model developed to mimic various stages of atherosclerosis (Zhang et al. 2020) (Fig. 5a). The vessel is composed of three layers, endothelial cells, smooth muscle cells, and fibroblasts. Atherosclerosis was induced by applying enzyme-modified low-density lipoprotein (eLDL) with/without tumor necrosis factor α (TNFα) to the engineered vessel. The perfusion of eLDL and TNFα was shown to reduce the vasoactivity of the vessel (the ability of the vessel to change its diameter in response to vasoactive agents), while increasing the production of nitride oxide, and the expression of pro-inflammatory signals (VCAM-1, ICAM-1, E-selectin, and TNFα) by the endothelial layer. This increased monocyte-endothelial adhesion and facilitated the trans-endothelial migration of monocytes. Histological staining of the vessels confirmed the formation of foam cells and uptake of eLDL within the fibroblast layer. Experiments also indicated the ability to partially recover the vessel by eliminating eLDL from the perfusion medium or injecting lovastatin co-enzyme or P2Y11 receptor inhibitor to the vessel. This further highlights the potential of microfluidic models to serve as drug discovery platforms to evaluate the efficacy of various anti-atherosclerosis treatments (Kim et al. 2014; Zheng et al. 2016) at a reduced time and cost compared to animal models.
Fig. 5.
Microfluidic vessel structures allowing for modeling various cardiovascular diseases or pathological flows: a atherosclerosis (Zhang et al. 2020), b vessel stenosis (Westein et al. 2013), c valve leaflet stenosis (Baratchi et al. 2020), d reversed flow (Tovar-Lopez et al. 2019), e vessel injury (Muthard and Diamond 2013), and f tumor angiogenesis (Sobrino et al. 2016)
Vessel stenosis (narrowing)
The chronic deposition of cholesterol and other lipids, cellular debris, calcium, and fibrin along the vessel walls leads to the development of atherosclerotic plaques, causing the gradual narrowing of the vessels (Gimbrone et al. 2000; Wootton and Ku 1999). The wall shear rate in a cylindrical tube is inversely proportional to the cube of its diameter () (Charalambos Vlachopoulos and Nichols 2011). This suggests the induction of high shear rates in narrowed vessels, which mediates biomechanical platelet aggregation (Rana et al. 2019) and determines cellular and particle margination (Ta et al. 2018).
Several microfluidic systems have been developed to recapitulate the biomechanical and biochemical microenvironment in narrowed vessels (Fig. 5b). This includes a straight channel containing a plague-like, semi-cylindrical barrier to mimic a stenosed vessel (Westein et al. 2013). Implementing an 80% stenosis model induced shear rates of > 8000 s–1, leading to a significant platelet aggregation at the post-stenosis region. The results indicated the vital role of von Willebrand factor (vWF) proteins in shear-induced platelet aggregation. Experiments also revealed the increased secretion of vWF by the endothelial cells located at the post-stenosis region, which further contributed to exacerbated platelet aggregation. Other stenotic geometries incorporating triangular (Tovar-Lopez et al. 2010), square (Tovar-Lopez et al. 2010), or trapezoid (Ha and Lee 2013; Jung and Yeom 2017) barriers have also been demonstrated. A recent study has incorporated a soft semi-cylindrical barrier to mimic the lipid core of an atherosclerotic plaque (Park et al. 2019). More complex stenosis structures involving multiple stenosed regions along one (Jung and Yeom 2017; Menon et al. 2017) or both sidewalls (Alsmadi et al. 2017; Ha and Lee 2013; Yazdani and Karniadakis 2016) have also been developed.
The low throughput of microfluidic channel experiments can limit their utility for clinical diagnostics and drug screening. To address this limitation, researchers have developed a microfluidic system, incorporating 12 long stenosed channels to run parallel experiments (Jain et al. 2016). Stenosed channels were made by reducing the width of the channels to 55% of their original size to induce shear rates of up to 2500 s−1 to emulate the conditions observed in patients with arteriosclerosis. Perfusion of blood through the channel led to the formation of fibrin networks and platelet aggregates at the post-stenosis region. Ex vivo experiments with a porcine model demonstrated the utility of the device for real-time monitoring of hemostasis and coagulopathy. Functionalization of the microfluidic channels with collagen (a well-known platelet agonist) reduced the clotting time from ∼ 30 to ∼ 10 min, and in turn reduced the blood volume required to be driven through the system. A similar strategy has been implemented for studying shear-induced platelet activation by integrating an array of micropatterned collagen spots into a multi-shear microfluidic device (Hansen et al. 2013).
Other innovative strategies have been implemented for mimicking the activation and adhesion of platelets in narrowed vessels. This includes a microfluidic system incorporating a magnetic stirrer to induce customized shear rate levels by simply adjusting the rotational speed of the stirrer (Xu et al. 2020). This enabled generating high, pathological shear levels suitable to activate platelets. The system utilized collagen-coated microbeads to facilitate the adhesion of activated platelets.
Microfluidics has also become a valuable tool to investigate the effects of new compounds on platelets and the coagulation system. This is of particular relevance, as there is a major medical need for new anti-platelet compounds that are not associated with bleeding complications (McFadyen et al. 2018).
Valve leaflet stenosis
Aortic valve stenosis due to the thickening and movement-restricting degeneration of the valve leaflets in aging adults imposes pathologically high shear stress levels on circulating blood cells (leukocytes and platelets) at the valve orifice (Osnabrugge et al. 2013). Circulating cells pass through the orifice ~ 1500 times each day and therefore are vulnerable to shear stress changes. Remarkably, monocytes are highly sensitive to shear gradients due to their large size (diameter > 25 μm) and their tendency to move close to the vessel walls and aortic valves. Recent research has shown increased activation of monocytes, characterized by elevated expression of proinflammatory markers and adhesion molecules, following the recirculation through microfluidic models of stenosed aortic valves (Baratchi et al. 2020) (Fig. 5c). The role of mechanical forces in regulating the adhesion, migration, and activation of immune cells has been comprehensively discussed in other reviews (Huse 2017; Vesperini et al. 2021).
Reversed flows
Sudden changes in the geometry of the vessels (occurring at the bifurcations, sharp curvatures, or localized expansions) disrupt the laminar, unidirectional flow pattern of blood and may lead to the generation of reversed flow patterns (also known as disturbed flows or vortices) within the vessel (Baratchi et al. 2017; Hahn and Schwartz 2009). Reversed flows are associated with flow separation and low (pathological) shear stress levels, which may activate the expression of pro-inflammatory markers and destabilize the endothelial barrier function, making it prone to atherosclerosis (Gimbrone et al. 2000).
Reversed flows have been studied using a glass model of carotid bifurcation (Zarins et al. 1983). This pioneering model was reconstructed based on measurements obtained from 57 patients with symptomatic atherosclerosis. Flow visualization along with detailed analysis of velocity profiles using laser-Doppler anemometry showed the formation of recirculating flows within the sinus at Reynolds numbers of more than 400. Similar effects have been observed using early versions of microfluidic flow chambers made of gasket rubbers utilizing vertical steps to generate recirculating flow regions (Chiu et al. 2003). More recently, researchers have investigated the endothelial dysfunction in response to oscillatory shear stress at the carotid arteries (Sei et al. 2017).
Despite such advances, the generation of disturbed flows was limited to the expanded region of the device, which in turn limited the number of stimulated cells. To overcome this limitation, researchers have developed a microfluidic system capable of producing multiple vortices at the predetermined locations of the device (Tovar-Lopez et al. 2019) (Fig. 5d). The system utilized an array of rectangular ridges patterned along the floor of a microfluidic channel. High-speed imaging along with numerical simulations revealed the formation of a pair of vortices between the neighboring ridges, the size of which was governed by the Reynolds number of the flow. The system facilitated studying the effect of pathological shear stress levels on the cytoskeletal orientation as well as nuclear shape and size of human aortic endothelial cells (HAECs).
Vessel injury
Platelet activation relies on the complex interaction between platelets and vessel wall, which leads to the adhesion, activation, and aggregation of platelets (Bye et al. 2016; Chen et al. 2014; Grover et al. 2018). Microfluidic models of the injured vessel facilitate the systematic study of such complex, multi-step, and dynamic interactions in a controlled manner, which cannot be achieved by animal models. An injured blood vessel has been replicated by interfacing a collagen scaffold with a microfluidic channel (Muthard and Diamond 2013) (Fig. 5e). The design was composed of a straight channel to serve as the blood vessel and a triangular scaffold loaded with fibrous matrix materials such as collagen, located along the sidewalls, to serve as the injured site. Notably, the system was equipped with several inlet ports to enable the wall shear stress and the pressure gradient across the scaffold (mimicking trans-thrombus permeation) to be modulated independently. The device allowed studying the dynamic formation of shear-induced platelet and thrombin along the collagen scaffold under controlled trans-thrombus pressure gradients. A similar design has been used for studying the thrombus formation around flow stagnation points (Herbig and Diamond 2017).
Platelet agonists such as thrombin, thromboxane, and adenosine diphosphate (ADP) play important roles in the activation of platelets (Yun et al. 2016). Hence, it is particularly important to study the function of platelet agonists in thrombus formation. The effect of platelet agonists has been studied by utilizing a multilayer microfluidic device incorporating porous membranes (Neeves and Diamond 2008). The porous membrane facilitated the release of ADP into the blood flow at desired rates. Confocal microscopy revealed that the size and height of the platelet aggregates strongly depend on the ADP flux. Scanning microscopy revealed the influence of the ADP flux on the morphology of the platelets. Platelet spreading and formation of filopodia was observed at the highest ADP flux. In comparison, the platelets did not spread at the lowest ADP flux and only adhered to the membrane. This configuration has also been used to study the effect of thrombin flux on fibrin deposition (Neeves et al. 2010) and endothelial cell permeability under flow (Kim et al. 2014; Young et al. 2010).
Tumor cell growth and invasion
Microfluidic angiogenesis models, discussed earlier (Kim et al. 2013; Nguyen et al. 2013), provide unparalleled opportunities for recapitulating and studying the vascularization of tumors (Sobrino et al. 2016) (Fig. 5f). Furthermore, such models enable the fundamental understanding of the molecular processes that lead to impairment of endothelial barrier function which may result in the trans-endothelial migration of tumor cells to enter the vasculature (intravasation) (Zervantonakis et al. 2012) or leave the vasculature (extravasation) (Michelle et al. 2017). Such models also facilitate studying the circulation of tumor cells through the vasculature (Michelle et al. 2017). This provides unique opportunities to observe the complex dynamics of tumor cells within the vasculature, which drives the lodgement, dislodgement, aggregation, and arrest of tumor cells (Soleimani et al. 2018). This fundamental knowledge is essential to develop therapeutic agents to suppress the growth and invasion of tumors (Kim et al. 2015).
Exchange of molecules across the vessel walls
Recirculation of blood through the vast network of arteries, veins, and capillaries facilitates the exchange of nutrition, proteins, hormones, enzymes, ions, waste, and gasses between the blood flow and the surrounding body organs/tissues (Stanfield 2016). Examples include the secretion, filtration, and reabsorption of vital substances via the liver, kidneys, and small intestine and the delivery of nutrition, water, and glucose to the brain as well as blood oxygenation via the lungs, to name a few. Microfluidic technologies provide unique platforms for studying the exchange of various molecules and materials between the blood vessels and the surrounding organs/tissues, as outlined in Table 4 and briefly described in this section.
Table 4.
Microfluidic models for studying the exchange of molecules across the vessel walls
| Organ | References |
|---|---|
| Kidney | (Ishahak et al. 2020; Lin et al. 2019; Vedula et al. 2017) |
| Liver | (Deng et al. 2019; Li et al. 2018) |
| Small intestine | (Jalili-Firoozinezhad et al. 2018; Kim and Kim 2018) |
| Blood-brain barrier | (Achyuta et al. 2013; Bang et al. 2017; Bonakdar et al. 2017; Booth and Kim 2012; Szydzik et al. 2016) |
| Alveoli (lungs) | (Grigoryan et al. 2019; Ishahak et al. 2020) |
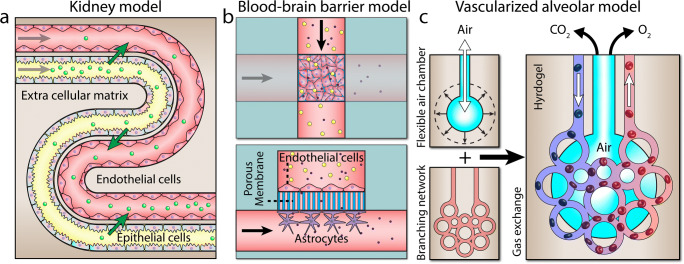
Renal reabsorption in kidneys
A vascularized proximal tubule model has been developed to study renal reabsorption in kidneys (Lin et al. 2019) (Fig. 6a). The model was composed of two parallel serpentine channels patterned ~ 70 μm apart with one channel coated with proximal tubule epithelial cells and the other coated with glomerular microvascular endothelial cells. The channels were embedded in a highly permeable ECM to facilitate the exchange of solutes across the tubular-vascular units. The utility of the system was proven by demonstrating the uptake of albumin and inulin by the proximal tubules and the reabsorption of glucose by the vasculature as a function of time.
Fig. 6.
Microfluidic vessel structures allowing for studying a renal reabsorption in kidney proximal tubules (Lin et al. 2019), b permeability of the blood-brain barrier (Booth and Kim 2012), and c oxygenation of blood across the alveoli (Grigoryan et al. 2019)
Blood-brain barrier
A multilayered microfluidic has been fabricated to investigate the selective permeability of the blood-brain barrier to various compounds (Booth and Kim 2012) (Fig. 6b). The device is composed of two perpendicular microfluidic channels, patterned on top of each other to serve as the lumenal and ablumenal sides of the neurovascular unit, which were interfaced via a polycarbonate porous membrane with a thickness of 10 μm and a pore size of 0.4 μm. Mouse brain endothelial cells and astrocytes were cultured on the lumenal and ablumenal sides of the porous membrane, respectively. The incorporation of embedded electrodes enabled monitoring of trans-endothelial electrical resistance (TEER) across the porous membrane. This allowed measuring the transient changes of barrier permeability in response to histamine.
Blood oxygenation in alveoli
A vascularized alveolar model has been fabricated to better mimic the process of gas exchange within the lungs (Grigoryan et al. 2019) (Fig. 6c). This model is composed of 3D polyhedral tessellations (to mimic air sacks) surrounded by a highly branched vascular network. The cyclic ventilation of the oxygen expanded the air sacs bringing them into contact with the adjacent vessels, facilitating the diffusive exchange of gas between the air sacs and the vessels. The expansion of air sacs also led to the compression of the vessels and redirection of flow to adjacent vessels, which in turn enhanced intravascular mixing and accelerated the uptake of oxygen by red blood cells (RBCs), as evidenced by the increasing oxygen saturation (SO2) of the RBCs and the changing of the color of RBCs from dark red (deoxygenated) to bright red (oxygenated) when passing through the system. This work offers a huge potential for studying pulmonary heart disease caused by abnormal gas exchange across the lungs (Han et al. 2007).
Conclusions and future directions
Microfluidic technologies provide unique analytical tools to explore the complex mechanobiology of the human circulatory system. These technologies aim to recapitulate the structural, biophysical, and biochemical properties of the human heart and vascular network and have enabled us to better understand how these organs function, which parameters contribute to the deterioration of these organs, and whether drugs and chemical compounds can reverse their deterioration. Owing to these features, these technologies can bridge between basic science researchers who use simple, inexpensive yet unrealistic cell culture models and clinicians who use complex, costly yet realistic animal models. Several microfluidic devices have demonstrated the effectiveness of such models in cardiovascular research, as discussed in the previous sections. To further advance these technologies, we propose the following steps to be taken in future research:
Better recapitulation of the biophysical properties of blood vessels
The inability of current methods to recapitulate the biophysical properties of blood vessels is among the key reasons hindering the widespread application of microfluidic vessel models. To address this limitation, improved strategies are required to ensure complex vessel structures (curved, bifurcated, complex networks with varying diameters, circular cross sections) can be fabricated, the biomechanical properties of the elastomers and hydrogels used for replicating the vessel structures closely match the properties of the vessels, relevant biological layers (endothelial cells, ECM, smooth muscle cells) can be arranged across the vessel, various flow dynamics (laminar, disturbed, turbulent, constant, pulsatile) can be generated inside the vessels, and tailored biomechanical loads (shear stress, pressure, compression, stretch) can be recreated within the vessel.
Modular, multi-component systems
The human circulatory system is an interconnected system composed of several organs (Fig. 1). While microfluidic models of individual cardiovascular organs facilitate a better understanding of the biology of the organs under highly controlled physiological and pathological conditions, the disconnection between various organs can significantly limit the ability of such models. The versatility and reliability of such microfluidic models can be substantially increased by designing modular, connectable multi-organ devices, which can be arranged in customized configurations (Fernandes et al. 2018). Several designs have been developed to address this requirement. This includes the reconfigurable microfluidic platforms utilizing self-aligning magnetic interconnects (Ong et al. 2019), screw interconnects (Chen et al. 2020), and interlocking Lego-like blocks (Owens and Hart 2018). Organ-on-chip modules can also be coupled using a pioneering robotic liquid-handling system (Novak et al. 2020). The system is equipped with peristaltic pumps for the continuous perfusion of the organ-on-chip modules and pipette pumps for linking one module to another along with a compact microscope for imaging of the modules. The system offers huge potentials for screening drugs (Herland et al. 2020).
Easier and more affordable systems
The adoption of simple, inexpensive, self-sufficient, maintenance-free components can reduce the complexity and cost of experimental setups and in turn facilitate the widespread application of microfluidic technologies in mechanobiology/cardiovascular research (Guck 2019; Mohammed, et al., 2019; Thurgood et al. 2019; Thurgood et al. 2018; Zhu et al. 2019).
Alternative materials
Most microfluidic systems are made of silicone-based polymers such as PDMS (Westein et al. 2013) or hydrogels such as collagen (Bertassoni et al. 2014). Such materials offer ease of fabrication, biocompatibility, elasticity, and transparency. Furthermore, hydrogels provide a soft tissue–like structure, which supports the proliferation, maturation, migration, and differentiation of cells and allows transport of nutrients, cellular waste, and tissue factors through their porous structure (Spicer 2020; Zhu and Marchant 2011). To ensure the microfluidic systems can recapitulate the human circulatory system, the physical properties (elasticity, porosity, roughness, thermal conductivity) and surface chemistry of such systems should closely match the biophysical properties of the relevant organs within the human circularity system. This requires the development of a new class of elastomers and hydrogels, which fulfill this requirement. These materials should preferably be photo-polymerizable to accelerate the fabrication process. A close collaboration between microfluidics, tissue engineering, and materials experts can facilitate the development of such materials.
New avenues for tracking and understanding of cardiovascular diseases
Wearable sensors (Khoshmanesh et al. 2021) have enabled the non-invasive and continuous monitoring of heart rate, heart rhythm, blood pressure, and oxygen saturation levels as well as various biomarkers corresponding to various cardiovascular diseases (Nahavandi et al. 2014). The integration and interpretation of these large data sets using machine learning algorithms enable better understanding of the heterogeneity of complex, multifactorial cardiovascular diseases in individuals or in a large population of patients (Hedman et al. 2020; Shameer et al. 2018). The adoption of this data to microfluidic vessel or cardiac models will enable us to design more realistic, patient-specific microfluidic assays aimed at detecting, monitoring, or preventing cardiovascular diseases.
Acknowledgements
E.P. acknowledges the National Health and Medical Research Council (NHMRC) for funding “The Australian Centre for Electromagnetic Bioeffects Research” (APP1135076). K.P. acknowledges the NHMRC for a L3 Investigator Fellowship support (GNT1174098). S.B. acknowledges the Australian Research Council (ARC) for Discovery Grants (DE170100239 and DP200101248). K.K. acknowledges the ARC for Discovery Grant (DP180102049).
Author contribution
N.N., P.T., N.C.S., and S.C. wrote the manuscript; E.P., S.B., and K.K. supervised the students; E.P., K.P., S.B., and K.K. generated the idea and wrote, reviewed, and edited the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Baratchi and Khashayar Khoshmanesh contributed equally to this work.
Contributor Information
Sara Baratchi, Email: Sara.Baratchi@rmit.edu.au.
Khashayar Khoshmanesh, Email: Khashayar.Khoshmanesh@rmit.edu.au.
References
- Abudupataer M, Chen N, Yan S, et al. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed Microdevices. 2019;22:10. doi: 10.1007/s10544-019-0460-3. [DOI] [PubMed] [Google Scholar]
- Achyuta AKH, Conway AJ, Crouse RB, et al. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–553. doi: 10.1039/C2LC41033H. [DOI] [PubMed] [Google Scholar]
- Aermes C, Hayn A, Fischer T, Mierke CT. Environmentally controlled magnetic nano-tweezer for living cells and extracellular matrices. Sci Rep. 2020;10:13453. doi: 10.1038/s41598-020-70428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari E, Spychalski GB, Rangharajan KK, Prakash S, Song JW. Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model. Lab Chip. 2018;18:1084–1093. doi: 10.1039/C8LC00130H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsmadi NZ, Shapiro SJ, Lewis CS, et al. Constricted microfluidic devices to study the effects of transient high shear exposure on platelets. Biomicrofluidics. 2017;11:064105. doi: 10.1063/1.4989386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbore C, Perego L, Sergides M, Capitanio M. Probing force in living cells with optical tweezers: from single-molecule mechanics to cell mechanotransduction. Biophys Rev. 2019;11:765–782. doi: 10.1007/s12551-019-00599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung A, Bhullar IS, Theprungsirikul J, et al. 3D cardiac μtissues within a microfluidic device with real-time contractile stress readout. Lab Chip. 2016;16:153–162. doi: 10.1039/C5LC00820D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai A, Li R, Chen W. The cellular mechanobiology of aging: from biology to mechanics. Ann N Y Acad Sci. 2021;1491:3–24. doi: 10.1111/nyas.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguru UM, Sundaresan L, Manivannan J, et al. Disturbed flow mediated modulation of shear forces on endothelial plane: a proposed model for studying endothelium around atherosclerotic plaques. Sci Rep. 2016;6:27304. doi: 10.1038/srep27304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Lee S-R, Ko J, et al. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci Rep. 2017;7:8083. doi: 10.1038/s41598-017-07416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Tovar-Lopez FJ, Khoshmanesh K, et al. Examination of the role of transient receptor potential vanilloid type 4 in endothelial responses to shear forces. Biomicrofluidics. 2014;8:044117. doi: 10.1063/1.4893272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Khoshmanesh K, Woodman OL, et al. Molecular sensors of blood flow in endothelial cells. Trends Mol Med. 2017;23:850–868. doi: 10.1016/j.molmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Baratchi S, Zaldivia MTK, Wallert M, et al. TAVI Represents an anti-inflammatory therapy via reduction of shear stress induced Piezo-1-mediated monocyte activation. Circulation. 2020;142:1092–1105. doi: 10.1161/CIRCULATIONAHA.120.045536. [DOI] [PubMed] [Google Scholar]
- Bersini S, Yazdi IK, Talò G, et al. Cell-microenvironment interactions and architectures in microvascular systems. Biotechnol Adv. 2016;34:1113–1130. doi: 10.1016/j.biotechadv.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/C4LC00030G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdar M, Graybill PM, Davalos RV. A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 2017;7:42811–42818. doi: 10.1039/C7RA07603G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- Brown AC, Stabenfeldt SE, Ahn B, et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater. 2014;13:1108. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye AP, Unsworth AJ, Gibbins JM. Platelet signaling: a complex interplay between inhibitory and activatory networks. J Thromb Haemost. 2016;14:918–930. doi: 10.1111/jth.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P, Fan H, Liu Z, He J-Q. Small mammalian animal models of heart disease. American Journal of Cardiovascular Disease. 2016;6:70–80. [PMC free article] [PubMed] [Google Scholar]
- Charalambos Vlachopoulos MOR, Nichols WW (2011) McDonald’s blood flow in arteries: theoretical, experimental and clinical principles. CRC Press
- Chen LJ, Kaji H. Modeling angiogenesis with micro- and nanotechnology. Lab Chip. 2017;17:4186–4219. doi: 10.1039/C7LC00774D. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corey SJ, Kim OV, Alber MS. Systems biology of platelet-vessel wall interactions. Adv Exp Med Biol. 2014;844:85–98. doi: 10.1007/978-1-4939-2095-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tang M, Huang D, et al. Real-time observation of leukocyte-endothelium interactions in tissue-engineered blood vessel. Lab Chip. 2018;18:2047–2054. doi: 10.1039/C8LC00202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Mo D, Gong M. 3D Printed reconfigurable modular microfluidic system for generating gel microspheres. Micromachines. 2020;11:224. doi: 10.3390/mi11020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-Y, Heilman S, Wasserman M, et al. A hydrogel-based microfluidic device for the studies of directed cell migration. Lab Chip. 2007;7:763–769. doi: 10.1039/b618463d. [DOI] [PubMed] [Google Scholar]
- Chiu J-J, Chen C-N, Lee P-L, et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. J Biomech. 2003;36:1883–1895. doi: 10.1016/S0021-9290(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Convery N, Gadegaard N. 30 years of microfluidics. Micro and Nano Engineering. 2019;2:76–91. doi: 10.1016/j.mne.2019.01.003. [DOI] [Google Scholar]
- Costa PF, Albers HJ, Linssen JEA, et al. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip. 2017;17:2785–2792. doi: 10.1039/C7LC00202E. [DOI] [PubMed] [Google Scholar]
- Cross VL, Zheng Y, Won Choi N, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lizarrondo SM, Gakuba C, Herbig BA, et al. Potent thrombolytic effect of N-acetylcysteine on arterial thrombi. Circulation. 2017;136:646–660. doi: 10.1161/CIRCULATIONAHA.117.027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witt SM, Swieringa F, Cavill R, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. doi: 10.1038/ncomms5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Zhang X, Chen Z, et al. A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. Biomicrofluidics. 2019;13:024101. doi: 10.1063/1.5070088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Kapetanovic E, Hockaday LA, Butcher JT. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014;10:1836–1846. doi: 10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval K, Grover H, Han L-H, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32:266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Editorial Of men, not mice. Nat Med. 2013;19:379–379. doi: 10.1038/nm.3163. [DOI] [PubMed] [Google Scholar]
- Estrada R, Giridharan GA, Nguyen M-D, et al. Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal Chem. 2011;83:3170–3177. doi: 10.1021/ac2002998. [DOI] [PubMed] [Google Scholar]
- Feinberg AW, Feigel A, Shevkoplyas SS, et al. Muscular thin films for building actuators and powering devices. Science. 2007;317:1366–1370. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- Fenech M, Girod V, Claveria V, et al. Microfluidic blood vasculature replicas using backside lithography. Lab Chip. 2019;19:2096–2106. doi: 10.1039/C9LC00254E. [DOI] [PubMed] [Google Scholar]
- Fernandes AC, Gernaey KV, Krühne U. Connecting worlds – a view on microfluidics for a wider application. Biotechnol Adv. 2018;36:1341–1366. doi: 10.1016/j.biotechadv.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Flanagan TC, Cornelissen C, Koch S, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28:3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Gimbrone MAJ, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesisa. Ann N Y Acad Sci. 2000;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Grebenyuk S, Ranga A. Engineering organoid vascularization. Frontiers in Bioengineering and. Biotechnology. 2019;7:39. doi: 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11:4165–4173. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover SP, Bergmeier W, Mackman N. Platelet signaling pathways and new inhibitors. Arterioscler Thromb Vasc Biol. 2018;38:e28–e35. doi: 10.1161/ATVBAHA.118.310224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck J. Some thoughts on the future of cell mechanics. Biophys Rev. 2019;11:667–670. doi: 10.1007/s12551-019-00597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Petrich BG, Shattil SJ, et al. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip. 2008;8:1486–1495. doi: 10.1039/b804795b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Lee SJ. Hemodynamic features and platelet aggregation in a stenosed microchannel. Microvasc Res. 2013;90:96–105. doi: 10.1016/j.mvr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- Hansen RR, Wufsus AR, Barton ST, et al. High content evaluation of shear dependent platelet function in a microfluidic flow assay. Ann Biomed Eng. 2013;41:250–262. doi: 10.1007/s10439-012-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- Hedman ÅK, Hage C, Sharma A, et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart. 2020;106:342. doi: 10.1136/heartjnl-2019-315481. [DOI] [PubMed] [Google Scholar]
- Herbig BA, Diamond SL. Thrombi produced in stagnation point flows have a core–shell structure. Cell Mol Bioeng. 2017;10:515–521. doi: 10.1007/s12195-017-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herland A, Maoz BM, Das D, et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nature Biomedical Engineering. 2020;4:421–436. doi: 10.1038/s41551-019-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Vera R, O’Callaghan P, Fatsis-Kavalopoulos N, Kreuger J. Modular microfluidic systems cast from 3D-printed molds for imaging leukocyte adherence to differentially treated endothelial cultures. Sci Rep. 2019;9:11321. doi: 10.1038/s41598-019-47475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockaday LA, Kang KH, Colangelo NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005–035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li Y, Li J, Chen H. Effects of altered blood flow induced by the muscle pump on thrombosis in a microfluidic venous valve model. Lab Chip. 2020;20:2473–2481. doi: 10.1039/D0LC00287A. [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17:679–690. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishahak M, Hill J, Amin Q, et al. Modular microphysiological system for modeling of biologic barrier function. Frontiers in Bioengineering and. Biotechnology. 2020;8:581163. doi: 10.3389/fbioe.2020.581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape — the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Graveline A, Waterhouse A, et al. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun. 2016;7:10176. doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S, Prantil-Baun R, Jiang A, et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human gut-on-a-chip. Cell Death Dis. 2018;9:223. doi: 10.1038/s41419-018-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska E, Tomecka E, Jesion I. Heart-on-a-chip based on stem cell biology. Biosens Bioelectron. 2016;75:67–81. doi: 10.1016/j.bios.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZH, Liu YL, Fan WT, Huang WH. Integrating flexible electrochemical sensor into microfluidic chip for simulating and monitoring vascular mechanotransduction. Small. 2020;16:1903204. doi: 10.1002/smll.201903204. [DOI] [PubMed] [Google Scholar]
- Jung SY, Yeom E. Microfluidic measurement for blood flow and platelet adhesion around a stenotic channel: effects of tile size on the detection of platelet adhesion in a correlation map. Biomicrofluidics. 2017;11:024119. doi: 10.1063/1.4982605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble H, Vadivelu R, Barton M, et al. An electromagnetically actuated double-sided cell-stretching device for mechanobiology research. Micromachines. 2017;8:256. doi: 10.3390/mi8080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshmanesh F, Thurgood P, Pirogova E, Nahavandi S, Baratchi S. Wearable sensors: at the frontier of personalised health monitoring, smart prosthetics and assistive technologies. Biosens Bioelectron. 2021;176:112946. doi: 10.1016/j.bios.2020.112946. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim G. Intestinal villi model with blood capillaries fabricated using collagen-based bioink and dual-cell-printing process. ACS Appl Mater Interfaces. 2018;10:41185–41196. doi: 10.1021/acsami.8b17410. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci. 2014;111:1078. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kasuya J, Jeon J, Chung S, Kamm RD. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip. 2015;15:301–310. doi: 10.1039/C4LC00866A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Chung M, Ahn J, Lee S, Jeon NL. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip. 2016;16:4189–4199. doi: 10.1039/C6LC00910G. [DOI] [PubMed] [Google Scholar]
- Kim S, Chung M, Jeon NL. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials. 2016;78:115–128. doi: 10.1016/j.biomaterials.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Fläschner G, Alsteens D, et al. Atomic force microscopy-based mechanobiology. Nature Reviews Physics. 2019;1:41–57. doi: 10.1038/s42254-018-0001-7. [DOI] [Google Scholar]
- Kwon S, Kurmashev A, Lee MS, Kang JH. An inflammatory vascular endothelium-mimicking microfluidic device to enable leukocyte rolling and adhesion for rapid infection diagnosis. Biosens Bioelectron. 2020;168:112558. doi: 10.1016/j.bios.2020.112558. [DOI] [PubMed] [Google Scholar]
- Lai A, Chen YC, Cox CD, et al. Analyzing the shear-induced sensitization of mechanosensitive ion channel Piezo-1 in human aortic endothelial cells. J Cell Physiol. 2021;236:2976–2987. doi: 10.1002/jcp.30056. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 2016;34:112–121. doi: 10.1016/j.biotechadv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Lee H, Park W, Ryu H, Jeon NL. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics. 2014;8:054102–054102. doi: 10.1063/1.4894595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- Li M, Ku DN, Forest CR. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip. 2012;12:1355–1362. doi: 10.1039/c2lc21145a. [DOI] [PubMed] [Google Scholar]
- Li X, George SM, Vernetti L, Gough AH, Taylor DL. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip. 2018;18:2614–2631. doi: 10.1039/C8LC00418H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu C, Wang P, et al. Indoor nanoscale particulate matter-induced coagulation abnormality based on a human 3D microvascular model on a microfluidic chip. Journal of Nanobiotechnology. 2019;17:20. doi: 10.1186/s12951-019-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NYC, Homan KA, Robinson SS, et al. Renal reabsorption in 3D vascularized proximal tubule models. Proc Natl Acad Sci. 2019;116:5399–5404. doi: 10.1073/pnas.1815208116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Rubin J, Deng Y, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- Liu H, Bolonduro OA, Hu N, et al. Heart-on-a-chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett. 2020;20:2585–2593. doi: 10.1021/acs.nanolett.0c00076. [DOI] [PubMed] [Google Scholar]
- Liu L, He F, Yu Y, Wang Y. Application of FRET biosensors in mechanobiology and mechanopharmacological screening. Frontiers in Bioengineering and. Biotechnology. 2020;8:595497. doi: 10.3389/fbioe.2020.595497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy B, Esch DJP, Shuler ML, Stokol T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng A. 2011;17:2965–2971. doi: 10.1089/ten.tea.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JM, Lam RHW, Weng S, Sun Y, Fu J. A silicone-based stretchable micropost array membrane for monitoring live-cell subcellular cytoskeletal response. Lab Chip. 2012;12:731–740. doi: 10.1039/C2LC20896B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino RG, Myers DR, Ahn B, et al. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci Rep. 2015;5:12401. doi: 10.1038/srep12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano A, Conficconi C, Lemme M, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16:599–610. doi: 10.1039/C5LC01356A. [DOI] [PubMed] [Google Scholar]
- Mathur A, Loskill P, Shao K, et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur T, Singh KA, Pandian NKR, et al. Organ-on-chips made of blood: endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips. Lab Chip. 2019;19:2500–2511. doi: 10.1039/C9LC00469F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer CW, Pointon A, Long CJ, et al. On the potential of in vitro organ-chip models to define temporal pharmacokinetic-pharmacodynamic relationships. Sci Rep. 2019;9:9619. doi: 10.1038/s41598-019-45656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen JD, Schaff M, Peter K. Current and future antiplatelet therapies: emphasis on preserving haemostasis. Nat Rev Cardiol. 2018;15:181–191. doi: 10.1038/nrcardio.2017.206. [DOI] [PubMed] [Google Scholar]
- Menon NV, Tay HM, Wee SN, Li KHH, Hou HW. Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip. 2017;17:2960–2968. doi: 10.1039/C7LC00607A. [DOI] [PubMed] [Google Scholar]
- Menon NV, Tay HM, Pang KT, et al. A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioengineering. 2018;2:016103. doi: 10.1063/1.4993762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelle BC, Jordan AW, Julia F, et al. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat Protoc. 2017;12:865. doi: 10.1038/nprot.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielin F, Serena E, Pavan P, Elvassore N. Microfluidic-assisted cyclic mechanical stimulation affects cellular membrane integrity in a human muscular dystrophy in vitro model. RSC Adv. 2015;5:98429–98439. doi: 10.1039/C5RA16957G. [DOI] [Google Scholar]
- Mohammed D, Versaevel M, Bruyère C, et al. Innovative tools for mechanobiology: unraveling outside-in and inside-out mechanotransduction. Frontiers in Bioengineering and Biotechnology. 2019;7:162. doi: 10.3389/fbioe.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed M, Thurgood P, Gilliam C, et al. Studying the response of aortic endothelial cells under pulsatile flow using a compact microfluidic system. Anal Chem. 2019;91:12077–12084. doi: 10.1021/acs.analchem.9b03247. [DOI] [PubMed] [Google Scholar]
- Moraes C, Likhitpanichkul M, Lam CJ, et al. Microdevice array-based identification of distinct mechanobiological response profiles in layer-specific valve interstitial cells. Integr Biol. 2013;5:673–680. doi: 10.1039/c3ib20254b. [DOI] [PubMed] [Google Scholar]
- Muehleder S, Ovsianikov A, Zipperle J, Redl H, Holnthoner W. Connections matter: channeled hydrogels to improve vascularization. Frontiers in Bioengineering and Biotechnology. 2014;2:52. doi: 10.3389/fbioe.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip. 2013;13:1883–1891. doi: 10.1039/c3lc41332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahavandi S, Baratchi S, Soffe R, et al. Microfluidic platforms for biomarker analysis. Lab Chip. 2014;14:1496–1514. doi: 10.1039/C3LC51124C. [DOI] [PubMed] [Google Scholar]
- Neeves KB, Diamond SL. A membrane-based microfluidic device for controlling the flux of platelet agonists into flowing blood. Lab Chip. 2008;8:701–709. doi: 10.1039/b717824g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeves KB, Illing DAR, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D-HT, Stapleton SC, Yang MT, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci. 2013;110:6712. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Thurgood P, Zhu JY, et al. “Do-it-in-classroom” fabrication of microfluidic systems by replica moulding of pasta structures. Biomicrofluidics. 2018;12:044115. doi: 10.1063/1.5042684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Thurgood P, Arash A, et al. Inertial microfluidics with integrated vortex generators using liquid metal droplets as fugitive ink. Adv Funct Mater. 2019;29:1901998. doi: 10.1002/adfm.201901998. [DOI] [Google Scholar]
- Novak R, Ingram M, Marquez S, et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nature Biomedical Engineering. 2020;4:407–420. doi: 10.1038/s41551-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Ryu H, Tahk D, et al. “Open-top” microfluidic device for in vitro three-dimensional capillary beds. Lab Chip. 2017;17:3405–3414. doi: 10.1039/C7LC00646B. [DOI] [PubMed] [Google Scholar]
- Ong SG, Huber BC, Lee WH, et al. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation. 2015;132:762–771. doi: 10.1161/CIRCULATIONAHA.114.015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong LJY, Ching T, Chong LH, et al. Self-aligning Tetris-Like (TILE) modular microfluidic platform for mimicking multi-organ interactions. Lab Chip. 2019;19:2178–2191. doi: 10.1039/C9LC00160C. [DOI] [PubMed] [Google Scholar]
- Osnabrugge RLJ, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Owens CE, Hart AJ. High-precision modular microfluidics by micromilling of interlocking injection-molded blocks. Lab Chip. 2018;18:890–901. doi: 10.1039/C7LC00951H. [DOI] [PubMed] [Google Scholar]
- Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69:1952–1967. doi: 10.1016/j.jacc.2017.01.064. [DOI] [PubMed] [Google Scholar]
- Parekh DP, Ladd C, Panich L, Moussa K, Dickey MD. 3D printing of liquid metals as fugitive inks for fabrication of 3D microfluidic channels. Lab Chip. 2016;16:1812–1820. doi: 10.1039/C6LC00198J. [DOI] [PubMed] [Google Scholar]
- Park MH, Chhai P & Rhee K (2019) Analysis of flow and wall deformation in a stenotic flexible channel containing a soft core, simulating atherosclerotic arteries. International Journal of Precision Engineering and Manufacturing.
- Polacheck WJ, Kutys ML, Tefft JB, Chen CS. Microfabricated blood vessels for modeling the vascular transport barrier. Nat Protoc. 2019;14:1425–1454. doi: 10.1038/s41596-019-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zheng Y, Hu J, et al. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface. 2014;11:20130852. doi: 10.1098/rsif.2013.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Westein E, Be N, Hagemeyer CE. Shear-dependent platelet aggregation: mechanisms and therapeutic opportunities. Frontiers in Cardiovascular Medicine. 2019;6:141. doi: 10.3389/fcvm.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault C, Dheeman DS, Hochstetter A. Microfluidic devices for drug assays. High Throughput. 2018;7:18. doi: 10.3390/ht7020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J, Zhang YS, Pitrez PR et al (2017) Biomechanical strain exacerbates inflammation on a Progeria-on-a-Chip Model. Small 13 [DOI] [PMC free article] [PubMed]
- Ryu H, Oh S, Lee HJ, et al. Engineering a blood vessel network module for body-on-a-chip applications. Journal of Laboratory Automation. 2015;20:296–301. doi: 10.1177/2211068214562831. [DOI] [PubMed] [Google Scholar]
- Salehi SS, Shamloo A, Hannani SK. Microfluidic technologies to engineer mesenchymal stem cell aggregates—applications and benefits. Biophys Rev. 2020;12:123–133. doi: 10.1007/s12551-020-00613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen AT, Zhang J, Madejski GR et al (2019) Ultrathin dual-scale nano- and microporous membranes for vascular transmigration models. Small 15 [DOI] [PMC free article] [PubMed]
- Santos A, Fernández-Friera L, Villalba M et al. (2015) Cardiovascular imaging: what have we learned from animal models? Frontiers in Pharmacology 6. [DOI] [PMC free article] [PubMed]
- Sato K, Nitta M, Ogawa A. A microfluidic cell stretch device to investigate the effects of stretching stress on artery smooth muscle cell proliferation in pulmonary arterial hypertension. Inventions. 2019;4:1. doi: 10.3390/inventions4010001. [DOI] [Google Scholar]
- Sei YJ, Ahn SI, Virtue T, Kim T, Kim Y. Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Sci Rep. 2017;7:10019. doi: 10.1038/s41598-017-10636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- Seo J, Conegliano D, Farrell M, et al. A microengineered model of RBC transfusion-induced pulmonary vascular injury. Sci Rep. 2017;7:3413. doi: 10.1038/s41598-017-03597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156–1164. doi: 10.1136/heartjnl-2017-311198. [DOI] [PubMed] [Google Scholar]
- Shao J, Wu L, Wu J, et al. Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip. 2009;9:3118–3125. doi: 10.1039/b909312e. [DOI] [PubMed] [Google Scholar]
- Shao Y, Mann JM, Chen W, Fu J. Global architecture of the F-actin cytoskeleton regulates cell shape-dependent endothelial mechanotransduction. Integr Biol. 2014;6:300–311. doi: 10.1039/c3ib40223a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton SE & Kamm RD. In Biomechanics of coronary atherosclerotic plaque vol. 4 (eds Jacques Ohayon, Gérard Finet, & Roderic Ivan Pettigrew) 303-319 (Academic Press, 2020).
- Shimizu A, Goh WH, Itai S, et al. ECM-based microchannel for culturingin vitrovascular tissues with simultaneous perfusion and stretch. Lab Chip. 2020;20:1917–1927. doi: 10.1039/D0LC00254B. [DOI] [PubMed] [Google Scholar]
- Shin Y, Lim S, Kim J, et al. Emulating endothelial dysfunction by implementing an early atherosclerotic microenvironment within a microfluidic chip. Lab Chip. 2019;19:3664–3677. doi: 10.1039/C9LC00352E. [DOI] [PubMed] [Google Scholar]
- Sinha R, Le Gac S, Verdonschot N, et al. Endothelial cell alignment as a result of anisotropic strain and flow induced shear stress combinations. Sci Rep. 2016;6:29510. doi: 10.1038/srep29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniadecki NJ, Anguelouch A, Yang MT, et al. Magnetic microposts as an approach to apply forces to living cells. Proc Natl Acad Sci. 2007;104:14553–14558. doi: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino A, Phan DTT, Datta R, et al. 3D microtumors in vitro supported by perfused vascular networks. Sci Rep. 2016;6:31589. doi: 10.1038/srep31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani S, Shamsi M, Ghazani MA, et al. Translational models of tumor angiogenesis: a nexus of in silico and in vitro models. Biotechnol Adv. 2018;36:880–893. doi: 10.1016/j.biotechadv.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Spicer CD. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem. 2020;11:184–219. doi: 10.1039/C9PY01021A. [DOI] [Google Scholar]
- Stanfield CL (2016) Principles of human physiology. Global Edition.
- Sun T, Shi Q, Liang Q, et al. Fabrication of vascular smooth muscle-like tissues based on self-organization of circumferentially aligned cells in microengineered hydrogels. Lab Chip. 2020;20:3120–3131. doi: 10.1039/D0LC00544D. [DOI] [PubMed] [Google Scholar]
- Swiatlowska P, Sanchez-Alonso JL, Wright PT, Novak P, Gorelik J. Microtubules regulate cardiomyocyte transversal Young’s modulus. Proc Natl Acad Sci. 2020;117:2764–2766. doi: 10.1073/pnas.1917171117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydzik C, Niego B, Dalzell G, et al. Fabrication of complex PDMS microfluidic structures and embedded functional substrates by one-step injection moulding. RSC Adv. 2016;6:87988–87994. doi: 10.1039/C6RA20688C. [DOI] [Google Scholar]
- Szydzik C, Brazilek RJ, Khoshmanesh K, et al. Elastomeric microvalve geometry affects haemocompatibility. Lab Chip. 2018;18:1778–1792. doi: 10.1039/C7LC01320E. [DOI] [PubMed] [Google Scholar]
- Ta HT, Truong NP, Whittaker AK, Davis TP, Peter K. The effects of particle size, shape, density and flow characteristics on particle margination to vascular walls in cardiovascular diseases. Expert Opinion on Drug Delivery. 2018;15:33–45. doi: 10.1080/17425247.2017.1316262. [DOI] [PubMed] [Google Scholar]
- Tan W, Scott D, Belchenko D, Qi HJ, Xiao L. Development and evaluation of microdevices for studying anisotropic biaxial cyclic stretch on cells. Biomed Microdevices. 2008;10:869–882. doi: 10.1007/s10544-008-9201-8. [DOI] [PubMed] [Google Scholar]
- Tay A, Pavesi A, Yazdi SR, Lim CT, Warkiani ME. Advances in microfluidics in combating infectious diseases. Biotechnol Adv. 2016;34:404–421. doi: 10.1016/j.biotechadv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Daniel Ou-Yang H, Lowe-Krentz L, Muzykantov VR, Liu Y. Biomimetic channel modeling local vascular dynamics of pro-inflammatory endothelial changes. Biomicrofluidics. 2016;10:014101–014101. doi: 10.1063/1.4936672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurgood P, Zhu JY, Nguyen N, et al. A self-sufficient pressure pump using latex balloons for microfluidic applications. Lab Chip. 2018;18:2730–2740. doi: 10.1039/C8LC00471D. [DOI] [PubMed] [Google Scholar]
- Thurgood P, Suarez SA, Chen S, et al. Self-sufficient, low-cost microfluidic pumps utilising reinforced balloons. Lab Chip. 2019;19:2885–2896. doi: 10.1039/C9LC00618D. [DOI] [PubMed] [Google Scholar]
- Tovar-Lopez FJ, Rosengarten G, Westein E, et al. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 2010;10:291–302. doi: 10.1039/B916757A. [DOI] [PubMed] [Google Scholar]
- Tovar-Lopez FJ, Rosengarten G, Nasabi M et al. (2013) An investigation on platelet transport during thrombus formation at micro-scale stenosis. PLoS ONE 8. [DOI] [PMC free article] [PubMed]
- Tovar-Lopez F, Thurgood P, Gilliam C, et al. A microfluidic system for studying the effects of disturbed flow on endothelial cells. Frontiers in Bioengineering and Biotechnology. 2019;7:81. doi: 10.3389/fbioe.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang HG, Rashdan NA, Whitelaw CBA, et al. Large animal models of cardiovascular disease. Cell Biochem Funct. 2016;34:113–132. doi: 10.1002/cbf.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvirkun D, Grichine A, Duperray A, Misbah C, Bureau L. Microvasculature on a chip: study of the endothelial surface layer and the flow structure of red blood cells. Sci Rep. 2017;7:45036. doi: 10.1038/srep45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm MW, Odijk M, Frimat J-P, et al. Direct quantification of transendothelial electrical resistance in organs-on-chips. Biosens Bioelectron. 2016;85:924–929. doi: 10.1016/j.bios.2016.06.014. [DOI] [PubMed] [Google Scholar]
- van Dijk CGM, Brandt MM, Poulis N, et al. A new microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix. Lab Chip. 2020;20:1827–1844. doi: 10.1039/D0LC00059K. [DOI] [PubMed] [Google Scholar]
- van Engeland NCA, Pollet AMAO, den Toonder JMJ, et al. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip. 2018;18:1607–1620. doi: 10.1039/C8LC00286J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedula EM, Alonso JL, Arnaout MA, Charest JL. A microfluidic renal proximal tubule with active reabsorptive function. PLoS One. 2017;12:e0184330. doi: 10.1371/journal.pone.0184330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesperini D, Montalvo G, Qu B, Lautenschläger F. Characterization of immune cell migration using microfabrication. Biophys Rev. 2021;13:185–202. doi: 10.1007/s12551-021-00787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter Boron EB (2016) Medical physiology, 3rd, 3rd edn. Elsevier
- Wang YI, Shuler ML. UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems. Lab Chip. 2018;18:2563–2574. doi: 10.1039/C8LC00394G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westein E, Van Der Meer AD, Kuijpers MJE, et al. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A. 2013;110:1357–1362. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JF, Simmons CA. Microfluidic assay for the on-chip electrochemical measurement of cell monolayer permeability. Lab Chip. 2019;19:1060–1070. doi: 10.1039/C8LC01321G. [DOI] [PubMed] [Google Scholar]
- Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng. 1999;1:299–329. doi: 10.1146/annurev.bioeng.1.1.299. [DOI] [PubMed] [Google Scholar]
- Xu S, Piao J, Lee B, Lim C, Shin S. Platelet thrombus formation by upstream activation and downstream adhesion of platelets in a microfluidic system. Biosens Bioelectron. 2020;165:112395. doi: 10.1016/j.bios.2020.112395. [DOI] [PubMed] [Google Scholar]
- Yan S, Li Y, Zhao Q, et al. Liquid metal-based amalgamation-assisted lithography for fabrication of complex channels with diverse structures and configurations. Lab Chip. 2018;18:785–792. doi: 10.1039/C8LC00047F. [DOI] [PubMed] [Google Scholar]
- Yazdani A, Karniadakis GE. Sub-cellular modeling of platelet transport in blood flow through microchannels with constriction. Soft Matter. 2016;12:4339–4351. doi: 10.1039/C6SM00154H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EWK, Watson MWL, Srigunapalan S, Wheeler AR, Simmons CA. Technique for real-time measurements of endothelial permeability in a microfluidic membrane chip using laser-induced fluorescence detection. Anal Chem. 2010;82:808–816. doi: 10.1021/ac901560w. [DOI] [PubMed] [Google Scholar]
- Yun S-H, Sim E-H, Goh R-Y, Park J-I, Han J-Y. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016;2016:9060143. doi: 10.1155/2016/9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:13. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.RES.53.4.502. [DOI] [PubMed] [Google Scholar]
- Zervantonakis IK, Hughes-Alford SK, Charest JL, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci. 2012;109:13515. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu Y, Ozbolat IT. Direct bioprinting of vessel-like tubular microfluidic channels. Journal of Nanotechnology in Engineering and Medicine. 2013;4:0210011–0210017. doi: 10.1115/1.4024398. [DOI] [Google Scholar]
- Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bishawi M, Zhang G, et al. Modeling early stage atherosclerosis in a primary human vascular microphysiological system. Nat Commun. 2020;11:5426. doi: 10.1038/s41467-020-19197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Jiang B, Wang D, et al. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip. 2012;12:3441–3450. doi: 10.1039/c2lc40173h. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chen J, López JA. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat Commun. 2015;6:7858. doi: 10.1038/ncomms8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang R, Jiang B, et al. An early-stage atherosclerosis research model based on microfluidics. Small. 2016;12:2022–2034. doi: 10.1002/smll.201503241. [DOI] [PubMed] [Google Scholar]
- Zhou J, Niklason LE. Microfluidic artificial “vessels” for dynamic mechanical stimulation of mesenchymal stem cells. Integr Biol. 2012;4:1487–1497. doi: 10.1039/c2ib00171c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Review of Medical Devices. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Suarez SA, Thurgood P, et al. Reconfigurable, self-sufficient convective heat exchanger for temperature control of microfluidic systems. Anal Chem. 2019;91:15784–15790. doi: 10.1021/acs.analchem.9b04066. [DOI] [PubMed] [Google Scholar]